(C) 2013 Robert J.B. Hoare. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The phylogeny of the mainly Australian nepticulid genus Pectinivalva Scoble, 1983 is investigated on the basis of morphology, and a division into three monophyletic subgenera is proposed on the basis of these results. These subgenera (Pectinivalva, Casanovula Hoare, subgen. n. and Menurella Hoare, subgen. n. ) are described and diagnosed, the described species of Pectinivalva are assigned to them, and representative new species are described in each: Pectinivalva (Pectinivalva) mystaconota Hoare, sp. n., Pectinivalva (Casanovula) brevipalpa Hoare, sp. n., Pectinivalva (Casanovula) minotaurus Hoare, sp. n., Pectinivalva (Menurella) scotodes Hoare, sp. n., Pectinivalva (Menurella) acmenae Hoare, sp. n., Pectinivalva (Menurella) xenadelpha Van Nieukerken & Hoare, sp. n., Pectinivalva (Menurella) quintiniae Hoare & Van Nieukerken, sp. n., and Pectinivalva (Menurella) tribulatrix Van Nieukerken & Hoare, sp. n. Pectinivalva (Menurella) quintiniae (from Quintinia verdonii, Paracryphiaceae) is the first known member of the genus with a host-plant not belonging to Myrtaceae. Pectinivalva (Menurella) xenadelpha from Mt Gunung Lumut, Kalimantan, Borneo, is the first pectinivalvine reported from outside Australia. Keys to the subgenera of Nepticulidae known from Australia, based on adults, male and female genitalia, and larvae, are presented. Host-plant relationships of Pectinivalva are discussed with relation to the phylogeny, and a list of known host-plants of Pectinivalva, including hosts of undescribed species, is presented. DNA barcodes are provided for most of the new and several unnamed species.
Pectinivalva, Myrtaceae, Eucalyptus, Paracryphiaceae, rain forest, Borneo, Indonesia, Australia, keys, hostplants, DNA barcodes
The subfamily Pectinivalvinae was described by
Preparation techniques. Rearing techniques, and techniques of slide preparation for larvae, pupal exuviae, adult heads, wing venation and genitalia followed
Morphological terminology. Terminology follows
Measurements of genitalia were taken directly with a calibrated eyepiece graticule, or from photographs taken with a Zeiss Axioskop using Axiovision software (see below) and rounded off to the nearest 5μm.
Hostplant names follow the Australian Plant Census (Australian Plant Census 2012) and were checked with APNI (Australian National Botanic Gardens 2011). Hostplant classification for Myrtaceae follows Wilson (2005).
Repositories. The following abbreviations have been used for institutions mentioned in the text:
anic Australian National Insect Collection, CSIRO Entomology, Canberra, Australia.
fmnh Finnish Museum of Natural History, Helsinki, Finland.
rmnh Naturalis Biodiversity Center, Leiden, The Netherlands.
mzb Museum Zoologicum Bogoriense, Research Center for Biology, Indonesian Insititute of Sciences, Cibinong, Indonesia
Material. Type material is cited with the descriptions. All material studied, including rearing records of species that have not been treated and voucher specimens for DNA barcoding, are listed in the Excel sheet in the Online Appendix 3. We have treated specimens with a genitalia slide or other preparation and DNA vouchers as individual records for each species. Other records combine all adults from a single rearing or other single collection event. While compiling this datasheet, the authors no longer had direct access to the ANIC collection in Canberra, and in some cases our notes were not sufficient to enter all fields. This explains why for some slides ‘sex unknown’ is given, and uncertainties about exactly how many specimens were collected on a given date.
Illustrations. Photographs of moths by B.E. Rhode in Auckland (species described by RJBH) were taken with a Nikon Ri1 digital camera mounted on a Leica M205 A stereo-microscope. Series of images for each specimen were subsequently montaged using Helicon Focus and Zerene Stacker software packages. Post-editing work, i.e. replacing backgrounds, ‘removing’ pins, and inserting scale bars was done in Photoshop.
Photographs of moths, leafmines (jointly described species) and all genitalia slides were taken by EJvN with a Zeiss AxioCam (HR or MR5) digital camera attached respectively to a Zeiss Stemi SV11 stereo-microscope and a Zeiss Axioskop H, using Carl Zeiss AxioVision software (version 4), for some photographs using the module “Extended focus”. Manipulation of photographs, using Adobe Photoshop® was kept to a minimum: disturbing conspicuous shades, protruding parts of pins, dust and air bubbles in slides were removed or obscured, black backgrounds smoothened.
Drawings were prepared by RJBH, using a drawing tube. Drawings on one plate are not necessarily in the same scale.
DNA barcodes. DNA was extracted from caterpillars or from dry adult abdomens. DNA extraction from larvae was usually destructive; from abdomens and some larvae the non-destructive protocol by
An NJ tree was prepared with Paup 4.0b10 for Windows (
Cladistic analysis. Choice of terminal taxa: Species were chosen with two overall aims in mind: (a) to cover the morphological diversity of Pectinivalva as far as possible, and (b) to cover the host-plant range as fully as possible so that inferences could be made about the evolution of host-plant choice in the genus. Only species for which both sexes of the adults were available were chosen: this criterion excluded most of the previously described species. Again, for the most part, species were chosen for which preserved larvae and mines were available; however, the early stages of Pectinivalva caenodora (Meyrick, 1906) are unknown. The early stages of Pectinivalva commoni Scoble, 1983 have not been preserved, but a very similar species (Pectinivalva 142: see Appendix 3) with genitalia very close to those of Pectinivalva commoni was reared during the course of this study and the scores for the immature stages of Pectinivalva commoni given here are based on the study of this second species.
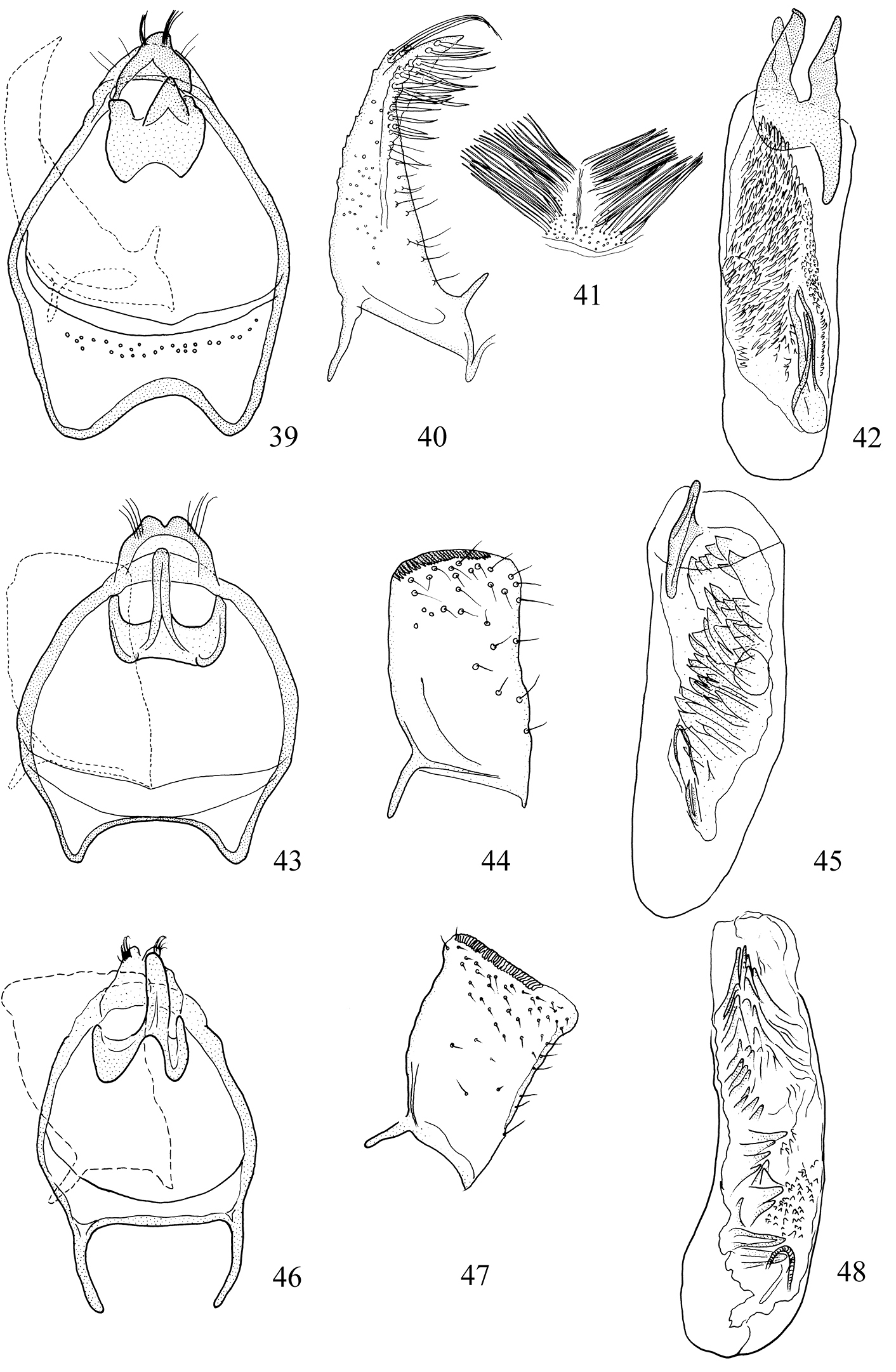
Details of material examined for species not described in this paper are given in the online Appendix 3. Undescribed species which have been reared are referred to here by their generic name and a rearing number. One new species that has not beenreared was also included: this is Pectinivalva mystaconota sp. n. Pectinivalva mystaconota diverges in morphology from most typical Pectinivalva species in that the pectinifer on the male valva is replaced by rows of strong flattened setae (Fig. 40).
The results of an unpublished phylogenetic analysis including most subgenera of Nepticulidae (Hoare & van Nieukerken, in prep.) and molecular studies (van Nieukerken et al. in prep) suggest that Roscidotoga represents the sister-group of Pectinivalva (cf.
Cladistic analysis. A maximum parsimony analysis with bootstrap was carried out with Paup 4.0b10 for Windows (
Characters were traced onto the most parsimonious trees using the program Mesquite 2.7 (
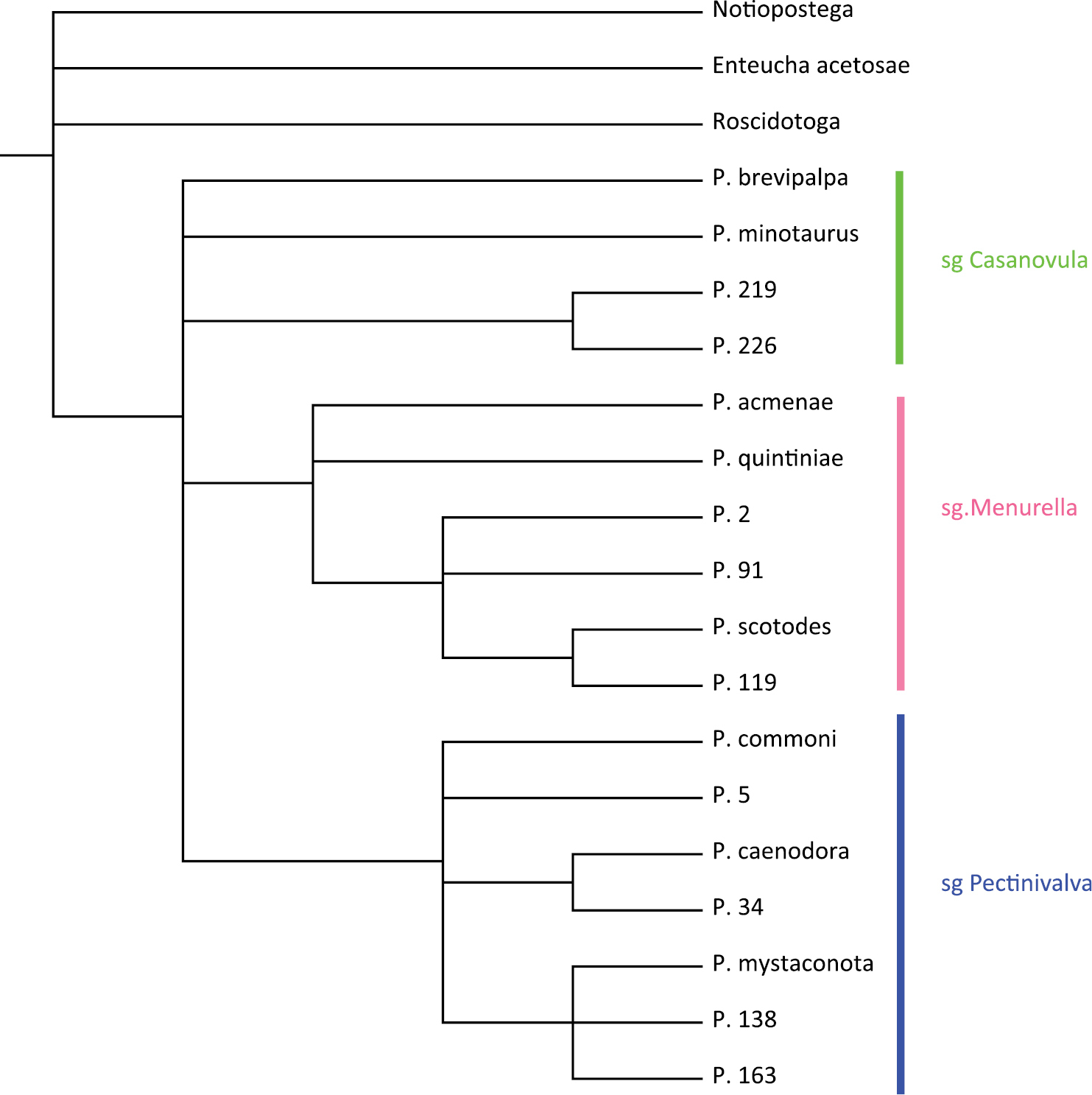
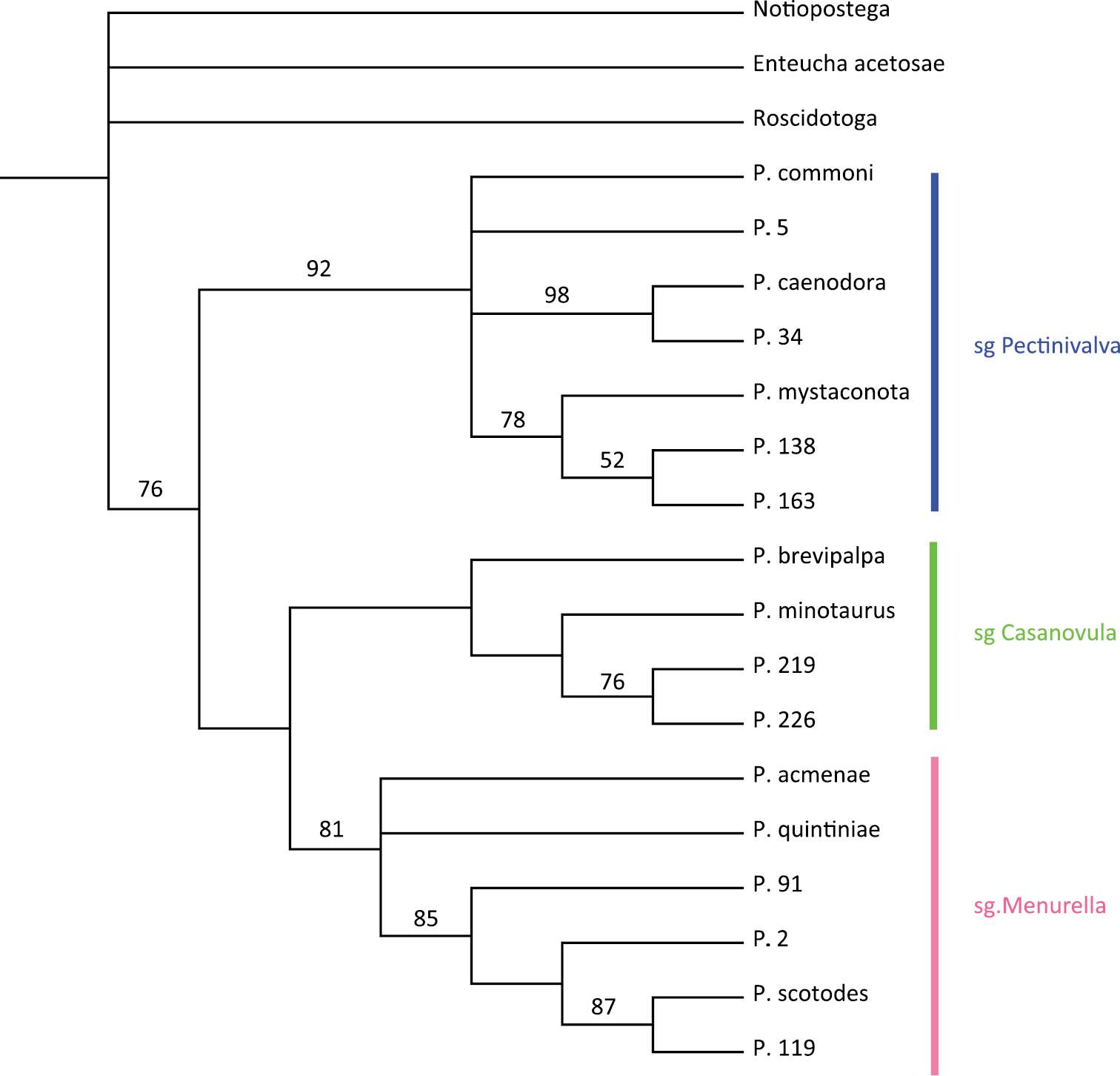
A heuristic search in PAUP produced 147 equally most parsimonious trees of length 60 with CI = 0.6667, RI = 0.8450 and RC = 0.5633. The strict consensus tree is presented in Fig. 1 (for bootstrap support see Fig. 2). Pectinivalva is consistently recovered as a monophyletic group with moderate support (bootstrap=76%), and within the genus, the three subgenera are almost always recovered, with strong support for Pectinivalva s.s. (92%) and Menurella (81%) (Fig. 1), but no support for Casanovula. Pectinivalva brevipalpa is the most problematic species, remaining in an unresolved polytomy with the three subgenera in the strict consensus tree. The 50% majority rule consensus tree is presented in Fig. 2, with bootstrap values; in this Pectinivalva brevipalpa forms a clade with Pectinivalva minotaurus, P. 219 and P. 226 in Casanovula. This placement is argued for below, and the 50% majority rule tree is used as the basis for the list of apomorphies given here, even without bootstrap support for Casanovula.
The monophyly of the genus Pectinivalva is supported by the following apomorphies:
(10–1) Uncus with a pair of well-defined tufts of setae.
(16–1) Vestibulum of female genitalia with a pair of lateral sclerites. The sclerites are absent in the clade Pectinivalva mystaconota + Pectinivalva 138 + Pectinivalva 163; this is unambiguously reconstructed as a secondary loss.
(17–1) Corpus bursae of female genitalia with extensive pectinations. This character is somewhat weak, since pectinations are present in many Nepticulinae (e.g. Stigmella and Acalyptris spp.). The pectinations are reduced in extent in most species of Menurella and lost independently in Pectinivalva 138 + Pectinivalva 163 and in Pectinivalva quintiniae.
(18–0 ACC) Signum of corpus bursae well-sclerotised and sparsely toothed. Since Casanovula and all outgroup taxa lack a signum, the basal state of this character in Pectinivalva is ambiguous. However, the form of the signum in Pectinivalva s. str. resembles that in Pectinivalva (Menurella) acmenae and Pectinivalva (Menurella) quintiniae, and similar signa do not occur elsewhere in Nepticulidae, so a single origin of this signum type is considered most likely.
(20–1) Anterior margin of T2 of abdomen with sclerotization interrupted. This character is paralleled in some genera in Trifurculini.
(23–1) Posterior lobes of larval head with sclerotization interrupted. This character is not known elsewhere in Nepticulidae. The posterior lobes are continuously sclerotized in Pectinivalva 119, a presumed reversion to the ancestral state.
The monophyly of Pectinivalva s. str. has very strong bootstrap support (92%). The group shares the following apomorphies:
(22–1) Larval head elongate and pyriform. The larva of Pectinivalva (Menurella) brevipalpa has a similar head-shape (Fig. 105); this is a presumed parallelism (see below).
(25–1) Larval mesothorax with only one pair of D setae (D1 absent). This character is constant within the group, but paralleled in Pectinivalva (Casanovula) 226 and Pectinivalva (Menurella) 91, and in many Nepticulinae (e.g., Simplimorpha, Stigmella spp., Ectoedemia (Fomoria) spp.).
(26–1) Cuticle of larva lacking spines. The larvae of all other species of Pectinivalva and Roscidotoga, and all known opostegid larvae, have a spinose cuticle. The spines are reduced or modified in some genera of Nepticulinae (e.g. the Ectoedemia (Fomoria) vannifera group: see
(27–1) Cuticle of larva with sculptured texture. This is a unique condition within the Nepticuloidea. It could be argued that characters 26 and 27 are not independent and that the sculpturing of the cuticle in Pectinivalva s. str. is homologous to the spines of other Nepticulidae. However, there are two reasons for rejecting this idea. Firstly, the sculpturing is particularly marked on the prothorax, where other species of Pectinivalvinae lack any spines (although some Nepticulinae have a spiny larval prothorax). Secondly, the sculpturing is present on the prothoracic sternite, an area that is never spined in other nepticulids. The two characters are therefore considered to be independent.
The following possible apomorphies are problematic:
(5–0) Forewing venation with R2+3 present. This is recovered as an unambiguous apomorphy of Pectinivalva s. str. in the analysis, since all other Pectinivalva species and all three outgroup taxa lack R2+3. However, loss of veins probably occurs rather easily in the evolution of these tiny moths, and independent losses are likely. Regaining a lost vein may not be impossible as supposed, e.g., by
(15–0 DEL) Cathrema of aedeagus supported by 2 or 3 interconnected sclerites. This form of cathrema is paralleled in Pectinivalva brevipalpa, and therefore could equally be interpreted as an apomorphy of Pectinivalva as a whole, with subsequent reduction in Pectinivalva minotaurus + Pectinivalva 219 + Pectinivalva 226 (ACC) and fusion into a sclerotised tube in Menurella.
The subgenera Menurella and Casanovula together form a monophyletic group supported by the following synapomorphies (but without bootstrap support):
(9–1) Uncus bifid. The undivided, hood-like or V- or Y-shaped uncus of most Nepticulidae (including Pectinivalva s. str.) appears to be the plesiomorphic state in the family (cf.
(21–1) Larval antenna 2-segmented (second and third segments fused). The antenna in Pectinivalva s. str. and in Roscidotoga is 3-segmented, as it is in most Lepidoptera. The basal state of this character for Pectinivalva is recovered as ambiguous, due to the reduced larval antennae of two of the outgroups (Notiopostega with two segments and Enteucha with one). However, independent reductions in the antenna are considered far more likely than the regaining of a lost segment, so this is regarded as a robust synapomorphy of the two groups. In Pectinivalva quintiniae, a further reduction to one segment has occurred, in parallel with Nepticulinae.
(24–1) Prothoracic sternite of larva much longer than broad. The Opostegidae lack a ventral prothoracic sclerotization, but most Nepticulidae, including Roscidotoga and Pectinivalva s. str., have a rather broad sclerite in this position.
If presence of vein R2+3 in Pectinivalva s str. is considered plesiomorphic, its loss (5–1) constitutes another probable synapomorphy of Menurella plus Casanovula, paralleled in Roscidotoga and Enteucha.
The monophyly of Casanovula has no bootstrap support. However, the last two of the three characters listed below are both unique within Pectinivalva and constant within this clade, so they are considered sufficient evidence to name this clade as a subgenus. The hostplant range is also distinctive (see below). Monophyly of the subgenus is supported by the following apomorphies:
(1–1 ACC) Basal flagellar segments of male antenna expanded and flattened. The flattened antenna occurs in Pectinivalva brevipalpa and Pectinivalva minotaurus, but not in the other two species of the group included in the analysis. Since this antennal character is unique amongst Nepticulidae, it seems very unlikely to have evolved independently in Pectinivalva brevipalpa and minotaurus, and is therefore best reconstructed as an apomorphy of Casanovula in the tree topology in Fig. 2, with subsequent loss in Pectinivalva 219 + Pectinivalva 226. The unresolved position of Pectinivalva brevipalpa in the strict consensus tree (Fig. 1) is due to the pyriform larval head (22–1), an apomorphy that it shares with Pectinivalva s. str. This is straightforwardly regarded as a parallelism, whereas the antennal character is not. For this reason, and because it shares the other two apomorphies listed below, Pectinivalva brevipalpa is confidently assigned to Casanovula.
