Citation: Silva GSC, Roxo FF, Britzke R, Oliveira C (2014) New species of the Pseudancistrus barbatus group (Siluriformes, Loricariidae) with comments on its biogeography and dispersal routes. ZooKeys 406: 1–23. doi: 10.3897/zookeys.406.7011
A new species of Pseudancistrus is described from the Tapajós Basin, and assigned to the P. barbatus group by having hypertrophied odontodes along the snout and lacking evertible cheek plates. The new species is distinguished from other species in that group (P. barbatus, P. corantijniensis, P. depressus and P. nigrescens) by its pattern of spots, length and color of snout odontodes, greater head depth, cleithral width, anal-fin spine length, peduncle depth and internares width. Molecular phylogenetic results corroborate placement of the new species in the Pseudancistrus barbatus group which is otherwise distributed in the Xingu Basin and rivers draining the Guyana Shield into the Atlantic Ocean. Topology tests strongly reject alternative hypotheses supporting close relationships with Guyanancistrus, Lithoxancistrus or the species Pseudancistrus pectegenitor, P. sidereus and P. genisetiger. Additionally, we propose two hypotheses on the distribution of the new species in the rio Tapajós, a Brazilian Shield drainage. The first one proposes that ancestral stock of the P. barbatus group was widely distributed throughout rivers draining the Guyana and Brazilian shields, and the species P. zawadzkii and Pseudancistrus sp. L17 are in the limit of the distribution for the group in Tapajós and Xingu rivers. The second hypothesis proposes that ancestral stock of the P. barbatus group was restricted to Guyana Shield rivers, and that headwater capture events permitted several dispersal routs through Guyana and Amazon rivers, permitted that the ancestral lineages of Pseudancistrus sp. L17 and P. zawadzkii reached the rivers of Amazon basin.
Ancistrini, freshwater, molecular phylogeny, F-reticulon 4, Brazilian Shield
Ancistrini is a highly diverse tribe of the subfamily Hypostominae, with 30 genera (
Species of the genus Pseudancistrus Bleeker, 1862 are distributed in the Orinoco, Amazon and Jaguaribe river systems, and rivers draining the Guyana Shield into the Atlantic Ocean.
In this paper, we present a formal description of a new species of Pseudancistrus from the Tapajós river basin. Additionally, we provide a phylogenetic context for the new species based on analysis of sequence data of F-reticulon 4 nuclear gene, and a brief discussion of biogeographic scenarios that may explain the distribution of the new species in the rio Tapajós and northern Brazilian Shield.
After capture, fish were anesthetized using 1% benzocaine in water, and either preserved in 95% ethanol for molecular studies or fixed in 10% formaldehyde for morphological studies. Vouchers and tissues were deposited in the collection of the Laboratório de Biologia e Genética de Peixes (LBP) and Museu de Zoologia da Universidade de São Paulo (MZUSP), Brazil, Muséum d’histoire naturelle de la ville de Genève (MHNG), Switzerland, Academy of Natural Sciences of Philadelphia (ANSP) and Auburn University (AUM), U.S.A., and Smithsonian Tropical Research Institute (STRI), Panama. Measurements and counts were taken on left side of specimens. Measurements follow
Total DNA was extracted from ethanol-preserved muscle, fin, and liver samples using the Wizard Genomic DNA Purification Kit (Promega, Madison, Wisconsin, U.S.A.). Partial sequences of F-reticulon 4 were amplified using polymerase chain reaction (PCR) with the following primers from
Amplifications were performed in a total volume of 12.5 μl containing 1.25 μl of 10X PCR buffer (20 mM Tris-HCl, pH 8.0, 40 mM NaCl, 2 mM Sodium Phosphate, 0.1 mM EDTA, 1 mM DTT, stabilizers, 50% (v/v) glycerol), 0.375 μl MgCl2 (50nM), 0.25 μl dNTPs (2 nM), 0.25 μl (each 5 mM primer), 0.05 μl Platinum® Taq DNA Polymerase (Invitrogen), 1 μl template DNA (50 ng), and 9.075 μl ddH2O. The nuclear markers were amplified in two PCR experiments; the first amplification using the primers Freticul4-D and Freticul4-R for 37–40 cycles (30 sec at 95°C, 30 sec at 48°C, and 135 sec at 72°C); and the second amplification using the primers Freticul4 D2, Freticul4 R2, and Freticul4 iR for 37–40 cycles (30 sec at 95°C, 30 sec at 53–54°C, and 135 sec at 72°C).
The products were then identified on a 1% agarose gel. The PCR products were purified using ExoSap-IT® (USB, Affymetrix Corporation, Cleveland, Ohio) following the manufacturer’s instructions. The purified PCR products were used to make a sequencing PCR using the BigDyeTM Terminator v 3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems- Life Technologies do Brasil Ltda, Vila Guarani, SP, Brazil). Subsequently, the amplified DNA was purified again and loaded onto a 3130-Genetic Analyzer automatic sequencer (Applied Biosystems), in the Instituto de Biociências, Universidade Estadual Paulista, Botucatu, São Paulo. Contigs were assembled and edited in BioEdit 7.0.9.0 (
The DNA sequences were aligned using ClustalW program implemented in DAMBE 5.2.31 (
Maximum-Likelihood (ML) analyses were performed using RAxML Web-Servers (Randomized Accelerated Maximum Likelihood,
Alternative tree topologies were evaluated in the program Treefinder (
http://zoobank.org/F244A7A4-253A-49B8-B027-16B640FDBCCF
http://species-id.net/wiki/Pseudancistrus_zawadzkii
Figure 1, Table 1MZUSP 115056, male, 116.4 mm SL. Brazil: Pará State: municipality of Itaituba: rio Tapajós (Amazon basin), 04°33'09.7"S, 56°17'59.6"W, 11 June 2012, R. Britzke and CEPTA’s team.
