(C) 2013 Vladimir Pešić. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Karucia sublacustrina a new species of freshwater snails (Hydrobiidae, Gastropoda) is described based on material collected from Skadar Lake (Montenegro, Albania). The new species belongs to monotypic genus Karucia gen. n. The shell morphology and body shape of the new genus resembles Radomaniola Szarowska, 2006 and Grossuana Radoman, 1973, from which it differs in the larger shells with relatively slim and a slightly, but clearly shouldered body whorl. The number of gastropods from Skadar Lake basin tallies now 50 species. The adjusted rate of gastropod endemicity for Skadar Lake basin is estimated to be 38%. By compiling faunal and taxonomic data we also aim to provide information of relevance as to conservation efforts.
Skadar Lake, gastropod endemism, taxonomy, ancient lake
The Skadar Lake system is a well-known hotspot of freshwater biodiversity (
During a recent survey of gastropod fauna of Skadar Lake one new hydrobiid genus was discovered and described in the present paper. Therewith, we aim to provide faunal information on Skadar Lake system gastropod diversity and endemism with relevance to conservation efforts.
Skadar Lake is the largest lake in the Balkan Peninsula with a surface area that seasonally fluctuates between 370 to 600 km2. Skadar Lake itself is located on the western Balkan with approximately two-third (229 km2) of its surface belonging to Montenegro and about one-third (142 km2) to Albania. The lake’s water level also varies seasonally from 4.7 to 9.8 m above sea level. The lake extends in the NW-SE direction, and it is approximately 44 km long. The Bojana River connects the lake with the Adriatic Sea, and the Drim River provides a link with the Ohrid Lake. The largest inflow is from the Morača, which provides about 62% of the lake’s water. A characteristic feature of Lake Skadar’s water balance is the high inflow from a number of temporary and permanent karstic springs, some of which are sublacustrine in cryptodepressians (so called ‘oko’). The Southern and southwestern sides of the lake are rocky, barren and steep, having bays in which the sublacustrine springs, are usually to be found. On the northern side there is an enormous inundated area, the boundaries of which change as water levels fluctuate.
