Citation: Janes MP, McFadden CS, Chanmethakul T (2014) A new species of Ovabunda (Octocorallia, Xeniidae) from the Andaman Sea, Thailand with notes on the biogeography of this genus. ZooKeys 431: 1–17. doi: 10.3897/zookeys.431.7751
A survey of xeniid octocorals was carried out in the waters off Southwestern Thailand in September, 2007. Microscopic investigation of the colonies revealed that three specimens belonged to the genus Ovabunda. Gross morphological examination is presented here accompanied by scanning electron micrographs of the sclerites. Molecular phylogenetic analysis showed identical genotypes at mtMutS, COI, and 28S rDNA for all three specimens and supports their generic assignment. Colony size and shape, sclerite size, and pinnule arrangement differ from nominal species of Ovabunda and thus a new species, O. andamanensis is introduced here. This work also presents a new eastern geographical record for the genus Ovabunda.
Cnidaria, Coelenterata, phylogeny, sclerites, SEM, soft coral, taxonomy
Ovabunda Alderslade, 2001 is a genus of tropical, shallow water zooxanthellate soft corals belonging to the family Xeniidae. They are an abundant component of benthic communities throughout the Red Sea (
Here we describe a new species of Ovabunda from recent collections in the Andaman Sea. Additionally, we report the first record of this genus outside of the eastern Indian Ocean and Red Sea.
All specimens were collected using SCUBA to a maximum depth of 10 meters. Photographs of living colonies were taken for each specimen. Specimens were fixed in 90% ethyl alcohol immediately after collection. The corals examined in this survey are deposited in the reference collection of the Phuket Marine Biological Center, Phuket, Thailand (PMBC).
Morphological examination of the preserved colonies was performed under a dissecting microscope at 20× power. Polyps were photographed; number of pinnule rows, pinnules along the outermost row and inner row when present for each specimen were recorded. Sclerites were prepared for light microscope examination (
Extraction of DNA from ethanol-preserved tissue samples, PCR amplification, and sequencing of two mitochondrial (mtMutS, COI + igr1) and a nuclear (28S rDNA) gene followed the protocols published in
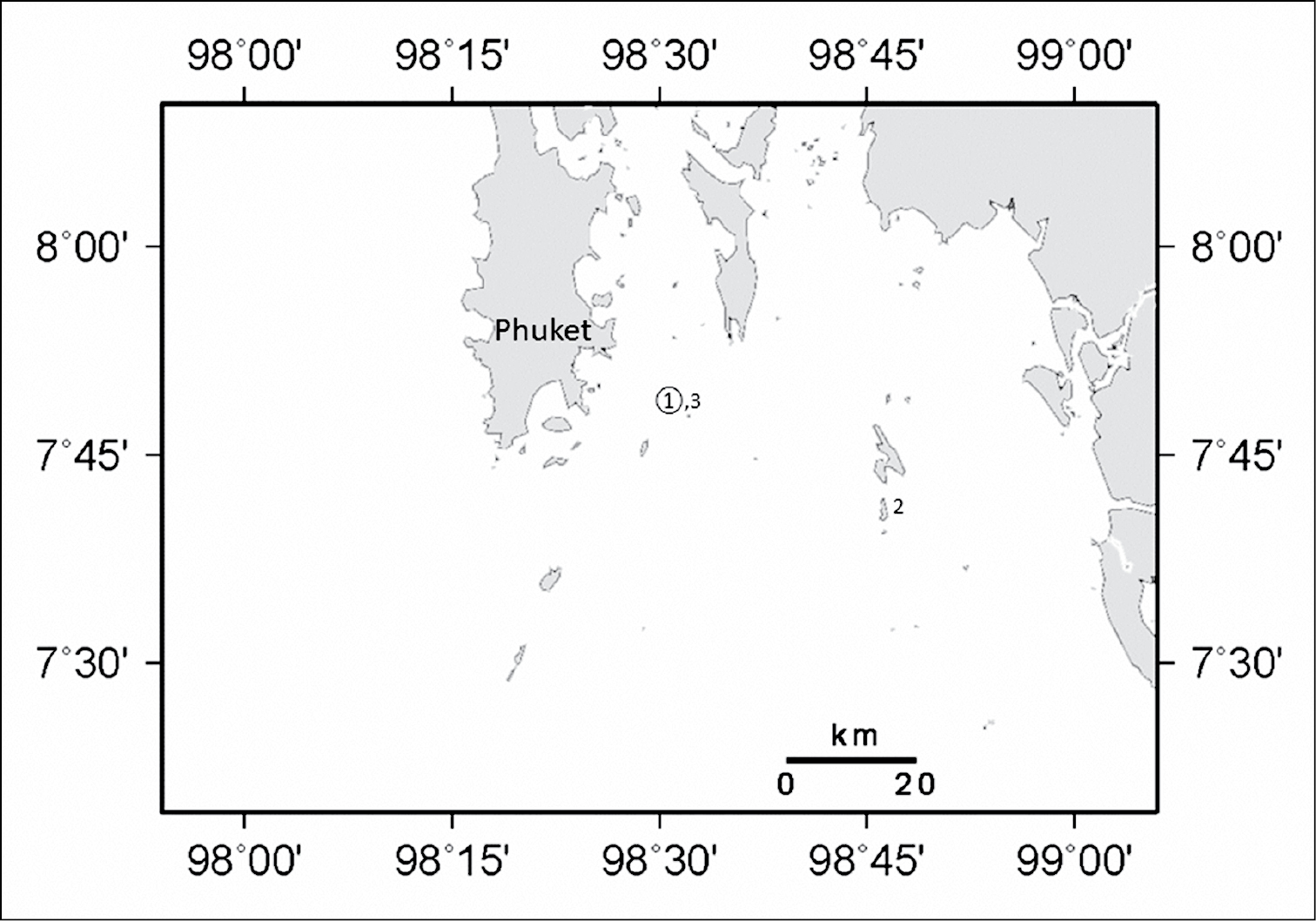
Holotype: PMBC 11860, Koh Doc Mai, Thailand, 07°47.76'N, 98°32.09'E, depth 8 meters, 26 September 2007 (1 colony), M. P. Janes collector. Paratypes: PMBC 11861, Koh Phi Phi, Hin Bida, Thailand, 07°39.20'N, 98°45.83'E, depth 10 meters, 28 September 2007 (1 colony), M. P. Janes collector. PMBC 11862, same data as the holotype.
