(C) Juan J. Morrone. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
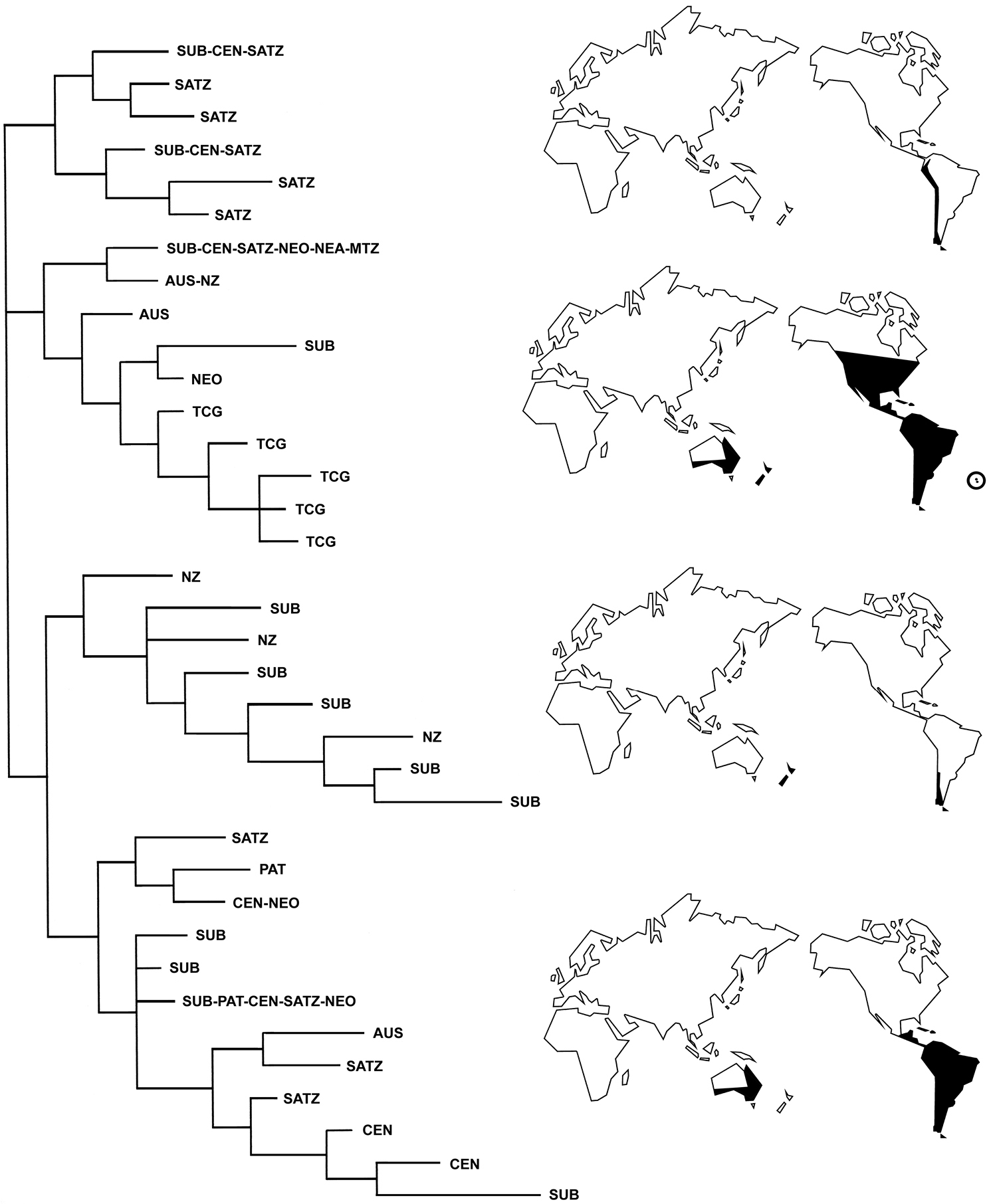
The phylogenetic relationships of the genera of Listroderini LeConte, 1876 are analyzed based on 58 morphological characters. The genera are grouped in four clades, which are given subtribal status: Macrostyphlina new subtribe (Adioristidius, Amathynetoides, Andesianellus, Macrostyphlus, Nacodius and Puranius), Palaechthina Brinck, 1948 (Anorthorhinus, Gunodes, Haversiella, Inaccodes, Listronotus, Neopachytychius, Palaechthus, Palaechtodes, Steriphus and Tristanodes), Falklandiina new subtribe (Falklandiellus, Falklandiopsis, Falklandius, Gromilus, Lanteriella, Liparogetus, Nestrius and Telurus), and Listroderina (Acroriellus, Acrorius, Acrostomus, Antarctobius, Germainiellus, Hyperoides, Lamiarhinus, Listroderes, Methypora, Philippius, Rupanius and Trachodema). The subtribes are characterized and keys to identify them and their genera are provided. Listroderini have four main biogeographical patterns: Andean (Macrostyphlina), Andean-New Zealand (Falklandiina), Andean-Neotropical-Australian (Listroderina) and Andean-Neotropical-Australian-New Zealand-Nearctic-Tristan da Cunha-Gough islands (Palaechthina). Geographical paralogy, particularly evident in the Subantarctic subregion of the Andean region, suggests that Listroderini are an ancient Gondwanic group, in which several extinction events might have obscured relationships among the areas.
