Citation: Li N-N, Toda MJ, Fu Z, Chen J-M, Li S-H, Gao J-J (2014) Taxonomy of the Colocasiomyia gigantea species group (Diptera, Drosophilidae), with descriptions of four new species from Yunnan, China. ZooKeys 406: 41–64. doi: 10.3897/zookeys.406.7176
Species of the genus Colocasiomyia de Meijere feed/breed on inflorescences/infructescences of the plants from the families Araceae, Arecaceae and Magnoliaceae. Although most of them utilize plants from the subfamily Aroideae of Araceae, three species of the recently established C. gigantea species group make use of plants of the subfamily Monsteroideae. We describe four new species of the gigantea group found from Yunnan, China: Colocasiomyia longifilamentata Li & Gao, sp. n., C. longivalva Li & Gao, sp. n., C. hailini Li & Gao, sp. n., and C. yini Li & Gao, sp. n. The species delimitation is proved in virtue of not only morphology but also DNA barcodes, i.e., sequences of the partial mitochondrial COI (cytochrome c oxidase subunit I) gene. Some nucleotide sites with fixed status in the alignment of the COI sequences (658 sites in length) are used as “pure” molecular diagnostic characters to delineate species in the gigantea group.
Adaptation, aroid, character-based barcoding, cohabitation, genetic distance, integrated taxonomy, Rhaphidophora clade
To date, as many as 90 species (of them only 25 species described) have been found in the genus Colocasiomyia de Meijere, 1914. All these species visit and breed on flowers of the families Araceae, Arecaceae or Magnoliaceae (
The gigantea group was recently erected by
Summary of the species of the Colocasiomyia gigantea species group.
| Species name | Distribution | Host plant | Reference |
|---|---|---|---|
| Colocasiomyia gigantea (Okada, 1987) | Java, Indonesia; Solomon Is. | Epipremnum pinnatum (L.) Engle | |
| Colocasiomyia scindapsae Fartyal & Toda, 2013 | Sabah, Malaysia | Scindapsus coriaceus Engler | Ditto |
| Colocasiomyia rhaphidophorae Gao & Toda, 2013 | Xishuangbanna, Yunnan, China | Rhaphidophora hookeri Schott | Ditto |
| Pu’er, Yunnan, China | Rhaphidophora decursiva (Roxb.) Schott | Present study | |
| Colocasiomyia longifilamentata sp. n. | Baoshan and Pu’er, Yunnan, China | Ditto | Ditto |
| Colocasiomyia longivalva sp. n. | Ditto | Ditto |
Ditto |
| Colocasiomyia hailini sp. n. | Ditto | Ditto | Ditto |
| Colocasiomyia yini sp. n. | Ditto | Ditto | Ditto |
a To be confirmed by further investigation (for details, see the “Remarks” section in the description of Colocasiomyia longivalva sp. n.)
Our recent field surveys in Yunnan Province, China brought new, insightful information on the evolution of flower-breeding habits in the gigantea group. We found four new species of this group visiting inflorescences of Rhaphidophora decursiva (Roxb.) Schott (Table 1); at least three of them were found breeding on inflorescences/infructescences of this plant. In Pu’er (central-southern part of Yunnan), Colocasiomyia rhaphidophorae cohabited with the above-mentioned three new species on inflorescences/infructescences of Rhaphidophora decursiva. Thus, the Chinese members of the gigantea group are mostly sympatric and overlapping in host plant selection with each other (cohabitation), in contrast to the allopatry and monopolization of host plant in the other members, Colocasiomyia gigantea and Colocasiomyia scindapsae.
The four new species of the gigantea species group are described here, based on species delimitation in virtue of morphological and molecular (DNA sequences of the mitochondrial cytochrome c oxidase subunit I gene, COI, as the DNA barcoding marker) characters.
Table 2 shows the fly samples/specimens involved in the present study. Most of them were collected from the field in southwestern China, Malaysia and Indonesia. Some were reared from inflorescences/infructescences of host plants; after dissection of inflorescences/infructescences under a stereoscopic microscope in laboratory, the fly eggs were isolated and transferred into Petri dishes with decayed pistils as food and then reared at 25 °C in an incubator until adults emerged.
Specimens of Colocasiomyia species used for DNA barcoding analysis.
| Species | Voucher #/GenBank accession number | Collection site |
|---|---|---|
| Colocasiomyia sulawesiana | Lot055 (DNA)/KJ700880 |
A |
| Colocasiomyia colocasiae | Lot072 (DNA)/KJ700879 |
B |
| Colocasiomyia xenalocasiae | 001627/KJ700881 | C |
| Colocasiomyia gigantea | Lot150 (DNA)/KJ700882 |
D |
| Colocasiomyia scindapsae | Lot036 (DNA)/KJ700886 |
B |
| Colocasiomyia rhaphidophorae | 000323/KJ700892 |
E |
| 001514 |
C | |
| Colocasiomyia longifilamentata sp. n. | 000349/KJ700902 |
F |
| 001515 |
C | |
| Colocasiomyia longivalva sp. n. | 000082/KJ700916 |
F |
| Colocasiomyia hailini sp. n. | 000355–0358/KJ700918–0921 |
F |
| 001448 |
C | |
| Colocasiomyia yini sp. n. | 000160/KJ700929 |
F |
| 001586 |
C |
a PCR/sequencing using the primers of
b PCR/sequencing using the primers of
c Adults obtained by laboratory rearing;
d Collection sites: A, Enrekang, South Sulawesi, Sulawesi, Indonesia; B, Park Headquarters, Mt. Kinabalu, Sabah, Malaysia; C, Yixiang, Pu’er, Yunnan, China; D, Bogor Botanical Garden, West Java, Indonesia; E, Menglun, Mengla, Xishuangbanna, Yunnan, China; F, Baihualing, Longyang, Baoshan, Yunnan, China
We followed the same method as in
A total of 54 individuals representing all the three known and four morphologically identified, putatively new species (Table 2) were subjected to DNA sequencing of the COI barcode fragments (
A total of 57 COI sequences (54 of the gigantea group and three of the cristata group) were determined. The sequences were edited in the SEQMAN module of the DNASTAR package (DNASTAR Inc. 1996), and aligned in MEGA5 (
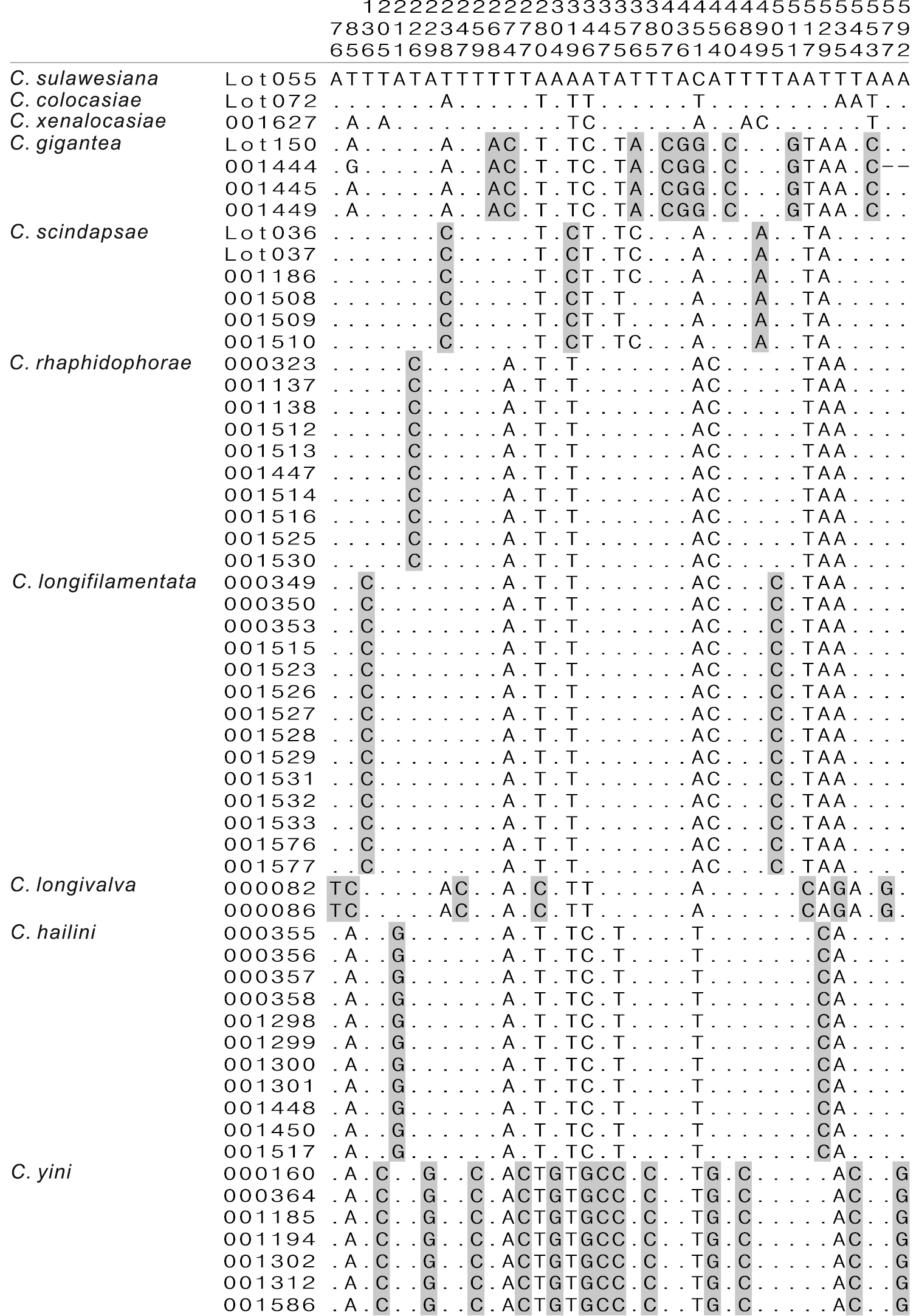
The alignment of the 57 COI sequences spanned 658 nucleotide sites in length, with 184 variable sites, among which 160 were parsimony informative. For the inter- and intraspecific K2P distances see Table 3. The largest intraspecific K2P distance in the gigantea group was found in Colocasiomyia scindapsae (= 0.0102), while the smallest interspecific one was found between Colocasiomyia rhaphidophorae and Colocasiomyia longifilamentata (= 0.0135). This implies that the “barcoding gap” (
Intra- and interspecific K2P distances (minimum–maximum) in the Colocasiomyia gigantea species group.