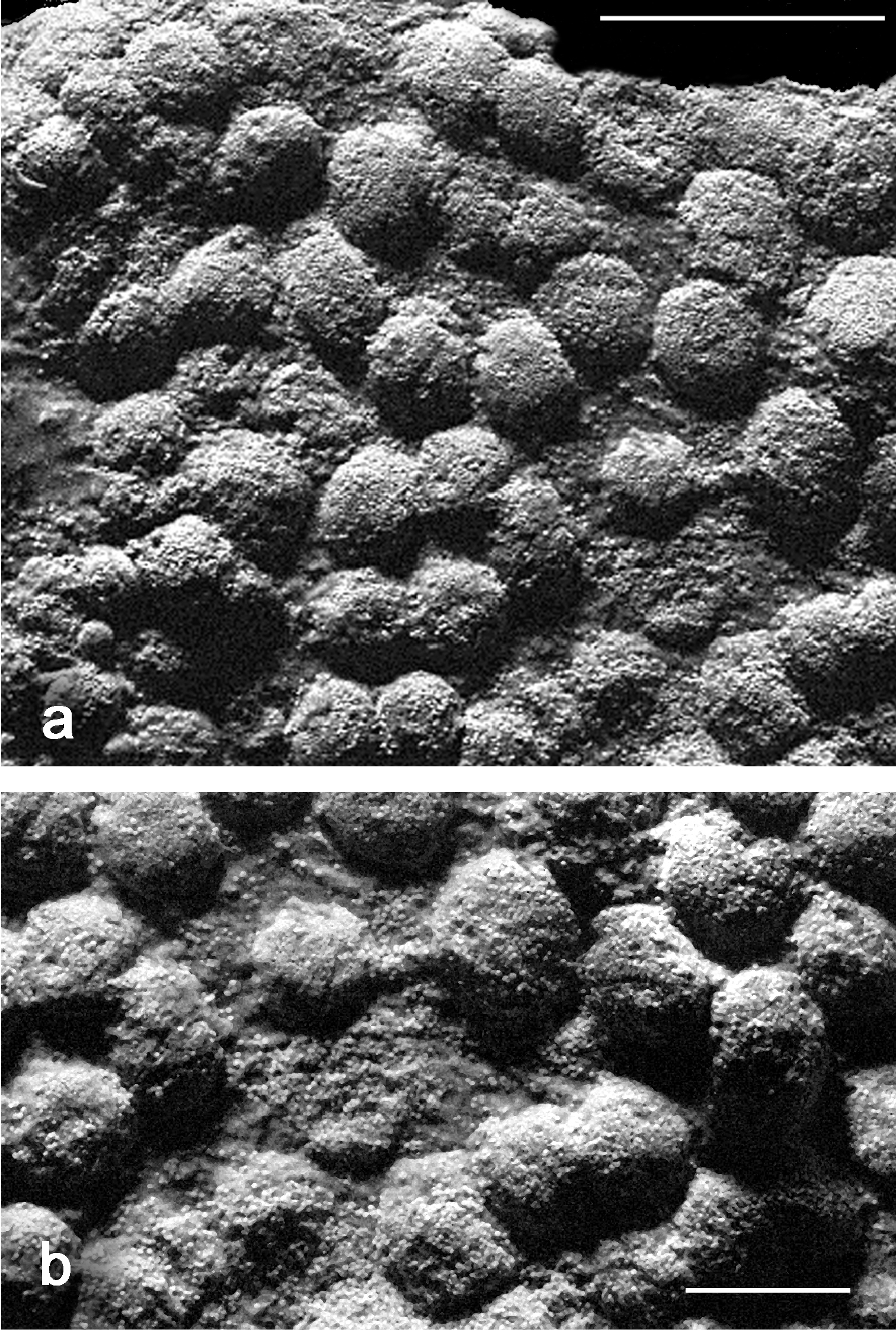
The holotype is comprised of multiple short, branched stalks sharing a common base attached to coral rock. In life, the holotype (Fig. 1a) is thickly beset with monomorphic polyps which were observed to be non-pulsatile. The tentacles are cylindrical, slender and up to 2.0 cm long (Fig. 1b). In a preserved state, the holotype consists of multiple stalks; two of the stalks are divided into two short branches (Fig. 2a). The stalks measure up to 8.0 mm tall and 5.0 mm wide at the base. A slightly convex capitulum is present at the distal end of the stalks from which moderately dense aggregations of polyps are growing. The polyp bodies are cylindrical, shrunken and measuring from their attachment at the capitulum to the base of the tentacle they are 1.5 mm long by 0.5 mm wide. Tentacles are slender, measuring up to 8.0 mm long by 0.2 mm wide with a blunt tip. There is one row of pinnules present on either side of the tentacle (Fig. 2b at arrow) with 17 to 19 pinnules in a row. The pinnules are barrel-like with a rounded tip and slight taper at the end. They measure 0.2 to 0.3 mm long by 0.1 mm wide. There is an open space on the tentacles between adjacent pinnules measuring 0.1 to 0.15 mm wide, nearly equal to their width. Zooxanthellate.