Brazil: Pará State: municipality of Itaituba: LBP 15045 (2 females, 97.9−128.7 mm SL), LBP 17724 (1 female, 87.5 mm SL), collected with holotype; LBP 16195 (1 male, 116.4 mm SL), rio Tracuá (trib. rio Tapajós), 04°28'11.2"S, 56°17'01.1"W.
Pseudancistrus zawadzkii is distinguished from all congeners, except species of the Pseudancistrus barbatus group, by presence of hypertrophied odontodes along the snout margin and the lack of evertible cheek plates. It further differs from two members of that group, Pseudancistrus barbatus and Pseudancistrus depressus, by having whitish spots that abruptly increase in size between the head (diameter 1.1−1.3 mm) and body (diameter 2.6−3.0 mm) (vs. whitish spots very small on whole body less than 1 mm), and snout odontodes yellowish (vs. snout odontodes reddish-brown). The new species differs from the other two members of the Pseudancistrus barbatus group, Pseudancistrus corantijniensis and Pseudancistrus nigrescens, by having odontodes along margin of snout increasing gradually in length from posterior of snout tip to cheek (vs. length of snout odontodes more uniform, smaller on tip of snout) and by having odontodes relatively longer on the most posterior portion of the nonevertible check plates (Fig. 1) (vs. odontodes shorter) (see fig. 3 in
Pseudancistrus zawadzkii, MZUSP 115056, holotype, male, 116.4 mm SL; Pará State, Tapajós river basin, Brazil.
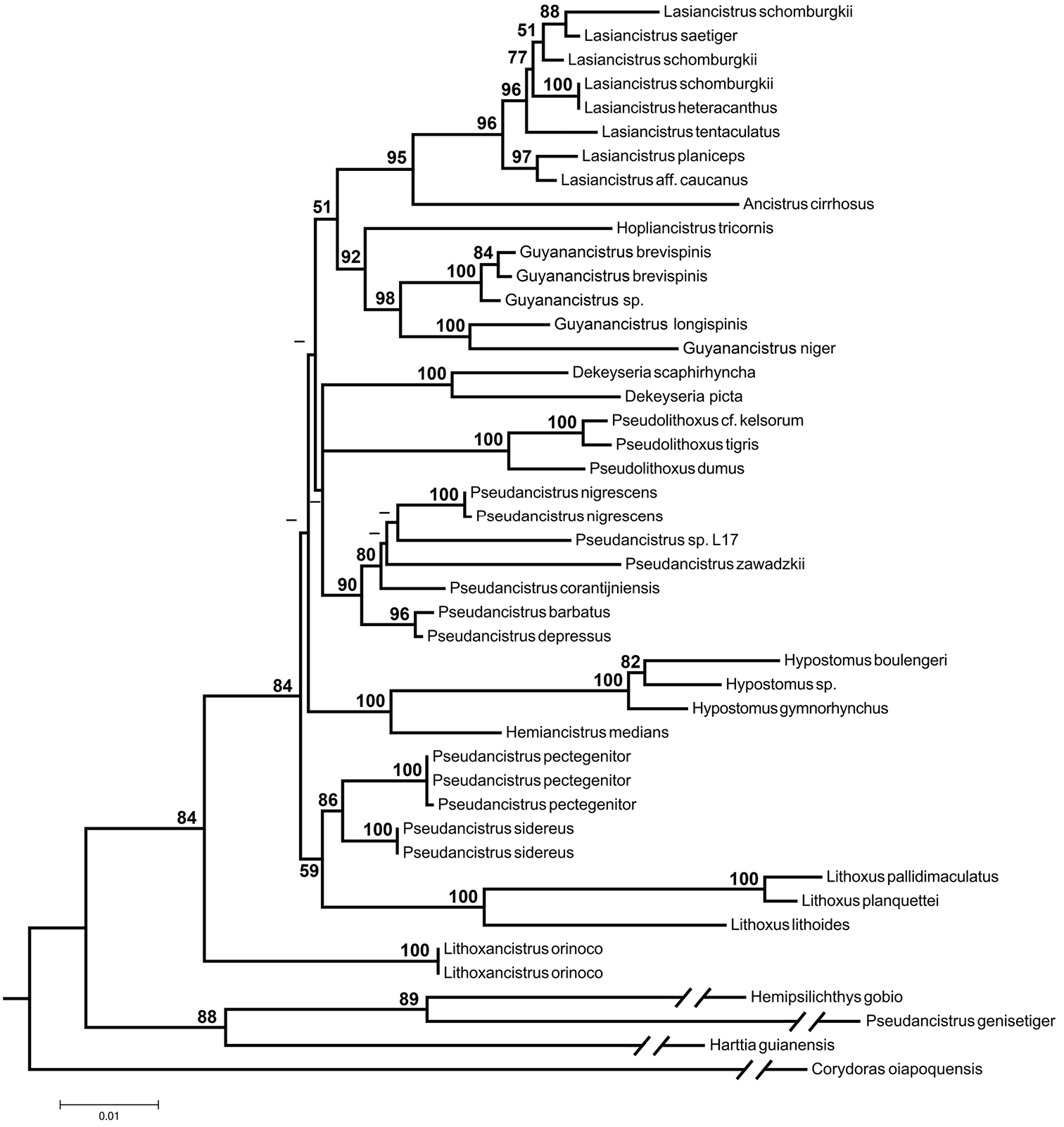
Maximum-likelihood tree based on nuclear gene sequence F-reticulon 4 (-lnL = 11470.59). Numbers next to nodes are bootstrap values based on 1, 000 pseudoreplicates. Values below 50% are not shown.
