Citation: Wang Q, Han X, Xue X-F, Hong X-Y (2014) Three new species of eriophyoid mites (Acari, Eriophyoidea) associated with Lauraceae in China. ZooKeys 406: 81–100. doi: 10.3897/zookeys.406.6897
In this paper, three new species of eriophyoid mites in the family Eriophyidae associated with Phoebe hunanensis Hand.–Mazz. (Lauraceae), namely Gammaphytoptus striatilobus sp. n., Phyllocoptes setalsolenidion sp. n., and Dechela phoebe sp. n. are described and illustrated. All are vagrants causing no apparent damage to the same host plants.
Acari, plant feeding, Prostigmata, taxonomy
Eriophyoidea is the lineage most highly adapted for plant feeding among the Acari. Among the vast array of eriophyoid taxa, patterns varying from narrow to extreme host specificity are far more prevalent, and repeatedly independent, than in other groups of phytophagous mites (
During July 2013, field surveys were conducted in Zhangjiajie National Forest Park of Hunan Province. We found three species from the same host Phoebe hunanensis Hand.–Mazz. (Lauraceae), this plant is native to South China naturally in the sheltered and moist places in valleys, under forests or by streams (
Phoebe hunanensis Hand.–Mazz. (Lauraceae) –The host plant in this study.
So far, no eriophyoid mite species have been described or reported from Phoebe hunanensis. Two species are, however, known from other Phoebe species, which are Bucculacus phoebus Huang, 2001a and Phyllocoptruta hungmaoensix Xue, Cheng & Hong, 2012. Furthermore, seven of the nine recognized Gammaphytoptus species and three Phyllocoptes species are found associated with Lauraceae. A key to known Gammaphytoptus and Phyllocoptes species is given.
Eriophyoid mites were collected from plants with the aid of hand-lens (30×). Eriophyoids, together with their host plants, were placed in vials and stored in 75% ethanol. Each vial was marked with the following collection data: specimen number, date, host plant species name, colour of living mites, sample location, collector name and relationship of mite to the host plant. Collection data were also recorded in a notebook and examples of host plant parts were kept in a plant specimen folder in a dry environment for further identification and reference.
The morphological terminology follows
http://zoobank.org/F691F812-36E8-4CB7-BB9C-844634DE98CD
http://species-id.net/wiki/Gammaphytoptus_striatilobus
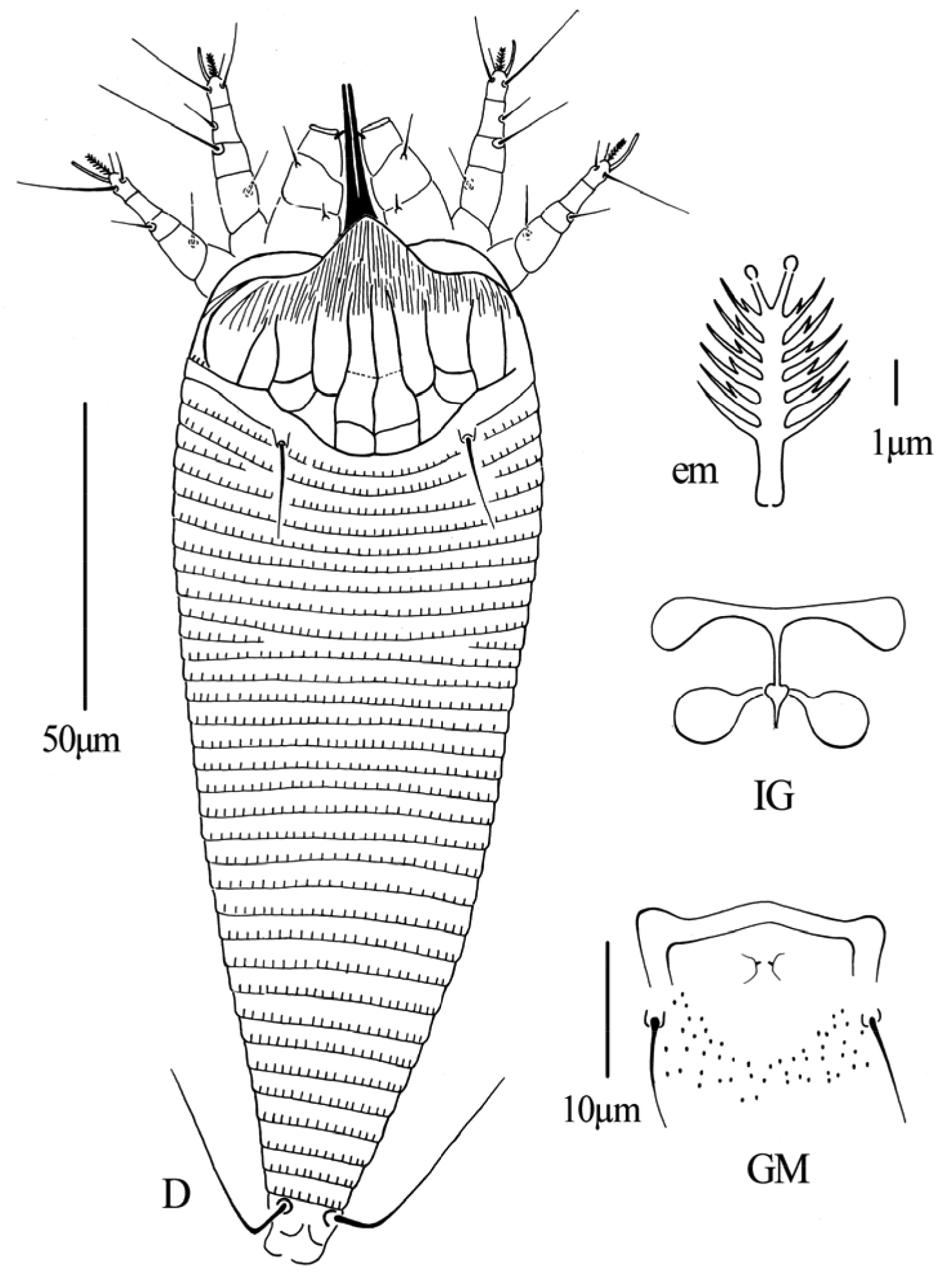
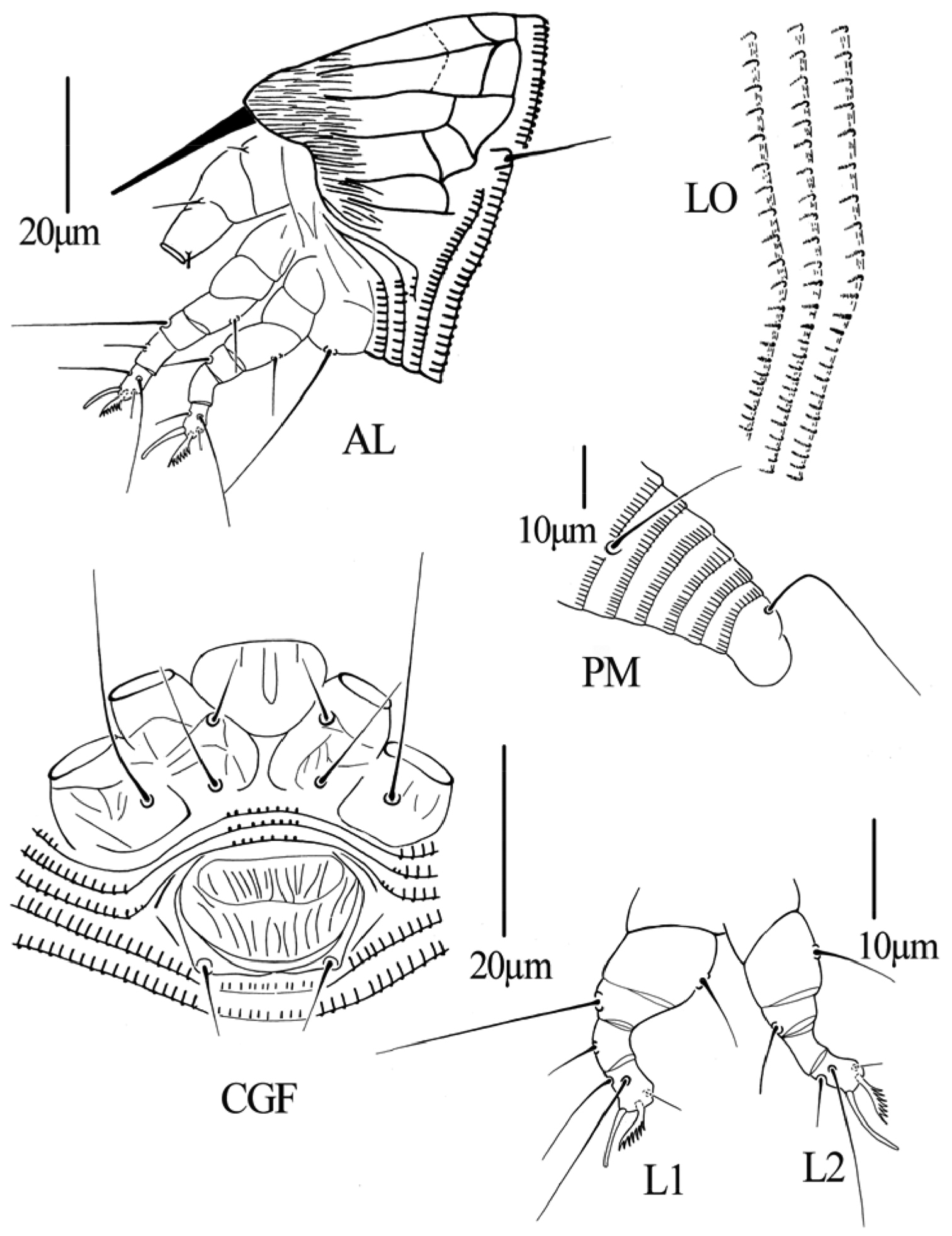
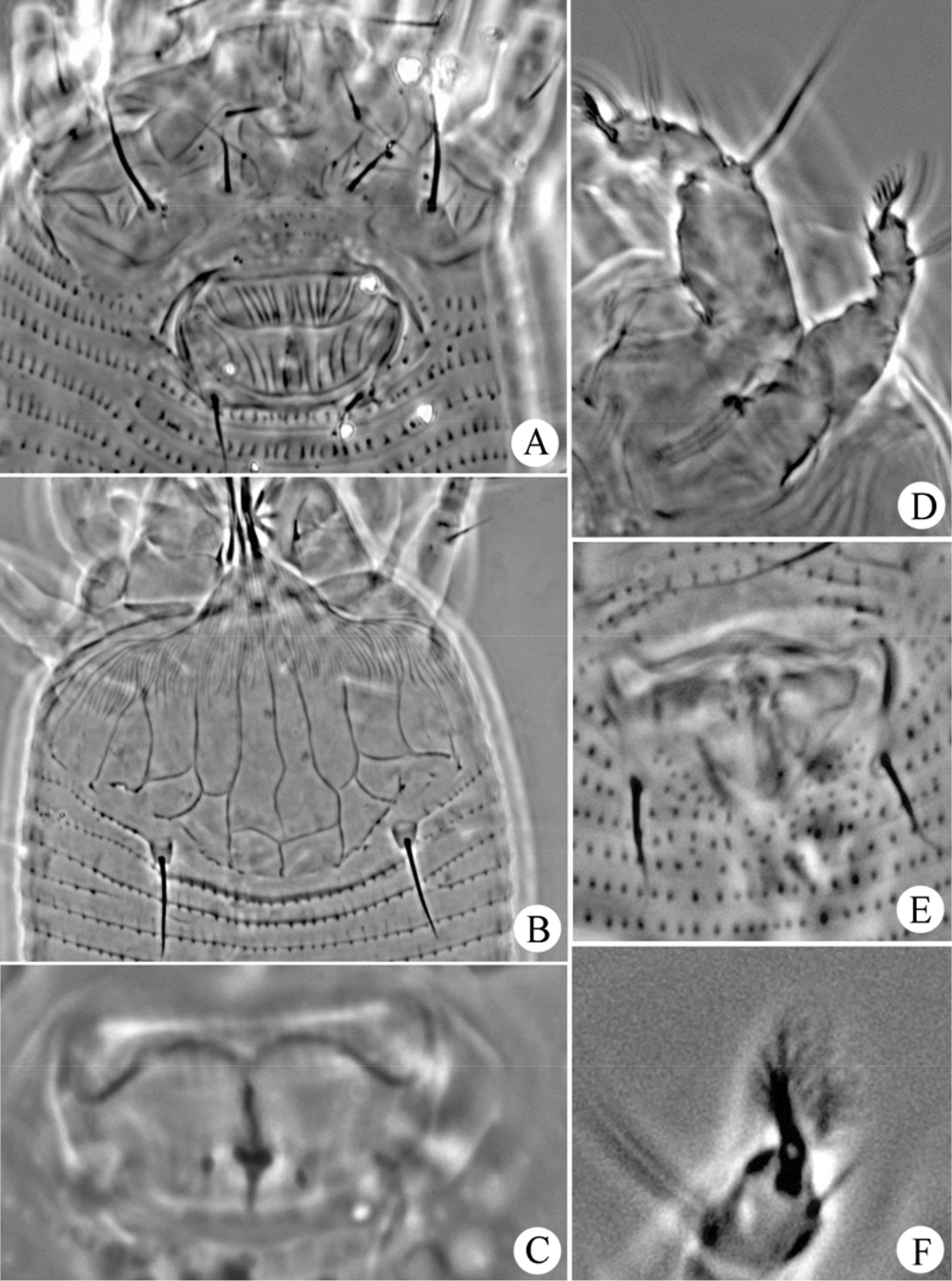