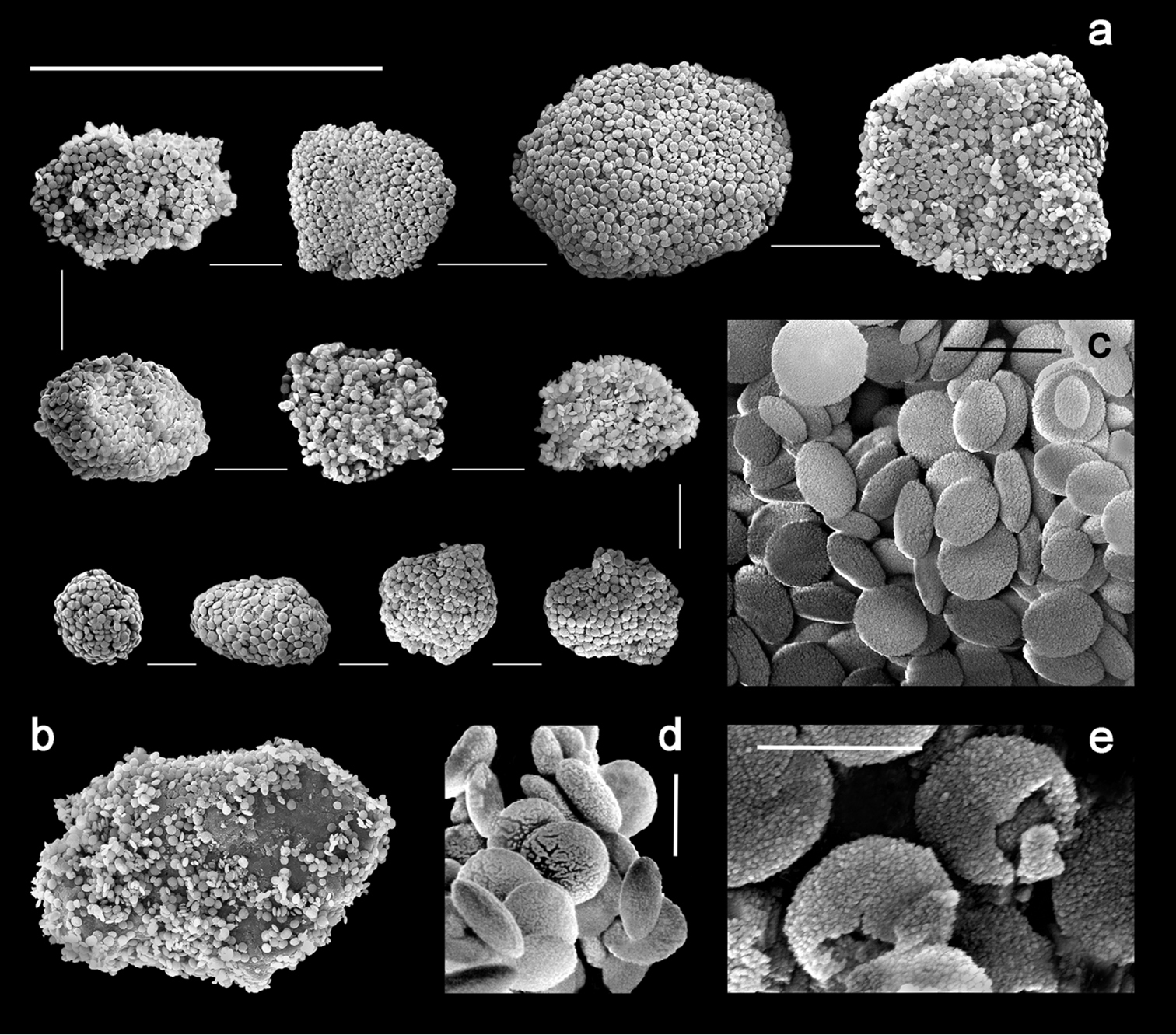
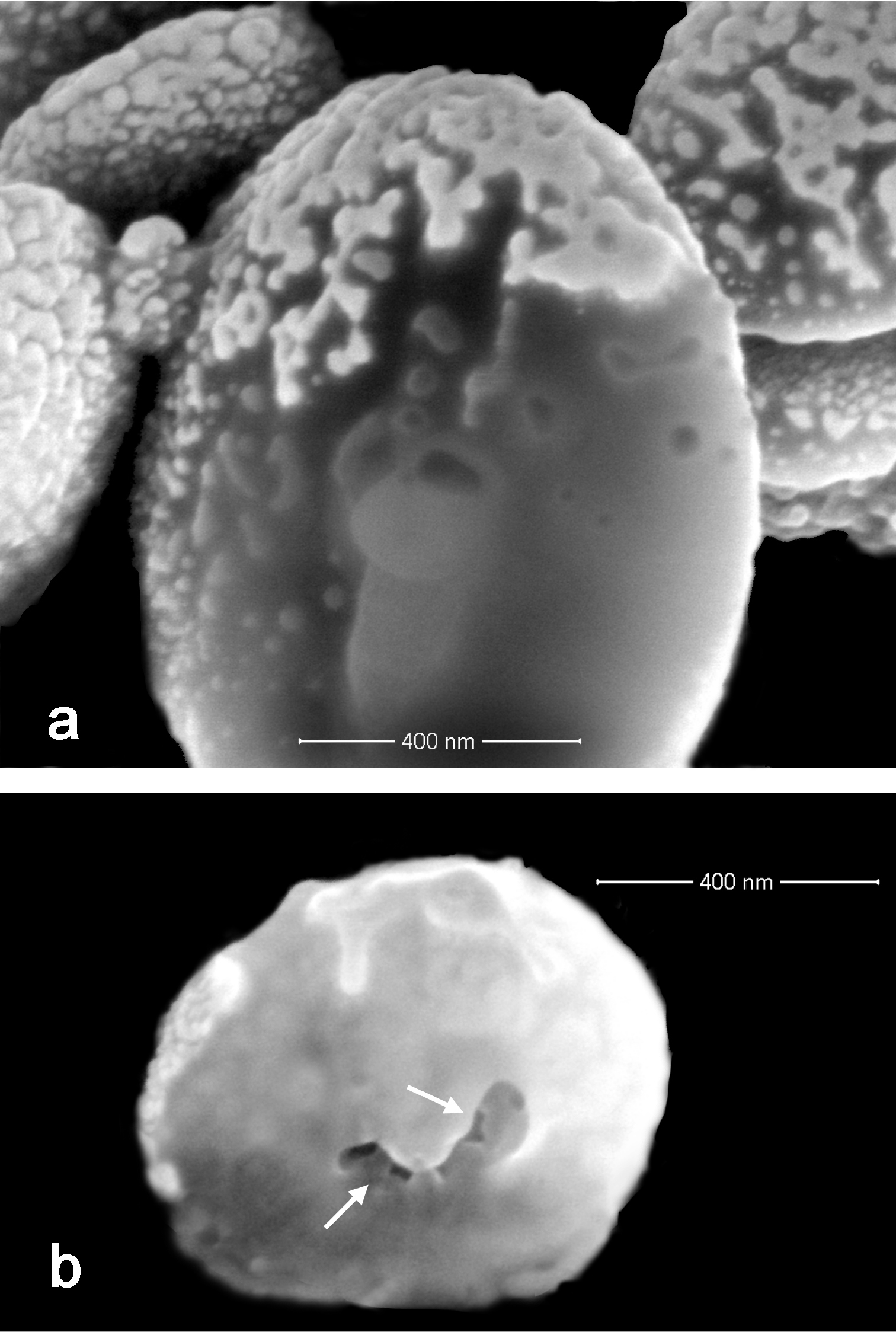
Sclerites are present in all parts of the holotype. They are moderately dense in the polyps (Fig. 3a–b) and fewer are found in the basal portion of the stalk. All of the sclerites are round to slightly oval or irregularly shaped spheroids (Fig. 4a). They measure from 0.010 to 0.018 mm in diameter on average with a few sclerites as large as 0.020 mm in maximal diameter and the smallest recorded at 0.005 mm in diameter. The sclerites are comprised of numerous, minute circular to egg-shaped corpuscular microscleres that quickly disassociate when extracted from the coral tissue with sodium hypochlorite. Rarely, a second form of sclerite can be found in the polyp tissue, which contains a solid calcite core coated with microscleres (Fig. 4b). The microscleres measure from 0.00035 to 0.00060 mm in diameter. SEM imaging revealed that they have a fine, granular surface ultrastructure (Fig. 4c) when viewed under moderately high magnification (×40, 000). The ultrastructure of the surface is comprised of a series of ridges, furrows, and coarse nodules (Fig. 4d) when examined at 65, 000 power. Most microscleres are intact but occasionally fractured ones are found (Fig. 4e). Broken microscleres reveal the presence of a cavity or cavities within. Further evidence of these can be seen in FIB imaging where the microsclere has been sliced longitudinally, demonstrating the depth of the furrows (Fig. 5a) and size of cavities (Fig. 5b) when magnified at 200, 000 power.
The preserved specimens are cream colored with whitish tentacles and light tan pinnules. Living colonies exhibit tan colored stalks; white polyp bodies and tentacles, and pinkish pinnules (Fig. 1a–d).
