(C) 2013 Bárbara L. V. Romera. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The present redescription of Diplodonta portesiana (d’Orbigny, 1846) is the first part of the revision of this genus in the East Atlantic. This species, despite being common in the Atlantic coast, remains poorly known. A detailed shell and anatomical study was conducted based not only on specimens from the type locality’s vicinities but also on samples from other regions. Diagnostic characters for Diplodonta portesiana includes: rounded shell with a small ligament; triangular, short and deep nymph; external micro ornamentation composed of small concavities in a concentric pattern; small adductor muscles; reduced pedal gape; pair of long hemipalps with a large area covered by folds; stomach with four ducts leading to digestive diverticula; and long intestine length. Our study suggests at least two new diagnostic characters to the genus: the two pair of muscles that controls the incurrent and excurrent openings and a residual ring-like tissue surrounding the anterior half of the posterior foot retractor muscle.
Diplodonta portesiana, Ungulinidae, anatomy, taxonomy, Atlantic Ocean
The ungulinid genus Diplodonta Bronn, 1831 [type: Venus lupinus Broochi, 1814; Italy, Piemonte, Andona Valey; subsequent designation by
The present paper is the first of a series concerned with the systematic revision of the West Atlantic Diplodonta. It focuses on Diplodonta portesiana (d’Orbigny, 1846), a common but usually misidentified regional species. The present redescription is based on type specimens and topotypes with a wide bulk of samples, and also extends the species’ definition to include its anatomy.
A complete list of examined material is presented after the description. The holotype was examined and the other ~40 samples contain individuals from several localities. Shell measures were taken with a digital caliper: length corresponds to the greatest distance between anterior and posterior margin; height is the longest distance between the dorsal umbo’s end and the ventral margin; width is the greatest straight line reaching from the right to the left valve. The dissected specimens were fixed in 70% ethanol and studied under a stereomicroscope by standard techniques (
The following abbreviations are used in the anatomical descriptions and figures: aa: anterior adductor muscle; an: anus; cc: cerebral connective; cg: cerebral ganglia; cn: ctenidial nerve; cp: cerebro-pedal connective; cv: cerebro-visceral connective; dd: digestive diverticula; dg: digestive gland; dh: dorsal hood; em: excurrent muscular wall; er: esophageal rim; es: esophagus; fp: posterior foot retractor muscle; fr: anterior foot retractor muscle; ft: foot; gi: gill; go: gonad; gs: gastric shield; gt: gill tissue; he: heart; id: inner demibranch; im: pair of incurrent channel muscle; in: intestine; ip: inner palp; ki: kidney; lp: labial palp; ld: left diverticula; lp: left pouch; mt: major typhlosole; na: anterior adductor muscle nerve; np: nephropore; nt: minor typhlosole; od: outer demibranch; op: outer palp; pa: posterior adductor muscle; pg: pedal ganglia; pm: pallial muscle; pn: pallial nerve; rd: right diverticula; rn: renal nerve; sm: pair of excurrent channel muscle; ss: style sac; st: stomach; vg: visceral ganglia; vm: visceral mass.
Institutional abbreviations: CENEMAR, Centro de Estudos Marinhos do Atlântico Sul, Porto Alegre, Brazil; CMAC, Museo Argentino Del Caracol, Argentina; MZSP, Museu de Zoologia da Universidade de São Paulo, Brazil; MZUCR, Museu de Zoologia da Costa Rica, Costa Rica; NHMUK, Natural History Museum of United Kingdom.