Phylogeny of Pectinivalvinae, based on 27 morphological characters of larva, pupa and adult, and 1 behavioural character of larva. Strict Consensus Tree of 147 equally most parsimonious trees (CI = 0.6667, RI = 0.8450 and RC = 0.5633). Outgroups are Notiopostega atrata Davis (Opostegidae), Enteucha acetosae (Stainton) and Roscidotoga callicomae Hoare (Nepticulidae).
Phylogeny of Pectinivalvinae, characters and outgroups as for Fig. 1. 50% Majority Rule Consensus Tree from 147 equally most parsimonious trees; bootstrap support (200 replicates) shown on branches.
(3–3) Forewing metallic, with shining fascia. Many Nepticulinae have a similar pattern, but as other species of Pectinivalva have drab, more or less unicolorous forewings, such coloration is apomorphic within the genus. One species of this group, not treated here (but included in the key to subgenera below) lacks a transverse fascia, but retains weakly metallic forewings with purplish reflections. Reflective coloration is known to correspond in Lepidoptera with diurnal activity, and members of this group have not been collected at light, in contrast with members of Pectinivalva s. str. and Menurella. The small eyes of the known species (interocular index less than 0.7) further confirm that the moths are diurnal.
(18–3 DEL) Corpus bursae of female genitalia lacking signum. All other species of Pectinivalva have a well developed signum. Although a signum is also lacking in many opostegids, including Notiopostega, and in the nepticulid outgroups Roscidotoga and Enteucha, the presence of a very similar form of signum (the ‘toothed band’) in Pectinivalva s. str. and in three plesiomorphic species of Menurella (Pectinivalva acmenae, Pectinivalva xenadelpha and Pectinivalva quintiniae) strongly suggests that the loss of the structure in Casanovula is apomorphic.
The monophyly of Menurella has strong bootstrap support (81%). Members of the group share the following apomorphies:
(12–1) Pectinifer of male valva with fewer than 20 elements. There are more than 20 elements in the pectinifer in all species of Pectinivalva s. str. and Casanovula that retain the structure, and usually more than 20 in the Opostegidae (e.g. 45 in Notiopostega atrata, 35 in Eosopostega issikii Davis, 1989). The pectinifer elements have probably been lost more than once in Menurella : they are absent, e.g., from Pectinivalva quintiniae and Pectinivalva warburtonensis (Wilson). Conversely, several species of Pectinivalva in ANIC (not treated here) clearly belong to Menurella on the basis of apomorphies 15–1, 16–2, 17–1 and 18–2 (see below), but have a pectinifer with more than 20 elements.
(15–1) Cathrema of aedeagus supported by a smooth sclerotized tube. The tube is always present within the group and not found in other pectinivalvines. It could possibly represent a fusion of the sclerites associated with the cathrema in Pectinivalva s. str. A similar smooth tubular structure is associated with the cathrema in species of Enteucha (see van
(16–2) Lateral sclerites of female vestibulum strongly developed. These sclerites are always narrow (occasionally absent) in Pectinivalva s. str. and Casanovula. In Pectinivalva acmenae they are still relatively narrow, but have deeply forked tips. In the remaining species of Menurella the sclerites are very broad and robust.
(17–1) Pectinations of corpus bursae of female restricted to posterior part of corpus. More extensive pectinations are found in most other species of Pectinivalva s. str. and Casanovula (except Pectinivalva 138 and Pectinivalva 163).
Within the three subgenera, somespecies-groups are recovered in all most parsimonious trees. Within Pectinivalva s. str., Pectinivalva caenodora and Pectinivalva 34 form a monophyletic unit with 98% bootstrap support (here referred to as the Pectinivalva caenodora group). At least one other species (Pectinivalva 89), not included in the current analysis, is known from this group. Pectinivalva caenodora and Pectinivalva 34 share the following apomorphies:
(3–1) Forewing with a pale costal streak. This streak is lacking in the third member of the group mentioned above.
(10–2) Tufts of setae on dorsum of uncus mounted on lobes. These lobes are found in other species of Pectinivalva s. str. which were not included in the current analysis and do not share the other synapomorpies of Pectinivalva caenodora + Pectinivalva 34: hence this apomorphy may define a broader species-group which includes the Pectinivalva caenodora group.
(11–1) Central element of gnathos broad and cordate. Shared by the third member of the Pectinivalva caenodora group, but again also present in a few other species of the Pectinivalva commoni group.
(14–1) Sublateral processes of transtilla strongly reduced.
(18–1) Signum continuously toothed.
Pectinivalva 163, Pectinivalva 138 and Pectinivalva mystaconota (bootstrap 78%) share the following apomorphies:
(6–1) Male hindwing strongly expanded at base. This is paralleled in Menurella in the group of species including Pectinivalva scotodes, Pectinivalva 2 and Pectinivalva 119.
(7–1) Male hindwing with androconial pocket. Again, this structure is present in the Menurella species listed above and in Pectinivalva 91.
(16–0) Vestibulum of female lacking lateral sclerites. The sclerites, which constitute an autapomorphy of Pectinivalva, are most parsimoniously regarded as secondarily lost in these species.
Pectinivalva 138 and Pectinivalva 163 (52% bootstrap support) share the following apomorphy:
(17–2) Pectinations of corpus bursae absent.
The position of Pectinivalva commoni and Pectinivalva 5 within Pectinivalva s. str. cannot be resolved on the basis of the characters used in the current analysis. Pectinivalva commoni most closely resembles the group formed by Pectinivalva 163 + Pectinivalva 138 + Pectinivalva mystaconota on the basis of head and forewing colour. It also shares with these species the presence of androconial scales on the male hindwing (
Within Casanovula, Pectinivalva minotaurus, Pectinivalva 219 and Pectinivalva 226 form a monophyletic group with 76% bootstrap support, based on the following synapomorphy:
(8–1) Anterior extension of vinculum H-shaped.
A further problematic synapomorphy is:
(15–2 ACC) Cathrema moderately developed, with 0–1 associated sclerites. Since this state is shared with Roscidotoga, the presumed sister-group of Pectinivalva, it may alternatively be a retained plesiomorphy (DEL) (see above, under Pectinivalva). In the latter case, Pectinivalva s. str.and Pectinivalva brevipalpa have independently converged on a weaker cathrema with 2–3 sclerites.
Pectinivalva 219 and Pectinivalva 226 (76% bootstrap support) share the following behavioural apomorphy:
(28–1) Exit hole a large slit; larva pupating in mine. This habit has not been observed in any other species of Pectinivalvinae.
Pectinivalva 226 has only one pair of D setae on the larval mesothorax (25–1); the state of this character is unknown in Pectinivalva 219, which was reared from pupae. It may prove to be a further apomorphy of this subgroup (paralleled in Pectinivalva s. str. and in P. 91).
Within Menurella, Pectinivalva acmenae and Pectinivalva quintiniae form an unresolved basal trichotomy with a clade containing the remaining species. The remaining species in the current analysis (Pectinivalva scotodes, Pectinivalva 2, Pectinivalva 91 and Pectinivalva 119; 85% bootstrap support) share the following apomorphies:
(7–1) Male hindwing with androconial pocket. This character is paralleled in Pectinivalva mystaconota + Pectinivalva 138 + Pectinivalva 163.
(13–1 ACC) Pectinifer elements of male valva broad and tooth-like. The pectinifer elements are narrow (13–0) in Pectinivalva 2, which is interpreted as a reversal. Alternatively, broad elements must have evolved independently in Pectinivalva 91 and in Pectinivalva scotodes + Pectinivalva 119.
(18–2) Signum in form of parallel spinules. This form of signum is highly characteristic of this group and not known elsewhere in Nepticulidae.
Pectinivalva tribulatrix (not included in the analysis) shares all these characters and also belongs to this group.
Pectinivalva scotodes, Pectinivalva 119 and Pectinivalva 2 form a monophyletic group supported by the following apomorphy (but without bootstrap support):
(6–1) Male hindwing strongly expanded at base. This character is paralleled in Pectinivalva mystaconota + Pectinivalva 138 + Pectinivalva 163 in Pectinivalva s. str.
Pectinivalva scotodes and Pectinivalva 119 are sister species amongst the sampled taxa (bootstrap support 87%) on the basis of the following synapomorphies:
(2–1) Sexual colour dimorphism pronounced (male forewing blackish; female forewing brown or yellowish).
(4–1) Male forewing with dorsal fringe of long narrow androconial scales.
Amongst the described species of Pectinivalva, Pectinivalva funeralis (Meyrick, 1906) and Pectinivalva libera (Meyrick, 1906) (each known only from the male) share apomorphy 4–1, and resemble Pectinivalva scotodes and Pectinivalva 119 in their male genitalia; they are therefore considered to belong to the same species group within Menurella.
The genus Pectinivalva falls into three monophyletic clades, each described and diagnosed as a subgenus of Pectinivalva below: subgenus Pectinivalva Scoble, 1983, subgenus Casanovula subgen. n. and subgenus Menurella subgen. n. It would be possible to regard these as informal species-groups. However, a more profound subdivision seems necessary, because all three subgenera contain recognizable species-groups (some of them outlined above), which are likely to be of use in the future, especially in the highly speciose subgenera Pectinivalva and Menurella. Two other possible classifications would have been consistent with the results of the cladistic analysis: (i) To give all three subgenera generic rank; (ii) to give Pectinivalva (sensu stricto) generic rank, and to erect a new genus containing Casanovula and Menurella as subgenera. Genera and subgenera are essentially artificial and subjective concepts: therefore the only criteria for judging between such classifications are considerations such as usefulness, informativeness and consistency of ranking within the family (i.e. a subgenus in Pectinivalva should be roughly equivalent to a subgenus in Ectoedemia, although sister-groups need not have the same taxonomic rank). Admittedly, the use of subgenera can be rather cumbersome; however, it also allows one to convey more information about the relationships of a species in its name. Because
The hypothesis that Roscidotoga falls outside Pectinivalva, and is not derived from within that genus, is corroborated by the cladistic analysis.
The host-plant relationships of Roscidotoga were discussed by
As stated before, almost all known host-plants of Pectinivalva belong to the Myrtaceae, but one species is known from Paracryphiaceae (Quintinia) (see below for discussion). A full list of known host-plants is provided in Appendix 2; this includes myrtaceous hosts from which no moths have been reared, but where nepticulid mines assumed to belong to Pectinivalva have been collected. The assumption is based on the fact that no other genera of Nepticulidae have been reared from Myrtaceae in Australia. The Myrtaceae is an old Gondwanan family, with a long history in the southern continents (
The discovery of one species of Pectinivalva (quintiniae) feeding on a host other than Myrtaceae is an interesting development. Pectinivalva quintiniae is in the subgenus Menurella, and although itapparently occupies a basal position in this subgenus (Fig. 2), it remains most parsimonious to assume that its host relationship is the result of a secondary shift from Myrtaceae. Pectinivalva quintiniae is a rainforest species. Quintinia verdonii F. Muell., the host-plant of Pectinivalva quintiniae, has been recently referred to the family Paracryphiaceae (APG III 2009).
Each subgenus of Pectinivalva has a distinctive host-plant range. Subgenus Pectinivalva has only been reared from species of Eucalyptus, the most speciose genus of Myrtaceae in Australia. Casanovula is the only subgenus without species on Eucalyptus: known host-plants are Tristaniopsis Peter G. Wilson, Lophostemon Peter G. Wilson and Melaleuca L. The widest range of host-plants is shown by members of Menurella: species have been reared from Leptospermum Forst. et f., Syzygium R.Br. ex Gaertn., Rhodomyrtus (DC.) Rchb., Angophora Cav., and Corymbia K.D. Hill & L.A.S. Johnson, as well as Eucalyptus. Leafmines found on the genera Pilidiostigma Burret and Gossia N.Snow & Guymer (both in Myrteae) and Syncarpia Ten. in Syncarpieae cannot yet been associated with one of the subgenera, although we predict that at least the mines on Myrteae most likely belong to Menurella.
In general the observation that closely-related moths tend to feed on closely-related plants (cf. van
The phylogeny suggests that the common ancestor of modern Pectinivalva was a Myrtaceae-feeder, and that the presence of a species on Quintinia is the result of a host-shift. It seems most likely that the split between Roscidotoga and Pectinivalva predates the Miocene aridification of Australia (ca. 24 - ca. 5 million years B.P.), and that the original hosts of Pectinivalva (like those of Roscidotoga) were rainforest plants. It is interesting that rainforest Myrtaceae still host species of subgenera Casanovula (Casanovula brevipalpa and Casanovula minotaurus) and Menurella (Menurella acmenae, Menurella xenadelpha, Menurella quintiniae and Menurella tribulatrix); the position of these species in the phylogeny is consistent with the hypothesis that their hosts may be ecologically and/or phylogenetically close to the original Pectinivalva host-plant.
The subgenus Pectinivalva appears to lack representatives on rainforest plants, and has only been reared from the sclerophyllous genus Eucalyptus. The earliest macrofossils currently accepted as belonging to Eucalyptus are leaves of Enteucha kitsoni Deane from the Berwick Quarry, Victoria; these date from the late Oligocene or very early Miocene (ca. 25 million years B.P.), a time when south-east Australia was probably beginning to dry climatically, though still dominated by rainforest (
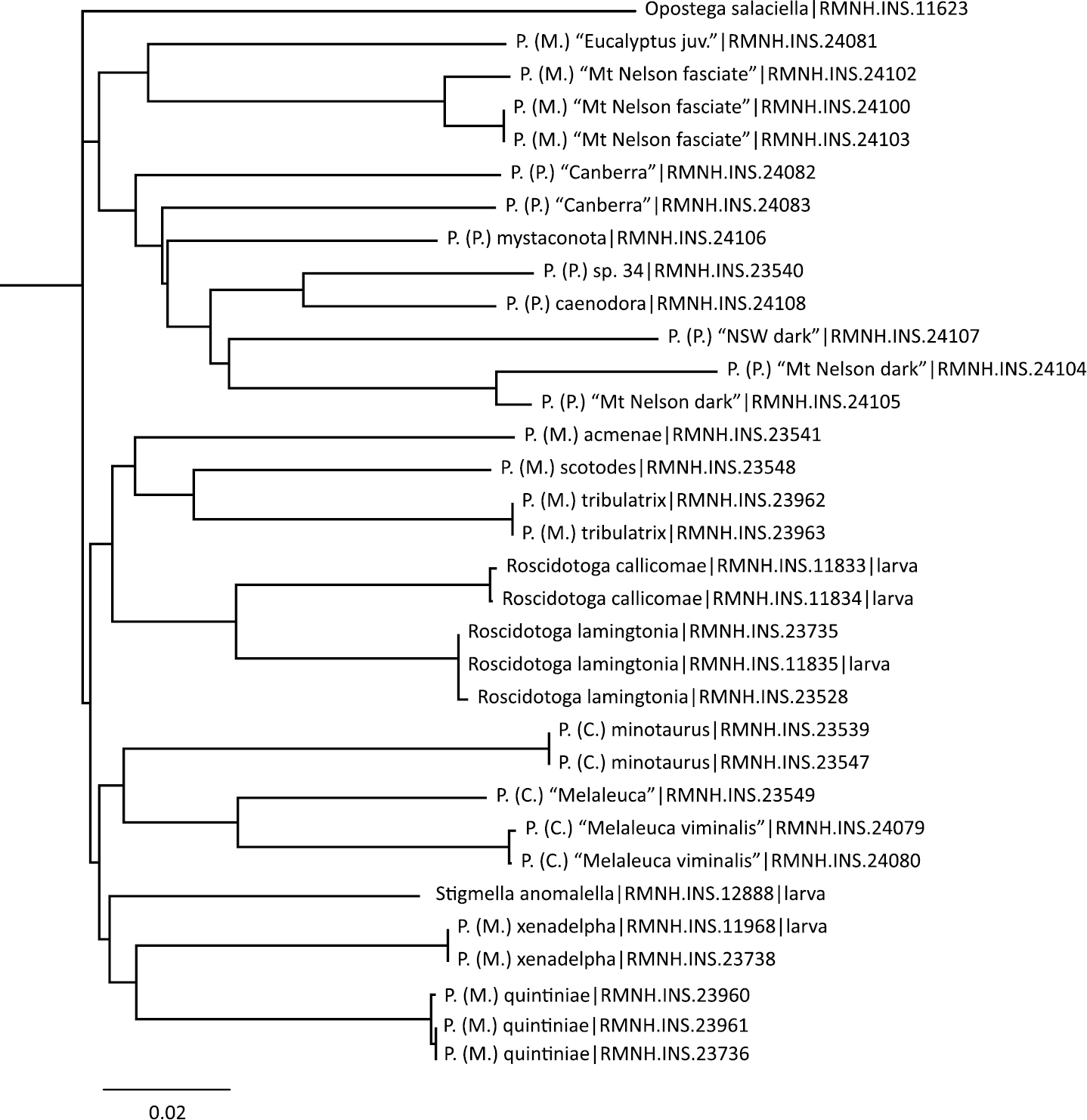
We provide the DNA barcodes (Fig. 3) for seven of the eight new species named in this paper (only missing Pectinivalva brevipalpa), and for one previously named species (Pectinivalva caenodora). In addition we include barcodes for a further nine or ten unnamed species that were available for barcoding. The main reason for including barcodes is to aid recognition of species and identification of immature stages without rearing. We do not intend to use these data for phylogenetic purposes, for which data on more genes are required.
Neighbor Joining Tree of DNA barcodes of Pectinivalvinae species, showing specimen registry numbers. Outgroup is Opostega salaciella (Treitschke, 1833). Stigmella anomalella (Goeze, 1783) chosen as extra outgroup, but groups here with Pectinivalva subgenus Menurella.
