Citation: Nedelchev S, Elshishka M, Lazarova S, Radoslavov G, Hristov P, Peneva V (2014) Calcaridorylaimus castaneae sp. n. (Nematoda, Dorylaimidae) from Bulgaria with an identification key to the species of the genus. ZooKeys 410: 41–61. doi: 10.3897/zookeys.410.6955
An unknown species belonging to the genusCalcaridorylaimus Andrássy, 1986 was collected from the litter of broadleaf forests dominated by Castanea sativa Mill. and mixed with Quercus daleshampii Ten. and Fagus sylvatica L. on Belasitsa Mountain, south-western Bulgaria. Calcaridorylaimus castaneae sp. n. is characterised by its long body (1.4–2.1 mm), lip region practically not offset, vulva transverse, short odontostyle (14.5–16 μm) and tail (75.5–110.5 μm, c=14.7–23.6; c’=2.9–4.4) in females and 38–46 μm long spicules with small spur before their distant end in males. It is most similar to C. andrassyi Ahmad & Shaheen, 2004, but differs in having transverse vs pore-like vulva and shorter spicules (38–46 μm vs 52–57 μm). An identification key to the species of the genus Calcaridorylaimus is proposed. Phylogenetic analyses were performed on 18S and D2-D3 expansion domains of 28S rRNA genes by Neighbor-Joining, Maximum Likelihood and Bayesian Inference methods. The phylograms inferred from 18S sequences showed closest relationships of the new species with some species belonging to the genus Mesodorylaimus. However, insufficient molecular data for members of both genera do not allow the phylogenetic relationships of Calcaridorylaimus and the new species described herein to be elucidated.
Taxonomy, morphology, 18S and D2-D3 rRNA genes, compendium
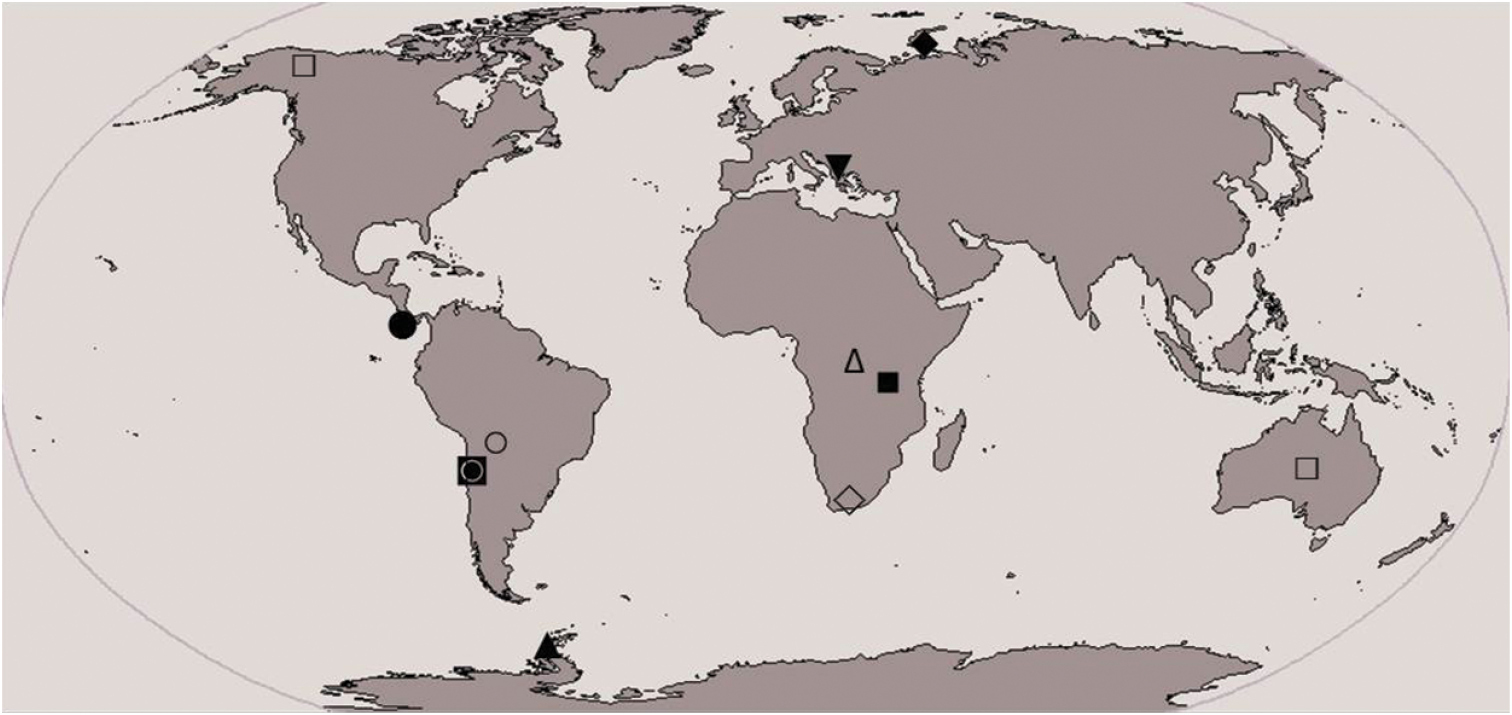
During an ecological study of chestnut forests on Belasitsa Mountain (2003-2005) an undescribed species belonging to the genus Calcaridorylaimus Andrássy, 1986 was recovered. The genus Calcaridorylaimus is represented by nine species worldwide: Calcaridorylaimus calcarifer Andrássy, 1986, Calcaridorylaimus promissus Andrássy, 1986, Calcaridorylaimus ruwenzorii (De Coninck, 1935) Andrássy, 1986, Calcaridorylaimus signatus (Loof, 1975) Andrássy, 1986, Calcaridorylaimus simillimus Andrássy, 1986, Calcaridorylaimus sirgeli Heyns & Meyer, 1995, Calcaridorylaimus arcticus Gagarin, 1997, Calcaridorylaimus andrassyi Ahmad & Shaheen, 2004 and Calcaridorylaimus beatus Andrássy, 2011. The genus is distributed mainly in the southern hemisphere: three species occur in Africa, two in South America and one each in Antarctic, Central America, and Europe, and Calcaridorylaimus promissus was recorded from Australia and Alaska, North America (
Distribution of Calcaridorylaimus spp. (■ Calcaridorylaimus calcarifer, ● Calcaridorylaimus andrassyi, ♦ Calcaridorylaimus arcticus, □ Calcaridorylaimus promissus, ▲ Calcaridorylaimus signatus, ○ Calcaridorylaimus simillimus, ♢ Calcaridorylaimus sirgeli, △ Calcaridorylaimus ruwenzorii, ◙ Calcaridorylaimus beatus, ▼ Calcaridorylaimus castaneae sp. n.).
The litter samples were collected in 2003 by the last author (VP) from three sites on Belasitsa Mountain representing different types of broadleaf forests dominated by Castanea sativa Mill. mixed with Quercus daleshampii Ten. and Fagus sylvatica L. (Forest Management Plan database, sub-compartments 104g, 140b and 146a). Subsequently, on 17.10.2012 new litter samples were collected by Dr Michaela Ilieva from one of these sites, sub-compartment 140b, in order to obtain fresh material for molecular studies. Nematodes were recovered from the litter using the Baermann funnel method. They were killed by heat (65 °C), fixed in TAF (Triethanolamine-formalin,
Genomic DNA was extracted from two female and two male worms using a standard nematode digestion protocol (
The sequences of the new species have been deposited in GenBank with the accession numbers KF717497 and KF717498 for the 18S and the D2-D3 rRNA genes, respectively. A BLAST (Basic Local Alignment Search Tool) search at NCBI (National Center for Biotechnology Information) was performed using the obtained sequences as queries to confirm their nematode origin and to identify the most closely related nematode sequences. The sequences revealing a similarity up to 97% and 85% with nematodes from various Dorylaimida families were included in the phylogenetic analyses of 18S and D2-D3 regions, respectively (
http://zoobank.org/9BF1D302-1986-47C5-B0D8-2F5DC75BD6D2
http://species-id.net/wiki/Calcaridorylaimus_castaneae
Figs 2–6See Table 1.