http://species-id.net/wiki/Diplodonta_portesiana
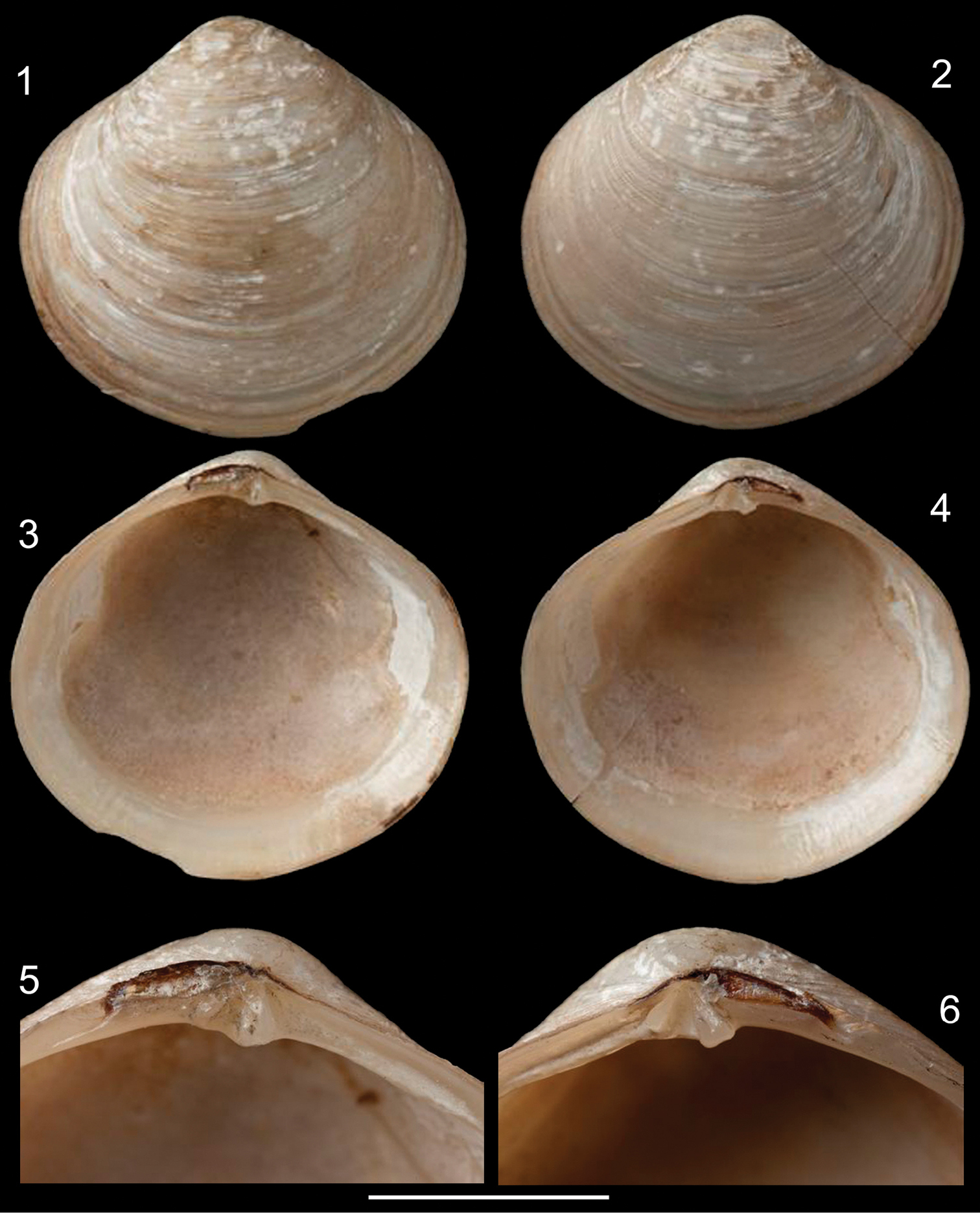
Figs 1–29Holotype NHMUK 1854.12.4.770 (Figs 1–6) (examined).
São Cristóvão Bay, Rio de Janeiro, Brazil.
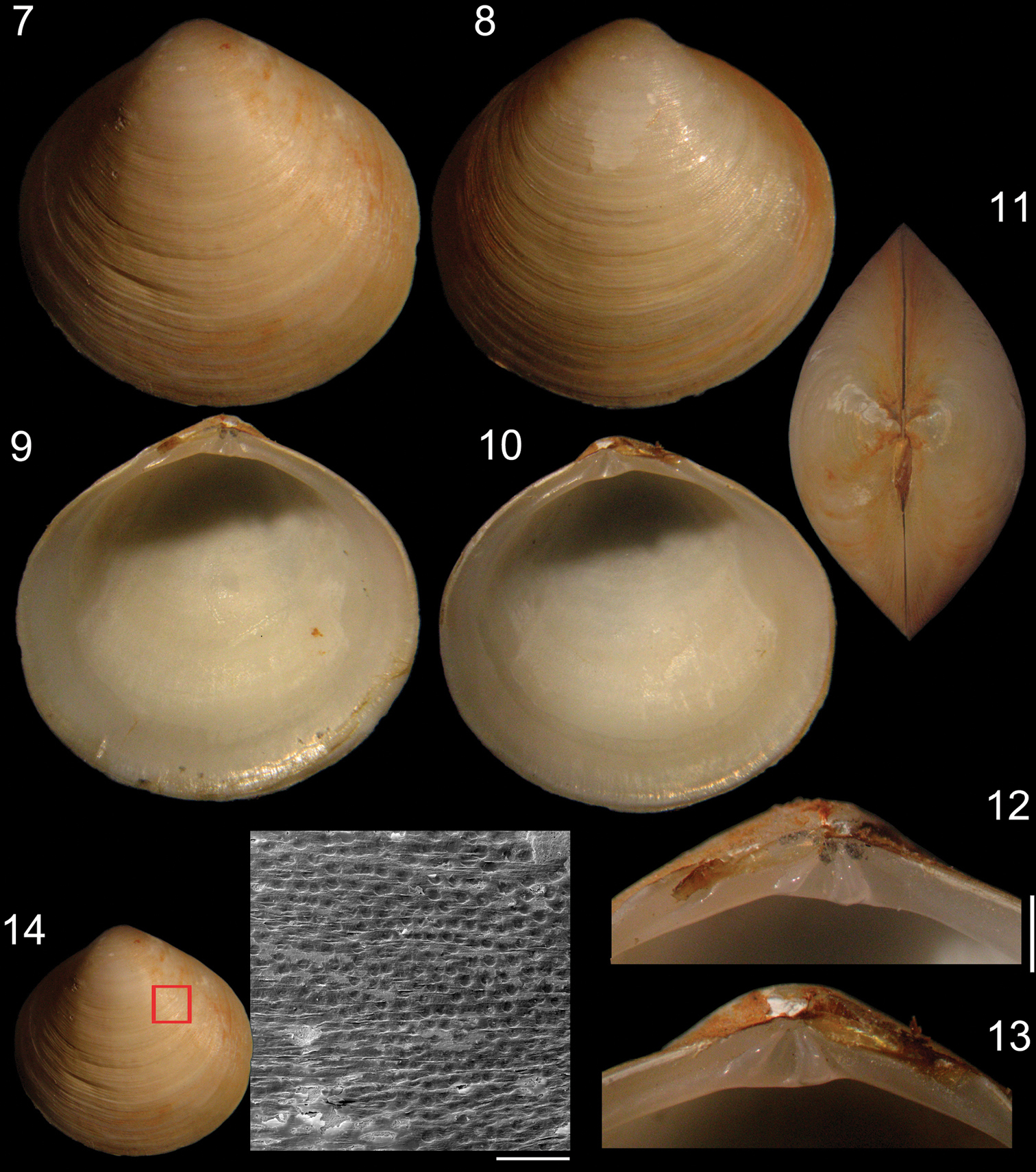
Shell (Figs 1–14): Rounded, centrally pointed, equivalve and inequilateral. Laterally inflated (Fig. 11), maximum inflation ~60% of total length. Externally covered by shallow concentric ribs. Pattern of horizontal aligned, small, rounded pits, slight parallel to concentric growth lines, only visible under SEM (Fig. 14). Color white, periostracum thin, translucent, rarely cream to dark brown close to edges. Valves fragile; internally opaque (Figs 9–10). Anterior adductor muscle scar reniform, ventral portion 2.5 times wider than dorsal portion; located between mid and dorsal thirds of shell height. Posterior adductor muscle scar elliptical, same distance from shell border as anterior adductor, located in median third of valve height. Pallial line entire and thin, away from shell border ~10% of valve height. Umbos low, ~5% of total shell height. Ligament parvincular, opisthodetic, ~20% of total shell length (Figs 11–13). Hinge heterodont, with two cardinal teeth, anterior tooth of left valve and posterior tooth of right valve bifid (Figs 12, 13). Dental shelf long, length equivalent to half of total dorsal margin length, close to cardinal teeth. Dorsal margin concave, forming groove at fusion with dental shelf. Lateral teeth absent. Nymph short, ~20% of total shell length, ~5 times wider than long, triangular. Lunule and escutcheon absent.