| Species | N |
Intraspecific distance | Interspecific distance | |||||
|---|---|---|---|---|---|---|---|---|
| Colocasiomyia gigantea | Colocasiomyia scindapsae | Colocasiomyia rhaphidophorae | Colocasiomyia longifilamentata sp. n. | Colocasiomyia longivalva sp. n. | Colocasiomyia hailini sp. n. | |||
| Colocasiomyia gigantea | 4 | 0–0.0058 | ||||||
| Colocasiomyia scindapsae | 6 | 0–0.0102 | 0.1027–0.1236 | |||||
| Colocasiomyia rhaphidophorae | 10 | 0–0.0054 | 0.1056–0.1148 | 0.0978–0.1156 | ||||
| Colocasiomyia longifilamentata sp. n. | 14 | 0–0.0099 | 0.1134–0.1259 | 0.0972–0.1088 | 0.0135–0.0282 | |||
| Colocasiomyia longivalva sp. n. | 2 | 0–0 | 0.1345–0.1370 | 0.0930–0.1046 | 0.0793–0.0860 | 0.0815–0.0834 | ||
| Colocasiomyia hailini sp. n. | 11 | 0–0.0069 | 0.1099–0.1179 | 0.1017–0.1230 | 0.0939–0.1038 | 0.0978–0.1086 | 0.1079–0.1125 | |
| Colocasiomyia yini sp. n. | 7 | 0–0.0034 | 0.1440–0.1483 | 0.1425–0.1664 | 0.1211–0.1359 | 0.1367–0.1484 | 0.1431–0.1468 | 0.0674–0.0758 |
a Number of sequences
Fig. 1 shows nucleotides at the sites where “pure” diagnostics for any species of the gigantea group are included. At least one diagnostic site was recognized for each species. For example, the site 226 is diagnostic for Colocasiomyia rhaphidophorae: this site has a fixed status of C (Cytosine) in this species, but T (Thymidine) in the other species. The sites 136 and 505 (both with fixed status of C) are diagnostic for Colocasiomyia longifilamentata.
Diagnostic nucleotide sites in the alignment of COI sequences of the Colocasiomyia gigantea group. Numbers at the top show the positions of the sites in the COI alignment (658-bp in length). Shaded sites are diagnostic for each species. Hyphens (-) indicate end missing data.
Included species. Colocasiomyia gigantea (Okada, 1987); Colocasiomyia rhaphidophorae Gao & Toda in
Diagnosis (
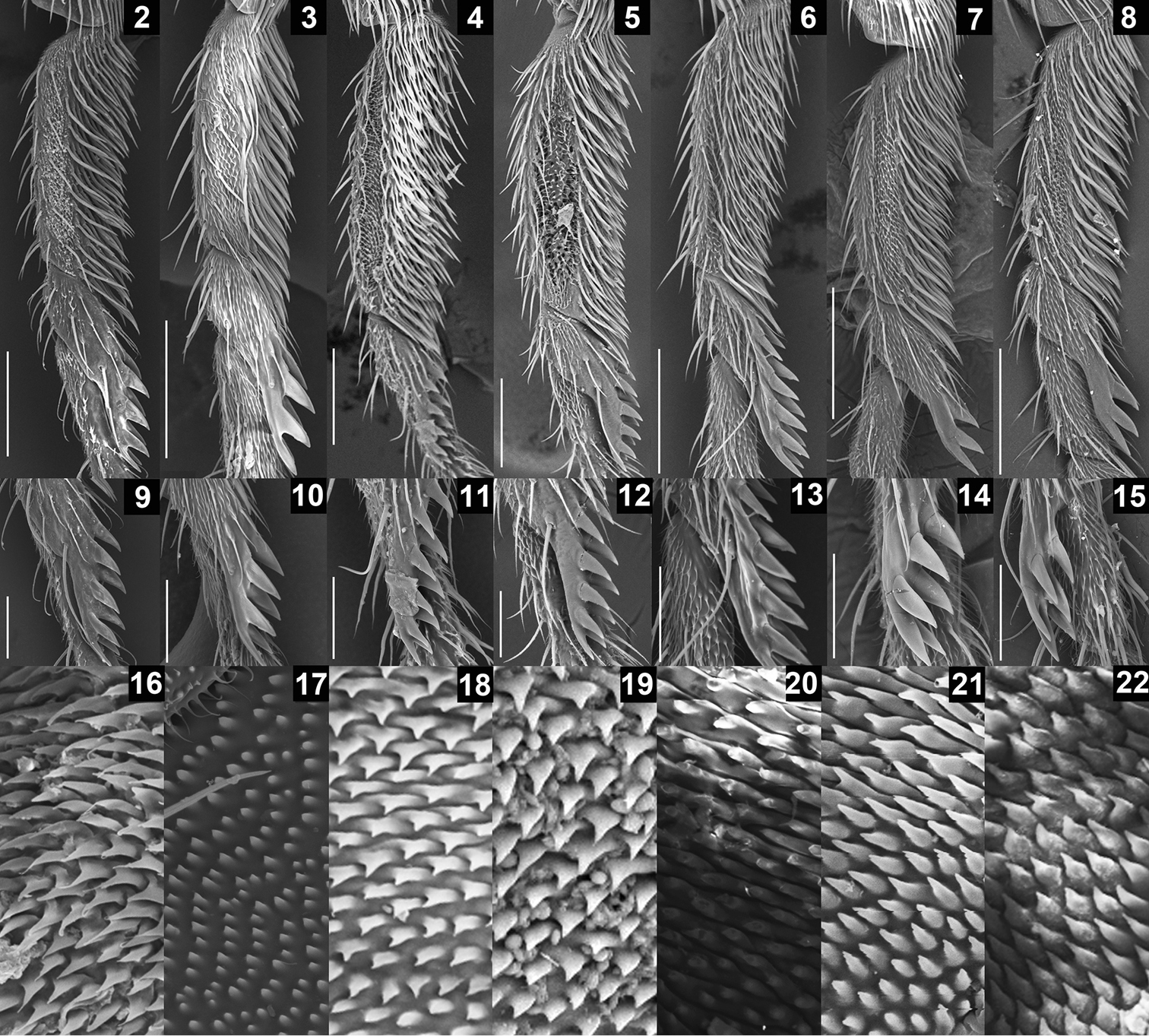
SEM photographs showing leg and oviscapt fine structures in the Colocasiomyia gigantea species group. Foreleg tarsomeres I and II (2–8), pegs on foreleg tarsomere II (9–15) and warts on basal part of lateral lobe or basal membrane of oviscapt (16–22) of Colocasiomyia gigantea (2, 9, 16), Colocasiomyia scindapsae (3, 10, 17), Colocasiomyia rhaphidophorae (4, 11, 18), Colocasiomyia longifilamentata sp. n. (5, 12, 19), Colocasiomyia longivalva sp. n. (6, 13, 20), Colocasiomyia hailini sp. n. (7, 14, 21) and Colocasiomyia yini sp. n. (8, 15, 22). Scale line = 0.1 mm in 2–8, 0.05 mm in 9–15. Figures 16–22 are in the same magnification, with the width corresponding to 30 µm.