Morphometric data presented in Table 1. In lateral view, dorsal profile convex from snout tip to dorsal-fin origin; straight, gradually descending from dorsal-fin origin to posterior insertion of adipose fin; straight, steeply ascending to insertion of caudal fin; ventral profile flat from snout tip to anal-fin origin; shallowly concave from anal-fin insertion to lower caudal-fin spine; greatest body depth at dorsal-fin origin. In dorsal view, greatest body width across cleithral region; snout broadly elliptical; body progressively narrowed from opercular region to caudal fin. Cross-section of body between pectoral and pelvic fins rounded dorsally and flattened ventrally; cross-section of caudal peduncle ellipsoid.
Morphometric data for Pseudancistrus zawadzkii.
| Pseudancistrus zawadzkii n = 5 | ||||
|---|---|---|---|---|
| Holotype | Range | Mean | SD | |
| Standard length (SL) | 116.4 | 128.7−87.5 | 109.5 | |
| Percents of SL | ||||
| Predorsal length | 43.3 | 43.1−46.1 | 44.5 | 1.3 |
| Head length | 36.6 | 32.9−37.8 | 36.3 | 1.9 |
| Head-dorsal length | 6.7 | 6.7−9.2 | 8.1 | 1.2 |
| Cleithral width | 35.2 | 35.2−38.0 | 36.7 | 1.2 |
| Head pectoral length | 30.5 | 29.6−32.2 | 30.9 | 0.9 |
| Thorax length | 23.5 | 21.2−23.5 | 22.5 | 1.1 |
| Pectoral-spine length | 31.5 | 31.3−33.2 | 31.9 | 0.7 |
| Abdominal length | 24.2 | 22.6−26.1 | 24.3 | 1.3 |
| Pelvic-spine length | 28.4 | 25.6−28.4 | 27.2 | 1.2 |
| Post-anal length | 31.2 | 29.6−31.2 | 30.5 | 0.7 |
| Anal-fin spine length | 12.5 | 11.9−13.8 | 12.6 | 0.7 |
| Dorsal pectoral depth | 27.3 | 26.6−30.7 | 28.6 | 1.7 |
| Dorsal spine length | 24.7 | 24.7−29.9 | 27.5 | 2.3 |
| Dorsal pelvic depth | 22.9 | 22.1−26.4 | 24.1 | 1.7 |
| Dorsal-fin base length | 31.2 | 29.1−31.2 | 30.0 | 1.0 |
| Dorsal-adipose distance | 11.2 | 10.5−13.7 | 11.6 | 1.2 |
| Adipose-spine length | 7.8 | 6.79−8.78 | 7.8 | 0.7 |
| Dorsal adipose caudal distance | 11.7 | 11.7−15.6 | 13.7 | 1.7 |
| Caudal peduncle depth | 12.5 | 12.5−14.2 | 13.3 | 0.6 |
| Ventral adipose caudal distance | 22.9 | 22.9−25.3 | 23.9 | 1.0 |
| Adipose anal distance | 21.3 | 18.5−21.3 | 19.8 | 1.0 |
| Dorsal-anal distance | 16.0 | 15.8−17.8 | 16.8 | 0.8 |
| Pelvic-dorsal distance | 29.5 | 22.0−29.5 | 22.5 | 2.7 |
| Percents of head length (HL) | ||||
| Head-eye length | 29.4 | 28.1−30.1 | 29.1 | 0.8 |
| Orbital diameter | 14.6 | 14.5−18.8 | 15.8 | 1.7 |
| Snout length | 63.2 | 63.2−70.5 | 66.8 | 3.1 |
| Internares width | 14.4 | 12.7−16.6 | 14.4 | 1.4 |
| Minimal interorbital distance | 28.8 | 28.8−35.7 | 32.2 | 2.5 |
| Mouth length | 53.8 | 52.0−60.6 | 55.7 | 3.5 |
| Barbel length | 14.0 | 7.6−14.0 | 10.6 | 2.6 |
| Dentary tooth cup length | 17.6 | 17.0−19.6 | 18.5 | 1.1 |
| Premaxillary tooth cup length | 17.8 | 17.2−19.2 | 18.2 | 0.7 |
| Head depth | 68.9 | 67.0−72.7 | 68.8 | 2.3 |
Body almost entirely covered by plates; ventral portions of head and abdomen and dorsal-fin base naked. Five lateral rows of dermal plates, dorsal plates 21−24, lateral mid-dorsal plates 19−21, lateral median plates 22−24, lateral mid-ventral plates 21−24, lateral ventral plates 18−20. Three predorsal plates; eight plates below dorsal-fin base; four plates between dorsal fin and adipose fin; five rows of plates on caudal peduncle. Dorsal spinelet present.
Body plates and cleithrum have minute odontodes. Odontodes slightly hypertrophied on pectoral-fin spines, becoming gradually larger towards tips. Numerous yellowish hypertrophied odontodes along lateral margins of head including snout; odontodes small on tip of snout, increasing gradually in length from anterolateral margin of snout to cheeks; longest odontodes on posterior most portion of non-evertible cheek plates. Eyes small (orbital diameter 14.5−18.8% of HL), dorsolaterally positioned. Oral disk transversely ellipsoid. Lower lip not reaching transverse line between gill openings. Lower lip covered with numerous small papillae. Maxillary barbel developed. Mouth relatively large. Premaxillary teeth 40−61 per ramus; dentary teeth 28−69 per ramus. Teeth bifid, medial cusp large and rounded, lateral cusp minute and pointed. Wide jaws, dentary bones forming an oblique angle, premaxillary bones almost co-linear.
