Citation: Farache FHA, Rasplus J-Y (2014) Revision of the Australasian genus Pseudidarnes Girault, 1927 (Hymenoptera, Agaonidae, Sycophaginae). ZooKeys 404: 31–70. doi: 10.3897/zookeys.404.7204
The species of Pseudidarnes are revised, and six species are described: P. acaudus Farache & Rasplus, sp. n.; P. astridae Farache & Rasplus, sp. n.; P. badiogeminus Farache & Rasplus, sp. n.; P. cooki Farache & Rasplus, sp. n.; P. kjellbergi Farache & Rasplus, sp. n.; P. laevis Farache & Rasplus, sp. n. Pseudidarnes minerva Girault, 1927 and P. flavicollis Bouček, 1988 are redescribed. A key to the species is provided as well as illustrations for all females and all known males (except the wingless male of P. minerva). We also provided further discussion on ecology, morphological patterns, and host taxonomy. Online dichotomous and multi-access interactive LUCID keys to all Pseudidarnes species are available at http://www.figweb.org/.
Chalcidoidea, Ficus, Moraceae, non-pollinating fig wasp, gall maker
Sycophaginae is one of the six subfamilies of chalcid wasps that are strictly associated with Ficus syconia. A recent phylogenetic analysis of the Chalcidoidea recovered Agaoninae (fig pollinators) + Sycophaginae as a monophyletic group (Agaonidae), whereas all other fig wasp subfamilies were included in Pteromalidae (
The Sycophaginae occur in all tropical regions of the world but are still poorly known, with about 60 described species and an estimated diversity of about 700 species (
The phylogeny of the Sycophaginae recovered by the analysis of multiple genes (
1) Eukoebelea (associated with Australasian Malvanthera fig trees) recovered as the sister lineage to all other genera.
2) A strongly supported clade of three genera, Pseudidarnes (Malvanthera) basal to Anidarnes Bouček, 1993 (associated with the New World Americana fig trees) plus the undescribed genus (associated with the Oriental Conosycea figs). A dichotomic key separating these three genera is provided by
3) A well-supported clade composed of two groups: Sycophaga (mostly associated with Australasian, oriental and afrotropical Sycomorus fig trees) and Idarnes Walker, 1843 (associated with the New World Americana figs).
The biogeography of the subfamily has been discussed by
Among the guilds that constitute fig wasp communities, Pseudidarnes species belong to the “large gall inducers” (
Pseudidarnes males are usually winged, but wingless males of Pseudidarnes minerva were recorded with very low frequencies (
In this paper we describe and illustrate six previously unknown species (two from Australia and four from Papua New Guinea). Redescriptions are also provided for Pseudidarnes minerva and Pseudidarnes flavicollis. We finally elaborated both dichotomous and interactive online keys to the known species of Pseudidarnes.
Maturing fig syconia were collected, opened, and transferred to tissue bags until the wasps emerge, which happens after a few hours - days. Wasps were killed using acetate and transferred to 70% ethanol. Most geographical coordinates and altitudes were estimated using label information. Field recorded coordinates were provided when available. Field-collected specimens were dehydrated through an ethanol and HMDS series (
Type and specimen depositories, and their respective curators are:
ASCI Australia, New South Wales, Orange, Orange Agricultural Institute, Agricultural Scientific Collections Unit (Peter Gillespie).
BMNH United Kingdom, London, The Natural History Museum [formerly British Museum (Natural History)](Natalie Dale-Skey Papilloud).
CBGP France, Montpellier. Centre de Biologie pour la Gestion des Populations (Emmanuelle Artige).
SAMC South Africa, Cape Town, Iziko South African Museum (Simon Van Noort).
To produce high quality images, some specimens were point-mounted on grey card in order to avoid loss of contrast caused by white background. Images were produced with an EntoVision Premium Portable Imaging System, comprising a Leica M16 zoom lens, a JVC KY-75U 3CCD digital camera and a portable computer workstation running EntoVision Imaging Suite software (GT Vision, Hagerstown, MD U.S.A.). Cartograph v5.6.0 (Microvision, Evry, France) software was subsequently used to merge an image series (representing about ten to twenty focal planes), producing a single image with increased depth of field. Illumination was achieved using a “quadrant” setup, with four fibre optic light guides stemming from two individual light sources (Leica CLS 150 X), similar to the one described by
http://species-id.net/wiki/Pseudidarnes
http://www.figweb.org/Fig_wasps/Agaonidae/Sycophaginae/Pseudidarnes/index.htm
Pseudidarnes minerva Girault, 1927, by monotypy.
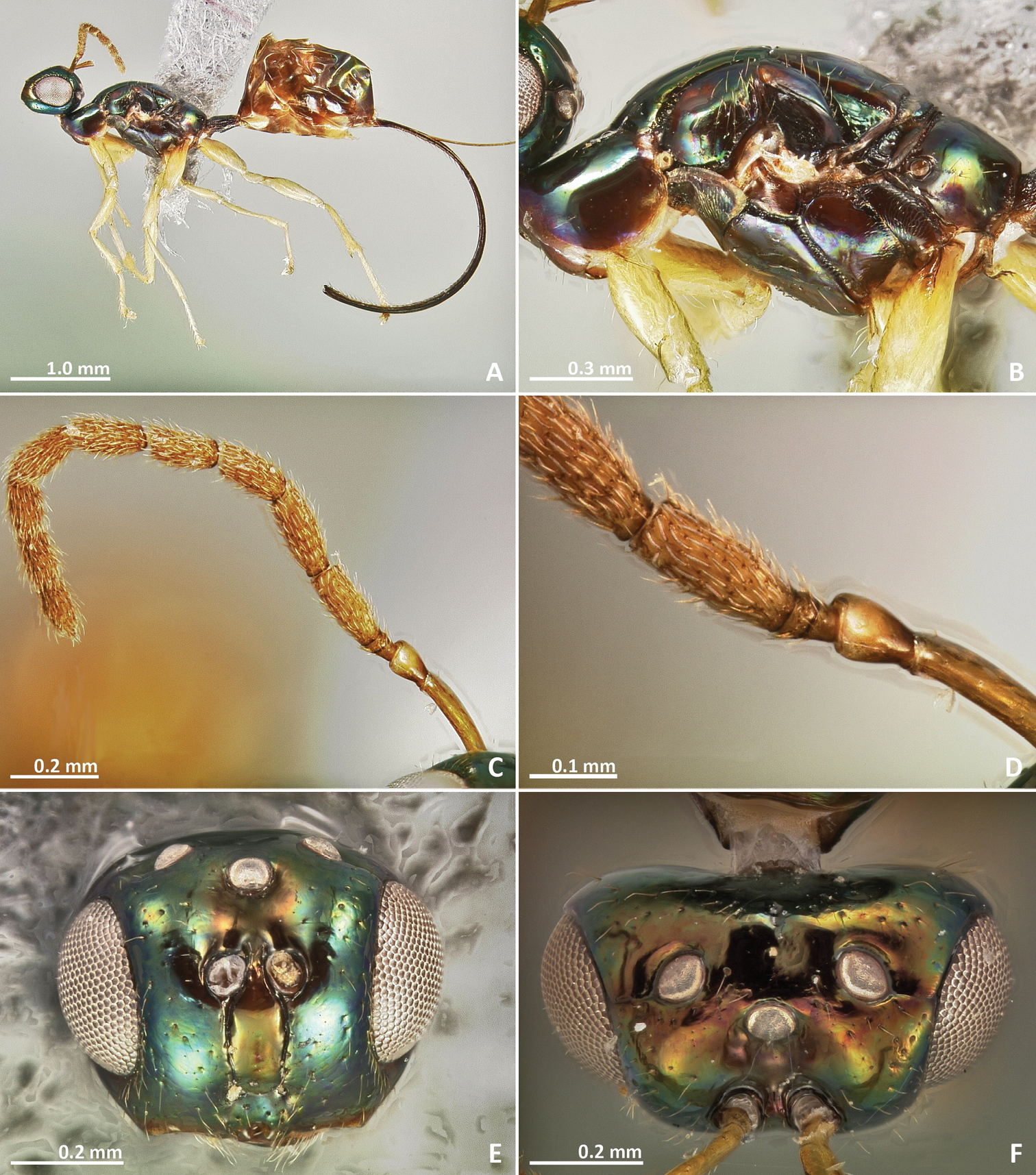
Female. Body length (excluding ovipositor) 2.3–3.7 mm. Body colour variable, yellow to dark brown, sometimes with green metallic tinge.