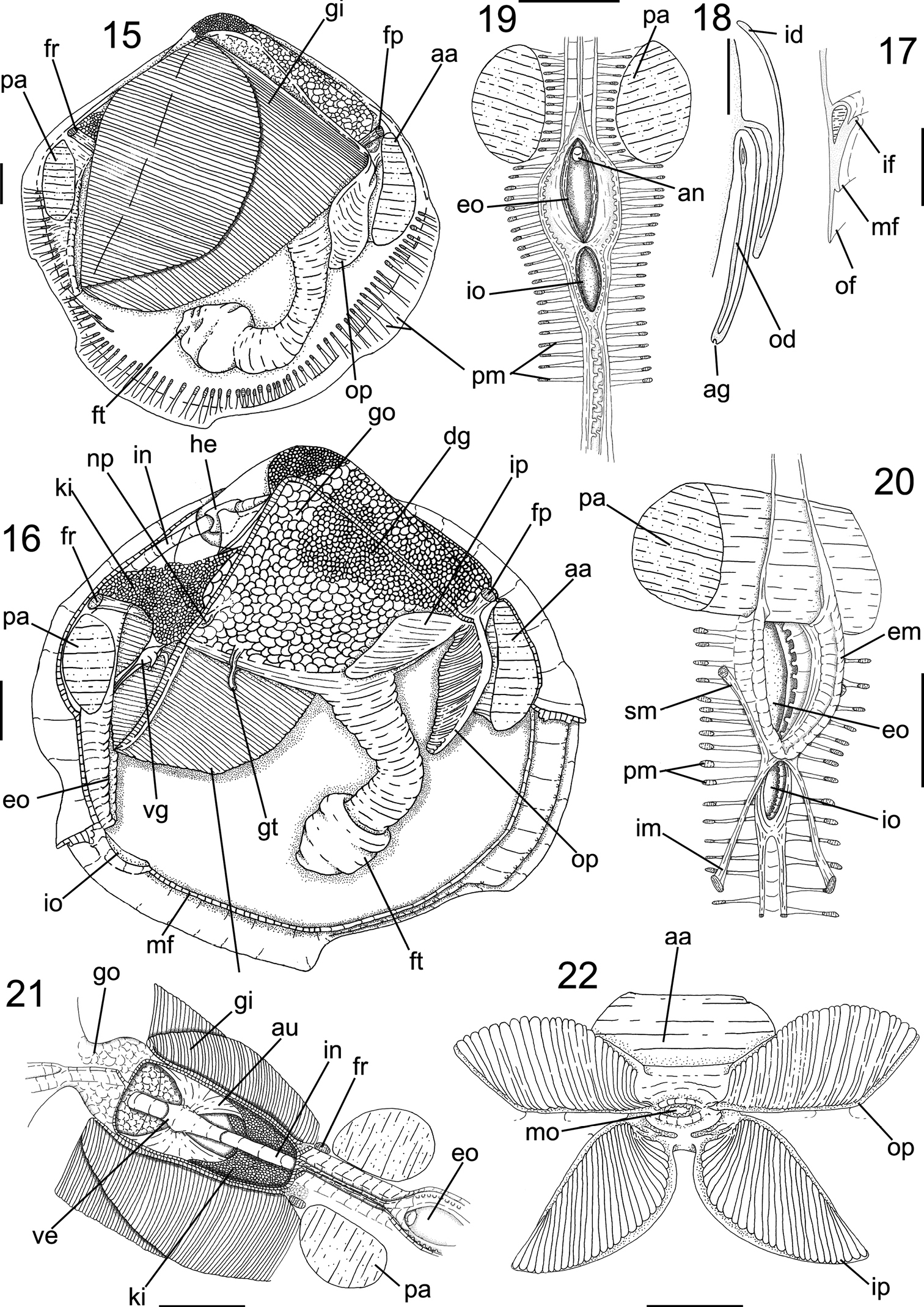
Main muscles (Figs 15, 16, 20, 23): Anterior adductor muscle reniform in section, ~2.6 times higher than wider, ventral half ~2.5 wider than dorsal half (Figs 15, 16, 23); occupying ~1/15 of internal shell volume, located between middle and dorsal third of shell height (Figs 15, 16, 20). Posterior adductor muscle ~40% smaller than anterior muscle, located at opposite end and parallel to anterior muscle (Figs 15, 16, 23). Pair of anterior foot retractor muscles oval in section and thin, originating dorso-posteriorly to anterior adductor muscle, insertion area ~1/20 of adductor insertion, length ~1/4 of total shell length, fusion of both branches occurring in its half-length (Figs 16, 23). Foot retractor muscles oval in section, slightly laterally compressed, thin, ~45% longer than anterior foot retractor muscles, inserting dorsally to anterior adductor muscle, in area equivalent to ~1/40 of anterior adductor insertion, both branches fusing in ventral quarter of shell length (Figs 16, 23). Incurrent and excurrent openings surrounded by two pairs of muscles (Fig. 20); pair of incurrent channel muscles bordering incurrent opening, inserting at dorsal ending of opening; long, straight and thin, equivalent to twice opening length (Fig. 20: im); pair of excurrent channel muscles narrow and thin, inserting below ventral ending of opening; bordering ~60% of excurrent opening length, ~30% shorter than pair of incurrent channel muscles (Fig. 20: sm).
Foot and byssus (Figs 15, 16, 23): Foot long, length ~70% of shell high; terminal bulb cylindrical, expanding ~30% beyond foot width, color orange to light brown; byssal groove and byssus absent.