Summarized geographical, physiographical, and hydrological characteristics of Skadar Lake (data from
| Location | 42˚03'–42˚21'N, 19˚03'–19˚30'E |
|---|---|
| Surface area min-max (mean), km2 | 370-530 (472) |
| Altitude (mean), m a.s.l | 5 |
| Length (maximum), km | 44 |
| Width (maximum), km | 14 |
| Depth (maximum), m | 8.3 |
| Depth (mean), m | 5.01 |
| Volume | 1.931.62×106m3 |
| Total drainage area, km2 | 5490 |
| Total length of coastline (including islands) L, km | 207 |
| Approximate length of lake outflow (Bojana River), km | 40 |
| Climate type | Csa (Koeppen) |
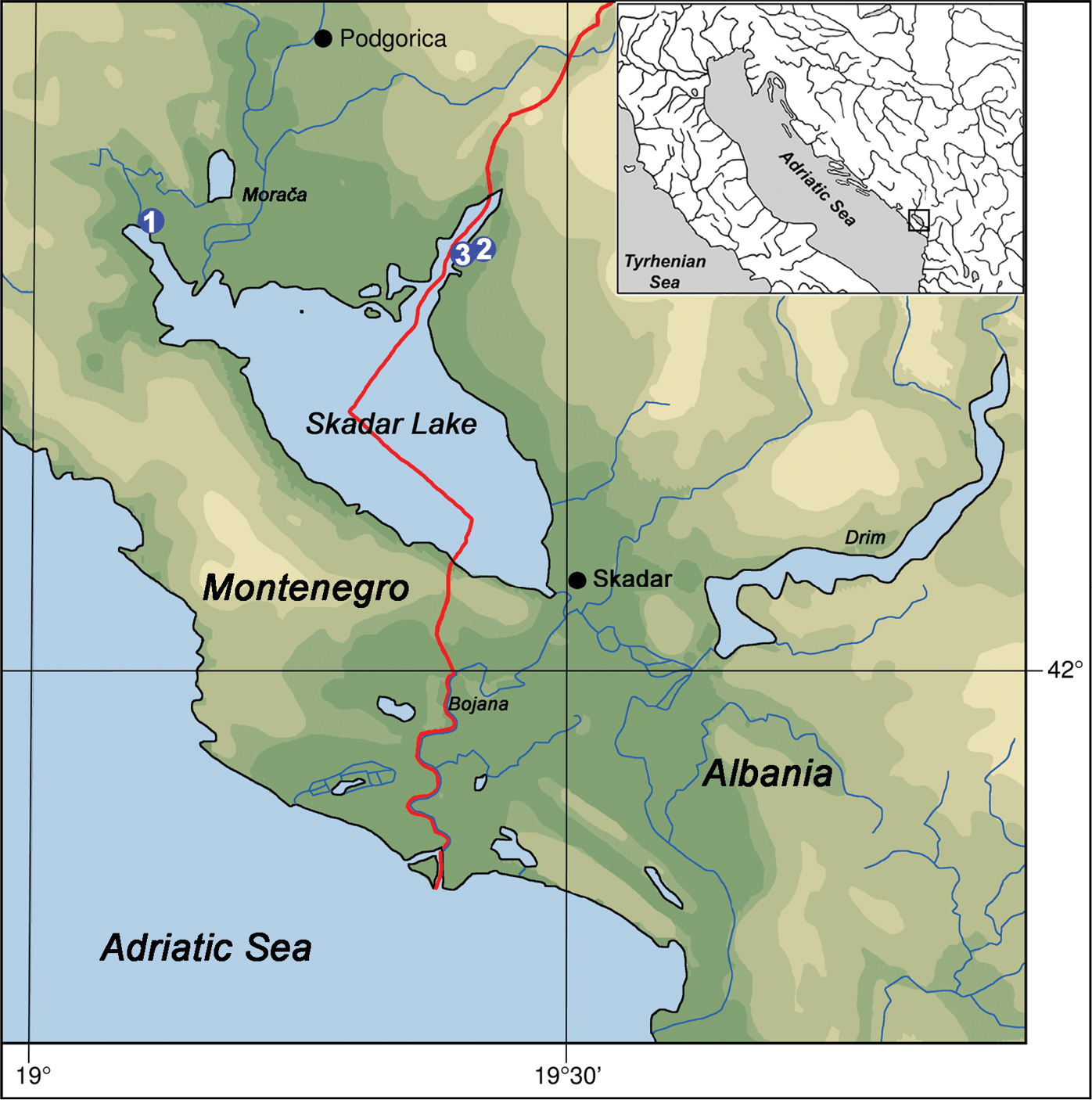
Map of Skadar Lake showing sampling localities of Karucia sublacustrina sp. n.: 1 sublacustrine spring Karuč, Montenegro 2 spring Syri i Sheganit, Albania 3 spring Syri i Hurdan, Albania.
During field work, gastropods were collected by hand netting, sorted on the spot from the living material andfixed with 80% ethanol. Shell morphometric variables (namely shell height and width) were measured using a stereo microscope (Zeiss). Shells and genital organs were photographed with a Leica digital camera system. The type material is stored in the Zoological Museum of Hamburg (ZMH).
Shell large and ovate-conical, with 4.5–5.5 slightly convex whorls. Body whorl relatively slim and prominent, slightly shouldered. The penis is tapered at the distal end and with a bi-lobed outgrowth on the left side.
Karucia sublacustrina sp. n.
The genus is named after the type locality.
The new genus appears to be close to Radomaniola Szarowska, 2006 and Grossuana Radoman, 1973, the hydrobioid genera bearing penis with a bi-lobed outgrowth on the left side and ovate-conical shell with more or less strongly developed last whorl (
urn:lsid:zoobank.org:act:33C8598A-CCB4-46C3-BABA-BDD429513743
http://species-id.net/wiki/Karucia_sublacustrina
Fig. 2a–d, 2kHolotype (ZMH 79651): Shell height 3.6 mm, shell width 2.3 mm; MONTENEGRO, Skadar Lake, sublacustrine spring Karuč, 42°21'30.84"N, 19°06'23.03"E, 15.xi.2012 Pešić. Paratypes: 8 ex. ZMH 79652; 20 ex. in coll. Glöer; same data and locality as holotype.
Montenegro, Skadar Lake, sublacustrine spring Karuč (Fig. 7a).