Diagnosis of the subgenera of Pectinivalva. Character states regarded as apomorphic are marked with an asterisk.<br/>
| Character | Pectinivalva (Pectinivalva) | Pectinivalva (Casanovula) | Pectinivalva (Menurella) |
|---|---|---|---|
| Forewing coloration | + unicolorous, or with costal streak | *purplish, usually with shining fascia | + unicolorous, or with white fascia or opposite spots |
| Forewing venation: R2+3 | Present | *Absent | *Absent |
| Male genitalia: uncus apex | Undivided | *Bifid | *Bifid |
| Male genitalia: vinculum | Rounded or weakly concave | *Strongly concave to H-shaped | Rounded to strongly concave |
| Male genitalia: cathrema | With 2–3 associated sclerites | Usually 1 or no associated sclerites<br/> (2–3 in brevipalpa) | *Supported by a sclerotized tube |
| Female genitalia: vestibulum | Lateral sclerites narrow or absent | Lateral sclerites narrow | *Lateral sclerites forked or broad |
| Female genitalia: signum | Longitudinal toothed band with lacunae | *Absent | *Oval toothed band or concentric ovals of parallel spinules |
| Larva: antenna | 3-segmented | *2-segmented | *2-segmented or 1-segmented |
| Larva: D setae of mesothorax | *1 pair | 2 pairs (occasionally 1) | 2 pairs (occasionally 1) |
| Larva: spinosity of cuticle | *Spines absent | Spines present | Spines present |
| Larva: texture of cuticle | *Sculptured and reticulate | Smooth | Smooth |
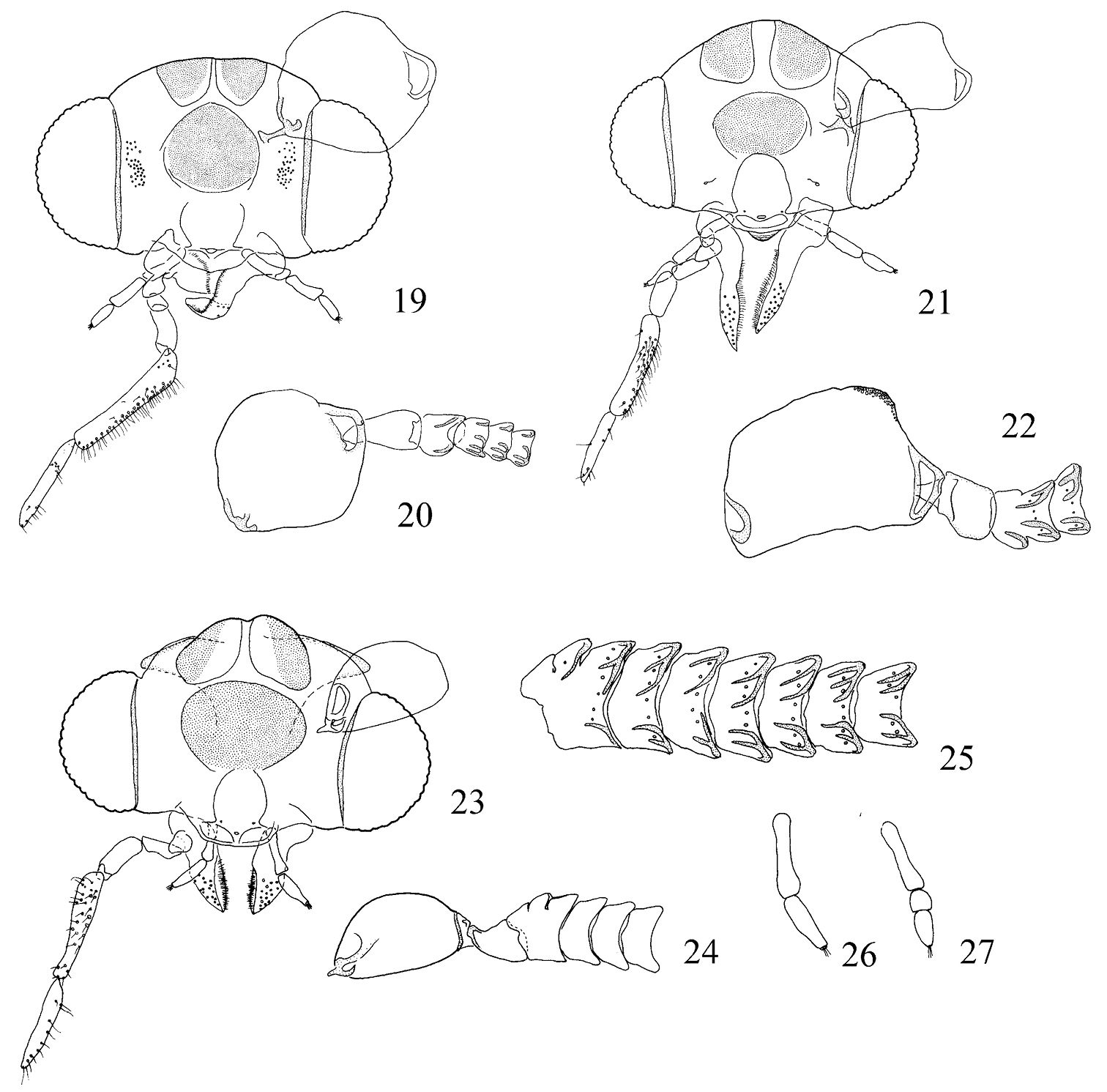
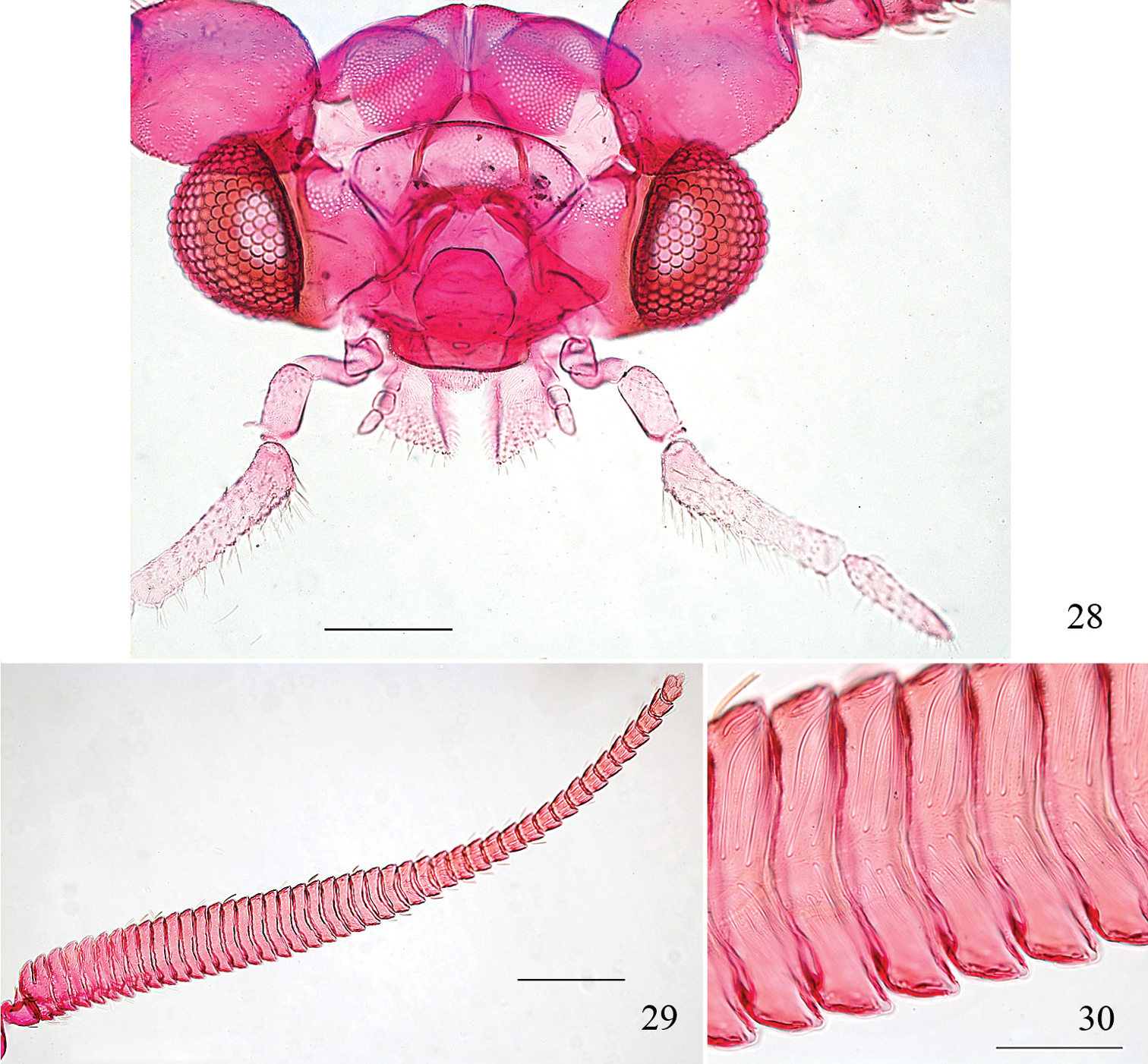
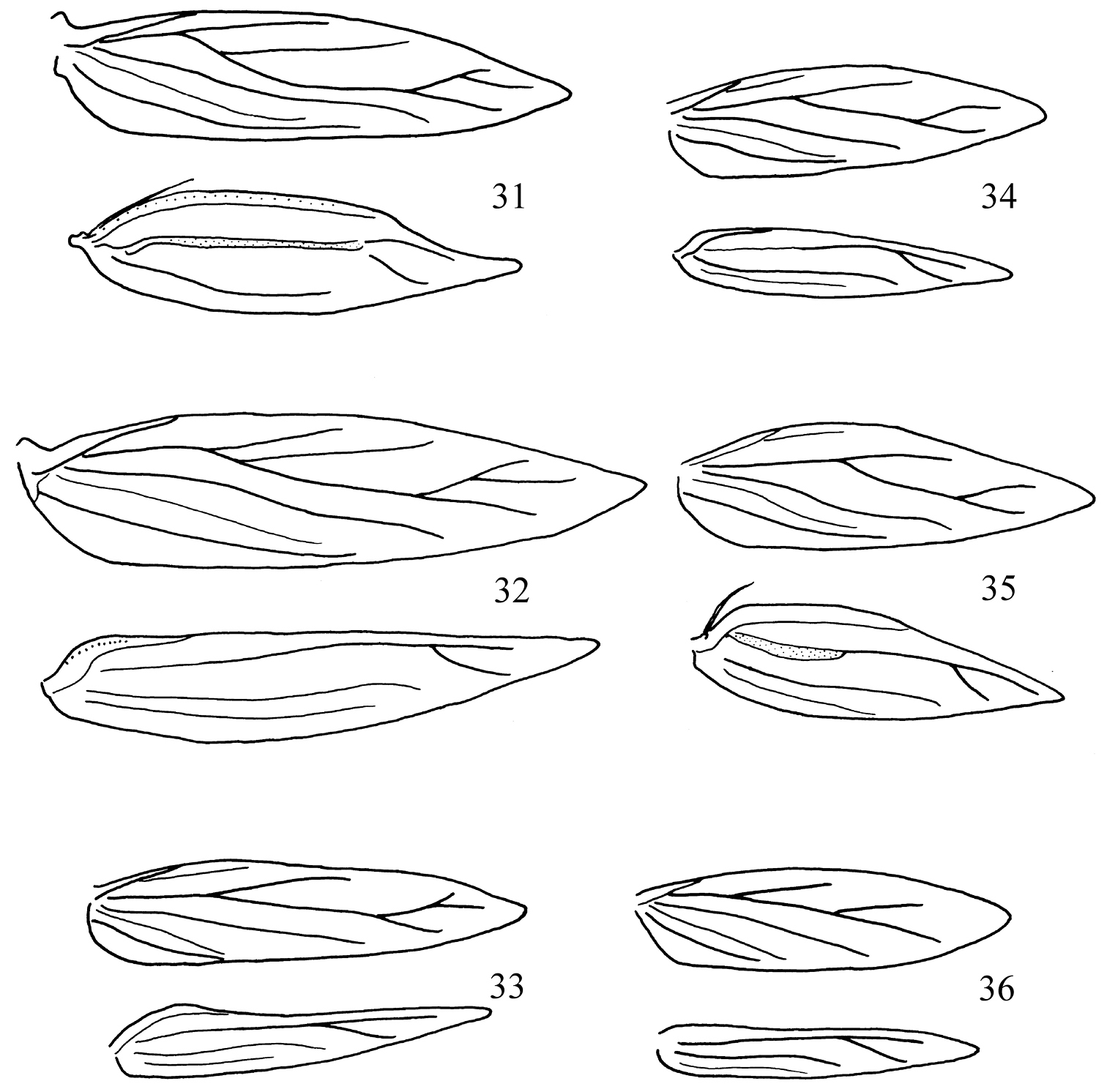
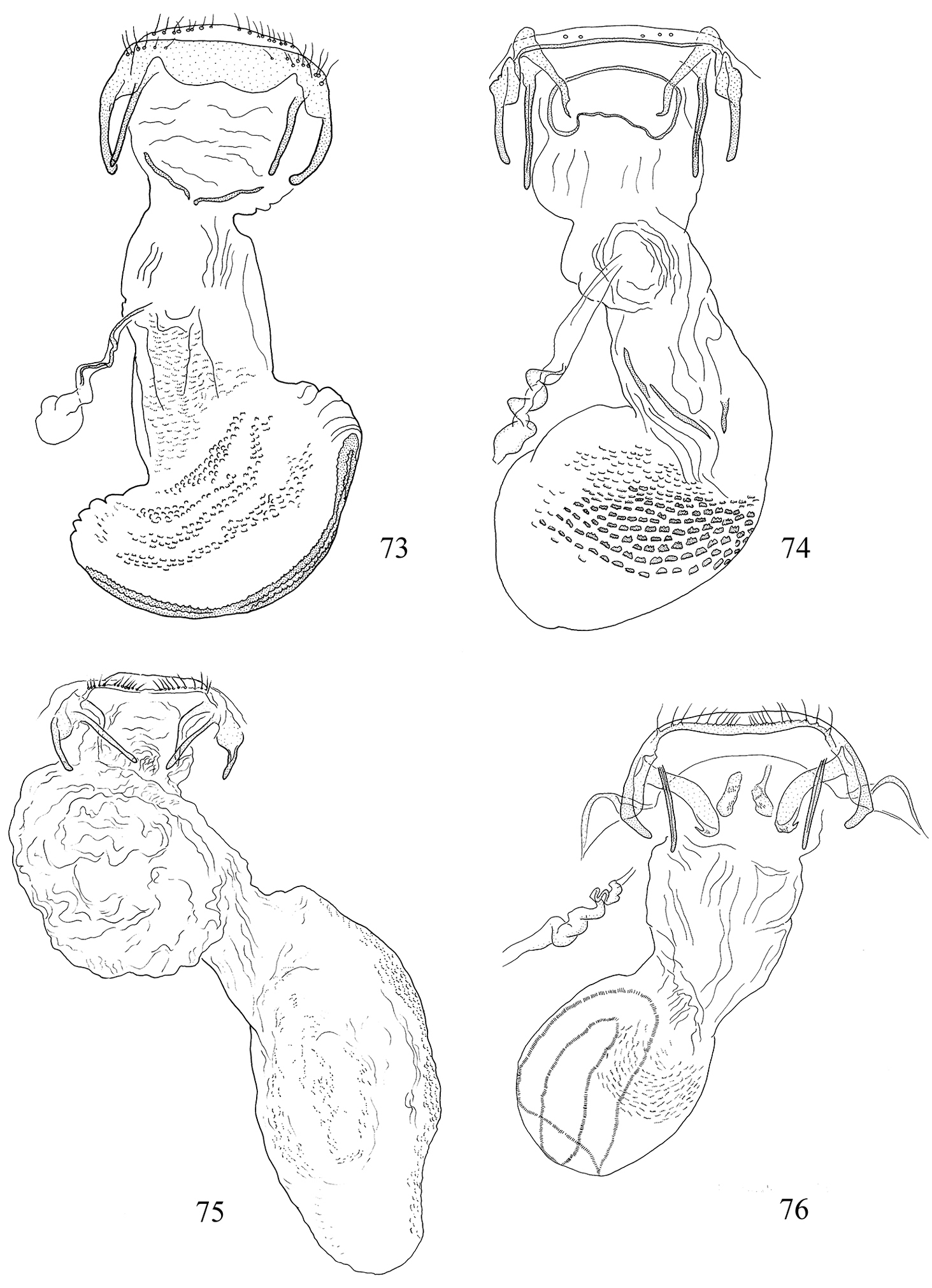
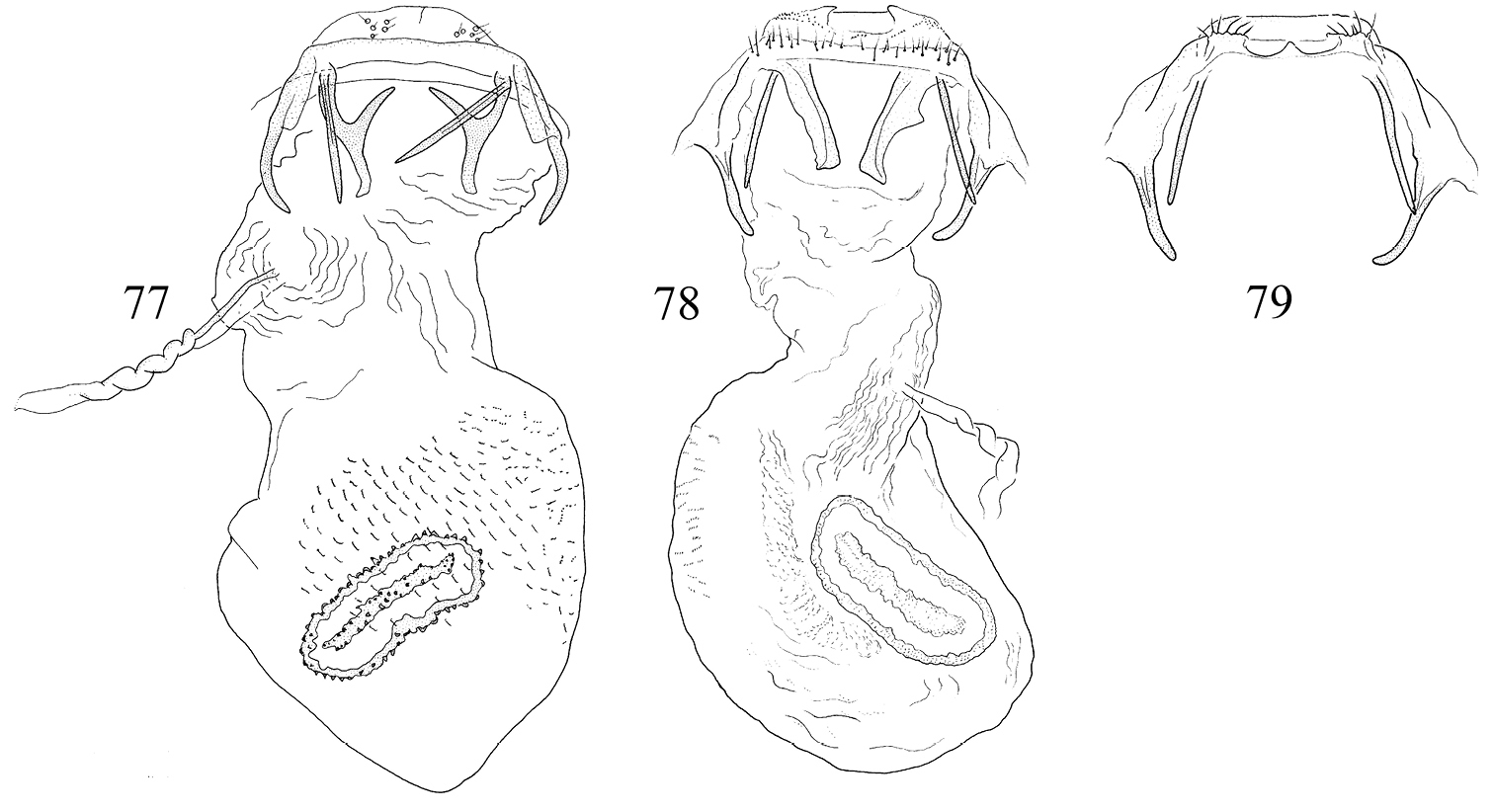
Adults. Head (Figs 19–30): Labial palpi 2- or 3-segmented; galeae short; maxillary palpi 5-segmented; antennae with sensillum vesiculocladum usually or always 5-branched (needs more detailed study in some species). Collar usually consisting of piliform scales. Forewing: underside sometimes with androconial scales in male; subdorsal retinaculum absent. Hindwing: upperside often with androconial scales in male. Wing venation (Figs 31–36): forewing without closed cell, Cu present, long; 1+2A unthickened, running obliquely from base of wing to meet dorsum before tornus; hindwing with trunk of Rs+M usually more or less deflected towards costa. Abdomen sometimes with specialized scales dorsally in male; S2a more or less pentagonal, usually with transverse rows of minute spines. Legs: fore-tibia of males sometimes thickened with specialized scales.
Male genitalia (Figs 39, 40, 42–72). Tegumen band-like, occasionally with lateral corners extended anteriorly into ‘shoulders’. Uncus either well-sclerotized and hood-like (Pectinivalva) or reduced (Roscidotoga). Gnathos (if present) with single central element. Valva usually with well-developed pectinifer. Aedeagus often with asymmetrical apical processes; striate thickening round base of ejaculatory duct (cathrema) weakly developed; vesica usually with numerous cornuti.
Female genitalia (Figs 73–103). S8 usually broadly squared off. Vestibulum often with lateral sclerites. Corpus bursae with single signum, or without signa.
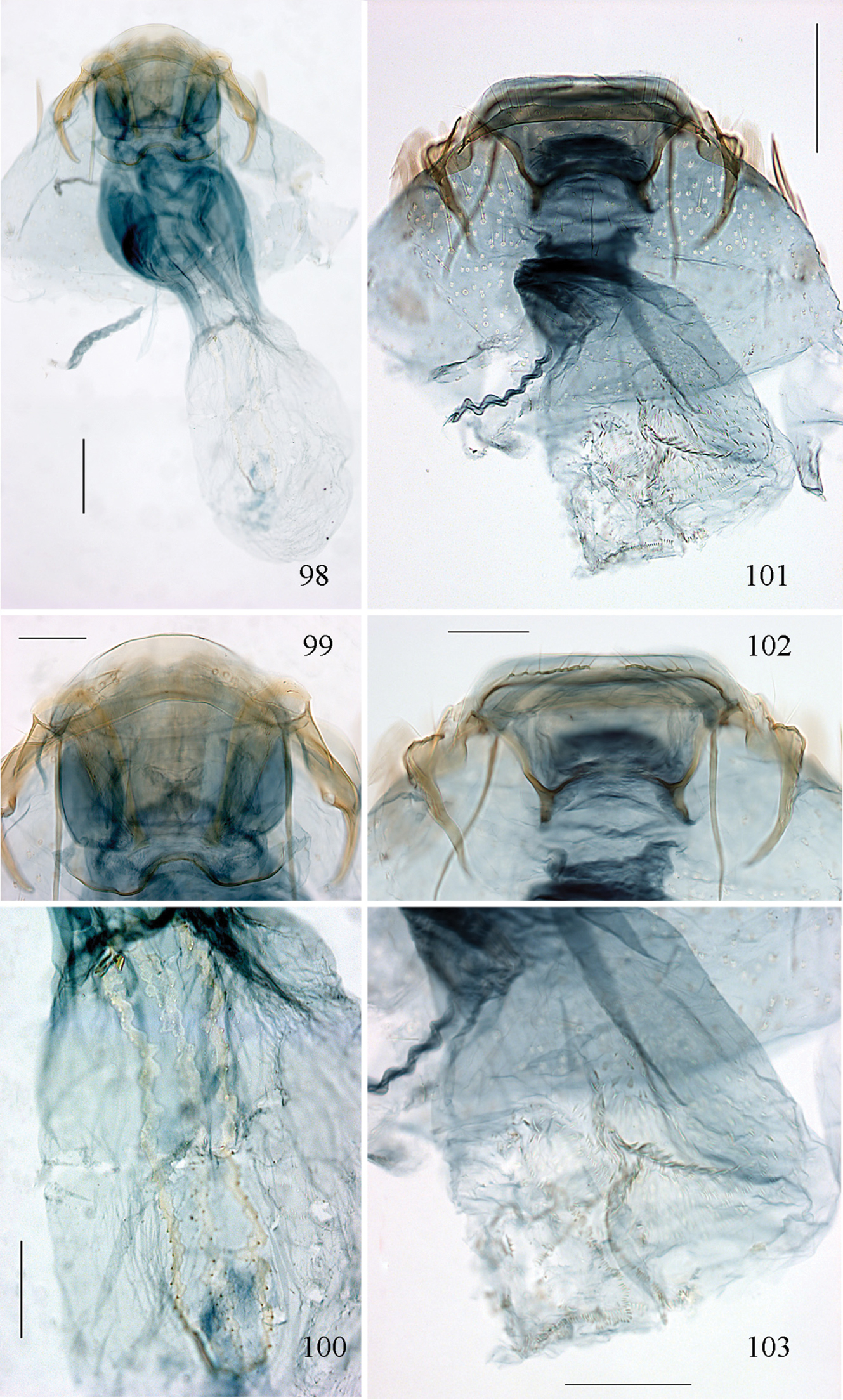
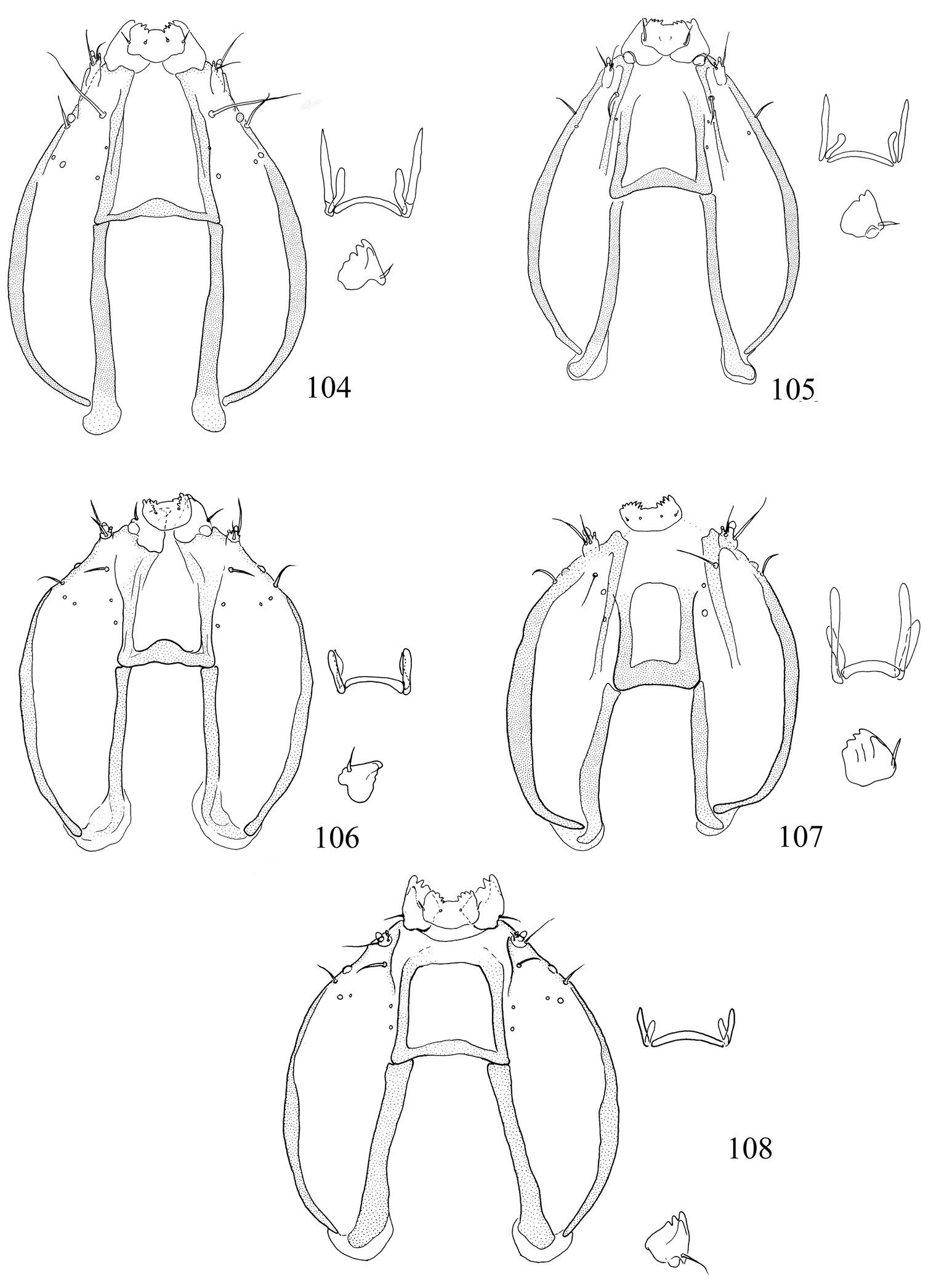
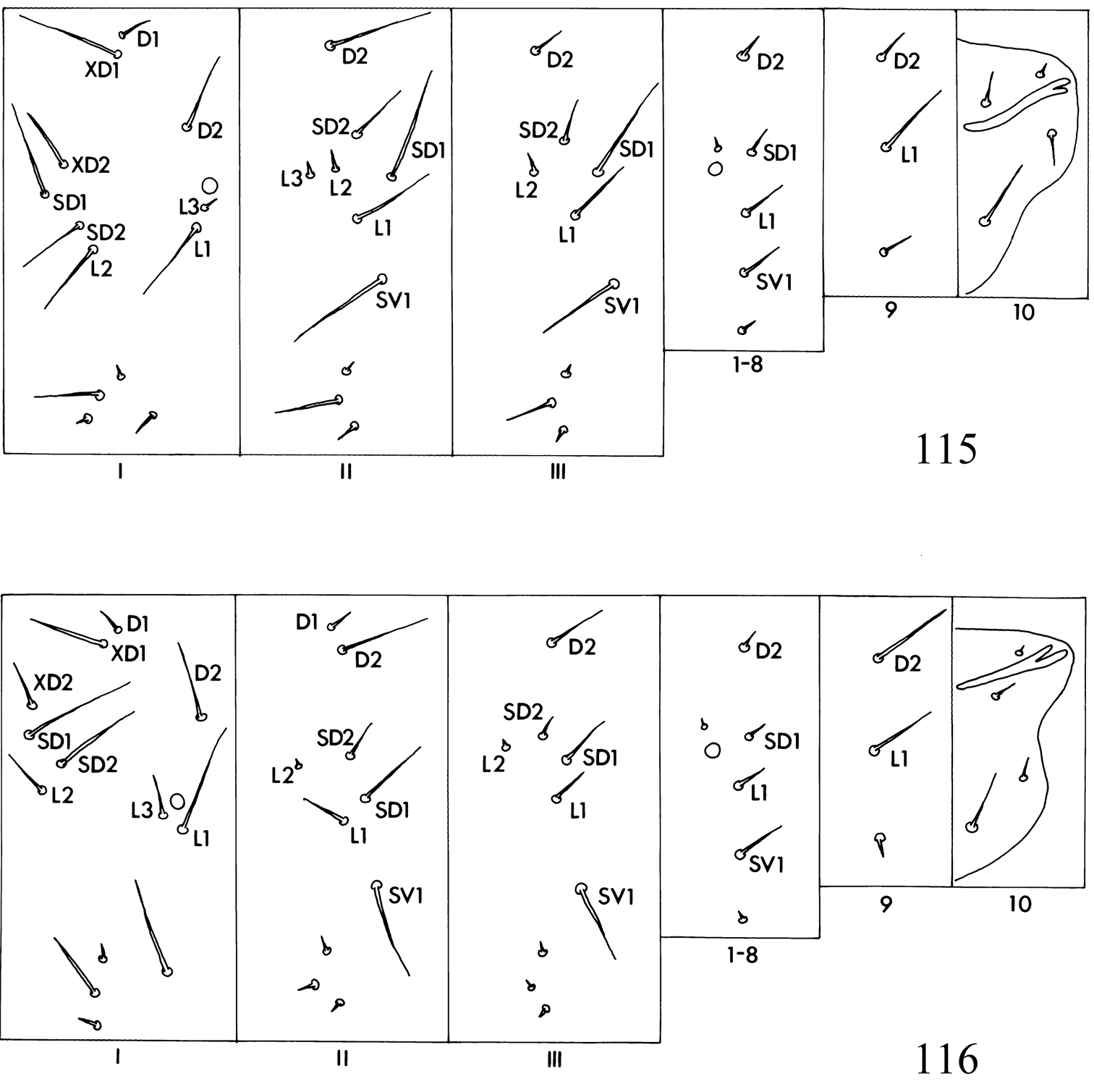
Larva. Head (Figs 104–108): antennae 2- or 3-segmented (1-segmented in Pectinivalva quintiniae); labial palpi 3-segmented; stipes with 2 setae; frontoclypeus approximately square or rectangular; anterior tentorial arms approximately 2 times as long as posterior. Chaetotaxy (Figs 115, 116): T1 with 13 pairs of setae; T2 with 10 or 11 pairs (3 setae ventral to SV1); T3 with 9 pairs (1 D seta and 2 L setae present); A1–8 with 6 pairs of setae; A9 with 3 pairs; A10 with 3 or 4 pairs. Anal rods apically pointed or forked.