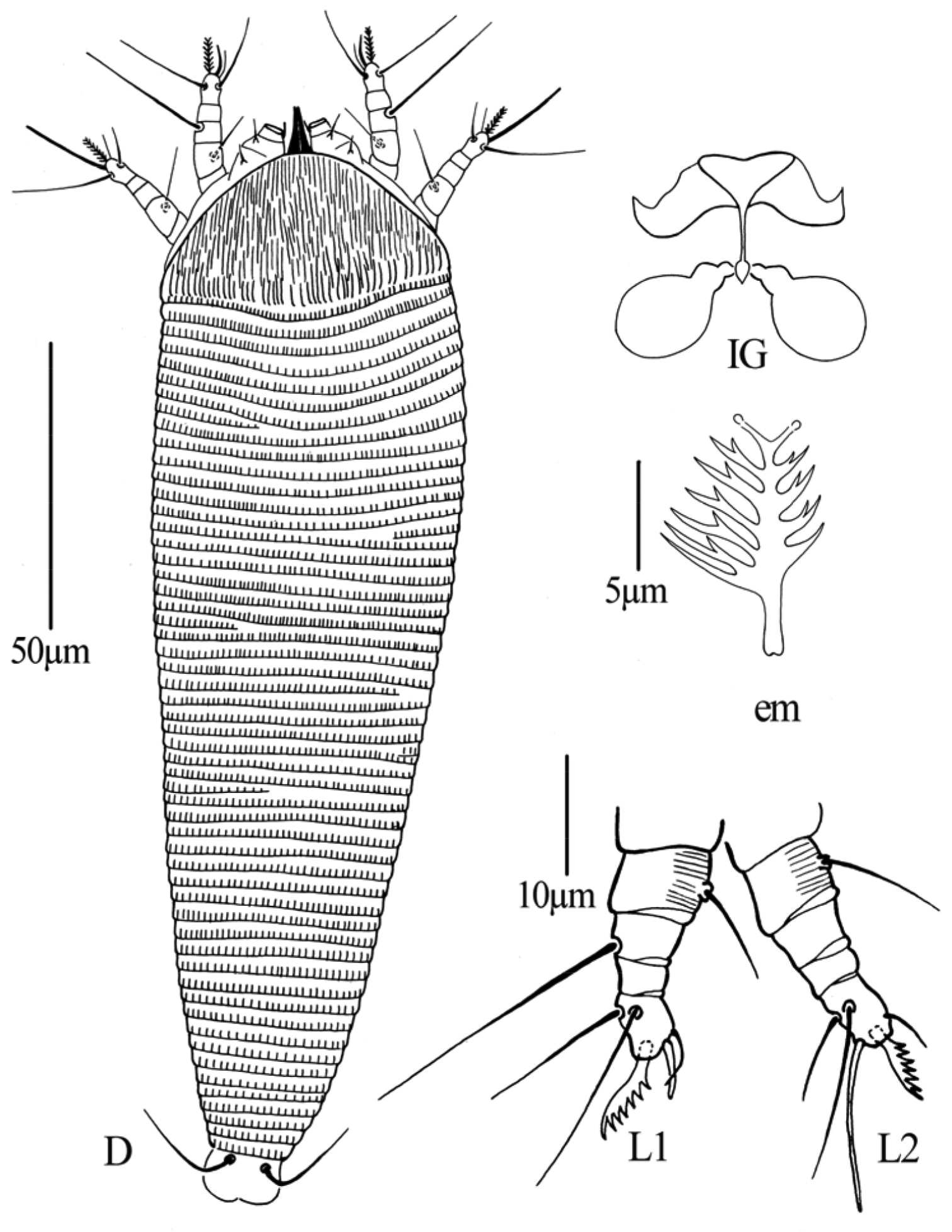
Figs 2–4FEMALE: (n=11). Body fusiform, 187 (187–200), 61 (55–61) wide, 56 (56–60) thick; light yellow. Gnathosoma 24 (20–24), projecting obliquely downwards, pedipalp coxal setae (ep) 2 (2–3), dorsal pedipalp genual setae (d) 7 (6–7), cheliceral stylets 24 (23–24). Prodorsal shield 40 (39–42), 50 (48–50) wide, median, admedian and submedian lines complete and parallel, connected with transverse lines, shield design with anterior part covered with striaes; anterior shield lobe present 8 (8–9). Scapular tubercles on rear shield margin, 32 (32–33) apart, scapular setae (sc) 16 (15–16), projecting posteriorly. Coxigenital region with 4 (3–4) semiannuli between coxae and genitalia. Coxal plates with irregular lines, anterolateral setae on coxisternum I (1b) 7 (6–7), 12 (12–13) apart, proximal setae on coxisternum I (1a) 14 (14–17), 11 (11–12) apart, proximal setae on coxisternum II (2a) 26 (26–27), 26 (24–26) apart. Prosternal apodeme absent. Leg I 27 (26–27), femur 9 (9–10), basiventral femoral setae (bv) 8 (8–9); genu 5 (4–5), antaxial genual setae (l’’) 23 (21–23); tibia 6 (5–6), paraxial tibial setae (l’) 6 (6–7), located at centre; tarsus 5 (5–6), paraxia, fastigial, tarsal setae (ft’) 11 (11–12), antaxial, fastigial, tarsal setae (ft’’) 17 (17–18), paraxial, unguinal, tarsal setae (u’) 4 (4–5); tarsal empodium (em) 4 (4–5), simple, 7-rayed, tarsal solenidion (ω) 6 (6–7), rod-like. Leg II 24 (24–26), femur 9 (9–10), basiventral femoral setae (bv) 9 (8–9); genu 4 (4–5), antaxial genual setae (l’’) 7 (6–7); tibia 5 (5–6); tarsus 5 (5–6), paraxia, fastigial, tarsal setae (ft’) 5 (5–6), antaxial, fastigial, tarsal setae (ft’’) 17 (16–17), paraxial, unguinal, tarsal setae (u’) 3 (3–4); tarsal empodium (em) 5 (5–6), simple, 7-rayed, tarsal solenidion (ω) 8 (7–8), rod-like. Opisthosoma dorsally with 34 (34–35) semiannuli, with elliptical microtubercles, ventrally with 49 (49–51) semiannuli, with elliptical microtubercles. Setae c2 12 (12–13) on ventral semiannulus 8 (8–9), 48 (48–50) apart; setae d 41 (40–43) on ventral semiannulus 19 (18–19), 40 (37–40) apart; setae e 11 (11–14) on ventral semiannulus 30 (30–31), 22 (20–22) apart, setae f 25 (24–25) on 6th ventral semiannulus from rear, 18 (18–20) apart. Setae h1 absent, h2 38 (37–38). Female genitalia 12 (12–15), 20 (20–22) wide, coverflap with two rows of ridges, the upper one with 14 (12–14) longitudinal ridges, the other with 12 (12–14) longitudinal ridges, setae 3a 8 (7–8), 15 (14–15) apart.
