Citation: Ribeiro-Costa CS, Vieira MK, Manfio, DKergoat GJ (2014) A remarkable new species group of green seed beetles from genus Amblycerus Thunberg (Coleoptera, Chrysomelidae, Bruchinae), with description of a new Brazilian species. ZooKeys 401: 31–44. doi: 10.3897/zookeys.401.6232
Representatives of the subfamily Bruchinae (Coleoptera: Chrysomelidae) are usually small and inconspicuous, with only a few species drawing the attention. Here we deal with several unusually colored species of Amblycerus Thunberg, 1815, one of the two most diverse bruchine genera in the Western hemisphere. We define the virens group that consists of five species whose bodies are covered with a green vestiture, including one new for science, Amblycerus medialis Ribeiro-Costa, Vieira & Manfio, sp. n. (Type locality: Brazil: Pará, Rondônia). This study also provides redescriptions, diagnoses, comparative notes, illustrations, geographic distribution records and a key to the species in this group.
Seed beetle, new species, taxonomy, key, Western hemisphere
Bruchinae Latreille, commonly known as seed beetles, is one of the 13 subfamilies of Chrysomelidae (
Members of Amblycerus are well defined and easily recognized by their subovate body, shallowly emarginate eyes, hind tibia without prominent lateral carinae, and the presence of two apical spurs on hind tibia (
To advance in the taxonomy and systematics of the virens group we propose a revision of the entire species group, including a redescription of Amblycerus virens and a description of a new Amblycerus species that also harbors this unusual green vestiture. We also provide an identification key, geographic distribution data and a diagnosis for the group based on comparisons of morphological characters used at group level in previous taxonomic and cladistic studies (
The material examined was loaned from museums/collections listed below (acronyms of museums/collections and curators’ names are also provided).
CNCI Canadian National Collection of Insects, Ottawa, Canada, (A. E. Davies);
CMNH Carnegie Museum of Natural History, Pittsburgh, United States, (R. Davidson);
DZUP Coleção de Entomologia Pe. J.S. Moure, Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Paraná, Brazil, (C. S. Ribeiro-Costa);
FSCA Florida State Collection of Arthropods, Gainesville, Florida, United States, (M. C. Thomas);
FIOC Fundação Instituto Oswaldo Cruz, Rio de Janeiro, Rio de Janeiro, Brazil, (J. Costa);
MZSP Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil, (S. Casari);
MPEG Museu Paraense Emílio Goeldi, Belém, Pará, Brazil, (O. T. Silveira);
BMNH The Natural History Museum, London, United Kingdom, (S. Shute);
USNM United States National Museum of Natural History, Washington D.C., United States, (A. Konstantinov and E. Roberts).
Most characters were observed from dry pinned insects. Male genitalia were studied following
The virens species group consists of five species: Amblycerus virens (Jekel, 1855), Amblycerus virescens Ribeiro-Costa, 1998, Amblycerus viridans Ribeiro-Costa, 1998, Amblycerus viridis Ribeiro-Costa, 1998 and Amblycerus medialis Ribeiro-Costa, Vieira & Manfio, sp. n. It can be distinguished from other Amblycerus species groups by combinations of characters that are listed below in the diagnosis.