Head. Face sculpture smooth to reticulate or slightly engraved, sometimes punctate. Antennae inserted well above to slightly below the middle line of compound eyes, but never very close to the clypeal margin. Toruli separated by one torulus diameter or less. Clypeal margin bilobed. Maxillary palpi composed of four segments. Labial palpi composed of three segments. Supraclypeal area delimited by subantennal grooves. Antenna with 13 segments (two anelli), and a 14th segment very short and unconspicuous. Funicular segments 1–2 × as long as wide.
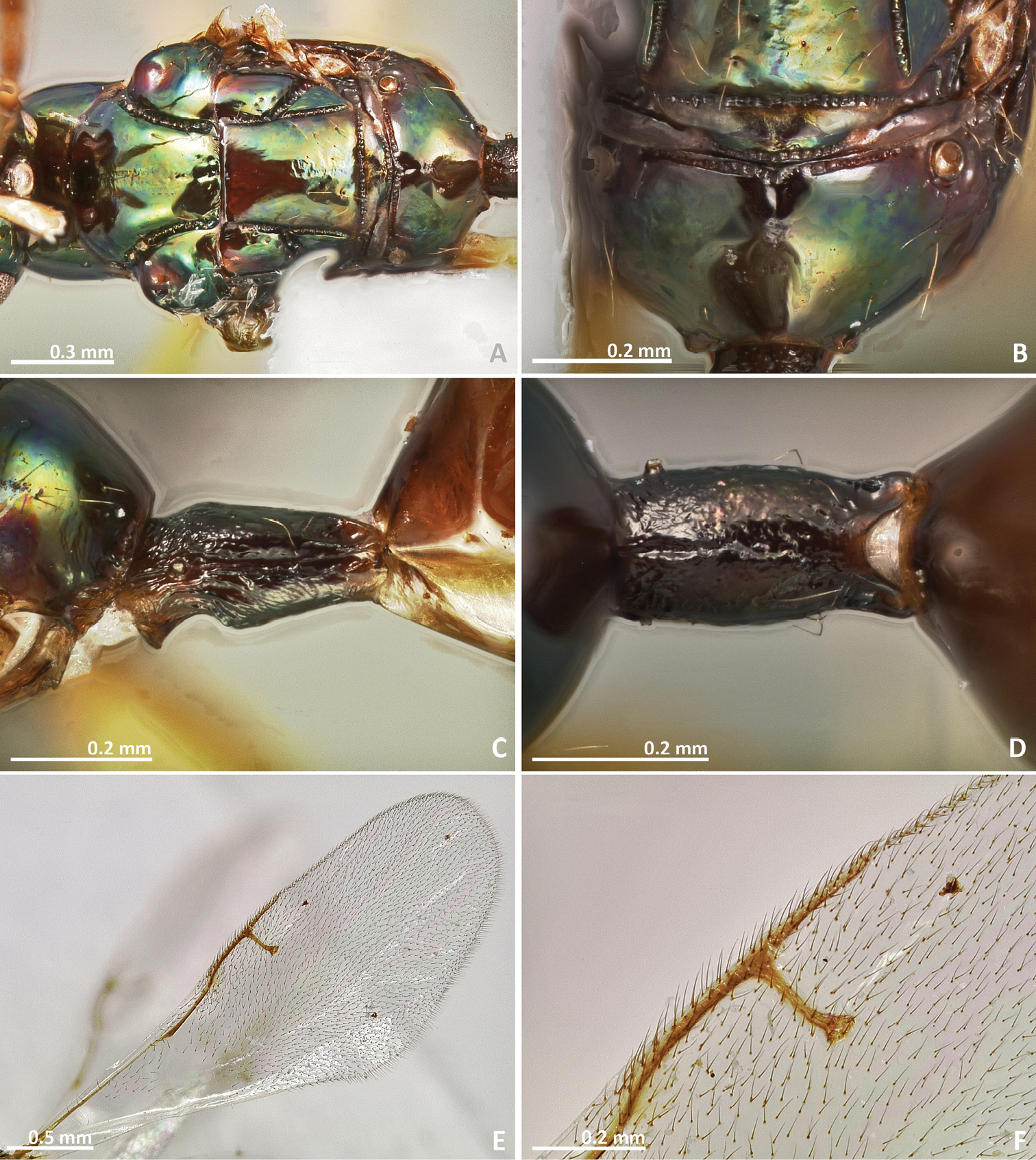
Mesosoma. Pronotum 1–2 × as long as high in lateral view. Notauli complete, deep and at least slightly crenulated. Mesoscutum as long as wide or longer than wide. Axilullar sulcus straight to slightly concave. Propodeum transverse, smooth to slightly reticulate and sometimes with a median sulcus. Wings hyaline, sometimes slightly infuscate medially. Marginal vein as long as stigmal vein, or longer. Postmarginal vein present (very short in Pseudidarnes cooki sp. n.). Marginal and postmarginal vein sometimes particularly widened (as Figs 4D, 6D, 8D, 10D & 16D).
Metasoma. First metasomal segment petiolate (petiole short in Pseudidarnes acaudus sp. n.). Ovipositor sheaths as long as body or shorter (very short in Pseudidarnes acaudus sp. n.).
Male. Very similar to female, usually slender and sometimes showing tinge variation. Male with very different colour patterns in Pseudidarnes astridae sp. n. (Figs 3–6).
Online dichotomous and multi-access interactive LUCID keys to Pseudidarnes species are available at: http://www.figweb.org/Fig_wasps/Agaonidae/Keys/index.htm
| 1 | Ovipositor sheaths extremely short, only weakly protruding beyond metasoma apex (Figure 1A). Pedicel elongated, slightly shorter than the scape (Fig. 1D). Mesosoma entirely brown (Fig. 2A) | Pseudidarnes acaudus sp. n. |
| – | Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Pedicel clearly shorter than the scape, at most 0.5 × the scape length. Mesosoma colour different, metallic or, when brown, with at least the pronotum yellow in lateral view | 2 |
| 2 | Pronotum yellow, without metallic tinge. Mesoscutum with irregular transverse striae (Fig. 4A, 8A, 16A). Marginal and postmarginal veins widened (Figs 4F, 8F, 16F). | 3 |
| – | Mesosoma metallic green, including pronotum. Mesoscutum smooth or reticulated. Marginal and postmarginal veins not widened | 5 |
| 3 | Head and mesosoma excluding pronotum with metallic tinge (Figs 15B, E, 16A). Propodeum with a well delimitated and carinulated median sulcus, extending to the posterior margin of the sclerite (Fig. 16B). Metascutellum crenulated (Fig. 16B) | Pseudidarnes flavicollis Bouček |
| – | Body without metallic tinge. Median sulcus of propodeum unconspicuous or absent. Metascutellum not crenulated | 4 |
| 4 | Mesosoma brown in dorsal view (Fig. 8A). Metascutellum with faint longitudinal striae (Figs 8B, 10B). Propodeum without median line (Figs 8B, 10B) | Pseudidarnes badiogeminus sp. n. |
| – | Mesosoma yellow in dorsal view (Fig. 4A, brown in males, but at least mesoscutellum yellow, Fig. 6A). Metascutellum and median area of propodeum with irregular transverse rugae (Figs 4B, 6B). | Pseudidarnes astridae sp. n. |
| 5 | Mesosoma entirely smooth and shiny (Figs 19B, 20A, 21B, 22A). First funicular segment 2 × as long as wide (Figs 19D, 21D). Distal antennomeres not forming a definite clava (Figs 19C, 21C). Propodeum with a very short median line (Figs 20B, 22B). | Pseudidarnes laevis sp. n. |
| – | Mesosoma sculpture mostly reticulate. First funicular segment ca. 1–1.5 × as long as wide. Distal antennomeres forming a definite clava. Propodeum medially with a deep carinulated sulcus, at least on the anterior half of the sclerite | 6 |
| 6 | Ovipositor sheaths short, about as long as the metasoma (Fig. 11A). Propodeum with a crenulated median sulcus extending to the posterior margin (Figs 12B, 14B) | Pseudidarnes cooki sp. n. |
| – | Ovipositor sheaths longer than metasoma. Propodeal median sulcus not reaching the posterior margin | 7 |
| 7 | Petiole transverse in dorsal view (Fig. 18D). Median sulcus of the propodeum broad and extending over the anterior half of the sclerite (Fig. 18B). Postmarginal vein shorter than the stigmal (Fig. 18F) | Pseudidarnes kjellbergi sp. n. |
| – | Petiole longer than wide in dorsal view (Figs 24D, 26D). Median sulcus of the propodeum extending over most of the propodeum length, not reaching the posterior margin (Figs 24B, 26B). Postmarginal veiwn as long as the stigmal (Figs 24F, 26F) | Pseudidarnes minerva Girault |
http://zoobank.org/ECCB10FF-4783-45C9-A97E-02E1E27600CF
http://species-id.net/wiki/Pseudidarnes_acaudus
Figures 1–2Holotype. ♀, PAPUA NEW GUINEA: Crater Mountain, -6.58°, 145.08°, 2000m, V.1990, McKee A., ex Ficus sp. (CBGP).