MALE: (n=7, dorsal view). Body fusiform, 169–190, 56–63 wide; light yellow. Gnathosoma 19–22, projecting obliquely downwards, pedipalp coxal setae (ep) 2–3, dorsal pedipalp genual setae (d) 5–6, cheliceral stylets 23–24. Prodorsal shield 37–40, 47–50 wide, median, admedian and submedian lines complete and parallel, connected with transverse lines, shield design with anterior part covered with striaes; anterior shield lobe present 8–9. Scapular tubercles on rear shield margin, 27–30 apart, scapular setae (sc) 15–16, projecting posteriorly. Coxigenital region with 4–5 semiannuli between coxae and genitalia. Coxal plates with irregular lines, anterolateral setae on coxisternum I (1b) 5–6, 11–12 apart, proximal setae on coxisternum I (1a) 12–15, 8–11 apart, proximal setae on coxisternum II (2a) 25–26, 23–25 apart. Prosternal apodeme absent. Leg I 25–26, femur 9–10, basiventral femoral setae (bv) 8–9; genu 4–5, antaxial genual setae (l’’) 18–22; tibia 5–6, paraxial tibial setae (l’) 5–6, located at centre; tarsus 5–6, paraxia, fastigial, tarsal setae (ft’) 11–12, antaxial, fastigial, tarsal setae (ft’’) 15–17, paraxial, unguinal, tarsal setae (u’) 4–5; tarsal empodium (em) 4–5, simple, 7-rayed, tarsal solenidion (ω) 5–6, rod-like. Leg II 24–26, femur 9–10, basiventral femoral setae (bv) 8–10; genu 3–4, antaxial genual setae (l’’) 6–7; tibia 5–6; tarsus 5–6, paraxia, fastigial, tarsal setae (ft’) 5–6, antaxial, fastigial, tarsal setae (ft’’) 16–17, paraxial, unguinal, tarsal setae (u’) 3–4; tarsal empodium (em) 4–5, simple, 7-rayed, tarsal solenidion (ω) 6–7, rod-like. Opisthosoma dorsally with 33–37 semiannuli, with elliptical microtubercles, ventrally with 47–50 semiannuli, with elliptical microtubercles. Setae c2 15–16 on ventral semiannulus 8–9, 51–57 apart; setae d 40–41 on ventral semiannulus 17–19, 30–35 apart; setae e 13–15 on ventral semiannulus 29–33, 18–19 apart, setae f 24–25 on 6th ventral semiannulus from rear, 20–23 apart. Setae h1 absent, h2 57–58. Male genitalia 16–18 wide, setae 3a 7–8, 15–16 apart.
Gammaphytoptus striatilobus sp. n.: D dorsal view of female em empodium IG female internal genitalia GM male genital region.
Gammaphytoptus striatilobus sp. n.: AL lateral view of anterior body region LO lateral view of annuli PM lateral view of posterior opisthosoma CGF female coxae and genitalia L1 leg I L2 leg II.
Gammaphytoptus striatilobus sp. n.: A coxae and female genitalia B prodorsal shield C female internal genitalia D leg I and leg II E male genitalia F empodium.