(data taken from Zoltán Fehér, Budapest; all material in the collection of the Hungarian Natural History Museum). ALBANIA: Malësi e Madhe district, Bajzë, Syri i Sheganit Spring by the Shkodër (Skadar) Lake, 42°16.360"N, 19°23.757'E, 15 m asl., 17.vi.2012 Fehér, Kovács & Murányi; Malësi e Madhe district, Bajzë, Syri i Hurdan spring lakes near Shkodër (Skadar) Lake, 10 m asl., 42°16.299'N, 19°23.941'E, 17.vi.2012 Fehér, Kovács & Murányi. MONTENEGRO: Cetinje municipality, Karuč, Karuč Spring by the Skadar Lake, 42°21.521'N, 19°06.375'E, 10 m asl., 15.vi.2012 Fehér, Karanović, Kovács, Murányi & Pešić.
Named after its occurence in sublacustrine spring.
The ovate-conical shell consists of 4.5-5.5 slightly convex whorls (Figs. 2a–b). The solid shell is yellowish and silky. The umbilicus is closed. The peristome is sharp and thickened at the columella (Fig. 2a). The aperture is ovoid, somewhat angled at the top. The operculum is orange (Fig. 2a). Body whorl relatively slim but prominent, a little shouldered. Shell height 3.2-4.1 mm, width 2.1-2.6 mm, aperture height 1.7 mm, width 1.4 mm.
The snout and the distal part of the tentacles are dark brown (Fig. 1c). Above the eyes the tentacles at their basis are orange. The penis is tapered at the distal end and bears a bi-lobed outgrowth on the left side (Fig. 2d, arrow).
As for the genus.
Montenegro, Skadar Lake area, old stillwater channel near the River Crnojevića and above the village Rijeka Crnojevića, 15 m asl., 42°21.297'N, 19°01.122' E, 10.xi.2012, Pešić.
This species was described by Wohlberedt (1901) from River Crnojevića as subspeces of Bithynia mostarensis. In the short original description, Wolhberedt (1901) stated that it differs from the nominal form by one additional whorl and the more acute spire. Later on,
In some females we found specimens with a pseudopenis, a very small, not completely developed penis. This phenomenon is found also in Bithynia danubialis Glöer & Georgiev, 2012, a species recently described from the Bulgarian part of the Danube (Glöer and Georgiev, 2012). It is worth to note that most of collected specimens were taken under stones and mud when the old stillwater channel was dry (Fig. 4A).
Comparative morphology of shell and penis in Bithynia radomani Glöer & Pešić, 2007 (a–b) Bithynia montenegrina (Wohlberedt, 1901) (c–d) and Bithynia hambergerae A. Reischütz, N. Reischütz & P.L. Reischütz, 2008 (e–f): a, c, e = shell, b, d, f = penis.
http://species-id.net/wiki/Bithynia_hambergerae
Fig. 3e–fMontenegro, Skadar Lake, Plavnica, River Plavnica, 42°17'03.76"N, 42°12'28.94"N, 15.vi.2012 Fehér, Karanović, Kovács, Murányi & Pešić.
Bithynia hambergerae was described by
Montenegro: Skadar Lake, sublacustrine spring Karuč, 42°21'30.84"N, 19°06'23.03"E, 10 m asl. Pešić; Skadar Lake, Božaj, pool near spring Vitoja, 42°19'30"N, 19°21'47"E, 8 m asl. Pešić
This species was a long time considered as subspecies of Lymnaea stagnalis (Linnaeus, 1758). From the latter species, Lymnaea raphidia can be easily distinguished by much slimmer spire (Fig. 4b). The preliminary phylogeographic study (
A. and P. Reischütz (
a Dry old stillwater channel near the River Crnojevića (September, 2012), sampling site of Bithynia montenegrina (Wohlberedt, 1901) b Lymnaea raphidia (Bourguignat, 1860) from Božaj, Montenegro.
Montenegro: Skadar Lake, River Gostiljska Reka, 42°17'09.05"N, 19°14'17.35"E, 25.iv.2008 Pešić; River Piva near Mratinje Dam, 43°16'23"N, 18°50'32"E, 20.viii.2010; Pljevlja town, spring in village Vrulja, 21.x. 2010 Pešić; River Zeta near Podgorica, vi. 1982, Glöer.