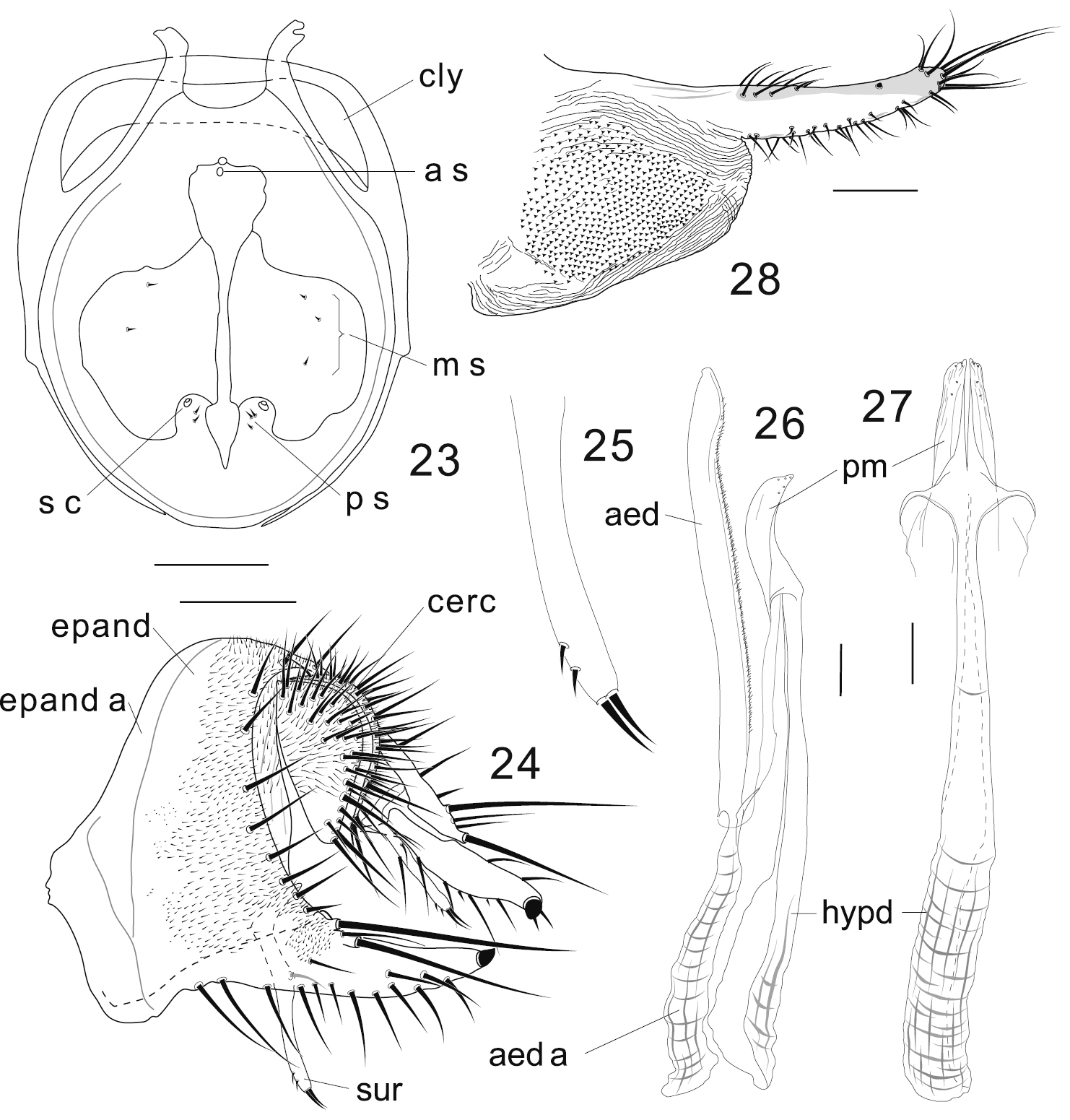
Colocasiomyia longifilamentata Li & Gao, sp. n. Adult male and female (paratypes) from Baihualing, Yunnan, China: 23 Cibarium and clypeus (dorsal view) 24 periphallic organs (posterolateral view) 25 apical part of surstylus 26 phallic organs (lateral view) 27 hypandrium and parameres (ventral view) 28 oviscapt (lateral view). Abbreviations: aed = aedeagus, aed a = aedeagal apodeme, a s = anterior sensilla, cerc = cercus, cly = clypeus, epand = epandrium, epand a = epandrial apodeme, hypd = hypandrium, m s = medial sensilla, ps = posterior sensilla, pm = paramere, s c = sensilla campaniformia, sur = surstylus. Scale lines = 0.1 mm.
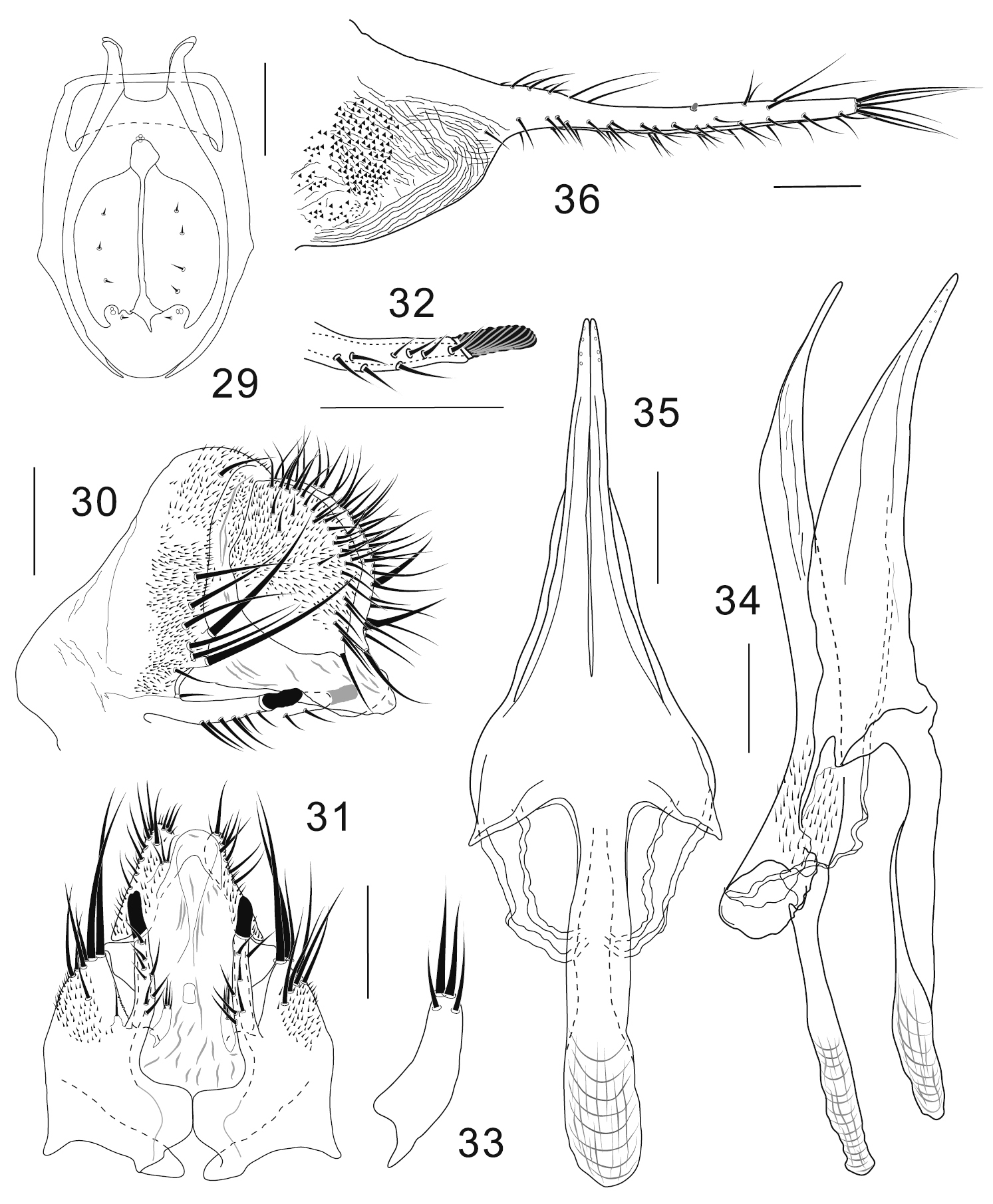
Colocasiomyia longivalva Li & Gao, sp. n. Adult male and female (paratypes) from Baihualing, Yunnan, China: 29 Cibarium and clypeus (dorsal view) 30 periphallic organs (posterolateral view) 31 periphallic organs (ventral view) 32 apical part of epandrial ventral lobe (ventral view) 33 surstylus (ventral view) 34 phallic organs (lateral view) 35 hypandrium and parameres (ventral view) 36 oviscapt (lateral view). Scale lines = 0.1 mm.