Cyclominae, weevils, Americas, Australia, New Zealand, Tristan da Cunha-Gough islands
Listroderini LeConte, 1876 are one of the largest tribes of Cyclominae (
Listroderini were originally assigned to the subfamily Cylydrorhininae (e.g.,
My objective is to analyse the cladistic relationships of the genera of Listroderini, especially to determine the phylogenetic placement of the genera from Australia, New Zealand and the Tristan da Cunha-Gough islands. I intend to provide a phylogenetic framework for future studies and to summarize the systematics and biogeography of the genera to date.
The studied specimens were provided by the following collections:
AMNH American Museum of Natural History, New York, USA.
AMPC Amyan MacFadyen, private collection, Coleraine, Northern Ireland.
ARPC Alexander Riedel, private collection, Friedberg, Germany.
BMNH The Natural History Museum, London, England.
BPBM Bernice P. Bishop Museum, Honolulu, USA.
CADIC Centro Austral de Investigaciones Científicas, Ushuaia, Argentina.
CBPC Carlos Bordón, private collection, Maracay, Venezuela.
CMNC Canadian Museum of Nature, Ottawa, Canada.
CNCI Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Canada.
CWOB Charles W. O’Brien private collection, Arizona, USA.
DEI Deutsches Entomologisches Institut, EberswaldeFinow, Germany.
DZUP Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Brazil.
FIML Fundación e Instituto Miguel Lillo, San Miguel de Tucumán, Argentina.
FMNH Field Museum of Natural History, Illinois, USA.
GJWC Guillermo J. Wibmer, private collection, Tallahassee, USA.
IADIZA Instituto Argentino de Investigaciones de las Zonas Áridas, Mendoza, Argentina.
ICNB Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Santafé de Bogotá, Colombia.
IPUM Instituto de la Patagonia, Universidad de Magallanes, Punta Arenas, Chile.
MACN Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
MCZ Museum of Comparative Zoology, Harvard University, Massachusetts, USA.
MHNS Museo Nacional de Historia Natural, Santiago, Chile.
MLP Museo de La Plata, La Plata, Argentina.
MNHN Museum National d´Histoire Naturelle, Paris, France.
MZFC Museo de Zoología “Alfonso L. Herrera”, Facultad de Ciencias, UNAM, Mexico City, Mexico.
NZAC New Zealand Arthropod Collection, Auckland, New Zealand.
SMTD Staatliches Museum für Tierkunde, Dresden, Germany.
USNM National Museum of Natural History, Washington D.C., USA.
ZMC Zoologisk Museum, Copenhagen, Denmark.
ZMHU Zoologische Museum der Humboldt Universität, Berlin, Germany.
Habitus drawing were made with a camera lucida attached to a stereoscopic microscope. Photographs were taken using a Scanning Electron Microscope at the Facultad de Ciencias, UNAM.
For the present study I examined species of the genera previously recognized for the tribe (