Cocoon. Usually reddish brown; usually spun outside the mine.
Pupa. Head: Clypeus squarish; frons with a pair of conspicuous setae posteriorly; labial palpi distinctly longer than maxillae. Eclosion more or less dorsal, so that suture between eyecaps and frons remains largely intact ventrally. Abdominal segments 2–8 each with 3–4 rows of spines on dorsum, and a prominent pair of dorsal setae.
Most known larvae of Pectinivalva are leaf-miners on Myrtales (Myrtaceae), one species is known from Paracryphiales (Paracryphiaceae); those of Roscidotoga are leaf-miners on Oxalidales (Cunoniaceae (including Eucryphiaceae) and Elaeocarpaceae).
Australia, Borneo. Probably more widespread in Australian and Oriental regions than currently known.
Roscidotoga Hoare, 2000a
Roscidotoga eucryphiae Hoare, 2000a
Roscidotoga callicomae Hoare, 2000a
Roscidotoga lamingtonia Van Nieukerken, Van den Berg & Hoare, 2011
Roscidotoga apphiripes Hoare, 2000a
Pectinivalva Scoble, 1983
Subgenus Pectinivalva (Pectinivalva)
Pectinivalva (Pectinivalva) caenodora (Meyrick, 1906)
Pectinivalva (Pectinivalva) chalcitis (Meyrick, 1906)
Pectinivalva (Pectinivalva) commoni Scoble, 1983
Pectinivalva (Pectinivalva) endocapna (Meyrick, 1906)
Pectinivalva (Pectinivalva) gilva (Meyrick, 1906)
Pectinivalva (Pectinivalva) melanotis (Meyrick, 1906)
Pectinivalva (Pectinivalva) mystaconota Hoare, sp. n.
Pectinivalva (Casanovula) Hoare, subgen. n.
Pectinivalva (Casanovula) brevipalpa Hoare, sp. n.
Pectinivalva (Casanovula) minotaurus Hoare, sp. n.
Pectinivalva (Menurella) Hoare, subgen. n.
Pectinivalva (Menurella) anazona (Meyrick, 1906)
Pectinivalva (Menurella) funeralis (Meyrick, 1906)
Pectinivalva (Menurella) libera (Meyrick, 1906)
Pectinivalva (Menurella) planetis (Meyrick, 1906)
Pectinivalva (Menurella) primigena (Meyrick, 1906)
Pectinivalva (Menurella) trepida (Meyrick, 1906)
Pectinivalva (Menurella) warburtonensis (Wilson, 1939)
Pectinivalva (Menurella) scotodes Hoare, sp. n.
Pectinivalva (Menurella) acmenae Hoare, sp. n.
Pectinivalva (Menurella) xenadelpha Van Nieukerken & Hoare, sp. n.
Pectinivalva (Menurella) quintiniae Hoare & Van Nieukerken, sp. n.
Pectinivalva (Menurella) tribulatrix Van Nieukerken & Hoare, sp. n.
Pectinivalva commoni Scoble, 1983, by original designation.
A large and diverse genus, here subdivided into three subgenera on the basis of the phylogenetic reconstruction presented above. The following overview of the morphology of the genus should be taken in conjunction with the more detailed descriptions of the subgenera given below. Because of the great number of species in the genus, a complete revision is impractical at present. Species have been selected for description in order to represent the range of host-plants, morphology and distribution so far known in Pectinivalva.
Adults. Head capsule (Figs 19–27): labial palpi 2- or 3-segmented. Underside of forewing and upperside of hindwing often with androconial scales in male. Costal bristles of male hindwing absent or replaced by lamellate scales. Legs: fore-tibia of male sometimes thickened with specialized scales. Upperside of abdomen sometimes with androconial scales in male. Anterior edge of T2 weakly sclerotized medially.
Male genitalia (Figs 39, 40, 42–72). Anterior extension of vinculum usually rather short. Lateral arms of vinculum occasionally more or less forked apically. Uncus hood-like, dorsally with a pair of well-defined tufts of strong setae. Gnathos present, 1 central element. Valva (Figs 40, 44, 47, 50, 53, 56) rounded, squarish or triangular, usually with well-developed pectinifer along distal edge. Transverse bar of transtilla usually absent. Juxta in the form of 2 elongate sclerotized flaps connecting bases of valvae with apex of aedeagus. Aedeagus very variable (see subfamily description).
Female genitalia (Figs 73–103). S8 usually very broad and squared off. Vestibulum usually with a pair of lateral sclerites associated with apophyses anteriores. Corpus bursae well sclerotized, without diverticulum, usually with single signum.
Larva. Head (Figs 104–108): antennae 2- or 3-segmented; posterior lobes usually not continuously sclerotized caudally. Chaetotaxy (Figs 115, 116): see subgeneric descriptions.
Pupa. As described for subfamily.
Most known larvae leaf-miners on Myrtaceae, with one species on Paracryphiaceae (Quintinia A. DC.).
Distinguished from Roscidotoga, the only other known genus of Pectinivalvinae, externally by the forewing pattern (without silver streak from mid-costa or suffusion of metallic scales towards apex); in the male genitalia by the presence of a gnathos and a well-sclerotized uncus with strong tufts of setae; and in the female genitalia by the simple (unexpanded) apophyses anteriores and the well-sclerotized corpus bursae, which lacks a diverticulum.
Australia (known from all states and territories), Borneo (a single species, Pectinivalva xenadelpha, described below).
Pectinivalva commoni Scoble, 1983: 13 (original designation and monotypy).
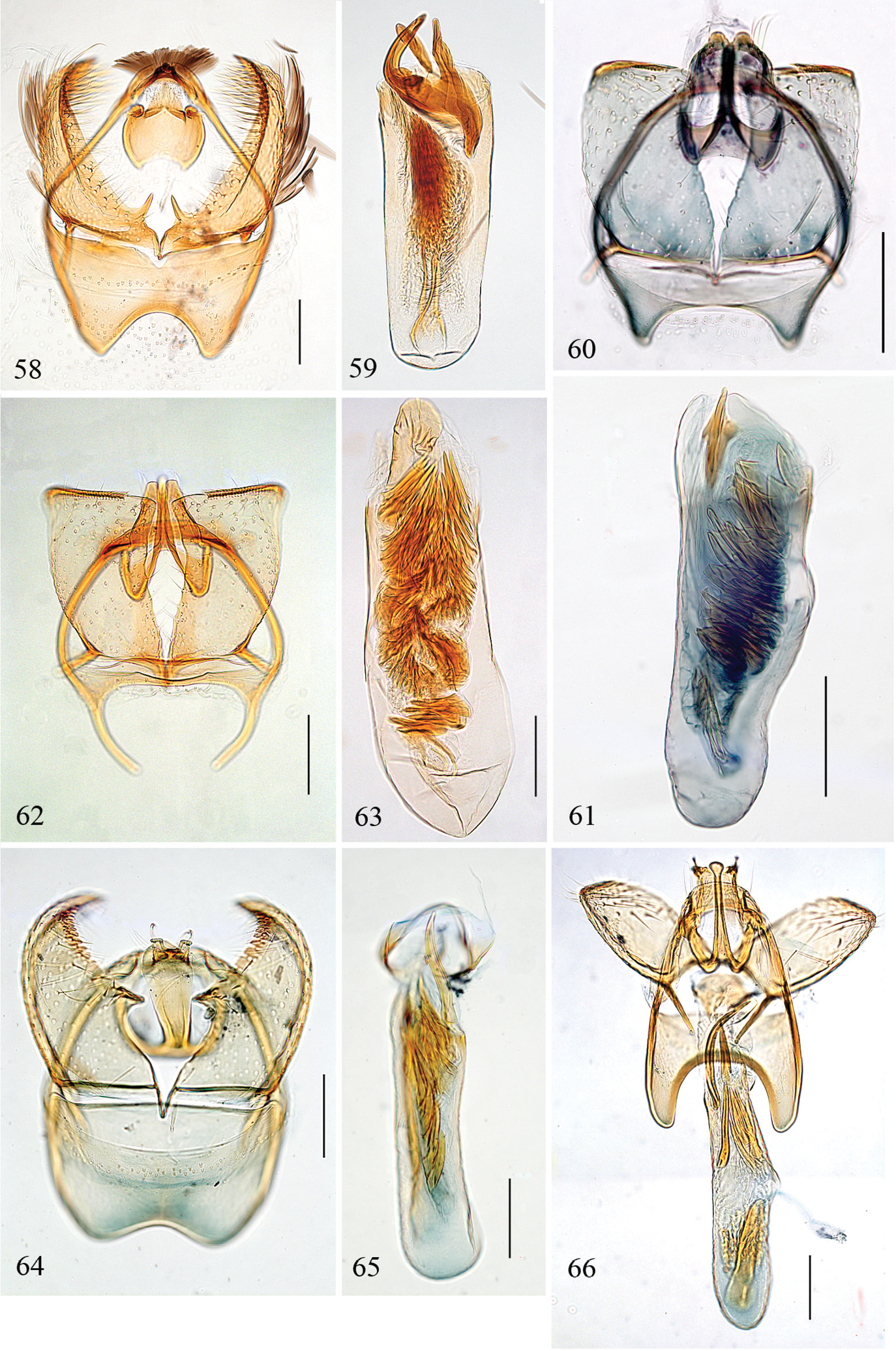
Adults. Head capsule (Fig. 19): labial palpi 3-segmented; interocular index 0.77–0.84. Antennae unmodified. Collar consisting of piliform scales (lamellate scales in one undescribed species). Wingspan ca. 4.5–8.4 mm. Forewing usually more or less unicolorous, greyish to fuscous, without transverse fascia, occasionally yellowish or with yellow costal streak. Hindwing in male often with longitudinal furrow (here termed ‘androconial pocket’) surrounded by androconial scales. Underside of forewing in male often with androconia. Wing venation (Figs 31, 32): R2+3 in forewing present. Abdomen usually without specialized scales; S2a always spinose. Fore-tibia of male usually thickened with blackish scales in those species with an androconial pocket.
Male genitalia (Figs 39, 40, 42, 58, 59). Lateral arms of vinculum not or weakly forked apically. Tegumen occasionally extended laterally. Valva either apically rounded, with conspicuous pectinifer of ca. 25–55 peg-like elements, or elongate and triangular, with pectinifer replaced by stiff setae. Sublateral processes sometimes reduced or absent. Aedeagus (Figs 42, 59): cathrema very weak, associated with 2 or 3 interconnected sclerites of variable length.
Female genitalia (Figs 73, 80–82). Lateral sclerites of vestibulum narrow, occasionally absent. Accessory sac absent. Signum an elongate toothed band with lacunae.
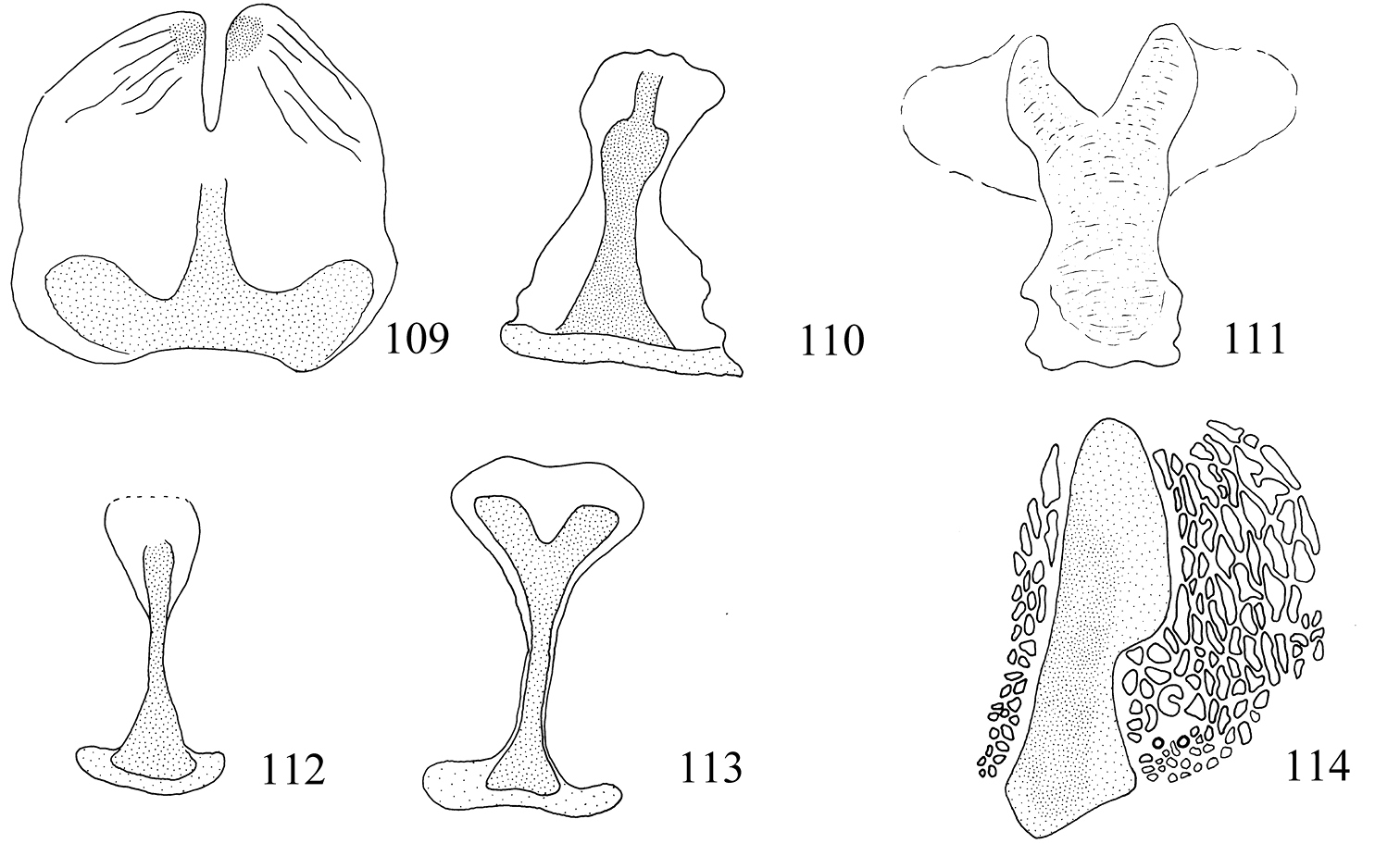
Larva. Head (Fig. 104): antennae 3-segmented, segments 2 and 3 each with 1 sensillum chaeticum and 1 sensillum basiconicum; head-shape distinctly elongate and pyriform. Thorax: prothoracic sternite (Fig. 109) broad, rounded or squarish; chaetotaxy (Fig. 115): T2 with 10 pairs of setae (1 pair of D setae). Abdomen: as described for subfamily. Cuticle of all segments completely lacking spines, and with raised reticulate texture, especially on prothorax.
Host-plants: Eucalyptus L’Hérit. spp. (Myrtaceae). Mine: usually a short gallery leading to a blotch; exit-hole a semicircular slit.
See Table 1.
Australia (known from all states and territories).
In addition to the six previously and newly described species, also approximately 65 undescribed species in the anic, of which the following, cited by their anic rearing numbers, have been studied in detail for the current work: Pectinivalva (Pectinivalva) 5; Pectinivalva (Pectinivalva) 34; Pectinivalva (Pectinivalva) 138; Pectinivalva (Pectinivalva) 142; Pectinivalva (Pectinivalva) 163.
Pectinivalva (Pectinivalva) is a relatively diverse subgenus, and could probably be subdivided into several species groups. One such group, the Pectinivalva (Pectinivalva) caenodora group, was diagnosed above. We do not propose to erect any further named species groups here, but we describe below a species that diverges strongly from most other members of the subgenus, and has several close relatives. Their placement in Pectinivalva (Pectinivalva) is argued for below, but as the larvae are as yet unknown, this decision may have to be revised.
Pectinivalva (Pectinivalva) is equivalent to the Pectinivalva commoni group of
urn:lsid:zoobank.org:act:D150BB4B-4D0A-41CE-830A-1B188F63DE6E
Holotype.♂, 35.16S, 149.06E, Black Mt., A.C.T., light trap, 26.iv.1963, I.F.B. Common. Genitalia slide 10164 (anic).Paratypes.Same locality and collector as holotype: 2♂, 28.xi.1957, 4♀, 9.xii.1957, 28.xi.1963, 14.ii.1964 and 19.ii.1964; same locality, blended light, R.J.B. Hoare: 2♂, 27.xi.1996 and 6.i.1997, slides 10161 and 12064 (anic); 6♂, Wellington, N.S.W., 28.x.1957, I.F.B. Common; 1♂, 1♀, 4 miles [6.5 km] SW of Gosford, N.S.W., 30, 31.iii.1965, I.F.B. Common, M.S. Upton; 1♂, 9 miles [14.5 km] NE of Windsor, N.S.W., 31.iii.1965, I.F.B. Common, M.S. Upton; 2♂, 220m, Mt Nelson, Hobart, Tasmania, m.v. light, 7.i.1980, 14.i.1981, P.B. McQuillan; 1♂, 42.56S 147.20E, Mt. Nelson, Tasmania, 330 m, 7.ii.2009, L. Kaila & J. Kullberg (fmnh), slides 11501–11503, 10165 (anic), EJvN 4106.
Male (Fig. 4). Wingspan 5.8–7.6 mm. Head capsule: labial palpi distinctly longer than galeae; maxillary palpi with ratio of segments from base approximately 0.3: 0.4: 0.6: 1.5: 1.0; interocular index 0.74. Frontal tuft orange; collar inconspicuous, white; eyecaps black, thinly scaled and almost transparent towards base; antennae blackish, 37–42 segments. Thorax and forewing blackish fuscous, weakly shining; cilia concolorous. Hindwing broadened at base, clothed in dark brown scales with iridescent reflections; an elongate androconial pocket in anterior ½ of wing, surrounded by shining granular blackish scales; cilia dark grey. Underside: forewing and hindwing dark fuscous; costa of hindwing with a series of blunt rectangular lamellate scales. Wing venation as in Fig. 31: base of R1 in forewing well separated from base of R2+3; trunk of Rs+M in hindwing not strongly deflected towards costa. Legs: fore-tibia somewhat thickened with blackish scales. Abdomen dark fuscous, with a moustache-like patch of hair-scales on T5 (Fig. 41).
Female (Fig. 5). Wingspan 7.5–8.0 mm. Similar to male, but head broader; antennae shorter, 30 segments; forewing somewhat broader; hindwing unmodified. Wing venation as in Fig. 32: base of R1 in forewing close to base of R2+3; trunk of Rs+M in hindwing strongly deflected towards costa. T5 of abdomen without hair-scales.
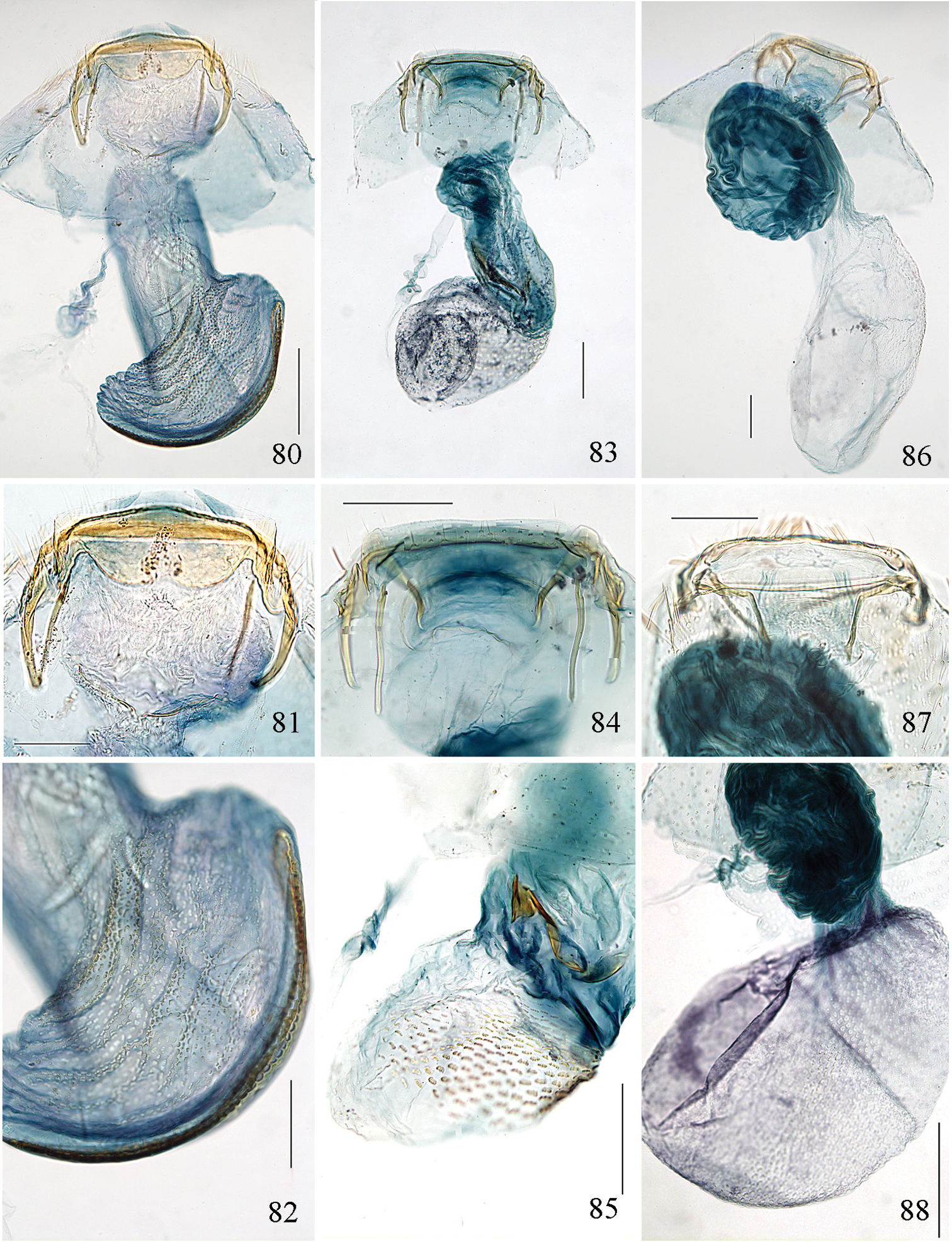
Male genitalia (Figs 39, 40, 42, 58, 59). Capsule ca. 480 μm long. Vinculum with anterior margin W-shaped; lateral arms and tegumen forming a triangle. Tegumen very narrow, caudally rounded. Uncus hood-like with well-sclerotized tip. Gnathos with enlarged basal plate, lateral arms slightly curved, central element short, triangular. Valva (Fig. 40) ca. 280–335 μm long, triangular and pointed; a spine-like process at base of medial edge; inner (dorsal) surface with numerous strong flattened setae in apical ½; exterior surface with a tuft of very robust, long setae extending beyond tip of valva (visible without dissection); sublateral processes well developed; pectinifer absent. Aedeagus (Figs 42, 59) ca. 550–575 μm including processes; 3 large blunt interconnected processes at apex, the left hand one curved; vesica with a large field of small cornuti, cathrema with 3 loosely interconnected elongate sclerites.