Morphometrics of Calcaridorylaimus castaneae sp. n., females and males, from Belasitsa Mountain. All measurements, unless indicated otherwise, in μm and in the form: mean ± SD (range).
| Habitat | Mixed broadleaf forest | Chestnut forest | |||||
|---|---|---|---|---|---|---|---|
| Locality and year of collection | 140b 2003 | 140b 2012 | 104g 2003 | ||||
| Characters | Holotype | Paratypes | |||||
| Females | Males | Females | Males | Females | Males | ||
| n | 40 | 20 | 10 | 10 | 18 | 12 | |
| L (mm) | 1.7 | 1.7±0.11 (1.5–2.0) |
1.6±0.08 (1.4–1.8) |
1.6±0.2 (1.4–1.8) |
1.6±0.1 (1.4–1.8) |
1.6±0.15 (1.4–2.1) |
1.5±0.06 (1.4–1.6) |
| a | 35.7 | 36.6±2.4 (32.5–42.7) |
37.9±2.3 (33.3–42.2) |
35.7±2.1 (32.2–38.7) |
40.0±2.3 (37.0–44.3) |
38.4±1.4 (35.7–40.6) |
38.1±2.0 (34.0–41.6) |
| b | 4.9 | 5.0±0.3 (4.5–5.7) |
4.7±0.2 (4.4–5.1) |
5.1±0.2 (4.8–5.6) |
5.1±0.4 (4.7–6.0) |
4.9±0.3 (4.4–5.5) |
4.6±0.2 (4.3–5.0) |
| c | 19.4 | 18.8±0.4 (16.0–22.0) |
74.4±7.4 (63.8–89.7) |
18.3±1.4 (16.8–21.0) |
66.7±6.9 (53.7–73.2) |
18.5±2.3 (14.7–23.6) |
72.2±7.0 (61.4–83.5) |
| c‘ | 3.9 | 3.6±0.4 (2.9–4.4) |
0.76±0.06 (0.67–0.84) |
3.5±0.4 (3.0–4.0) |
0.84±0.06 (0.77–0.95) |
3.6±0.3 (2.9–4.2) |
0.76±0.06 (0.68–0.87) |
| V/T % | 50.7 | 51.6±1.7 (47.9–54.8) |
66.3±3.2 (60–72) |
51.2±1.6 (49–54) |
59.5±2.5 (56–62.5) |
51.0±2.3 (46.5–54.5) |
62.8±4.3 (54–70) |
| G1% | 17 | 18.4±1.7 (14-22.6) |
- | 19.1±1.0 (18.1–20.7) |
- | 17.8±2.1 (13.8–21.7) |
- |
| G2% | 16 | 18.3±2.3 (14.1–24.4) |
- | 18.2±1.0 (16.6-19.1) |
- | 18.7±3 (14.5-26.5) |
- |
| Odontostyle | 14.5 | 15.0±0.4 (14.5–16) |
15.1±0.25 (14.5–16) |
15.1±0.2 (14.9–15.4) |
14.9±0.4 (14–15) |
15.0±0.2 (14.5–15) |
15±0.3 (14.5–16) |
| Odontophore | 26 | 24.8±2.2 (20–29) |
22.9±1.5 (21–27) |
21.6±2.1 (20–26) |
23.7±4.0 (20–28) |
27.2±1.6 (24–30) |
25.6±1.7 (23–28) |
| Spear | 41 | 39.8±2.2 (35–43) |
37.7±1.2 (35.5–41.5) |
36.8±2.0 (35–41) |
38.6±4.2 (34–43) |
41±1.4 (38–44) |
40.7±1.6 (38–43) |
| Neck length | 346 | 342±13.2 (310–366) |
338.6±8.8 (318–352) |
319.3±27.3 (272.5–352) |
315±31.4 (242–340) |
335.8±17 (306–376) |
336±11.9 (310–352) |
| Cardia length | 19 | 19±1.8 (16–24) |
19±2.1 (16–24) |
33.3±7.0 (22-41) |
29.6±5.8 (24–39) |
18.5±2.4 (15–25) |
19.9±3.8 (14.5–26) |
| Body width at: lip region |
12 | 12.3±0.3 (12–12.5) |
12.3±0.3 (12–12.5) |
12.4±0.5 (12–13) |
12.5±0.5 (12–13) |
12.3±0.4 (12–13) |
12.5±0.19 (12–13) |
| mid-body | 48 | 46.8±4.7 (39.5–55) |
42.8±2.3 (37–47) |
45.2±2.2 (41–48) |
40.1±2.7 (35–44.5) |
42±4.7 (37–58) |
40.7±2.2 (37–45) |
| anus | 22 | 24.9±1.8 (22–30) |
28.8±0.9 (27–30) |
25.7±1.1 (24.5–28.5) |
28.7±1.7 (25–30.5) |
24.8±1.3 (23–27) |
28.5±1.0 (27–30) |
| Lateral chord | 12 | 12.7±1.8 (10.5–18) |
10.1±1.3 (8–12.5) |
12.8±1.1 (11–15) |
10.4±1.4 (9-13) |
12.2±1.2 (10.5–16) |
10±0.9 (8.5–12) |
| Prerectum | 89 | 89.4±11 (68–117) |
184±17.2 (132–207) |
89.5±19.1 (64–104.5) |
155.5±24.6 (130-200) |
76.6±9.3 (60–96) |
165.4±10 (145–176) |
| Rectum | 41 | 37±2.4 (30–41) |
41±2.1 (37.5–44) |
34.5±4.2 (30–40.5) |
40.3±3.3 (34-43) |
39.6±1.4 (37–41) |
43.8±1.5 (41–46) |
| Tail | 88 | 90.9±7.1 (76–110) |
22.1±1.8 (19–25) |
88.7±8.5 (75.5–103) |
24.3±2.6 (20–28) |
90.1±9 (78–110.5) |
21.6±2.3 (18–26) |
| Spicules | - | - | 43±1 (41–45) |
- | 43.6±2.9 (38–46) |
- | 43.5±2 (39.5–45) |
| T-distance anterior end of anterior testis-cloaca (mm) | - | - | 1.1±0.1 (0.9–1.3) |
- | 0.96±0.11 (0.86-1.11) |
- | 1.0±0.1 (0.8–1.15) |
Female. Body slender, more or less curved ventrally. Cuticle ca 2 μm at anterior part of neck, 3 μm thick at midbody, 4.5–5.5 μm at postanal region; outer layer with fine transverse striae. Lateral chord ca 1/4 of body width. Three dorsal, two ventral and three lateral pores are observed in the spear area. Lip region practically not offset, lip region width ca 20% of its height. Lips partly fused, labial papilae slightly protruding. Body at proximal end of pharynx 3–4 times the width of the lip region diameter. Amphidial aperture 5–6 μm wide or about half the lip region width. Odontostyle 1.2–1.3 times the lip region diameter, aperture occupying 35–40% of its length. Odontophore simple 1.3–1.7 times odontostyle length. Guiding ring at 8.5–9 μm from anterior end. Nerve ring surrounding the pharynx at 36–39% of neck length from head end. Hemizonid and conspicuous excretory pore observed in the nerve ring region. Pharyngeal characters (five females and five males): pharynx beginning to widen at 56–60% and attaining its full width at 61–64% of neck length from anterior end. DO (for terminology see
| DO=60–64 | S1N1=78-81 | S2N=91–92 | K=73–84 |
| DN=62–66 | S1N2=82–85 | S2O=93–95 | K’=78–85 |
| DO–DN=2.2–3.8 | S1N1–S1N2=2.9–4.8. |