In addition to Ancylus fluviatilis we found another Ancylus sp. clearly different from the former species by the shell morphology. Already,
Ancylus recurvus can be easily distinguished from Ancylus fluviatilis by the shape of apex which is shifted forward reaching the border of the shell’s base (Fig. 5b). Furthermore, the apex in Ancylus recurvus is rounded and it is directed more straight, while in Ancylus fluviatilis it is acute and turned to the left side.
Shell: a Ancylus fluviatilis (topotype, Germany) b Ancylus recurvus (Zeta river, Montenegro).
For the Skadar Lake basin, a total of 54 extant gastropod taxa is reported (
At the scale of Skadar Lake, about 31 % of the gastropods (12 out of 39 species sampled in the lake) are endemic. At the scale of the Skadar Lake basin, 38% (19 species) of the total fauna appear to be endemic. Compared with two other famous ancient Balkan lakes (in parentheses % of gastropod endemism, data taken from
The on-site molluscan species diversity in the investigated area ranged from one to 14 species, with the highest diversity in the sublacustrine springs. Compared to the other investigated habitats of Skadar Lake lacustrine systems, Karuč was species rich. We found a subset of 14 gastropod species, including eight out of 19 proposed endemic taxa.
Taking lake surface areas into account,
The faunal relationships of malacofauna among the Balkan’s lakes were analysed by
Within the Skadar Lake basin, endemism occurs at different spatial scales: (a) species endemic to Skadar Lake and its sublacustrine springs, adjacent pools as well the mouths of the surrounding tributaries and its downstream parts, (b) species endemic to surrounding springs, (c) species endemic to underground waters (interstitial waters of the surrounding tributaries, and surrounding caves). An estimation of the degree of endemism in the late category show that many endemics are characteristic for the subterranean habitat (
Skadar Lake endemism occurs also at the genus level. Skadar Lake harbors only endemic and monotypic hydrobiid genus Karucia gen. n. Four other Balkan lakes, i.e., Ohrid, Trichonis, Prespa and Mikri Prespa, currently have one endemic genus each (
Despite the still scarce data on the biota of Skadar Lake (e.g.
Comparative species list and type of endemism of gastropods occurring in Skadar Lake basin. Levels of endemicity: Eskadar – endemic to Skadar Lake basin; Emontenegro – endemic to the southern and central part of Montenegro; Emontenegro+albania - endemic to Adriatic drainage of Montenegro and Albania; Emont.+alb.+gre. – endemic to Adriatic drainage of Montenegro, Albania and mainland Greece. Spatial scales of gastropod diversity: LH – species collected in Skadar Lake and its sublacustrine springs, adjacent pools and mouths of the surrounding tributaries (including its downstream part), SH – species collected in the surrounding spring habitat, GH – species living in the subterranean habitat (spr. – found in spring).
| Scale of endemism | LH | SH | GH | Red List Category (after Cuttelod et al. 2011) | |
|---|---|---|---|---|---|
| Neritomorpha | |||||
| Theodoxus fuviatilis (Linnaeus, 1758) | + | + | Least Concern | ||
| Caenogastropoda | |||||
| Viviparus mamillatus Küster, 1852 | Emont.+alb.+gre. | + | Data Deficient | ||
| Amphimelania holandrii (C. Pfeifer, 1828) | + | Least Concern | |||
| Bithynia zeta Glöer & Pešić, 2007 | Emontenegro+albania | + | Endangered | ||
| Bithynia radomani Glöer & Pešić, 2007 | Emontenegro+albania | + | + | Least Concern | |
| Bithynia skadarskii Glöer & Pešić, 2007 | Eskadar | + | Endangered | ||
| Bithynia montenegrina (Wohlberedt, 1901) | Eskadar | + | Data Deficient | ||
| Bithynia hambergerae Reischütz, N. Reischütz & P.L. Reischütz, 2008 | Eskadar | + | Data Deficient | ||
| Radomaniola curta curta (Küster, 1852) | Emontenegro+albania | + | Least concern | ||
| Radomaniola lacustris (Radoman, 1983) | Eskadar | + | Critically Endangered | ||
| Radomaniola elongata (Radoman, 1973) | Eskadar | + | Critically Endangered | ||
| Radomaniola montana (Radoman, 1973) | Emontenegro | + | Least Concern | ||
| Vinodolia scutarica (Radoman, 1973) | Eskadar | + | Endangered | ||