The 58 morphological characters used in the analysis were taken from external structures (53) and male and female genitalia (5). The distribution of character states is shown in the data matrix (Table 1). The characters and their corresponding character states are as follows:
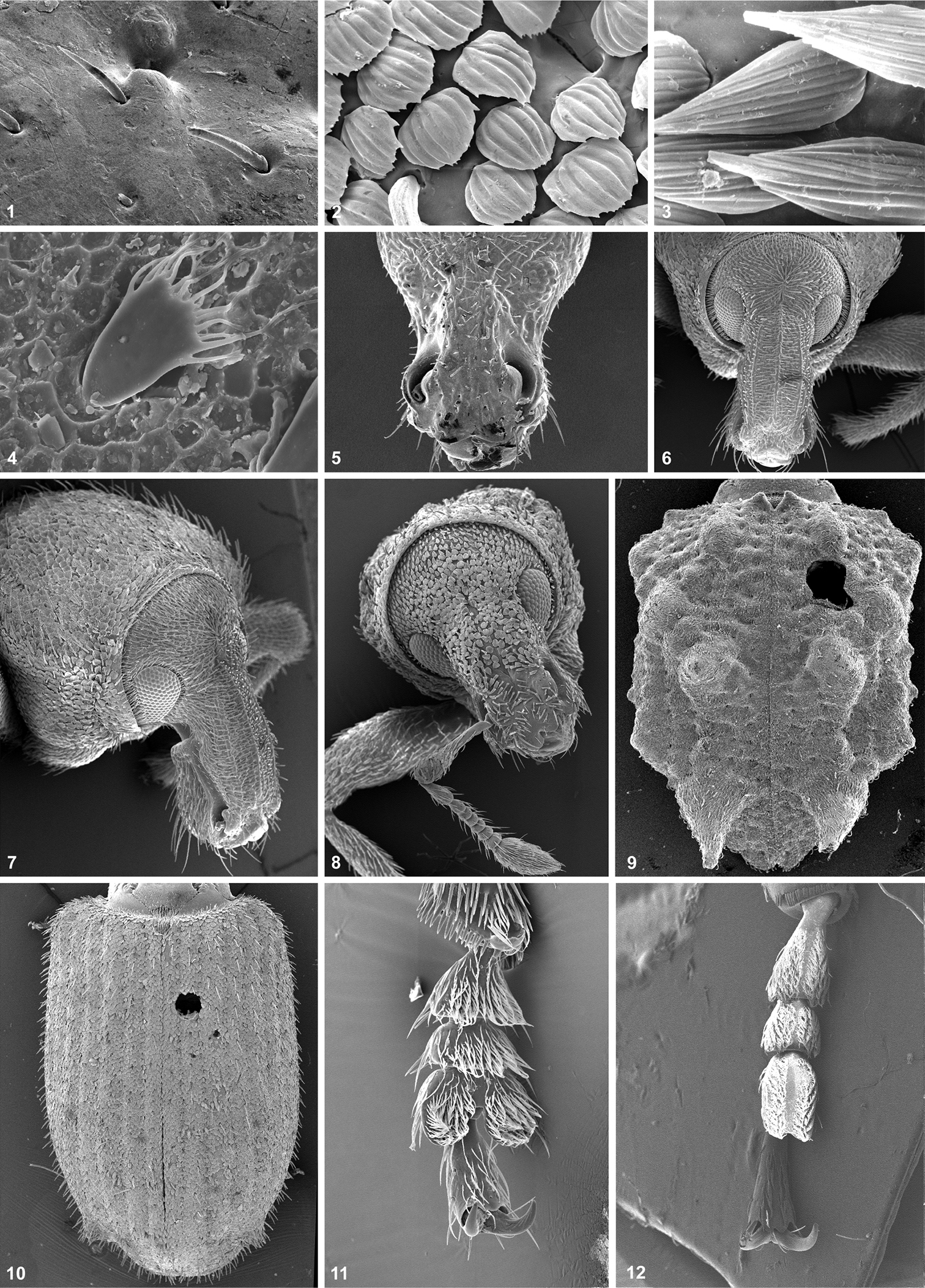
1 Body: length. (0) large to very large (> 15.0 mm); (1) medium-sized (7.1–14.9 mm); (2) small to very small (< 7.0 mm) [additive].
2 Vestiture: scales. (0) present; (1) absent.
3 Vestiture: scale shape. (0) seta-like (Fig. 1); (1) subcircular (Fig. 2); (2) lanceolate (Fig. 3); (3) with finger-like processes (Fig. 4) [non-additive].
4 Vestiture: setae. (0) present; (1) absent.
5 Rostrum: shape. (0) stout, very short (Fig. 5); (1) relatively stout, medium-sized, shorter than pronotum (Fig. 6); (2) slender, as long as or longer than pronotum [additive].
6 Rostrum: dorsal carinae. (0) present (Fig. 6); (1) absent (Figs 5, 8).
7 Scrobes: shape. (0) long, deep, sharply bordered, reaching eyes; (1) short, ill-defined, broad.
8 Epistome. (0) poorly demarcated; (1) raised.
9 Scrobes: position. (0) dorsolateral to dorsal; (1) lateral.
10 Suprascrobal keels. (0) absent; (1) present.
11 Scrobes: ventral tooth. (0) absent; (1) present (Fig. 7).
12 Pterygia. (0) simple, not exposed (Fig. 6); (1) auriculate, exposed (Fig. 5).
13 Mandibles. (0) with one apical cusp; (1) with two apical cusps.
14 Mandible and pharyngeal processes. (0); short and strong; (1) long and narrow.
15 Mandibles. (0) plurisetose (more than 4 setae); (1) paucisetose (1-4 setae).
16 Maxillary malae: teeth. (0) present; (1) absent.
17 Eyes: shape. (0) subcircular (Fig. 5); (1) transverse (Fig. 7).
18 Eyes: size. (0) large to medium (more than 30 facets); (1) small (10-25 facets); (2) very small (8 or fewer facets) [additive].
19 Eyes: position. (0) lateral (Fig. 6); (1) dorsal (Fig. 5).
20 Eyes: convexity. (0) strong; (1) slight; (2) flat [additive].
21 Antennal insertions. (0) distal; (1) at the middle of the rostrum.
22 Scapes: length. (0) long (surpassing posterior margin of eyes when resting in scrobe); (1) medium-sized (reaching eyes when resting in scrobe); (2) short (not reaching anterior margin of eyes when resting in scrobe) [additive].
23 Funicles: segment 1. (0) elongate; (1) globose.
24 Funicles: segments 2. (0) elongate; (1) globose.
25 Funicles: relative lengths of segments 1 and 2. (0) 1 longer than 2 (Fig. 8); (1) 1 subequal to or shorter than 2.
26 Funicles: segments 3–6. (0) elongate; (1) globose (Fig. 8).
27 Clubs: shape. (0) fusiform; (1) inflated.
28 Pronotum: shape. (0) subcircular; (1) transverse; (2) subtrapezoidal; (3) subquadrate; (4) subclyndrical [non-additive].