Paratype. ♀, same data as holotype, (CBGP).
Pedicel elongated, slightly shorter than the scape. Mesosoma entirely brown. Petiole short, transverse in dorsal view. Ovipositor sheaths extremely short, only weakly protruding beyond metasoma apex.
Female. Body length 2.6 mm. Metallic tinge absent or very feeble. Predominantly brown. Scape and pedicel yellow brown. Head darker than mesosoma. Petiole yellow. Legs predominantly yellow, coxae almost concolorous with mesosoma. Remaining leg segments predominantly yellow and brown.
Head. Antennae inserted far above the middle line of compound eyes. Scape slightly longer than pedicel. Pedicel very elongated (more than 2 × as long as wide), slender, and longer than first funicular segment. Anelli almost as long as wide, proximal anellus longer than wide. First funicular segment approximately 1.5 × as long as wide. Distal antennomeres not forming a distinct clava. Face sculpture slightly engraved. Face pilosity short and sparse. Supraclypeal area narrow, its delimiting sulci converging near epistomal groove, and its sculpture barely rugose. Lateral ocelli nearly 1 × its diameter far from the eye margin.
Mesosoma. Pronotum longer than high in lateral view. Mesoscutum slightly engraved reticulate. Frenal sulcus smooth. Mesepimeron sculpture mostly smooth, slightly engraved. Metascutellum very short and smooth, inconspicuous, and almost completely covered by frenum. Propodeum with a well delimited and slightly carinulated median sulcus, which extends to the posterior margin of the sclerite. Propodeum sculpture smooth, slightly rugose. Wings hyaline, with sparse pilosity. Marginal and postmarginal vein not particularly widened. Postmarginal vein as long as stigmal vein.
Metasoma. Petiole smooth and transverse in lateral view. Petiole dorsally without a longitudinal median sulcus. Ovipositor sheaths extremely short, only weakly protruding beyond metasomal apex.
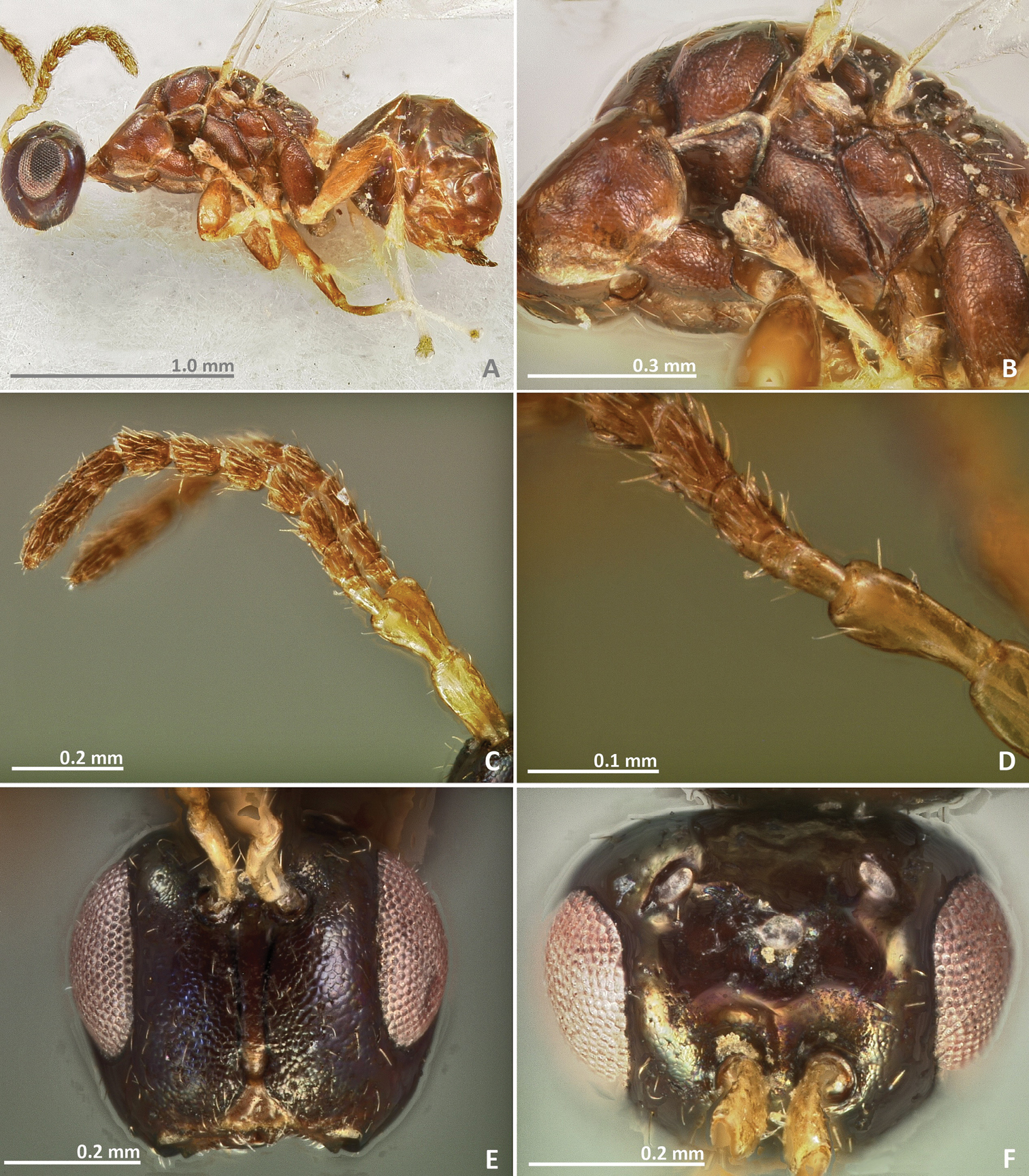
Pseudidarnes acaudus sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes acaudus sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Unknown.
The specific name refers to the short ovipositor sheaths exhibited by this species.