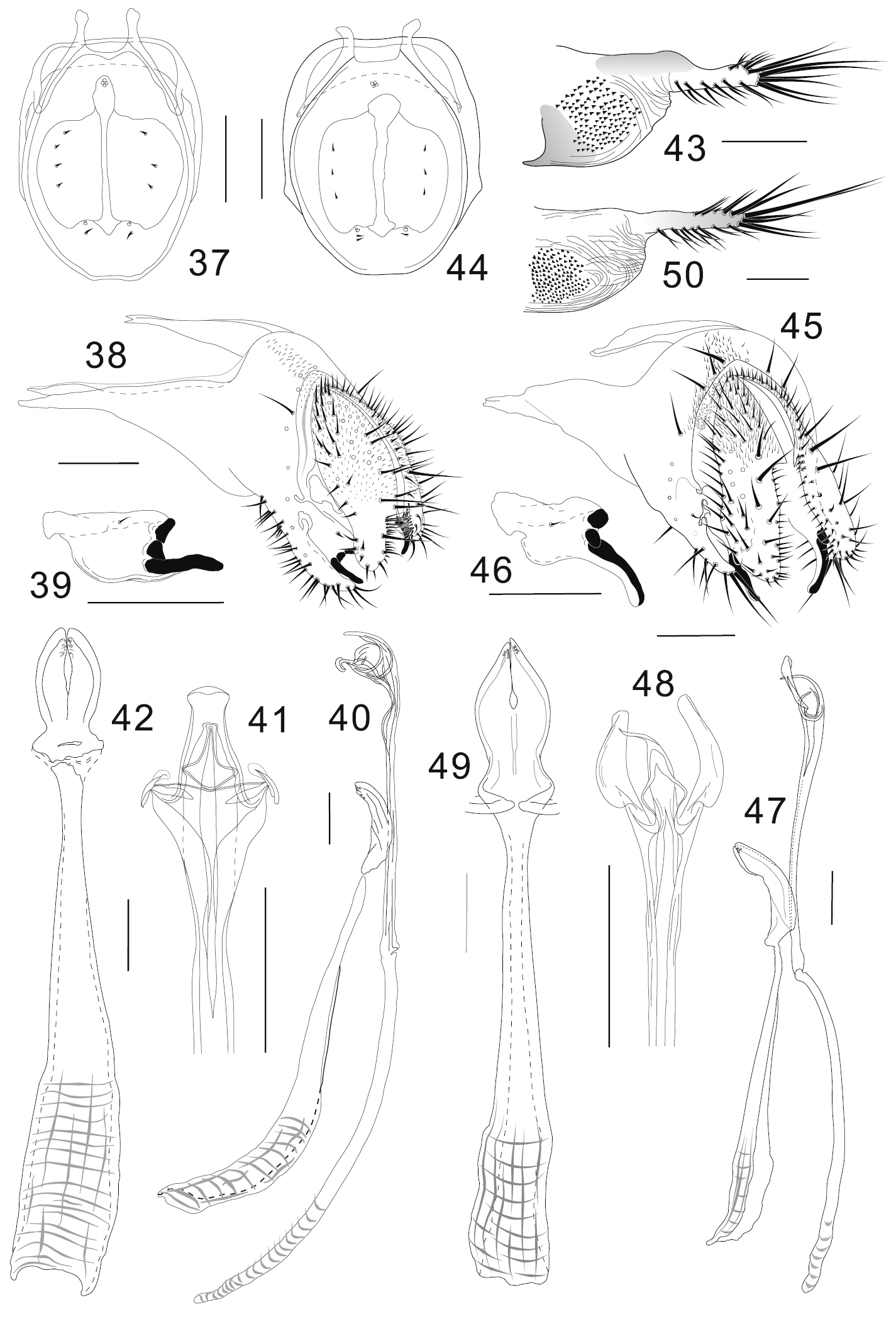
Colocasiomyia hailini Li & Gao, sp. n. (37–43) and Colocasiomyia yini Li & Gao, sp. n. (44–50). Adult males and females (paratypes) from Baihualing, Yunnan, China: 37, 44 Cibarium and clypeus (dorsal view) 38, 45 periphallic organs (posterolateral view) 39, 46 surstylus 40, 47 phallic organs (lateral view) 41, 48 apical part of aedeagus (ventral view) 42, 49 hypandrium and parameres (ventral view) 43, 50 oviscapt (lateral view). Scale lines = 0.1 mm.
Remarks. The characters described to be common among the three known species of the gigantea group by
http://zoobank.org/67051239-8955-4D5D-A7CE-9B575A4270A9
http://species-id.net/wiki/Colocasiomyia_longifilamentata
Figs 5, 12, 19, 23–28Holotype ♂ (No. 000068): CHINA: Baihualing, Longyang, Baoshan, Yunnan, 1500 m (25°17.19'N, 98°47.65'E), ex Rhaphidophora decursiva inflorescence at Stage III (the male phase: stamens appearing on the surface of spadix and dehiscing to release pollen), 16.vi.2011, JJ Gao (KIZ).
Paratypes: same data as holotype (7♂, 1♀: Nos 000069–78, 290, 291, 293); same but 16–17.vi.2011 (3♂, 1♀: Nos 000349, 350, 1133, 1134); same but 12.vii.2011 (1♂: No. 000168); same but 14.vii.2011 (1♂: No. 000353); same but from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected on 16.vi.2011, JJ Gao (4♂, 1♀: Nos 001597, 98, 1631–33); from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected from Yixiang, Simao, Pu’er, Yunnan, 1250 m (22°41.19'N, 101°7.77'E), on 12–13.xii.2012, JJ Gao and Z Fu (5♂, 5♀: Nos 001523, 26, 27–29, 31–33, 76, 77) (KIZ, SEHU).
Adult male. Head: Supracervical setae 12–15 per side. Dorsomedial arm of tentorial apodeme approximately 1/2 as long as dorsolateral arm. Eye red, somewhat roundish, with very sparse interfacetal setulae. Frontal vitta mat, black. First flagellomere not concave on inner margin. Facial carina trapeziform, medially wider than twice width of first flagellomere, as long as pedicel and first flagellomere combined. Palpus convex on ventrodistal portion. Cibarial posterior sensilla minute, 2 or 3 per side (Fig. 23). Labellum with 22 pseudotracheae per side.
Thorax: Scutum and scutellum glossy, blackish brown to black; thoracic pleura glossy, blackish brown. Acrostichal setulae in 6 rows.
Wing: Veins yellow. Halter grayish brown except for grayish yellow stalk.
Legs: Foreleg second tarsomere with 8–11 pegs (Figs 5, 12). Foreleg coxa large, with approximately 8 long setae on underside near attachment to trochanter. Small preapical dorsal setae present only on tibiae of hindlegs.
Abdomen: Tergites glossy, entirely blackish brown; II to VI+VII each bearing setulae and setae in approximately 3–4 transverse rows; setae of posteriormost row largest. Sternites pale brown to brown; VI posteriorly not bilobed.