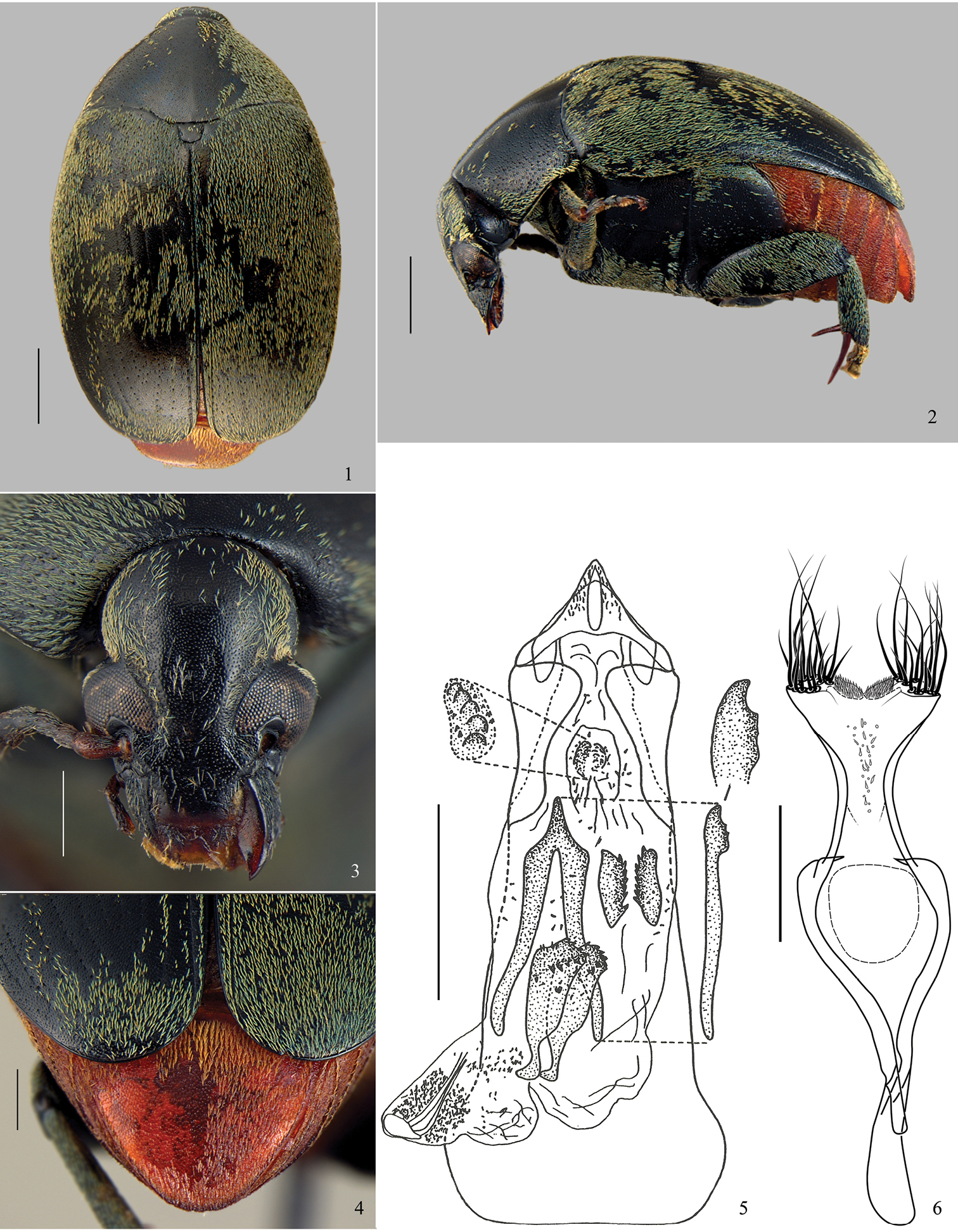
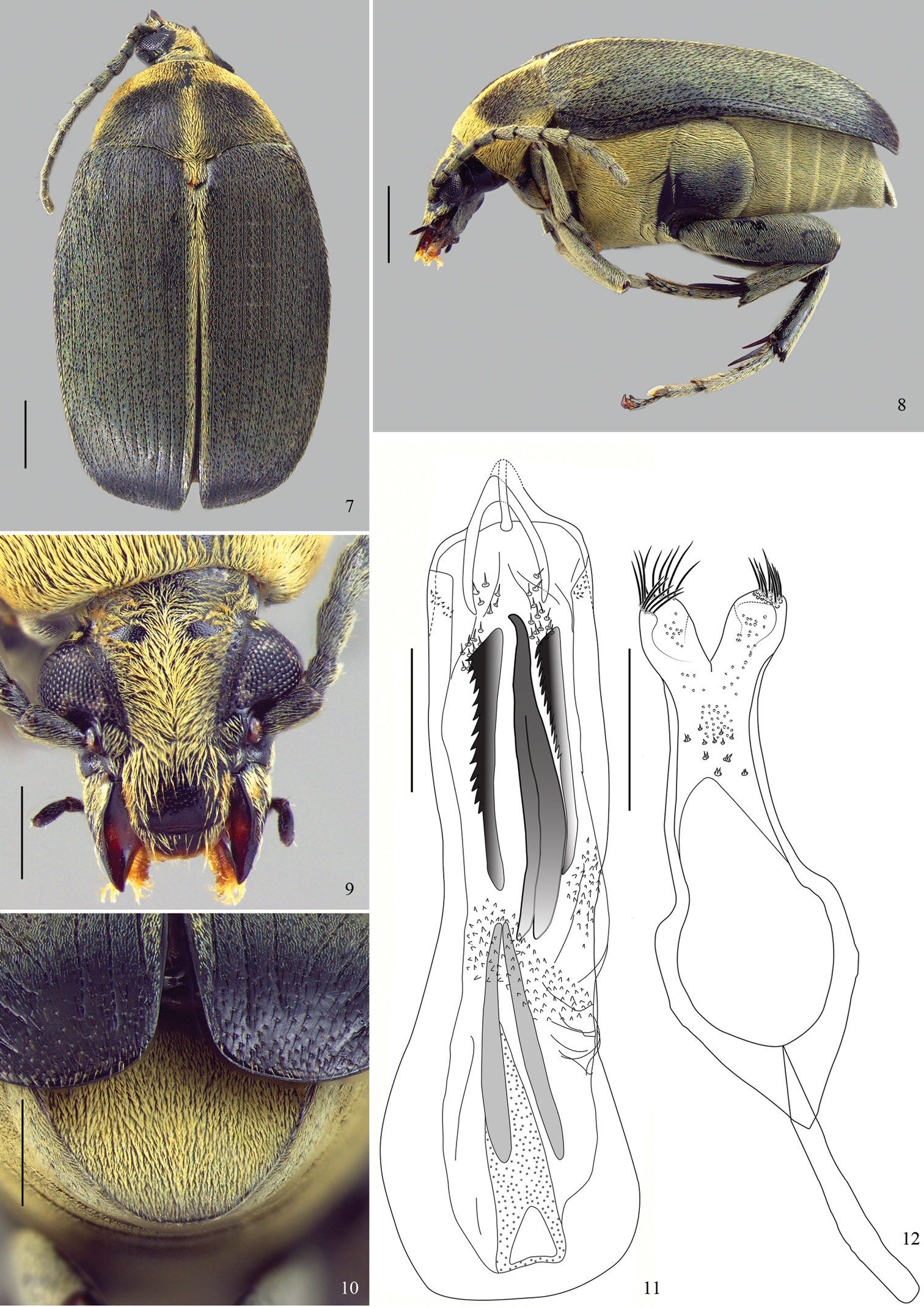
Figs 1–12
Diagnosis. Most of dorsum covered with a green vestiture (Figs 1, 7); pygidium with yellowish setae homogeneously distributed (Figs 4, 10). Head covered with fine and dense punctures, without frontal carina (Figs 3, 9); frontoclypeal suture indistinct (Fig. 3). Disc of pronotum semicircular (Figs 1, 7), with background covered with fine and dense punctures; lateral carina almost reaching the anterior margin of pronotum (Fig. 2); cervical sulcus absent; prosternal process not expanded beyond anterior coxae, slightly constricted between coxae. Metepisternum without transverse, fusiform, curved and striate file; metepisternal sulcus forming right angle. Metaventrite with moderately coarse and sparse punctures; median sulcus one-half as long as metaventrite. Pygidium with apical margin rounded (Figs 4, 10). Internal sac of male genitalia at the median region with a pair of blade sclerites with serrate margin and a wishbone-shaped unpaired sclerite (Figs 5, 11).
Amblycerus virens (Jekel, 1855), specimen male: 1 dorsal 2 lateral 3 head 4 pygidium 5 median lobe of male genitalia 6 tegmen of male genitalia. Scale bars = 1.0 mm (Figs 1–2); scale bars = 0.5 mm (Figs 3–4, 6); scale bar = 0.2 mm (Fig. 5).
Amblycerus medialis Ribeiro-Costa, Vieira & Manfio, sp. n., holotype male: 7 dorsal 8 lateral 9 head 10 pygidium 11 median lobe of male genitalia 12 tegmen of male genitalia. Scale bars = 1.0 mm (Figs 7–8); scale bars = 0.5 mm (Figs 9–12).