29 Pronotum: width. (0) larger than that of elytra; (1) smaller than that of elytra.
30 Pronotum: disc. (0) rugose; (1) smooth, polished.
31 Pronotum: tubercles. (0) absent; (1) present.
32 Postocular lobes. (0) present, well-developed; (1) present, slightly developed; (2) absent [additive].
33 Prosternum. (0) non-excavate; (1) excavate.
34 Metanepisternal sutures. (0) posteriorly fused or obliterated; (1) present, complete.
35 Scutellum. (0) not visible; (1) visible.
36 Elytra: shape. (0) oblong-oval (Fig. 10); (1) subrectangular (Fig. 9); (2) elongate-oval [non-additive].
37 Elytra. (0) not fused; (1) fused along interelytral suture.
38 Elytral disc. (0) convex; (1) slightly convex; (2) flat [additive].
39 Elytral intervals. (0) convex; (1) flat.
40 Elytral basal margin. (0) not raised; (1) raised, subcarinate.
41 Elytral humeri. (0) rounded; (1) subquadrate.
42 Elytral humeral tubercles. (0) absent; (1) present.
43 Several tubercles on elytral disc. (0) present, small, rounded; (1) absent; (2) present, strong (Fig. 9) [non-additive].
44 Series of three tubercles restricted to elytral interval 3. (0) absent; (1) present.
45 Series of declivital tubercles on elytra. (0) absent; (1) present.
46 Carina on elytral apical declivity. (0) absent; (1) present.
47 Anteapical elytral tubercle. (0) absent; (1) present.
48 Elytral apex, female. (0) not produced; (1) produced.
49 Femora: shape. (0) subcylindrical, clavate; (1) dorsoventrally compressed, clavate; (2) subcylindrical, markedly clavate [non-additive].
50 Tibiae: shape. (0) subcylindrical, laterally not expanded; (1) apically expanded.
51 Tibial spurs. (0) present; (1) absent.
52 Tarsomeres 3. (0) bilobed (Fig. 11); (1) subcylindrical (Fig. 12).
53 Ventrites 3 and 4, female. (0) combined shorter than 5; (1) combined longer than 5.
54 Aedeagus, lateral view. (0) robust; (1) slender.
55 Distal gonocoxites. (0) strongly sclerotized; (1) membranous.
56 Styli. (0) well-developed, claw-like; (1) well-developed, finger-like; (2) reduced to a few vibrissae [non-additive].
57 Apodeme of female sternum 8. (0) short (< 3 times longer than plate); (1) long (> 4 times longer than plate).
58 Plate of female sternum 8. (0) developed; (1) reduced.
The cladograms were constructed using software TNT (
Data matrix analysed. Character states of polymorphic taxa are indicated between square brackets.
| Epicthonius | 0000000000000000000000000000000000000000000000000000000000 |
| Hyomora | 1010000010001010100202000100000001000000001000000110000000 |
| Rhythirrinus | 1010000010001010100101000101001011110000001000100010000200 |
| Aphela | 21?0010010001010010201101100000001000000001000000010001200 |
| Telurus | 21?0011010011010000101000110000200100000001000010000101210 |
| Acroriellus | 2000101010001010100101000101000101100010000110000000001200 |
| Acrorius | 2000101010001010100101000101001101100100000010100000001100 |
| Acrostomus | 1000101110101010100100000003000101100010001000000000001[01]00 |
