Citation: Haarto A, Ståhls G (2014) When mtDNA COI is misleading: congruent signal of ITS2 molecular marker and morphology for North European Melanostoma Schiner, 1860 (Diptera, Syrphidae). ZooKeys 431: 93–134. doi: 10.3897/zookeys.431.7207
The northern European taxa of genus Melanostoma Schiner, 1860 (Syrphidae, Diptera) are revised. A longstanding question concerning the number of Melanostoma taxa occurring in northern Europe prompted us to contrast and compare their morphological and molecular variability. Particular uncertainty concerned the putative existence of a sibling species of Melanostoma mellinum, and the identity of the taxon Melanostoma dubium in northern Europe due to existence of morphologically similar dark forms of M. mellinum in the northern parts of its distributional range. Partial sequences of two DNA markers, the mitochondrial protein-coding gene cytochrome c oxidase subunit I (COI-3') and the nuclear second internal transcribed spacer (ITS2) were analysed separately under parsimony. The obtained COI-3'gene fragment showed taxon-specific haplotypes and haplotypes that were shared among the taxa. The ITS2 sequences presented genotypes unique to each species, and congruence with our independently established taxonomic entities. Based on congruent signal of the ITS2 sequences and study of morphological characters we establish the presence of four taxa in northern Europe: Melanostoma mellium (= M. dubium nec auctt., syn. n.), M. certum sp. n. (= M. dubium auctt.), M. mellarium stat. n. (= M. mellinum auctt. partim) and M. scalare. Lectotype designations were made for Musca mellina, Syrphus mellarius and Melanostoma mellinum var. melanatus.
The following synonymies were established: Melanostoma mellarium = Melanostoma melanatum syn. n.; Melanostoma mellinum = Scaeva dubia syn. n., Melanostoma tschernovi syn. n., and Melanostoma clausseni syn. n. Morphological circumscriptions of the taxa and an identification key are presented.
Melanostoma, taxonomy, ITS2, COI
The taxa of genus Melanostoma Schiner, 1860 (Diptera, Syrphidae, Syrphinae) are among the most abundant hoverflies in the northern Palaearctic region occurring in both undisturbed and human impacted woodlands and grasslands. The three presently recognized species on the European continent, Melanostoma dubium (Zetterstedt, 1838), Melanostoma mellinum (Linnaeus, 1758) and Melanostoma scalare (Fabricius, 1794) (
Species of the genus Melanostoma are medium-sized (5–9 mm) hover flies, dark coloured with a greenish or bluish tinge, usually with 1–4 pairs of variously shaped maculae on the abdomen. The genus Melanostoma is closely allied to genus Platycheirus Lepeletier & Serville, 1828. Both genera have bare eyes, a black face and scutellum, and antennae shorter than head. These genera have distinct shapes of surstyli and postgonites of the male genitalia (see e.g.
It has, however, long remained doubtful whether the present species definitions actually reflect the number of Melanostoma species in the continent (e.g.
The present study attempts to finally resolve the longstanding confusion regarding the species identities, nomenclature and circumscriptions for the Melanostoma taxa occurring in northern Europe in light of not previously utilised molecular characters and informative new morphological characteristics. We employed DNA sequence characters of a large fragment of the 3'–end of the first subunit of the mitochondrial protein coding cytochrome c oxidase gene (hereafter COI) and of the nuclear second internal transcribed spacer region (ITS2). This allowed us to explore the congruence of the morphologically delimited species with the DNA haplotypes of COI and genotypes of ITS2 and to evaluate if our morphological hypotheses were supported by the molecular data. At the same time this approach allowed to contrast the usefulness (signal) of the employed molecular markers (COI and ITS2) for resolving the taxonomy of closely related hover fly species. The COI gene has been a work-horse both for taxonomic and systematic studies of invertebrates including Diptera, as the 5'–fragment of the gene constitutes the core barcoding gene region for animals (
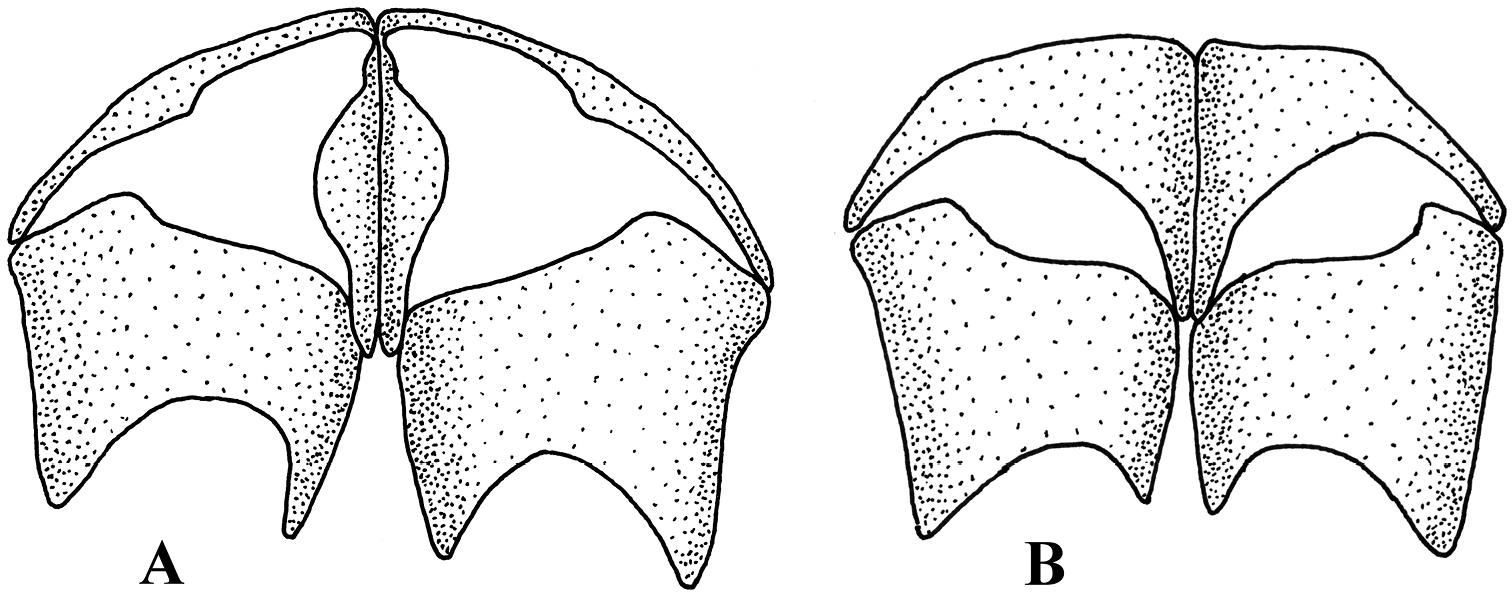
Shape of metasternum. A Melanostoma mellarium and B Platycheirus podagratus.
The characters used in the key, descriptions, and drawings employ the terminology established by
The original label information of the examined type material is captured between single quotes ('’), and labels are separated with a slash /. Depository institutions of each specimen are indicated between square brackets after the label information. The acronyms used for collections largely follow the
AHPC Antti Haarto personal collection, Turku, Finland
LSUK Linnean Collection of Insects, repository managed by the Linnean Society of London, London, UK
MNHN Muséum National d'Histoire Naturelle, Paris, France
MZH Finnish Museum of Natural History, Helsinki, Finland
MZL Museum of Zoology, Lund, Sweden
MZT Zoological Museum of the University of Turku, Finland
SKPC Sakari Kerppola personal collection, Helsinki, Finland
When necessary, a lectotype has been designated and labelled accordingly in order to fix the concept of the taxon in question and to ensure the universal and consistent interpretation of the same.
Images of external morphology (pinned specimens; 30–40 exposures; Canon EOS 40S digital camera) and male genitalia (submersed in ethanol; 20–30 exposures; Olympus E520 digital camera on Olympus S7X16 microscope) were taken using d-cell software vs 5.1 and composed using CombineZP software vs. 2 (
In addition to the DNA voucher specimens, abundant material of pinned flies of the M. mellinum sensu lato (112 males, 138 females) and Melanostoma scalare taxa (27 males, 25 females), from localities in northern Europe (coll. MZH, MZT and AHPC), were available for study of morphological characteristics (Table 1). For Melanostoma dubium only about ten pinned specimens of each sex were obtained for this study (coll. MZH, MZT and AHPC), including the DNA vouchers.
A comprehensive sample of specimens identified according to present concepts as Melanostoma dubium, Melanostoma mellinum and Melanostoma scalare from Fennoscandia were used for molecular work. Additionally, specimens of Melanostoma mellinum auctt. and Melanostoma scalare obtained from a broad geographical range across Europe were also available for molecular work, including one sample of Melanostoma incompletum from the Canary Islands (Table 1). Specimens of M. dubium, M. tschernovi, Melanostoma clausseni and Melanostoma mellinum from northern Siberia, Russia (provided and identified by A. V. Barkalov) were also subjected to molecular analyses. Specimens used for molecular study are listed in Table 1. Locality labels for samples from Finland include Finnish grid coordinates (ykj) (see http://www.maanmittauslaitos.fi/sites/default/files/Finnish_Coordinate_Systems.pdf). Added geographical coordinates in DMS are shown in square brackets. DNA voucher specimens were deposited in the MZH and labelled accordingly.
DNA was extracted from 1–3 legs of dry pinned or ethanol preserved specimens using the Nucleospin Tissue DNA extraction kit (Machery-Nagel, Düren, Germany) following the manufacturer’s protocols and then re-suspended in 50 µl of ultra-pure water. PCR reactions were carried out using GE Ready-to-Go PCR beads in 25 µl reaction aliquots containing 2–4 µl DNA extract, 1 µl of each primer (at 10 pmol/µl) and ultrapure water. Thermocycler conditions were initial denaturing at 95°C 2 min, 29 cycles of 30 s denaturing at 94°C, 30 s annealing at 49°C, 2 min extension at 72°C, followed by a final extension of 8 min at 72°C. The universally conserved primers used for amplifying and sequencing the COI 3'–fragment (ca 770 bp) were the forward primer C1-J-2183 [5'–CAACATTTATTTTGATTTTTTGG–3'] (alias JERRY) and reverse primer TL2-N-3014 [5'–TCCAATGCACTAATCTGCCATATTA–3'] (alias PAT) (
Amplified PCR products were electrophoresed on 1.5% agarose gels and treated with Exo-SapIT (USB Affymetrix, Ohio, USA) prior to sequencing. Both PCR primers were used for sequencing. The Big Dye Terminator Cycle Sequencing Kit (version 3.1) (Applied Biosystems, Foster City, CA, USA) was used on an ABI 3730 (Applied Biosystems, Foster City, CA, USA) genetic analyzer at the Sequencing Service Laboratory of the Finnish Institute for Molecular Medicine (ww.fimm.fi). The sequences were edited for base-calling errors and assembled using Sequencher™ (version 4.9) (Gene Codes Corporation, Ann Arbor, MI, USA). All new sequences were submitted to GenBank (see Table 1 for accession numbers).
The protein-coding COI gene was aligned manually and it was not necessary to include gaps in this alignment. The alignment of the ITS2 fragment was carried out using the E-INS-I strategy as implemented in MAFFT (
Platycheirus europaeus (Goeldlin, Maibach & Speight, 1990) (Diptera, Syrphidae) was used to root the trees. Single gene parsimony analyses were conducted for each gene region. Parsimony analysis was performed using NONA (
List of specimens used for molecular work including GenBank accession numbers.