Holotype female (slide number NJAUAcariEriHN128C.1; marked Holotype), from Phoebe hunanensis Hand.–Mazz. (Lauraceae), Zhangjiajie National Forest Park, Zhangjiajie City, Hunan Province, P.R. China, 29°20'41"N, 110°27'33"E, elevation 420m, 10 July 2013, coll. Qiong Wang, Xiao Han and Jingfeng Guo, deposited as a slide mounted specimen in the Arthropod/Mite Collection of the Department of Entomology, NJAU, Jiangsu Province, China. Paratypes 10 females and 7 males on 17 microscope slides (slide number NJAUAcariEriHN128C.2–128C.18), with the same data as holotype.
This species is vagrant on lower part of the leaf surface. No damage to the host plant was observed.
The specific designation “striatilobus” is from the character of prodorsal shield lobe (“lobus”) marked with parellel fine impressed lines (“striatus”), masculine in gender.
This new species is similar to Gammaphytoptus machilus Li, Wei & Wang, 2009, but can be differentiated from the latter by having: the design of prodorsal shield with anterior part covered with striaes (a prodorsal shield design with two rows of cells in Gammaphytoptus machilus); dorsal semiannuli with elliptical microtubercles (dorsal semiannuli smooth in Gammaphytoptus machilus) and ventral semiannuli with elliptical microtubercles (ventral semiannuli with rounded microtubercles in Gammaphytoptus machilus).
http://zoobank.org/3A5DC24D-760D-4E86-A6F2-FA640D8F52E3
http://species-id.net/wiki/Phyllocoptes_setalsolenidion
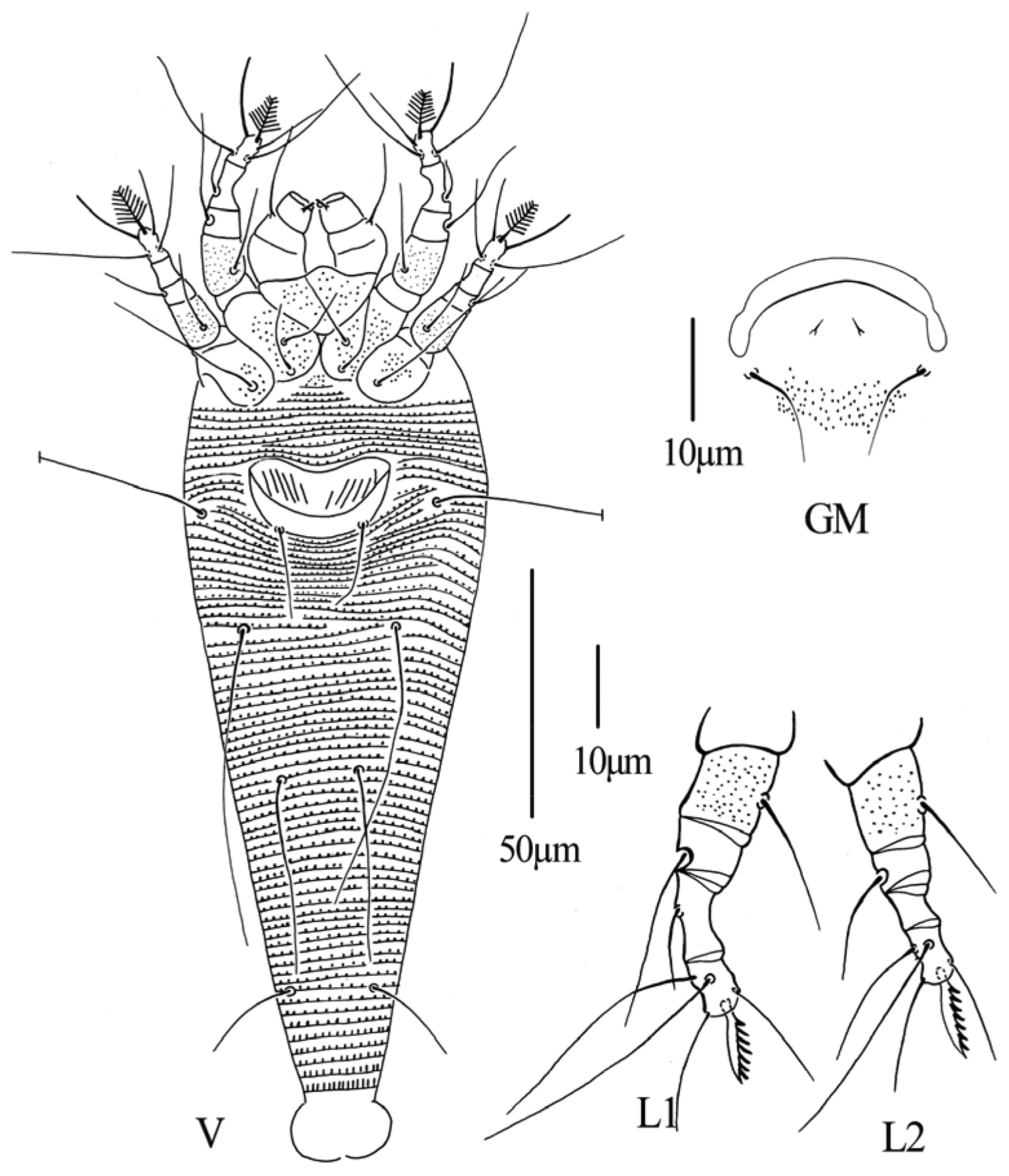
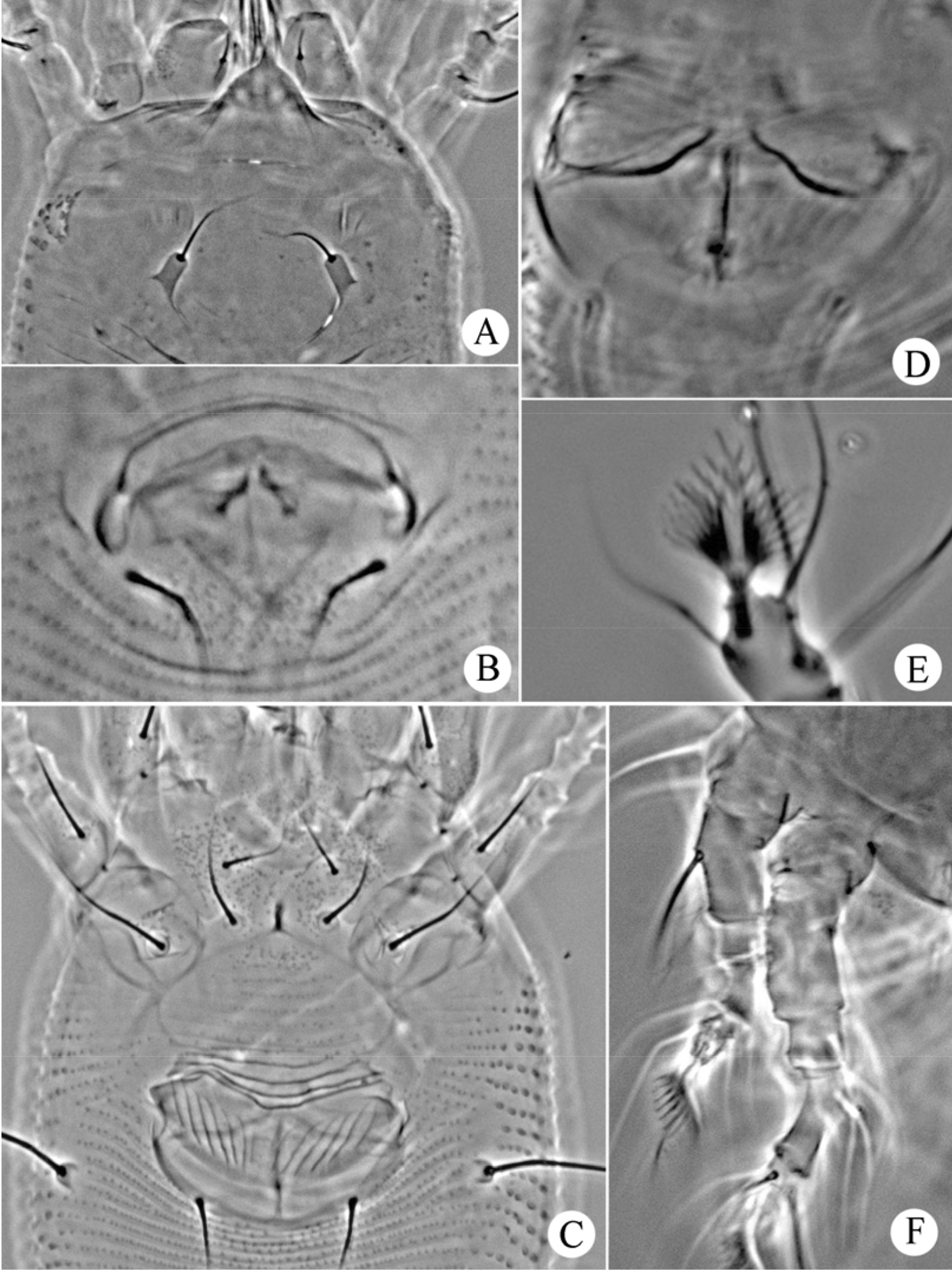
Figs 5–7FEMALE: (n=5, dorsal view). Body fusiform, 198 (186–198), 62 (59–65) wide; light yellow. Gnathosoma 29 (28–31), projecting obliquely downwards, pedipalp coxal setae (ep) 4 (4–5), dorsal pedipalp genual setae (d) 18 (18–21), cheliceral stylets 23 (20–24). Prodorsal shield 41 (41–42), 59 (59–60) wide, median, admedian and submedian lines absent, prodorsal shield with some short lines; anterior shield lobe 10 (10–14), acuminate, ending in a sharp point. Scapular tubercles 5 (5–6), ahead of rear shield margin, 19 (19–24) apart, scapular setae (sc) 10 (8–10), projecting centrad. Coxigenital region with 11 (10–11) semiannuli between coxae and genitalia. Coxal plates with fine granules, anterolateral setae on coxisternum I (1b) 15 (15–16), 12 (12–15) apart, proximal setae on coxisternum I (1a) 16 (15–16), 10 (10–11) apart, proximal setae on coxisternum II (2a) 33 (30–33), 25 (25–26) apart. Prosternal apodeme 4 (4–5). Leg I 37 (36–38), femur 12 (11–13), with fine granules, basiventral femoral setae (bv) 18 (18–20); genu 7 (6–7), antaxial genual setae (l’’) 22 (20–22); tibia 11 (10–11), paraxial tibial setae (l’) 10 (9–10), located at 1/3 from dorsal base; tarsus 7 (7–8), paraxia, fastigial, tarsal setae (ft’) 30 (29–30), antaxial, fastigial, tarsal setae (ft’’) 35 (32–35), paraxial, unguinal, tarsal setae (u’) 15 (15–17); tarsal empodium (em) 9 (8–9), simple, 8-rayed, tarsal solenidion (ω) 16 (16–17), seta-like. Leg II 31 (31–32), femur 10 (10–12), with fine granules, basiventral femoral setae (bv) 15 (15–16); genu 5 (4–5), antaxial genual setae (l’’) 14 (14–16); tibia 5 (5–6); tarsus 7 (5–7), paraxia, fastigial, tarsal setae (ft’) 18 (18–20), antaxial, fastigial, tarsal setae (ft’’) 28 (25–28), paraxial, unguinal, tarsal setae (u’) 14 (14–16); tarsal empodium (em) 10 (9–10), simple, 8-rayed, tarsal solenidion (ω) 15 (15–17), seta-like. Opisthosoma dorsally with 45 (45–48) semiannuli, smooth, ventrally with 70 (70–76) semiannuli, with small