Associated with an undetermined Ficus species collected in Papua New Guinea. Reared together with Pseudidarnes laevis sp. n., but less abundant than the later.
http://zoobank.org/FBBF3AC7-E935-40E8-AF15-FA87970689EC
http://species-id.net/wiki/Pseudidarnes_astridae
Figures 3–6Holotype. ♀, PAPUA NEW GUINEA: East New Britain: Raunsepna, North Baining Mountains, -4.433°, 151.783°, 1000m, 26.II.1999, Vaamonde CL, ex Ficus xylosycia CLV11 (CBGP).
Paratype. ♂ Same data as holotype (CBGP).
Body without metallic tinge. Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesoscutum with faint irregular transverse striae. Median area of metascutellum and median area of propodeum with irregular transverse rugae. Marginal and postmarginal vein widened.
Female. Body length 3.4 mm. Ovipositor sheaths length 1.8 mm. Metallic tinge absent. Predominantly yellow. Head dark brown. Mesepisternum, mesepimeron, and metapleuron predominantly brown.
Head. Antennae inserted far above the middle line of compound eyes. Scape more than 2 × as long as pedicel. Pedicel elongated, slender, but shorter than first funicular segment. Anelli almost as long as wide. First funicular segment 2 × as long as wide. Distal antennomeres not forming a distinct clava. Face sculpture smooth with sparse punctures, lower face slightly engraved. Face pilosity long and dense. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli 0.5 × its own diameter far from the eye margin.
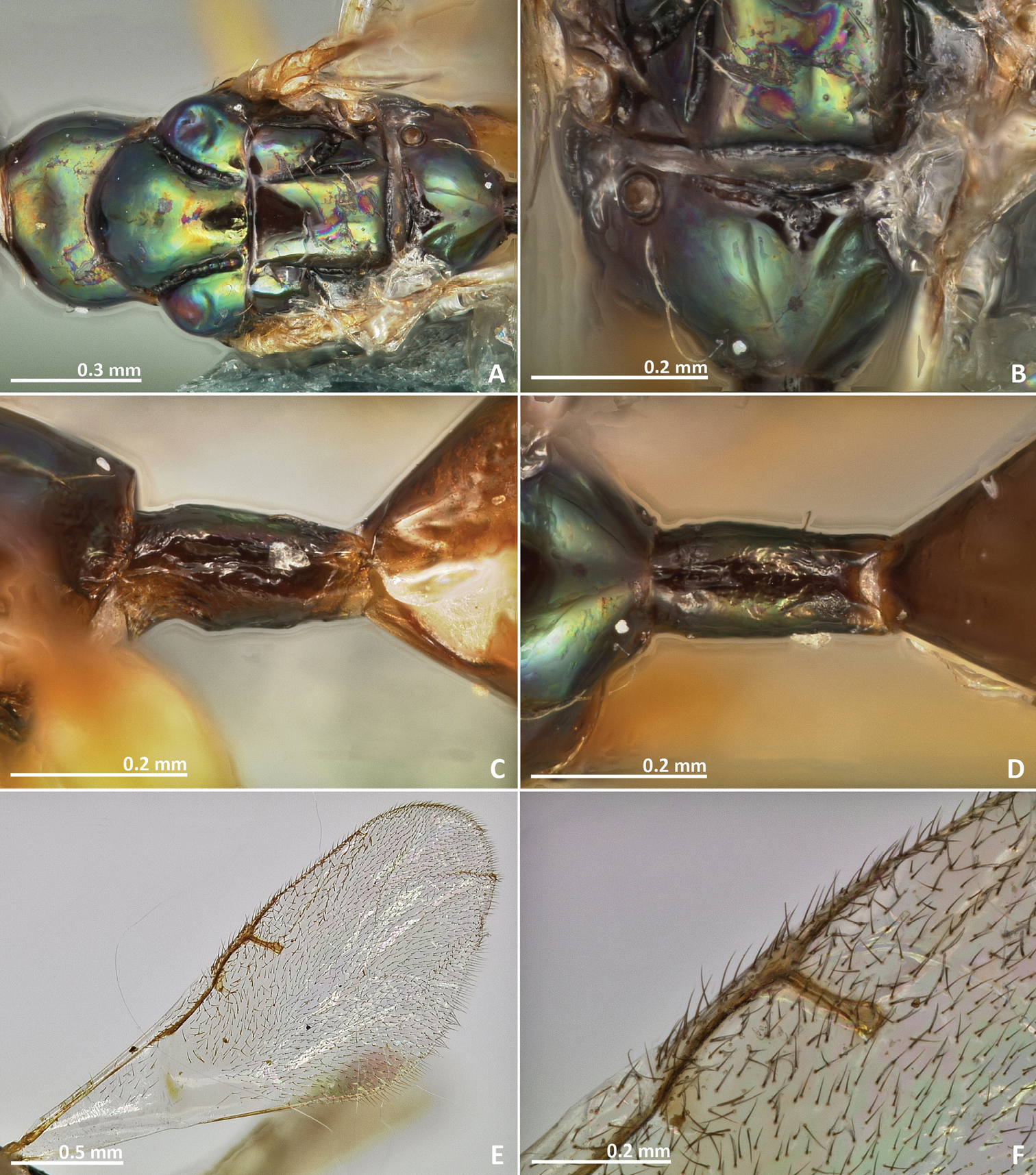
Mesosoma. Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesoscutum with faint transversal striae. Mesoscutellum smooth. Frenal sulcus sparsely crenulated. Mesepimeron sculpture mostly smooth, slightly striate. Metascutellum longer than frenum and smooth, with faint and irregular transverse rugae at the median line. Propodeum sculpture mostly smooth. Median line of propodeum with irregular transverse rugae. Wings with rather dense pilosity, and medially infuscate. Marginal and postmarginal vein widened. Postmarginal vein shorter than stigmal vein.
Metasoma. Petiole rugose, 1.7 × as long as high in lateral view. Petiole dorsally without a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 2.8 × hind tibia length and 0.5 × body length.
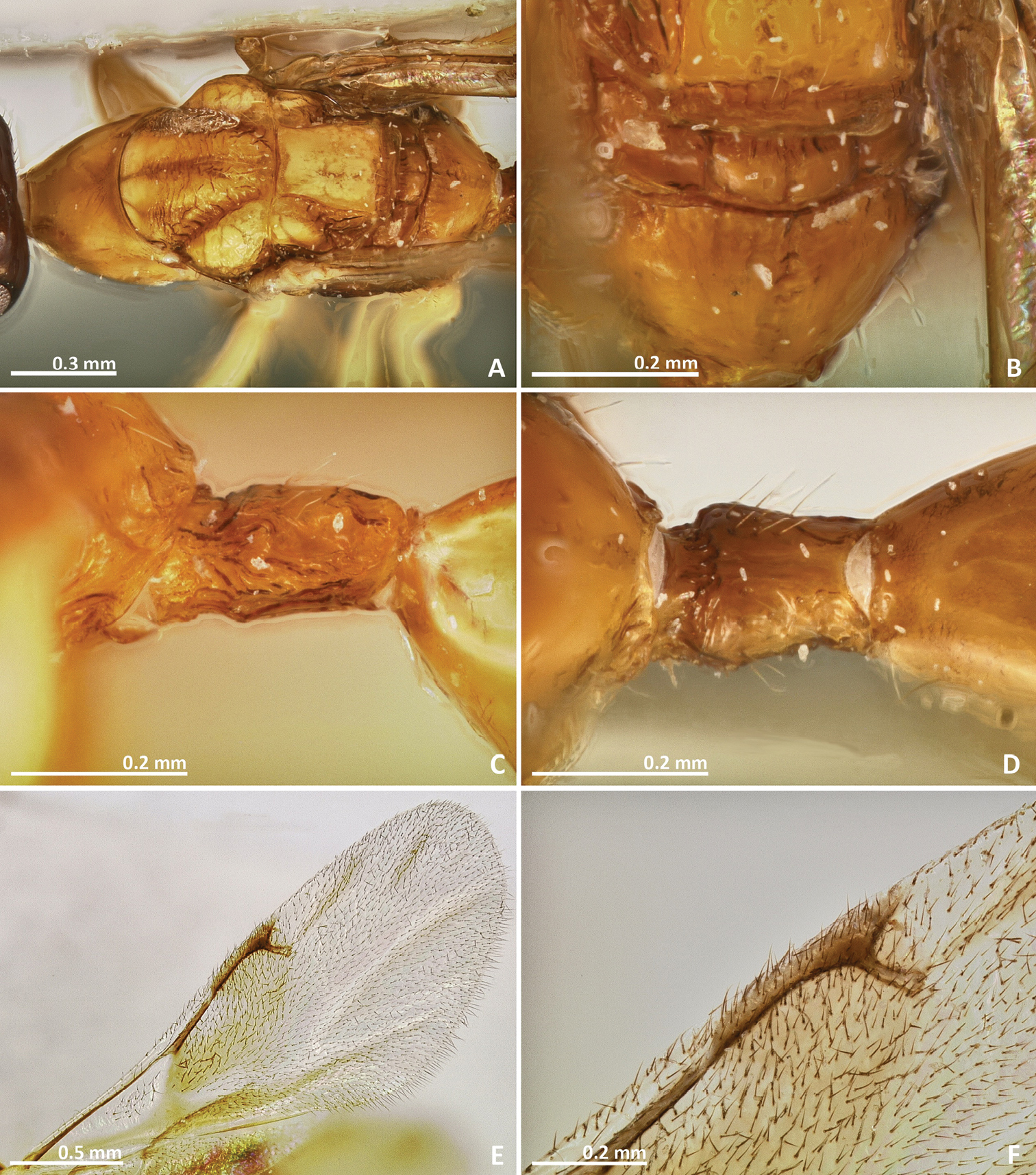
Pseudidarnes astridae sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes astridae sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Body length 4.2 mm. Characters agreeing with females, except the following. Body colour browner, mesoscutellum yellow in dorsal view. Posterior ocelli contiguous to the eye margin, and larger. Wing infuscation more pronounced.