The name is derived from the collection location, the Andaman Sea.
This species was collected from Koh Doc Mai (Fig. 6) and Koh Phi Phi, Hin Bida located along the eastern coast of Phuket Island, Thailand. Colonies occur in low abundance, spaced 1-2 meters apart on small ledges of vertical walls growing among sea fans, corallimorpharians, and Dendronephthya sp. soft corals. Ovabunda andamanensis sp. n. has also been observed in situ in the Mergui Archipelago, Myanmar (T. Chanmethakul, personal observation).
Both the holotype (PMBC 11860) and one paratype (PMBC 11861) were very similar in size (up to 7.0 cm in life), sclerite dimensions, exhibited non-pulsatile polyps, pinnule rows and number of pinnules per row. The other paratype (PMBC 11862) was smaller in life (Figs 1c, 2c), measuring up to 5.0 cm tall and exhibited tentacles that curved inward (Fig. 1d). It differed from the holotype in having shorter tentacles and two rows of 13–14 pinnules in each row (Fig. 2d) instead of one row with 17–19 pinnules.
In comparing the morphology of Ovabunda andamanensis sp. n. to nominal Ovabunda species colony size, sclerite size and shape, and pinnule arrangement were examined, with the later considered a more variable diagnostic character. Among species of Ovabunda described as having one or two rows of pinnules, O. arabica, O. biseriata, O. gohari, O. faraunensis and Ovabunda verseveldti most closely resemble Ovabunda andamanensis sp. n. Ovabunda gohari and Ovabunda verseveldti are similar to the holotype in having primarily one row of pinnules although occasionally two rows are present in polyps of Ovabunda gohari. The overall range of sclerite sizes is larger in Ovabunda gohari, 0.033–0.063 mm, compared to the 0.010–0.018 mm in Ovabunda andamanensis sp. n., there are 18–22 pinnules in a row compared to 17–19 pinnules in Ovabunda andamanensis sp. n., and Ovabunda gohari has pinnules spaced at 2–3 times the pinnule width along the tentacles whereas in Ovabunda andamanensis sp. n. pinnules are more closely set. According to
The pinnule number in Ovabunda faraunensis agrees well with the 17–19 pinnules observed in the holotype of Ovabunda andamanensis sp. n., however Ovabunda faraunensis has larger sclerites (
Among species of Xenia, Xenia puerto-galerae Roxas, 1933 most closely resembles Ovabunda andamanensis sp. n. The holotype is described by Roxas (1933) as branched, measuring 20.0 mm tall and 8.0 mm in diameter with polyps comprised of thick tentacles that are proportionately small and “two rows of slender pointed pinnules, fifteen to seventeen in a row”. The tentacles are 8.0 mm long by 1.0 mm wide at the base and pinnules measure 0.7 to 0.8 mm long by 0.2 to 0.3 mm wide. However, in the colony with two rows (PMBC 11862) in Ovabunda andamanensis sp. n., the stalks are smaller, 8.0 mm by 5.0 mm, the tentacles are narrower, 8.0 mm by 0.2 mm, the pinnules are smaller, 0.2 to 0.3 mm by 0.1 mm, and there are fewer pinnules (13 to 14). Most notable are the sclerites, which are described as “thin, oval discs 0.018 mm long and 0.018 to 0.0124 mm wide” in Xenia puerto-galerae compared to the 0.010 to 0.018 mm in diameter sphere shaped sclerites observed in Ovabunda andamanensis sp. n. Unfortunately, the location of the holotype of Xenia puerto-galerae remains unknown so a direct SEM comparison of the sclerites could not be performed.
In situ images of Ovabunda andamanensis sp. n. type material Holotype (PMBC 11860): a colony (smallest) b close up of polyps. Paratype (PMBC 11862): c colony (smallest) d close up of polyps.
Preserved specimens of Ovabunda andamanensis sp. n. Holotype (PMBC 11860): a colony b polyp. Paratype (PMBC 11862): c colony d polyp. Scale bars: a and c 10 mm; b and d 4 mm.
Ovabunda andamanensis sp. n. Holotype (PMBC 11860) sclerites: a–b uncoated ESEM wet mount images of tentacle sclerites in situ. Scale bars: a 0.04 mm; b 0.015 mm.
Ovabunda andamanensis sp. n. Holotype (PMBC 11860) SEM image of coated sclerites: a whole sclerite most common form b whole sclerite least common form c microscleres from a disintegrated sclerite d surface ultrastructure of loose microscleres e fractured microscleres. Scale bars: a–b 0.020 mm; c 0.001 mm; d–e 0.0005mm.
Ovabunda andamanensis sp. n. Holotype (PMBC 11860) sclerites: a FIB image of the surface ultrastructure of microscleres b FIB image showing interior cavities of a microsclere.
Distribution of Ovabunda andamanensis sp. n. in the Andaman Sea: 1 PMBC 11860 2 PMBC 11861 3 PMBC 11862. Circled number denotes holotype.