and rounded microtubercles set on rear annular margins, last 5th–6th semiannuli with elongated and linear tubercles. Setae c2 53 (53–55) on ventral semiannulus 14 (13–15), 49 (49–52) apart; setae d 59 (55–60) on ventral semiannulus 28 (26–28), 33 (32–33) apart; setae e 40 (39–42) on ventral semiannulus 42 (42–45), 15 (15–18) apart, setae f 21 (20–22) on 9th ventral semiannulus from rear, 17 (16–18) apart. Setae h1 4 (4–5), h2 25 (24–25). Female genitalia 14 (14–15), 26 (26–28) wide, coverflap with 14 (12–14) longitudinal ridges, setae 3a 20 (17–20), 17 (17–19) apart.
MALE: (n=1, dorsal view). Body fusiform, 169, 54 wide; light yellow. Gnathosoma 27, projecting obliquely downwards, pedipalp coxal setae (ep) 4, dorsal pedipalp genual setae (d) 18, cheliceral stylets 22. Prodorsal shield 42, 57 wide, median, admedian and submedian lines absent, prodorsal shield with some short lines; anterior shield lobe 12, acuminate, ending in a sharp point. Scapular tubercles 5 ahead of rear shield margin, 24 apart, scapular setae (sc) 8, projecting centrad. Coxigenital region with 9 semiannuli between coxae and genitalia. Coxal plates with fine granules, anterolateral setae on coxisternum I (1b) 12, 14 apart, proximal setae on coxisternum I (1a) 17, 10 apart, proximal setae on coxisternum II (2a) 24, 25 apart. Prosternal apodeme 4. Leg I 30, femur 11, with fine granules, basiventral femoral setae (bv) 15; genu 4, antaxial genual setae (l’’) 20; tibia 7, paraxial tibial setae (l’) 10, located at 1/3 from dorsal base; tarsus 6, paraxia, fastigial, tarsal setae (ft’) 27, antaxial, fastigial, tarsal setae (ft’’) 28, paraxial, unguinal, tarsal setae (u’)14; tarsal empodium (em) 8, simple, 8-rayed, tarsal solenidion (ω) 15, seta-like. Leg II 26, femur 10, with fine granules, basiventral femoral setae (bv) 13; genu 4, antaxial genual setae (l’’) 14; tibia 5; tarsus 6, paraxia, fastigial, tarsal setae (ft’)13, antaxial, fastigial, tarsal setae (ft’’) 23, paraxial, unguinal, tarsal setae (u’) 11; tarsal empodium (em) 7, simple, 8-rayed, tarsal solenidion (ω) 14, seta-like. Opisthosoma dorsally with 42 semiannuli, smooth, ventrally with 71 semiannuli, with small and rounded microtubercles set on rear annular margins, last 5th–6th semiannuli with elongated and linear tubercles. Setae c2 50 on ventral semiannulus 14, 40 apart; setae d 52 on ventral semiannulus 25, 30 apart; setae e 40 on ventral semiannulus 43, 15 apart, setae f 24 on 9th ventral semiannulus from rear, 17 apart. Setae h1 5, h2 22. Male genitalia 21 wide, setae 3a 11, 17 apart.
Phyllocoptes setalsolenidion sp. n.: D dorsal view of female em empodium IG female internal genitalia CGF female coxae and genitalia.
Phyllocoptes setalsolenidion sp. n.: V ventral view of female GM male genital region L1 leg I L2 leg II.
Phyllocoptes setalsolenidion sp. n.: A prodorsal shield B male genitalia C coxae and female genitalia D female internal genitalia E empodium F leg I and leg II.
Holotype female (slide number NJAUAcariEriHN128A.1; marked Holotype), from Phoebe hunanensis Hand.–Mazz. (Lauraceae), Zhangjiajie National Forest Park, Zhangjiajie City, Hunan Province, P.R. China, 29°20'41"N, 110°27'33"E, elevation 420m, 10 July 2013, coll. Qiong Wang, Xiao Han and Jingfeng Guo, deposited as a slide mounted specimen in the Arthropod/Mite Collection of the Department of Entomology, NJAU, Jiangsu Province, China. Paratypes 4 females and 1 male on 5 microscope slides (slide number NJAUAcariEriHN128A.2-128A.6), with the same data as holotype.