| Labcode | Taxon | Country | Finnish grid coordinates and /or geogr. coordinates | Province | Locality | Date | Collector | GenBank accession mtDNA COI | GenBank accession ITS2 |
|---|---|---|---|---|---|---|---|---|---|
| MZH_Y397 | Melanostoma certum | Finland | 7674:3253 69°2.59'N, 20°48.316'E | Le: Enontekiö | Kilpisjärvi | 6.VII.2005 | G. Ståhls & V. Milankov leg. | KJ848068 | KJ847974 |
| MZH_Y472 | Melanostoma certum | Finland | 7588:3334 68°20'N, 22°58"E | Le: Enontekiö | Vähäniva | 16.VI.2006 | E.M. & L. Laasonen leg. | KJ848069 | KJ847975 |
| MZH_Y491 | Melanostoma certum | Finland | 77224:34611 69°34'42"N, 25°59'49"E | Li: Inari | Akujoen risteys | 2.VII.2006 | I. Kakko leg. | NA | KJ847976 |
| MZH_Y643 | Melanostoma certum | Finland | 76865:2790 69°10'9"N, 21°25'22"E | Le: Enontekiö | Annjaloanji | 13.VII.2007 | A. Haarto leg. | NA | KJ847977 |
| MZH_Y1648 | Melanostoma certum | Finland | 76865:2790 69°10'9"N, 21°25'22"E | Le: Enontekiö | Annjaloanji | 22.VII.2005 | A. Haarto leg. | NA | KJ847978 |
| MZH_Y1872 | Melanostoma certum | Finland | 77042:34600 69°24'54"N, 25°58'36"E | Li: Utsjoki | Karigasniemi, Ailigas | 25.VI.2013 | K. Mattila leg. | KJ848107 | KJ847979 |
| MZH_Y395 | Melanostoma mellarium | Finland | 7674:3253 | Le: Enontekiö | Kilpisjärvi | 6.VII.2005 | G. Ståhls & V. Milankov leg. | KJ848078 | KJ847981 |
| MZH_Y396 | Melanostoma mellarium | Finland | 7674:3253 | Le: Enontekiö | Kilpisjärvi | 6.VII.2005 | G. Ståhls & V. Milankov leg. | NA | KJ847982 |
| MZH_Y407 | Melanostoma mellarium | Finland | 729:38:00 | Lkem: Kemi | Ajos | 15.VI.2004 | E.M. & L. Laasonen leg. | NA | KJ847983 |
| MZH_Y415 | Melanostoma mellarium | Finland | 67071:090 | Al: Eckerö | Skag | 1.VI.2005 | E.M. & L. Laasonen leg. | KJ848079 | KJ847985 |
| MZH_Y416 | Melanostoma mellarium | Finland | 708:38 | Ob: Sievi | Kiiskilä | 17.VI.2005 | E.M. & L. Laasonen leg. | KJ848080 | KJ847986 |
| MZH_Y417 | Melanostoma mellarium | Finland | 708:38 | Ob: Sievi | Kiiskilä | 17.VI.2005 | E.M. & L. Laasonen leg. | NA | KJ847987 |
| MZH_Y438 | Melanostoma mellarium | Finland | 67549:35144 | Sa: Luumäki | Päivärinne | 9.VI.2006 | J. Kahanpää leg. | NA | KJ847988 |
| MZH_Y439 | Melanostoma mellarium | Finland | 67549:35144 | Sa: Luumäki | Päivärinne | 9.VI.2006 | J. Kahanpää leg. | KJ848081 | KJ847989 |
| MZH_Y453 | Melanostoma mellarium | Finland | Li: Inari | KJ | 8.VII.2005 | E.M. & L. Laasonen leg. | NA | KJ847992 | |
| MZH_Y527 | Melanostoma mellarium | Norway | EIS 160 | FO Sör-Varanger | Pasvik, Skogfoss | 28.VI.2006 | T. R. Nielsen leg. | KJ848083 | KJ847993 |
| MZH_Y530 | Melanostoma mellarium | Norway | EIS 160 | FO Sör-Varanger | Pasvik, Skogfoss | 29.VI.2006 | T. R. Nielsen leg. | KJ848084 | KJ847994 |
| MZH_Y531 | Melanostoma mellarium | Norway | EIS 160 | FO Sör-Varanger | Pasvik, Fagermo | 28.VI.2006 | T. R. Nielsen leg. | NA | KJ847995 |
| MZH_Y612 | Melanostoma mellarium | Finland | 76178:35210 | Li: Ivalo | Näveriniemi | 5.VII.2007 | G. Ståhls leg. | NA | KJ847990 |
| MZH_Y621 | Melanostoma mellarium | Finland | 77586:35009 | Li: Utsjoki | roadside | 8.VII.2007 | G. Ståhls leg. | KJ848095 | KJ847996 |
| MZH_Y642 | Melanostoma mellarium | Luxembourg | Bonnerue meadow | L’Ourtie occidental, 229-78 | 21.V.2006 | W. van Steenis leg. | KJ848091 | KJ847991 | |
| MZH_Y646 | Melanostoma mellarium | Finland | 76764:2523 | Le: Enontekiö | Saana (koivikko) | 16.VII.2007 | A. Haarto leg. | NA | KJ847997 |
| MZH_Y647 | Melanostoma mellarium | Finland | 76764:2523 | Le: Enontekiö | Saana (koivikko) | 16.VII.2007 | A. Haarto leg. | KJ848092 | KJ847998 |
| MZH_Y770 | Melanostoma mellarium | Norway | EIS 169 | FÖ Sör-Varanger | Svanvik | 30.VI.2008 | T. R. Nielsen leg. | NS | KJ847984 |
| MZH_Y1650 | Melanostoma mellarium | Finland | 775:350 | Le: Enontekiö | Saana | 11.VII.2011 | E.M. & L. Laasonen leg. | NA | KJ847980 |
| MZH_Y399 | Melanostoma mellinum | Finland | 6771:255 | Ta: Lammi | Biol. station | 28.V.2005 | G. Ståhls leg. | NA | KJ847999 |
| MZH_Y400 | Melanostoma mellinum | Finland | 6771:255 | Ta: Lammi | Biol. station | 28.V.2005 | G. Ståhls leg. | NA | KJ848000 |
| MZH_Y405 | Melanostoma mellinum | Finland | 6682:108 | Al: Mariehamn | Espholm | 30.V.2005 | E. M. & L. Laasonen leg. | NA | KJ848001 |
| MZH_Y406 | Melanostoma mellinum | Finland | 7623:539 | Li: Inari | Heinäj. | 7.VII.2005 | E.M. & L. Laasonen leg. | NA | KJ848002 |
| MZH_Y409 | Melanostoma mellinum | Finland | Li: Utsjoki | Tsuomas | 5–6.VII.2005 | E.M. & L. Laasonen leg. | NA | KJ848003 | |
| MZH_Y410 | Melanostoma mellinum | Finland | 69103:5353 | Ta: Joroinen | 29.VI.2006 | A. Haarto leg. | KJ848072 | KJ848004 | |
| MZH_Y413 | Melanostoma mellinum | Finland | 6696:124 | Al: Sund | Bomarsund | 1.VI.2005 | E.M. & L. Laasonen leg. | NA | KJ848005 |
| MZH_Y414 | Melanostoma mellinum | Finland | 6696:124 | Al: Sund | Bomarsund | 1.VI.2005 | E.M. & L. Laasonen leg. | NA | KJ848006 |
| MZH_Y419 | Melanostoma mellinum | Finland | Li: Kiilopää | 16–24.VII.2005 | E.M. & L. Laasonen leg. | NA | KJ848007 | ||
| MZH_Y434 | Melanostoma mellinum | Finland | 6696:234 | Ab: Parainen | Petteby | 31.V.2006 | A. Haarto leg. | NA | KJ848008 |
| MZH_Y435 | Melanostoma mellinum | Finland | 6733:222 | Ab: Mietoinen | Perkko | 28.V.2006 | A. Haarto leg. | NA | KJ848009 |
| MZH_Y436 | Melanostoma mellinum | Finland | 6696:234 | Ab: Parainen | Petteby | 31.V.2006 | A. Haarto leg. | KJ848087 | KJ848010 |
| MZH_Y437 | Melanostoma mellinum | Finland | 6733:222 | Ab: Mietoinen | Perkko | 25.V.2006 | A. Haarto leg. | NA | KJ848011 |
| MZH_Y442 | Melanostoma mellinum | Finland | Ok: Kuhmo | Härkäniementie | 18.VIII.2006 | G. Ståhls leg. | KJ848071 | KJ848012 | |
| MZH_Y451 | Melanostoma mellinum | Finland | Le: Kilpisjärvi | VII.2005 | G. Ståhls leg. | NA | KJ848013 | ||
| MZH_Y452 | Melanostoma mellinum | Finland | 7747:472 | Li: Utsjoki | 30.VI.2005 | E.M. & L. Laasonen leg. | NA | KJ848014 | |
| MZH_Y456 | Melanostoma mellinum | Sweden | Uppland | Järfälla | VIII.2006 | H. Bartsch leg. | KJ848085 | KJ848015 | |
| MZH_Y457 | Melanostoma mellinum | Sweden | Uppland | Järfälla | VIII.2006 | H. Bartsch leg. | KJ848074 | KJ848016 | |
| MZH_Y475 | Melanostoma mellinum | Finland | 664:18 | Ab: Korpo | Utö | 28.7.2006 | A. Haarto leg. | NA | KJ848017 |
| MZH_Y479 | Melanostoma mellinum | Netherlands | RD 128-566 | Breukelen | Overholland | 5.V.2006 | W. van Steenis leg. | KJ848086 | KJ848018 |
| MZH_Y480 | Melanostoma mellinum | Netherlands | RD 128-464 | Breukelen | Niejenrode | 5.V.2006 | W. van Steenis leg. | KJ848075 | KJ848019 |
| MZH_Y488 | Melanostoma mellinum | Finland | 77042:34600 | Li: Utsjoki | Kaivojoki, Karigasniemi | 01.VII.2006 | I. Kakko leg. | KJ848076 | KJ848020 |
| MZH_Y489 | Melanostoma mellinum | Finland | 76954:34829 | Li: Utsjoki | Kaamasmukka | 01.VII.2006 | I. Kakko leg. | KJ848077 | KJ848021 |
| MZH_Y490 | Melanostoma mellinum | Finland | 77224:34611 | Li: Inari | Akujoen risteys | 02.VII.2006 | I. Kakko leg. | NA | KJ848022 |
| MZH_Y528 | Melanostoma mellinum | Norway | EIS 160 | FO Sör-Varanger | Pasvik, Skogfoss | 27.VI.2006 | T. R. Nielsen leg. | KJ848073 | KJ848023 |
| MZH_Y529 | Melanostoma mellinum | Norway | EIS 160 | FO Sör-Varanger | Pasvik, Skogfoss | 28.VI.2006 | T. R. Nielsen leg. | NA | KJ848024 |
| MZH_Y593 | Melanostoma mellinum | Italy | Sardinia | Prov. Sassari | 3.VI.2007 | C. Kehlmaier leg. | NA | KJ848025 | |
| MZH_Y611 | Melanostoma mellinum | Finland | 75944:35160 | Li: Saariselkä | Kaunispäänoja | 5.VII.2007 | G. Ståhls leg. | NA | KJ848034 |
| MZH_Y613 | Melanostoma mellinum | Finland | 77426:35005 | Li: Utsjoki | Kevo, Kutuniemi | 9.VII.2007 | G. Ståhls leg. | NA | KJ848035 |
| MZH_Y614 | Melanostoma mellinum | Finland | 77422:34997 | Li: Utsjoki | Kevonsuu | 11.VII.2007 | G. Ståhls leg. | NA | KJ848036 |
| MZH_Y619 | Melanostoma mellinum | Finland | 77586:35009 | Li: Kaunispäänoja | roadside | 5.VII.07 | A. Ssymank leg. | NA | KJ848026 |
| MZH_Y620 | Melanostoma mellinum | Finland | 76872:2807 | Li: Utsjoki | roadside | 8.VII.2007 | G. Ståhls leg. | KJ848094 | KJ848027 |
| MZH_Y644 | Melanostoma mellinum | Finland | 76865:2790 | Le: Enontekiö | Toskaljoki | 16.VII.2007 | A. Haarto leg. | NA | KJ848029 |
| MZH_Y645 | Melanostoma mellinum | Finland | 76764:2523 | Le: Enontekiö | Annjaloanji | 13.VII.2007 | A. Haarto leg. | NA | KJ848030 |
| MZH_Y647 | Melanostoma mellarium | Finland | 7678:251 | Le: Enontekiö | Saana (koivikko) | 16.VII.2007 | A. Haarto leg. | NA | NA |
| MZH_Y648 | Melanostoma mellinum | Finland | 7678:251 | Le: Enontekiö | Siilasjärvi | 11.VII..2007 | A. Haarto leg. | KJ848093 | KJ848031 |
| MZH_Y649 | Melanostoma mellinum | Finland | 7678:251 | Le: Enontekiö | Siilasjärvi | 11.VII..2007 | A. Haarto leg. | NA | KJ848032 |
| MZH_Y697 | Melanostoma mellinum | Finland | 7594:516 | Li: Inari | K-oja | 5.VII..2007 | E.M. & L. Laasonen leg. | KJ848070 | KJ848033 |
| MZH_Y770 | Melanostoma mellinum | Norway | EIS 169 | FÖ Sör-Varanger | Svanvik | 30.VI.2008 | leg. T.R. Nielsen | KJ848099 | KJ848037 |
| MZH_Y1586 | Melanostoma mellinum | Finland | 75916:38516 | Li: Inari | 30.VI.2011 | E.M. & L. Laasonen leg. | NA | KJ848038 | |
| MZH_Y1613 | Melanostoma mellinum | Finland | 75916:85162 | Li: Inari | 30.VI.2011 | E.M. & L.Laasonen leg. | NA | KJ848039 | |
| MZH_Y1625 | Melanostoma mellinum | Russia | Taimyr | 114 km from Khatangi at river Kotyi | 22.VI.2010 | A.V. Barkalov leg. | KJ848096 | KJ848040 | |
| MZH_Y1626 | Melanostoma mellinum | Russia | Taimyr | 114 km from Khatangi at river Kotyi | 22.VI.2010 | A.V. Barkalov leg. | KJ848097 | KJ848041 | |
| MZH_Y1627 | Melanostoma dubium | Russia | Taimyr | 114 km from Khatangi at river Kotyi | 22.VI.2010 | A.V. Barkalov leg. | KJ848098 | KJ848042 | |
| MZH_Y1628 | Melanostoma dubium | Russia | Taimyr | 114 km from Khatangi at river Kotyi | 22.VI.2010 | A.V. Barkalov leg. | NA | KJ848043 | |
| MZH_Y1629 | Melanostoma clausseni Barkalov, 2009 Paratype | Russia | republ. Altai | Ulaganskij raion | 1–4.VII.2008 | R. Dudko leg. | NA | KJ848060 | |
| MZH_Y1630 | Melanostoma tschernovi Barkalov, 2009 | Russia | Taimyr | Shore of Zakharova Rassocha | 3.VII.2011 | A. V. Barkalov leg. | NA | KJ848061 | |
| MZH_Y1631 | Melanostoma tschernovi Barkalov, 2009 | Russia | Taimyr | Shore of Zakharova Rassocha | 3.VII.2011 | A. V. Barkalov leg. | NA | KJ848063 | |
| MZH_Y1660 | Melanostoma mellinum | Greece | Olymp | 18.V.2011 | A. Vujic leg. | KJ848102 | KJ848044 | ||
| MZH_Y1661 | Melanostoma mellinum | Greece | Samos | 17.IV.2011 | A. Vujic & S. Radenkovic leg. | KJ848103 | KJ848045 | ||
| MZH_Y1662 | Melanostoma mellinum | Serbia | Tara | 5.VIII.2010 | A. Vujic leg. | KJ848105 | KJ848046 | ||
| MZH_Y1664 | Melanostoma mellinum | Greece | Olymp | 18.V.2011 | A. Vujic leg. | KJ848104 | KJ848047 | ||
| MZH_Y1785 | Melanostoma tschernovi Barkalov, 2009 | Russia | 73°24'N, 80°39'E | NW Tajmyr península | 6.VII.2012 | A.V. Barkalov leg. | NA | KJ848065 | |
| MZH_Y1786 | Melanostoma tschernovi Barkalov, 2009 | Russia | 73°24'N, 80°39'E | NW Tajmyr península | 16.VII.2012 | A.V. Barkalov leg. | KJ848100 | KJ848064 | |
| MZH_Y1871 | Melanostoma mellinum | Turkey | Bozdag mnt | 16.IX.2013 | G. Ståhls leg. | KJ848106 | KJ848056 | ||
| MZH_Y1880 | Melanostoma mellinum | Russia | Chukotka, | near river Anadyr | 23.VII.2013 | A. V. Barkalov leg. | KJ848101 | KJ848059 | |
| MZH_E61 | Melanostoma mellinum | Cyprus | Almirolibado | 31.V.–2.VI.2012 | S. Dimitriou leg. | KJ848109 | KJ848057 | ||
| MZH_E62 | Melanostoma mellinum | Cyprus | Almirolibado | 31.V.–2.VI.2012 | S. Dimitriou leg. | KJ848108 | KJ848058 | ||
| MZH_Y398 | Melanostoma scalare | Finland | 7663:149 | Le: Kilpisjärvi | 6.VII.2005 | G. Ståhls & V. Milankov leg. | NA | KJ848048 | |
| MZH_Y401 | Melanostoma scalare | Hungary | W Somlo | Weingut | 10.IX.2005 | E.M. & L. Laasonen leg. | NA | KJ848052 | |
| MZH_Y402 | Melanostoma scalare | Finland | 68744:5732 | Ta: Rantasalmi, | Hiltula | 30.VI.2006 | A. Haarto leg. | NA | KJ848049 |
| MZH_Y403 | Melanostoma scalare | Finland | 68744:5732 | Ta: Rantasalmi, | Hiltula | 30.VI.2006 | A. Haarto leg. | NA | KJ848050 |
| MZH_Y404 | Melanostoma scalare | Finland | 6682:108 | Al: Mariehamn | Espholm | 30.V.2005 | E. Laasonen leg. | NA | KJ848051 |
| MZH_Y441 | Melanostoma scalare | Finland | Ok: Kuhmo | Lentuankoski | 15.VIII.2006 | G. Ståhls leg. | KJ848082 | KJ848053 | |
| MZH_Y594 | Melanostoma scalare | Italy | 8°35'586"E, 40°10'377"N | Sardinia | Prov. Oristano, Il Montiferru | 8.IV.2007 | leg. C. Kehlmaier | KJ848090 | KJ848055 |
| MZH_Y641 | Melanostoma scalare | Netherlands | RD 128-463 | Breukelen | Nijenrode | 23.IV.2006 | W. van Steenis leg. | KJ848089 | KJ848054 |
| MZH_Y1838 | Melanostoma incompletum Becker, 1908 | Spain | Canary islands | Tenerife, 3 km S Los Realejos | 16.II.2013 | M. Reemer leg. | NA | KJ848066 | |
| MZH_Y443 | Platycheirus europaeus Goeldlin, Maibach & Speight, 1990 | Finland | Ok: Kuhmo | Lentuankoski | 15.VIII.2006 | G. Ståhls leg. | KJ848067 | KJ847973 |
Due to the geographic and taxonomic focus of the present study, the following type material of Melanostoma dubium and Melanostoma mellinum and part of their currently recognised synonyms were studied.