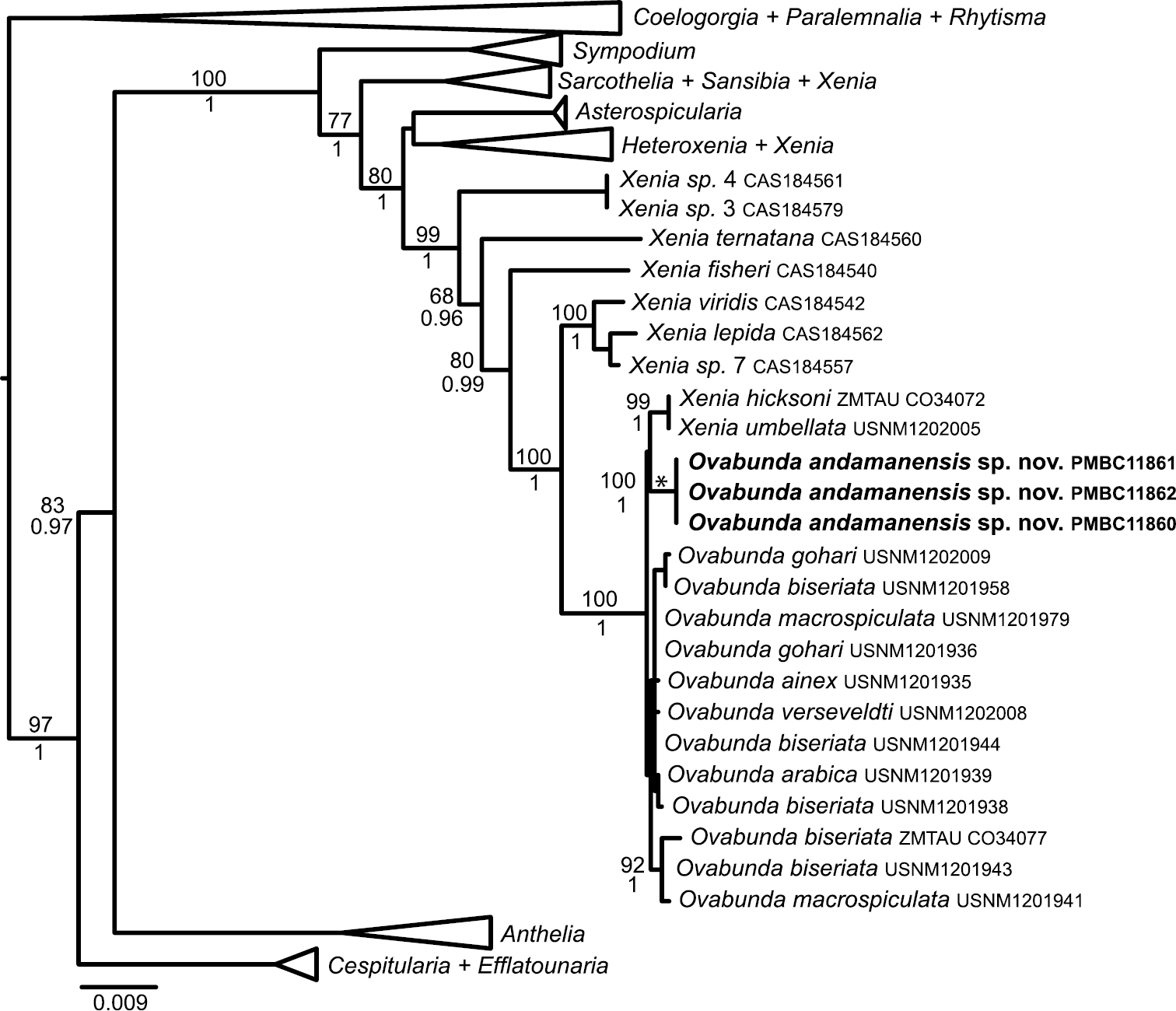
All three Ovabunda specimens in this collection had identical genotypes at mtMutS, COI + igr1 and 28S rDNA. Phylogenetic analyses placed them in a well-supported clade with all other species of Ovabunda as well as several species of Xenia from the Red Sea (Fig. 7). Within that clade, only four MOTUs were distinguished by an average genetic distance of 0.3% or greater. The three Thai specimens belonged to a MOTU that was separated from all other species by 0.5%. The two species of Xenia (Xenia umbellata, Xenia hicksoni) found within the clade belonged to a MOTU that was separated from Red Sea Ovabunda species by 0.4%. All Red Sea Ovabunda species belonged to just two MOTUs that were separated by 0.3%: the group of [USNM1201941, USNM1201943 and ZMTAUCO34077] and all others. Based on its sclerite size, colony size and form, pinnule arrangement, and unique phylogenetic position, we hereby designate a new species Ovabunda andamanensis sp. n. for our material.
Maximum likelihood reconstruction of family Xeniidae based on a partitioned analysis of mtMutS, COI and 28S rDNA sequences (2255 bp). Numbers above nodes are bootstrap percentages (100 replicates) from ML analyses; numbers below nodes are Bayesian posterior probabilities. Some clades have been collapsed to triangles to facilitate readability.