Female genitalia (Figs 73, 80–82). Total length ca. 935 μm. T9 not forming distinct anal papillae, 8–9 setae on each side. T8 with ca. 9–10 setae on each side. Apophyses anteriores slightly longer than posteriores (Fig. 81). Lateral sclerites of vestibulum absent. Corpus bursae with elongate posterior portion and oval anterior portion; anterior portion with strong transverse folds and numerous strong close-set pectinations. Signum (Fig. 82) an elongate weakly toothed band along anterior edge of corpus.
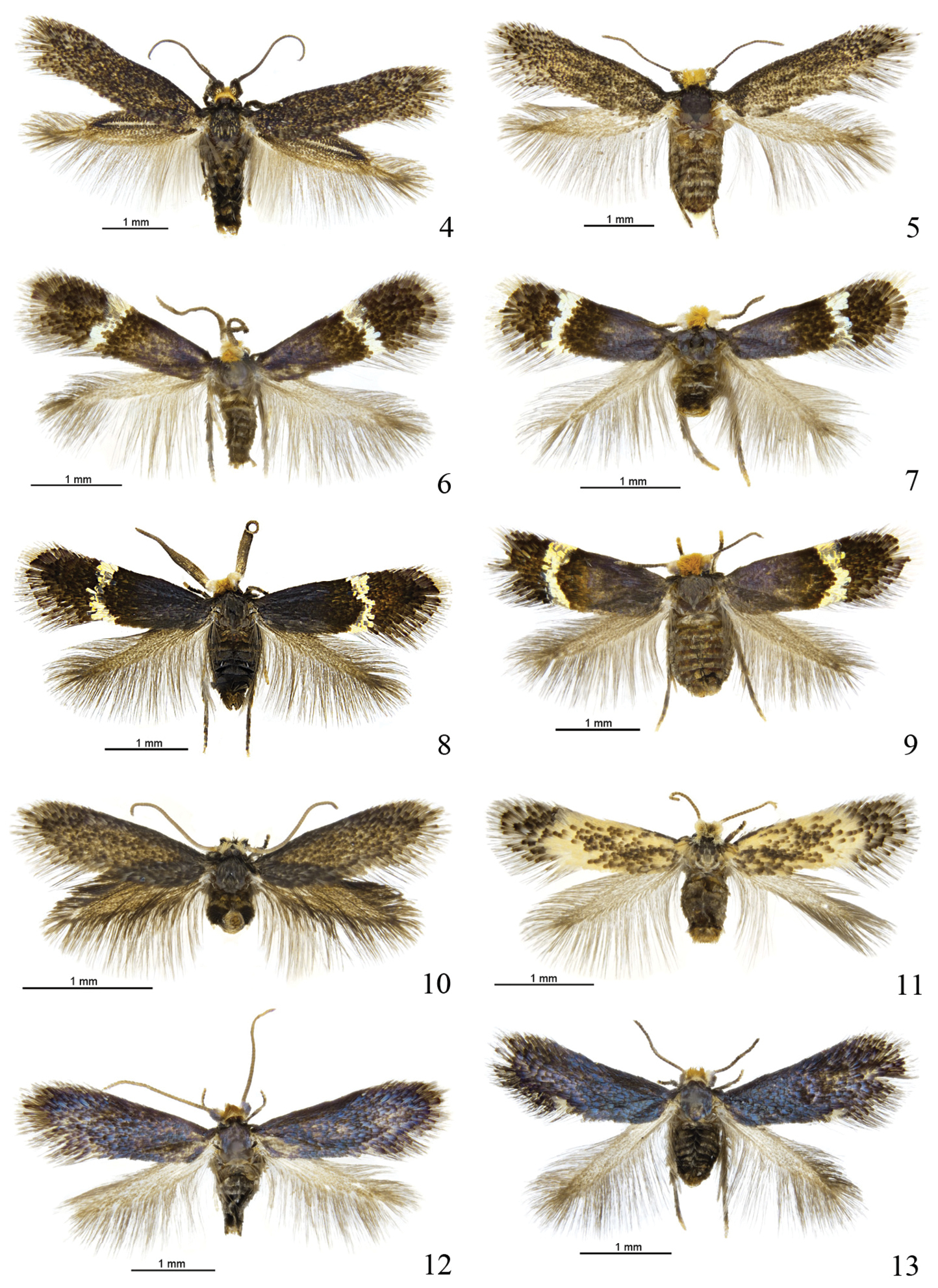
Pectinivalva spp., adults. 4 Pectinivalva (Pectinivalva) mystaconota, male paratype, Black Mt., A.C.T., 27.xi.1996 5 Pectinivalva (Pectinivalva) mystaconota, female paratype, 4 miles [6.5 km] SW of Gosford, N.S.W., 30.iii.1965 6 Pectinivalva (Casanovula) brevipalpa, male paratype, Fitzroy Falls, N.S.W., emg. 28.x.1996 7 Pectinivalva (Casanovula) brevipalpa female paratype, Fitzroy Falls, emg. 9.xii.1996 8 Pectinivalva (Casanovula) minotaurus male paratype, Leslie St., Toowoomba, Qld, emg. 8–9.ii.1996 9 Pectinivalva (Casanovula) minotaurus female paratype, Leslie St., emg. 19.ii.1996 10 Pectinivalva (Menurella) scotodes. male paratype, Leslie St, Toowoomba, Qld, emg. 8.x.1995 11 Pectinivalva (Menurella) scotodes female paratype, McAfee’s Lookout, Brisbane Forest Park, Qld, emg. 1.x.1995 12 Pectinivalva (Menurella) acmenae male paratype, Mt Dromedary, N.S.W., emg. 22.x.1995 13 Pectinivalva (Menurella) acmenae female paratype, Kioloa State Forest, N.S.W., emg. 11.x.1995.
Pectinivalva (Menurella) spp., adults. 14 Pectinivalva (Menurella) xenadelpha, female holotype, Indonesia, Kalimantan, Gunung Lumut, emg. 15.xii.2005 15 Pectinivalva (Menurella) quintiniae, male holotype, Tullawalal, Lamington N.P., Qld, emg. 25.ix.-6.x.2004 16 Pectinivalva (Menurella) quintiniae, female paratype, Tullalwalal, emg. 25.ix.–6.x.2004 17 Pectinivalva (Menurella) tribulatrix, male holotype, Cape Tribulation, Qld, emg. 8.ix.2004 18 Pectinivalva (Menurella) tribulatrix, female paratype, Cape Tribulation, emg. 8.ix.2004. Scales 1 mm.
Both sexes can be distinguished from similar members of the subgenus by the black eyecaps with their weakly scaled transparent bases. In addition, the combination of the pointed valvae with their long, strong setae (visible in undissected males) and the moustache-like patch of hair-scales on T5 of the abdomen is characteristic of the male. In the female genitalia, the absence of lateral sclerites in the vestibulum and the transversely rugose corpus bursaeare diagnostic.
Collected in scattered localities in eastern Australia from Wellington, N.S.W. south to Mt Nelson, Hobart, Tasmania; presumably widespread, but not yet known from Victoria.
RMNH.INS.24106, Genbank KC292479
The specific name is derived from the Greek mystax (a moustache) and notos (a back) and refers to the tuft of hair-scales on T5 in the male. It is an adjective.
Several species related to Pectinivalva (Pectinivalva) mystaconota are known: all lack a pectinifer and have more or less dense tufts of setae on the dorsal surface of the valva. The group seems to be best represented in Western Australia. As the larvae are unknown, and the only definite apomorphies for the subgenus Pectinivalva are characters of the larva, the assignment of this group to the subgenus remains to be confirmed. The undivided uncus and the form of the sclerites associated with the cathrema in the male genitalia, and the form of the signum in the female genitalia, are characteristic of the subgenus Pectinivalva, but these features may be plesiomorphic within the genus as a whole. However, in the most parsimonious trees resulting from the cladistic analysis presented above, Pectinivalva mystaconota was placed as sister species to Pectinivalva (Pectinivalva) 138 + Pectinivalva (Pectinivalva) 163. For these reasons, it is here placed in the subgenus Pectinivalva.
Pectinivalva (Casanovula) brevipalpa sp. n.
Adults. Head capsule (Figs 23–30): labial palpi either normal, 3-segmented, or with segments 2 and 3 reduced (Fig. 27), or 2-segmented (Fig. 26); interocular index 0.55–0.68. Antennae sometimes dilated and flattened at base. Head colour blackish or orange; eyecaps white, often blackish posteriorly. Collar consisting of piliform scales. Wingspan 3.8–6.0 mm. Forewing with dark blue or purplish lustre and (except in one species) transverse shining silver or pale gold fascia. Hindwing without androconial pocket. Underside of male forewing without androconia. Wing venation (Fig. 33): R2+3 in forewing absent. Upperside of abdomen in male sometimes with specialized lamellate scales; S2a strongly spinose or without spines. Legs: fore-tibia of male not thickened above with scales.
Pectinivalva spp, adult male heads, anterior view. 19 Pectinivalva (Pectinivalva) 138, head 20 Pectinivalva (Pectinivalva) 138 antennal base 21 Pectinivalva (Menurella) scotodes, head 22 Pectinivalva (Menurella) scotodes antennal base 23–26 Pectinivalva (Casanovula) brevipalpa: 23 head 24 antennal base 25 flagellomeres 1–7, showing sensillum vesiculocladum 26 labial palpus 27 Pectinivalva (Casanovula) minotaurus labial palpus.
Pectinivalva (Casanovula) minotaurus, adult male head, anterior view. 28 Head 29 whole antenna, excluding scape 30 portion of basal ½ of flagellum, showing sensillum vesiculocladum. All from slide ANIC11325. Scales 100 μm (28), 200 μm (29), 50 μm (30).
Pectinivalva spp., wing venation. 31 Pectinivalva (Pectinivalva) mystaconota, male 32 Pectinivalva (Pectinivalva) mystaconota, female 33 Pectinivalva (Casanovula) brevipalpa male 34 Pectinivalva (Menurella) scotodes female 35 Pectinivalva (Menurella) scotodes male 36 Pectinivalva (Menurella) acmenae female.
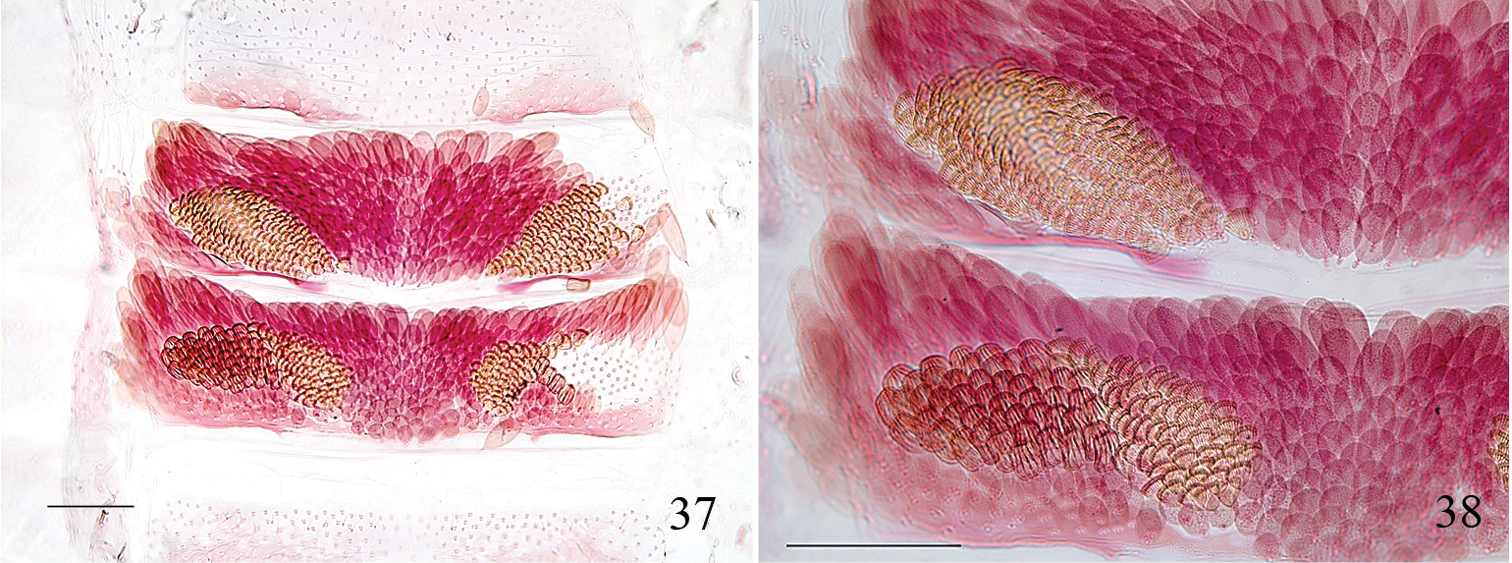
Pectinivalva (Casanovula) minotaurus, male androconia. 37 Abdominal tergites 4–5, partially descaled 38 close-up of androconia on one side of T4–5, showing the two distinct types. Slide ANIC11325, scales 100 μm.
Male genitalia(Figs 43–48, 60–63). Anterior extension of vinculum more or less H-shaped, with strong medial excavation. Tegumen simple. Uncus apically bifid, basally with 2 weakly sclerotized areas. Gnathos central element narrow and pointed. Valva (Figs 44, 47) relatively stout, apically rounded or squared off, pectinifer consisting of ca. 22–29more or less peg-like elements. Aedeagus (Figs 45, 48, 61, 63): vesica with numerous small cornuti; cathrema moderately weak, with or without associated sclerites.
Female genitalia (Figs 74, 75, 83–88). Lateral sclerites of vestibulum present, narrow. Accessory sac more or less developed. Corpus bursae with numerous pectinations, signum absent.
Larva. Head (Figs 105, 106): antennae 2-segmented; segment 2 with 1 pair of sensilla chaetica and 1 pair of sensilla basiconica; head-shape cordate or pyriform. Thorax: prothoracic sternite (Figs 110, 111) more or less narrow, subtriangular; chaetotaxy (Fig. 116): T2 with 10 or 11 pairs of setae (2 pairs of D setae (except in Pectinivalva (Casanovula) 226, which has 1 pair of D setae); L3 present or absent). Abdomen: as described for subfamily. Cuticle not textured, all segments except T1 and A10 with covering of fine spines.
Pectinivalva spp., male androconia and genitalia, ventral view. 39–42 Pectinivalva (Pectinivalva) mystaconota: 39 genital capsule 40 left valva 41 androconial scales on T5 42 aedeagus 43–45 Pectinivalva (Casanovula) brevipalpa: 43 genital capsule 44 left valva 45 aedeagus 46–48 Pectinivalva (Casanovula) minotaurus: 46 genital capsule 47 left valva 48 aedeagus.
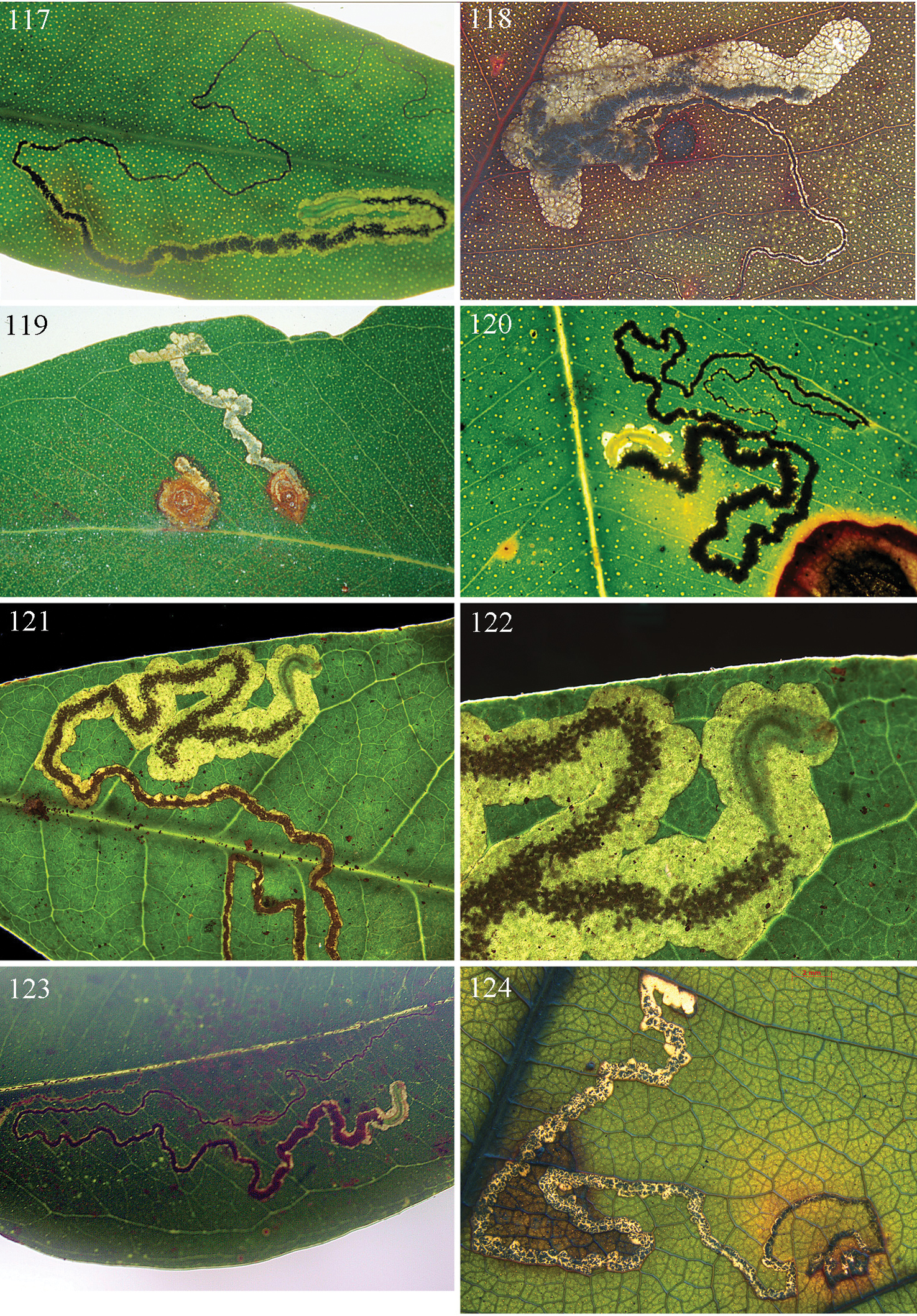
Host-plants: Lophostemon Peter G. Wilson spp., Tristaniopsis Peter G. Wilson spp., and Melaleuca L. spp. (including species formerly assigned to Callistemon R. Br.) (all Myrtaceae). Mine (Figs 117, 118): either a narrow gallery more or less filled with frass, or a gallery expanding into a blotch; exit-hole a small semicircular slit, a small semicircular hole, or a large slit: in the last case larva pupating in mine.
See Table 1.
Known only from eastern Australia: Queensland, N.S.W., A.C.T. and Tasmania; to be expected in Victoria.
The subgenus is named (in the diminutive) after the famous Italian adventurer and philanderer Giacomo Casanova, in reference to the unusual sexual ornamentation of the males of some species (e.g. Pectinivalva (Casanovula) minotaurus sp. n., in which the male has strongly dilated antennae and specialized scales on the abdomen upperside). It is considered feminine (in spite of its derivation) to accord with the gender of Pectinivalva.
No previously described species are referable to this subgenus. The following species are described below: Pectinivalva (Casanovula) brevipalpa sp. n. and Pectinivalva (Casanovula) minotaurus sp. n. Also at least five undescribed species in the anic, of which the following, cited by their anic rearing numbers, have been studied in detail for the current work: Pectinivalva (Casanovula) 219; Pectinivalva (Casanovula) 226.
This is the least speciose of the three subgenera of Pectinivalva. Two species-groups can conveniently be recognised. In the Pectinivalva (Casanovula) brevipalpa group (not monophyletic according to the cladistic analysis), the antenna of the male is dilated and flattened at the base, the vertex bears a pair of sclerotized crests, and the labial palpus is modified, with segments 2 and 3 reduced or fused. Abdominal sternite 2a is strongly spinose. The host-plants are Tristaniopsis and Lophostemon spp. and pupation is outside the mine. In addition to Pectinivalva brevipalpa and Pectinivalva minotaurus, a single undescribed species (Pectinivalva 41, feeding on Lophostemon confertus), is referable to this species group. In the Pectinivalva (Casanovula) 219 group, the labial palpi and the vertex are unmodified; S2a lacks spines; the host-plants belong to Melaleuca (including Callistemon), and pupation may be within the mine. About four species are known in this species group.
urn:lsid:zoobank.org:act:E17B15B1-A20D-4AE6-A4B6-C4F3E970640B
Holotype. ♂, 34.39S, 150.29E, Fitzroy Falls, N.S.W., emg. 25.x.1996, Tristaniopsis collina, R.J.B. Hoare. Genitalia slide 12111 (anic). Paratypes. 6♂, 5♀, same data as holotype, emg. 17.x.-9.xii.1996; 2♂, 4♀, 34.48S, 150.34E, Cambewarra Lookout, N.S.W., Tristaniopsis collina, emg. 15.x.-22.xii.1995, R.J.B. Hoare and E.S. Nielsen; 1♂, 1♀, 35.37S, 150.16E, 1 km SE of East Lynne, Kioloa State Forest, N.S.W., emg. 26, 28.x.1995, Tristaniopsis collina, R.J.B. Hoare; 1♀, Clyde Mt., N.S.W., [host unidentified], emg. 28.x.1963, I.F.B. Common. Slides 11237, 11238, 11328, 12065, 12136 (anic).
Male (Fig. 6). Wingspan 4.3–5.9 mm. Head capsule (Figs 23–26): labial palpi reduced, 2-segmented; maxillary palpi with ratio of segments from base approximately 0.2: 0.4: 0.5: 1.2: 1.0; interocular index 0.67; vertex with a pair of sclerotized crests. Frontal tuft ferruginous; collar white; eyecaps white; antennae with basal segments dilated and flattened, gradually tapering, shining lead-grey, whitish beneath, ca. 32 segments. Thorax and tegulae dark fuscous with purplish reflections. Forewing to 2/3 dark fuscous with purplish reflections; a shining silver to pale golden fascia at 2/3, slightly broader on costa, apex of wing dark fuscous without reflections; cilia pale grey beyond a line of fuscous-tipped scales. Hindwing grey, unmodified; cilia grey. Abdomen lead-grey, slightly shining.
Female (Fig. 7). Wingspan 4.3–5.2 mm. Similar to male, but antennae not dilated at base, 18 segments.
Male genitalia (Figs 43–45, 60, 61). Capsule ca. 250 μm. Anterior extension of vinculum with semicircular excavation. Uncus squarish, bilobed, with a tuft of 5–6 setae arising from dorsal side of each lobe near tip, centre of uncus with two weakly sclerotized ‘windows’. Gnathos with elongate central element and short lateral arms. Valva (Fig. 44) ca. 190 μm, squarish; pectinifer consisting of ca. 27 narrow elements. Transtilla absent. Aedeagus (Fig. 45, 61) 360 μm, a single rather broad, blunt spine at apex on left. Vesica with numerous close-set rather broad cornuti.
Female genitalia (Fig. 74, 83–85). Total length 520 μm. T9 with 7 setae on each side. Apophyses anteriores rather narrow with slightly incurved tips; apophyses posteriores slightly narrower and longer than anteriores. Lateral sclerotizations of vestibulum narrow, bent inwards, tips unmodified. Ductus spermathecae with 1 indistinct convolution. Posterior part of corpus bursae very convoluted; anterior part with many coarse pectinations; 2 or 3 indistinct elongate sclerotizations ½ way down corpus.
Larva. Green. Head (Fig. 105) elongate, pyriform; length of head ca. 410 μm; width ca. 295 μm. Thorax: prothoracic sternite as in Fig. 110. Chaetotaxy (Fig. 116) as described for subgenus; T2 with 10 pairs of setae (L3 absent); A10 with 4 pairs. Anal rods distinctly forked posteriorly.
Host plant: Tristaniopsis collina Peter G. Wilson & Waterhouse (Myrtaceae). Egg: on underside of leaf. Mine (Fig. 117): commences as very long narrow gallery either filled with greenish frass or with black linear frass, broadens rather abruptly into gallery with central line of black frass; exit-hole on upperside, a semicircular slit. Cocoon: reddish brown. Occupied mines have been collected on 25 June, 1 July, 13 July and 3 August.
The male is superficially similar to that of Pectinivalva (Casanovula) minotaurus, but differs in its much less strongly expanded antennae. The male of Pectinivalva (Casanovula) minotaurus also differs in having shell-like androconial scales on the upperside of the abdomen, visible on dissection, and a more distinctly H-shaped vinculum (Figs 46, 62). The female of Pectinivalva brevipalpa is also very similiar to that of minotaurus but can be distinguished on dissection by the presence of the indistinct sclerites ½ way down the corpus bursae.