Terminalia (Figs 24–27): Epandrium pubescent except for anterior margin, antero- and postero-ventral portion and large apodeme lobe, with 6–7 setae per side near posterior margin; anteroventral portion of epandrium curved inward, apically articulated to lateral arm of hypandrium; posteroventral lobe well developed, narrowly prolonged, scabbard-like, apically with a large peg, latero-ventrally with 12–13 short to moderate setae, dorso-subbasally with 2 very long, apically diverged setae extending almost beyond its tip, and 2 shorter ones (Fig. 24). Cercus crescent, pubescent on dorsal 2/3, with approximately 31 long setae, including 3 distinctly longer ones: 2 on ventral apex and 1 on subventral portion (Fig. 24). Membrane between epandrium and cercus pubescent dorsally to laterally. Surstylus entirely narrow, downward elongated, with only trichoid setae: 2 long at apex, 1 long on submedial inner surface, and 2 small setulae on subapical outer surface (Figs 24, 25). Median piece of 10th sternite somewhat anchor-shaped in posterior view, medially forming longitudinal ridge, laterally with broad flank. Paramere somewhat blade-like in lateral view, apically with 5 minute sensilla along edge (Figs 26, 27). Aedeagus nearly entirely separated into a pair of lateral lobes ventrally connected by subbasally to subapically densely pubescent membrane, slightly curved ventrad subapically, somewhat pointed apically; apodeme proceeding nearly along aedeagal axis, shorter than aedeagus, but longer than its 1/2; aedeagal basal processes connecting dorsobasal corners of aedeagus and lateral arms of hypandrium (Fig. 26).
Measurements (holotype / range in 6♂ paratypes, in mm): BL (straight distance from anterior edge of pedicel to tip of abdomen) = 2.76 / 2.45–2.75, ThL (medial distance from anterior notal margin to apex of scutellum) = 1.29 / 1.17–1.37, WL (distance from humeral cross vein to wing apex) = 2.49 / 2.31–2.74, WW (maximum wing width) = 1.08 / 0.96–1.07.
Indices (holotype / range in 6♂ paratypes): FW/HW (frontal width / head width) = 0.55 / 0.55−0.57, ch/o (maximum width of gena / maximum diameter of eye) = 0.52 / 0.44−0.63, prorb (proclinate orbital seta / posterior reclinate orbital seta in length) = 1.55 / 1.34–1.67, rcorb (anterior reclinate orbital seta / posterior reclinate orbital seta in length) = 0.49 / 0.38–0.56, orbito (distance between proclinate and posterior reclinate orbital setae / distance between inner vertical and posterior reclinate orbital setae) = 0.71 / 0.70−0.83, vb (subvibrissal seta / vibrissa in length) = 0.33 / 0.24–0.39, dcl (anterior dorsocentral seta / posterior dorsocentral seta in length) = 0.51 / 0.53–0.68, presctl (prescutellar seta / posterior dorsocentral seta in length) = 0.46 / 0.45–0.54, sctl (basal scutellar seta / apical scutellar seta in length) = 0.71 / 0.63–0.74, sterno (anterior katepisternal seta / posterior katepisternal seta in length) = 0.72 / 0.68–0.80, mid katepisternal seta indistinguishable from the other fine setae, dcp (distance between ipsilateral dorsocentral setae / distance between anterior dorsocentral setae) = 1.07 / 0.85−1.31, sctlp (distance between ipsilateral scutellar setae / distance between apical scutellar setae) = 1.10 / 0.94−1.39, C (2nd costal section between subcostal break and R2+3 / 3rd costal section between R2+3 and R4+5) = 2.56 / 2.28−2.75, 4c (3rd costal section between R2+3 and R4+5 / M1 between r-m and dm-cu) = 0.81 / 0.76−0.98, 4v (M1 between dm-cu and wing margin / M1 between r-m and dm-cu) = 1.44 / 1.37−1.70, 5× (CuA1 between dm-cu and wing margin / dm-cu between M1 and CuA1) = 0.88 / 0.75−0.82, ac (3rd costal section between R2+3 and R4+5 / distance between distal ends of R4+5 and M1) = 3.44 /2.81–4.13, M (CuA1 between dm-cu and wing margin / M1 between r-m and dm-cu) = 0.16 / 0.14–0.19, C3F (length of heavy setation in 3rd costal section / length of 3rd costal section) = 0.79 / 0.74–0.83.
Female. Head, thorax, wing and legs as in male.
Terminalia: Tergite VII mid-dorsally not constricted; VIII entirely pubescent, with 5 setae in transverse (against body axis) row on discolored posteroventral portion. Oviscapt distally narrowing; distal narrow portion as long as proximal, broad portion, with approximately 15, 6 and 3 trichoid ovisensilla per side on ventral, dorsal and apical margins, respectively, and a tiny, peg-like ovisensillum near dorsosubapical margin (Fig. 28).
Measurements (range in 5♀ paratypes, in mm): BL = 2.66–3.30, ThL = 1.33–1.47, WL = 2.40–2.90, WW = 1.03–1.21.
Indices (range in 5♀ paratypes): FW/HW = 0.55−0.60, ch/o = 0.51−0.59, prorb = 1.35–1.56, rcorb = 0.42−0.78, orbito = 0.65−0.80, vb = 0.28−0.39 (4♀), dcl = 0.46−0.63, presctl = 0.45–0.56, sctl = 0.65−0.74 (4♀), sterno = 0.44−0.77, dcp = 0.92−1.00, sctlp = 1.04−1.20, C = 2.21−2.68, 4c = 0.79−0.90, 4v = 1.44−1.53, 5x = 0.68−0.97, ac = 2.73−3.59, M = 0.17–0.19, C3F = 0.78–0.86.
Filaments 2, approximately 1.8–2.4 times as long as length of egg body.
The specific name “longifilamentata” refers to the long filaments of egg.
China (Yunnan).
Although this species closely resembles Colocasiomyia rhaphidophorae in the external morphology and structures of male and female terminalia, it can be easily distinguished from the latter by the epandrium having several setae on the dorsal to lateral portion (Fig. 24) (Colocasiomyia rhaphidophorae lacking setae there).
http://zoobank.org/01776174-A214-4450-8AD6-8B17016CAA15
http://species-id.net/wiki/Colocasiomyia_longivalva
Figs 6, 13, 20, 29–36Holotype ♂ (No. 000079): CHINA: Baihualing, Longyang, Baoshan, Yunnan, 1500 m (25°17.19'N, 98°47.65'E), ex Rhaphidophora decursiva inflorescence at Stage III, 17.vi.2011, JJ Gao (KIZ).
Paratypes: same data as holotype but 16.vi.2011 (2♂, 8♀: Nos 000080–87, 171, 172); same but 15.vi.2011 (1♂: No. 000170) (KIZ, SEHU).
Adult male. Head: Supracervical setae 13 per side. Dorsomedial arm of tentorial apodeme 1/3 as long as dorsolateral arm. Eye red, somewhat roundish, with very sparse interfacetal setulae. Frontal vitta mat, black. First flagellomere not concave on inner margin. Facial carina trapeziform, medially wider than twice width of first flagellomere, as long as pedicel and first flagellomere combined. Palpus convex on ventrodistal portion. Cibarial posterior sensilla minute, 1 per side (Fig. 29). Labellum with 20 pseudotracheae per side.
Thorax: Scutum and scutellum glossy, black; thoracic pleura glossy, blackish brown. Acrostichal setulae in 6 rows.
Wing: Veins yellow. Halter grayish brown except for grayish yellow stalk.
Legs: Foreleg second tarsomere with 8–10 pegs (Figs 6, 13). Foreleg coxa large, with approximately 8 long setae on underside near attachment to trochanter. Small preapical dorsal setae present only on tibiae of hindlegs.
Abdomen: Tergites glossy, entirely dark brown; II to VI+VII each bearing setulae and setae irregularly arranged; setae of posteriormost row largest. Sternites grayish yellow; VI posteriorly not bilobed.
Terminalia (Figs 30–35): Epandrium notched above insertion of ventral lobe, pubescent except for anterolateral margin to ventral portion and ventral lobe, with 7–8 setae per side along ventral margin of ventral lobe and approximately 10 setae (including 2 thickest ones located just above and 2 shortest ones just on subventral notch) along posterior margin; anteroventral portion curved inward, apically articulated to lateral arm of hypandrium (Figs 30, 31); ventral lobe prolonged like rod, apically with grooved, finger-like peg (Figs 30–32). Surstylus basally articulated to inner, basal corner of epandrial ventral lobe, 1/3 as long as epandrial ventral lobe, distally nearly parallel with epandrial ventral lobe, with 2 trichoid setae apically and 2 trichoid, thinner setae on ventral, subapical surface (Figs 31, 33). Cercus large, somewhat rhombic, wider than 1/2 its (dorsoventral) height, pubescent on dorsal 2/3, with approximately 33 setae mostly distributed near posterior margin, including slightly prominent one at caudoventral apex (Fig. 30). Membrane between epandrium and cercus pubescent dorsally to laterally. Tenth sternite folded into two lateral lobes connected with each other caudodorsally; lateral lobe triangularly extended anterodorsally, fused with membrane between epandrium and cercus. Hypandrium narrow plate-like, posteriorly T-shaped, with lateral arms fused to membranous, aedeagal basal processes (Figs 34, 35). Parameres long, coalescent to hypandrium, triangular in ventral view, apically with 4 minute sensilla arranged in a row, basally fused to each other (Figs 34, 35). Aedeagus nearly entirely separated into a pair of lateral lobes, pubescent basally, bent ventrad subapically, narrowly pointed at apex; aedeagal apodeme rod-like, 1/2 as long as aedeagus (Fig. 34); aedeagal basal processes membranous, connecting dorsobasal corners of aedeagus and lateral arms of hypandrium.