Mantle (Figs 15–17): Mantle lobes symmetrical, thin, translucent, colorless. Pallial muscles strong and short, distributed evenly in ventral side of mantle lobe, length ~1/10 animal height (Fig. 15). Mantle border with three folds (Fig. 17): outer fold long and thin, ~1/3 of shell thickness, ~15 times higher than wide; middle fold short, twice wider and ~7.5 times shorter than outer fold; inner fold short, with twice as wide and ~5 times shorter than outer fold. Periostracum produced between outer and middle fold. Middle fold with 60 pairs of papillae, bordering all ventral border (Fig. 16); papilla twice taller than wide, tip rounded, separated by area equivalent to 5 times each papilla width. Mantle lobes partially free, fused in ~35% of total mantle lobes length, by inner fold; from incurrent opening to middle portion of animal’s length (Fig. 16: mf); free portion extending until ventral surface of ventral half of anterior adductor muscle, forming pedal gape, corresponding to ~55% of total shell length. Incurrent and excurrent openings formed of fusion of third mantle fold; flanked by ~30 pairs of papillae (Fig. 19: eo); excurrent opening ~1/4 of total shell length; incurrent opening ~60% of total excurrent opening length. No well-developed siphons; except for thick muscular wall internally at excurrent opening, acting like socket to demibranchs (Fig. 20: em). Small striped “ring like” tissue surrounding middle portion of posterior foot retractor muscle, below gill insertion at visceral mass (Fig. 16: gt).