| Vinodolia gluhodolica (Radoman, 1973) | Eskadar | +(spr.) | Endangered | ||
| Vinodolia matjasici (Bole, 1961) | Eskadar | +(spr.) | Critically Endangered | ||
| Vinodolia zetaevalis (Radoman, 1973) | Eskadar | + | Data Deficient | ||
| Bracenica spiridoni Radoman, 1973 | Eskadar | +(spr.) | Endangered | ||
| Karucia sublacustrina sp. n. | Eskadar | + | |||
| Antibaria notata (Frauenfeld, 1865) | Emontenegro | + | Least Concern | ||
| Litthabitella chilodia (Westerlund 1886) | + | Least Concern | |||
| Plagigeyeria montenigrina Bole, 1961 | Eskadar | + | Critically Endangered | ||
| Plagigeyeriazetaprotogona vitojaReischütz & Reischütz, 2008 | Eskadar | +(spr.) | Endangered | ||
| Pyrgula annulata (Linnaeus, 1767) | + | Least Concern | |||
| Heterobranchia | |||||
| Valvata cristata O.F. Müller, 1774 | + | Least Concern | |||
| Valvata montenegrina Glöer & Pešić, 2008 | Eskadar | + | Endangered | ||
| Valvata piscinalis (O.F. Müller, 1774) | + | Least Concern | |||
| Acroloxus lacustris (Linnaeus, 1758) | + | Least Concern | |||
| Galba truncatula (O.F. Müller, 1774) | + | Least Concern | |||
| Stagnicola montenegrinus Glöer & Pešić, 2009 | + | Near Threatened | |||
| Radix auricularia (Linnaeus, 1758) | + | Least Concern | |||
| Radix labiata (Rossmässler, 1835) | + | Least Concern | |||
| Radix balthica (Linnaeus, 1758) | + | Least Concern | |||
| Radix skutaris Glöer & Pešić, 2007 | Eskadar | + | + | Endangered | |
| Lymnaea raphidia (Bourguignat, 1860) | Emontenegro+albania | + | |||
| Lymnaea stagnalis (Linnaeus, 1758) | + | Least Concern | |||
| Haitia acuta (Draparnaud, 1805) | + | Least Concern | |||
| Bathyomphalus contortus (Linnaeus, 1758) | + | Least Concern | |||
| Planorbarius corneus (Linnaeus, 1758) | + | Least Concern | |||
| Planorbis vitojensis Glöer & Pešić, 2010 | Eskadar | + | |||
| Gyraulus crista (Linnaeus, 1758) | + | Least Concern | |||
| Gyraulus ioanis Glöer & Pešić, 2007 | Eskadar | + | Critically Endangered | ||
| Gyraulus meierbrooki Glöer & Pešić, 2007 | Eskadar | + | Endangered | ||
| Gyraulus shasi Glöer & Pešić, 2007 | Eskadar | + | Critically Endangered | ||
| Gyraulus cf. piscinarum (Bourguignat, 1852) | + | Not Aplicable | |||
| Anisus vortex (Linnaeus, 1758) | + | Least Concern | |||
| Hippeutis complanatus (Linnaeus, 1758) | + | Least Concern | |||
| Segmentina nitida (O.F. Müller, 1774) | + | Least Concern | |||
| Ferrissia fragilis (Tryon, 1863) | + | ||||
| Ancylus fuviatilis (O.F. Müller, 1774) | + | + | Least Concern | ||
| Ancylus recurvus Martens, 1873 | + | + |
Most authors agree that the Skadar Lake basin is of tectonic origin (e.g., Laska et al. 1981,
It should be noted that some other crenobiontic hydrobiid species, inhabitants of neighbouring springs can be also found in lacustrine habitat, at least part of the year as these spring are regularly flooded by the lake water (Fig. 7b). A year study in the spring on the island Vranjina (Fig. 7f), the locus typicus of crenobiontic species Radomaniola elongata(Radoman), shows that connection between eucrenon and the lake and consenquently lake water regime is the main factor influnded changes in the benthic assemblages (
Nonetheless, information on the potential timing of phylogenetic events on the key endemic taxa in Skadar Lake are still lacking, so the exact limnological origin and the origin of faunal or floral elements of Skadar Lake remain uncertain. Despite the limited knowledge about the lake’s evolutionary history
Endemic gastropod species occurring in the Skadar Lake basin – I part. a Vinodolia matjasici (Bole, 1961) b Radomaniola curta curta (Küster, 1852) c Vinodolia scutarica (Radoman, 1973) d Radomaniola montana (Radoman, 1973) e Radomaniola elongata (Radoman, 1973) f Radomaniola lacustris (Radoman, 1983) g Bracenica spiridoni Radoman, 1973 h Valvata montenegrina Glöer & Pešić, 2008 i Bithynia radomani Glöer & Pešić, 2007 j Bithynia hambergerae A. Reischütz, N. Reischütz & P.L. Reischütz, 2008 k Karucia sublacustrina n. gen. n. sp. l Bithynia zeta Glöer & Pešić, 2007 m Bithynia montenegrina (Wohlberedt, 1901) n Bithynia skadarskii Glöer & Pešić, 2007 o Viviparus mamillatus Küster, 1852.