Dorsal fin II, 7, origin approximately at midpoint between pectoral- and pelvic-fin origins, last dorsal-fin ray reaching adipose fin when depressed. Pectoral fin I, 6, spine tip curved inward, covered with enlarged odontodes distally; depressed tip reaching one-third length of pelvic-fin spine. Pelvic fin I, 5, spine tip curved inward, almost reaching anal-fin origin when depressed. Anal fin I, 5, spine tip straight, reaching seventh plate posterior to its origin. Caudal fin I, 7−I, 7, distal margin concave, inferior lobe longer than superior. Adipose fin with lightly curved spine, preceded by single median preadipose plate.
Ground color dark greenish-brown on dorsum and sides of body, becoming dark brown posteriorly, and lighter brown ventrally. Anterior portion of head to posterior margin of orbits with many small, crowded, yellow spots; spots becoming abruptly larger on posterior portion of head, continuing on body, becoming slightly and gradually larger towards caudal peduncle. Dorsal plate series usually with two large spots per plate. Mid-dorsal plates usually with one large spot per plate. Lateral median plates with one large spot per plate. Mid-ventral plates and ventral plates with one large spot per plate. Dorsal-fin spine, rays and membranes with large round large spots. Adipose-fin with two large spots on spine and membrane. Pectoral, pelvic, anal and caudal fin with numerous and similarly sized yellow spots. Hypertrophied odontodes along head margin yellowish (Fig. 3).
Pseudancistrus zawadzkii, live specimen, LBP 15045, paratype, female, 128.7 mm SL, Tapajós river, Pará State, Brazil.
Similar to pattern described for living individuals, but with ground color dark brown, and spots pale tan (Fig. 1).
Males possess a papilla posterior to urogenital opening, an attribute absent in females. Both sexes in Pseudancistrus zawadzkii exhibit highly hypertrophied odontodes along snout margin, similar to others species of Pseudancistrus (
Specific name is in honor of Cláudio Henrique Zawadzki, professor at Universidade Estadual de Maringá (UEM), Maringá, Paraná State, Brazil, in recognition of his dedication and remarkable contributions to the study of the family Loricariidae.
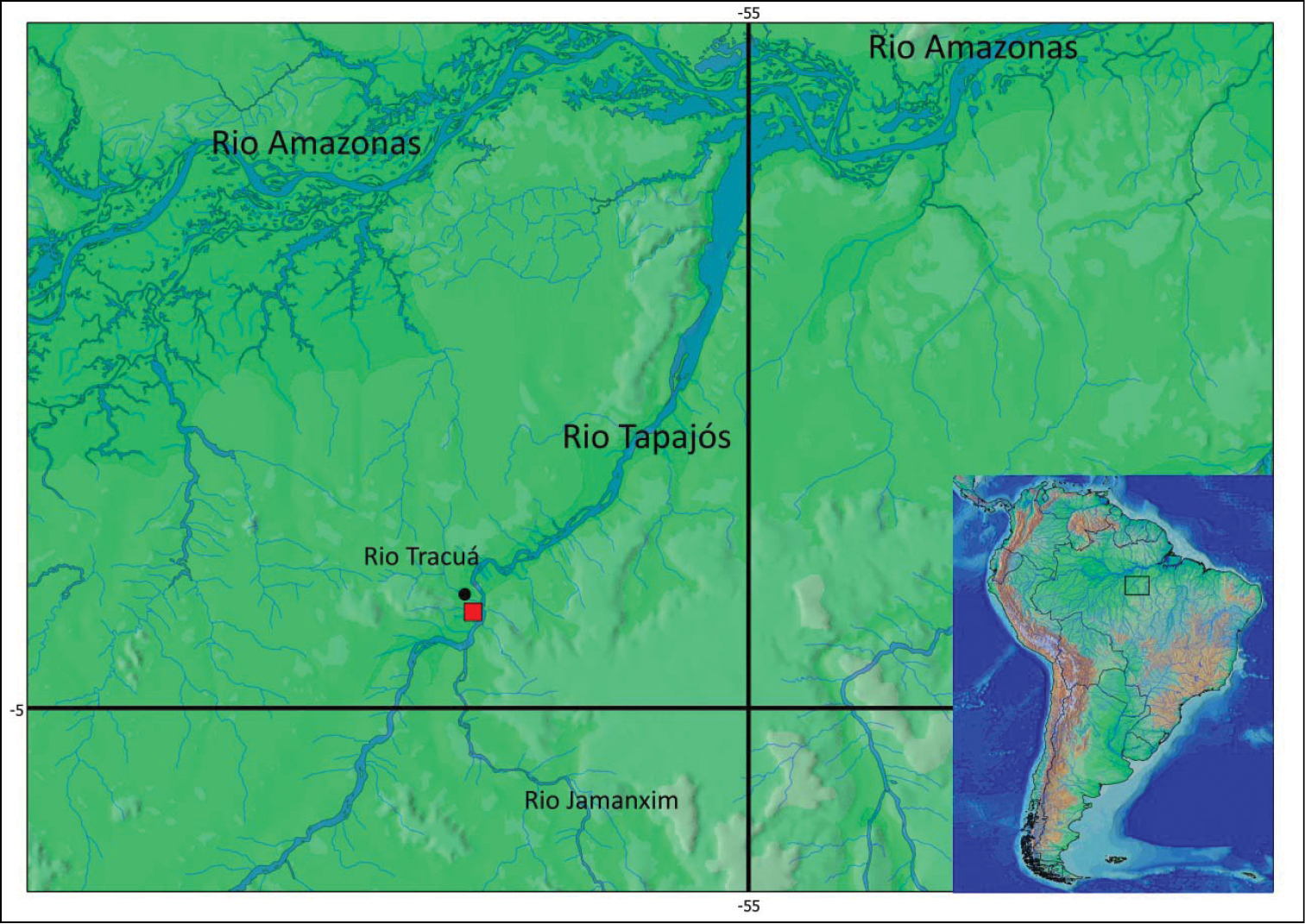
Pseudancistrus zawadzkii is known from rio Tapajós (04°33'10"S, 56°18'W) and rio Tracuá (04°28'11"S, 56°17'01"W), municipality of Itaituba, all from rio Tapajós basin, Pará State, Brazil. (see Fig. 4 for distribution map of type species localities).