Cardia conoid, variable in length, microvilli visible only here. Genital system didelphic-amphidelphic, ovaries reflexed, reaching rarely the vulva level. Oviducts and ovaries very long compared to uteri. Uteri not differentiated, short, anterior 96-125 μm and posterior 95-135 μm long, in one female with no sperm inside the genital system uteri shorter, 83 μm each, sphincter between uterus and pars dilatata oviductus well developed. Sperm present in uteri and pars dilatata oviductus. Synchronous uterine eggs 1–3, measuring 72–78 × 32–44 μm. Vulva transverse. Vagina extending to 60–70% inwards: pars proximalis 9–13 μm wide, 14.5–19.5 μm long, pars refringens more or less rounded trapezoid, 10–12.5 μm wide, 4–6 μm deep; pars distalis approx. 1.5 μm (terminology following
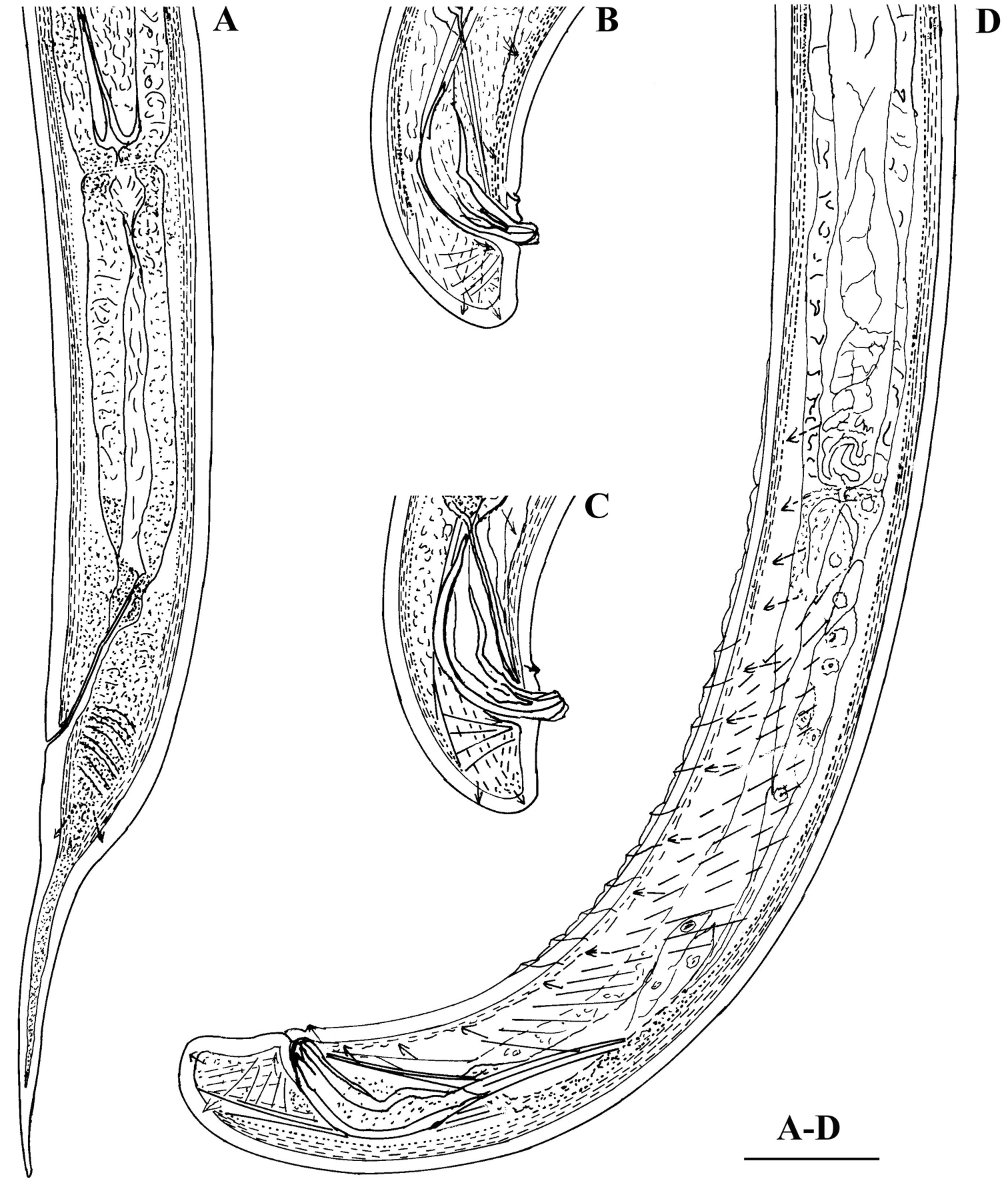
Male. General morphology similar to that of female, body curved ventrally in J-shape when fixed. Genital system diorchic, testes opposed, well developed. Spicules dorylaimoid, with double contour on dorsal arm, 1.3–1.6 times the corresponding body diameter long; ventral arm smaller than dorsal. A spur present dorsally before the distal tip, distinctly visible in extruded spicules. Lateral guiding pieces 9-11 μm long or ca. 23 % spicule length. In addition to adcloacal pair seven to twelve (mostly nine or ten), regularly spaced ventromedian supplements present (9 supplements in 8 specimens; 10 suppl. – in 7, and 7, 8 and 12 each in one specimen). Prerectum 4.5–7.5 times the corresponding body diameter long, extending 0.7–1.8 body widths anterior to the supplement series. Tail dorsally conoid and broadly rounded. One subdorsal and one subterminal pair of caudal pores.
Calcaridorylaimus castaneae sp. n. differs from all species in the genus by a combination of the following characters: long body (1.4–2.1 mm), lip region practically not offset, short odontostyle (14.5–16 μm in females and 14-16 μm in males) and short female tail (75.5–110.5 μm, c=14.7–23.6; c’=2.9–4.4); vulva transverse, 38–46 μm long spicules with spur before its distal end. The new species is most similar to Calcaridorylaimus andrassyi from which it can be differentiated by having transverse vulva vs pore-like and shorter spicules (38–46 μm vs 52–57 μm). Further, Calcaridorylaimus castaneae differs from Calcaridorylaimus ruwenzorii by having shorter odontostyle (14.5-16 μm vs 19.5-25 μm), and tail (75.5-110.5 μm vs 160 μm, higher c (c=14.7-23.6 vs c=10) and lower c’ (c’=2.9–4.4 vs 7) values in females. It can be differentiated from Calcaridorylaimus arcticus, Calcaridorylaimus beatus, Calcaridorylaimus calcarifer and Calcaridorylaimus signatus by having different vulva shape (transverse vs longitudinal) and shorter spicules (38–46 μm vs 57–67 μm; 48–55 μm; 52–54 μm and 72 μm). From Calcaridorylaimus sirgeli it differs by having transverse vulva vs pore-like, higher c (c=14.7–23.6 vs c=9.7–11.3) and lower c’ (c’=2.9–4.4 vs c’=5.3–6.7) values. Finally, Calcaridorylaimus castaneae differs from Calcaridorylaimus promissus and Calcaridorylaimus simillimus by having longer odontostyle (14.5–16 μm vs 13 μm and 11 μm) and shorter tail (75.5–110.5 μm vs 158–178 μm and 175 μm).
Presence of the conspicuous excretory pore observed in Calcaridorylaimus castaneae is unusual for members of Dorylaimida, however, similar structure has been mentioned only for two Longidorus species, namely Longidorus macrosoma Hooper, 1961 and Longidorus carniolensis Širca et al., 2011 (
Belasitsa Mountain, south-western Bulgaria, litter from old broadleaf forest (100-140 years) dominated by Castanea sativa, mixed with Quercus daleshampii and Fagus sylvatica. Site is located in the vicinity of Belasitsa hut, 41°22'12"N, 23°11'12"E (sub-compartment 140b). Second locality: young sweet chestnut forest (30-40 years old) near Belasitsa village (sub-compartment 104g).
Holotype and 80 paratype females and 47 males deposited in the nematode collection of the Institute of Biodiversity and Ecosystem Research, Sofia, Bulgaria. Other paratypes deposited as follows: four females and two males in the Nematode Collection of the Foodland Environment Research Agency, Sand Hutton, UK (former Rothamsted Nematode Collection); three females and three males in the USDA Nematode Collection, Beltsville, Maryland, USA; two females and two males in the Riverside Nematode Collection, University of California, Riverside, USA; four females, and four males in the Wageningen Nematode Collection (WANECO), Wageningen, the Netherlands; four females and three males in the Nematode Collection of the Zoology Museum of the Ghent University, Belgium.
The scientific name is derived from the generic name of dominant tree species, the sweet chestnut tree (Castanea) in the forest where this nematode was found.