Melanostoma mellinum (Linnaeus, 1758)
Musca mellina Linnaeus, 1758
Syrphus mellarius Meigen, 1822
This taxon was described based on an unknown number of males and females. The type locality is “Nord de la France”. In MNHN collections two female syntype specimens exist, one with labels ’Meigen 1486 40 / Syrphus mellarius female'and another female labelled ’Meigen 1486 40 / mellinum type’. The first mentioned female is a specimen of M. scalare. We herewith designate the second female as the lectotype of Syrphus mellarius Meigen, 1822 and have labelled it accordingly. Based on our results (see below) it is hereafter named Melanostoma mellarium (Meigen, 1822), stat. n.
Syrphus melliturgus Meigen, 1822
Type locality “Nord de la France”. In MNHN, only one specimen remains labelled ’Meigen 1482 40 / Syrphus melliturgus male’. Of the pinned specimen only the thorax with legs and both wings remains. The identity of the specimen cannot be ascertained.
Syrphus minutus Macquart, 1829
The taxon was described based on a single male. The type locality was not given. The type was not found at MNHN, but apparently exists in the collections of Musée d’Histoire Naturelle de Lille, France (curator P. de Bleeckere, pers. comm.) where some Macquart types remain/were deposited. Type material was not available for this study.
Syrphus unicolor Macquart, 1829
Macquart mentioned several females, with black abdomen. The type locality was not given. Syntypes were not found in MNHN, but an unknown number of syntypes apparently exist in the collections of Musée d’Histoire Naturelle de Lille, France (curator P. de Bleeckere, pers. comm.) where some Macquart types remain/were deposited. Syntypes could not actually be studied.
Melanostoma mellinum var. angustatoides Kanervo, 1934
Melanostoma mellinum var. melanatus Kanervo, 1934
This taxon was recognized based on three females from Haukilampi locality (Murmansk region, Russia) with “completely melanic abdomen”. We have studied the two female specimens found in MZT and labelled one as lectotype. The lectotype has the following labels: ‘Haukilampi, 28.4.28'/ ‘Lectotype Melanostoma mellinum var. melanatus Kanervo, Haarto & Ståhls des. 2014’. Both specimens belong to Melanostoma mellarium (syn. n.).
Melanostoma mellinum var. obscuripes Kanervo, 1934
Melanostoma dubium (Zetterstedt, 1838)
In the Catalogue of Palaearctic Diptera,
Scaeva dubia Zetterstedt, 1838
The nominate form, var. a, was described based on females from Torne lappmark, Lycksele lappmark and Åsele lappmark, northern Sweden. A second form, var. b, was described based on females from Lycksele lappmark, northern Sweden, and Finnmark, northern Norway.
Syrphus unicolor Rondani, 1857
This is a junior primary homonym of Syrphus unicolor Macquart, 1829.
Chilosia (Anocheila) freyi Hellén, 1949
This taxon was described from northern Finland. The holotype female deposited in MZH is damaged, only thorax, wings and legs remain. It certainly is a species of Melanostoma, but we are unable to identify this taxon with certainty.
Pachyspyria flavitibia Enderlein, 1938 and Pachyspyria sexpunctatum Enderlein, 1938 were described as variations of Scaeva dubia. Both species names accent morphological characteristics (yellow tibiae, and abdomen with six maculae/patches) that are not diagnostic for Melanostoma dubium auctt. Furthermore, their type localities are central European. The type materials were not studied by us. The names cannot be accepted as synonyms of Melanostoma dubium auctt., and we cannot place them in synonymy with any Melanostoma taxon.
Accordingly, the taxon identified by authors as Melanostoma dubium (Melanostoma dubium auctt. nec Zetterstedt) is a different taxon from the Melanostoma mellinum taxon. The lectotype of Melanostoma dubium (Zetterstedt) is a synonym of Melanostoma mellinum. Taking the above presented information into consideration, the Melanostoma dubium auctt. nec Zetterstedt taxon is in need of a new name and type designation.
We successfully obtained mtDNA COI sequences for 41 ingroup Melanostoma specimens with 743 bp unambiguous sequence alignment, and ITS2 for 93 ingroup terminals with sequence length variation among all ingroup taxa between 400–404 bp with a total dataset alignment of 409 bp.
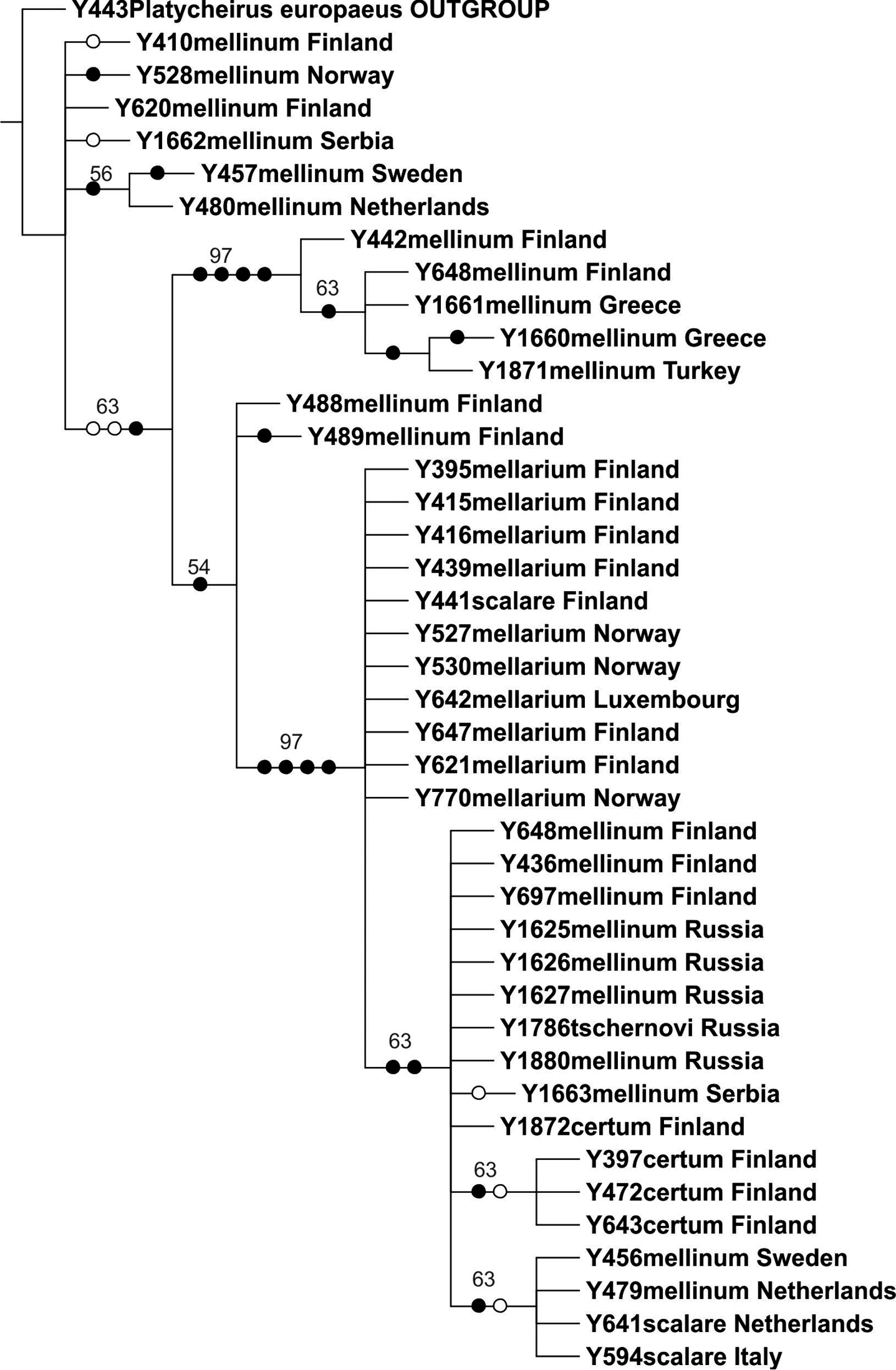
The COI dataset comprised 18 parsimony informative characters. The parsimony analysis of the COI gene resulted in 72 equally parsimonious trees of 98 steps; the strict consensus tree is shown in Fig. 2 (taxa labelled according to new results). The COI gene 3'–fragment contained 18 variable sites (Table 2). We recorded two haplotypes for Melanostoma certum sp. n., one unique and one shared with Melanostoma mellinum, and 16 haplotypes for Melanostoma mellinum (in traditional sense) (Melanostoma mellinum specimens with uncorrected sequence divergence < 1%), one of which was shared with Melanostoma certum sp. n., and another one shared with Melanostoma scalare (Fig. 2, Table 2). Melanostoma mellarium had one haplotype which was shared with Melanostoma scalare, thus Melanostoma scalare showed no unique haplotypes for the COI gene region for the present dataset. All sequences of Melanostoma tschernovi, Melanostoma dubium and Melanostoma mellinum (no sequence obtained for Melanostoma clausseni) obtained from Russia clustered among Melanostoma mellinum samples.