Map showing the type locality (red square) of Pseudancistrus zawadzkii at rio Tapajós, 04°33'09.7"S, 56°17'59.6"W, and paratype locality (black circle) at rio Tracuá, Tapajós river basin, 04°28'11.2"S, 56°17'01.1"W.
The rio Tapajós, and rio Tracuá where Pseudancistrus zawadzkii occurs are clear water rivers, varying from medium to large size, with rocky outcrops forming small waterfalls and substrates of rocks and sand (Fig. 5).
a Habitat at type locality of Pseudancistrus zawadzkii: rio Tapajós, municipality of Itaituba, Pará State, Brazil b habitat at paratype locality: rio Tracuá, Tapajós river basin, municipality of Itaituba, Pará State, Brazil.
Partial sequences of the nuclear gene F-reticulon 4 (RTN4) were obtained in this study and from GenBank for 44 specimens representing 35 Loricariidae species and the new species Pseudancistrus zawadzkii (Table 3). We included samples of the four lineages of Pseudancistrus proposed by
Evolutionary relationships among species of Pseudancistrus sensu lato and other members of Otothyrini are similar between our ML phylogenetic tree (-lnL = 11470.59) and the one proposed by
The new species Pseudancistrus zawadzkii possesses hypertrophied odontodes along the snout margin and lacks evertible cheek plates.
Likelihood-based tests for alternative topologies. SH and AU are probability values obtained from the Shimodaira-Hasegawa and the Approximately Unbiased tests (
| Test | Topology | - Ln L | ∆ - Ln L | ELW | SH | AU |
|---|---|---|---|---|---|---|
| ML | 11910.81 | |||||
| 1 | Pseudancistrus zawadzkii sister group to Pseudancistrus pectegenitor + Pseudancistrus sidereus |
11952.41 | 41.60 | <0.001* | 0.021* | <0.001* |
| 2 | Pseudancistrus zawadzkii sister group to Guyanancistrus members |
11962.24 | 51.43 | <0.001* | 0.011* | <0.001* |
| 3 | Pseudancistrus zawadzkii sister group to Lithoxancistrus members |
11966.25 | 55.44 | <0.001* | <0.001* | <0.001* |
| 4 | Pseudancistrus zawadzkii sister group to Pseudancistrus genisetiger |
12033.30 | 122.49 | <0.001* | <0.001* | <0.001* |
a The alternative topology was defined as the ML tree forcing the desired relationship.
Taxa list, specimen and sequence data analyzed in the present study (n=44). Institutional acronyms follow
| Species | Catalog Number | Field Number | GenBank Nº F-RTN4 | Ref. |
|---|---|---|---|---|
| Corydoras oiapoquensis | MHNG 2682.023 | GF06-186 | GU210997 | Alexandrou et al. (2011) |
| Hemipsilichthys gobio | LBP 2368 | 15363 | EU817547 | |
| Harttia guianensis | MHNG 2643.016 | GF00–351 | FJ013232 | |
| Hypostomus sp. | MHNG 2721.062 | PE08-198 | JN855790 | |
| Hypostomus boulengeri (Eigenmann & Kennedy, 1903) | MHNG 2519.23 | ASU7 | EU817560 | |
| Hypostomus gymnorhynchus (Norman, 1926) | MHNG 2621.098 | SU01-160 | JN855789 | |
| Ancistrus cirrhosis (Valenciennes, 1836) | MHNG 2645.037 | MUS 202 | HM623638 | Rodriguez et al. (2011) |
| Dekeyseria picta (Kner, 1854) | MHNG 2588.046 | MUS 162 | JN855755 | |