Pallial cavity (Figs 15–16, 18, 22): Occupying half of inner shell volume (Fig. 16). Labial palps small, ~1/30 of inner shell area, triangular; external surface smooth; outer and inner hemipalps similar in size, ~7% longer and ~60% wider than anterior adductor muscle insertion area (Figs 16, 22); outer hemipalp connected to mantle lobe by dorsal border, in ~2/5 of total length; inner hemipalp connected to visceral mass by dorsal border, in ~1/4 of this total length; internal surface of both hemipalps covered by 30 transversal folds; outer hemipalp folds high and rounded in section, covering ~95% of surface, thin smooth area in dorsal margin, corresponding to 1/25 of total surface of hemipalp; folds of inner hemipalp flattened in section and centrally grooved in length; covering ~80% of internal surface of hemipalp, forming two thin smooth areas at ventral and dorsal borders, corresponding 1/8 of total surface of hemipalp; near mouth folds decreasing until forming small grooves converging at middle region of palps, towards to mouth (Fig. 22). Gills large, area ~16 times wider than outer hemipalp area, ~1/3 of total valve area (Figs 16, 18); with two demibranchs; outer demibranch fusiform, twice longer than wide; folded upon ~1/3 of own total area; covering pericardium and kidney; connected to mantle lobe by ~15% of length of postero-dorsal border; inner demibranch triangular, ~3 times longer than wide; folded upon half of total demibranch area; ~45% of external surface covered by outer demibranch; connection to visceral sac by cilia; gill filaments with rounded tips. Suprabranchial chamber volume ~3/5 of infrabranchial chamber volume (Fig. 16).
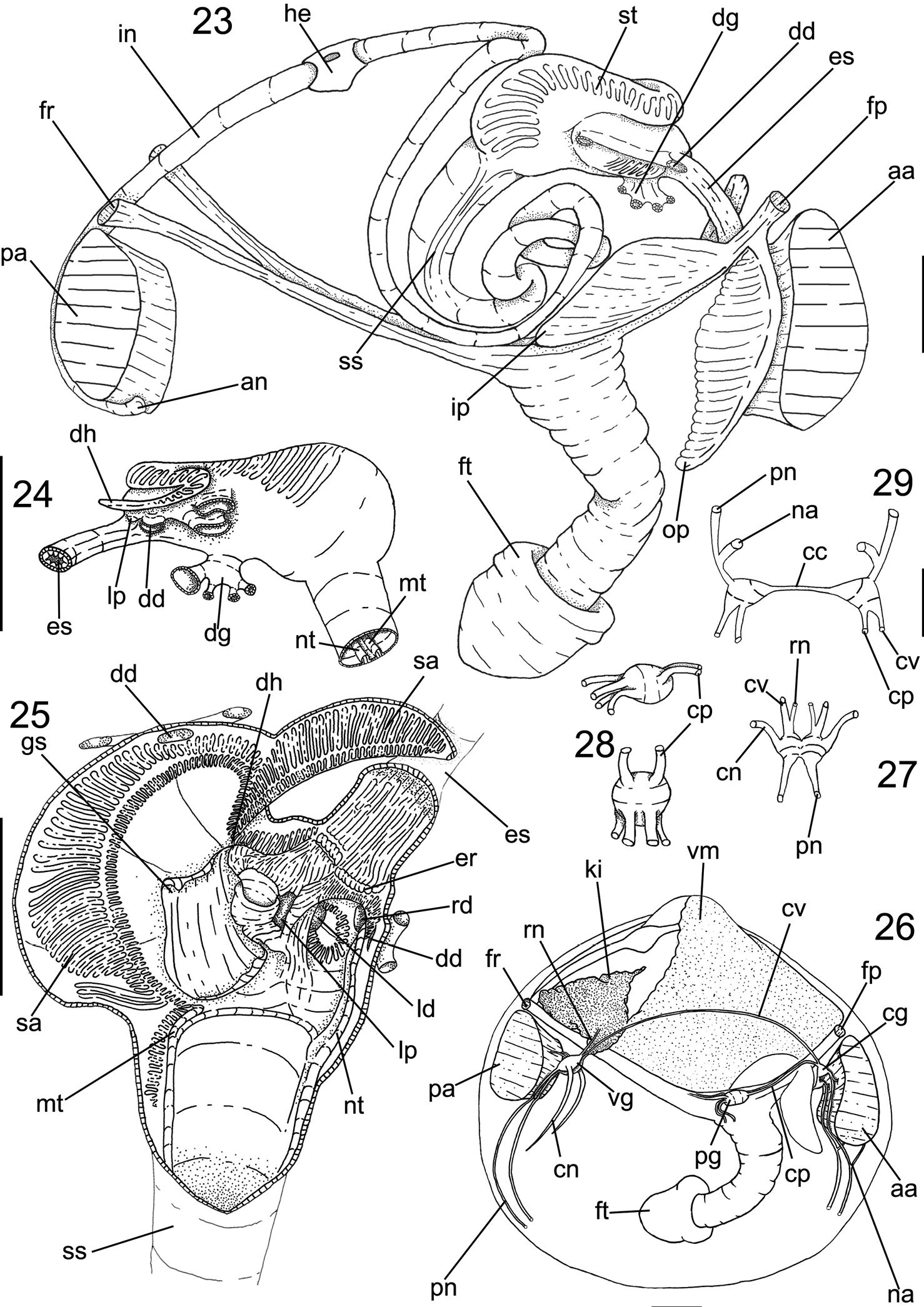
Visceral mass (Figs 16, 23): Visceral sac occupying half of inner shell volume; triangular, inflated, located dorsally between pair of anterior foot retractors and pair of posterior foot retractor, ~3 times larger than muscular base; ~35% of antero-dorsal region filled with greenish brown digestive gland, remainder areas filled with cream colored gonads. Stomach and style sac located vertically at central portion of visceral sac (Fig. 23).