Pseudidarnes astridae sp. n. male. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes astridae sp. n. male. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
The specific name is dedicated to our friend Astrid Cruaud for the long long walks we share together in the jungles of the world, trying to find fig trees.
Reared from syconia of Ficus xylosycia Diels. Ficus xylosycia hosted three Pseudidarnes species. Pseudidarnes badiogeminus sp. n. was collected together with Pseudidarnes astridae sp. n. in New Britain, whereas Pseudidarnes flavicollis Bouček was collected in Bulolo. We are convinced that the host identification is correct for both samplings and that the guilds of non-pollinating fig wasps may vary with geography.
http://zoobank.org/0EA058A5-E760-4114-8CBD-72DF1D488803
http://species-id.net/wiki/Pseudidarnes_badiogeminus
Figures 7–10Holotype. ♀, PAPUA NEW GUINEA: East New Britain: Raunsepna, North Baining Mountains, -4.433°, 151.783°, 1000m, 26.II.1999, Vaamonde CL, ex Ficus xylosycia CLV11 (CBGP).
Paratype. 1♀ 1♂ same data as holotype (CBGP).
Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesosoma brown in dorsal view. Mesoscutum with irregular transverse rugae. Metascutellum with faint longitudinal striae. Marginal and postmarginal veins widened. Median line of propodeum absent
Female. Body length 3 mm. Ovipositor sheaths length 1.6 mm. Metallic tinge absent. Body colour predominantly brown. Scape and pedicel yellow. Flagellomeres yellow brown. Head dark brown. Pronotum yellow brown laterally. Legs yellow, coxae browner.
Head. Antennae inserted far above the middle line of compound eyes. Scape nearly 2 × as long as pedicel. Pedicel elongated, slender, and as long as first funicular segment. Proximal anellus longer than wide. First funicular segment 2 × as long as wide. Distal antennomeres not forming a distinct clava. Face sculpture engraved, slightly rugose. Face pilosity short and sparse. Supraclypeal area narrow, its delimiting sulci converging near epistomal groove, and its sculpture barely rugose. Lateral ocelli 0.5 × its own diameter far from the eye margin.
Mesosoma. Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesoscutum transversally striate. Mesoscutellum mostly smooth. Frenal sulcus with shallow crenulation. Mesepimeron sculpture mostly smooth, slightly striate. Metascutellum longer than frenum, with faint longitudinal striae. Propodeum smooth, without median line. Wings with rather dense pilosity, and medially infuscate. Marginal and postmarginal vein widened. Postmarginal vein longer than stigmal vein.
Metasoma. Petiole slightly rugose, 1.7 × as long as high in lateral view. Petiole dorsally without a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 2.7 × hind tibia length and 0.5 × body length.
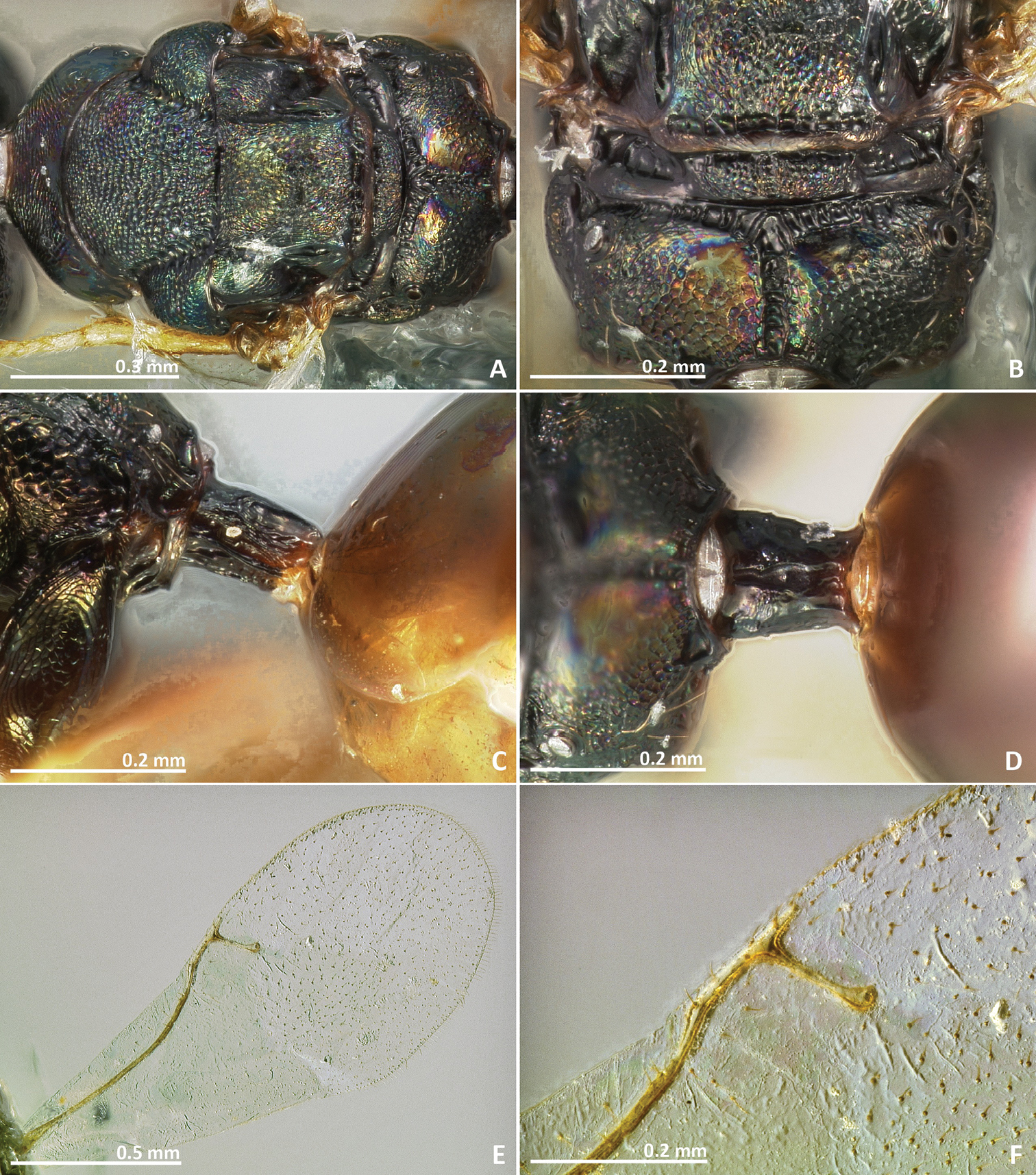
Pseudidarnes badiogeminus sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes badiogeminus sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Body length 3.2 mm. Characters agreeing with females, except the following: ocelli larger and contiguous to the eye margin. Pedicel slightly shorter.