| Dekeyseria scaphirhyncha (Kner, 1854) | AUM 43874 | V5528 | JN855756 | |
| Hemiancistrus medians (Kner, 1854) | MHNG 2664.078 | GF00-084 | JF747011 | Fisch-Muller et al. (2012) |
| Guyanancistrus brevispinis | MHNG 2725.099 | GF00-103 | JN855772 | |
| Guyanancistrus brevispinis | MHNG 2621.073 | SU01-121 | JN855773 | |
| Guyanancistrus longispinis | MHNG 2725.100 | GF99-204 | JN855757 | |
| Guyanancistrus niger | MHNG 2722.089 | GF99-185 | JN855759 | |
| Guyanancistrus sp. | MHNG 2679.099 | MUS 300 | JN855774 | |
| Hopliancistrus tricornis Isbrücker & Nijssen, 1989 | MHNG 2588.051 | MUS 146 | JN855765 | |
| Lasiancistrus aff. caucanus | MHNG 2586.043 | MUS 118 | JN855786 | |
| Lasiancistrus heteracanthus (Günther, 1869) | MHNG 2613.037 | CA 013 | JN855787 | |
| Lasiancistrus planiceps (Meek & Hildebrand, 1913) | STRI-01805 | Stri 3526 | JN855785 | |
| Lasiancistrus saetiger |
MHNG 2602.016 | BR98-148 | JN855754 | |
| Lasiancistrus schomburgkii (Günther, 1869) | MHNG 2651.009 | PE08-719 | JN855782 | |
| Lasiancistrus schomburgkii | MHNG 2651.068 | GY04-308 | JN855783 | |
| Lasiancistrus schomburgkii | MHNG 2710.055 | PE08-277 | JN855784 | |
| Lasiancistrus tentaculatus Armbruster, 2005 | MhnG uncat. | MUS 573 | JN855788 | |
| Lithoxus lithoides Eigenmann, 1912 | MHNG 2651.087 | GY04-136 | JN855777 | |
| Lasiancistrus pallidimaculatus Boeseman, 1982 | MHNG 2621.066 | SU01-096 | JN855778 | |
| Lasiancistrus planquettei Boeseman, 1982 | MHNG 2722.060 | GF03-055 | JN855779 | |
| Lithoxancistrus orinoco | AUM 43725 | V5246 | JN855766 | |
| Lithoxancistrus orinoco | AUM 42179 | P4527 | JN855767 | |
| Pseudancistrus barbatus | MHNG 2653.059 | GF00-074 | JN855761 | |
| Pseudancistrus corantijniensis | MHNG 2672.092 | SU05-296 | JN855781 | |
| Pseudancistrus depressus | MHNG 2674.026 | SU05-020 | JN855780 | |
| Pseudancistrus genisetiger | MHNG 2593.061 | MUS 173 | JN855764 | |
| Pseudancistrus nigrescens | MHNG 2651.069 | GY04-313 | JN855770 | |
| Pseudancistrus nigrescens | MHNG 2650.087 | GY04-260 | JN855771 | |
| Pseudancistrus pectegenitor | AUM 42202 | V5363 | JN855769 | |
| Pseudancistrus pectegenitor | ANSP 182801 | V5433 | JN855768 | |
| Pseudancistrus sidereus | AUM 43443 | P4871 | JN855775 | |
| Pseudancistrus sidereus | AUM 42180 | P4537 | JN855776 | |
| Pseudancistrus zawadzkii | LBP 15045 | 61628 | KJ028080 | Present study |
| Pseudancistrus sp. L17 | MHNG 2586.046 | MuS 132 | JN855763 | |
| Pseudolithoxus cf. kelsorum | MHNG 2679.043 | MUS 260 | JN855762 | |
| Pseudancistrus dumus (Armbruster & Provenzano, 2000) | MHNG 2708.080 | MUS 288 | JN855760 | |
| Pseudancistrus tigris (Armbruster & Provenzano, 2000) | AUM 42215 | V5292 | JN855758 |