New South Wales.
The specific name is derived from the Latin brevis (short) and palpus (the sensitive palm of the hand: hence, in zoology, a palp) and refers to the reduced, 2-segmented labial palpi of the adult male. It is an adjective.
urn:lsid:zoobank.org:act:72FAEDB0-F837-4C78-90E3-3ABB6F805085
Holotype. ♂, 27.36S 151.59E, Leslie St., Toowoomba, Qld, emg. 19.ii.1996, Lophostemon confertus, R.J.B. Hoare, I.F.B. Common. Paratypes. 2♂, 6♀, same data as holotype, emg. 2.-27.ii., 1.iii.1996, slide 11325 (anic); 11♂, 17♀, same locality, emg. 2.i.-2.ii.2001, Lophostemon confertus, R.J.B. Hoare, C. van den Berg, genitalia slides CvdB110, EvN 3539, 3547 (rmnh); 3♀, 27.33S, 151.59E, Prince Henry Heights, Toowoomba, Queensland, emg. 15, 18.ii.1986, Lophostemon confertus, I.F.B. Common, slide 10209 (anic); 1♂, 1♀, Brisbane, Queensland, emg. 30.xii.1957, Lophostemon suaveolens, I.F.B. Common, slides 11507, 11582 (anic); 1♂, Goodna, [Queensland], 8.iv.1906, [A.J. Turner], slide 11506 (anic).
Male (Fig. 8). Wingspan 4.7–5.5 mm. Head capsule (Figs 27–30): labial palpi 3-segmented; segment 2 reduced, maxillary palpi with ratio of segments from base approximately 0.3: 0.8: 0.6: 1.7: 1.0; interocular index 0.57; vertex with a pair of sclerotized crests. Frontal tuft ferruginous; collar ferruginous; eyecaps white, posteriorly leaden; antennae with flagellomeres in basal ½ greatly dilated and flattened, tapering beyond this, shining lead-grey, yellowish beneath, ca. 43–48 segments. Thorax and tegulae dark fuscous with purplish reflections. Forewing to ½ dark fuscous with bluish and purplish reflections; beyond this dark fuscous with bronzy reflections; a shining pale golden fascia at 2/3, apex of wing at base of cilia with purplish reflections; cilia grey beyond a line of fuscous-tipped scales, pale brownish around apex. Hindwing grey, unmodified; cilia grey. Abdomen with T2–3 shining brassy golden, remaining tergites shining dark leaden with green and violet reflections; T4 laterally with contiguous groups of androconial scales of two types: inner scales scallop-shaped, finely ridged; outer scales calyx-shaped, coarsely ribbed; T5 with similar area of androconia consisting entirely of scallop-shaped scales (Figs 37, 38), these showing as velvet black crescents on abdomen in situ.
Female (Fig. 9). Wingspan 4.7–5.8 mm. Similar to male, but antennae not dilated at base, ca 21–24 segments; abdomen entirely leaden with brassy reflections.
Male genitalia (Figs 46–48, 62, 63). Capsule ca. 360–375 μm. Anterior extension of vinculum reduced to curved lateral struts, i.e. vinculum anteriorly H-shaped. Uncus subtriangular, bilobed, with a compact tuft of setae arising from dorsal side of each lobe near tip. Gnathos with elongate central element and short lateral arms. Valva (Fig. 47) ca. 235 μm, squarish, caudal margin very straight; pectinifer consisting of ca. 29 narrow elements. Transtilla absent. Aedeagus (Figs 48, 63) 545 μm, with single broad, blunt apical process. Vesica with numerous close-set spine-like cornuti in several groups.
Female genitalia (Fig. 75, 86–88). Total length 800–880 μm. T9 with ca 9 setae on each side. Apophyses anteriores reduced to rounded stubs; apophyses posteriores narrow, much longer than anteriores. Lateral sclerotizations of vestibulum narrow, bent inwards, tips squared off. Ductus spermathecae with 1 ½ convolutions. Posterior part of corpus bursae very convoluted; anterior part with many coarse pectinations in right half; left half with a few fine pectinations only; no further sclerotizations in corpus.
Larva. Green. Head (Fig. 106) parallel-sided; length of head ca. 250 μm; width ca. 215 μm. Thorax: prothoracic sternite as in Fig. 111. Chaetotaxy as described for subgenus; T2 with 11 pairs of setae (L3 present), A10 probably with 3 pairs (but 1 pair possibly lost in slide examined). Anal rods distinctly forked posteriorly.
Host plants: Lophostemon confertus (R.Br.) Peter G.Wilson & J.T.Waterh.and Lophostemon suaveolens (Sol. ex Gaertn.) Peter G.Wilson & J.T.Waterh. (Myrtaceae). Egg: invariably on upperside of leaf. Mine (Fig. 118): commences as very long narrow gallery with black linear frass, leaving narrow clear margins, broadens rather abruptly into an irregular wide gallery or elongate blotch, sometimes with gallery parts, with central line of black frass or in the case of the blotch, frass concentrated on one or both sides; exit-hole on underside, an almost circular hole. Cocoon (Fig. 125): dark reddish brown. Occupied mines have been collected on 6 and 17 July and 15 August. A male pupa (pharate adult) is shown in Figs 126, 127.
Very similar externally to Pectinivalva (Casanovula) brevipalpa in both sexes; diagnostic characters are listed under that species.
Southern Queensland.
RMNH.INS.23539, Genbank KC292478 and RMNH.INS.23547, Genbank KC292477, both identical.
The species is named after the famous beast of Greek mythology, the Minotaur. The name (a noun in apposition) refers to the extraordinarily expanded and flattened male antennae, which are likened to the Minotaur’s horns.
The antennae of the male are the most strongly modified of any known species of Nepticulidae. Although many male-specific head structures in other insects are utilized in male-male competitive interactions over mates (e.g. the lateral cephalic projections of Phytalmia spp, Tephritidae (
Pectinivalva (Menurella) scotodes sp. n.
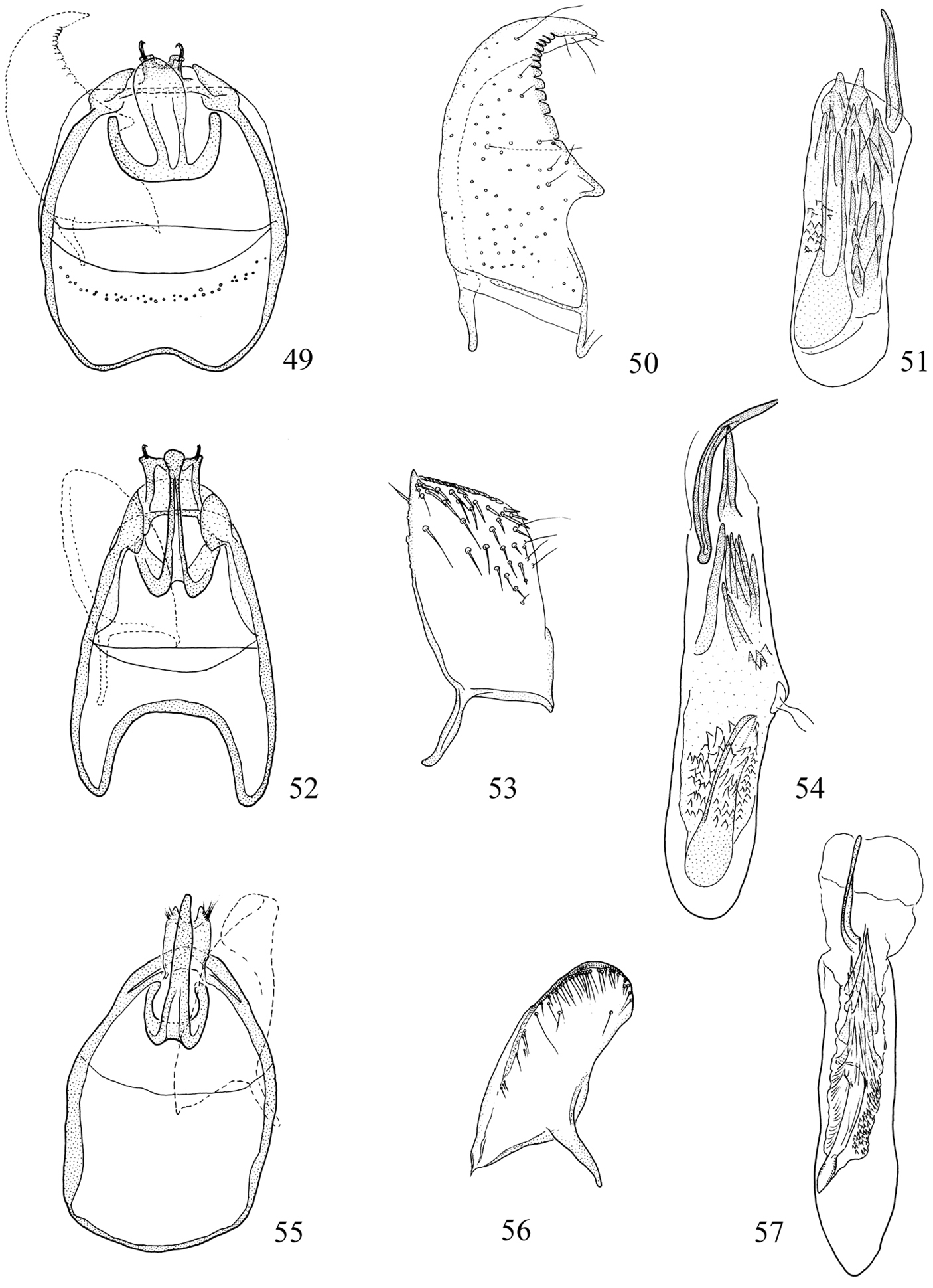
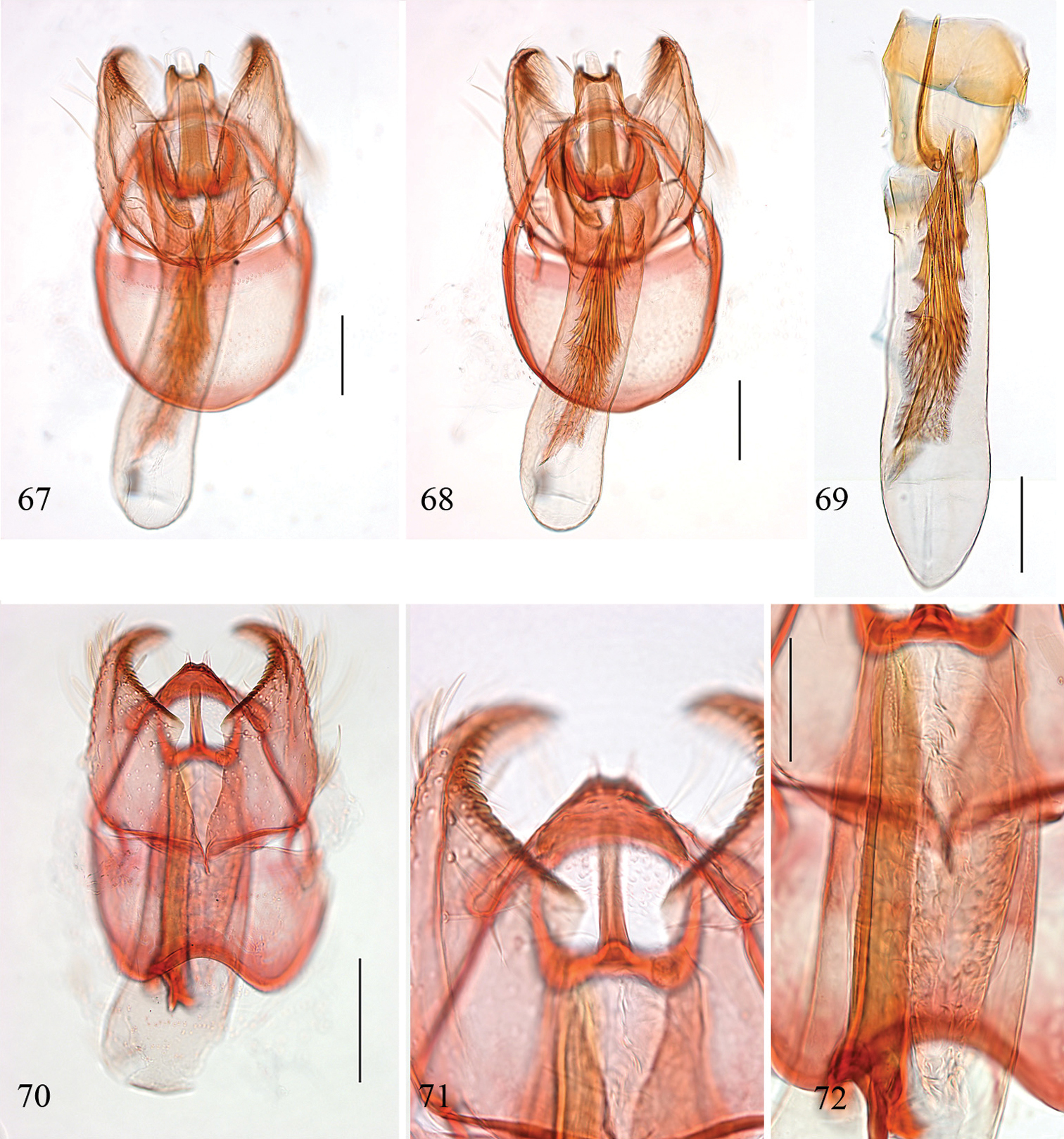
Adults. Head capsule (Figs 21, 22): labial palpi 3-segmented; interocular index 0.49–0.84. Antennae in male occasionally broadened in middle, or with pedicel and segment 1 of flagellum modified as in Fig. 22. Collar consisting of piliform scales (lamellate scales in one undescribed species). Wingspan3.2–7.0 mm. Thorax and forewing usually unicolorous greyish to fuscous, or with transverse pale fascia or opposite pale spots on costa and tornus at 2/3, occasionally yellowish with dark markings. Costa of forewing in male sometimes with a tuft of very narrow stiff scales towards base. Hindwing of male occasionally expanded at base; androconial pocket often present. Underside of forewing in male sometimes with androconia. Wing venation (Figs 34–36): R2+3 in forewing absent. Abdomen: S2a with or without spines. Legs: fore-tibia in male of those species with androconial pocket usually thickened above with blackish scales.
Male genitalia (Figs 49–57, 64–72). Vinculum with lateral arms often conspicuously forked apically; the lower branchsupporting the uncus and the upper branch the tegumen. Uncus apically bifid. Valva (Figs 50, 53, 56) variable in shape; pectinifer with ca. 9–34 (usually fewer than 20) peg-like, spine-like or broad tooth-like elements, or reduced to a thickening along caudal edge of valva. Transverse bar of transtilla absent (present in Pectinivalva (Menurella) 119). Aedeagus (Figs 51, 54, 57, 65, 66, 69, 72): cathrema associated with the apex of a long tubular sclerotization.
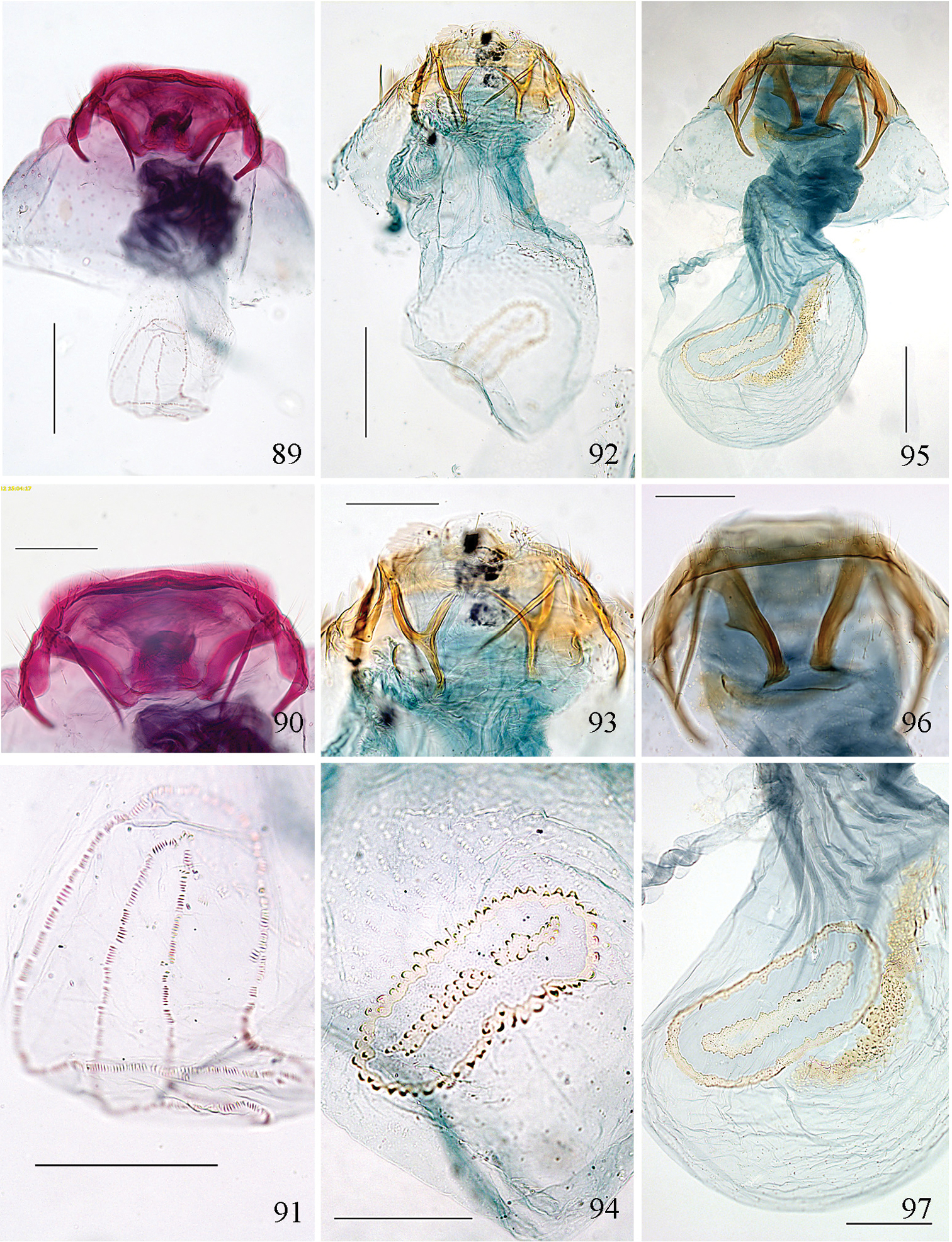
Pectinivalva (Menurella) spp., male genitalia, ventral view. 49–51 Pectinivalva (Menurella) scotodes: 49 genital capsule 50 left valva 51 aedeagus 52–54 Pectinivalva (Menurella) acmenae: 52 genital capsule 53 left valva 54 aedeagus 55–57 Pectinivalva (Menurella) quintiniae: 55 genital capsule 56 left valva 57 aedeagus.
Pectinivalva spp., male genitalia, ventral view. 58, 59 Pectinivalva (Pectinivalva) mystaconota, paratype, slide EJvN 4106: 58 genital capsule 59 aedeagus 60, 61 Pectinivalva (Casanovula) brevipalpa, holotype, slide ANIC12111: 60 genital capsule 61 aedeagus 62, 63 Pectinivalva (Casanovula) minotaurus, paratype, slide CvdB110: 62 genital capsule 63 aedeagus 64, 65 Pectinivalva (Menurella) scotodes, paratype, slide ANIC11262: 64 genital capsule 65 aedeagus 66 Pectinivalva (Menurella) acmenae, paratype, slide ANIC10213 66 genital capsule with aedeagus almost in situ. Scales 100 μm (59 same scale as 58).
Pectinivalva (Menurella) spp., male genitalia, ventral view. 67–69 Pectinivalva (Menurella) quintiniae, holotype, slide ANIC18720 and paratype, slide EJvN3736 (69): 67, 68 genitalia with aedeagus in situ 69 aedeagus 70–72 Pectinivalva (Menurella) tribulatrix, holotype, slide ANIC18721: 70 genitalia with aedeagus in situ 71 close-up of gnathos and pectinifer 72 close-up of aedeagus showing tubular sclerite associated with cathrema. Scales 100 μm, 50 μm (71, 72).
Female genitalia (Figs 76–79, 89–103). Lateral sclerites of vestibulum present: forked or thickened. Accessory sac absent. Corpus bursae with extent of pectinations more or less reduced; signum either a small weakly toothed band (Pectinivalva (Menurella) acmenae, Pectinivalva (Menurella) xenadelpha and Pectinivalva (Menurella) quintiniae) or 2 concentric ovals of fence-like marks.
Larva. Head (Figs 107, 108) always more or less cordate, never pyriform. Chaetotaxy: T2 with 10 or 11 pairs of setae (D1 usually present; L3 present or absent). Otherwise not distinguished from that of Pectinivalva (Casanovula).
Host-plants: Syzygium R.Br. ex Gaertn.species (formerly in Acmena), Leptospermum Forst. et f. spp., Angophora Cav. spp., Corymbia maculata (Hook.) K.D. Hill & L.A.S. Johnson, Rhodomyrtus macrocarpa Benth., Eucalyptus spp., probably other Myrtaceae, and with one species on Paracryphiaceae (Quintinia). Mine (Figs 119–124): usually a narrow gallery more or less filled with frass, occasionally a very short gallery leading to and enveloped by a blotch; exit-hole usually a small semicircular hole.
See Table 1.
Australia (known from all states and territories), Indonesia (Borneo: Kalimantan).
The subgeneric name is the diminutive of Menura, the genus to which the lyre-bird belongs. It stems from a fancied resemblance between the uncus in some species of the group and the tail of the male lyre-bird. It should be treated as feminine.
In addition to eleven described and new species, also approximately 70 undescribed species in the anic, of which the following, cited by their anic rearing numbers, have been studied in detail for the current work: Pectinivalva (Menurella) 2; Pectinivalva (Menurella) 91; Pectinivalva (Menurella) 119.
Menurella is the most diverse of the three subgenera of Pectinivalva, in terms of numbers of species, morphology and host-plant choice. Below we describe the type species Pectinivalva (Menurella) scotodes, three morphologically unusual rainforest species Pectinivalvaacmenae, Pectinivalva xenadelpha, Pectinivalva tribulatrix, and Pectinivalva quintiniae with unusual morphology and host-plant.
Pectinivalva (Menurella) is equivalent to the Pectinivalva funeralis group of
urn:lsid:zoobank.org:act:62D9A762-334E-4FDE-A191-0282BED3FC18
Holotype. ♂, 27.36S, 151.59E, Leslie St., Toowoomba, Queensland, emg. 8.x.1995, Eucalyptus pilularis, R.J.B. Hoare, I.F.B. Common. Paratypes. 6♂, 4♀, same data as holotype, emg. 3–10.x., 9.xi., 13.xii.1995, slides 11330, 12066 (anic); 5♂, 4♀, 27.36S, 151.59E, J.E. Duggan Park, Leslie St., Toowoomba, Queensland, 6.vii.2000, emg. 11.viii.-27.ix.2000, Eucalyptus pilularis, R.J.B. Hoare, C. van den Berg, bred in NL, slide EJvN3548 (rmnh); 4♂, 2♀, McAfee’s Lookout, Brisbane Forest Park, Queensland, emg. 1–10.x.1995, Eucalyptus carnea, R.J.B. Hoare, slides 11262, 11263 (anic).4♂, 3♀, Lisarow, N.S.W., emg. ix.1954, ix.-x.1955, x.1956, Eucalyptus acmenoides, K.M. Moore; 1♀, Mollymook, N.S.W., 21.xii.1996, emg. 28.i.1997, Eucalyptus pilularis, R.J.B. Hoare.
Male (Fig. 10). Wingspan 5.2–5.7 mm. Head capsule (Figs 21, 22): labial palpi distinctly shorter than galeae; maxillary palpi with ratio of segments from base approximately 0.4: 0.3: 0.4: 1.4: 1.0; interocular index 0.69; scape slightly expanded posteriorly into a setose ‘bump’; 1st 2 flagellar segments of antenna fused and narrower than remaining segments so that base of flagellum appears slightly invaginated posteriorly. Frontal tuft black, collar white; eyecaps white, black-bordered posteriorly beneath; antennae shining grey, ca. 42 segments. Thorax and forewing entirely blackish brown; a row of long blackish androconial scales projecting from dorsum; cilia shining dark brown, cilia-line indistinct. Hindwing rather broad, dark brown, with a small narrow androconial pocket basally; cilia shining blackish. Underside: forewing dark brown, costa black with a knob of black granular scales at base forming retinaculum; hindwing dark brown with blackish lamellate scales along basal ½ of costa. Wing venation as in Fig. 35. Legs: fore-tibia thickened above with blackish scales. Abdomen shining blackish.