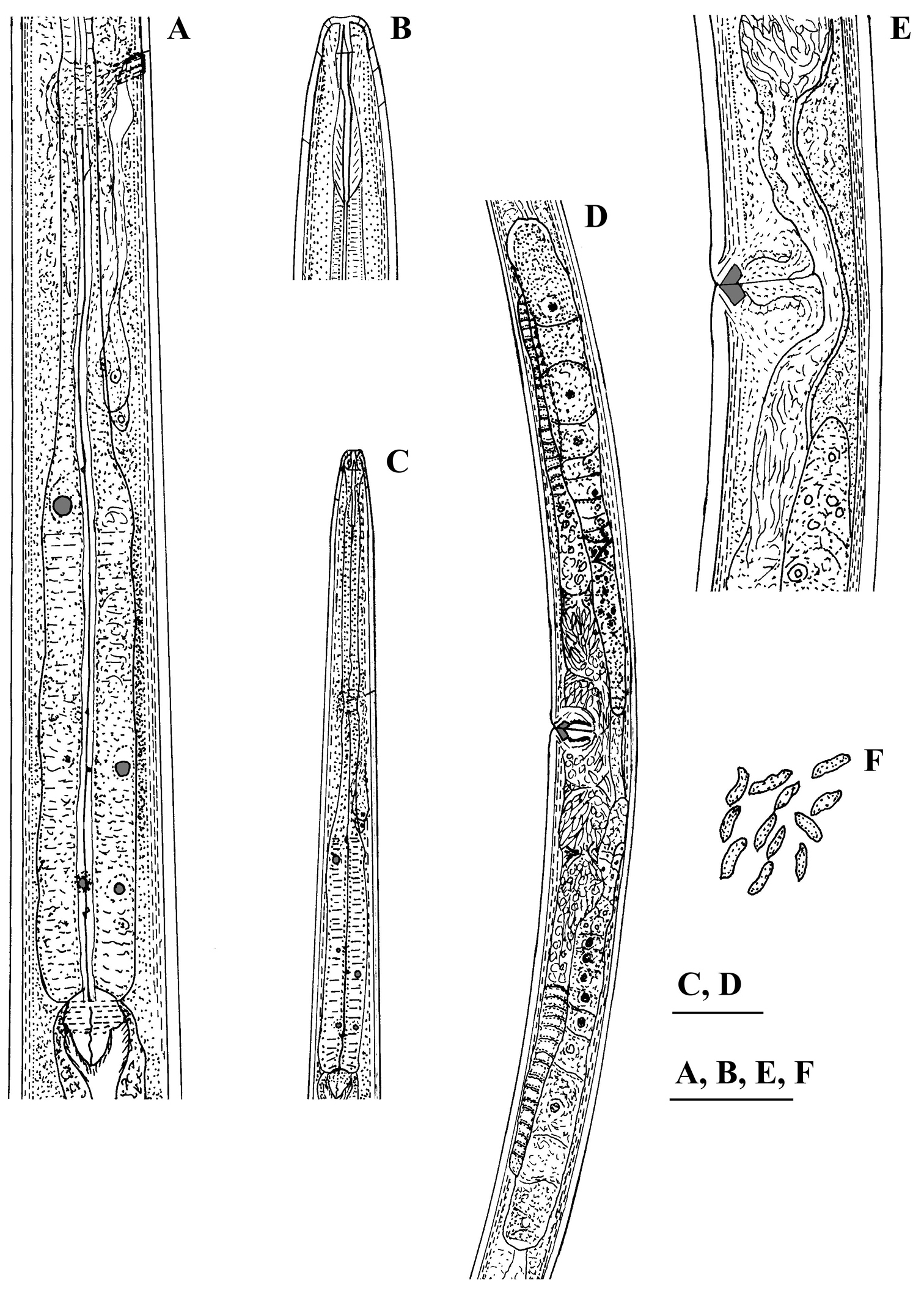
Calcaridorylaimus castaneae sp. n. Female: A Pharyngeal gland nuclei B Anterior region C Pharyngeal region D Genital system E Vulval region F Sperm cells in uterus. Scale bars: A, B, E, F – 30 μm; C, D – 50 μm.
Calcaridorylaimus castaneae sp. n. Female: A Posterior region. Male: B, C Extruded spicules with supplements D Posterior region. Scale bar: A–D – 30 μm.
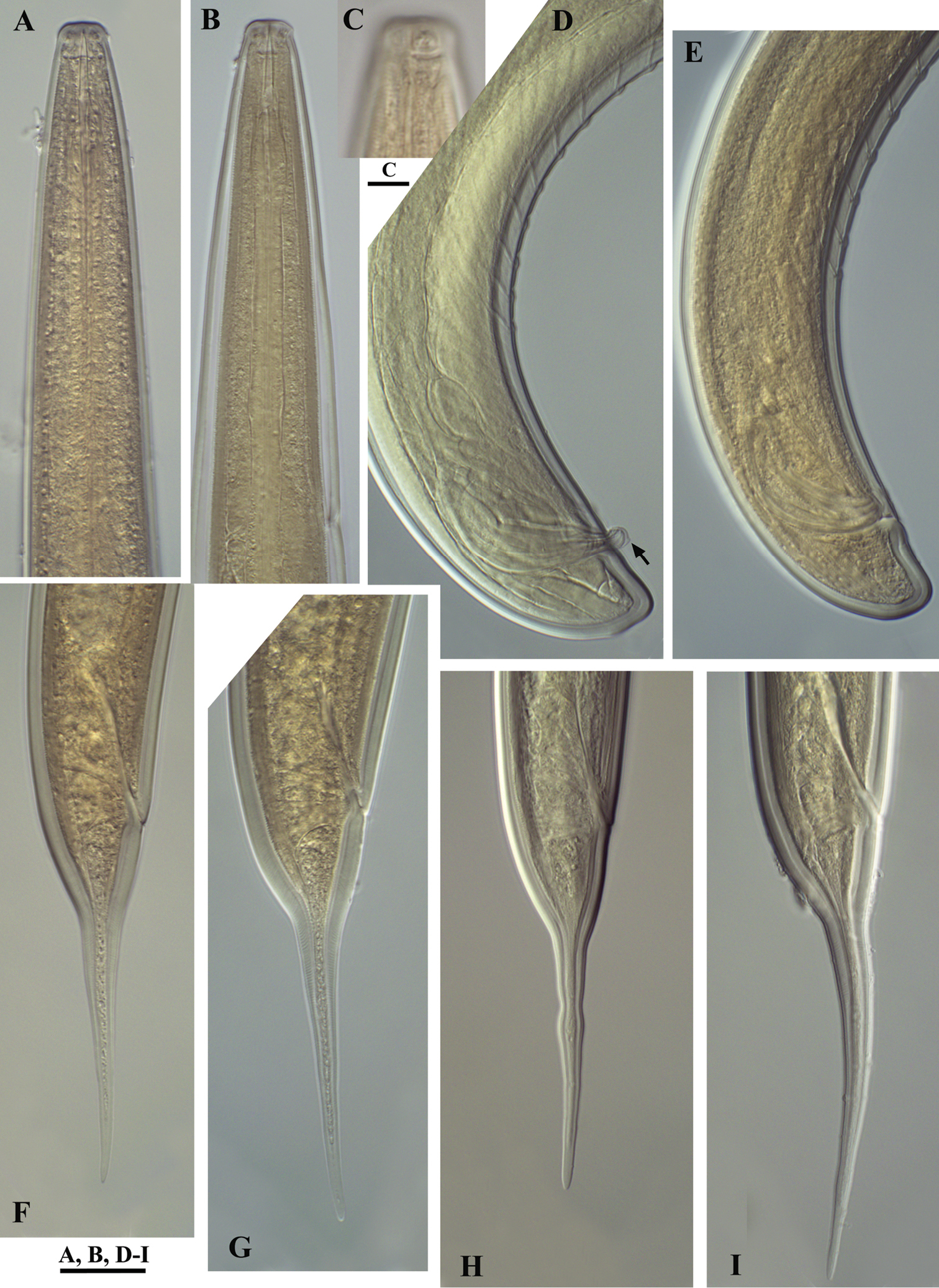
Calcaridorylaimus castaneae sp. n. Female: A Anterior end C Amphid F–I Tail shapes Male B Anterior end D Posterior end with extruded spicules, arrow indicating the spur E Posterior end. Scale bars: A, B, D–I – 20 μm; C – 6 μm.