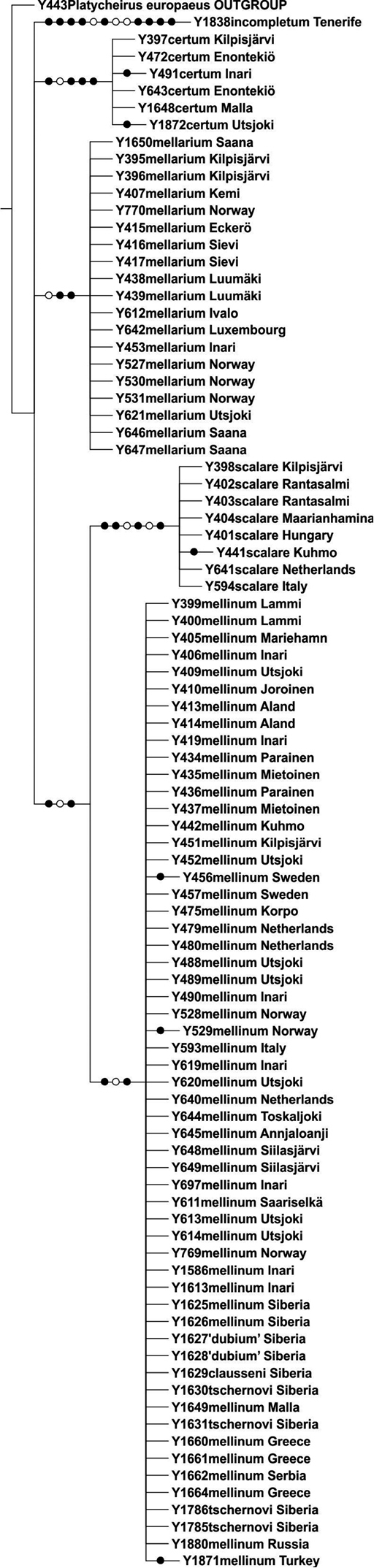
The parsimony analysis of the ITS2 marker resulted in two equally parsimonious trees of 155 steps, and the strict consensus tree is shown in Fig. 3. The ITS2 marker showed very low intraspecific variability (0.4%), and interspecific variability ranged between 2.6–6.0%. The ITS2 tree resolved the included Melanostoma specimens as five non-overlapping clades, with no samples exhibiting shared genotypes between the taxa. Again, all Russian samples (this time including Melanostoma clausseni) clustered within the Melanostoma mellinum clade (Fig. 3).
Strict consensus tree resulting from parsimony analysis of mtDNA COI gene. Filled circles denote unambiguous nucleotide changes, open circles ambiguous changes. Bootstrap support values indicated above branches.
MtDNA COI gene 3'–fragment, haplotype variation of 18 non-continuous sites among ingroup samples. Haplotypes are indicated with Roman numerals for each taxon. Samples listed by country, for Finnish sample localities North, Central or South Finland is indicated. The colors indicate haplotypes shared by two taxa.
| Code | Taxon | 020 | 050 | 071 | 110 | 140 | 206 | 272 | 281 | 284 | 332 | 419 | 434 | 521 | 548 | 578 | 635 | 699 | 737 | Haplotype number | Locality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZH_Y397 | certum | T | A | T | T | T | C | A | T | G | T | A | C | C | - | - | - | - | - | I | Finland: N |
| MZH_Y472 | certum | T | A | T | T | T | C | A | T | G | T | A | C | C | A | C | G | A | T | I | Finland: N |
| MZH_Y643 | certum | T | A | T | T | T | C | A | T | G | T | A | C | C | A | C | G | A | T | I | Finland: N |
| MZH_Y1872 | certum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | II | Finland: N |
| MZH_Y648 | mellinum | T | A | A | C | A | C | A | C | A | G | G | T | C | A | T | A | A | C | I | Finland: N |
| MZH_E62 | mellinum | T | A | A | C | A | T | A | C | A | G | G | T | C | A | T | A | A | C | II | Cyprus |
| MZH_Y442 | mellinum | T | A | A | C | A | T | A | C | A | G | G | T | C | A | T | A | A | T | III | Finland: C |
| S564 | mellinum | T | A | A | C | A | T | A | C | A | T | G | T | C | A | T | A | A | T | IV | Netherlands |
| MZH_Y1871 | mellinum | T | A | A | C | A | T | A | C | A | A | G | T | C | A | T | A | A | C | V | Turkey |
| MZH_Y1660 | mellinum | T | A | A | T | A | T | A | C | A | A | G | T | C | A | T | G | A | C | VI | Greece |
| MZH_Y1661 | mellinum | T | A | A | T | A | T | A | C | A | G | G | T | C | A | T | G | A | C | VII | Greece |
| MZH_Y410 | mellinum | C | A | A | T | A | T | A | T | A | T | A | T | T | A | T | A | A | T | VIII | Finland: C |
| MZH_Y419 | mellinum | C | G | A | T | A | T | A | T | A | T | A | T | T | A | T | A | A | T | IX | Finland: N |
| MZH_Y528 | mellinum | C | G | A | T | A | T | A | T | A | T | A | T | T | A | T | A | A | T | IX | Norway |
| MZH_Y457 | mellinum | C | G | A | T | A | T | A | T | A | T | A | T | T | G | T | A | A | T | X | Sweden |
| MZH_Y480 | mellinum | C | G | A | T | A | T | A | T | A | T | A | T | T | G | T | A | A | T | X | Netherlands |
| MZH_Y488 | mellinum | T | A | A | T | A | T | A | T | A | T | A | T | C | A | T | G | A | T | XI | Finland: N |
| MZH_Y489 | mellinum | T | A | A | T | A | T | A | T | A | T | A | T | C | A | T | G | A | T | XI | Finland: N |
| MZH_Y697 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Finland: N |
| MZH_Y436 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Finland: S |
| MZH_Y437 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Finland: S |
| MZH_Y1625 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | n | T | XII | Russia |
| MZH_Y1626 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | n | T | XII | Russia |
| MZH_Y1627 | ’dubium’ | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | n | T | XII | Russia |
| MZH_Y1880 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Russia |
| MZH_Y1786 | ’tschernovi’ | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Russia |
| MZH_Y1880 | mellinum | T | A | T | T | T | C | A | T | G | T | A | T | C | A | C | G | G | T | XII | Russia |
| MZH_Y456 | mellinum | T | A | T | T | T | C | G | T | G | T | A | T | C | A | C | G | A | T | XIII | Sweden |
| MZH_Y479 | mellinum | T | A | T | T | T | C | G | T | G | T | A | T | C | A | C | G | A | T | XIII | Netherlands |