Pseudancistrus zawadzkii, Pseudancistrus corantijniensis, and Pseudancistrus nigrescens share the presence of whitish colored snout odontodes and a dark colored body covered with white spots. The new species can be easily distinguished from Pseudancistrus corantijniensis and Pseudancistrus nigrescens by having large hypertrophied odontodes on the posteriormost portion of the non-evertible check plates, and marginal odontodes that increase gradually in length from tip of snout to cheeks. Pseudancistrus barbatus and Pseudancistrus depressus share reddish-brown snout odontodes, a probable synapomorphy, and are the sister group to Pseudancistrus zawadzkii, Pseudancistrus corantijniensis and Pseudancistrus nigrescens.
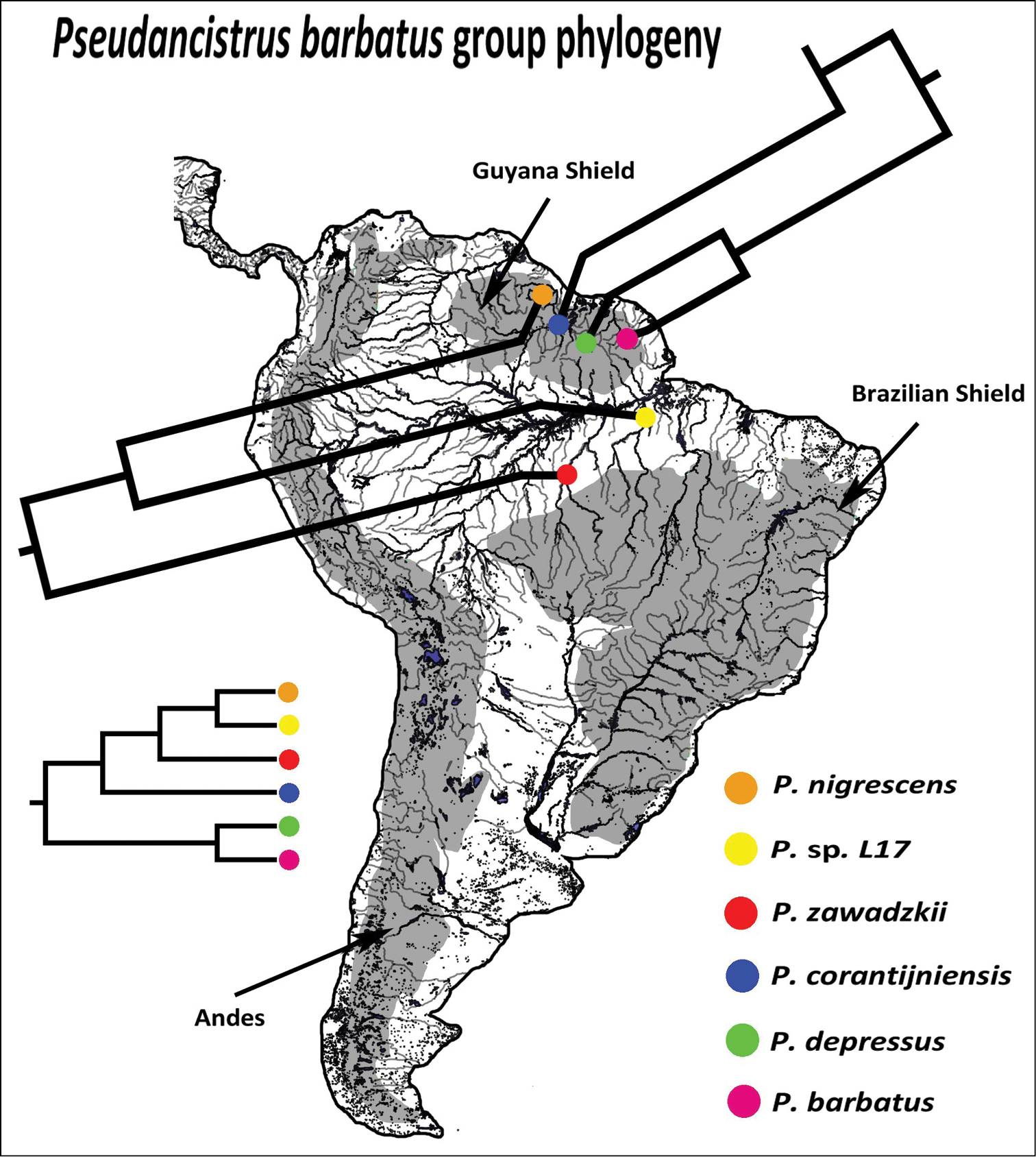
Named species of the Pseudancistrus barbatus group are distributed in rivers draining to Guyana Shield into the Atlantic Ocean, and the new species described herein is from Tapajós river draining of Brazilian Shield into the Amazon. In our phylogeny, species from the eastern Guyana Shield (Pseudancistrus barbatus and Pseudancistrus depressus) form a clade sister to a group composed of species from the western Guyana Shield (Pseudancistrus corantijniensis and Pseudancistrus nigrescens) and Amazon basin (Pseudancistrus zawadzkii and Pseudancistrus sp. L17) (Fig. 6). Therefore, based on this interpretation and our results of phylogenetic analysis, we suggested two hypotheses that could generate the distribution pattern of Pseudancistrus barbatus group extant-species. The first hypothesis is that the ancestral stock of the Pseudancistrus barbatus group was widely distributed through all Guyana Shield rivers and Amazon Brazilian Shield rivers, and the species Pseudancistrus zawadzkii and Pseudancistrus sp. L17 are in the limit of the distribution for the group in Tapajós and Xingu rivers, respectively.
Distribution and phylogenetic relationships of species of the Pseudancistrus barbatus group based on F-reticulon 4 gene. Based in our first hypothesis of extand-species distribution of this group the ancestral was widespread through all Guyana Shield rivers and Amazon Brazilian Shield rivers, the species Pseudancistrus zawadzkii and Pseudancistrus sp. L17 are in the limited distribution of this group in Tapajós and Xingu rivers, drainages of Brazilian Shield into Amazon.
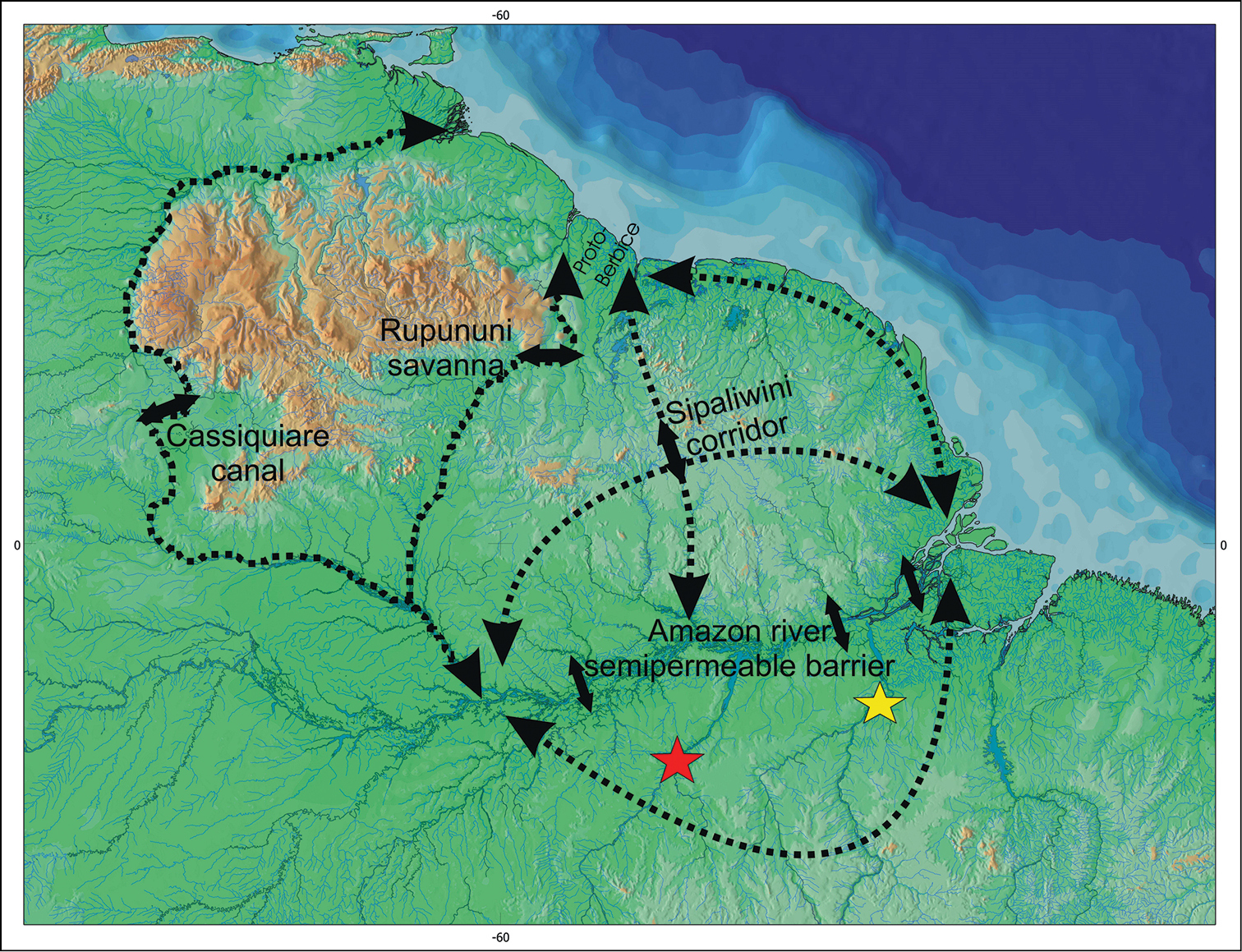
The second hypothesis suggests that the ancestral stock of Pseudancistrus barbatus group should have been distributed through Guyana Shield rivers and there existed several dispersal routes through Guyana and Amazon rivers, permitting that the ancestral lineages of Pseudancistrus sp. L17 and Pseudancistrus zawadzkii reached the rivers of Amazon basin (see Fig. 7 for dispersal routes). Therefore, examples of connections and areas of movement among Guyana drainages and the north tributaries of Amazon basin was reported by several authors: (1) the Rupununi portal, an example of seasonal connection among Takutu and Rupununi rivers (Armbruster and Werneke 2005;
Hypothesized dispersal routs between basins of the Guiana Shield and Amazon Shield of ancestror of the Pseudancistrus barbatus group (based on