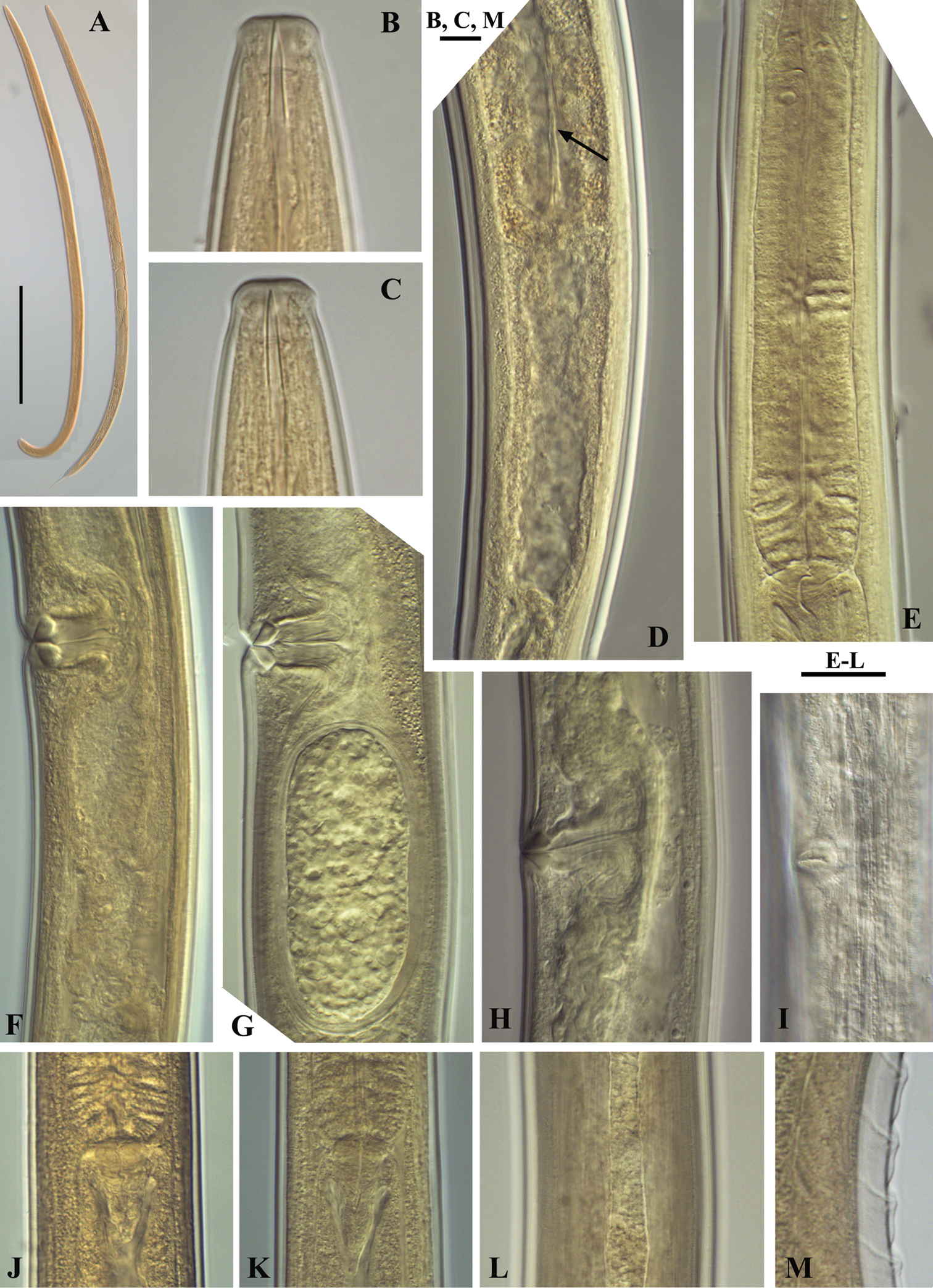
Calcaridorylaimus castaneae sp. n. Female: A Entire body B Lip region D Prerectum, arrow pointing tongue-like valve E Pharyngeal bulb F Vulval region with posterior uterus G Vulval region with egg in posterior uterus H, I Vulval region J Cardia L Lateral field. Male: A Entire body C Lip region K Cardia M Supplements. Scale bars: A – 200 μm; B, C, M – 6 μm; E–L – 20 μm.
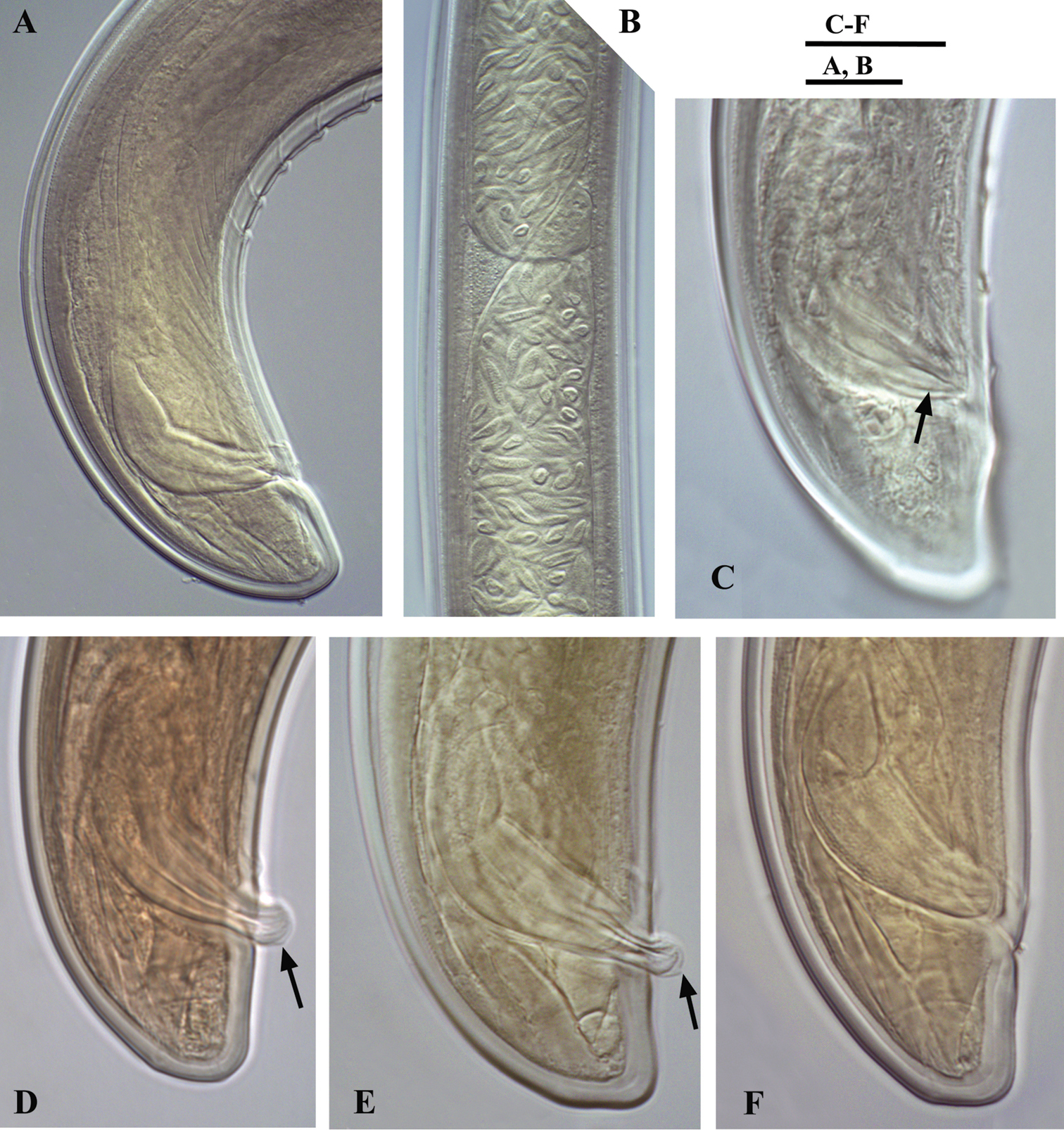
Calcaridorylaimus castaneae sp. n. Male: A Posterior end B Sperm cells in testis C–F Spicular region C Lateral piece of spicules D, E Extruded spicules, arrows pointing the spur F Spicules in the body. Scale bars: A, B – 20 μm; C–F – 18 μm.