| MZH_Y594 | scalare | T | A | T | T | T | C | G | T | G | T | A | T | C | A | C | G | A | T | XIII | Italy, Sard. |
| MZH_Y641 | scalare | T | A | T | T | T | C | G | T | G | T | A | T | C | A | C | G | A | T | XIII | Netherlands |
| MZH_Y1663 | mellinum | T | A | T | C | T | C | A | T | G | T | A | T | C | A | C | G | A | T | XIII | Serbia |
| MZH_Y1662 | mellinum | T | G | A | C | A | T | A | T | A | T | A | T | T | A | T | G | A | T | XIV | Serbia |
| MZH_E61 | mellinum | T | A | T | T | A | T | G | T | G | T | A | T | C | A | C | A | A | T | XV | Cyprus |
| MZH_Y395 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Finland: N |
| MZH_Y415 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Finland: S |
| MZH_Y416 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Finland: C |
| MZH_Y439 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Finland: C |
| MZH_Y527 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Norway |
| MZH_Y530 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Norway |
| MZH_Y770 | mellarium | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Norway |
| MZH_Y441 | scalare | T | A | T | T | T | C | A | T | A | T | A | T | C | A | C | G | A | T | I | Finland: C |
Strict consensus tree resulting from parsimony analysis of nuclear ITS2 gene region. Filled circles denote unambiguous nucleotide changes, open circles ambiguous changes. Samples from Finland labelled with locality names, from elsewhere with country name (for more information see Table 1). Bootstrap support values indicated above branches.
The description is based on
Head: Eyes bare. Frontal triangle of male blackish, shining or with variable amount of dusting. Frons of female blackish, mostly shining with a pair of triangular dusted maculae above lunule. The size of these maculae varies and they are medially separated or confluent. Face and shallow facial tubercle blackish, shining or with a variable amount of dusting. Lunule black and shiny. Antenna varying from totally dark brown to largely yellow with brown dorsal margin of basoflagellomere.
Thorax: Scutum blackish, shining, usually with slight dusting anteriorly and laterally. Pili on scutum predominantly yellowish or whitish, rarely partly or totally blackish. Scutellum shining. Pleura mostly bare, blackish, shining or with variable amount of dusting. Katepimeron with widely separated dorsal and ventral pile patches. Metasternum consists of only a narrowly sclerotized anterior and median stripe. Wing: Usually totally microtrichose, at most with small bare areas around base of cell BM.
Legs: Coxa blackish. Metacoxa without posterior pile tuft. Femur, tibia and tarsus slender without outstanding pile or bristles. Coloration varies from almost totally yellow to almost totally dark brown.
Male abdomen: Nearly parallel sided, two to five times as long as greatest width. Terga 2–4 usually with sub-rectangular yellow maculae, but maculae sometimes darkened and/or reduced to various extent. Yellow maculae shining or with various amount of dusting. Maculae on tergum 2 separated from anterior margin. Maculae on terga 3–4 usually reaching anterior margins. Maculae on terga 2–4 usually reaching lateral margins and separated from posterior margins.
Female abdomen: Shape varying from nearly parallel sided to oval; two to four times as long as its greatest width. Terga 2–5 usually with yellow maculae but these maculae sometimes darkened and/or reduced to some extent. Maculae on tergum 2 roundish and separated from the margins. Terga 3–4 with anterior triangular maculae narrowly reaching lateral margins. Tergum 5 with or without anterolateral maculae.
Antenna. A Melanostoma scalare, male and B Melanostoma certum, male.
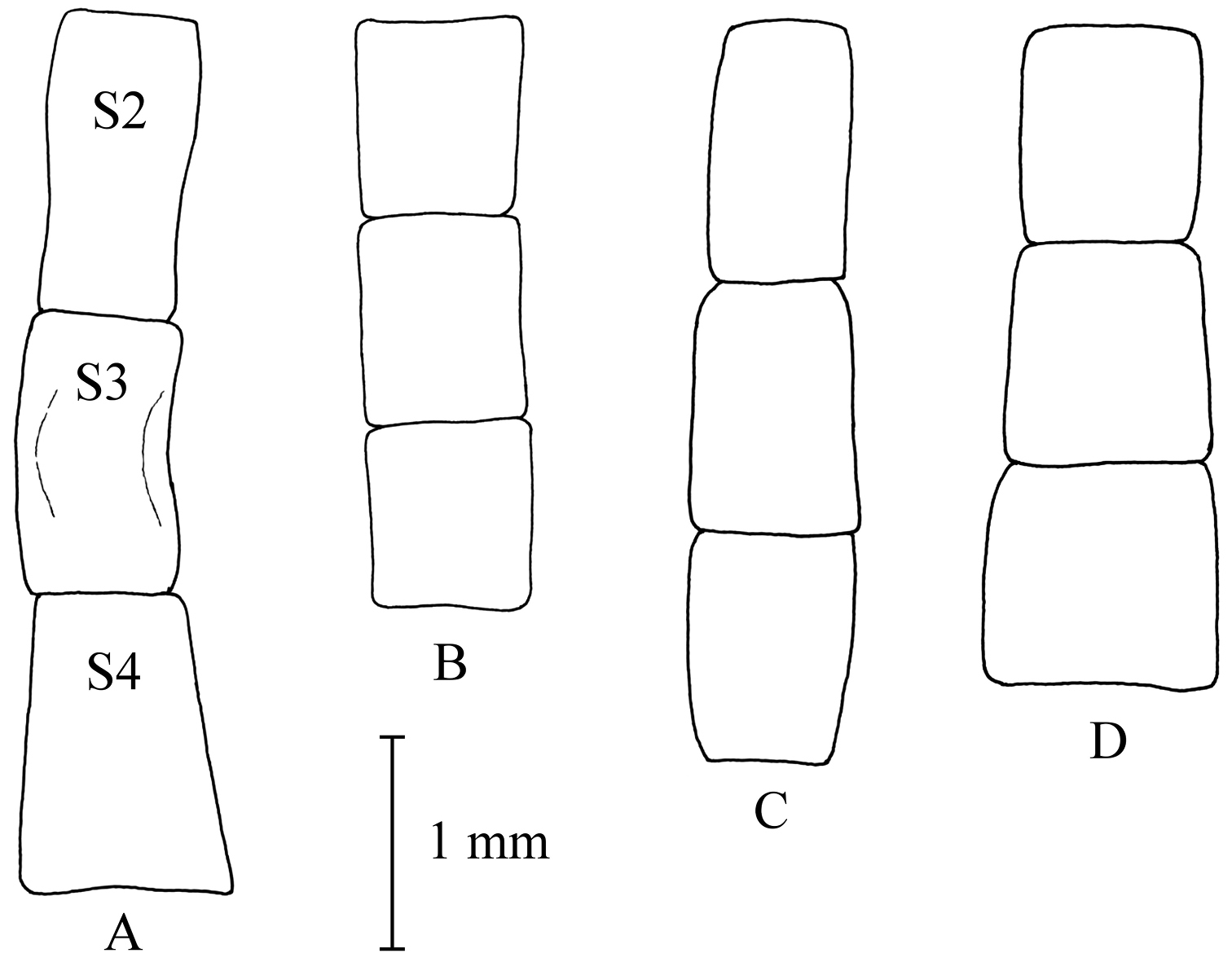
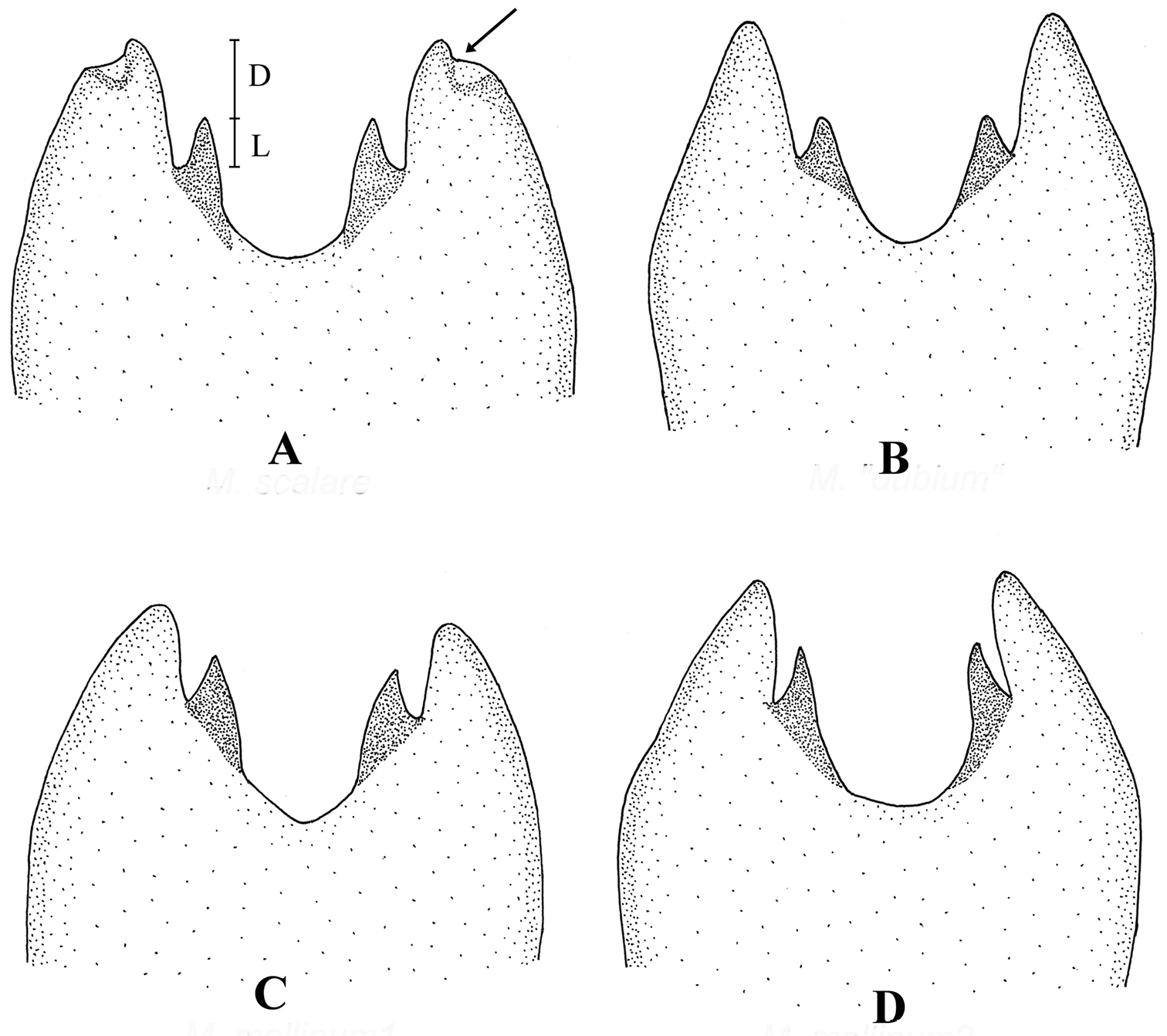
Shape of male sterna 2–4. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
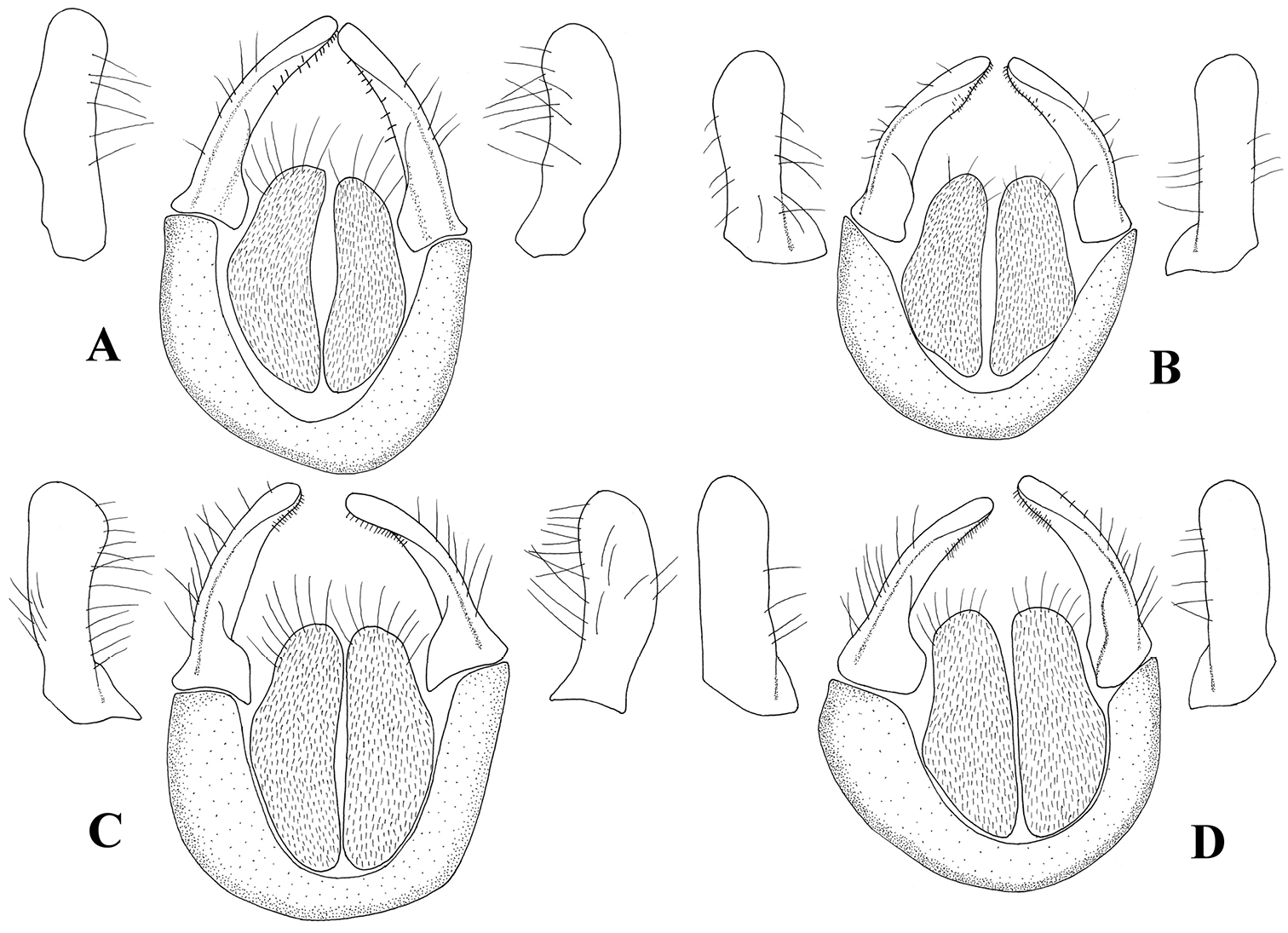
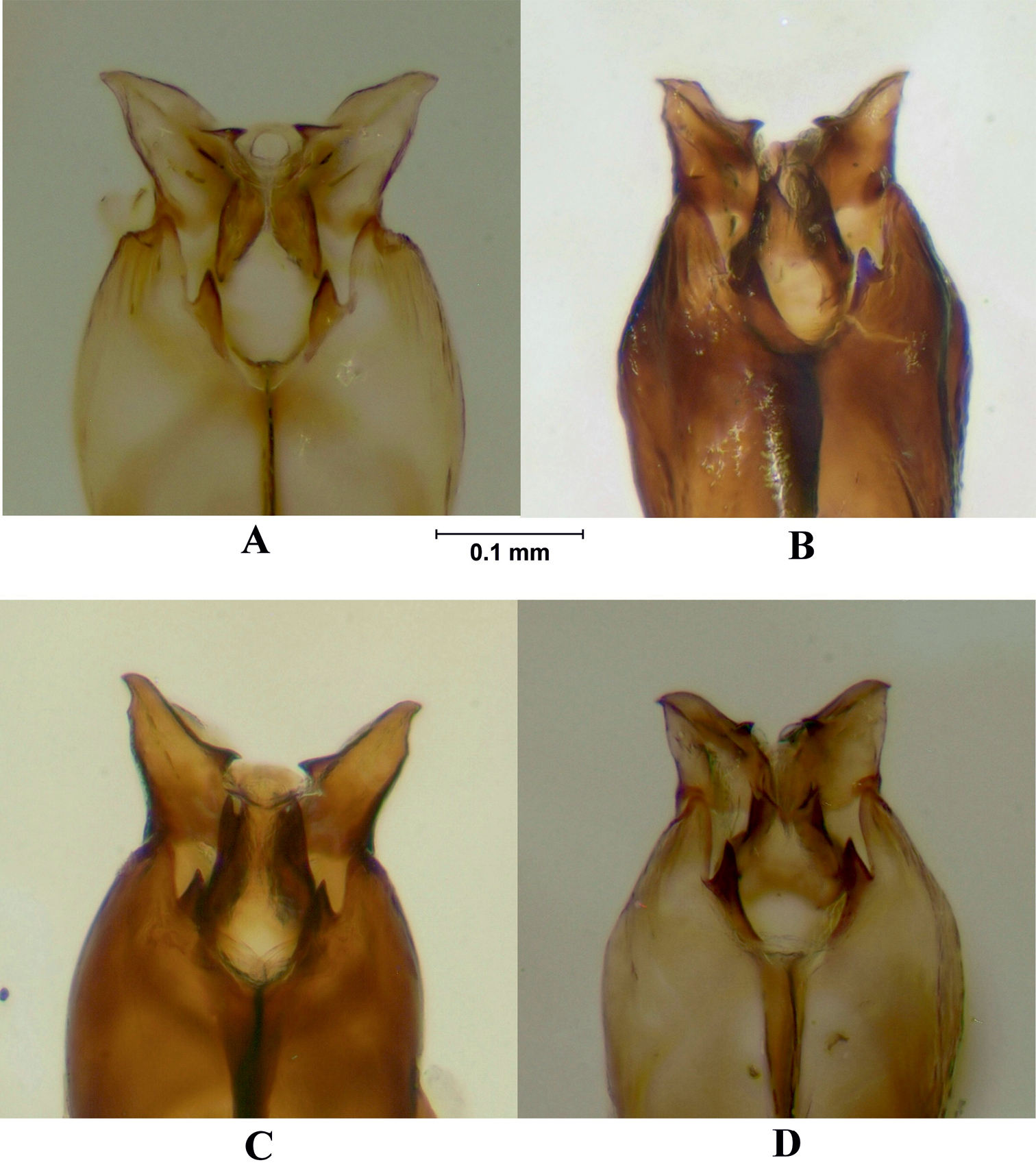
Cerci and surstyli, dorsal view and surstyli, lateral view. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
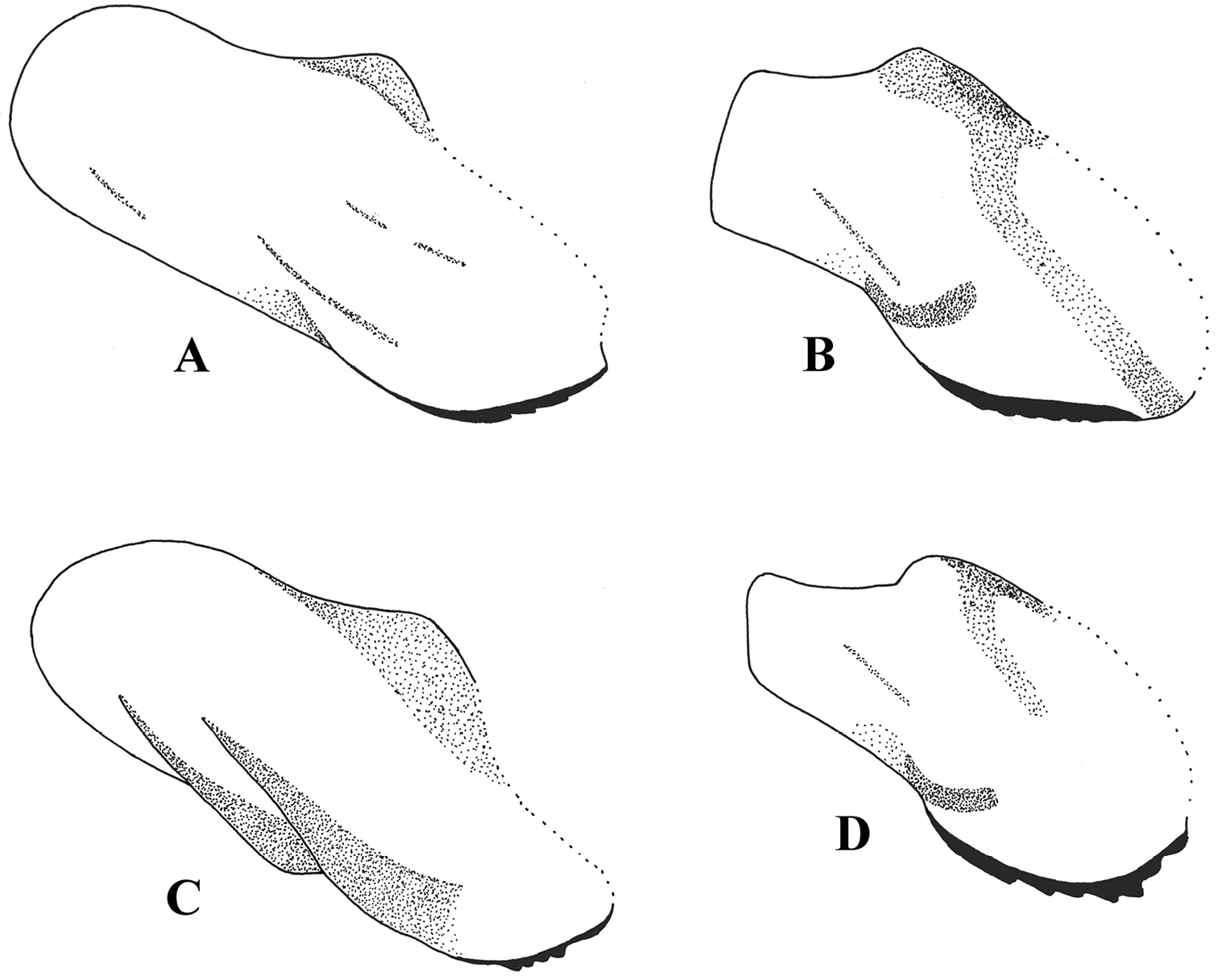
Postgonite and anterior part of hypandrium, lateral view. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
http://zoobank.org/843A2625-9859-4486-9FEA-04865F72F4CE
FINLAND: Le: Enontekiö, Annjaloanji, [69°10'9"N, 21°25'22"E], ykj76865:2790.