Octocorals are thought to exhibit their greatest species richness in the Indo-Malayan region consisting of Indonesia, the Philippines and New Guinea (
This new record from Thailand increases the know distribution of Ovabunda in the Indian Ocean approximately 5000 km eastward. Both the holotype and one paratype of Ovabunda andamanensis sp. n. were collected from Koh Doc Mai (Fig. 6) located along the eastern coast of Phuket Island. An additional paratype was collected from Koh Phi Phi, Hin Bida located at the southern end of the Gulf of Thailand. Xeniids have been recorded previously from the Andaman Sea (
The gross morphology, sclerite dimensions, and molecular results all support the description of Ovabunda andamanensis sp. n. as a new species. The range of intraspecific variability among xeniid species is poorly known. Living colonies of Ovabunda andamanensis sp. n. were observed to be smaller and less numerous than Ovabunda colonies found on the coral reefs in the northern Gulf of Aqaba (M. Janes, personal observation) and the largest sclerites found in Ovabunda andamanensis sp. n. had a maximal diameter of 0.020 mm, notably smaller than the 0.035–0.040 mm maximal diameter recorded by
The results of our study suggest both the holotype and two paratypes of Ovabunda andamanensis sp. n. are the same species which can exhibit one or two rows of pinnules. Previous authors have suggested the number of rows of pinnules can be variable within a species and is therefore not a highly reliable taxonomic character. Early on both
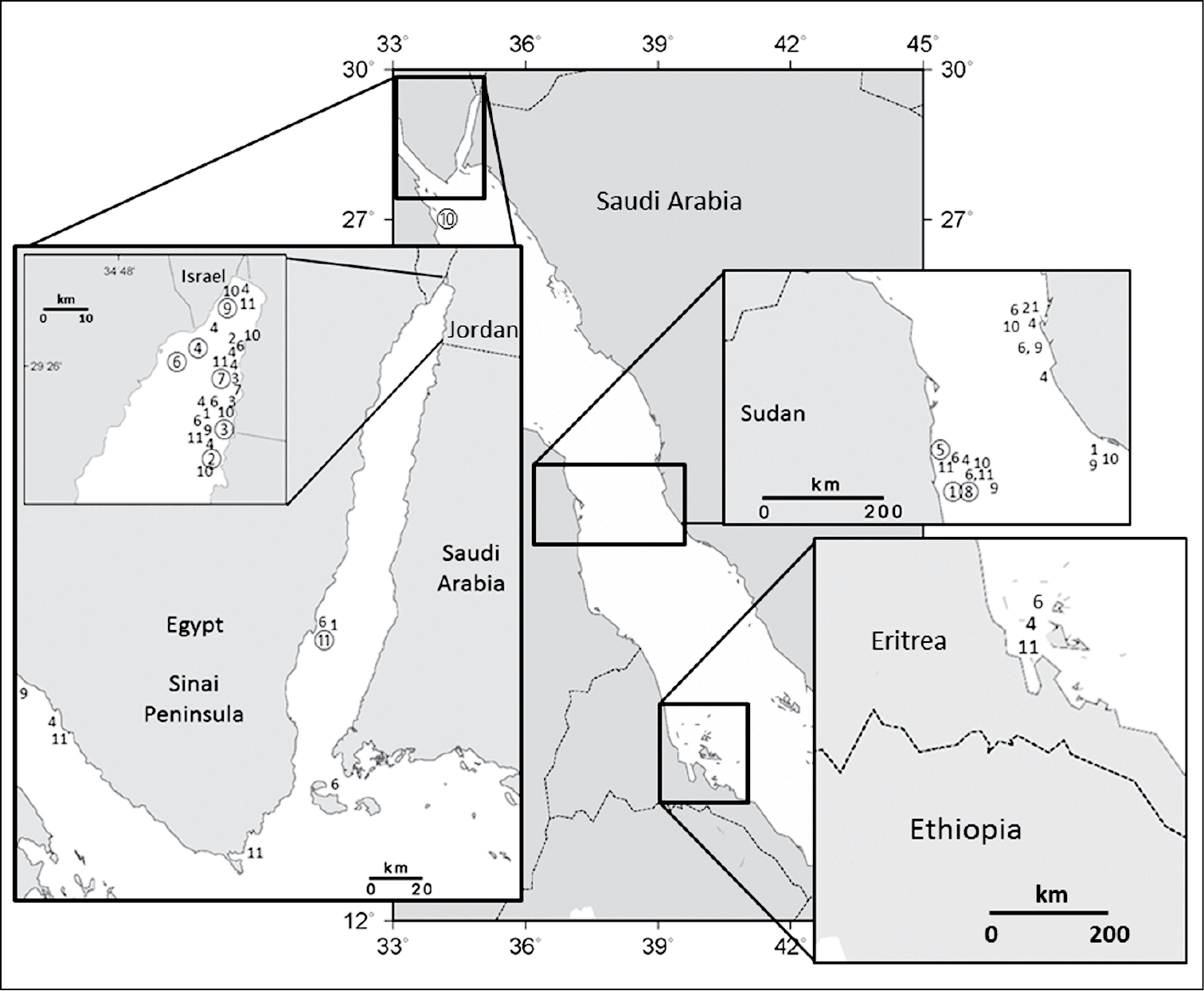
Distribution of Ovabunda species in the Red Sea: 1 Ovabunda ainex 2 Ovabunda arabica 3 Ovabunda benayahui 4 Ovabunda biseriata; 5 Ovabunda crenata; 6 Ovabunda faraunensis; 7 Ovabunda gohari 8 Ovabunda hamsina 9 Ovabunda impulsatilla 10 Ovabunda macrospiculata 11 Ovabunda verseveldti. Circled number denotes holotype.