Comparative notes. Within the virens group, Amblycerus virens, Amblycerus virescens, Amblycerus viridans and Amblycerus viridis, share more morphological similarities to each other than with Amblycerus medialis Ribeiro-Costa, Vieira & Manfio, sp. n. The most obvious difference between them is the fact that for Amblycerus virens, Amblycerus virescens, Amblycerus viridans and Amblycerus viridis, the pubescence on pronotum and elytra is not variegated and does not present stripes.
In comparison with other Amblycerus species, it is worthy to note that Amblycerus medialis presents two long, serrate blades in the internal sac of male genitalia. Interestingly, these serrate blades (character 24(1), pg. 7,
Sexual dimorphism. Sexual dimorphism was not observed even in the shape of the apex of the last abdominal ventrite.
Geographical distribution. Neotropical region, although the species from this group are more commonly distributed between the North of French Guiana to Midwest Brazil.
Host plants. This species group does not have known host plants records.
| 1 | Eyes prominent laterally ( |
2 |
| 1’ | Eyes flat laterally ( |
3 |
| 2 (1) | Pronotum and elytra with mid strip of vestiture (Fig. 7), antennae serrate from 4 to 10 antennomere (Fig. 8), ocular index: 2, 04–2, 22 | Amblycerus medialis Ribeiro-Costa, Vieira & Manfio, sp. n. |
| 2’ | Pronotum with uniform pattern of vestiture, lacking stripes (Fig. 1), antennae moderately serrate from 5 to 10 antennomere ( |
4 |
| 3 (1’) | Median lobe of male genitalia with wishbone sclerite as long as the blade sclerites ( |
Amblycerus virescens Ribeiro-Costa, 1998 |
| 3’ | Median lobe of male genitalia with wishbone sclerite more than half of the length of the blade sclerites ( |
Amblycerus viridans Ribeiro-Costa, 1998 |
| 4 (2’) | Median lobe of male genitalia with blade sclerites longer than wishbone sclerite, denticulate from the apex to half its length ( |
Amblycerus viridis Ribeiro-Costa, 1998 |
| 4’ | Median lobe of male genitalia with blade sclerites about one half of wishbone sclerite length, with denticles restricted to subapical region (Fig. 5) | Amblycerus virens (Jekel, 1855) |
http://species-id.net/wiki/Amblycerus_virens
Figs 1–6BL: 5.6 mm; BW: 3.84 mm
Integument (Figs 1–4). Body mostly black, mouth parts and antennomeres 1 and 2 brown to yellowish, apical spurs of hind tibiae reddish brown, pygidium and abdomen rufous with golden shine.
Vestiture (Figs 1–4). Pronotum, elytra and thorax covered with greenish setae, abdomen and pygidium with yellowish setae, both not variegated.
Head (Fig. 3). Covered with fine and dense punctures. Frons without frontal carina. Eye finely faceted, moderately prominent laterally. Ocular index: 3.11; ocular sinus: 0.63; postocular lobe 0.33 the eye length. Antenna not reaching anterior margin of hind coxa; moderately serrate from antennomeres 5-10 (
Prothorax. Pronotum semicircular; covered with fine and dense punctures, moderately coarse punctures intermixed on lateral areas (Fig. 1). Lateral carina almost reaching the anterior margin of pronotum (Fig. 2). Cervical sulcus absent. Prosternal process not expanded beyond anterior coxae; flat and slightly constricted between coxae.
Mesothorax and metathorax. Scutellum as long as wide; round or unidentate at apex (Fig. 1). Elytron with striae 1 and 10 moderately impressed; 2, 3, 8 and 9 weakly impressed until the third apical region of elytron then only isolated punctures representing striae; 4–7 striae formed only by isolated punctures; 4 and 5 anastomosed before the fusion of 6+7. Interstriae with moderately coarse and sparse punctures. Metaventrite moderately protuberant (Fig. 2) with moderately coarse and sparse punctures; median sulcus one-half as long as metaventrite. Metepisternum with moderately coarse and sparse punctures; metepisternal sulcus forming straight angle, with transverse axis straight and not reaching lateral margin of metepisternum. Mid coxae lower than anterior coxae, in lateral view (Fig. 2). Hind femur about 2.5 times longer than wide. Hind coxae with moderately coarse and sparse punctures. Hind tibia lateral spur about 1.5 times the length of median spur; 1-tarsomere about 1.5 the length of the lateral spur and 2.5 times the median spur.