| 1 | Odontostyle 19.5–25 µm long | Calcaridorylaimus ruwenzorii |
| – | Odontostyle shorter, ≤17 µm | 2 |
| 2 | c’=11 | Calcaridorylaimus simillimus |
| – | c’<9 | 3 |
| 3 | c’=2.2–2.4 | Calcaridorylaimus beatus |
| – | c’≥ 2.9 | 4 |
| 4 | c≤11 | 5 |
| – | c>11 | 7 |
| 5 | V=45–47, supplements 10–13 | Calcaridorylaimus promissus |
| – | V>49, supplements 6–9 | 6 |
| 6 | Vulva longitudinal, spicules 52–54 µm | Calcaridorylaimus calcarifer |
| – | Vulva pore-like, spicules 39–45 µm | Calcaridorylaimus sirgeli |
| 7 | Lip region 14–17 µm wide, vulva longitudinal | 8 |
| – | Lip region 11–13 µm wide, vulva not longitudinal | 9 |
| 8 | Tail 120–171 µm long, L=1.6–2.27, spicules 57–67 | Calcaridorylaimus arcticus |
| – | Tail 100 µm long, L=1.3–1.7, spicules 72 | Calcaridorylaimus signatus |
| 9 | Vulva pore-like, spicules 52–57 | Calcaridorylaimus andrassyi |
| – | Vulva transverse, spicules 38–46 | Calcaridorylaimus castaneae |
Main morphological and morphometrical data of Calcaridorylaimus species, habitat type and distribution.
| Species | Body length (mm) | a | c | c’ | V% | Odontostyle | Lip region width | Tail | Vulva shape | Spicule length | Supple-ments | Habitat | Distribution | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcaridorylaimus andrassyi ♀ ♂ |
1.8 (1.7–1.9) 1.5 (1.5–1.6) |
44 (42–46) 39 (34–45) |
15 (14–16) 78 (76–81) |
4.4 (4.1–4.7) 0.66 (0.6–0.7) |
49 (48–52) |
15 (15–16) 15 |
12 (11.5–12) 12 (11.5–12.5) |
113 (105–120) 20 (18.5–21.5) |
P | 54 (52–57) |
10 |
forest | Costa Rica | |
| Calcaridorylaimus arcticus | 1.6–2.3 1.84–2.24 |
24–34 24–34 |
13–16 53–75 |
3.5–5.8 0.6–0.7 |
49–53.4 | 14–17 14–17 |
15–17 15–17 |
139 (120–171) 36 (29–48) |
L | 63 (57–67) |
10–12 |
aquatic, fresh-water | Russian Arctic | |
| Calcaridorylaimus beatus | 1.28–1.30 1.36–1.48 |
24–29 28–33 |
19–23 57–72 |
2.2–2.4 0.6–0.8 |
51–52 | 15–16 |
14–15 | 60–68 | L | 48–55 | 13–14 |
Moist grassy soil, 4500 m asl | Chile | |
| Calcaridorylaimus calcarifer | 1.18–1.30 0.95–1.07 |
30–32 26–28 |
7.8–10.3 45–46 |
6–8 0.7–0.8 |
49–52 | 13–14 | 10–12 | 115–167 21–23 |
L | 52–54 |
8–9 |
rain forest soil | Republic of Congo | |
| Calcaridorylaimus castaneae sp. n. | 1.4–2.1 1.4–1.8 |
32.2–42.7 33.3–44.3 |
16.0–23.6 53.7–89.7 |
2.9–4.4 0.7–0.95 |
46.5–54.8 | 14.5–16 14–16 |
12–13 12–13 |
75.5–110.5 18–28 |
T | 38–46 |
7–12 |
broadleaf forest, litter | Bulgaria | Present study |
| Calcaridorylaimus promissus | 1.28–1.37 1.02–1.10 |
36–38 28–30 |
7.7–8.4 47–57 |
8.4–9.0 0.8–0.9 |
45–47 |
13 |
10–11 - |
158–178 18–22 |
T | 50–53 |
10–13 |
wet moss from a rock, forest | Australia | |
| **Calcaridorylaimus promissus | 1.6–1.65 1.55 |
34–37 35 |
9–11 90 |
7–8 ? |