Female (Fig. 11). Wingspan 5.0–5.2 mm. Head: frontal tuft brownish, collar white; eyecaps shining white, unmodified, antennae shining grey, ca. 24 segments, basal flagellar segments unmodified. Thorax and forewing paler than in male, yellowish overlain more or less extensively with brownish fuscous scales, leaving following markings yellow: a diffuse streak just beneath costa reaching ½ way along wing and diffuse opposite spots on costa and tornus at 2/3, cilia grey with moderately distinct cilia-line. Hindwing narrower than in male, grey; cilia grey. Underside: forewing shining dark brown; hindwing shining grey. Wing venation as in Fig. 34. Legs unmodified. Abdomen shining dark grey, paler beneath.
Male genitalia (Figs 49–51, 64, 65). Capsule ca. 370 μm long. Vinculum with slight anterior excavation; lateral arms inconspicuously forked apically, the caudal bifurcations from each side uniting to form straight bar along base of tegumen. Tegumen narrow, lateral corners produced anteriorly into distinct ‘shoulders’. Uncus small, boat-shaped. Gnathos central element long, spatulate. Valva (Fig. 50) ca. 245 μm long, reaching well beyond tegumen, strongly curved; medial edge smoothly excavated and ending in triangular projection; pectinifer consisting of 12 broad, blunt elements; dorsal surface towards apex with long setae. Juxta consisting of paired plate-like sclerites. Aedeagus (Fig. 51, 65) ca. 455 μm long; a spine-like process projecting from apex on right in ventral view; vesica with ca. 20 rather large cornuti, the 2 apical ones with very broad bases; sclerotized tube supporting cathrema very long, 2/3 length of aedeagus.
Female genitalia (Fig. 76, 89–91). Total length ca. 640 μm. T9 with ca. 10–11 setae on each side. Apophyses posteriores slightly longer than anteriores; apophyses anteriores curved inwards. Segment 7 produced laterally into 2 small evaginations either side of apophyses anteriores. Lateral sclerites of vestibulum broad, their apices associated with a pair of roughened irregular sclerotizations in centre of vestibulum. Ductus bursae strongly folded. Ductus spermathecae with ca. 3–4 poorly defined convolutions. Corpus bursae rounded; a field of concentrically arranged pectinations in posterior ½ on one side; signum a pair of concentric ovals of fence-like spines.
Larva. Appearing translucent whitish or yellowish in mine, becoming dull purplish white on vacating. Head as in Fig. 107; length of head ca. 345 μm; width ca. 270 μm. Thorax: prothoracic sternite (Fig. 112) narrow, I-shaped. Chaetotaxy and spinosity: T2 with 10 pairs of setae (L3 absent); otherwise as described for subgenus Casanovula. Anal rods slightly forked posteriorly.
Pectinivalva spp., female genitalia, ventral view. 73 Pectinivalva (Pectinivalva) mystaconota 74 Pectinivalva (Casanovula) brevipalpa 75 Pectinivalva (Casanovula) minotaurus 76 Pectinivalva (Menurella) scotodes.
Pectinivalva (Menurella) spp., female genitalia, ventral view. 77 Pectinivalva (Menurella) acmenae 78 Pectinivalva (Menurella) quintiniae 79 Pectinivalva (Menurella) quintiniae, apophyses and papillae anales.
Pectinivalva spp., female genitalia, ventral view. 80–82 Pectinivalva (Pectinivalva) mystaconota, paratype, slide ANIC10161 83–85 Pectinivalva (Casanovula) brevipalpa, paratypes, slides ANIC11328, ANIC11238 (85) 86–88 Pectinivalva (Casanovula) minotaurus, paratypes, slides ANIC11327 (86), ANIC10209. Scales 100 μm, 200 μm (80, 88).
Pectinivalva(Menurella) spp., female genitalia, ventral view. 89–91 Pectinivalva (Menurella) scotodes, paratype, slideANIC11263 92–94 Pectinivalva (Menurella) acmenae, paratype, slideANIC11242 95–97 Pectinivalva (Menurella) quintiniae, paratype, slide EJvN3961. Scales 200 μm (89, 92, 95), 100 μm.
Host plants: Eucalyptus pilularis Smith, Enteucha carnea R. Baker, Enteucha acmenoides Schauer and probably Enteucha saligna Smith (see below) (Myrtaceae). Egg: on upperside of leaf. Mine (Fig. 119): commences as a tight spiral around the egg, causing a raised red-brown spot on the leaf about 3–5 mm in diameter; later broadens into a more or less contorted linear gallery with black frass leaving narrow clear margins; exit-hole on leaf underside, a crescentic hole. Often several mines to a leaf. Cocoon: reddish brown. Occupied mines were collected on 17 and 20 July 1995, and 21 Dec 1996, and have also been recorded in January, March, April, June and August (Moore 1966).
The male of Pectinivalva (Menurella) scotodes resembles those of Pectinivalva (Menurella) funeralis (Meyrick), Pectinivalva (Menurella) libera (Meyrick) and Pectinivalva (Menurella) 119. It can be distinguished from all of these by its black head-tuft. The brown and yellow wing pattern of the female is distinctive amongst known species (although the females of Pectinivalva (Menurella) funeralis and Pectinivalva (Menurella) libera are unknown). The unusual larval mine of Pectinivalva (Menurella) scotodes appears to be diagnostic.
N.S.W, Southern Queensland.
RMNH.INS.23548, Genbank KC292483.
The specific name (an adjective) is derived from the Greek skotodes, meaning either ‘dark’ or ‘dizzy’. It refers both to the blackish coloration of the adult male moth, and to the habit of the young larva, which mines in tight circles.
The two female paratypes reared from mines on Eucalyptus carnea collected near Brisbane have a sparser scattering of brown scales on the yellow ground colour than the females reared from Enteucha pilularis. However, no other differences have been observed between specimens from these two host-plants, and the mines also appear to be identical.
This species was first collected by K.M. Moore, who described and illustrated the mine (Moore 1966: figs 15, 15A). There are specimens in the anic (here designated paratypes) reared by him in the 1950’s. The host-plant is indicated on the labels only by a rearing number; mines from his herbarium with the corresponding number are all in leaves of Eucalyptus acmenoides. He also recorded mines on Enteucha saligna, but no specimens reared from this host-plant have been located. Moore referred to this species as ‘Nepticula sp. 3’ and regarded it as related to Nepticula gilva Meyrick. He was probably misled by the wing-pattern of the female of Pectinivalva (Menurella) scotodes, which bears some resemblance to that of Pectinivalva (Pectinivalva) gilva: he would not have seen the type specimen of Pectinivalva (Pectinivalva) gilva in the BMNH. The two species are not closely related and belong to different subgenera of Pectinivalva.
urn:lsid:zoobank.org:act:6720E8F8-1B68-45B0-B6EE-A94F9D8F0ADA
Holotype. ♂, 35.37S, 150.16E, 1 km SE of East Lynne, Kioloa State Forest, N.S.W., Acmena smithii, emg. 12.x.1995, R.J.B. Hoare. Paratypes. 3♂, 3♀, same data as holotype, emg. 10–21.x.1995; 2♂, 36.19S, 150.03E, Mt Dromedary, N.S.W., emg. 22, 24.x.1995, R.J.B. Hoare, E.S. Nielsen and M.J. Matthews, genitalia slides 10213, 11242 (anic); 2♂, 28.42S, 153.37E, Broken Head NR, N.S.W., 13.vii.2000, emg. 15–18.viii.2000, Acmena smithii, R.J.B. Hoare, C. van den Berg, bred in NL, slide EJvN3541 (rmnh).
Male (Fig. 12). Wingspan 4.5–5.5 mm. Head: frontal tuft ferruginous; collar inconspicuous, consisting of white, grey-tipped scales; eyecaps anteriorly white, posteriorly shining grey with bluish reflections; antennae shining dark grey, whitish beneath, ca. 35 segments. Thorax, tegulae and forewing uniform shining dark grey with strong blue reflections; an inconspicuous tornal spot consisting of a few white scales; cilia dark grey. Hindwing unmodified, pale grey; cilia pale grey. Underside: forewing grey with faint brassy reflections; hindwing grey. Abdomen shining dark grey; anal tuft inconspicuous, dark grey.
Female (Fig. 13). Wingspan 5.2–5.6 mm. Similar to male, but antenna with 23–25 segments, and forewing rather broader. Wing venation as in Fig. 36. Abdominal tip not as broad and ‘square’ as in females of other Pectinivalva spp.
Male genitalia(Figs 52–54, 66). Capsule ca. 425 μm long, forming a narrow triangle. Anterior edge of vinculum excavated in a half-oblong. Tegumen rounded, with ventral extensions on each side overlapping lateral arms of gnathos. Uncus rectangular, bilobed, lobes slightly produced, with 3 setae on each. Gnathos central element long, reaching just beyond uncus, ending in small swelling. Valva (Fig. 53) ca. 210 μm long, squarish, more rounded caudally and produced into a short point at exterior corner of apex; apical ½ with numerous spine-like setae on dorsal surface; pectinifer consisting of ca. 18 spine-like elements. Long sublateral processes present. Juxta a weak subcircular plate. Aedeagus (Figs 54, 66) ca. 510 μm long, a curved spine arising towards apex on left, a shorter spine to right of this one, a third spine in line with second and anterior to it. Vesica basally with cathrema surrounded by a field of many broad, short cornuti; a separate field of ca. 9 long narrow cornuti above opening of ejaculatory duct.
Female genitalia(Fig. 77, 92–94). Total length ca. 760 μm. T9 prominent, with a group of 5–6 setae on each side. Apophyses anteriores rather narrow, curved inwards; apophyses posteriores narrow, straight, approximately equal in length to anteriores. Lateral sclerotizations of vestibulum strongly developed, forked, the bifurcations diverging widely, anterior pair blunt, posterior pair pointed. Ductus spermathecae with 4½ convolutions. Posterior part of corpus broad, folded, without markings; anterior part rounded, with rows of inconspicuous pectinations; signum consisting of broken linear sclerotization surrounded by oval sclerotized ring with blunt dentitions.
Larva. Green. Length of head ca. 440 μm; width ca. 350 μm. Thorax: prothoracic sternite in shape of Y with expanded base (Fig. 113); an additional small roundish sclerite on each side of this and antero-dorsal to SV and V group of setae. Chaetotaxy and spinosity: T2 with 11 pairs of setae (L3 present); otherwise as described for subgenus Casanovula. Anal rods distinctly forked posteriorly.
Host plant: Syzygium smithii (Poir.) Nied. (Myrtaceae) (formerly Acmena smithii), common lilly pilly. Egg: almost invariably on upperside, usually near leaf margin. Mine (Fig. 120): a long, very narrow contorted gallery, filled with brown frass apart from irregular crenulations along mine edge; exit-hole on underside, a semicircular hole. Cocoon: reddish brown. Occupied mines were collected on 30 July and 3 August.
This is the one of three known species of Pectinivalva in which the forewings have a bluish lustre but no transverse fascia. The others are Pectinivalva xenadelpha and Pectinivalva quintiniae, both described and diagnosed below. There is an undescribed Australian species of Stigmella which sometimes occurs together with Pectinivalva (Menurella) acmenae, and in which the forewings are similarly unicolorous dark blue; however, the Stigmella species is distinctly larger (wingspan 6–8 mm), and has a collar consisting of white lamellate scales; its larva is a leaf-miner on Baloghia inophylla (G. Forster) P. Green (Euphorbiaceae).
New South Wales. Vacated mines probably of this species were seen abundantly along the coast near Manley, Sydney.
RMNH.INS.23541, Genbank KC292474.
The specific name is derived from the former host-plant genus, and is a noun in the genitive. Because the moth is referred to under this manuscript name in the first author’s unpublished thesis, we have chosen to retain it for consistency, in spite of the change in classification of the host-plant.
The host-plant genus of Pectinivalva (Menurella) acmenae, Syzygium, is not closely related to other myrtaceous hosts from which Pectinivalva species have been reared in Australia, and belongs to the tribe Syzygieae (
urn:lsid:zoobank.org:act:3F7C0ED7-9A5A-4A4D-8D5D-FFB1DE384D85
Holotype. ♀, INDONESIA (Kalim. Timur), Pasir distr.: Gunung Lumut Prot. For., Gunung Lumut, ridge SW of summit, 18–20.xi.2005, 50M LD864441, 950 m, leafmines, undisturbed Acmena dominated low forest, on Acmena acuminatissima (Blume) Merr. & L.M. Perry, emg. 15.xii.2005, RMNH/EvN no 2005185–1, E.J. van Nieukerken, genitalia slide EvN 3738 (mzb).
Additional material: leafmines, larvae, same locality (rmnh).
Male. Unknown.
Female (Fig. 14, 129). Wingspan 4.0 mm. Head: frontal tuft pale ferruginous; collar inconspicuous, white; eyecaps anteriorly white, posteriorly shining grey with bluish reflections; antennae shining dark grey, 24 segments. Thorax, tegulae and forewing uniform shining dark grey with weak blue reflections; cilia dark grey. Hindwing pale grey; cilia pale grey. Underside: forewing grey with faint brassy reflections; hindwing grey. Abdomen shining dark grey, abdominal tip as in acmenae.
Female genitalia (Figs 98–100). Total length ca. 770 μm. T9 prominent, with a group of 5 setae on each side. Apophyses anteriores rather narrow, curved inwards; apophyses posteriores narrow, straight, longer than anteriores. Lateral sclerotizations of vestibulum strongly developed, but not forked. Ductus spermathecae with ca 6 close set convolutions. Posterior part of corpus normal, slightly folded, without markings; anterior part rounded, with rows of inconspicuous pectinations; signum consisting of broken linear sclerotization surrounded by oval sclerotized ring with blunt dentitions.
Pectinivalva (Menurella) spp., female genitalia, ventral view. 98–100 Pectinivalva (Menurella) xenadelpha, holotype, slide EJvN3738 101–103 Pectinivalva (Menurella) tribulatrix, paratype, slide EJvN3963. Scales 100 μm, 50 μm (99, 100, 102).
Larva. Not preserved.
Host-plant: Syzygium acuminatissimum (Blume) A. DC. (Myrtaceae) (formerly Acmena acuminatissima), very closely related to the Australian Syzygium smithii (
Very similar externally to Pectinivalva (Menurella) acmenae from Australia, but lacks the pale tornal spot of that species. In the genitalia, the lateral sclerites of the vestibulum are not forked (as they are in acmenae), and the apophyses posteriores are distinctly longer than the apophyses anteriores (same length in acmenae).
Borneo, East Kalimantan: Gunung Lumut.
The species name (a noun in apposition) derives from the Greek xenos (stranger, foreigner) and adelpha (sister) and refers to the close relationship to acmenae as well as the great geographical distance between this and other known Pectinivalva species.
RMNH.INS.23738 (holotype), Genbank KC292487 and RMNH.INS.11968 (larva), genbank KC292486, both identical.
We choose to describe this species here, even on the basis of a single female, to be able to record the genus from outside Australia. The detailed knowledge of its life history and three DNA markers (including CO1 barcode) will make future association with males straightforward.
urn:lsid:zoobank.org:act:0D0B16C5-745C-433A-9571-169793F72486
Holotype. ♂, Tullawalal, Lamington National Park, Qld, [UTM: 56J NP188794], la. 19.viii.2004, 900–940 m, [rainforest], emg. 25.ix.-6.x.2004, Quintinia verdonii, E.J. van Nieukerken, R.J.B. Hoare, RMNH/EvN no. 2004100, genitalia slide 18720 (anic) (= EJvN 3960). Paratypes. 2♂, 6♀, same data as holotype, genitalia slides ♂: EvN 3736, ♀: EvN 3961, 3993 (anic, rmnh); 2♀, 28.28S, 153.07E, Bar Mountain, Border Ranges Nat. Pk, N.S.W., emg. 21–23.viii.2000, Quintinia verdonii, C. van den Berg, R.J.B. Hoare (anic, rmnh).
Additional material: leafmines from same localities.
Male (Fig. 15). Wingspan 4.7–4.8 mm. Head: frontal tuft ferruginous; collar inconspicuous, consisting of shining pale grey scales; eyecaps basally white, exteriorly shining grey with violet reflections; antennae shining dark grey, whitish beneath, ca. 35–38 segments. Thorax, tegulae and forewing uniform shining fuscous with strong blue to violet reflections; cilia greyish fuscous. Hindwing unmodified, grey; cilia grey. Underside: forewing dark greyish fuscous; hindwing grey. Abdomen shining dark greyish fuscous; anal tuft inconspicuous, fuscous.
Female (Figs 16, 128). Wingspan 5.0–5.8 mm. Similar to male, but antenna with ca. 27 segments, and forewing rather broader.
Male genitalia (Figs 55–57, 67–69). Capsule ca. 425–465 μm long, ovoid. Anterior edge of vinculum rounded, without excavation. Tegumen rounded, without ventral extensions. Uncus rectangular, bilobed, lobes strongly produced, with ca. 6 setae on each. Gnathos central element long, reaching just beyond uncus, tapering apically. Valva (Fig. 56) ca. 305–320 μm long, rounded caudally; apically fringed with numerous spine-like setae on dorsal surface; pectinifer absent, but apex of valva thickened and well-sclerotized. Long sublateral processes present. Juxta a subrectangular plate. Aedeagus (Figs 57, 69) ca. 455–480 μm long, a curved spine arising towards apex on left. Vesica basally with many broad, short cornuti, grading into field of much larger longer cornuti towards apex.
Female genitalia (Fig. 78, 79, 95–97). Total length ca. 950 μm. T9 produced on each side into prominent anal papillae, each with a group of 5–6 setae. Apophyses anteriores moderately narrow, curved inwards; apophyses posteriores narrow, straight, distinctly shorter than anteriores. Lateral sclerotizations of vestibulum strongly developed, thick, not forked but with outer tooth-like process at ca. ½ length. Ductus spermathecae with 2½ convolutions. Posterior part of corpus broad, folded, without markings; anterior part rounded, with faint pectinations; signum consisting of broken linear sclerotization surrounded by oval sclerotized ring with blunt dentitions; an elongate band of scobination opposite signum.
Larva. Green. Head as in Fig. 108; length of head ca. 410 μm; width ca. 450 μm. Thorax: prothoracic sternite hourglass-shaped; no additional sclerites. Chaetotaxy and spinosity: T2 with 11 pairs of setae (L3 present); otherwise as described for subgenus Casanovula. Anal rods not forked posteriorly.
Pectinivalva spp., larval heads, dorsal view (head capsule to left, tentorium above right, mandible below right). 104 Pectinivalva (Pectinivalva) 138 105 Pectinivalva (Casanovula) brevipalpa 106 Pectinivalva (Casanovula) minotaurus 107 Pectinivalva (Menurella) scotodes 108 Pectinivalva (Menurella) quintiniae.
Host plant: Quintinia verdonii F. Muell. (Paracryphiaceae). Egg: on either side of leaf. Mine (Fig. 122): a long, meandering gallery, central line of blackish frass taking up most of mine width except near end where gallery broadens and frass takes up only ½ width; exit-hole on underside, a semicircular to oval hole. Cocoon: reddish brown. Occupied mines have been collected on 13 July and 19 August.
Pectinivalva spp., larval prothoracic sclerites. 109–113 Prosternites: 109 Pectinivalva (Pectinivalva) 138 110 Pectinivalva (Casanovula) brevipalpa 111 Pectinivalva (Casanovula) minotaurus 112 Pectinivalva (Menurella) scotodes 113 Pectinivalva (Enteucha) acmenae. 114 Pectinivalva (Pectinivalva) 5, dorsal sclerite, showing surrounding reticulate cuticle; rings indicate positions of setae D1 and XD1.
Pectinivalva spp., larval chaetotaxy. 115 Pectinivalva (Pectinivalva) 138 116 Pectinivalva (Casanovula) brevipalpa.
Pectinivalva spp., larval leaf-mines. 117 Pectinivalva (Casanovula) brevipalpa on Tristaniopsis collina 118 Pectinivalva (Casanovula) minotaurus vacated mine on Lophostemon confertus 119 Pectinivalva (Menurella) scotodes on Eucalyptus pilularis 120 Pectinivalva (Menurella) acmenae on Syzygium smithii 121, 122 Pectinivalva (Menurella) quintiniae on Quintinia verdonii 123 Pectinivalva (Menurella) xenadelpha on Syzygium acuminatissimum 124 Pectinivalva (Menurella) tribulatrix vacated mine on Rhodomyrtus macrocarpa.
Pectinivalva (Casanovula) and Pectinivalva (Menurella) spp., cocoon, pupa, live adults. 125 Pectinivalva (Casanovula) minotaurus cocoon 126 Pectinivalva (Casanovula) minotaurus male pupa (pharate adult), ventral view 127 Pectinivalva (Casanovula) minotaurus male pupa (pharate adult), dorsal view 128 Pectinivalva (Menurella) quintiniae live adult male 129 Pectinivalva (Menurella) xenadelpha, female holotype, live.
Superficially very similar to Pectinivalva (Menurella) acmenae, but lacking the pale tornal forewing spot of that species.
Northern N.S.W. (Border Ranges National Park); Southern Queensland, (Lamington National Park).
RMNH.INS.23736, Genbank KC292482, RMNH.INS.23960 (holotype), Genbank KC292481 and RMNH.INS.23961, Genbank KC292480, with one variable nucleotide.
The specific name (a noun in the genitive) is derived from the host-plant genus.
Currently this is the only species of Pectinivalva known from a host-plant that does not belong to Myrtaceae. Quintinia was formerly placed in Escalloniaceae, but is now assigned to the small family Paracryphiaceae (e.g.,
urn:lsid:zoobank.org:act:A3AF88EF-778D-4D82-B333-24DC81DEE3EA
Holotype. ♂, Cape Tribulation, Queensland, [UTM: 55K CC365219], la. 23.vii.2004, [coastal rainforest], emg. 8.ix.2004, Rhodomyrtus macrocarpa, E.J. van Nieukerken, RMNH/EvN no 2004017, genitalia slide 18721 (anic) (= EvN 3962). Paratype. ♀, same data as holotype, genitalia slide EvN 3963 (rmnh).
Additional material: many leafmines from type locality, and 2 km south.
Male (Fig. 17). Wingspan 3.5 mm, forewing length 1.5 mm. Head: frontal tuft yellow to ferruginous, collar white; eyecaps basally white, exteriorly grey; antennae grey, 25 segments. Thorax and forewing entirely shining grey fuscous, cilia-line indistinct. Hindwing basally wide, grey, with androconial pocket in basal half; cilia grey. Underside: forewing and hindwing dark brown. Abdomen grey brown, with small white anal tufts.
Female (Fig. 18). Wingspan 3.2 mm, forewing length 1.4 mm. Head: as male, but eyecaps shining white, no grey, antennae with 17 segments. Coloration as male, but hindwing narrower, grey. Abdomen shining dark grey, wide blunt abdominal tip.
Male genitalia (Figs 70–72). Capsule ca. 235 μm long, ovoid. Anterior edge of vinculum with shallow excavation. Tegumen rounded, without ventral extensions. Uncus triangular, slightly indented in middle, lobes with ca. 3–4 setae on each. Gnathos central element long, not reaching beyond uncus, parallel edges, rounded tip. Valva ca. 190 μm long, reaching well beyond tegumen, strongly curved; medial edge slightly excavated and ending in obtuse angle; pectinifer consisting of 15–16 broad, blunt elements; dorsal surface towards apex with long setae. Sublateral processes short. Juxta not visible. Aedeagus (Fig. 72) ca. 280 μm long; tubelike sclerite associated with cathrema ca 2/3 aedeagus length, anteriorly bilobed; vesica otherwise with a few small cornuti.
Female genitalia (Fig. 101–103). Total length ca. 335 μm. T9 produced on each side into prominent anal papillae, each with a group of 7 setae. Apophyses anteriores moderately narrow, curved inwards; apophyses posteriores narrow, straight, longer than anteriores. Lateral sclerotizations of vestibulum strongly developed, forked, the bifurcations diverging widely. Ductus spermathecae with 6 convolutions. Corpus small, about as long as wide, folded, covered with many pectinations; signum of concentric bands of fence-like spinules, indistinct.
Larva. Green. Fieldnotes state that it feeds with dorsum upwards, which may be incorrect. Larva not preserved.
Host-plant: Rhodomyrtus macrocarpa Benth., finger cherry (Myrtaceae). Many mines and three larvae were collected on the ca. 20 cm long leaves of seedling shrubs. Egg: on either side of leaf. Mine (Fig. 124): a narrow, long gallery, either completely meandering, or partly straight and following a major vein; frass black, broken and dispersed over total gallery width, not leaving clear margins; edges of gallery not straight, irregular; exit-hole on underside, a semicircular to oval hole. Cocoon reddish brown. Occupied mines have been collected on 22 July.