Pseudidarnes badiogeminus sp. n. male. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes badiogeminus sp. n. male. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
The specific name refers to the similarity of Pseudidarnes badiogeminus with Pseudidarnes astridae, but showing different colour.
Collected from syconia of Ficus xylosycia. See Pseudidarnes astridae for further information.
http://zoobank.org/2C54B54B-5925-45BD-A1CA-1440704749C8
http://species-id.net/wiki/Pseudidarnes_cooki
Figures 11–14Holotype. ♀, AUSTRALIA: Queensland: Cairns, Rex Lookout, -16.65°, 145.56°, 100m, 13.I.1999, Rasplus J.Y., ex Ficus obliqua (CBGP).
Paratypes. AUSTRALIA: Queensland: Cairns, Rex Lookout, -16.65°, 145.56°, 100m, 7♀, 1♂, 13.I.1999, Rasplus J.Y., ex. Ficus obliqua (CBGP), North of Cairns, Costal road, -16.65°, 145.56°, 100m, 1♀, 27.X.2005, Jousselin E. & Coeur d’Acier A., ex Ficus obliqua, n° JRAS01422 (CBGP), Port Douglas, -16.483230°, 145.464058°, 10m, 3♀, 28.X.2005, Jousselin E. & Coeur d’Acier A., ex Ficus obliqua, n° JRAS01429 (1 ♀ CBGP, 1 ♀ BMNH, 1 ♀ SAMC).
Metallic tinge present at least in some body regions. Mesosoma sculpture mostly reticulate. Propodeum with a crenulated median sulcus extending to the posterior margin. Postmarginal vein shorter than stigmal vein. Ovipositor sheaths short, about as long as the metasoma.
Female. Body length 2.3 mm. Ovipositor sheaths length 0.9 mm. Metallic tinge present at least in some body regions. Predominantly dark green. Antennae brown. Coxae almost concolorous with mesosoma. Femora brown. Tibiae and tarsi predominantly yellow. Metatibia proximally yellow brown. Metasoma predominantly brown.
Head. Antennae inserted just above the middle line of compound eyes. Scape nearly 3 × as long as pedicel. Pedicel almost as long as wide, pyriform, and shorter than first funicular segment. Anelli transverse. First funicular segment approximately 1.5 × as long as wide. Distal antennomeres forming a distinctive clava. Face sculpture reticulate. Face pilosity short and sparse, becoming longer near oral margin and eyes. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli 1 × its own diameter far from the eye margin.
Mesosoma. Pronotum short, nearly as long as high in lateral view. Mesoscutum strongly reticulate. Mesoscutellum reticulate. Frenal sulcus densely crenulated. Mesepimeron sculpture reticulate. Metascutellum longer than frenum, reticulate. Propodeum with a well delimited and carinulated median sulcus, extending to the posterior margin of the sclerite. Propodeum sculpture reticulate, smooth near the proximal region of median line of propodeum. Wings hyaline, with sparse pilosity. Marginal and postmarginal vein not particularly widened. Postmarginal vein shorter than stigmal vein.
Metasoma. Petiole rugose, 1.5 × as long as high in lateral view. Petiole dorsally with a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 2.25 × hind tibia length, 0.4 × body length.
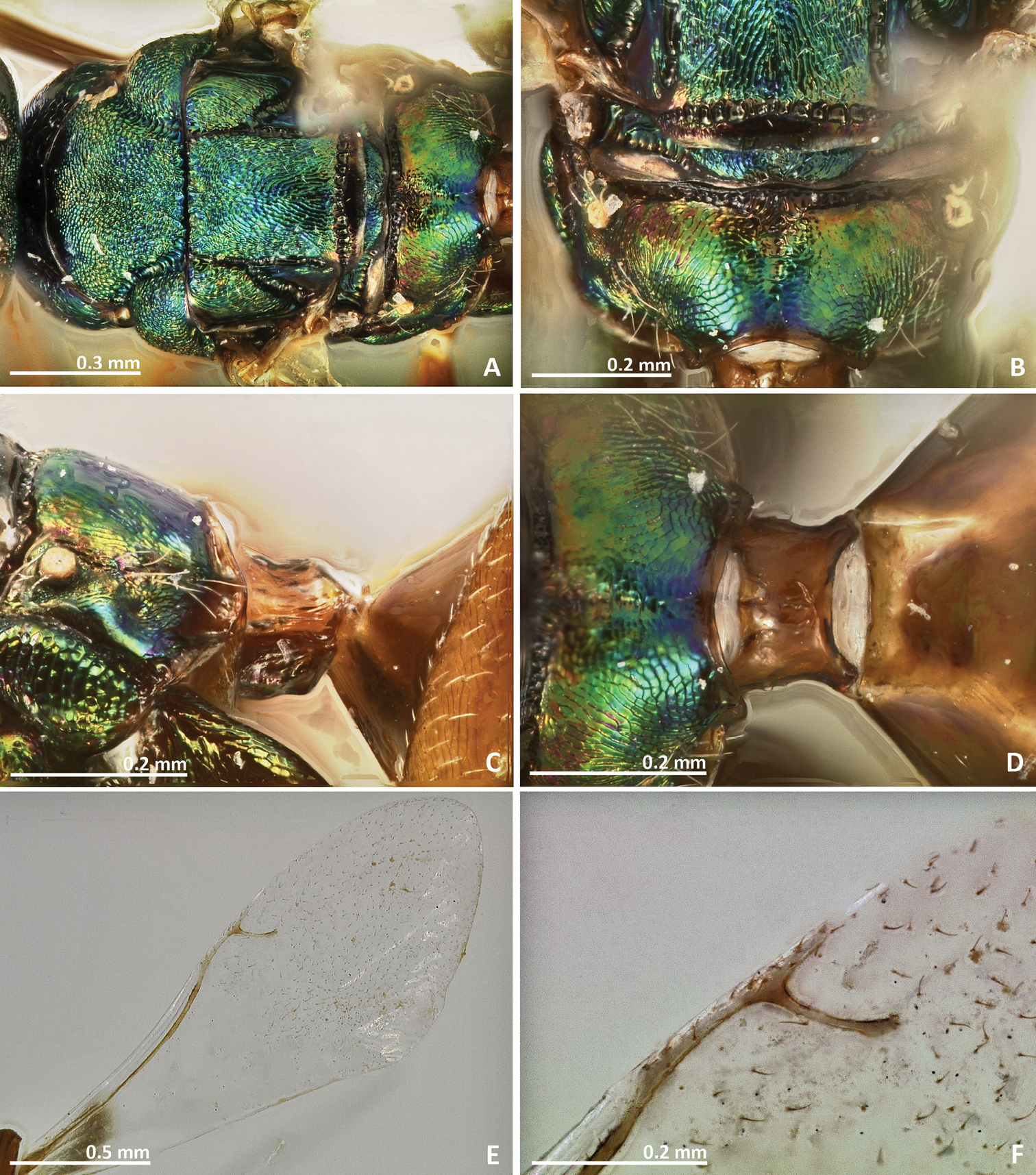
Pseudidarnes cooki sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes cooki sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Body length 2.4 mm. Characters agreeing with the females, except the following. Body slender. Antenna more yellow and inserted at the middle line of compound eyes or slightly below. Ocelli larger. Body sculpture fainter. Petiole more brown. Wings more pilose.