Circulatory and excretory systems (Figs 16, 21): Pericardium located dorso-posteriorly of visceral sac, between umbonal cavity and dorsal kidney surface, occupying ~1/4 of visceral mass volume (Fig. 16). Pair of auricles antero-posteriorly elongated, connected to central axis of gills in 1/5 of gills total length; walls thick and translucent. Ventricle elongated, with thick walls; located at central portion of pericardium; surrounding ~1/3 of intestinal length crossing pericardium; connected to auricles at central portion of lateral walls (Fig. 21). Kidney located postero-ventrally in visceral mass (Fig. 16: ki), below posterior end of pericardium and dorsal surface of posterior foot retractor muscles, color light brown; shape triangular , occupying ~1/4 of visceral mass volume. Nephropore rounded, located at posterior portion of visceral mass, at ~10% of visceral mass height, opening in suprabranchial chamber (Fig. 16: np).
Digestive system (Figs 22–25): Palps and digestive glands described above. Mouth small, located centrally at palps intersection, with small lips (Fig. 22). Esophagus short and narrow, ~1/5 of total length of visceral sac and ~1/10 of high (Fig. 23), cylindrical; not touching anterior adductor muscle, crossing anterior third of anterior foot retractor muscles, parallel to anterior foot retractors (Fig. 23); inner surface covered by longitudinal folds until stomach entry, forming esophageal rim (Fig. 25); connected to anterior surface of stomach. Stomach of medium size, occupying ~1/5 of visceral sac volume, elliptical, located anteriorly at umbo; ~60% of visceral sac length and ~1/3 of high (Fig. 23: st); anterior portion ~30% wider than anterior portion. Pair of ducts to digestive diverticula located ventrally at anterior half of stomach, turned ventrally; digestive diverticula ducts connected to lateral walls of anterior half of stomach; one digestive diverticula duct connected at right wall, dorsally at esophagus line; one digestive diverticula duct connected at left wall, anteriorly to left pouch. Dorsal hood elongated and narrow, ~1/4 of stomach total length, ~4 times longer than wide, pointed anteriorly. Left pouch located below anterior ending of dorsal hood, anteriorly to digestive diverticula ducts; shallow and small, occupying area ~1/10 of surface of stomach (Fig. 24); internally anterior half of gastric chamber partially divided in ventral and dorsal portion by septum (Fig. 25); originating at anterior portion of right wall until dorsal hood entrance, septum length ~1/5 of total stomach length; dorsal surface covered by two sorting areas. Inner surface of stomach mostly smooth, with three distinct sorting areas; first sorting area starting at dorsal region of anterior portion, jointly to dorsal region of esophageal rim; heading dorsally until dorsal hood entrance and folding itself, covering posterior half of dorsal surface of gastric septum, narrow and long, composed of short and transverse folds with equivalent length and width; second sorting area starting on anterior half of dorsal surface of septum; heading from right side to dorsal hood, short and wide, composed of transverse folds twice longer than wide if compared with folds of first sorting area; third sorting area starting inside dorsal hood, running on latero-dorsally posterior right portion of stomach, entering style sac and attenuating. Gastric shield in central region of dorsal wall of stomach, occupying ~25% of total gastric area, translucent and iridescent, with two lateral projections, anterior projection penetrate left pouch and posterior projection penetrate dorsal hood; two gastric ridges narrow and tall running towards style sac, on ventral gastric surface, typhlosoles originating from each ridge. Long ridge originating in internal right wall of left digestive diverticula, leaving posteriorly and entering anteriorly in right side of left diverticula, forming semi-circle, touching left internal wall of diverticula and leaving anteriorly from ventral side of diverticula, on surface of anterior gastric portion, entering in anterior portion of right diverticula; bordering right lateral wall and leaving posteriorly, running on ventral wall of stomach until style sac opening, forming minor typhlosole (Fig. 25: nt) at ventral surface of sac. Small ridge running on ventro-posterior region of stomach, circular shape, flanking style sac entrance, running on ventral surface of sac forming major typhlosole (Fig. 25: mt). Style sac (ss) connecting ventrally to dorsal portion of stomach; conic; narrowing towards dorsal region of visceral sac, ~3.5 longer than wide; occupying ~1/6 of visceral sac total volume, height half and width ~1/10 of visceral sac high (Fig. 23). Intestine narrow and long, originating in level where typhlosoles decreasing in shape at internal surface of style sac (Fig. 23); running towards ventral portion of visceral sac, below central portion of stomach; with three overlapping loops, last one covering dorsally other two; following to posterior portion of visceral sac, parallel to style sac; forming loop on dorso-posterior surface of stomach, leaving visceral sac, crossing pericardium and posterior surface of kidney, crossing between pair posterior foot retractor muscles; touching entire posterior surface of posterior adductor muscle (Fig. 23); intestine total length ~10 times longer than style sac. Anus simple, sessile, opening at ventral region of posterior adductor muscle.