Holotype: male, pinned, deposited in MZH. Original labels: ‘Finland, [69°10'9"N, 21°25'22"E], ykj76865:2790, Le: Enontekiö, Annjaloanji, 13.7.2007, A. Haarto leg. / DNA voucher specimen MZH_Y643, G. Ståhls, FMNH, Helsinki, Finland / Holotype Melanostoma certum Haarto & Ståhls 2013’. Paratypes: 1 male, FINLAND: EnL: Enontekiö Korkea Jehkas lampi, [69°4'39"N, 20°50'58"E], ykj76785:2553, 20.7.2005, K. Mattila leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [AHPC]; 1 male, FINLAND: Li: Utsjoki Pulmanki, [70°2'26"N, 27°53'53"E], ykj 77739:5344, 5.7.2004, J. Kahanpää leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [MZT]; 1 female, FINLAND: Li: Utsjoki, Karigasniemi, Ailigas, [69°24'51"N, 25°58'45"E], ykj77041:4601, 6.VII.2004, J. Kahanpää leg. / Melanostoma dubium A. Haarto det. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [MZH]; 1 female, FINLAND: Le: Enontekiö Annjaloanji, [69°10'9"N, 21°25'39"E], ykj76865:2790, 13.7.2007 (puro), A. Haarto leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [AHPC]; 1 female, FINLAND: Le: Enontekiö Jogasjärvi, [69°9'58"N, 21°27'50"E], ykj76860:2806, 11–16.7.2007, malaise, R. Jussila leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [MZT]; 1 female FINLAND: Le: Enontekiö Bumbovarri, [69°11'N 21 29'E], ykj7686:328, 9.7.2007, J.-P. Kaitila & M. Rantala leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [SKPC]; 1 female FINLAND: Le: Enontekiö Annjaloanji, [69°10'N, 21°26'E], ykj7686:328, 12.7.2007, J.-P. Kaitila & M. Rantala leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [SKPC]; 1 female FINLAND: Le: Enontekiö Toskaljoki, [69°10'34"N, 21°27'34"E], ykj76871:328, 12.7.2008, J.-P. Kaitila & M. Rantala leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [SKPC]; 1 male FINLAND: Le: Enontekiö Toskaljoki, [69°10'34''N, 21°27'34''E], 76871:328, 11.7.2008, J.-P. Kaitila & M. Rantala leg. / Paratype Melanostoma certum Haarto & Ståhls 2013’, [SKPC]; and six DNA voucher paratype specimens in MZH as listed in Table 1.
Head: Colour greyish black. Angle of approximation of eyes 85°–100°. Eye contiguity about as long as frontal triangle. Ocellar triangle slightly longer than wide with dark pile and indistinctly grey dusted. Occiput very narrow and usually with pale pile. Frontal triangle with indistinct or thin grey dusting. Lateral parts of frontal triangle with dark pile. Face shining with indistinct or thin grey dusting. Lateral parts of face with dark and pale pile. Gena about as wide as basoflagellomere and with greyish dusting. Antenna dark brown. Basoflagellomere about 1.3 times as long as wide. Arista brown and almost twice as long as basoflagellomere. Arista with extremely short pile, longest pile at the most half width of arista at base (Fig. 4B).
Thorax: Scutum shining greyish black except for thin greyish dusting at anterior margin. Scutum with erect whitish pile and with shorter semi-adpressed whitish pile on anterior margin. Anterior part of scutum with long erect pile which length at least third of length of scutellum. Postpronotum totally covered by thin greyish dusting. Notopleuron with thin greyish dusting. Scutellum shining greyish black, with whitish erect pile at its dorsum, hind margin and ventral side. Pleura greyish black with thin greyish dusting. Pleura with pale erect pile. Calypter brownish with pale brownish pile at edge. Halter yellow, with slightly darkened base of stem. Wing: Completely microtrichose, with slightly brownish ting. Stigma yellowish brown.
Legs: Coxa black with grey dusting. Trochanter dark brown. Femur usually mainly black except narrowly yellow apically. Tibia usually mainly dark brown except narrowly yellow bases and apices. Tarsus usually dark brown. Leg with pale and dark pile mixed.
Abdomen: Terga dark brown or black with dense or weak brownish dusting. Tergum 2 without or with pair of yellow roundish maculae. Terga 3 and 4 with pair of yellow subrectangular maculae. Terga 1 and 2 laterally with long whitish pile. Terga with pale and usually some dark semi-adpressed pile outside of yellow maculae. Only pale pile on yellow maculae. Terga 2, 3 and 4, each about 1.2 times as long as its width. Sterna weakly dusted and with pale semi-adpressed pile. Sternum 2 about 1.4 times as long as wide at its posterior margin. Sternum 3 about 1.3 times as long as wide at its anterior margin. Sternum 4 about 1.3 times as long as wide at its anterior margin. Shape of sterna 2–4 is shown in Fig. 5B. Male genitalia: Cercus and surstylus as in Fig. 6B. Postgonite short and without distinct ridges laterally (Figs 7B, 8B). Postgonite ventrally as in Fig. 9B. Margin of hypandrium near postgonites with short triangular projections, index DL more than 2.2 (Figs 10B, 11B).
Similar to male, but differs as follows:
Head: Frons shining except greyish dusted triangles which usually are medially confluent. Ventral to the dusted triangles the thinly dusted area is usually reaching the lateral area of lunule. Frons at level of front ocellus slightly narrower than length of antenna. Dorsal part of frons with dark pile and ventral part of frons with pale pile. Occiput as broad as two diameters of an ocellus and usually with pale pile.
Thorax: Scutum and scutellum with short pale pile. Calypter pale brownish with whitish pile at edge.
Legs: Coloration similar to male.
Abdomen: Terga 1 and 2 laterally with long whitish pile. Terga 2–5 dorsally with whitish semi-adpressed pile and always without yellow maculae. Terga 2 and 3 each about 0.5 times as long as wide at its posterior margin. Tergum 4 about 0.6 times as long as wide at its posterior margin. Sternum 2 about 0.6 times as long as wide at its posterior margin. Sternum 3 about 0.6 times as long as wide at its anterior margin. Sternum 4 at least 0.7 times as long as wide at its anterior margin. Sterna 3 and 4 almost parallel sided, rarely slightly broadened towards posterior margins. Shape of sterna 2–4 is shown in Fig. 12B, C.
(4 males and 6 females): Body 5–7 mm.
All verified specimens originate from North European localities north of 68°N, and almost all specimens are sampled at or above the tree line.
The word certum means clear, defined, and is to be treated as adjective in neutrum.
Postgonite, lateral view. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
Postgonite, ventral view. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
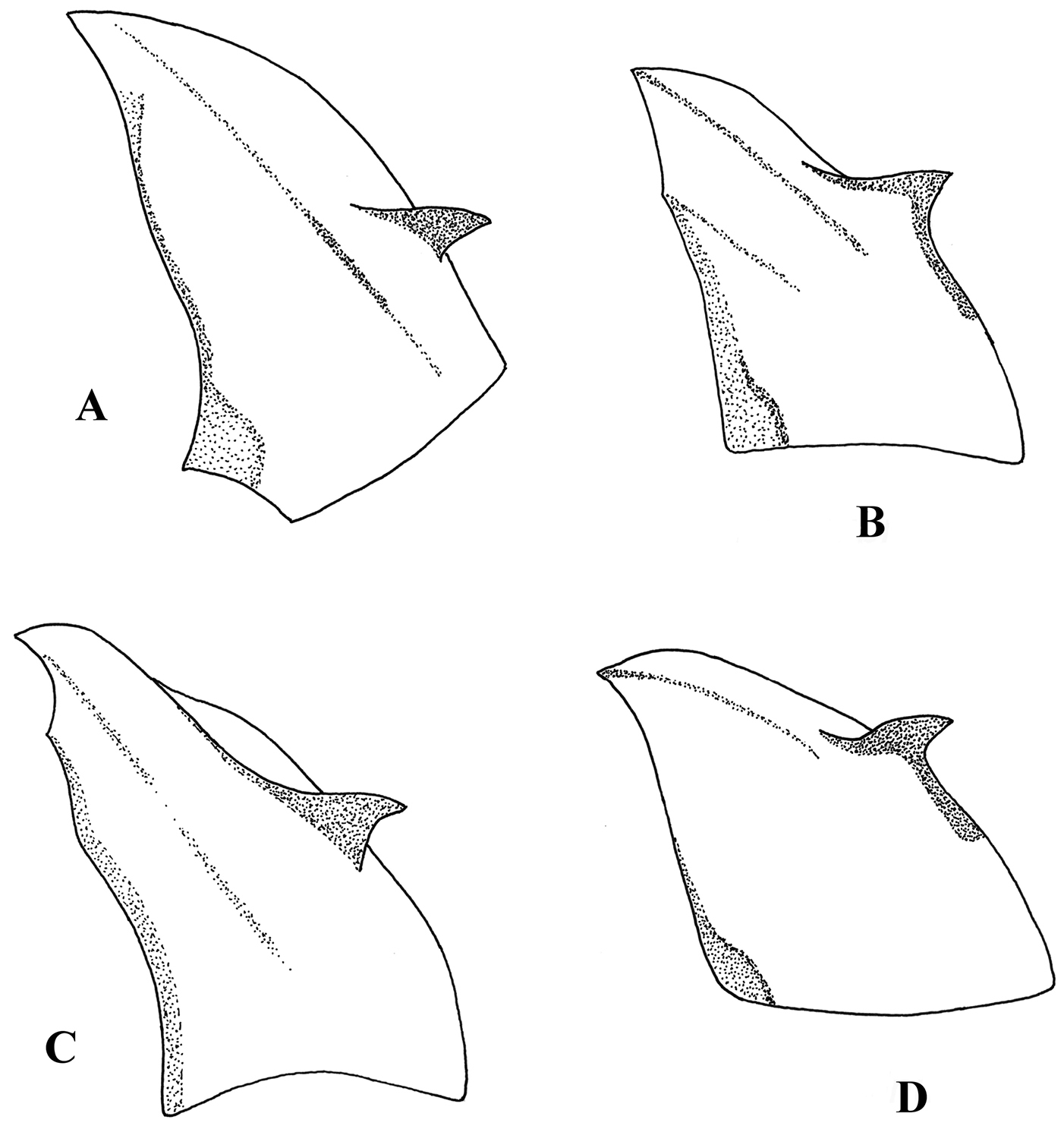
Hypandrium, lateral view. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
Hypandrium, ventral view, shape of postgonites A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium and D Melanostoma mellinum.
The locality of the lectotype is not indicated in the original label.
Lectotype of Melanostoma mellarium: female, pinned, deposited in MNHN. Original label: ’Meigen 1486 40 / mellinum type’. Here the lectotype is designated to fix the concept of Melanostoma mellarium (Meigen) and to ensure the universal and consistent interpretation of the same. Labelled: ‘LECTOTYPE Melanostoma mellarium (Meigen, 1822), Haarto & Ståhls des. 2013’. Images from MNHN.
of Melanostoma mellinum var. melanatus: female, pinned, deposited in MZT. Original label: ‘Haukilampi, 28.4.28'‘Lectotype Melanostoma mellinum var. melanatus Kanervo, Haarto & Ståhls des. 2014’.
DNA voucher specimens in MZH (Table 1) 17 males and 30 females in MZH; 25 male and 25 female specimens in AHPC.
Head: Colour black. Angle of approximation of eyes 80°–90°. Eye contiguity about as long as frontal triangle. Ocellar triangle slightly longer than wide with dark pile and with indistinct grey dusting. Occiput very narrow and dorsally with dark pile and laterally with pale pile. Frontal triangle shining with indistinct grey dusting. Lateral parts of frontal triangle with dark pile. Face shining with indistinct grey dusting. Lateral parts of face with pale and dark pile. Gena about as wide as basoflagellomere and with thin greyish dusting. Antenna mainly dark brown, basoflagellomere usually with a yellow spot baso-ventrally. Basoflagellomere about 1.3 times as long as its width. Arista usually brown and about twice as long as basoflagellomere. Longest pile of arista at most half width of arista at base as in Fig. 4B.
Thorax: Scutum shining black except for thin greyish dusting at anterior margin. Scutum usually with pale brown and dark erect pile and with shorter semi-adpressed pale pile on anterior margin. Pile rarely mainly dark on scutum. Anterior part of scutum with short erect pile which length about fourth part of length of scutellum. Postpronotum totally covered by thin greyish dusting. Notopleuron covered by indistinct greyish dusting. Scutellum shining black with pale and dark erect pile at its dorsum and posterior margin. Scutellum only pale pile at its ventral side. Pleura black and usually with only thin grey dusting and usually more distinctly shining on posterior part of anepisternum, anterior part of anepimeron and dorsal part of katepisternum. Pleura with pale erect pile. Calypter brownish with pale brownish pile on margin. Halter yellow with slightly darkened base of stem. Wing: Usually completely microtrichose, rarely with small bare area on base of cell BM. Membrane with slightly brownish ting. Stigma yellowish brown.