Vagrant on lower part of the leaf surface. No damage to the host plant was observed.
The specific designation setalsolenidion is derived from the shape (setal) of the tarsal solenidion. It is regarded as a noun phrase regardless of the gender and part of speech.
This new species is similar to Phyllocoptes machilus Wei, Xie & Chen, 2006, but can be differentiated from the latter mainly by possessing: prodorsal shield lacking median, admedian and submedian lines (with median line incomplete, present on the anterior and rear 1/5 respectively, admedian lines complete, forming a network in Phyllocoptes machilus); anterior shield lobe acuminate, ending in a sharp point (with small frontal lobe in Phyllocoptes machilus); femur having fine granules (femur smooth in Phyllocoptes machilus) and tarsal empodium 8-rayed, tarsal solenidion seta-like (tarsal empodium 4-rayed, tarsal solenidion knobbed).
http://zoobank.org/E018B236-5BB5-485C-A921-66AC407D15A8
http://species-id.net/wiki/Dechela_phoebe
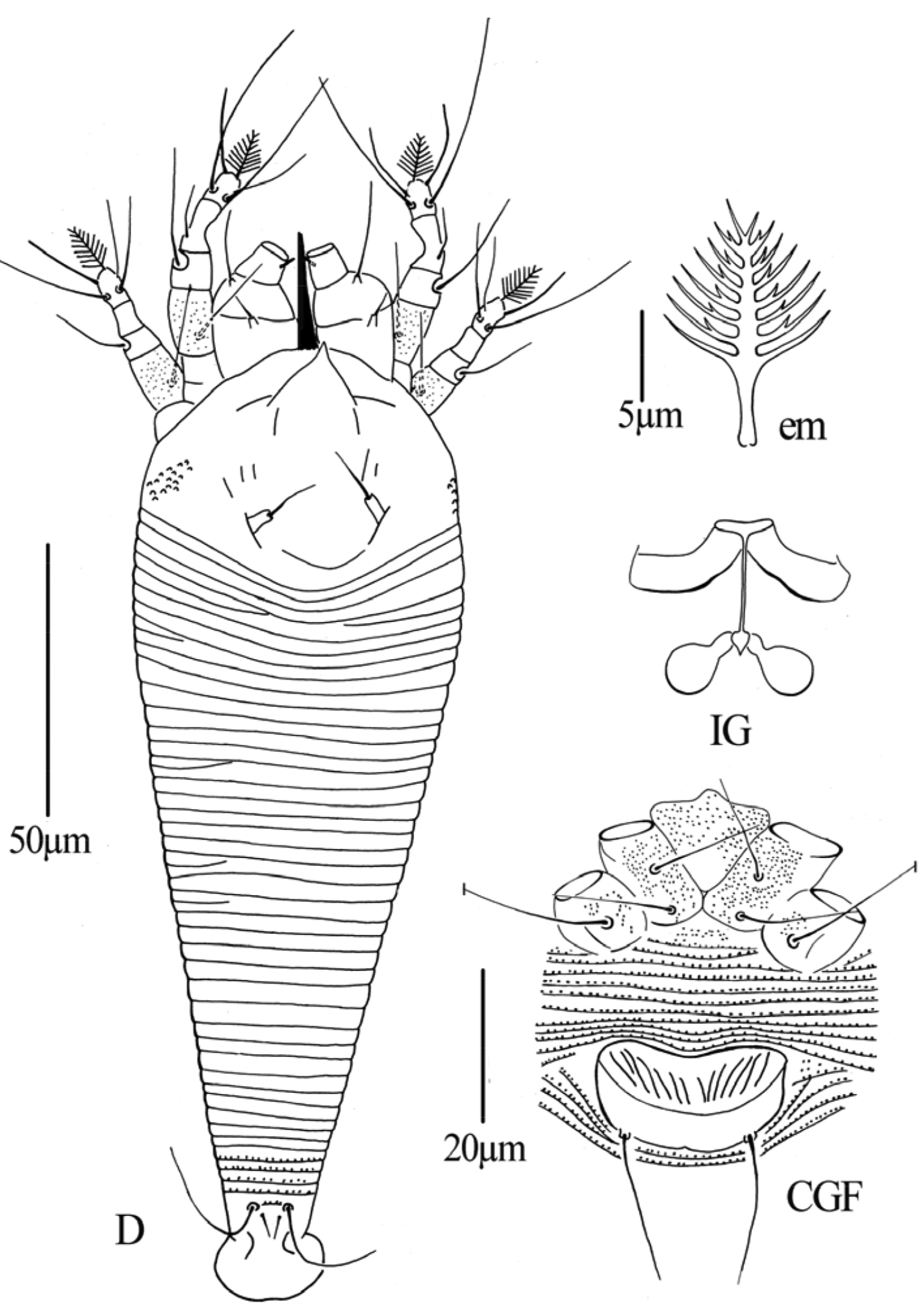
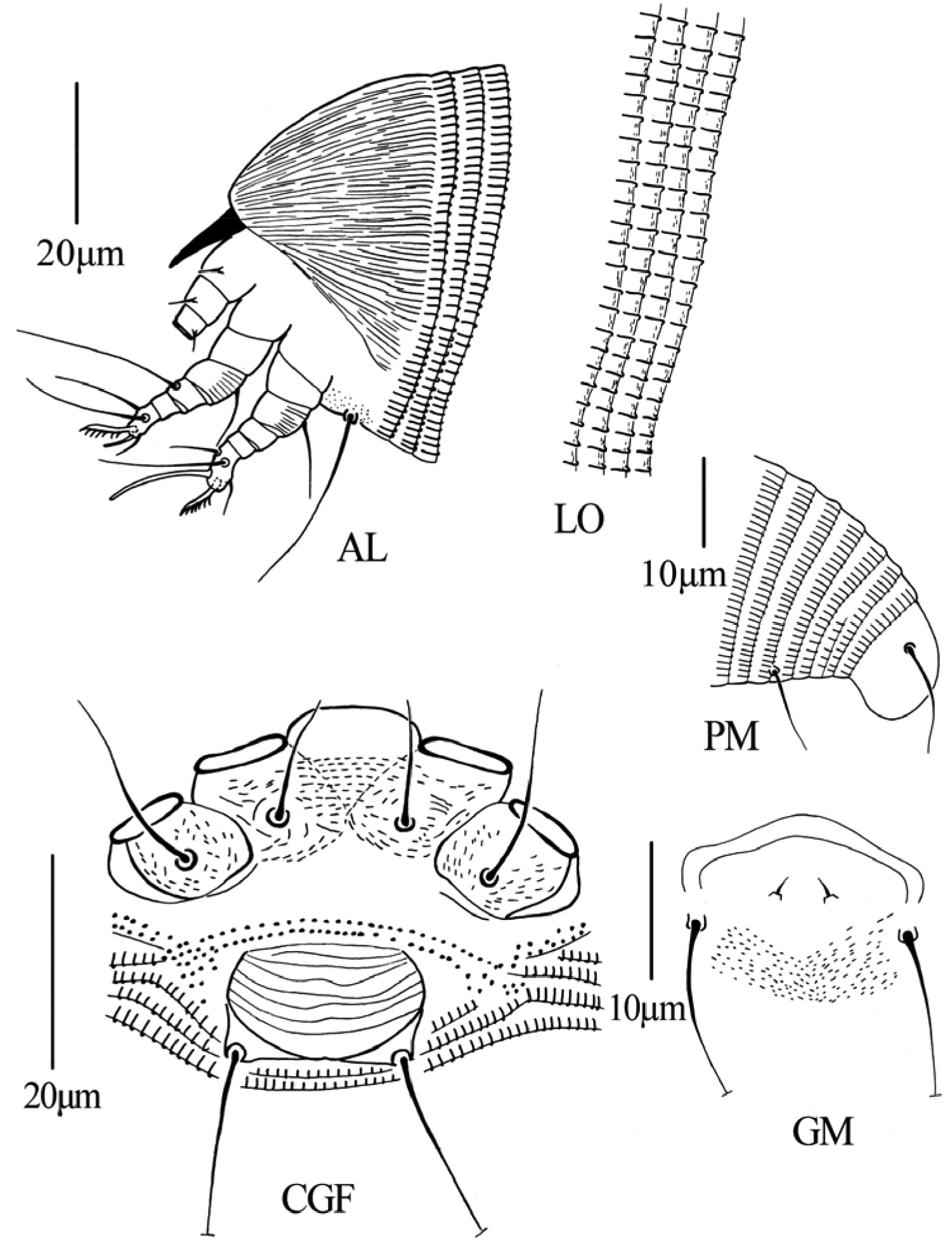
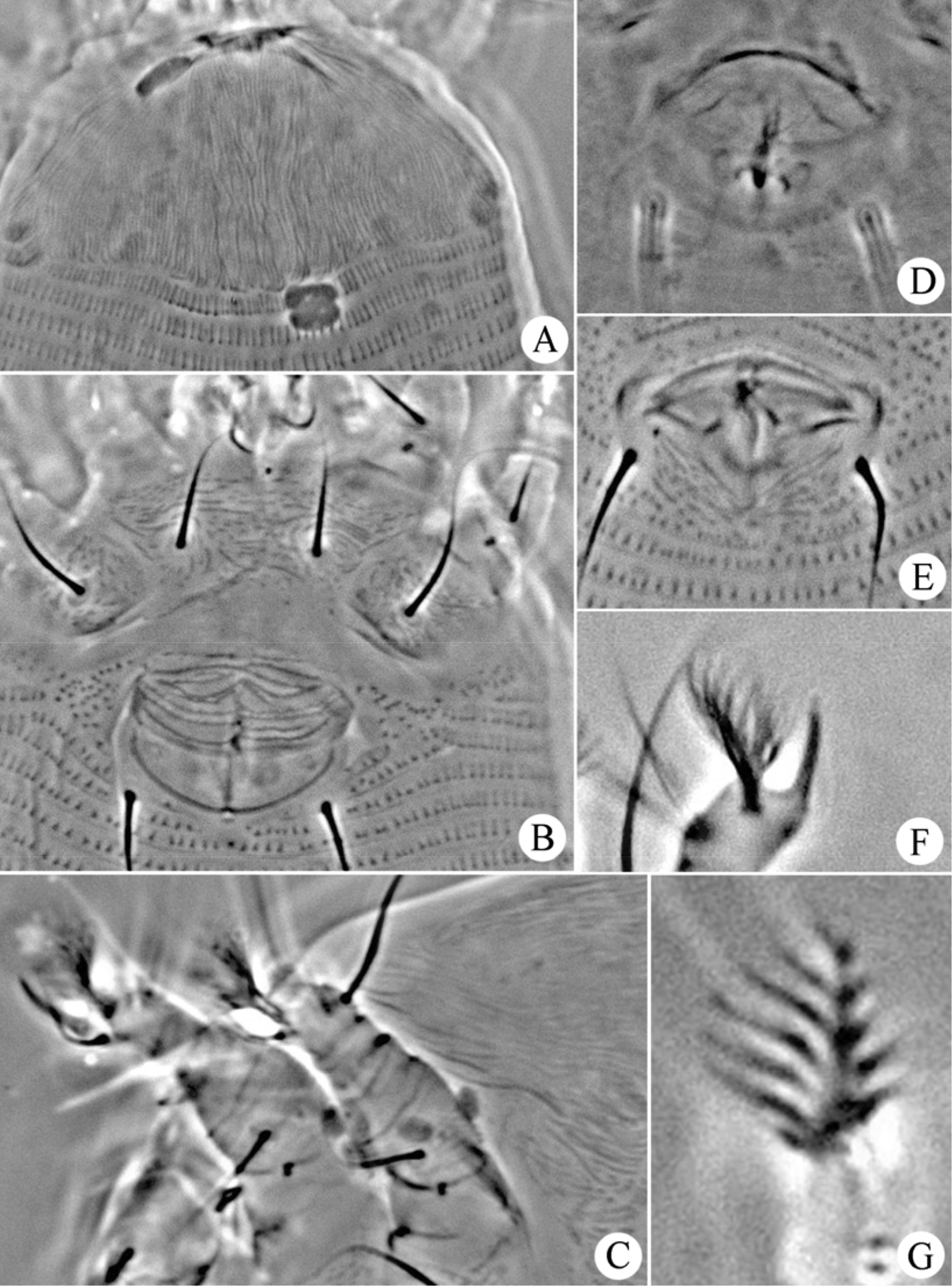
Figs 8–10FEMALE: (n=13). Body vermiform, 187 (183–192), 60 (55–60) wide, 62 (57–62) thick; light yellow. Gnathosoma 15 (15–18), projecting obliquely downwards, pedipalp coxal setae (ep) 3 (2–3), dorsal pedipalp genual setae (d) 4 (4–5), cheliceral stylets 12 (12–14). Prodorsal shield 27 (26–30), 51 (45–51) wide, covered with short lines; anterior shield lobe absent. Scapular tubercles and scapular setae absent. Coxigenital region with 2 (2–3) indistinct semiannuli between coxae and genitalia. Coxal plates with minute lines, anterolateral setae on coxisternum I (1b) absent, proximal