Pseudidarnes cooki sp. n. male. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes cooki sp. n. male. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
The specific name is dedicated to our friend and colleague Dr. James Cook, in recognition of his amazing contribution to our knowledge of fig wasps.
This species is strictly associated with Ficus obliqua G. Forst. and was studied by
http://species-id.net/wiki/Pseudidarnes_flavicollis
Figures 15–16Holotype. ♀, PAPUA NEW GUINEA: Bulolo: Manki area, -5.37°, 144.18°, 700m, 22.VII.1981, Roberts H., ex Ficus xylosycia (BMNH) [examined].
Paratype. ♀, same data as holotype (BMNH) [examined].
Head and mesosoma excluding pronotum with metallic tinge. Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesoscutum with irregular transverse rugae. Marginal and postmarginal veins widened. Propodeum with a well delimitated and carinulated median sulcus, extending to the posterior margin of the sclerite. Metascutellum as well as lateral panel of metanotum crenulated.
Female. Body length 3.3 mm. Ovipositor sheaths length 1.6 mm. Metallic tinge present at least in some body regions. Predominantly yellow and green. Scape and pedicel yellow. Flagellomeres brown. Head green, green red near vertex. Pronotum yellow. Prepectus yellow brown. Remaining mesosoma geen to dark green, propodeum green brown. Legs predominantly yellow, metacoxa proximally brown. Petiole dark brown. Metasoma predominantly yellow, dorsally brown.
Head. Antennae inserted far above the middle line of compound eyes. Scape nearly 2 × as long as pedicel. Pedicel elongated, slender, and shorter than first funicular segment. Anelli transverse. First funicular segment 2 × as long as wide. Distal antennomeres not forming a distinct clava. Face sculpture smooth with sparse punctures, lower face with engraved transverse striae. Face pilosity short and sparse, becoming longer near oral margin and eyes. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli contiguous to the eye margin.
Mesosoma. Pronotum long, nearly 1.5–2 × as long as high in lateral view. Mesoscutum transversally striate. Mesoscutellum smooth. Frenal sulcus densely crenulated. Mesepimeron sculpture slightly striate. Metascutellum as well as lateral panel of metanotum crenulated, metascutellum longer than frenum. Propodeum with a well delimited and carinulated median sulcus, extending to the posterior margin of the sclerite. Propodeum sculpture mostly smooth, slightly rugose laterally. Wings hyaline, with rather dense pilosity. Marginal and postmarginal vein widened. Postmarginal vein longer than stigmal vein.
Metasoma. Petiole rugose, 2 × as long as high in lateral view. Petiole dorsally without a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 2.7 × hind tibia length, 0.5 × body length.
Pseudidarnes flavicollis Bouček, 1988, paratype, female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes flavicollis Bouček, 1988, paratype, female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Unknown.
Collected from syconia of Ficus xylosycia Diels. See Pseudidarnes astridae biology section.
http://zoobank.org/1DF43475-A3A3-431B-8B59-C3810F83970F
http://species-id.net/wiki/Pseudidarnes_kjellbergi
Figures 17–18Holotype. ♀, AUSTRALIA: Kununarra:, -15.8319°, 128.8564°, 80m, 20.X.1997, Dixon, D., ex Ficus platypoda, n° PhD 455 (CBGP).
Paratype. ♀, same data as holotype (CBGP).
Mesosoma metallic green. Mesosoma sculpture mostly reticulate. Median sulcus of the propodeum extending over the anterior half of the sclerite. Postmarginal vein shorter than the stigmal. Petiole transverse in dorsal view. Ovipositor sheaths longer than metasoma.
Female. Body length 3.1 mm. Ovipositor sheaths length 2.4 mm. Metallic tinge present at least in some body regions. Predominantly green. Antenna yellow brown. Coxae brown. Femora and tibiae predominantly brown. Tarsi yellow. Petiole brown. Metasoma green brown.
Head. Antennae inserted at the middle line of compound eyes. Scape nearly 3 × as long as pedicel. Pedicel almost as long as wide, pyriform, and as long as first funicular segment. Anelli transverse. First funicular segment approximately as long as wide. Distal antennomeres forming a distinctive clava. Face sculpture reticulate. Face pilosity short and sparse. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli nearly 1 × its diameter far from the eye margin.
Mesosoma. Pronotum short, nearly as long as high in lateral view. Mesoscutum strongly reticulate. Mesoscutellum reticulate. Frenal sulcus densely crenulated. Mesepimeron sculpture slightly reticulate ventrally, becoming smooth in its medial and upper region. Metascutellum as long as frenum, reticulate. Propodeum with a broad crenulated median line extending over the anterior half of the sclerite. Median line very faint or absent in the posterior half of the propodeum. Propodeum sculpture engraved reticulate. Wings hyaline, with sparse pilosity. Marginal and postmarginal vein not particularly widened. Postmarginal vein shorter than stigmal vein.
Metasoma. Petiole slightly rugose, and transverse in lateral view. Petiole dorsally without a longitudinal median sulcus Ovipositor sheaths long, distinctly protruding beyond metasomal apex. Ovipositor sheaths length 3.8 × hind tibia length, 0.7 × body length.
Pseudidarnes kjellbergi sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes kjellbergi sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Unknown.
The specific name is dedicated to our friend and colleague Dr. Finn Kjellberg, in recognition of his excellent work in fig wasps.
Reared from syconia of Ficus platypoda (Miq.) A. Cunn. ex Miq.
http://zoobank.org/475EA63C-EDE3-4BE9-819E-616FBEE94879
http://species-id.net/wiki/Pseudidarnes_laevis
Figures 19–22Holotype. ♀, PAPUA NEW GUINEA: Crater Mountain -6.58°, 145.08°, 2000m, V.1990, McKee A., ex Ficus sp., n° AM 451 (CBGP).
Paratypes. PAPUA NEW GUINEA: Crater Mountain -6.58°, 145.08°, 2000m, 20♀, 20♂, V.1990, McKee A., ex Ficus sp., n° AM 451 (17♀, 18♂ CBGP, 1♀, 1♂ BMNH, 1♀, 1♂ SAMC, 1♀ RPSP), 6♀, 1♂, V.1990, McKee A., ex Ficus sp., n° AM 550 (CBGP).
Mesosoma metallic green, entirely smooth and shiny. First funicular segment 2 × as long as wide. Distal antennomeres not forming a definite clava.
Female. Body length 3.7 mm. Ovipositor sheaths length 3.9 mm. Metallic tinge present at least in some body regions. Predominantly dark green. Antennae yellow brown. Legs yellow. Petiole dark brown. Metasoma predominantly brown, slightly green.
Head. Antennae inserted at the middle line of compound eyes or slightly above. Scape nearly 3 × as long as pedicel. Pedicel almost as long as wide, shorter than first funicular segment. Anelli transverse. First funicular segment 2 × as long as wide. Distal antennomeres not forming a distinct clava. Face sculpture smooth, with very sparse punctures. Face pilosity short and sparse. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli nearly 1 × its diameter far from the eye margin.
Mesosoma. Pronotum longer than high in lateral view. Mesoscutum mostly smooth. Mesoscutellum smooth. Frenal sulcus densely crenulated. Mesepimeron sculpture mostly smooth. Metascutellum smooth, very short, inconspicuous, and almost completely covered by frenum. Propodeum with a vestigial median line not extending from the beginning of the proximal region. Propodeum sculpture smooth. Wings hyaline, with rather dense pilosity. Marginal and postmarginal vein not particularly widened. Postmarginal vein as long as stigmal vein, or slightly longer.
Metasoma. Petiole 2 × as long as high in lateral view. Petiole sculpture in lateral view slightly rugose. Petiole dorsally with a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 4.3 × hind tibia length, as long as body.
Pseudidarnes laevis sp. n. female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes laevis sp. n. female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Body length 3 mm. Characters agreeing with the females, except the following. Body slender. Anelli more transverse. Ocelli slightly larger than the female’s ocelli. Wing more pilose.