Additionally, the mainstream of Amazon River can act as a permeable barrier for endemic taxa on the respective Guiana and Brazilian shields. Several genera known to tolerate more lowland conditions (e.g. Ancistrus Kner, 1854, Lasiancistrus, and Hypostomus Lacepéde, 1803) may be able to cross the Amazon basin, but such dispersal is unlikely among most species of Ancistrini (
Also, the dispersal routes around adjacent drainages of southern and northern Guyana Shield and northern parts of the Brazilian Shield could allow the dispersal of the ancestral form of Pseudancistrus zawadzkii and Pseudancistrus sp. L17, as well as others ancestral species of the Pseudancistrus barbatus group and even species of Ancistrini (
Pseudancistrus barbatus (Valencienes, 1840): ANSP 177366, 2, 76.5−103.7 mm SL, Burro Burro river, Water Dog Falls, Essequibo river basin, Guyana. ANSP 189119, 3, 75.1−151.5 mm SL, Lawa river, Sipalawini, Suriname. Pseudancistrus brevispinis (Heitmans, Nijssen & Isbrücker, 1983): ANSP 189128, 3, 56.8−125.7 mm SL, Marowini river, Sipalawini, Suriname. Pseudancistrus nigrescens Eigenmann, 1912: ANSP 177379, 5, 96.4−133.5 mm SL, Burro Burro river, Water Dog Falls, Essequibo river basin, Guyana. Pseudancistrus orinoco (Isbrücker, Nijssen & Cala, 1988): ANSP 160600, 6, 68.0−78.5 mm SL, Orinoco river, Venezuela. Pseudancistrus pectegenitor Lujan, Armbruster & Sabaj, 2007: ANSP 190755, 1, 206, 2 mm SL, Ventuari river, Orinoco river basin, Venezuela. Pseudancistrus sidereus Armbruster, 2004b: ANSP 185321, 4, 148.6−154.1 mm SL, Casiquiari river, Venezuela. Pseudancistrus sp. L17: LBP 16551, 2, 75.3−101.0 mm SL; rio Xingu, Altamira, Pará State, Amazon river basin, Brazil. ANSP 193074, 3, 51.7−188.7 mm SL, Xingu river, Altamira, Pará State, Amazon river basin, Brazil. Pseudancistrus sp. ANSP 191153, 6, 49.2−75.7 mm SL, Ventuari river, Orinoco river basin, Venezuela.
We are grateful to M.H. Sabaj Pérez (Academy of Natural Sciences of Philadelphia) and J.S. Albert (University of Louisiana at Lafayette) for loans of specimens and curatorial assistance; to CEPTA’s team (Centro de Pesquisa Treinamento em Aquicultura - formerly Centro Nacional de Pesquisa e Conservação de Peixes Continentais) for help collecting specimens; B. Waltz and again to M.H. Sabaj Pérez for reading the manuscript and providing valuable suggestions. Fishes collected in accordance with Brazilian laws, under a permanent scientific collecting license issued to Dr. Claudio Oliveira by IcmBio/CEPTA. Research supported by Brazilian agencies FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, proc. 2010/01610-9 to FFR, proc. 2012/01622-2 to GSCS and proc. 2011/00269-4 to RB), MCT/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Comparative material from rio Xingu made available by iXingu Project funded by the U.S. National Science Foundation (DEB-1257813).