Legs: Coxa black with grey dusting. Trochanter dark brown. Femur usually mainly black except yellow apical part. Tibia usually mainly yellow with dark brown ring varying size. Metatibia usually with a longer dark ring than other tibiae. Tarsus dark brown except mesotarsus with two basal segments yellow. Leg with pale and dark pile mixed.
Abdomen: Terga dark brown or black with weak greyish dusting. Tergum 2 with pair of yellow oval maculae. Terga 3 and 4 with pair of yellow subrectangular maculae. Terga 1 and 2 laterally with long pale pile. Terga with varying amount of dark and pale semi-adpressed pile outside of yellow maculae. Only pale pile on yellow maculae. Terga 2, 3 and 4 each about 1.4 times as long as its width. Sterna with weak dusting and with pale semi-adpressed pile. Sternum 2 about 1.6 times as long as its width at its posterior margin. Sternum 3 about 1.5 times as long as its width at its anterior margin. Sternum 4 about 1.4 times as long as its width at its anterior margin. Shape of sterna 2–4 are shown in Fig. 5C. Male genitalia: Cercus and surstylus as in Fig. 6C. Postgonite long and with distinct ridges laterally (Figs 7C, 8C). Postgonite ventrally in Fig. 9C. The hypandrial, margin at postgonites with long triangular projections, index DL less than 1.2 (Figs 10C, 11C).
Similar to male, but differs as follows:
Head: Frons shining except greyish dusted triangles. Frons at level of front ocellus slightly narrower than length of antenna. Dorsal part of frons with dark pile and ventral part of frons with pale pile. Occipital orbit as broad as two diameters of an ocellus and usually dorsally with pale and dark pile and laterally with pale pile. Scape and pedicel brown or yellowish brown.
Thorax: Scutum and scutellum with short pale pile. Calypter whitish yellow with whitish pile at edge. Wing: Indistinctly brownish tinged. Stigma pale yellowish brown.
Legs: Femur and tibia usually mainly yellow with dark brown ring varying size. Metaleg usually largely darker than other leg.
Abdomen: Terga indistinctly grey dusted. Tergum 2 without or with a pair of small yellow oval maculae. Terga 3 and 4 usually with a pair of small yellow elongated triangular maculae. Tergum 5 at anterior margin without or with pair of short yellow maculae. Tergum 2 about 0.6 times as long as its width at its posterior margin. Tergum 3 about 0.7 times as long as its width at its posterior margin. Tergum 4 about 0.9 times as long as its width at its posterior margin. Sternum 2 about 0.8 times as long as its width at its posterior margin. Sternum 3 about 0.8 times as long as its width at its anterior margin. Sternum 4 about 0.8 times as long as its width at its anterior margin. Shape of sterna 2–4 are shown in Fig. 12D.
(25 males and 25 females): Body 7–9 mm.
We have verified specimens from Fennoscandia and central Europe, but data for a more detailed distributional map is presently not available.
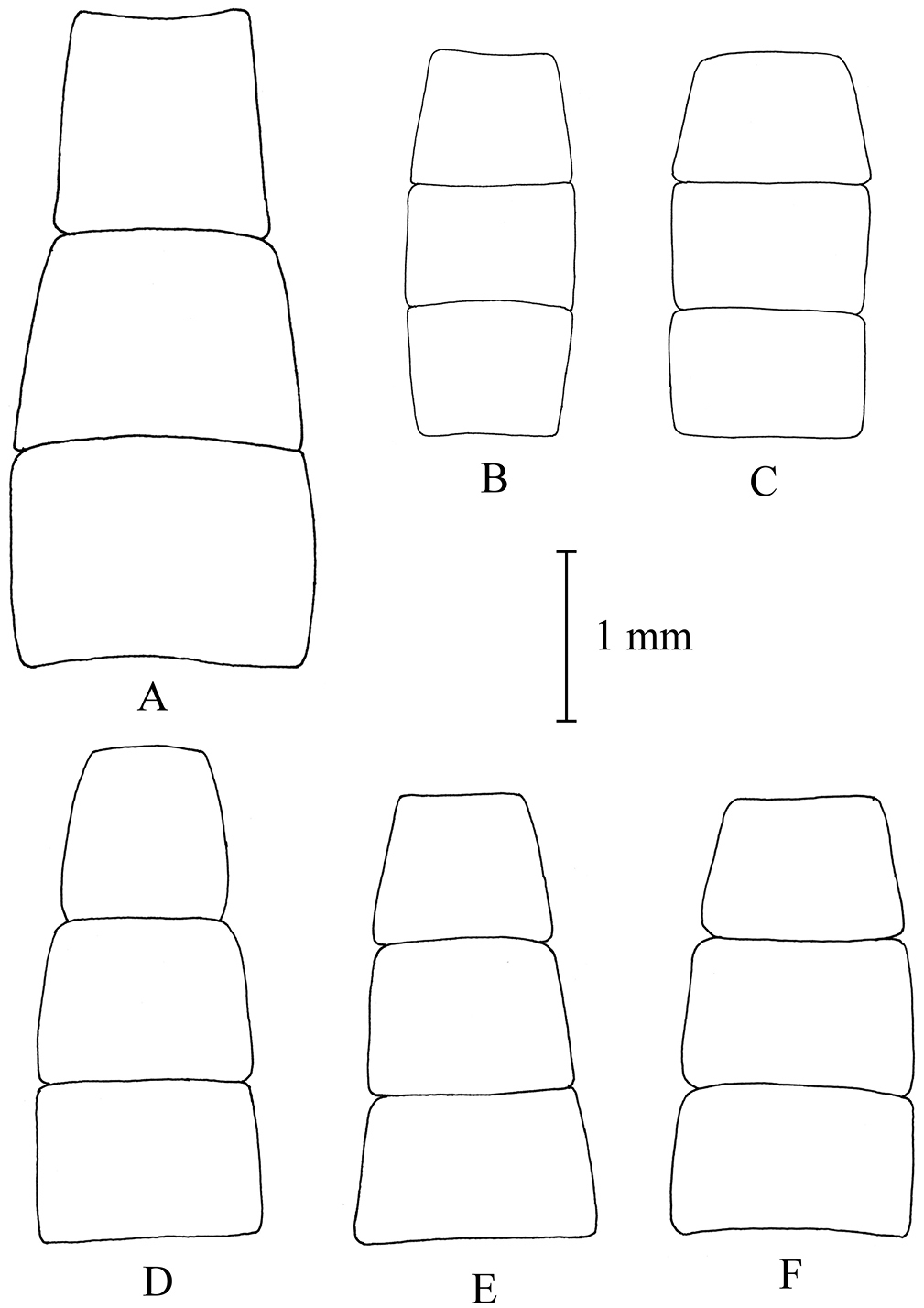
Shape of female sterna 2–4. A Melanostoma scalare, B and C Melanostoma certum D Melanostoma mellarium and E and F Melanostoma mellinum.
Dorsal view of female head. Position of posterior ocellus as compared to the hind eye line of female. A Melanostoma mellarium and B Melanostoma mellinum.
Abdomen of female. A Melanostoma scalare B Melanostoma certum C Melanostoma mellarium D Melanostoma mellinum.
The locality of the lectotype is not indicated in the original label.
Lectotype of Melanostoma mellinum: female, pinned, deposited in LSUK. We designate the specimen with collection number LINN 5304 as lectotype of Musca mellina Linnaeus, 1758. The lectotype bears no original label. Images: http://www.linnean-online.org/22691/.
Lectotype of Syrphus melliturgus: male, pinned, deposited in MNHN. Original label: ’Meigen 1482 40 / Syrphus melliturgus male’. Of the specimen only the thorax with legs and both wings remains. The identity of the specimen cannot be ascertained, but we accept the synonymy.
The type of Syrphus minutum Macquart, 1829 and syntypes of Syrphus unicolor Macquart, 1829 apparently exist at Musée d’Histoire Naturelle, Lille, France, but could not be studied.
Melanostoma mellinum var. melanatus (type material deposited in MZT) is here synonymized with Melanostoma mellarium.
The types of Melanostoma mellinum var. angustatoides Kanervo, 1934 are lost (see section Type studies).
The lectotype of Scaeva dubia Zetterstedt, 1838, original label ’S. dubia f Juckasjärvi'(in MZL) was studied, as well as one syntype (see section Type studies).
A male paratype of Melanostoma clausseni Barkalov, 2009 from the type locality (Russia, Altai, Ulaganskii region, Kuraiskii, 2500-2800m) was provided for study by A. V. Barkalov, and the taxon is here synonymized with Melanostoma mellinum. The type materials of remaining synonyms have not been studied.
DNA voucher specimens in MZH (Table 1); 85 males and 100 females in MZH; 25 male and 25 female specimens in AHPC.
Head: Colour brownish black. Angle of approximation of eyes 80°–90°. Eye contiguity about as long as frontal triangle. Ocellar triangle slightly longer than wide with dark pile and with thin grey dusting. Occiput very narrow and dorsally usually with dark pile and laterally with pale pile. Frontal triangle with indistinct or thin grey dusting. Lateral parts of frontal triangle with dark pile. Face shining with indistinct or thin grey dusting. Lateral parts of face with pile which colour varying from completely pale to almost completely dark. Gena about as wide as basoflagellomere and with greyish dusting. Antenna mainly dark brown, usually basoflagellomere with yellow spot basally at ventral side. Basoflagellomere about 1.4 times as long as its width. Arista usually yellowish brown and about twice as long as basoflagellomere. Longest pile of arista at most half width of arista at base as in Fig. 4B.
Thorax: Scutum shining brownish black except for thin greyish dusting at anterior margin. Scutum with erect pile and with shorter semi-adpressed usually mainly pale pile on anterior margin. Pile rarely mainly dark on scutum. Anterior part of scutum almost always with short erect pile which length about fourth part of length of scutellum. Postpronotum totally covered by thin greyish dusting. Notopleuron usually covered by thin greyish dusting. Scutellum shining brownish black. Scutum and scutellum with pile which colour varying from totally pale brown to almost totally dark. Pleura brownish black and usually with thinly grey dusting. Pleura with pale or brownish erect pile. Calypter brownish with pale brownish pile at edge. Halter yellow with slightly darkened base of stem.
Wing: Usually completely microtrichose, rarely with small bare area on base of cell BM. Membrane with indistinct brownish ting. Stigma usually yellowish brown.
Legs: Coxa black with grey dusting. Trochanter dark brown. Femur usually mainly black except yellow apical part. Tibia usually mainly yellow with dark brown ring varying size. Metatibia usually with a longer dark ring than other tibiae. Tarsus dark brown except mesotarsus with the two basal segments yellow. Leg with pale and dark pile mixed.
Abdomen: Terga dark brown or black with weak greyish dusting. Tergum 2 with pair of yellow oval maculae. Terga 3 and 4 with pair of yellow elongated maculae. Terga 1 and 2 laterally with long pale pile. Terga with varying amount of dark and pale semi-adpressed pile outside of yellow maculae. Only pale pile on yellow maculae. Terga 2, 3 and 4 each about as long as wide. Sterna with weak dusting and with pale semi-adpressed pile. Sternum 2 about 1.3 times as long as its width at its posterior margin. Sternum 3 about 1.2 times as long as its width at its anterior margin. Sternum 4 about as long as its width at its anterior margin. Shape of sterna 2–4 are shown in Fig. 5D.
Male genitalia: Cercus and surstylus (Fig. 6D). Postgonite short and without distinct ridges laterally (Figs 7D, 8D). Postgonite ventrally in Fig. 9D. The hypandrial margin at postgonites with long triangular projections, index DL less than 1.2 (Figs 10D, 11D).
Similar to male, but differs as follows:
Head: Frons shining except greyish dusted triangles. Ventral to the dusted triangles the thinly dusted area is not connected to the sides of lunule. Frons at level of front ocellus slightly narrower than length of antenna. Dorsal part of frons with dark pile and ventral part of frons with pale pile. Occiput as broad as two diameters of an ocellus and usually dorsally with pale and dark pile and laterally with pale pile.
Thorax: Scutum and scutellum with short pale pile. Calypter whitish yellow with whitish pile at edge.
Legs: Coloration of femur varies from mainly yellow to mainly dark. Metaleg usually largely darker than other leg.
Abdomen: Some specimens have all terga dorsally only with pale yellowish semi-adpressed pile. Tergum 2 without or with pair of small yellow oval maculae. Terga 3 and 4 with pair of yellow elongated triangular maculae of varying size or yellow maculae lacking. Tergum 5 at anterior margin without or with pair of short yellow maculae. Tergum 2 about 0.5 times as long as its width at its posterior margin. Tergum 3 about 0.5 times as long as its width at its posterior margin. Tergum 4 about 0.6 times as long as its width at its posterior margin. Sternum 2 about 0.6 times as long as its width at its posterior margin. Sternum 3 about 0.6 times as long as its width at its anterior margin. Sternum 4 about 0.6 times as long as its width at its anterior margin. Sterna 3 and 4 with posterior margin of sternum distinctly broader than width of anterior margin of sternum. Sternum 4 is at most slightly longer than sternum 3. Shape of sterna 2–4 are shown in Fig. 12E, F.
(25 males and 25 females): Body 6–8 mm.
Types were not studied.
DNA voucher specimens in MZH from Hungary, Italy, the Netherlands and Finland (MZH); 16 male and 20 female specimens from Luxembourg, Netherlands, Serbia, Sweden (MZH); 25 male and 25 female specimens in AHPC.