| Adioristidius | 2000100011001010110101000104000100100000001000000000011110 |
| Amathynetoides | 2000100011001010110101000100010101100010001000000000011110 |
| Andesianellus | 21?0100011001010020201000114000200100001001000000000001110 |
| Anorthorhinus | 2000200010001010100102000004000101100000001000000000001100 |
| Antarctobius | 10[01]0101010001010100101000100000201100000001000100000001[12]00 |
| Falklandiellus | 2010011010011010100101000101000200100000001010000000001100 |
| Falklandiopsis | 21?0011010011010101201000100000101100000011010002000001100 |
| Falklandius | 20?0011000011010011200010110000200100000001000000000001110 |
| Germainiellus | 1000101010001010100101000101000101100000001000100000001100 |
| Gromilus | 2000101010011010100200000004000101100000000000000000001100 |
| Gunodes | 1010200010001010100102001110000101100010001000000000001100 |
| Haversiella | 2011210010001011100112000100000201120010001000000010001201 |
| Hyperoides | 1020101010001010100100000101000101100010001000000000011100 |
| Inaccodes | 2000200010001010100102000110000101100010001000000000001100 |
| Lamiarhinus | 2000101010001010100100000001001101111000102010100000001100 |
| Lanteriella | 21?00110000110100212000101000102001000100010000011000?12?0 |
| Liparogetus | 1000001010011010100111000113000201100000001000000010001100 |
| Listroderes | [12]010101010[01]01010100101000101000101100000001000100000001[01]00 |
| Listronotus | [12]010[12]00010001010100101001104000101100000001000100000001100 |
| Macrostyphlus | 2010100011001010100101000114000200100000000000000000011110 |
| Methypora | 2010100010001010100102000104000101110200000000110000001100 |
| Nacodius | 2000100011001010100100000100010201100010001000000000011100 |
| Neopachytychius | 2010200010001110100102000100000101100010001000000000001100 |
| Nestrius | 2000101000011010010200000114000200000000001000000000001100 |
| Palaechthus | 1000210010001010100102001112000101120010001000000000001100 |
| Palaechtodes | 1000200010001010100102001114000101120010001000000000001100 |
| Philippius | 0030101010001010110201000100101101011200101010100011001100 |
| Puranius | 2010100011001010100101000111000101100010000000000000011110 |
| Rhigopsidius | 1030000110001010100201000101001011110000102010100000000000 |
| Rupanius | 2000100010001010100101000101001101110100002001000000001200 |
| Steriphus | 1010200010001010100100000100000101120000001000100000001100 |
| Trachodema | 2030101010001010100100000101001101100100102010100000001100 |
| Tristanodes | 2000200010001010100102001114000101120010001000000000001100 |
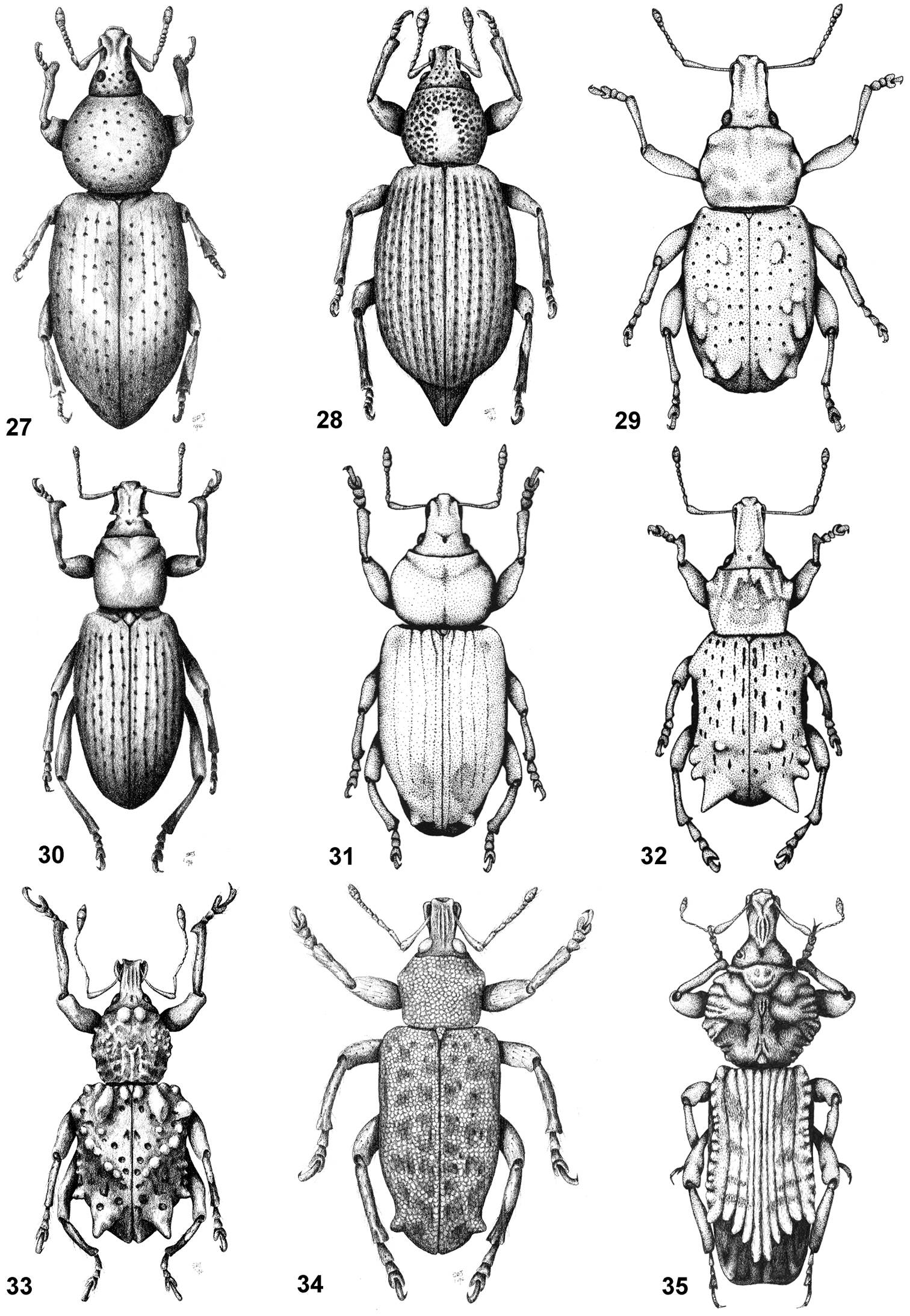
Some of the characters analysed. 1 Seta-like scales 2 subcircular scales 3 lanceolate scales 4 scales with finger-like processes 5, 6, 8 face and rostrum, dorsal view 7 face and rostrum, lateral view 8, 9 elytra, dorsal view 11, 12 tarsomere 3, ventral view. 1, 5 Falklandius antarcticus; 2, 8, 11 Falklandiellus suffodens; 3 Hyperoides subcinctus; 4, 12 Philippius superbus; 6, 7, 10 Listroderes costirostris; 9 Lamiarhinus aelficus.