Abdomen (Fig. 4). Ventrites with moderately coarse and dense punctures; last ventrite as long as 4-ventrite. Pygidium one-third covered by the elytra; apical margin rounded, with moderately coarse and dense punctures.
Male genitalia. Median lobe (Fig. 5) about 4.15 times its widest at apical region; ventral valve with lateral margins straight; dorsal valve with lateral margins concave and acuminate apex. Internal sac (Fig. 5) in the apical region without anterior sclerites; a pair of tuberculate median sclerites; a pair of ovoid and denticulate posterior sclerites. Median region with a pair of sinuous blade sclerites, sinuous at base and denticulate at apex; a long wishbone sclerite, about two times longer than the blade sclerites, curved and denticulate at apex and with stems moderately separated. Basal region of sac without sclerites; apical and median regions with several spines. Tegmen (Fig. 6) slightly emarginated between lateral lobes expanded.
Syntype studied by the first author and deposited in BMNH, sex undetermined, labels: ‘Type H. T.’ [round, white with red margin]; ‘Cayenna’ [white]; ‘ex. Mus. W. W. S.’ [white]; ‘Type’ [white]; ‘53272’ [white]; ‘Fry Coll. 1905. 100’ [white]; ‘Spermophagus virens Dj. n. sp. Cayen’; ‘SYNTYPE/Spermophagus/virens Jekel, 1855/Ribeiro-Costa, C. S.’
BRAZIL: Amazonas: 1 male specimen, São Gabriel, Rio Negro, 9.X.1927, J.F. Zikán, (FIOC). Pará: 1 male specimen, Santarém, VII.1919, S. M. Klages(CARN). FRENCH GUIANA: Bélvédère de Saül: 1 male specimen, Mont Itoupé, 30.III.2010, P. H. D. leg. (DZUP).
Brazil (Amazonas and Pará), Fench Guiana.
Amblycerus virens differs from the other species of the group by the length of the lateral spur of hind tibia (2.4 times the length of median; Fig. 2) (for other species the length of the lateral spur of hind tibia is less than 1.85 times the length of median); the internal sac of male genitalia has small blade sclerites (Fig. 5) (other species in the group have long blade sclerites).
This species shares with Amblycerus viridis and Amblycerus medialis the prominent eyes (Figs 3, 9), postocular lobe narrower and metaventrite protuberant in lateral view (Figs 2) but the male genitalia do not show closer similarities among these species. Amblycerus virens and Amblycerus virescens share a long wishbone sclerite comparing to the blades in the internal sac of male genitalia (Fig. 5;
http://species-id.net/wiki/Amblycerus_virescens
Brazil (Amazonas, Amapá and Goiás).
This species can be distinguished from all other of virens group by the internal sac of male genitalia that has a pair of subtriangular sclerites with denticulate margins (
Amblycerus virescens and Amblycerus viridans have no salient eyes (
http://species-id.net/wiki/Amblycerus_viridans
Brazil (Mato Grosso).
Amblycerus viridans differs from all other species in the group by the structure of the internal sac of male genitalia, which includes a pair of sclerites formed by dense denticles (
http://species-id.net/wiki/Amblycerus_viridis
Brazil (Mato Grosso).
Amblycerus viridis differs from the other species in the group by its shorter hind femur, which is 2.32 times longer than its width (in others species the ratio is superior to 2.5 times). Additional information on external and internal similarities is also presented in the sections dedicated to Amblycerus virens and Amblycerus viridans.
http://zoobank.org/D819155A-D242-4BE0-813B-B5B258DD4554
http://species-id.net/wiki/Amblycerus_medialis
Figs 7–12BL: 6.3 mm; BW: 3.78 mm
Integument color (Figs 7–10). Body black except mouth parts brownish; apical spurs of hind tibiae brownish to black.
Vestiture (Figs 7–10). Pronotum with a predominantly green vestiture but also with yellowish setae on the anterior margin, lateral areas and median line; elytra with a predominantly green vestiture but also with yellowish setae on 1 interstria; thorax and abdomen covered with pale yellowish setae.