Endemic gastropod species occurring in the Skadar Lake basin – II part. a Gyraulus meierbrooki Glöer & Pešić, 2007 b Gyraulus ioanis Glöer & Pešić, 2007 c Gyraulus shasi Glöer & Pešić, 2007 d Planorbis vitojensis Glöer & Pešić, 2010 e Radix skutaris Glöer & Pešić, 2007 f Lymnaea raphidia (Bourguignat, 1860).
Skadar Lake basin and selected characteristic habitat types. a sublacustrine spring Karuč (the locus typicus of Karucia sublacustrina sp. n.) b spring Vitoja flooded by the lake water (December 2010) c View from NE of the lake (River Crnojevića) d View from Malo Blato with eutrophic conditions and Phragmites belt e Šasko Lake (the water of the lake comes from the Bojana River but the communication with the river and the Skadar Lake regulatly interrupred during summer months) – the locus typicus of Gyraulus shasi and Gyraulus ioanis f spring (captured for the local drinking) on the island Vranjina, the locus typicus of Radomaniola elongata. Photos. V. Pešić.
Ancient lakes are among the most vulnerable and threatened ecosystems (
From a conservation point of view, it is necessary to assess the current status of the endemic species as well to estimate the faunal change during the past decades. However, in most cases this cannot be assessed adequately due to insufficient data so the additional molluscs surveys are necessary even though the species-level taxonomy of many genera are still under discussion (e.g.
Because access to, and sampling in hypogean habitats are difficult, subterranean hydrobiid gastropods have been collected mainly from very few living animals or from empty shells only, and often outside the subterranean networks in springs which flows directly out of the ground (
Effects of human-induced environmental changes are especially evident for sublacustrine springs, with eutrophication and using for water supplying (e.g., sublacustrine spring Karuč) being the most serious threats. Changes are recognizable in the whole ecosystem, for example, by the species loss and invasion by nonnative species. Some endemic species have already gone extinct (e.g., endemic fish Chondrostoma scodrensis Elvira, 1987, see
These circumstances and the reported decline in endemic gastropod diversity, should trigger efforts to save this sensitive lake ecosystem. The IUCN Red List of Threatened Species (Cuttelod et al. 2011) includes 21 endemic species from the Skadar Lake basin. Six of them are assessed as Critically Endangered, 9 as Endangered, 3 as Data Deficient and 3 as Least Concern in the IUCN Red List of endangered species (see: Table 2). Furthermore, the seven species: Vinodolia scutarica, Vinodolia matjasici, Vinodolia zetaevalis, Radomaniola lacustris, Radomaniola elongata, Bracenica spiridoni and Valvata montenegrina are protected in Montenegro by national legislation (Službeni list RCG, br. 76/06,
We would like to thank to Dr Zoltán Fehér (Hungarian Natural History Museum, Budapest) for providing his data of Karucia sublacustrina and part of the material considered in this study. Furthermore, we are grateful to Dr Eike Neubert (Switzerland), Dr Zoltán Fehér and anonymous referee for their careful work and valuable comments. The senior author is indebted to the following colleagues for assistance and company during field work: Sead Hadžiablahović, Bogić Gligorović, Ana Pavićević and Tom Karanović, This study was supported by the research project CBFEcoMTG from the Ministry of Science, Montenegro. Dr David Walker reviewed the English.