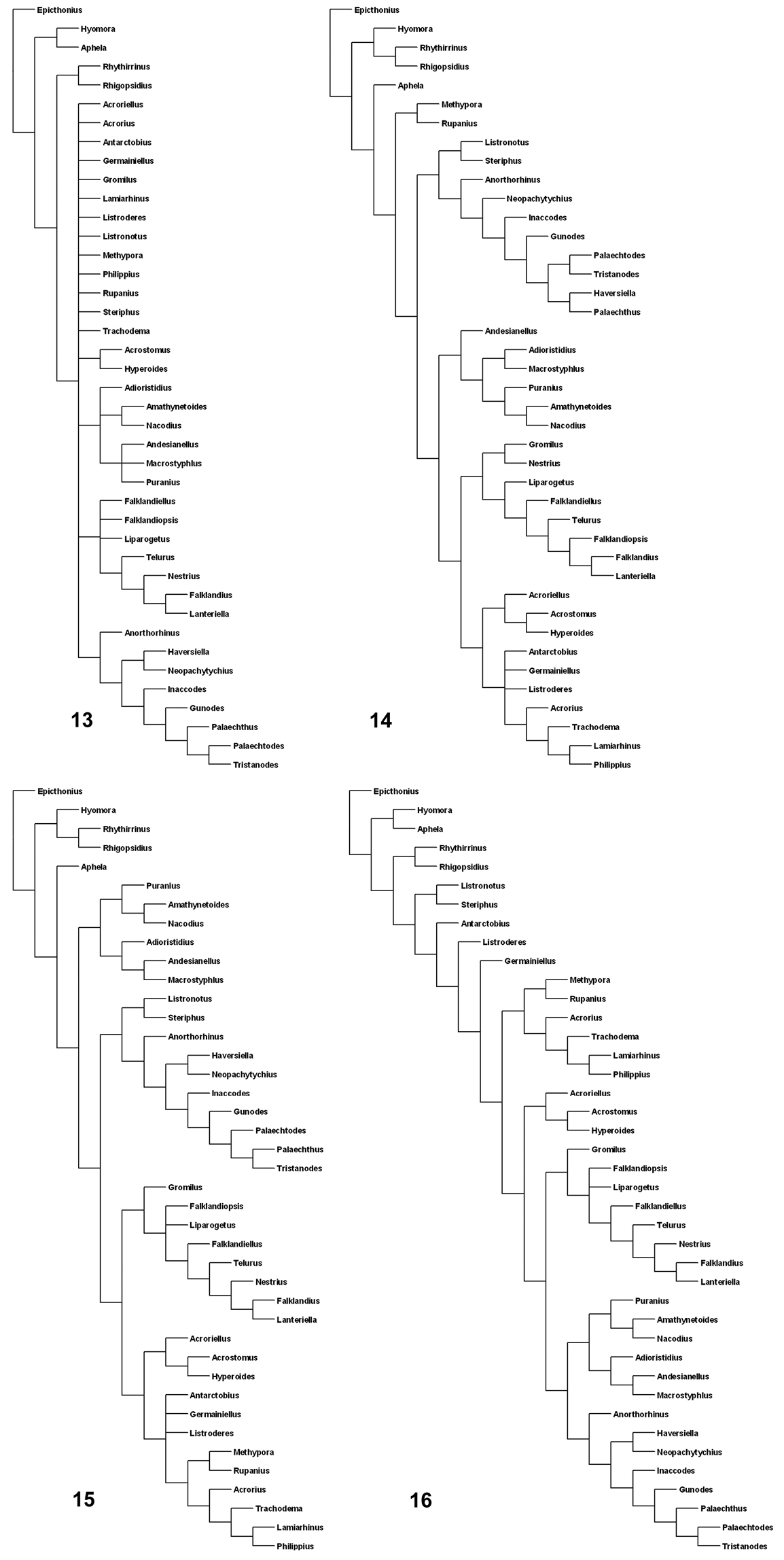
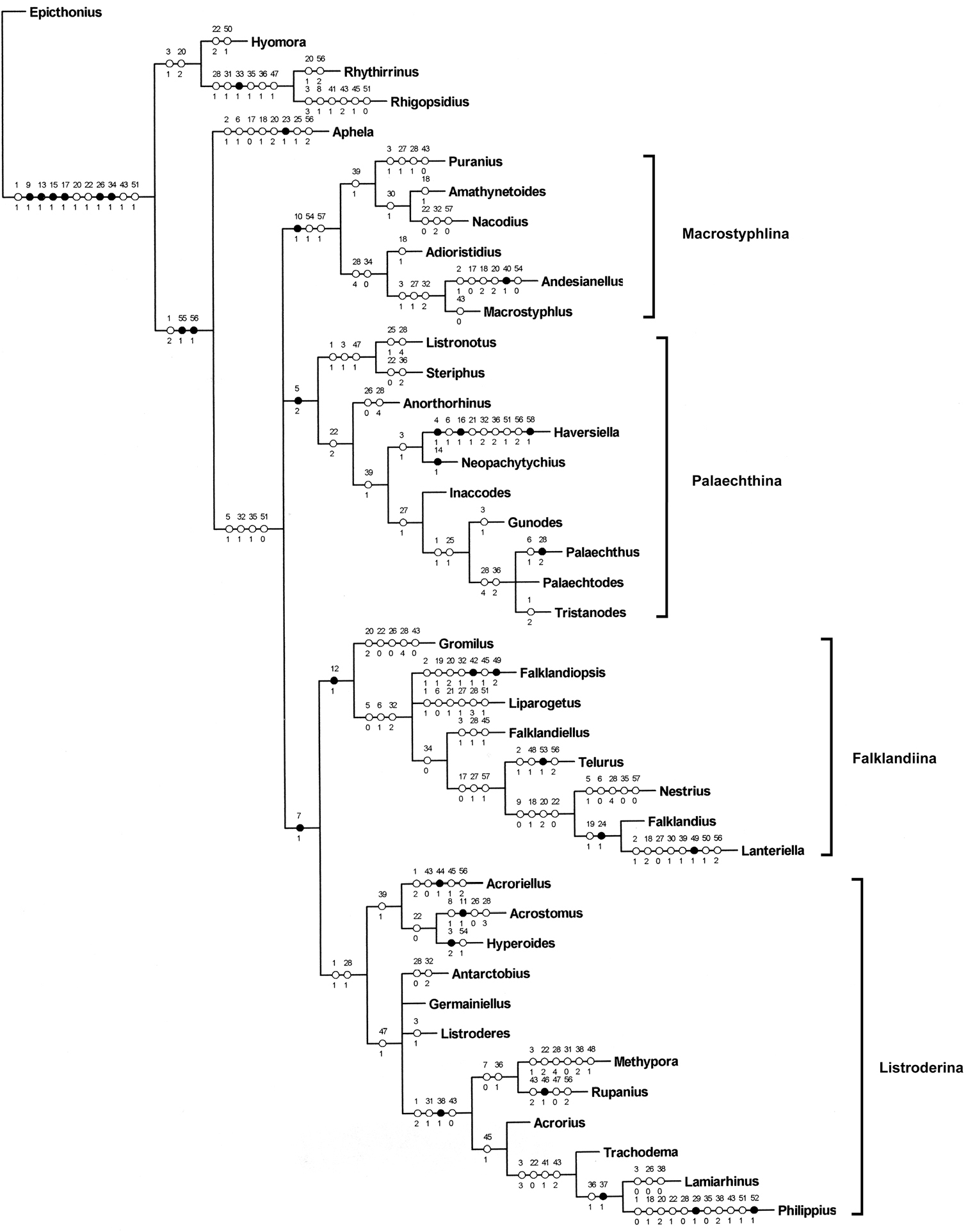
The analysis of the data matrix (Table 1) under equal weights and with different concavity constants led to different cladograms: 100 cladograms under equal weights (Fig. 13); three cladograms with k=3 (Fig. 14); six cladograms with k= 6 (Fig. 15); and two cladograms with k= 12 (Fig. 16). In all the analyses the tribe Listroderini is recovered as a monophyletic taxon. Rhigopsidius, previously placed by
I consider that the results of the analysis with k= 6 are not as extreme as the others and show more clearly the four main clades, which are treated herein as subtribes (Fig. 17):
1 Macrostyphlina new subtribe: genera Adioristidius, Amathynetoides, Andesianellus, Macrostyphlus, Nacodius and Puranius.
2 Palaechthina Brinck, 1948: genera Anorthorhinus, Gunodes, Haversiella, Inaccodes, Listronotus, Neopachytychius, Palaechthus, Palaechtodes, Steriphus and Tristanodes.
3 Falklandiina new subtribe: genera Falklandiellus, Falklandiopsis, Falklandius, Gromilus, Lanteriella, Liparogetus, Nestrius and Telurus.
4 Listroderina LeConte, 1876: genera Acroriellus, Acrorius, Acrostomus, Antarctobius, Germainiellus, Hyperoides, Lamiarhinus, Listroderes, Methypora, Philippius, Rupanius and Trachodema.
Consensus cladograms of the different analyses. 13 equal weights 14 k=3 15 k=6 16 k=12.
Consensus cladgrma of the cladograms obtained with k=6 with character state changes indicated.
Listroderes Schönherr, 1826.