Head (Fig. 9) covered with fine and dense punctures. Frons without frontal carina. Eyes moderately faceted, strongly prominent laterally. Ocular index: 2.23; ocular sinus: 0.78; postocular lobe 0.34 the eye length. Antennae not reaching anterior margin of hind coxa (Fig. 8); serrated from 4 to 10 antennomeres; from 3 to 11 antennomeres 1.94 longer than wider; 11 antennomere with truncate apex (Fig. 8). Frontoclypeal suture indistinct. Clypeus covered with fine and dense punctures except on a narrow band on apical portion. Labrum with few fine punctures on basal margin.
Prothorax (Fig. 7). Pronotum semicircular; covered with fine and dense punctures, moderately coarse punctures intermixed all over pronotum. Lateral carina complete, almost reaching the anterior margin of pronotum. Cervical sulcus absent. Prosternal process longer than anterior coxae, gently arched between coxae and slightly constricted between coxae.
Mesothorax and Metathorax. Scutellum longer than wide with tridentate apex. Elytron with striae moderately impressed, not fused apically. Interstriae with moderately coarse and dense punctures (Fig. 7). Metaventrite slightly protuberant with moderately coarse and sparse punctures; median sulcus one-half as long as metaventrite. Metepisternum without punctures; metepisternal sulcus forming straight angle, with transverse axis straight and reaching lateral margin of metepisternum. Mid coxae lower than anterior coxae, in lateral view (Fig. 8). Surface of hind coxae without punctures. Hind femur 3 times longer than wide. Hind tibia lateral spur about 1.5 times the length of median spur, and 1-tarsomere about 1.5 the length of lateral spur and 2.5 times the median spur.
Abdomen (Fig. 10). Ventrites finely punctulate, the last about 2 times wider than the 4-ventrite; pygidium one-half covered by the elytra, with apical margin rounded, with fine punctures.
Male genitalia (Figs 11–12). Median lobe (Fig. 11) about 5.43 times its widest at apical region; ventral valve with lateral margins concave, dorsal valve with lateral margins straight. Internal sac (Fig. 11) in the apical region without sclerites. Median region with a pair of straight blade sclerites, one side denticulate; wishbone sclerite as long as blade sclerites, curved at apex and stems moderately separated. Basal region with a sclerite with long stems gradually approximated. Apical and median regions with several spines and denticles. Tegmen (Fig. 12) deeply emarginated, about 0.35 times the length of the expanded lateral lobes.
Holotype deposited in FSCA, male, with labels: ‘BRAZIL: Rondônia 62/ km SW Ariquemes, nr/ Fzda. Rancho Grande/ 8-20-XI-1994 JE Eger/ MV & Black Lights’[white, printed in black]; ‘FSCA’ [green, printed in black]; ‘HOLOTYPE/ Amblycerus medialis/ Ribeiro-Costa, Vieira & Manfio, / [white with red margin, printed in black] (FSCA). 1 paratype deposited in CNCI, female, with labels: ‘BRAZIL, Pará ♀/ Faz. Taperinha/ XI-19-22-1969/ JM & BA Campbell’ [white, printed in black]; ‘CNC’ [white with green line in the middle, printed in black]; ‘PARATYPE/ Amblycerus medialis/ Ribeiro-Costa, Vieira & Manfio/ [white with yellow margin, printed in black] (CNCI).
Brazil (Pará and Rondônia).
Amblycerus medialis can be easily separated from others species in the group by the presence of yellow pubescent stripes on the pronotum and elytra (Fig. 7) (others species are exclusively with a green vestiture); antennomeres about 2 times as long as wide (Figs 7–8) (others wider than long).
Additional information on external and internal similarities is also presented in the sections dedicated to Amblycerus virens and Amblycerus viridans.
The specific name refers to the median line on dorsum.
C. S. Ribeiro-Costa is very grateful to Dr John M. Kingsolver† who was the first to guide her on the studies of bruchines, this fascinating group of insects and, always gave support on her researches. We also thanks the curators/institutions for loaning specimens, Stéphane Brule for sending one species from virens group; the TAXon line – Rede Paranaense de Coleções Biológicas, Departamento de Zoologia (DZUP), Universidade Federal do Paraná (UFPR) for the photos; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing scholarships to the authors. And, finally, we thank the anonymous reviewers who provided many valuable suggestions for improving the final version. Contribution number 1897 DZUP, UFPR, Brazil.