setae on coxisternum I (1a) 12 (12–15), 13 (11–13) apart, proximal setae on coxisternum II (2a) 19 (18–21), 28 (27–29) apart. Prosternal apodeme absent. Leg I 21 (20–22), femur 6 (6–7), with some dash lines on ventral part, basiventral femoral setae (bv) 9 (9–11); genu 4 (3–4), antaxial genual setae (l’’) 24 (22–24); tibia 3 (2–3), paraxial tibial setae (l’) absent; tarsus 5 (5–6), paraxia, fastigial, tarsal setae (ft’) 13 (13–15), antaxial, fastigial, tarsal setae (ft’’) 17 (16–18), paraxial, unguinal, tarsal setae (u’) 5 (5–7); tarsal empodium (em) 7 (7–8), simple, 7-rayed outside, 5-rayed inside, tarsal solenidion (ω) 5 (5–6), rod-like, located below empodia. Leg II 18 (18–19), femur 6 (5–6), with some dash lines on ventral part, basiventral femoral setae (bv) 10 (10–11); genu 4 (3–4), antaxial genual setae (l’’) absent; tibia 2 (2–3); tarsus 6 (5–6), paraxia, fastigial, tarsal setae (ft’) 7 (7–8), antaxial, fastigial, tarsal setae (ft’’) 18 (18–23), paraxial, unguinal, tarsal setae (u’) 5 (4–5); tarsal empodium (em) 6 (6–7), simple, 7-rayed outside, 5-rayed inside, tarsal solenidion (ω) 15 (15–16), rod-like. Opisthosoma dorsally with 55 (55–57) annuli, with elliptical microtubercles, ventrally with 56 (56–58) annuli, with elliptical microtubercles. Setae c2 10 (10–11) on ventral annulus 8 (7–9), 48 (48–50) apart; setae d 53 (50–55) on ventral annulus 16 (16–18), 38 (38–40) apart; setae e 50 (50–52) on ventral annulus 32 (31–32), 26 (26–27) apart, setae f 15 (15–16) on 6th ventral annulus from rear, 12 (11–12) apart. Setae h1 absent, h2 21 (20–23). Female genitalia 12 (12–14), 19 (18–19) wide, coverflap with transverse dashes, setae 3a 30 (27–30), 16 (15–16) apart.