Genital system (Fig. 16): Gonads cream- colored, in granular aspect. Pair of gonoducts connected to gonad acini along posterior portion of visceral sac. Genital pore simple, located at posterior region of visceral sac, opening inside renal chamber.
Central nervous system (Figs 26–29): Pair of cerebral ganglia (cg) surrounding dorsal surface of anterior portion of esophagus (Fig. 29); dorsally at external surface of outer labial palp; triangular shaped, longer than wide; length half of esophagus length; each ganglion with ~1/3 of a transverse section of esophagus; cerebral commissure ~1/7 longer than ganglia total length; from anterior portion connecting anterior adductor muscle nerve (na), touching posterior surface of anterior adductor muscle, bifurcating in two branches; internal branch penetrating dorso posterior third of muscle and leaving at ventral surface of muscle; outer branch bordering posterior surface of anterior muscle, both branches fusing at ventral region of anterior muscle (Fig. 26); two connectives originating dorsally in ganglia, anteriorly to cerebro-visceral connective (cv) crossing visceral mass, touching gonopore dorsally, bordering anterior portion of kidney and connecting dorsally at visceral ganglia, connecting posteriorly cerebro-pedal connective (cp) running immersed in pedal muscles, connecting to anterior region of pedal ganglia. Pair of visceral ganglia (vg) fusiform, small, length same than height, ~1/2 of cerebral ganglia length, partially fused at median portion, with slightly central groove (Fig. 27); located ventrally to kidney, parallel to posterior adductor muscle, in dorsal tip connecting cerebra-visceral connective (described above) and renal nerve (rn), penetrating into kidney area; laterally originating ctenidial nerves (cn) running thought central axis of posterior portion of gills; dorsally originating posterior adductor muscle nerve, penetrating middle region of anterior surface of posterior muscle; at ventral tip originating pallial nerve (pn), touching anterior surface of ventral portion of posterior adductor muscle, running parallel to incurrent and excurrent apertures and mantle border, diffusing at mantle lobe board (Fig. 26). Pair of pedal ganglia (pg) oval, longer than wide, totally fused with each other, similar length and height of cerebral ganglia (Fig. 28); located internally to posterior retractor pedal muscles, above foot insertion, in anterior tip connecting cerebro-pedal connectives from cerebral ganglia; in posterior tip connecting two pairs of nerves, dorsal pair running towards posterior region, inside posterior foot retractor muscles; postero-ventral nerves curved to ventral region, running internally foot.
Diplodonta portesiana. Holotype (NHMUK 1854.12.4.770; L: 20 mm; H: 17 mm). 1 Left valve, external view 2 Right valve, external view 3 Left valve, internal view 4 Right valve, internal view 5 Left hinge detail 6 Right hinge detail. Scale: 2 mm.
Specimen of Diplodonta portesiana (MZSP 22747, L: 13.4 mm, H: 13.1 mm, width: 7.7 mm). 7 Left valve, external view 8 Right valve, external view 9 Left valve, internal view 10 Right valve, internal view 11 Dorsal view 12 Left hinge detail 13 Right hinge detail 14 External surface of shell under SEM; Scale: 2 mm, except 14: 200 µm.
Anatomy of Diplodonta portesiana. 15 Right view, right mantle lobe removed 16 Same view, gill removed 17 Mantle border, transverse section in median portion 18 Gill, transverse section in middle portion 19 Incurrent and excurrent openings, posterior view 20 Same, anterior-slightly right view 21 Pericardium region, dorsal view 22 Labial palps, ventral view, hemipalps deflected; Scale: 2 mm, except 17, 18: 1mm.