One of the smallest Pectinivalva species we know, recognised by unmarked greyish fuscous wings, grey edged scape in male and androconial pocket on male hindwing.
Northern Queensland, Cape Tribulation.
RMNH.INS.23962 (holotype), Genbank KC292484 and RMNH.INS.23963, Genbank KC292485, identical.
The species name is a noun in apposition, from the Latin tribulare, to press: hence tribulatio, distress, trouble, tribulatrix, one who causes trouble. It refers partly to the type locality (Cape Tribulation), and partly to difficulties the authors encountered in identifying the hostplant.
This species stands out from its relatives amongst the ‘derived’ species of Menurella (those with broad tooth-like pectinifer elements) in its hostplant Rhodomyrtus, which belongs to the tribe Myrteae; other members of this group feed on Eucalypteae.
The keys presented here are only intended for the identification of nepticulid specimens taken in Australia, and will not necessarily work for material captured elsewhere. They are based on the extensive collection of Nepticulidae in ANIC, with associated larval material. Although the keys should work for all Australian nepticulids so far known, it should be noted that our knowledge of the fauna is still very incomplete and there may possibly be species which will key out incorrectly or not at all.
| 1 | Forewing with vein 1+2A unthickened | 2 |
| – | Forewing with vein 1+2A thickened | 7 |
| 2 | Forewing with apical suffusion of pale bluish or silver scales | Roscidotoga |
| – | Forewing without apical suffusion of pale bluish or silver scales | 3 |
| 3 | Vein R2+3 of forewing present | Pectinivalva (Pectinivalva) |
| – | Vein R2+3 of forewing absent | 4 |
| 4 | Forewing without bluish or purplish lustre | Pectinivalva (Menurella) (part) |
| – | Forewing with bluish or purplish lustre | 5 |
| 5 | Forewing with transverse fascia | Pectinivalva (Casanovula) (part) |
| – | Forewing without transverse fascia | 6 |
| 6 | Forewing lustre bluish | Pectinivalva (Menurella)1 |
| – | Forewing lustre purplish | Pectinivalva (Casanovula)2 |
| 7 | Collar consisting of lamellate scales | 8 |
| – | Collar consisting of piliform scales | 9 |
| 8 | Vein Cu of forewing absent; closed cell present (vestigial) | Acalyptris |
| – | Vein Cu of forewing present; closed cell absent | Stigmella |
| 9 | Vein M of hindwing 2-branched; male hindwing underside with raised androconia | Trifurcula (Glaucolepis) |
| – | Vein M of hindwing 1-branched; male hindwing underside without raised scales | Ectoedemia (Fomoria) |
1 Two species of Pectinivalva (Menurella) key out here: Pectinivalva (Menurella) acmenae and Pectinivalva (Menurella) quintiniae.
2 One undescribed species of Pectinivalva (Casanovula) lacks a fascia and keys out here.
| 1 | Gnathos present, not reduced | 2 |
| – | Gnathos strongly reduced or absent | Roscidotoga |
| 2 | Gnathos with 1 central element | 3 |
| – | Gnathos with 2 central elements | Stigmella (part) |
| 3 | Uncus dorsally with 2 well-defined tufts of setae | 4 |
| – | Uncus without well-defined tufts of setae | 6 |
| 4 | Uncus pointed, undivided | Pectinivalva (Pectinivalva) |
| – | Uncus bifid | 5 |
| 5 | Cathrema associated with a smooth tubular sclerotization | Pectinivalva (Menurella) |
| – | Cathrema without associated sclerites, or sclerites not forming a smooth tube | Pectinivalva (Casanovula) |
| 6 | Transverse bar of transtilla membranous or absent | 7 |
| – | Transverse bar of transtilla present | 8 |
| 7 | Valvae widely separated at base; aedeagus with carinate processes | Acalyptris |
| – | Valvae close basally; aedeagus lacking carinae | Trifurcula (Glaucolepis) |
| 8 | Valvae widely separated at base; vinculum with posterior membranous extension; aedeagus with carinae | Ectoedemia (Fomoria) |
| – | Valvae close basally; vinculum unmodified; aedeagus lacking carinae | Stigmella3 |
3 One undescribed Stigmella species from South Australia, which has a gnathos with a single central element, keys out here.
| 1 | Signum present | 2 |
| – | Signum absent | 7 |
| 2 | One signum, not reticulate | 3 |
| – | Two reticulate signa | 5 |
| 3 | Signum a pair of concentric ovals of fence-like pectinations | Pectinivalva (Menurella) (part) |
| – | Signum a toothed band | 4 |
| 4 | Lateral sclerites of vestibulum forked or with exterior tooth at ½; signum small, rounded | Pectinivalva (Menurella)4 |
| – | Lateral sclerites (if present) not forked, no exterior tooth at ½; signum long, narrow | Pectinivalva (Pectinivalva) |
| 5 | Margins of signa crenulate | Acalyptris |
| – | Margins of signa smooth | 6 |
| 6 | Vestibulum with complex sclerotizations | Ectoedemia (Fomoria) |
| – | Vestibulum without sclerotizations | Trifurcula (Glaucolepis)5 |
| 7 | Apophyses anteriores expanded basally; corpus bursae weakly sclerotized, with diverticulum | Roscidotoga |
| – | Apophyses normal; corpus bursae well or moderately sclerotized; no diverticulum | 8 |
| 8 | Vestibulum with lateral sclerites | Pectinivalva (Casanovula) |
| – | Vestibulum without lateral sclerites | Stigmella |
4 Two species of Pectinivalva (Menurella) key out here: Pectinivalva (Menurella) acmenae and Pectinivalva (Menurella) quintiniae.
5 No females of Trifurcula have yet been captured in Australia, and the distinction used here is based on Holarctic members of the genus (see
| 1 | Antenna 3-segmented | 2 |
| – | Antenna 2- or 1-segmented | 3 |
| 2 | Cuticle spinose, with smooth texture; mesothorax with 2 D setae | Roscidotoga |
| – | Cuticle without spines, sculptured; mesothorax with 1 D seta | Pectinivalva (Pectinivalva) |
| 3 | Antenna 2-segmented | 4 |
| – | Antenna 1-segmented | 5 |
| 4 | Hostplant Lophostemon, Tristaniopsis, or Melaleuca | Pectinivalva (Casanovula)6 |
| – | Hostplant another myrtaceous genus, or Quintinia | Pectinivalva (Menurella)6 |
| 5 | Sensilla of antenna arranged in a cross | Stigmella |
| – | Sensilla not arranged in a cross | 6 |
| 6 | Mesothorax with 1 D seta | Ectoedemia (Fomoria) |
| – | Mesothorax with 2 D setae | 7 |
| 7 | Labial palpus 2-segmented | Acalyptris |
| – | Labial palpus 3-segmented | Trifurcula (Glaucolepis)7 |
6 No constant morphological differences have been found between larvae of Pectinivalva (Casanovula) and those of Pectinivalva (Menurella).
7 No larva of Trifurcula has been found in Australia, and the distinction used here is based on the description of European species in
This paper was begun as part of RJBH’s PhD studies at the Australian National University (Division of Botany and Zoology) and CSIRO Entomology, Canberra, Australia in 1995–1998. He would especially like to acknowledge his supervisors Penny Gullan and the late Ebbe Nielsen for their help and enthusiastic encouragement during this time. Much practical help during the period of the PhD, and subsequently with loans and other advice was provided by Marianne Horak, Ted Edwards, Vanna Rangsi and You-Ning Su of ANIC (CSIRO Entomology). For identification of host-plants of Nepticulidae, especially the extremely difficult Eucalyptus spp., RJBH is very grateful to Mike Crisp, Tom Hartley, Ian Telford, Tony Bean and Ian Brooker. For permits for fieldwork, he acknowledges the National Parks Services of New South Wales, Victoria and Tasmania, and the Forestry Commissions of New South Wales and Queensland. Other colleagues and friends in Australia assisted with fieldwork, photography, hospitality and advice of diverse kinds, and especial thanks are due to Marcus Matthews, Hanna Reichel, the late Ian Common, Klaus Sattler (visiting ANIC from BMNH), Keith Herbert, Anne Hastings, Pete Cranston, Laurence Mound, Kim Pullen, Matt Colloff, and Chris Reid. Many thanks to Kees van den Berg, who accompanied RJBH on a highly enjoyable and productive field-trip to Queensland and northern New South Wales in July 2000, and subsequently reared the valuable material collected. Work on the manuscript was revived in 2009 under the auspices of a Naturalis Temminck Fellowship, and RJBH gratefully acknowledges the funding provided under this programme for his stay in Leiden that year to collaborate with EJvN. For highly skilled photography of Pectinivalva adults of species described by RJBH, he is indebted to Birgit Rhode, and to the late Rosa Henderson for help with scanning and editing of drawings. Leonie Clunie kindly assisted with entering data for the online Appendix 3 of material examined. The completion of this research was supported by Core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment’s Science and Innovation Group.
We are grateful to EJvN´s colleagues Dick Groenenberg, Camiel Doorenweerd for carrying out molecular analyses and to BOLD staff. Queensland Parks is acknowledged for permission for fieldwork in 2004, and Tropenbos Indonesia (Balikpapan) and participants in the 2005 Biodiversity Assessment expedition to Gunung Lumut, Kalimantan, Indonesia are acknowledged for the opportunity to study leafminers and for their pleasant company.
Wuu Kuang Soh (Dublin, Ireland) kindly identified Syzygium acuminatissimum and W. (Bill) J.F. McDonald (Brisbane, Australia) is acknowledged for the identification of various Queensland plants on the basis of photographs of vouchers that we sent him. We thank Lauri Kaila (Helsinki) for the loan of the rich material that he, Jaakko Kullberg and Marko Mutanen collected during their trips in Australia.
All characters were treated as unordered. Figure numbers are given only as exemplars of character states and not all illustrations of each state are necessarily indicated.
1 Shape of male antenna: (0) flagellum of even width throughout; (1) basal flagellar segments expanded and flattened (Figs 24, 25, 29, 30).
2 Sexual colour dimorphism: (0) not pronounced; (1) pronounced (Figs 10, 11).
(3) Forewing pattern of male: (0) more or less unicolorous (Fig. 4); (1) dark with a pale costal streak (Hoare et al. 1997: fig. 1); (2) dark with a pair of opposite pale spots or a pale fascia; (3) metallic with a shining fascia (Fig. 6, 8); (4) metallic with silver spots (Hoare 2000a: figs 1–3).
(4) Androconial scales of male forewing: (0) absent; (1) present as a long dorsal fringe (Hoare et al. 1997: fig. 20).
(5) Forewing venation: (0) R2+3 present (Figs 31, 32); (1) R2+3 absent (Figs 33–36).
(6) Shape of male hindwing: (0) more or less lanceolate (Fig. 33); (1) strongly expanded at base (Figs 31, 35).
(7) Androconial pocket of male hindwing: (0) absent; (1) present (Figs 31, 35).
(8) Male genitalia — anterior extension of vinculum: (0) rounded or smoothly concave (Fig. 39); (1) H-shaped (Fig. 46).
(9) Male genitalia — uncus: (0) undivided (Fig. 39); (1) bifid (Figs 43, 46).
Notiopostega atrata has a weakly developed uncus which is very slightly indented apically (Davis 1989: fig. 274): this species has been scored ‘?’ for this character.
(10) Male genitalia — uncus: (0) without well-defined tufts of setae; (1) a pair of well-defined tufts present (Figs 39, 43); (2) tufts present, arising from lobes on dorsal surface of uncus (Hoare et al. 1997: fig. 39).
(11) Male genitalia — central element of gnathos: (0) narrow (at least basally) or triangular (Fig. 39, 43, 46); (1) broad and cordate (Hoare et al. 1997: fig. 38).
The gnathos in Notiopostega atrata is reduced and takes the form of a narrow band. Roscidotoga callicomae lacks a gnathos. Both species are scored ‘?’ for this character.
(12) Male genitalia — number of elements on pectinifer of valva: (0) 20 or more (Fig. 44, 47); (1) fewer than 20 (Fig. 50); (2) pectinifer strongly reduced or absent (Figs 40, 56).
(13) Male genitalia — shape of pectinifer elements: (0) peg- or spine-like (Figs 44, 53); (1) broad and tooth-like (Fig. 50).
(14) Male genitalia — sublateral processes of transtilla: (0) well-developed (Figs 40, 44); (1) strongly reduced (Hoare et al. 1997: fig. 38).
(15) Male genitalia — cathrema of aedeagus: (0) weakly developed, supported by 2 or 3 interconnected sclerites (Fig. 42) (1) moderately developed, supported by a smooth sclerotized tube (Fig. 51, 72); (2) moderately developed, with 1 short sclerite, or without associated sclerotizations (Fig. 48); (3) absent.
The structure of the cathrema and its associated sclerites in Pectinivalva is often obscured by cornuti and hard to observe in conventional slide preparations, and the character coding adopted here is somewhat provisional in nature. Further detailed studies, perhaps of everted vesicas, are desirable.
(16) Female genitalia — lateral sclerites of vestibulum: (0) absent (Fig. 73); (1) present, narrow (Fig. 74); (2) present, strongly developed (Fig. 78).
In Pectinivalva acmenae, the lateral sclerites are relatively narrow but have very strongly forked tips (Fig. 77): this species has been tentatively scored (2) for this character.
(17) Female genitalia — pectinations on corpus bursae: (0) extensive (Fig. 74); (1) restricted to a field on the posterior part of the corpus (Fig. 76); (2) very reduced or absent (Fig. 78).
(18) Female genitalia — form of signum: (0) well-sclerotized, sparsely toothed (Fig. 77); (1) well-sclerotized, continuously toothed (Hoare et al. 1997: fig. 73); (2) sclerotization reduced to parallel spinules (Fig. 76); (3) absent (Fig. 75).
(19) Female genitalia — shape of signum: (0) narrow, band-like (Fig. 73); (1) broad, oval (Fig. 76).
(20) Anterior edge of tergite 2 of abdomen: (0) continuously well sclerotized; (1) weakly sclerotized medially (Hoare 2000a: fig. 9).
(21) Larval antenna: (0) 3-segmented (Hoare 2000a: fig. 36); (1) 2-segmented; (2) 1-segmented.
(22) Shape of larval head: (0) anteriorly unmodified, more or less cordate (Fig. 106–108); (1) anteriorly elongate, more or less pyriform (Figs 104, 105).
(23) Posterior lobes of larval head: (0) continuously sclerotized (Hoare 2000a: fig. 29); (1) with sclerotization interrupted (Figs 104–108).
(24) Shape of prothoracic sternite of larva: (0) approximately as long as broad (Fig. 109); (1) much longer than broad (Figs 112, 113).
The larva of Notiopostega atrata lacks a prothoracic sternite, and this species has therefore been scored ‘?’ for this character.
(25) Number of mesothoracic D setae of larva: (0) 2 pairs (Fig. 116); (1) 1 pair (Fig. 115).
(26) Spinosity of larval cuticle: (0) cuticle spiny; (1) cuticle lacking spines.
(27) Texture of larval cuticle: (0) smooth; (1) sculptured (Fig. 114).
(28) Exit-hole of larval mine: (0) a small slit or hole (Fig. 118), larva leaves mine to pupate; (1) a large slit, larva pupating in mine.
| 0000 00000 11111 1111 12 22222 222 | |
| 1234 56789 01234 5678 90 12345 678 | |
| Notiopostega | 0000 1000? 0?001 3023 ?0 100?0 000 |
| Enteucha acetosae | 0030 10000 002?0 3023 ?0 20000 000 |
| Roscidotoga callicomae | 0040 10000 0?2?0 2023 ?0 00000 000 |
| Pectinivalva acmenae | 0000 10001 10100 1210 11 10110 000 |
| Pectinivalva brevipalpa | 1030 10001 10000 0103 ?1 11110 000 |
| Pectinivalva caenodora | 0010 00000 21001 0101 01 ????? ??? |
| Pectinivalva commoni | 0000 00000 10000 0100 01 01101 110 |
| Pectinivalva minotaurus | 1030 10011 10000 2103 ?1 10110 000 |
| Pectinivalva mystaconota | 0000 01100 102?0 0000 01 ????? ??? |
| Pectinivalva scotodes | 0101 11101 10110 1212 11 10110 000 |
| Pectinivalva quintiniae | 0000 10001 102?0 1220 11 20110 000 |
| Pectinivalva 2 | 0000 11101 10100 1212 11 10110 000 |
| Pectinivalva 5 | 0000 00000 10000 0100 01 01101 110 |
| Pectinivalva 34 | 0010 00000 21001 0101 01 01101 110 |
| Pectinivalva 91 | 0020 10101 10110 1212 11 10111 000 |
| Pectinivalva 119 | 0101 11101 10110 1212 11 10010 000 |
| Pectinivalva 138 | 0000 01100 10000 0020 01 01101 110 |
| Pectinivalva 163 | 0000 01100 10000 0020 01 01101 110 |
| Pectinivalva 219 | 0030 10011 10000 2103 ?1 ????? ??1 |
| Pectinivalva 226 | 0030 10011 10000 2103 ?1 10111 001 |
Myrtaceae
Lophostemoneae
Lophostemon ?confertus (R.Br.) Peter G.Wilson & J.T.Waterh.
Pectinivalva (Casanovula) 113
Lophostemon confertus (R.Br.) Peter G.Wilson & J.T.Waterh.
Pectinivalva (Casanovula) minotaurus, Pectinivalva (Casanovula) 41
Lophostemon suaveolens (Sol. ex Gaertn.) Peter G.Wilson & J.T.Waterh.
Pectinivalva (Casanovula) minotaurus
Melaleuceae
Melaleuca citrina (Curtis) Dum.Cours (= Callistemon citrinus)
Pectinivalva (Casanovula) 219
Melaleuca salicina Craven (= Callistemon salignum)
Pectinivalva (Casanovula) A98
Melaleuca squarrosa Donn ex Sm.
Pectinivalva (Casanovula) 226
Melaleuca viminalis (Sol. ex Gaertn.) Byrnes (= Callistemon viminalis)
Pectinivalva (Casanovula) sp EvN2004116
Melaleuca sp. (= Callistemon sp.)
Pectinivalva (Casanovula) 174
Syncarpieae
Syncarpia glomulifera (Sm.) Nied.
Pectinivalva sp. (RH198) (vacated mines only)
Eucalypteae
Angophora costata (Gaertn.) Britten
Pectinivalva (Menurella) Q1, Pectinivalva (Menurella) 119
Angophora floribunda (Sm.) Sweet
Pectinivalva (Menurella) Q2, Pectinivalva (Menurella) Angophora 1 (CvdB_Loc IV.21.sp1), Pectinivalva (Menurella) Angophora 2 (CvdB_Loc IV.21.sp2)
Corymbia ficifolia (F.Muell.) K.D.Hill & L.A.S.Johnson
Pectinivalva (Menurella) 127
Corymbia maculata (Hook.) K.D. Hill & L.A.S. Johnson
Pectinivalva (Menurella) 29
Corymbia torelliana (F.Muell.) K.D.Hill & L.A.S.Johnson (=Eucalyptus torelliana)
Pectinivalva sp. (RH114) (vacated mines only)
Eucalyptus acmenoides Schauer
Pectinivalva sp. (RH158) (vacated mines only)
Eucalyptus blakelyi Maiden
Pectinivalva (Menurella) scotodes
Eucalyptus ?blakelyi Maiden
Pectinivalva (Pectinivalva) 78
Eucalyptus bridgesiana R.T.Baker
Pectinivalva (Menurella) 91, Pectinivalva (Menurella) 92, Pectinivalva (Menurella) 123, Pectinivalva (Pectinivalva) 138, Pectinivalva (Pectinivalva) 148a, Pectinivalva (Pectinivalva) 163
Eucalyptus carnea R.T. Baker
Pectinivalva (Menurella) scotodes, Pectinivalva (Pectinivalva) 225
Eucalyptus cyanophylla Brooker
Pectinivalva (Menurella) 76
Eucalyptus cypellocarpa L.A.S. Johnson
Pectinivalva (Pectinivalva) 89, Pectinivalva (Pectinivalva) 90
Eucalyptus ?delegatensis R.T. Baker
Pectinivalva (Pectinivalva) commoni
Eucalyptus dives Schauer
Pectinivalva (Menurella) 91
Eucalyptus elata Dehnh.
Pectinivalva (Pectinivalva) 100
Eucalyptus ?globoidea Blakely
Pectinivalva (Pectinivalva) 153
Eucalyptus globulus Labill.
Pectinivalva (Menurella) Q3, Pectinivalva (Menurella) 91, Pectinivalva (Pectinivalva) A87, Pectinivalva (Pectinivalva) 148a
Eucalyptus goniocalyx F.Muell. ex Miq.
Pectinivalva (Pectinivalva) 148b
Eucalyptus grandis W. Hill
Pectinivalva (Pectinivalva) 148a
Eucalyptus leptophylla F.Muell. ex Miq.
Pectinivalva (Menurella) 91
Eucalyptus macarthurii H.Deane & Maiden
Pectinivalva (Pectinivalva) A87, Pectinivalva (Menurella) 150
Eucalyptus mannifera Mudie
Pectinivalva (Pectinivalva) 78, Pectinivalva (Menurella) 151
Eucalyptus melliodora A.Cunn. ex Schauer
Pectinivalva (Menurella) 23, Pectinivalva (Menurella) 136
Eucalyptus microcorys F. Muell.
Pectinivalva (Menurella) 23
Eucalyptus ?muelleriana A.W. Howitt
Pectinivalva (Menurella) 91
Eucalyptus nitida Hook. f.
Pectinivalva (Menurella) 227
Eucalyptus oleosa F.Muell. ex Miq.
Pectinivalva sp. (RH169) (vacated mines only)
Eucalyptus pilularis Sm.
Pectinivalva (Menurella) scotodes, Pectinivalva (Menurella) 91
Eucalyptus polyanthemos Schauer
Pectinivalva (Menurella) 21
Eucalyptus rubida H.Deane & Maiden
Pectinivalva (Pectinivalva) 139
Eucalyptus saligna Sm.
Pectinivalva (Menurella) KM7
Eucalyptus ?saligna Sm.
Pectinivalva (Pectinivalva) 5
Eucalyptus socialis F.Muell. ex Miq.
Pectinivalva sp. (RH168) (vacated mines only)
Eucalyptus tereticornis Sm.
Pectinivalva (Pectinivalva) 68
Eucalyptus viminalis Labill.
Pectinivalva (Menurella) 143, Pectinivalva (Pectinivalva) 78, Pectinivalva (Pectinivalva) 142
Leptospermeae
Leptospermum ?glaucescens S.Schauer
Pectinivalva (Menurella) 211
Leptospermum laevigatum (Gaertn.) F.Muell.
Pectinivalva (Menurella) A85
Leptospermum morrisonii
Pectinivalva (Menurella) 189
Leptospermum scoparium Joy Thomps.
Pectinivalva (Menurella) 216
Leptospermum trinervium (Sm.) Joy Thomps.
Pectinivalva (Menurella) 2, Pectinivalva (Menurella) 175
Kanieae
Tristaniopsis collina Peter G. Wilson & Waterhouse
Pectinivalva (Casanovula) brevipalpa
Syzygieae
Syzygium acuminatissimum (Blume) A. DC. (= Acmena acuminatissima)
Pectinivalva (Menurella) xenadelpha
Syzygium ingens (F.Muell. ex C.Moore) Craven & Biffin (= Acmena brachyandra)
Pectinivalva (Menurella) cf acmenae (EvN2004099) (vacated mines only)
Syzygium smithii (Poir.) Nied. (= Acmena smithii)
Pectinivalva (Menurella) acmenae
Myrteae
Gossia bidwillii (Benth.) N.Snow & Guymer (= Austromyrtus bidwillii)
Pectinivalva sp. (RH46) (vacated mines only)
Pilidiostigma tropicum L.S.Sm.
Pectinivalva sp. (RH106) (vacated mines only)
Rhodomyrtus macrocarpa Benth.
Pectinivalva (Menurella) tribulatrix
Paracryphiaceae
Quintinia verdonii F. Muell.
Pectinivalva (Menurella) quintiniae
Specimen locality data of Pectinivalva Scoble (Lepidoptera: Nepticulidae) and outgroup taxa: examined specimens, DNA vouchers and host records. (doi: 10.3897/zookeys.278.4743.app) File format: Microsoft Excel (xls).
Explanation note: The records comprise all material studied, including rearing records of species that have not been treated and voucher specimens for DNA barcoding. We have treated specimens with a genitalia slide or other preparation and DNA vouchers as individual records for each species. Other records combine all adults from a single rearing or other single collection event. While compiling this datasheet, the authors no longer had direct access to the ANIC collection in Canberra, and in some cases our notes were not sufficient to enter all fields. This explains why for some slides ‘sex unknown’ is given, and uncertainties about exactly how many specimens were collected on a given date.