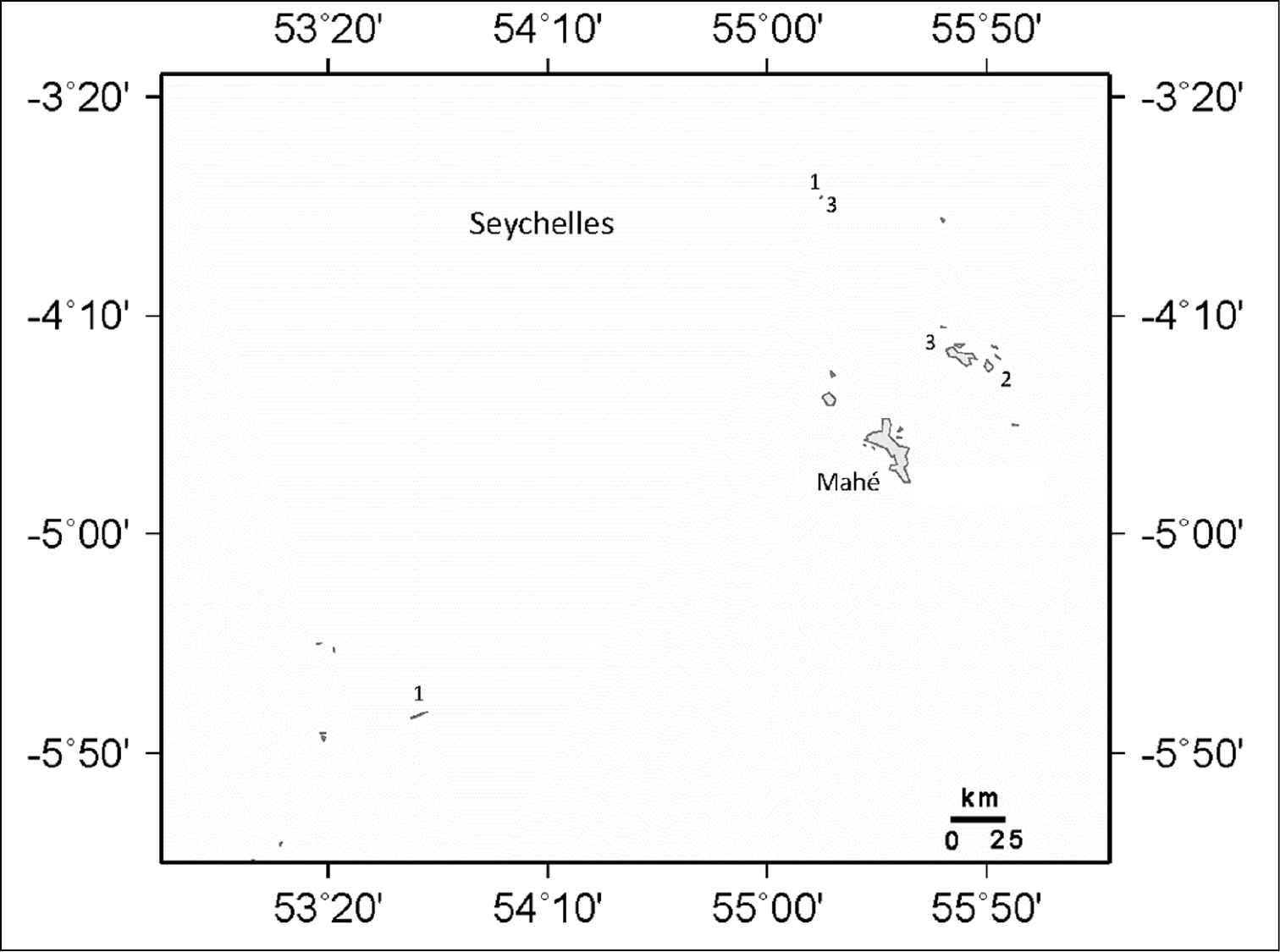
Distribution of Ovabunda species in the Seychelles Islands: 1 Ovabunda impulsatilla 2 Ovabunda benayahui 3 Ovabunda hamsina.
We are grateful for the generous support provided by Charatsee Aungtonya for curatorial assistance; Zhenquan Liu and Kenneth Mossman for SEM operations; Dr. Karl Weiss and the LeRoy Eyring Center for Solid State Science, Arizona State University for use of their microscopy facilities. Principle funding and laboratory facilities were provided by AquaTouch, Phoenix, Arizona USA. Our sincere thanks to Dr. Phil Alderslade and an anonymous reviewer whose comments improved this manuscript; also to Dr. Yehuda Benayahu and the organizers of the International Conference on Coelenterate Biology, 1-6 December 2013, Eilat, Israel for the opportunity to present this work.
Supplement S1.
Authors: Catherine S. McFadden
Data type: specimens data
Explanation note: Specimens and sequences included in molecular phylogenetic analysis of xeniids.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Link: doi: 10.3897/zookeys.431.7751.app1