Head: Colour bluish black. Angle of approximation of eyes 80°–90°. Eye contiguity about as long as length of frontal triangle. Ocellar triangle slightly longer than wide with dark and pale pile and with thin grey dusting. Occiput very narrow and with pale pile. Frontal triangle with dense grey or yellowish grey dusting except area above lunule with thin dusting. Lateral parts of frontal triangle with pale pile. Face with dense grey or yellowish grey dusting except shiny facial tubercle. Lateral parts of face with pale pile. Gena about as wide as basoflagellomere and with dense greyish dusting. Antenna mainly yellow, anterodorsal margin of basoflagellomere distinctly brown. Basoflagellomere about 1.5 times as long as wide. Arista yellowish brown and about twice as long as length of basoflagellomere. Arista short pubescent with pile about as long as width of base of arista (Fig. 4A).
Thorax: Scutum shining bluish black except for thin greyish dusting at anterior margin. Scutum with pale yellow erect pile and with shorter pale semi-adpressed pile on anterior margin. Anterior part of scutum with long erect pale pile which length at most third of length of scutellum. Postpronotum totally covered with dense greyish dusting. Notopleuron covered with greyish dusting. Scutellum shining bluish black with pale erect pile at its dorsum, hind margin and ventral side. Pleura bluish black with grey or yellowish grey dusting. Pleura with pale erect pile. Calypter whitish yellow with whitish pile at edge. Halter yellow with slightly darkened base of stem.
Wing: Microtrichose except for cell BM basally narrowly bare, with indistinct brownish ting. Stigma pale yellowish brown.
Legs: Coxa black with grey dusting. Trochanter yellowish brown. Femur and tibia yellow and brown in varying extent. Tarsus yellowish brown. Metaleg usually darker than other leg. Leg with all pile pale.
Abdomen: Terga dark brown or black with weak greyish dusting. Tergum 2 with yellow long oval maculae. Terga 3 and 4 with a pair of long yellow subrectangular maculae. Terga 1 and 2 laterally with long pale pile. Terga with dark and pale semi-adpressed pile. Pale pile on yellow maculae. Terga 2, 3 and 4 each about twice as long as wide. Sterna with weak dusting and with pale semi-adpressed pile. Sternum 2 about 2.5 times as long as its width at its posterior margin. Sternum 3 about twice as long as its width at its anterior margin. Sternum 4 nearly twice as long as its width at its anterior margin. Shape of sterna 2–4 are shown in Fig. 5A.
Male genitalia: Cercus and surstylus (Fig. 6A). Postgonite long and without distinct ridges laterally (Figs 7A, 8A). Postgonite ventrally in Fig. 9A. The hypandrial margin at postgonites with short triangular projections, index DL about 1.5 (Figs 10A, 11A).
Similar to male, but differs as follows:
Head: Frons shining except greyish dusted triangles which narrowly connected to dusted area of face. Frons at level of front ocellus about as broad as length of antenna. Occiput as broad as two diameters of an ocellus.
Thorax: Scutum with short pale pile.
Abdomen: Tergum 2 with pair of yellow oval maculae. Terga 3 and 4 with a pair of yellow elongated triangular maculae. Tergum 5 at anterior margin with a pair of short yellow maculae. Tergum 2 about 0.6 times as long as its width at its posterior margin. Tergum 3 about 0.7 times as long as its width at its posterior margin. Tergum 4 about 0.9 times as long as its width at its posterior margin. Sternum 2 about 0.8 times as long as its width at its posterior margin. Sternum 3 about 0.8 times as long as its width at its anterior margin. Sternum 4 about 0.9 times as long as its width at its anterior margin. Shape of sterna 2-4 are shown in Fig. 12A.
(25 males and 25 females): Body 7–9 mm.
A very common and abundant species, known from the whole Palaearctic area and Northern Africa.
The species in the genus Melanostoma are highly variable in colour and dusting (pollinosity, microtrichosity), and none of the species can be identified solely based on the pale colour and dusting patterns of the abdomen or colouring of legs. The only Fennoscandian species of Melanostoma that seems to be quite stable in its coloration is Melanostoma scalare, but this taxon is also easily distinguished from its congeners based on other characteristics.
Melanostoma scalare can be easily told apart from the other Melanostoma species by its pilose arista (Fig. 4A), densely dusted face and long abdomen (Fig. 14B). Melanostoma certum sp. n. is the relatively smallest and darkest species of the genus. Males of Melanostoma certum can be separated from Melanostoma mellarium and Melanostoma mellinum by the presence of only long (whitish) pile on scutum (Fig. 16B) while Melanostoma mellarium and Melanostoma mellinum have short dark or pale yellowish pile on scutum (Fig. 16A). Females of Melanostoma certum can be partly separated from Melanostoma mellarium and Melanostoma mellinum by the combination of the totally dark abdomen and presence of only whitish pile on abdomen. The female specimens of Melanostoma mellarium and Melanostoma mellinum with pale pilose and dark integument of abdomen (melanic females) have sterna 3 and 4 distinctly broadened towards their posterior margins (Fig. 12D–F), while Melanostoma certum has sterna 3 and 4 evenly broad (Fig. 12B–C). Although typical specimens of Melanostoma mellarium have shiny, indistinctly greyish dusted pleura, some Melanostoma mellarium have thinly, but distinctly, greyish dusted pleura as in Melanostoma certum and typical Melanostoma mellinum. Lastly, Melanostoma mellarium has a longer abdomen than Melanostoma certum and Melanostoma mellinum. Therefore, a reliable identification of M. mellarium, M. mellinum and usually Melanostoma certum implies the study of the length and width proportions of terga and shapes of sterna.
Males (external morphological features)
| 1 | Arista with pile about as long as width of base of arista (Fig. 4A). Abdomen with sternum 2 about 2.5 times as long as its width at its posterior margin and sternum 3 nearly twice as long as its width at its anterior margin (Fig. 5A) | Melanostoma scalare (Fabricius, 1794) |
| – | Arista with pile shorter than half width of base of arista (Fig. 4B). Abdomen with sternum 2 at most twice as long as its width at its posterior margin and sternum 3 at most 1.6 times as long as its width at its anterior margin (Fig. 5B, C, D) | 2 |
| 2 | Anterior part of scutum with long whitish pile at least a third of the length of scutellum (Fig. 16B). Angle of approximation of eyes 85°–100°. Usually terga 2–4 distinctly longer than wide. Sternum 2 at most 1.5 times as long as its width at its posterior margin (Fig. 5B). Sternum 3 at most 1.4 times as long as its width at its anterior margin (Fig. 5B). Pleura usually densely yellowish grey dusted and almost matt | Melanostoma certum sp. n. |
| – | Anterior part of scutum with pile of variable colour and shorter, about a quarter of the length of scutellum (Fig. 16A). Angle of approximation of eyes 80°–90°. If anterior part of scutum with long whitish pile then terga 2–4 about as long as wide | 3 |
| 3 | Usually terga 2–4 about as long as wide. Pleura usually densely yellowish grey dusted and almost matt. Sternum 2 about 1.3 times as long as its width at its posterior margin (Fig. 5D). Sternum 3 about 1.2 times as long as its width at its anterior margin (Fig. 5D). Anterior part of scutum with short mainly pale yellowish pile mixed with variable amount of dark pile | Melanostoma mellinum (Linnaeus, 1758) |
| – | Usually of terga 2–4 distinctly longer than wide. Pleura usually distinctly shining on posterior part of anepisternum, anterior part of anepimeron and dorsal part of katepisternum. Sternum 2 at least 1.6 times as long as its width at its posterior margin (Fig. 5C). Sternum 3 about 1.5 times as long as its width at its anterior margin (Fig. 5C). Anterior part of scutum with variable ratios of short pale yellowish and dark pile | Melanostoma mellarium (Meigen, 1822), stat. n. |
Males (genitalia characteristics)
| 1 | Index DL more than 2.2 (Figs 10B, 11B) | Melanostoma certum sp. n. |
| – | Index DL less than 1.7 (Figs 10A, C, D, 11A, C, D) | 2 |
| 2 | Postgonite short (Figs 7D, 8D) | Melanostoma mellinum (Linnaeus, 1758) |
| – | Postgonite long (Figs 7A, C, 8A, C) | 3 |
| 3 | Index DL about 1.5 (Figs 10A, 11A). Postgonite without distinct ridges laterally (Figs 7A, 8A) | Melanostoma scalare (Fabricius, 1794) |
| – | Index DL less than 1.2 (Figs 10C, 11C). Postgonite with distinct ridges laterally (Figs 7C, 8C) | Melanostoma mellarium (Meigen, 1822), stat. n. |
Females (external morphological features)
| 1 | Arista with pile about as long as width of base of arista (Fig. 4A). Cell BM basally without microtrichia. Face except facial knob with distinct grey dusting | Melanostoma scalare (Fabricius, 1794) |
| – | Arista with pile shorter than half width of base of arista (Fig. 4B). Wing almost always entirely covered with microtrichia. Face shining with weak greyish dusting | 2 |
| 2 | Posterior ocellus in front of the hind eye line (Fig. 13A). Abdomen long with nearly parallel sides. Total length of terga 2, 3 and 4 at least 1.9 times as long as width of posterior margin of tergum 3 (Fig. 14C) (Difficult feature because lateral margins of terga turn under abdomen). Tergum 3 usually almost as long as wide. Pleura usually partly distinctly shining on posterior part of anepisternum, anterior part of anepimeron and dorsal part of katepisternum | Melanostoma mellarium (Meigen, 1822), stat. n. |
| – | Posterior ocellus about at the level of the hind eye line (Fig. 13B). Abdomen short, narrowly or broadly oval. Total length of terga 2, 3 and 4 at most 1.7 as long as width of posterior margin of tergum 3 (Fig. 14B, D). Tergum 3 usually distinctly shorter than its width. Pleura usually densely yellowish grey dusted and almost matt | 3 |
| 3 | Terga black without yellow maculae and with whitish semi-adpressed pile. Sterna 3 and 4 almost evenly broad, rarely slightly broadened towards posterior margins (Figs 12B, 12C). Frons thinly dusted laterad of lunule (Fig. 15B) | Melanostoma certum sp. n. |
| – | Terga black with or without yellow maculae and with pale yellowish semi-adpressed pile usually mixed with dark pile. Sterna 3 and 4 with posterior margin of sternum distinctly broader than width of anterior margin of sternum (Figs 12E, 12F). Frons shiny laterad of lunule (Fig. 15A) | Melanostoma mellinum (Linnaeus, 1758) |
The COI gene 3'–region did not present haplotypes unique to each morphologically identified species, e.g. the Melanostoma scalare taxon, which is morphologically well defined, only showed COI haplotypes shared with other taxa (Fig. 2, Table 2). The ITS2 marker, however, was resolved into five unique sequence clusters (Fig. 3). The new morphological characteristics identified for the Melanostoma taxa occurring in northern Europe are fully consistent with the information from the ITS2 gene region. Thus, based on the ITS2 spacer region and the congruent morphology, and the type studies discussed above, we recognize four taxa in Northern Europe as follows, Melanostoma certum sp. n., Melanostoma mellinum (Linnaeus, 1758), Melanostoma mellarium (Meigen, 1822), stat. n., and Melanostoma scalare (Fabricius, 1794). All species and specimens originating from Russia, Siberia (males and females of Melanostoma clausseni Barkalov, Melanostoma tschernovi Barkalov, Melanostoma dubium and Melanostoma mellinum) that were send for molecular study by Dr. A.V. Barkalov (see Table 1) were identified by Dr. A.V. Barkalov and compared with the types and other materials in his possession. The materials included one paratype, a male of Melanostoma clausseni Barkalov, other specimens of Melanostoma clausseni and Melanostoma tschernovi used for molecular study were not types but most specimens originated from areas close to the type localities. Comparison of the external morphology and male genitalia for these materials (including the paratype of Melanostoma clausseni) with the specimens of the Melanostoma spp. taxa treated in this study, all fit within the morphological variation of Melanostoma mellinum and present identical ITS2 marker sequences. The descriptions of the Melanostoma clausseni and Melanostoma tschernovi species do not describe differences of male genitalia between these taxa, nor do the descriptions indicate genitalia differences with Melanostoma dubium or Melanostoma mellinum.
The process of delimiting and identifying species is potentially better understood if based on comprehensively studied morphology in conjunction with information from DNA sequences of independent loci, and including samples/specimens from as broad geographical distributions as possible. This approach was possible in this particular group as most of the studied taxa of this group are abundant and widely distributed, but only morphology and one genetic marker agree while the COI gene fragment was proved to be uninformative. A high number of haplotypes for the 3'–fragment of the COI gene was recorded (Table 2). Most species exhibited shared haplotypes with another species (Table 2). This could result from incomplete lineage sorting in a recently diverged taxon and / or mitochondrial introgression. The hypothesis of incomplete lineage sorting is plausible since ancestral variability may have been maintained in Europe where the taxa of the genus are widely distributed and copious.
The nuclear ITS2 gene region is still less applied than mtDNA genes (e.g. COI, COII, cytB) for resolving or delimiting closely related taxa. We found that the ITS2 amplified well only for ‘fresh'specimens of <3 years. In this study the ITS2 marker provided complete concordance with our independently established morphological hypothesis for North European Melanostoma spp.
That integumental expression of pale (yellow to red) colour patterns of hoverfly abdomen can be temperature dependent as shown for taxa of Eupeodes Osten Sacken, 1877 (
We thank Dr. Christophe Daugeron and Emmanuel Delfosse (MNHN, Paris), Rune Bygebjerg (MZL, Lund), Veikko Rinne and Anssi Teräs (MZT, Turku) for help with locating materials in collections and providing images, and Anatolij V. Barkalov, Jere Kahanpää, Iiro Kakko, Christian Kehlmaier, Erkki M. and Leena Laasonen, Keijo Mattila, Tore R. Nielsen, Menno Reemer, Axel Ssymank, Wouter van Steenis and Ante Vujić for generously providing specimens for molecular study. This study was partly funded by the Finnish Ministry of Environment project on Deficiently known forest species (PUTTE).