Measurements (holotype / range in 3♂ paratypes, in mm): BL = 3.30 / 2.40−3.20, ThL = 1.40 / 1.18−1.42, WL = 2.80 / 2.50−2.88, WW =1.09 / 1.03−1.20.
Indices (holotype / range in 3♂ paratypes): FW/HW = 0.49 / 0.48−0.50, ch/o = 0.51 / 0.53−0.54, prorb = 1.04 / 1.25−1.49, rcorb = 0.44 / 0.44−0.50, orbito = 0.74 / 0.56−0.66, vb = 0.47 / 0.38−0.50, dcl = 0.50 / 0.51−0.59, presctl = 0.50 / 0.51−0.59, sctl = 0.91 / 0.65−0.77, sterno = 0.65 / 0.56−0.66, mid katepisternal seta indistinguishable from other fine setae, dcp = 1.08 / 0.86−1.07, sctlp = 1.23 / 1.10−1.26, C = 1.76 / 1.79−1.83, 4c = 1.16 / 1.18−1.19, 4v = 1.83 / 1.85−1.95, 5x = 0.98 / 0.99−1.18, ac = 4.54 / 3.84−4.08, M = 0.14 / 0.15−0.17, C3F = 0.83 / 0.84−0.88.
Female. Head, thorax, wing and legs as in male.
Terminalia: Tergite VII mid-dorsally not constricted; VIII pubescent nearly entirely, with 3−4 setae in a transverse row on unpubescent medio-posterior portion. Oviscapt distal narrow portion twice as long as proximal, broad portion, with approximately 18, 6 and 3 trichoid ovisensilla per side on ventral, dorsal and apical margins, respectively, and tiny, peg-like ovisensillum near mid-dorsal margin (Fig. 36).
Measurements (range in 8♀ paratypes, in mm): BL = 2.80−3.21 (7♀), ThL = 1.26−1.47, WL = 2.67−2.90, WW =1.08−1.26.
Indices (range in 8♀ paratypes): FW/HW = 0.49−0.52, ch/o = 0.49−0.54, prorb = 0.97−1.25, rcorb = 0.34−0.57, orbito = 0.56−0.73, vb = 0.43−0.64, dcl = 0.49−0.56, presctl = 0.49−0.56, sctl = 0.64−0.87 (7♀), sterno = 0.63−0.87, dcp = 0.82−1.06, sctlp = 1.10−1.31, C = 1.78−2.06, 4c = 1.05−1.23, 4v = 1.81−1.99, 5x = 0.88−1.18, ac = 3.55−4.36, M = 0.14−0.18, C3F = 0.84−0.89.
China (Yunnan).
Pertaining to the long oviscapt valva.
Adults of this species were very rarely captured from inflorescences of Rhaphidophora decursiva. So far we have never get any adult of this species by laboratory rearing from the host inflorescences/infructescences. This species is distinguished from the other members of the gigantea group: epandrium somewhat notched above insertion of ventral lobe; epandrial ventral lobe prolonged like rod, apically with grooved, finger-like peg (Figs 30–32); surstylus 1/3 as long as epandrial ventral lobe, distally nearly parallel with the latter (Figs 31, 33); paramere large, as long as hypandrium (Figs 34, 35); distal narrow portion of oviscapt twice as long as proximal, broad portion (Fig. 36).
http://zoobank.org/8F467F73-9310-499B-875B-AB023BCD6992
http://species-id.net/wiki/Colocasiomyia_hailini
Figs 7, 14, 21, 37–43Holotype ♂ (No. 001641): CHINA: Baihualing, Longyang, Baoshan, Yunnan, 1500 m (25°17.27'N, 98°48.7'E), ex Rhaphidophora decursiva inflorescence at Stage III, 29.vi.2006, JT Yin (KIZ).
Paratypes: same data as holotype (11♂, 27♀: Nos 001634–40, 42–49, 51–73); same but (25°17.19'N, 98°47.65'E) (2♂, 2♀: Nos 001674, 75, 1135, 1136), 19.vi.2011, JJ Gao; same but (25°17.19'N, 98°47.65'E) (4♂: Nos 000355–58), 12–15.vii.2011, JJ Gao; same but (25°17.19'N, 98°47.65'E) (2♂, 2♀: Nos 001298–301), from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected on 3–9.viii.2012, JJ Gao, Z Fu, NN Li, JM Chen and SS Li; Yixiang, Simao, Pu’er, Yunnan (22°41.19'N, 101°7.77'E) (2♂, 1♀: Nos 001448, 50, 1517), from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected on 12–13.xii.2012, JJ Gao and Z Fu (KIZ, SEHU).
Adult male. Head: Supracervical setae approximately 7 per side. Dorsomedial arm of tentorial apodeme 1/3 as long as dorsolateral arm. Eye red, somewhat roundish, with very sparse interfacetal setulae. Frontal vitta mat black. First flagellomere concave on inner margin. Facial carina trapeziform, medially wider than twice width of first flagellomere, as long as pedicel and first flagellomere combined. Palpus convex on ventrodistal portion. Cibarial posterior sensilla minute, 1 per side (Fig. 37). Labellum with 11 pseudotracheae per side.
Thorax: Scutum and scutellum glossy, blackish brown to black; thoracic pleura glossy, blackish brown. Acrostichal setulae in 4 rows.
Wing: Veins yellow. Halter grayish brown except for grayish yellow stalk.
Legs: Foreleg second tarsomere with 6 pegs (Figs 7, 14). Foreleg coxa large, with approximately 15 long setae on underside near attachment to trochanter. Small preapical dorsal setae present only on tibiae of hindlegs.
Abdomen: Tergites glossy, entirely dark brown; II to VI+VII each bearing setulae and setae in approximately 3 transverse rows; setae of posteriormost row largest. Sternites pale brown to brown; VI posteriorly not bilobed.
Terminalia (Figs 38–42): Epandrium pubescent dorsomedially only, with 6 setae per side near posterior margin, 15–16 setae per side on ventral portion and 23–24 setae as thick as upper cercal ones along ventral margin of ventral lobe; apodeme well developed into distally tapering, triangular extension strongly projected anteriad, twice as long as epandrial width, broadly sclerotized along dorsal and ventral margins (Fig. 38); anteroventral portion of epandrium curved inward, apically articulated to lateral arm of hypandrium. Surstylus broad, basally narrowly fused to basal corner of epandrial ventral lobe, dorsally broadly sclerotized, with 1 short, trichoid seta on upper medial portion of outer surface and 3 large, peg-like prensisetae on distal margin; lowest prensiseta nearly as long as width of surstylus, curved inwards and slightly downwards, especially in distal 1/3, narrowly edged by caudoventral portion of surstylus only along its basal portion (Figs 38, 39). Cercus semilunar, narrower than 1/2 its (dorsoventral) height, pubescent on dorsal 2/3, with approximately 48 setae (including prominent one twice as long as others) all over and approximately 45 setulae on caudoventral, inner margin; ventral slightly incurved lobe 1/3–1/4 of cercal height (Fig. 38). Membrane between epandrium and cercus pubescent dorsally to laterally. Median piece of 10th sternite cordiform in posterior view, moderately sclerotized; lateral piece somewhat cuneiform, narrowing anteriad, connected to inner, basal corner of epandrial ventral lobe with membranous tissue. Hypandrium long, narrow, plate-like, anteriorly widened (Fig. 42). Parameres somewhat semilunar in ventral view, basally fused to each other, apically with 5 or 6 minute sensilla in small medioapical patch (Figs 40, 42). Aedeagus nearly entirely separated into a pair of lateral lobes, nearly straight, apically trilobed; lobes curved ventrad and connected with each other by tendon-like membranous structures (Figs 40, 41); apodeme rod-like, arched in lateral view, longer than aedeagus (Fig. 40).
Measurements (holotype / range in 10♂ paratypes, in mm): BL = 2.01 / 1.85−2.14, ThL = 0.86 / 0.76−0.94, WL = 1.79 / 1.59−1.89, WW = 0.79 / 0.72−0.85.