50–51 | 14–15 | ?- | 150–160 ? |
? | 50 |
9–11 |
moss from swampy soil | Alaska | |
| Calcaridorylaimus ruwenzorii | 1.6 1.3–1.5 |
32 35–40 |
10 44–74 |
7 | 47 | 19.5–25 | 12–14 | 160 | ? | 44 |
7–8 |
Republic of Congo | ||
| Calcaridorylaimus signatus | 1.3–1.7 1.7 |
25–33 29 |
12.0–18.0 61 |
2.9–4.2 0.6* |
49–56 - |
16–18 17 |
14* - |
100* 24* |
L | 72 |
12 |
wet moss | Antarctic | |
| Calcaridorylaimus simillimus | 1.3 1.14 |
43 33 |
7.4 90 |
11 0.7 |
50 | 11.5 | 9.5–10 | 175 12 |
T | 45 |
11 |
Stipa sp., near lake Titikaka | Bolivia | |
| Calcaridorylaimus sirgeli | 1.34 (1.1–1.5) 1.36 (1.2–1.5) |
33.2 (30–36) 37.7 (35–38) |
10.6 (9.7–11.3) 77 (63–85) |
6.1 (5.3–6.7) 0.7 (0.6-.8) |
53.4 (52–56) |
13.6 (12–15) 13.1 (12.5–14) |
11.5 (10–12.5) 11.3 (10–12.5) |
127 (105–148) 17.9 (15–22) |
P | 42.4 (39–45) |
6–9 |
moist soil under fynbos and mosses | South Africa |
Shape of vulva: L – longitudinal; P – pore like; T – transverse; when average values are present ranges in parentheses; * from the drawing; ** Calcaridorylaimus promissus from Alaska (
The sequences of D2-D3 expansion domains of 28S and 18S ribosomal DNA from two female and two male nematodes have been processed. No inter-individual variability within both domains have been observed, thus only two consensus sequences for each of the genes (787 and 1699 bp long) have been submitted to GenBank (accession numbers KF717498 and KF717497). A BLAST search for D2-D3 region showed highest similarity (88%) to the sequence of Labronema vulvapapillatum clone 2 (AY592997,
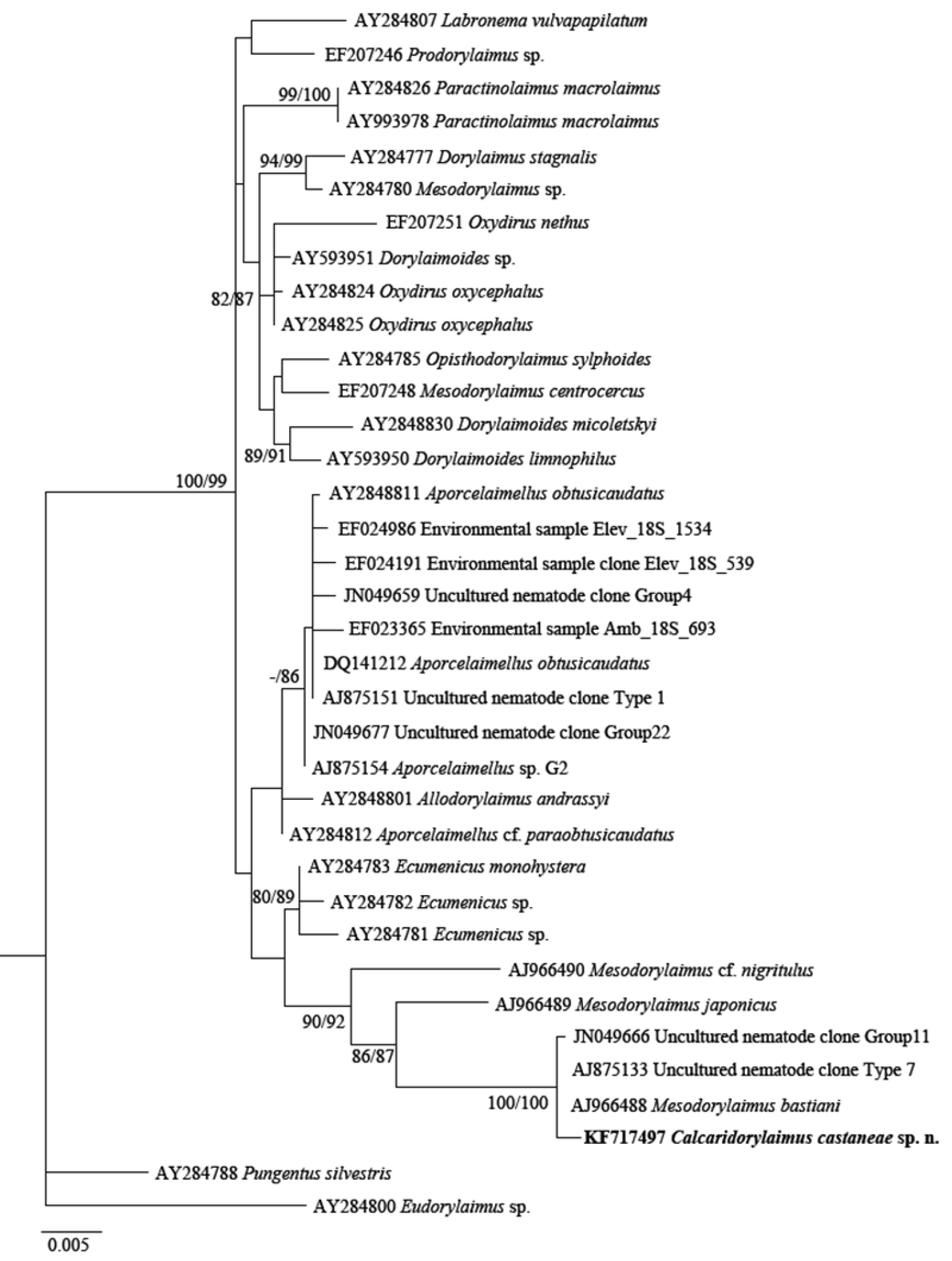
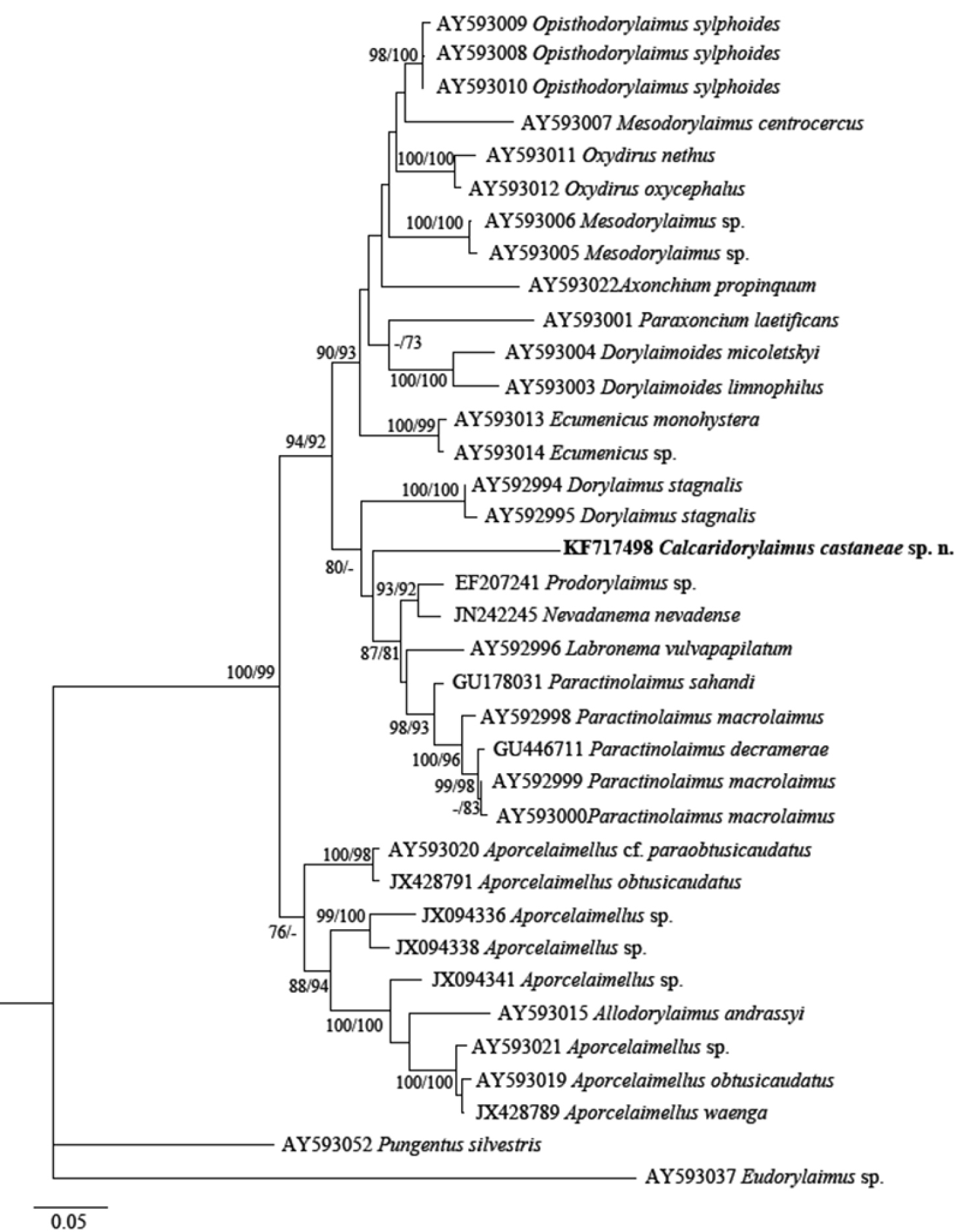
The phylogenetic analyses based on 18S rDNA and D2-D3 of 28S rDNA sequences from various dorylaimid species with the highest matches of the BLAST search (up to 97% and 85%, respectively) were aligned along with our sequence. The phylograms obtained by NJ, ML and BI methods showed similar topology and differed only in the positions of poorly supported clades. The BI trees (Figs 7 and 8) with posterior probabilities higher than 0.8 and NJ-ML trees with bootstrap values above 70% are presented (Figs 9 and 10). The new species has clustered in a well-supported group with several Mesodorylaimus spp. (Mesodorylaimus bastiani (Bütschli, 1873), Mesodorylaimus japonicus (Cobb in Thorne and Swanger, 1936) and Mesodorylaimus cf. nigritulus (Schneider, 1937)) and two unidentified nematodes from environmental samples in the 18S rDNA phylogenetic reconstructions (Figs 7 and 9); and to more distantly related species from various dorylaimid genera and families (Prodorylaimus, Labronema, Nevadanema, Paractinolaimus) in the partial D2-D3 LSU reconstructions (Figs 8 and 10).