Anatomy of Diplodonta portesiana. 23 Digestive system as in situ, right view, foot and main muscles also shown 24 Stomach, left view 25 Internal stomach surface, right view, dorsal gastric wall sectioned longitudinally and deflected 26 Nervous system and topology of other main structures, right view 27 Visceral ganglia, ventral view 28 Pedal ganglia, superior figure in right view, inferior figure in ventral view 29 Cerebral ganglia, anterior view; Scale: 23, 26: 2 mm; 24, 25: 1 mm; 27–29: 0, 5 mm.
Infaunal, in muddy sands; from 0 to 68 m depth.
(length, height and width in mm). MZSP 22747 (Figs 7–14): 13.4 by 7.1 by 7.7; MZSP 105725 #1: 16.4 by 16.1 by 10.1; CENEMAR: 17.6 by 17.3 by 11.7.
From Limón, Cahuita, Costa Rica, to, Bombinhas, Santa Catarina, Brazil.
Holotype HMUK 1854.12.4.770 (d’Orbigny col. 1842); COSTA RICA. Limón; Refugio Nacional de Vida Silvestre, Gandoca- Manzanillo, between Gandoca and Rio Sixaola river mouth, 12 m depth, 09°34'37.8966"N, 82°34'33.0309"W, MZUCR- INB0003816046, 3 valves (T: 181-S. Avilla/Lote: 73904, A. Berrocal id., S. Ávila col. 12/vi/2003); Cahuíta, National Park, 1 km to east of Puerto Vargas. 12 m depth, 9°43'33.3681"N, 82°48'31.5178"W, MZUCR- INB0003414093, 2 valves (S. Ávila id, S. Ávila col. 09/vi/20010); 3 km to North east of Cahuita, reef out, 12 m depth. 9°45'50.3530"N, 82°49'29.9092"W, MZUCR- INB0003411964, 2 valves, (S. Ávila id., J. Magaña col. 11/vi/2001); Punta Cahuita, 8 m depth, 9°44'58.2370"N, 82°49'10.5220"W, MZUCR- INB0001494593, 1 valve (J. Espinosa id., Y. Camacho col. 24/v/1998). BRAZIL. Bahia; Salvador, Todos os Santos bay, MZSP 105725, 1 specimen (CETESB col. ii/2000). Rio de Janeiro; Angra dos Reis, Ilha Grande bay, 7.5 m depth, MZSP 22747, 9 specimens (Emília sta. 61, Ilha Grande Project, 10/xii/1965); 9.6m depth, MZSP 22780, 4 specimens, (Ilha Grande Project, Penna- Neme col. 25/vii/1966); Sepetiba bay, Itacuruça Island, MNRJ 12458, 8 specimens. Santa Catarina; Bombinhas, 3–5 m depth, CENEMAR, 2 specimens, (in shrimp net, J. Tarasconi col., 03/iv/ 1994); 5-10 m depth, CMAC, 3 valves (J. Tarasconi col, 1995); 8–10 m depth, CMAC, 2 valves (trawled by shrimpers, J. Tarasconi col. x/1994).
Below, characters described on this study are compared between classical anatomical studies on genus Diplodonta. Lastly, general aspects concerning the species are analyzed, improving current data aboutthe genus.
Shell: The original description by
Muscular system: A first description on the species’ adductor muscles was offered by
Foot: The foot shape is one of the most outstanding characters in Ungulinidae. It was described by
Mantle: The current translucent mantle and the pedal gape, common to the genus (Mittré, 1850), was found in Diplodonta portesiana. A concentration of glandular cells below the main rejection tract, i.e., between the outer and middle folds of the mantle, and no differences between the papillae of the middle and inner folds have been found in histological studies of ungulinids (
Pallial cavity:
Circulatory system: A ventricle and intestine crossing was noticed by
Digestive system: The digestive structures are delicate and mostly covered by the gonads and the digestive gland. The most striking character in the digestive system is the stomach, complex, possessing several sorting areas and folds.
Central nervous system:
As stated above, a revision of the genus Diplodonta is still in progress, and several diagnostic morphological and shell characters have already been found. This paper, with a more complete description of Diplodonta portesiana, is only the first step of this effort. The other species already are under study in order to improve the taxonomy of Diplodonta in the near future. The further publications will allow more comparisons and discussion of the taxonomical features, from species to family levels.
The authors wish to thank Kathie Way, John Taylor, Phil Hurst and Andreia Salvador, NHMUK, by pictures of the Diplodonta portesiana type material; José C. Tarasconi, CENEMAR, for lent material from the South Brazil locality; Ênio Mattos and Phillip Lenktaitis from Laboratório de Miscroscopia Eletrônica da Universidade de São Paulo, by the help in SEM pictures; Yolanda Camacho, MZUCR, by lent material from the central coast of East Atlantic; Daniel Forcelli, who lent samples from the South Atlantic of Argentina coast; Rodrigo B. Salvador (Staatliches Museum für Naturkunde Stuttgart) for the revision of English and precious tips to improve the text. The present study was partly supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), proc. #2010/11401-8 (Bárbara L.V. Romera) and proc. #2010/11253-9 (Carlo M. Cunha).