Indices (holotype / range in 10♂ paratypes): FW/HW = 0.54 / 0.53−0.56, ch/o = 0.48 / 0.46−0.55, prorb = broken / 1.08 (1♂), rcorb = 0.61 / 0.56−0.59 (6♂), orbito = 0.86 / 0.63−0.85, vb = 0.31 / 0.30−0.36, dcl = 0.52 / 0.54−0.62 (6♂), presctl = 0.38 / 0.30−0.39 (9♂), sctl = 0.75 / 0.59−0.77 (9♂), sterno = 0.75 / 0.72−0.94, mid katepisternal seta indistinguishable from other fine setae, dcp = 0.92 / 0.82−1.02, sctlp = 1.00 / 0.92−1.12, C = 1.90 / 1.72−2.12, 4c = 1.18 / 0.89−1.21, 4v = 1.79 / 1.28−1.75, 5x = 1.15 / 0.94−1.17, ac = 3.56 /3.04−3.59, M = 0.19 / 0.19−0.21, C3F = 0.48 / 0.35−0.53.
Female. Head, thorax, wing and legs as in male.
Terminalia: Tergite VII mid-dorsally not constricted; VIII pubescent nearly entirely, with 4–5 setae in a longitudinal row on unpubescent, discolored, posteroventral portion. Oviscapt distal elongation constricted dorsally at basal 1/3, apically somewhat truncate, with 6, 9 and 3 trichoid ovisensilla per side on distal 1/3 of dorsal margin, entire ventral margin, and at apex, respectively (Fig. 43).
Measurements (range in 10♀ paratypes, in mm): BL = 2.43−2.95, ThL = 0.89−1.22, WL = 1.90−2.48, WW = 0.85−1.07.
Indices (range in 10♀ paratypes): FW/HW = 0.55−0.59, ch/o = 0.50−0.62, prorb broken, rcorb = 0.40−0.52 (3♀), orbito = 0.68−0.91, vb = 0.29−0.35, dcl = 0.43−0.57 (6♀), presctl = 0.29−0.37 (7♀), sctl = 0.69−0.77 (9♀), sterno = 0.74−0.91, dcp = 0.90−1.09, sctlp = 1.00−1.12, C = 1.83−2.23, 4c = 1.00−1.14, 4v = 1.50−1.86, 5x = 0.87−1.19, ac = 3.28−4.00, M = 0.16−0.20, C3F = 0.38−0.59.
China (Yunnan).
The specific name “hailini” is a patronym in honor of the emeritus Professor Hai-lin Wang of the Southwest Forestry University, China.
This species resembles the following species, Colocasiomyia yini sp. n., in overall outer morphology, but can be distinguished from the latter by 1) C3F < 2/3 (C3F ≥ 2/3 in yini sp. n.); 2) lowest, peg-like prensiseta on distal margin of surstylus curved inwards and slightly downwards, especially in distal 1/3, narrowly edged by caudoventral portion of surstylus only along its basal portion (Fig. 39) [in Colocasiomyia yini sp. n., lowest peg-like prensiseta strongly curved downwards in distal half, narrowly edged by caudoventral portion of surstylus along its whole length (Figs 46)]; 3) ventral slightly incurved lobe of cercus 1/3–1/4 of cercal height (Fig. 38) [in Colocasiomyia yini, ventral lobe of cercus approximately 1/2 of cercal height (Fig. 45)]; 4) distal, narrow part of oviscapt constricted dorsally at basal 1/3 (Fig. 43) [in Colocasiomyia yini sp. n., distal, narrow part of oviscapt almost even-edged (Fig. 50)].
http://zoobank.org/0A22EA8C-6EF6-48DF-B46C-B946CEE327E9
http://species-id.net/wiki/Colocasiomyia_yini
Figs 8, 15, 22, 44–50Holotype ♂ (No. 000159): CHINA: Baihualing, Longyang, Baoshan, Yunnan, 1500 m (25°17.19'N, 98°47.65'E), ex Rhaphidophora decursiva inflorescence at Stage III, 12.vii.2011, JJ Gao (KIZ).
Paratypes. Same data as holotype (3♂, 2♀: Nos 000160, 165-167, 178); same but 16.vi.2011 (1♂, 2♀: Nos 000161-163); same but (25°17.27'N, 98°48.7'E), 29.vi.2006 (1♀: No. 001650), JT Yin; same but 13.vii.2011 (1♀: No. 000164); same but 14.vii.2011 (1♂: No. 000169); same but from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected on 23–24.ix.2012, JJ Gao, Z Fu and JM Chen (1♀: No. 001185); from laboratory rearing of eggs in infructescences of Rhaphidophora decursiva collected from Yixiang, Simao, Pu’er, Yunnan (22°41.19'N, 101°7.77'E) on 12–13.xii.2012, JJ Gao and Z Fu (1♀: No. 001586) (KIZ, SEHU).
Adult male. Head: Supracervical setae 11 per side. Dorsomedial arm of tentorial apodeme 1/3 as long as dorsolateral arm. Eye red, somewhat roundish, with very sparse interfacetal setulae. Frontal vitta mat black. First flagellomere not concave on inner margin. Facial carina trapeziform, medially wider than twice width of first flagellomere, as long as pedicel and first flagellomere combined. Palpus convex on ventrodistal portion. Cibarial posterior sensilla minute, 1 or 2 per side (Fig. 44). Labellum with 11 pseudotracheae per side.
Thorax: Scutum and scutellum glossy, black; thoracic pleura glossy, blackish brown. Acrostichal setulae in 4 rows.
Wing: Veins yellow. Halter dark brown except for grayish yellow stalk.
Legs: Foreleg second tarsomere with 6 pegs (Figs 8, 15). Foreleg coxa large, with approximately 10 long setae on underside near attachment to trochanter. Small preapical dorsal setae present only on tibiae of hindlegs.
Abdomen: Tergites glossy, entirely dark brown; II to VI+VII each bearing setulae and setae in approximately 3 transverse rows; setae of posteriormost row largest. Sternites II–V pale brown; VI blackish brown, and bilobed posteriorly.
Terminalia (Figs 45–49): Epandrium with 6 setae per side from lateral portion to middorsal, posterior margin, 14 setae per side in ventral portion and 15–16 setae as thick as upper cercal ones along ventral margin of ventral lobe; apodeme well developed into distally tapering, triangular extension strongly projected anteriad, twice as long as epandrial width, broadly sclerotized along dorsal and ventral margins (Fig. 45); anteroventral portion of epandrium curved inward, apically articulated to lateral arm of hypandrium (Fig. 45). Surstylus basally fused to basal corner of epandrial ventral lobe, dorsally broadly sclerotized, with 1 short, trichoid seta on upper medial portion, and 3 large, peg-like prensisetae on distal margin; lowest prensiseta slightly longer than width of surstylus, strongly curved downwards in distal half, narrowly edged by caudoventral portion of surstylus along its whole portion (Figs 45, 46). Cercus oblong, narrower than 1/2 its (dorsoventral) height, pubescent on dorsal 1/2, with approximately 58 setae (including one distinctively longer than others) all over and approximately 26 setulae on caudoventral, inner margin; ventral lobe approximately 1/2 of cercal height (Fig. 45). Membrane between epandrium and cercus pubescent dorsally to laterally. Median piece of 10th sternite rhombic in posterior view, moderately sclerotized; lateral piece somewhat cuneiform, narrowing anteriad, connected to inner, basal corner of epandrial ventral lobe with membranous tissue. Hypandrium long, narrow, plate-like, anteriorly widened, posteriorly T-shaped, with lateral arms fused to membranous aedeagal basal processes (Figs 47, 49). Parameres somewhat semilunar in ventral view, basally fused to each other, apically with 6 minute sensilla in small patch (Figs 47, 49). Aedeagus nearly entirely separated into a pair of lateral lobes, slightly bent, apically trilobed; median lobe curved ventrad and connected with lateral ones by tendon-like membranous structures (Figs 47, 48); apodeme rod-like, arched in lateral view, as long as aedeagus (Fig. 47); aedeagal basal processes membranous, connecting dorsobasal corners of aedeagus and posterolateral expansions of hypandrium.
Measurements (holotype / range in 5♂ paratypes, in mm): BL = 2.66 / 2.20−2.56 (4♂), ThL = 1.12 / 0.90−1.05, WL = 2.27 / 1.87−2.27, WW =1.03 / 0.85−1.00.