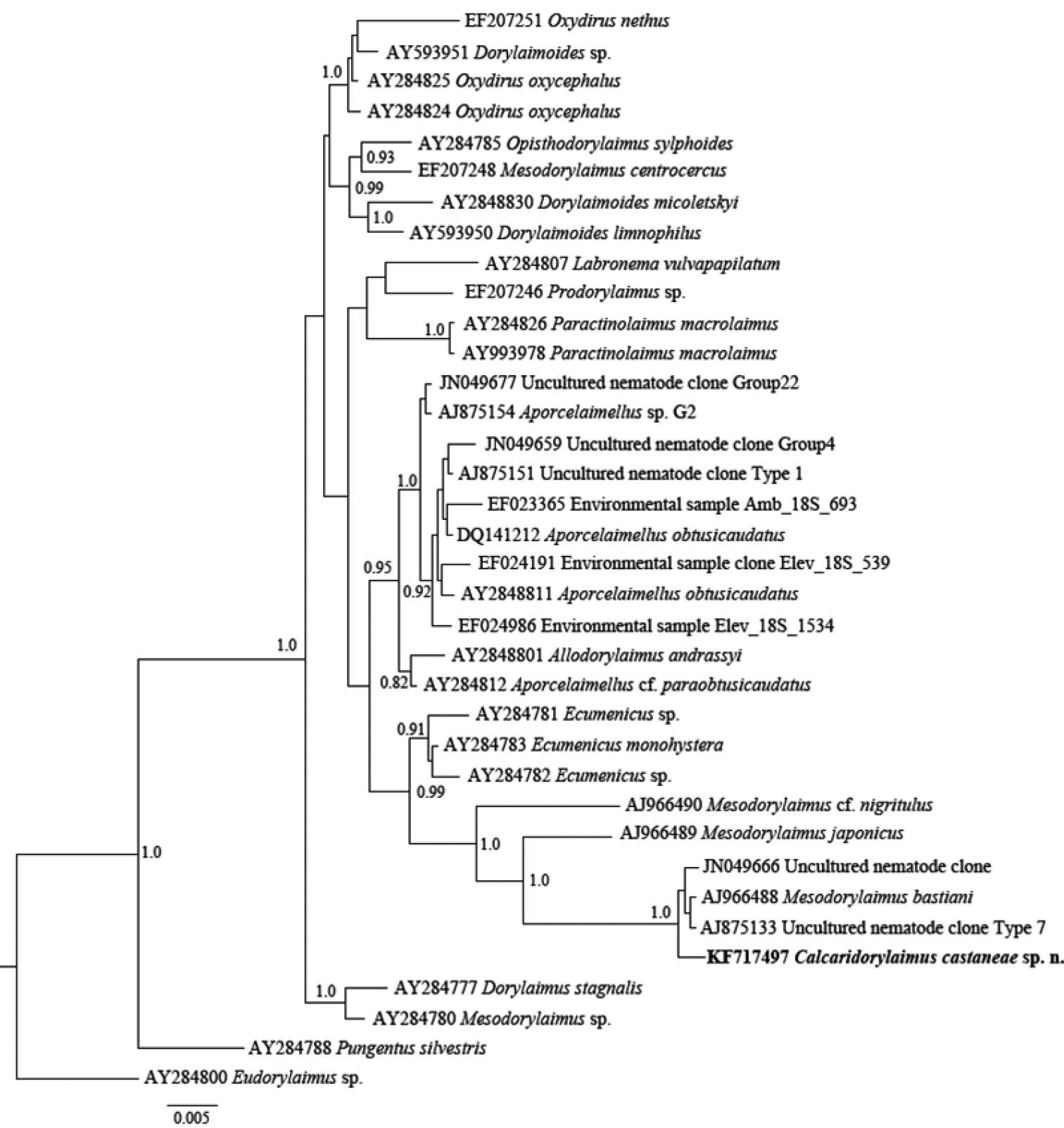
Phylogenetic relationships of Calcaridorylaimus castaneae sp. n. and its closest species for 18S rRNA gene. Bayesian Inference strict consensus tree acquired under GTR+G model. Posterior probabilities higher than 0.8 are presented.
Phylogenetic relationships of Calcaridorylaimus castaneae sp. n. and its closest species for D2-D3 expansion segments of the 28S rRNA gene. Bayesian Inference strict consensus tree acquired under GTR+G model. Posterior probabilities higher than 0.8 are presented.
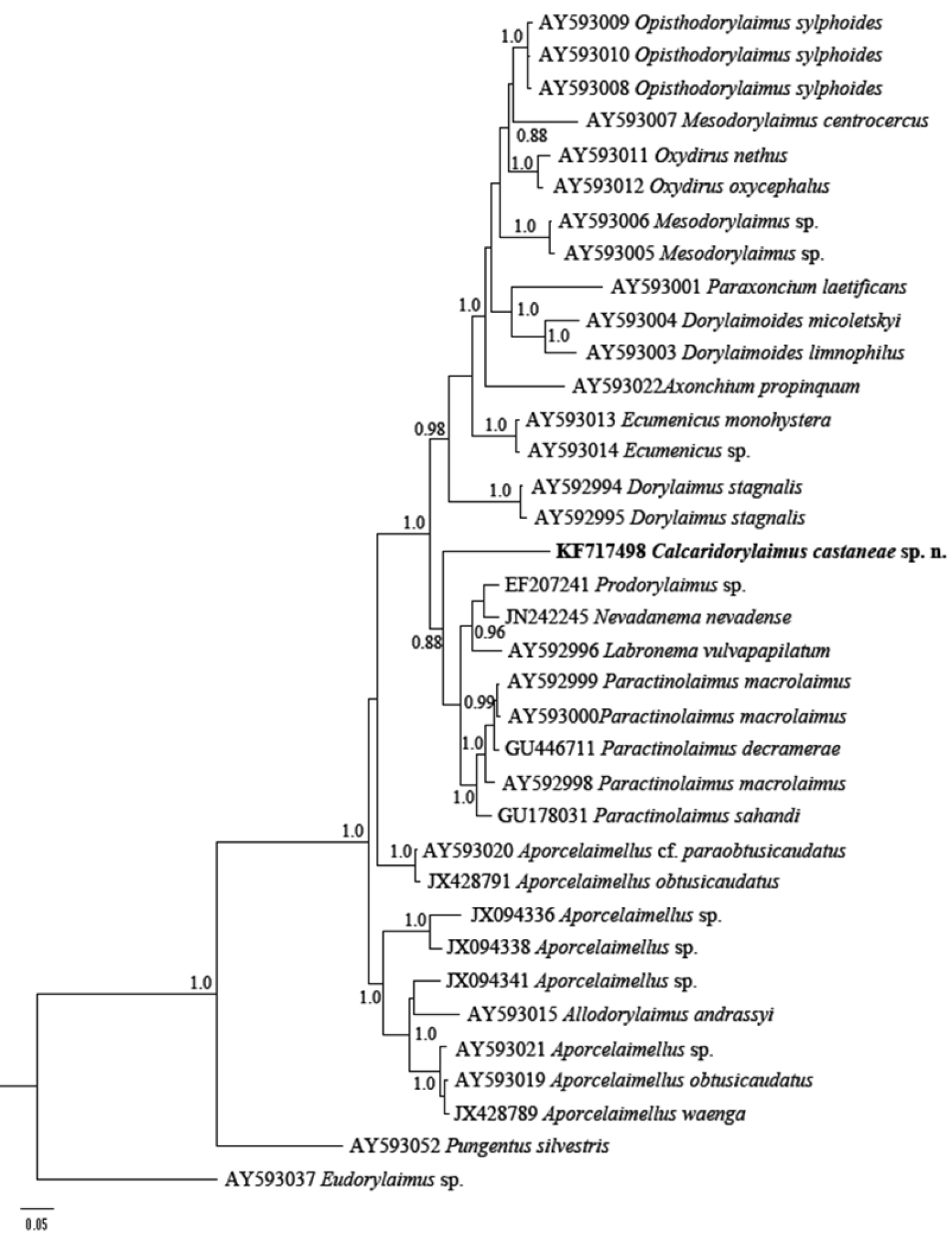
Phylogenetic relationships of Calcaridorylaimus castaneae sp. n. and its closest species for 18S rRNA gene. Tree acquired with Neighbor Joining and Maximum Likelihood (GTR+G model) methods. Bootstrap values higher than 70% are presented.
Phylogenetic relationships of Calcaridorylaimus castaneae sp. n. and its closest species for D2-D3 expansion segments of the 28S rRNA gene. Tree acquired with Neighbor Joining and Maximum Likelihood (GTR+G model) methods. Bootstrap values higher than 70% are presented.
The genus Calcaridorylaimus was erected by
The authors thanks Dr Michaela Ilieva from Institute of Biodiversity and Ecosystem Research for collecting additional litter samples from a sweet chestnut forest. This study was partly funded by the project ANIDIV, supported by the Bulgarian Academy of Sciences and project Assessment of the dynamics of structural and functional parameters of chestnut ecosystems in Belasitsa Mountain; funded by Ministry of Agriculture and Forests, Bulgaria. The authors are thankful to Dr Nathalie Yonow from Swansea University, Wales, UK for critical reading of the manuscript and helpful suggestions.