MALE: (n=2, dorsal view). Body vermiform, 175–192, 48–54 wide; light yellow. Gnathosoma 20–21, projecting obliquely downwards, pedipalp coxal setae (ep) 2–3, dorsal pedipalp genual setae (d) 4–5, cheliceral stylets 10–13. Prodorsal shield 25–27, 40–50 wide, covered with short lines; anterior shield lobe absent. Scapular tubercles and scapular setae absent. Coxigenital region with 2–3 indistinct semiannuli between coxae and genitalia. Coxal plates with minute lines, anterolateral setae on coxisternum I (1b) absent, proximal setae on coxisternum I (1a) 12–13, 9–10 apart, proximal setae on coxisternum II (2a) 17–20, 24–25 apart. Prosternal apodeme absent. Leg I 17–20, femur 6–7, with some dash lines on ventral part, basiventral femoral setae (bv) 8–9; genu 3–4, antaxial genual setae (l’’) 20–21; tibia 3–4, paraxial tibial setae (l’) absent; tarsus 4–5, paraxia, fastigial, tarsal setae (ft’) 10–11, antaxial, fastigial, tarsal setae (ft’’) 14–16, paraxial, unguinal, tarsal setae (u’) 5–6; tarsal empodium (em) 6–7, simple, 7-rayed outside, 5-rayed inside, tarsal solenidion (ω) 5–6, rod-like, located below empodia. Leg II 17–20, femur 5–6, with some dash lines on ventral part, basiventral femoral setae (bv) 8–9; genu 2–3, antaxial genual setae (l’’) absent; tibia 2–3; tarsus 5–6, paraxia, fastigial, tarsal setae (ft’) 6–7, antaxial, fastigial, tarsal setae (ft’’) 18–19, paraxial, unguinal, tarsal setae (u’) 4–5; tarsal empodium (em) 5–6, simple, 7-rayed outside, 5-rayed inside, tarsal solenidion (ω) 13–15, rod-like. Opisthosoma dorsally with 54–56 annuli, with elliptical microtubercles, ventrally with 56–57 annuli, with elliptical microtubercles. Setae c2 15–16 on ventral annulus 8–9, 40–41 apart; setae d 43–45 on ventral annulus 16–17, 30–34 apart; setae e 43–44 on ventral annulus 30–32, 23–24 apart, setae f 15–16 on 6th ventral annulus from rear, 10–11 apart. Setae h1 absent, h2 26–27. Male genitalia 18–19 wide, setae 3a 26–30, 15–16 apart.
Dechela phoebe sp. n.: D dorsal view of female IG female internal genitalia em empodium L1 Leg I L2 leg II.
Dechela phoebe sp. n.: AL lateral view of anterior body LO lateral view of annuli PM lateral view of posterior opisthosoma CGF female coxae and genitalia GM male genital region.
Dechela phoebe sp. n.: A prodorsal shield B coxae and female genitalia C leg I and leg II D female internal genitalia E male genitalia F tarsal solenidion of leg I G empodium.
13 females and 2 males on 15 microscope slides (slide number NJAUAcariEriHN128B.1-128B.15), from Phoebe hunanensis Hand.–Mazz. (Lauraceae), Zhangjiajie National Forest Park, Zhangjiajie City, Hunan Province, P.R. China, 29°20'41"N, 110°27'33"E, elevation 420m, 10 July 2013, coll. Qiong Wang, Xiao Han and Jingfeng Guo, deposited as a slide mounted specimen in the Arthropod/Mite Collection of the Department of Entomology, NJAU, Jiangsu Province, China.
Vagrant on lower part of the leaf surface. No damage to the host plant was observed.
The specific designation Phoebe is derived from the generic name of the host plant; feminine in gender.
This new species is very similar to Dechela epelis Keifer, 1965, but some quantitative characters can be used to separate them (Table 1).
The differential diagnosis between Dechela epelis, Keifer and Dechela phoebe sp. n.
| Characters | Dechela epelis Keifer | Dechela phoebe sp. n. |
|---|---|---|
| body length | 175–190 | 187 (183–192) |
| body width | 42–45 | 60 (55–60) |
| gnathosoma length | 19 | 15 (15–18) |
| d | 3.5 | 4 (4–5) |
| shield length | 26 | 27 (26–30) |
| shield width | 32 | 51 (45–51) |
| anterior shield lobe | present | absent |
| coxisternal area | coxae with curved lines of granules or short dashes | coxal plates with minute lines |
| leg I | 20–21 | 21 (20–22) |
| tibia I/l’ | 3/absent | 3 (2–3)/absent |
| tarsus I/ω | 5, tarsal solenidion 4 straight or slightly curved laterally | 5 (5–6), tarsal solenidion 5 (5–6), slightly curved laterally |
| em I | 7-rayed on outside, 5-rayed inside | 7 (7–8), 7-rayed on outside, 5-rayed inside |
| leg II | 20 | 18 (18–19) |
| tibia II | 2 | 2 (2–3) |
| tarsus II/ω | 5, tarsal solenidion 10 straight | 6 (5–6), tarsal solenidion 15 (15–16) straight |
| em II | 7-rayed on outside, 5-rayed inside | 6 (6–7), 7-rayed on outside, 5-rayed inside |
| dorsal annuli | 62 | 55 (55–57) |
| ventral annuli | 62 | 56 (56–58) |
| c2 | 15, on 6–8 annuli behind shield, projecting up and forward | 10 (10–11), on 8 (7–9) annuli from coxae |
| d | 36, on 19 annuli | 53 (50–55), on 16 (16–18) annuli |
| e | 42, on 37 annuli | 50 (50–52), on 32 (31–32) annuli |
| f | 14, on 4–5 from rear | 15 (15–16), on 6 from rear |
| h1 | absent | absent |
| female genitalia/3a | 16 wide, 11 long; coverflap with transverse and gently curved lines of granules and dashes; 13 long | 19 (18–19) wide, 12 (12–14) long; coverflap with transverse dashes; 30 (27–30) long |
| host plant | Bixa sp. (Bixaceae) | Phoebe hunanensis Hand.–Mazz. (Lauraceae) |
| 1 | Female genitalia appressed to coxae, ridges on female coverflap in two uneven ranks | 2 |
| – | Female genitalia not appressed to coxae, ridges on female coverflap in one rank | 9 |
| 2 | The anterior part of prodorsal shield design covered with striaes | Gammaphytoptus striatilobus sp. n. |
| – | Prodorsal shield design without short lines | 3 |
| 3 | Dorsal annuli smooth | Gammaphytoptus machilus Li, Wei & Wang, 2009 |
| – | Dorsal annuli with microtubercles | 4 |
| 4 | Empodia 6-rayed or 7-rayed | 5 |
| – | Empodia 5-rayed | 6 |
| 5 | Empodia 6-rayed, prodorsal shield pattern of part longitudinal and part network lines | Gammaphytoptus camphorae Keifer, 1939 |
| – | Empodia 7-rayed, prodorsal shield without median line and submedian, admedian lines complete | Gammaphytoptus commune Huang & Wang, 2009 |
| 6 | Prodorsal shield design with median line complete | 7 |
| – | Prodorsal shield design with median line incomplete | 8 |
| 7 | Prodorsal shield design complex and anteriorly with a number of cells | Gammaphytoptus bengalensis Das & Chakrabarti, 1985 |
| – | Prodorsal shield design simple and with a number of longitudinal parallel lines | Gammaphytoptus litseasis Ghosh & Chakrabarti, 1982 |
| 8 | Setae h1 present | Gammaphytoptus zuihoensus Huang & Wang, 2004 |
| – | Setae h1 absent | Gammaphytoptus litseaus Huang, 2001b |
| 9 | Dosal annuli smooth | Phyllocoptes setalsolenidion sp. n. |
| – | Dosal annuli with microtubercles | 10 |
| 10 | Empodia 4-rayed | Phyllocoptes machilus Wei, Xie & Chen, 2006 |
| – | Empodia 5-rayed | 11 |
| 11 | Coxae with short curved lines and dashes | Phyllocoptes linderafolius Styer, 1975 |
| – | Coxae with granular lines | Phyllocoptes sassafrasella Keifer, 1959 |
This research was funded by the National Natural Science Foundation of China (No. 31172132). We thank Hao-Sen Li of NJAU for reviewing an earlier draft of this manuscript. We are very grateful to Radmila Petanović and an anonymous reviewer for their valuable comments on the manuscript. We also thank Professor Ri-Ming Hao of the College of Horticulture at NJAU for identifying host plants.