Indices (holotype / range in 5♂ paratypes): FW/HW = 0.53 / 0.50−0.56, ch/o = 0.54 / 0.47−0.59, prorb = 1.04 / 0.97−1.20, rcorb = 0.42/ 0.48−0.55, orbito = 0.58 / 0.56−0.68, vb = 0.46 / 0.40−0.52, dcl = 0.50 / 0.47−0.50, presctl = 0.34 / 0.24−0.37, sctl = 0.62 / 0.57−0.69 (4♂), sterno = 0.79 / 0.79−1.21 (3♂), mid katepisternal seta indistinguishable from other fine setae, dcp = 0.88 / 0.80−0.90, sctlp = 1.15 / 0.90−1.03, C = 2.35 / 2.04−2.52, 4c = 0.96 / 0.95−1.07, 4v = 1.67 / 1.63−1.92, 5x = 1.02 / 1.06−1.27, ac = 3.75 / 3.00−3.68, M = 0.15 / 0.16−0.18, C3F = 0.77 / 0.69−0.80.
Female. Head, thorax, wing and legs as in male.
Terminalia: Tergite VII mid-dorsally not constricted; VIII pubescent nearly entirely, with 5−6 setae in a transverse row on unpubescent, discolored, posteroventral portion. Oviscapt distal elongation almost smooth on dorsal margin, with 5, 7 and 3 trichoid ovisensilla per side on distal 1/2 of dorsal margin, entire ventral margin and at apex, respectively, of distal elongation (Fig. 50).
Measurements (range in 5♀ paratypes, in mm): BL = 2.05−2.86 (4♀), ThL = 0.93−1.20, WL = 2.20−2.60, WW = 0.86−1.05.
Indices (range in 5♀ paratypes): FW/HW = 0.53−0.56, ch/o = 0.43−0.60, prorb = 0.88−1.11, rcorb = 0.28−0.51, orbito = 0.60−0.70, vb = 0.29−0.45, dcl = 0.46−0.52, presctl = 0.26−0.34, sctl = 0.50−0.65 (4♀), sterno = 0.76−0.83 (4♀), dcp = 0.81−0.84, sctlp = 0.85−1.13, C = 2.20−2.34, 4c = 0.96−1.03, 4v = 1.67−1.92, 5x = 0.91−1.23, ac = 3.12−4.76, M = 0.13−0.18, C3F = 0.65−0.74.
China (Yunnan).
In honor of Mr Jian-Tao Yin of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.
Much fewer adults of this species were collected from inflorescences of Rhaphidophora decursiva in comparison to Colocasiomyia hailini sp. n. and Colocasiomyia longifilamentata sp. n. Breeding of this species on Rhaphidophora decursiva was confirmed by laboratory rearing of eggs laid on inflorescences of the host plant. See the Remarks for Colocasiomyia hailini sp. n. with respect to morphological differences from it.
In this key, the numbers of figures of
| 1 | Labellum with 16 pseudotracheae per side. Aedeagal apodeme distinctly longer than aedeagus (“Fig. 4C”). Distal, narrow part of oviscapt narrowing and gently curved ventrad, apically arrowhead-shaped (“Fig. 4E”) | Colocasiomyia scindapsae Fartyal & Toda |
| – | Labellum with ≤ 14 or ≥ 20 pseudotracheae per side. Aedeagal apodeme as long as or shorter than aedeagus (Figs 26, 34, 40, 47; “Figs 2F, 3D”). Distal narrow part of oviscapt truncate apically, or curved dorsad if not truncate | 2 |
| 2 | Labellum with 14 pseudotracheae per side. Distal, narrow part of oviscapt broadly truncate apically, much shorter than proximal, broad part (“Fig. 2H”) | Colocasiomyia gigantea (Okada) |
| − | Labellum with 11 or ≥ 20 pseudotracheae per side. Distal, narrow part of oviscapt not or only slightly truncate apically, curved dorsad apically, longer or only slightly shorter than proximal, broad part (Figs 28, 36, 43, 50) | 3 |
| 3 | Labellum with 11 pseudotracheae per side. Foreleg tarsomere II with 6 pegs (Figs 7, 8, 14, 15). Acrostichal setulae in 4 rows | 4 |
| − | Labellum with ≥ 20 pseudotracheae per side. Foreleg tarsomere II with ≥ 8 pegs (Figs 2–6, 9–13). Acrostichal setulae in 6 rows | 5 |
| 4 | Wing C3F index < 2/3. Distance between antennal sockets same as socket width. Distal, narrow part of oviscapt constricted subbasally on dorsal margin (Fig. 43) | Colocasiomyia hailini Li & Gao, sp. n. |
| − | Wing C3F index > 2/3. Distance between antennal sockets larger than socket width. Distal, narrow part of oviscapt finger-like, not constricted subbasally on dorsal margin (Fig. 50) | Colocasiomyia yini Li & Gao, sp. n. |
| 5 | Epandrium notched above basal corner of epandrial ventral lobe; ventral lobe prolonged like rod, apically with grooved, finger-like peg (Figs 30–32). Surstylus 1/3 as long as epandrial ventral lobe, distally nearly parallel with the latter (Figs 31, 33). Hypandrium as long as paramere (Figs 34, 35). Distal, narrow part of oviscapt twice as long as proximal, broad part (Fig. 36) | Colocasiomyia longivalva Li & Gao, sp. n. |
| – | Epandrium not notched along posterior margin; ventral lobe narrowing distally, apically with ungrooved, apically pointed peg (Fig. 24; “Fig. 3C”). Surstylus entirely narrow, elongated downward, as long as epandrial ventral lobe (Fig. 25; “Fig. 3C”). Hypandrium 3–4 times as long as paramere (Figs 26, 27; “Fig. 3D, E”). Distal, narrow part of oviscapt as long as or shorter than proximal, broad part (Fig. 28; “Fig. 3G”) | 6 |
| 6 | Posterior margin of epandrium without setae (“Fig. 3C”). Palpus dark, apically swollen | Colocasiomyia rhaphidophorae Gao & Toda |
| – | Posterior margin of epandrium with several setae (Fig. 24). Palpus pale, apically not swollen | Colocasiomyia longifilamentata Li & Gao, sp. n. |
DNA barcoding has been innovated to facilitate works of not only taxonomists but also non-experts in specimen identification (http://ibol.org/about-us/background/). Even for expert taxonomists, it is not trivial to identify pre-imaginal stages or a mass of adult individuals of closely related species in Drosophilidae. In Colocasiomyia, it is known in a number of cases, including that given by the present study, that closely related species cohabit on the same host plant. To study mechanisms for cohabitation in such systems, DNA barcoding approaches should be very useful in species identification, especially for pre-imaginal stages. However, the “barcoding gap” between intra- and interspecific genetic distances based on COI sequences is too flimsy to validate the objective, distance-based species delimitation in the gigantea group. Similarly, in some previous studies, distance-based DNA barcoding based on COI sequences did not work well for species delimitation due to the overlapping of inter- and intraspecific distances (see
Unlike the other Colocasiomyia species, all the species of the gigantea group breed on (Colocasiomyia longivalva at least visits) inflorescences/infructescences of the subfamily Monsteroideae (Araceae). All of the host plants are hemiepiphytic, bisexual-flowered climbers belonging to the Rhaphidophora clade (
The monsteroid plants as hosts of the gigantea group are quite different in the structure of spadix and the fruiting process from the Aroideae known as hosts of other Colocasiomyia species groups.
So far, our knowledge about the biogeography of the gigantea group is apparently very limited with respect to the known geographical distribution of respective host plants. Colocasiomyia scindapsae has been recorded only from Mt. Kinabalu (Sabah), Colocasiomyia gigantea from Bogor (West Java) and Solomon Is., and the others from Yunnan, China. The ranges of their host plant species are wider: Scindapsus coriaceus is distributed in Indonesia and Malaysia (http://gwannon.com/species/Scindapsus-coriaceus), Rhaphidophora hookeri in China, Myanmar and Vietnam (http://www.gwannon.com/species/Rhaphidophora-hookeri), Rhaphidophora decursiva in Himalaya, India and Indonesia (http://en.hortipedia.com/wiki/Raphidophora_decursiva), and Epipremnum pinnatum being native to New Guinea, the Malay Archipelago and the Pacific Islands (http://en.hortipedia.com/wiki/Epipremnum_pinnatum) but currently distributed almost all over the Oriental and Australasian Region (
We thank Mr RS Lin and GM Li (the Baihualing Village) for their help in the field work, and Mr R He (Research Institute of Resources Insects, the Chinese Academy of Forestry) for his help in SEM photographing. This work was supported by the National Science Foundation of China (No. 31160429), the fund of the Ministry of Science and Technology of China (No. 2011FY120200 and 2012FY110800), and Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (Nos 21570085 and 24370033).