Pseudidarnes laevis sp. n. male. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes laevis sp. n. male. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
The specific name refers to the smooth body sculpturation exhibited by this species.
See Pseudidarnes acaudus biology section.
http://species-id.net/wiki/Pseudidarnes_minerva
Figures 23–27Holotype. ♀, AUSTRALIA: Queensland: Brisbane and Lake Manchester, -27.48°, 152.76°, 67m, [no date], ex Ficus rubiginosa (ASCI) [examined].
AUSTRALIA: Queensland: Amity, -27.39°, 153.44°, 5m, 7♀, 23.I.1999, Rasplus J.Y. & Meusnier, S, ex Ficus rubiginosa, n° JRAS00722 (CBGP), Ballina, -28.89°, 153.56°, 5m, 7♀, 3♂, 25.I.1999, Rasplus J.Y. & Meusnier, S, ex Ficus rubiginosa, n° JRAS00726 (CBGP), 1♀, 25.I.1999, Rasplus J.Y., ex Ficus rubiginosa, n° JRAS00727_01 (CBGP), Mount Molloy, -16.67°, 145.33°, 400m, 1♂, 25.X.2005, Jousselin E. & Coeur d’Acier A., ex Ficus rubiginosa, n° JRAS01418_28 (CBGP), Yungaburra, -17.27°, 145.58°, 700m, 5♀, 15.I.1999, Rasplus J.Y. & Meusnier, S, ex Ficus rubiginosa, n° JRAS00690 (CBGP); Victoria: Melbourne, -37.81°, 144.96°, 20m, 2♀, 2♂, I.1995, Cook J., ex Ficus rubiginosa (CBGP).
Mesosoma metallic green, mostly reticulate. Median sulcus of the propodeum extending over most of the propodeum length, not reaching the posterior margin. Postmarginal vein as long as the stigmal. Petiole longer than wide in dorsal view. Ovipositor sheaths longer than metasoma.
Body length 2.6 mm. Ovipositor sheaths length 2.33 mm.
Coloration. Metallic tinge present at least in some body regions. Predominantly green. Scape yellow. Petiole yellow brown. Flagellomeres brown. Coxae almost concolorous with mesosoma. Femora brown. Tibiae and tarsi predominantly yellow. Metatibia proximally browner. Petiole brown. Metasoma browner dorsally.
Head. Antennae inserted at or slightly below the middle line of compound eyes. Scape nearly 3 × as long as pedicel. Pedicel almost as long as wide, pyriform, and as long as first funicular segment. Anelli transverse. First funicular segment longer than wide to approximately as long as wide. Distal antennomeres forming a distinctive clava. Face sculpture reticulate. Face pilosity short and sparse. Supraclypeal area wide, its delimiting sulci not converging near epistomal groove, and its sculpture mostly smooth. Lateral ocelli nearly one diameter far from the eye margin.
Mesosoma. Pronotum short, nearly as long as high in lateral view, or slightly longer than high. Mesoscutum reticulate. Mesoscutellum engraved. Frenal sulcus densely crenulated. Mesepimeron sculpture reticulate. Metascutellum very short, inconspicuous, and almost completely covered by frenum. Propodeum with a carinulated longitudinal median line, extending over most of the propodeum length, not reaching the posterior margin. Propodeum sculpture engraved reticulate, smooth near the proximal region of median line of propodeum. Petiole 1.5 × as long as high in lateral view. Wings hyaline, with sparse pilosity. Marginal and postmarginal vein not particularly widened. Postmarginal vein as long as stigmal vein, or slightly shorter.
Metasoma. Petiole sculpture in lateral view slightly rugose. Petiole dorsally with a longitudinal median sulcus. Ovipositor sheaths long, distinctly protruding beyond metasoma apex. Ovipositor sheaths length 4.4 × hind tibia length, 0.9 × body length.
Pseudidarnes minerva Girault, 1927 female. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes minerva Girault, 1927 female. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Male. Body length 2.3 mm. Characters agreeing with the females, except the following. body slender. Coxae brown, not concolorous with mesosoma. Anelli more transverse than female. Head sculpture engraved. Ocelli ca. 2 × the diameter of the female’s. Body sculpture fainter. Wing more pilose.
Wingless males were described for this species (
Pseudidarnes minerva Girault, 1927, male. A habitus lateral B mesosoma lateral C antenna D anelli E head, anterior view F vertex, dorsal view.
Pseudidarnes minerva Girault, 1927, male. A mesosoma dorsal B propodeum dorsal C petiole lateral view D petiole dorsal view E wing F detail of venation.
Pseudidarnes minerva Girault, 1927 holotype female. A habitus lateral B mesosoma in dorsal view.
Reared from Ficus rubiginosa Desf. ex Vent. syconia. Ficus rubiginosa is pollinated by Pleistodontes imperialis Saunders. Usually collected in low abundances, but sometimes they are quite abundant, at least in Eastern Australia and Auckland (as seen by
SOLOMON ISLANDS: Gatokae: Mbulo island, -8.76°, 158.28°, 100m, 1♂, 20.II.2019, Cruaud A & Rasplus JY, ex Ficus baola, n°JRAS02523_02 (CBGP).
Collected in Ficus baola C. C. Berg.
Two specimens belonging to this species were included in the phylogenetic analysis by
Due to their low abundance and relative rarity, some species were described here from very small series. Nevertheless, many specimens of Pseudidarnes laevis were collected from the same sample of figs, which indicates that they can sometimes show high infestation rates. This pattern is also observed in Anidarnes, which are also large gallers, and are usually found at low abundances (
Here we collected and analysed wasps from both sexes in five from the eight studied species. Pseudidarnes males were similar to the females, in contrast to many other wasps associated with fig inflorescences, which are sexually dimorphic and show wingless males. Nevertheless, wingless males occur in very low abundance for Pseudidarnes minerva (
The eight Pseudidarnes species were collected from five different hosts. Ficus xylosycia hosted three species, namely Pseudidarnes astridae, Pseudidarnes badiogeminus, and Pseudidarnes flavicollis. The former two species were reared together in the same sample. An undetermined fig species also hosted two species, Pseudidarnes laevis and Pseudidarnes acaudus (reared in a same sample). Despite the fact that more than one species may share the same fig, we did not find any Pseudidarnes species occurring in more than one host, which possibly indicates that they are host specific.
Pseudidarnes acaudus is the most divergent of all collected species, and is easily recognizable by its extremely short ovipositor sheaths. The other species can be separated into two morphological groups that correspond well to their geography and to their host association. Papuan species show a slender mesosoma, long funicular segments, and body sculpture that is mostly smooth, while Australian species have a short and robust mesosoma, shorter funicular segments, and a reticulate body sculpture. The taxonomy of the section Malvanthera is also geographically consistent since two of its subsections (namely Malvantherae and Platypodeae) are primarily Australian, while subsection Hesperidiiformes has its diversity centre in New Guinea (
This is the first revisionary treatment of Pseudidarnes. We believe that, due to the lack of previous careful sampling, several Pseudidarnes species remain to be discovered especially in New Guinea, but also in Australia. We hope that this work will encourage discovery and further studies on the biology of Pseudidarnes species.
We are greatly indebted to Armelle Coeur d'acier, James Cook, Dale Dixon, Emmanuelle Jousselin, and Carlos L. Vaamonde for contributing with samples. For assistance in the development of online keys we express our gratitude to Simon van Noort. We also express our sincere gratitude to Astrid Cruaud and Serge Meusnier for kind sampling assistance to JYR. For the loan of specimens we thank the following curators: Natalie Dale-Skey Papilloud (BMNH, London, U.K.) and Peter Gillespie (ASCI, Australia). The Synthesys project. http://www.synthesys.info/ funded the stay of JYR at the NMW and at NCB Naturalis. FHAF was funded by FAPESP (grants 2010/51158-5 and 2012/19815-1).