Very small to very large (1.0–22.8 mm); integument reddish brown (black in Acrostomus); vestiture consisting mostly of dense scales and setae (rarely only scales or setae), setae on rostrum and pronotum directed anteriad or mesad, on elytra posteriad; rostrum stout and very short to slender, as long as or longer than pronotum; scrobes usually lateral; epistome poorly demarcated, rarely raised (Acrostomus); eyes usually large, flat, transverse or subcircular; mandibles with two apical cusps and paucisetose (1-4 setae); antennae with funicle 7-segmented, segments 1 and usually 2 elongate, clubs fusiform or inflated; prothorax with or without postocular lobes; prosternum long, non-excavate; elytra oblong-oval, elongate-oval or subrectangular; tibiae mucronate, generally with spurs (when present pro- and mesotibiae with 1 spur and metatibiae with 1–2 spurs); claws divaricate, simple or with slight basal swelling; aedeagus with tegmen lacking parameres (reduced in Methypora); distal gonocoxites membranous, generally simple, with large, apical or subapical stylus carrying a tuft of setae, but occasionally without stylus and apex of gonocoxite flattened and bent outwards.
Listroderini were formerly considered as related to Rhythirrinini (
Larvae of Listroderini are generally oligophagous ectophytic root-feeders (
| 1 | Rostrum slender, as long as or longer than pronotum (except shorter than pronotum in some species of Listronotus); scrobes long, sharply bordered, reaching eyes; funicular segment 1 usually subequal to or shorter than 2; commonly associated with aquatic or semiaquatic plants | Palaechthina |
| – | Rostrum stout or relatively stout, shorter than pronotum; scrobes usually short, ill-defined, broad; funicular segment 1 longer than 2; associated to terrestrial plants | 2 |
| 2 | Rostral dorsal carinae usually absent; pterygia auriculate, exposed (Fig. 5) | Falklandiina |
| – | Rostral dorsal carinae present; pterygia simple, not exposed (Fig. 6) | 3 |
| 3 | Scrobes short, ill-defined, broad, lacking suprascrobal keel; elytra with intervals convex, with anteapical tubercle (except for Rupanius) | Listroderina |
| – | Scrobes long, deep, sharply bordered, reaching eyes, with suprascrobal keel; elytra with intervals usually flat, lacking anteapical tubercle | Macrostyphlina |
Macrostyphlus Kirsch, 1889.
Scrobes long, deep, sharply bordered, reaching eyes, with suprascrobal keel; elytra oblong-oval, with intervals usually flat, lacking anteapical tubercle.
This new subtribe, which basically corresponds to the Macrostyphlus generic group of
| 1 | Postocular lobes present | 2 |
| – | Postocular lobes absent | 4 |
| 2 | Pronotum transverse to strongly transverse | Puranius (Fig. 19) |
| – | Pronotum subcircular or subcylindrical | 3 |
| 3 | Pronotum subcircular with subparallel flanks, disc smooth, polished; metanepisternal sutures present, complete; elytra with intervals flat | Amathynetoides |
| – | Pronotum subcylindrical, disc rugose; metanepisternal sutures posteriorly fused or obliterated; elytra with intervals convex | Adioristidius (Fig. 18) |
| 4 | Vestiture consisting of subcircular scales and setae; elytra with small, rounded tubercles | Macrostyphlus |
| – | Vestiture consisting of seta-like scales and setae or only setae; elytra lacking tubercles | 5 |
| 5 | Vestiture consisting of seta-like scales and setae; eyes large, slightly convex; pronotum disc smooth, polished; basal elytral margin not raised | Nacodius |
| – | Vestiture consisting of setae only; eyes very small, microphthalmic (8 or fewer facets), flat; pronotum disc rugose; basal elytral margin raised, subcarinate | Andesianellus |
http://species-id.net/wiki/Adioristidius
Fig. 18Adioristus similaris Voss, 1954.
Small to very small (1.5–4.1 mm); vestiture consisting of seta-like scales and setae; antennal clubs fusiform; pronotum subcylindrical, disc rugose; metanepisternal sutures posteriorly fused or obliterated; elytral intervals convex.
Adioristidius is the sister genus of Macrostyphlus-Andesianellus.
Adioristidius anchonoideus (Hustache, 1938); Adioristidius carinicollis (Voss, 1954); Adioristidius chilensis Morrone, 1994; Adioristidius costulatus (Hustache, 1938); Adioristidius crassirostris (Hustache, 1938); Adioristidius cuprisquameus (Voss, 1954); Adioristidius granulatus (Hustache, 1938); Adioristidius hirsutus Morrone, 1994; Adioristidius hydanius Morrone, 1994; Adioristidius jorgei Morrone, 1994; Adioristidius lidiae Morrone, 1994; Adioristidius manu Morrone, 1994; Adioristidius morio (Voss, 1954); Adioristidius nivalis (Kuschel, 1949); Adioristidius pampaensis (Voss, 1954); Adioristidius peruvianus (Voss, 1954); Adioristidius puncticollis (Hustache, 1938); Adioristidius scrobicollis (Voss, 1954); Adioristidius similaris (Voss, 1954); Adioristidius subimpressus (Voss, 1954); Adioristidius subtuberculatus (Voss, 1954); Adioristidius sulcicollis (Hustache, 1938); Adioristidius tuberculatus (Voss, 1954); Adioristidius variegatus (Voss, 1954).
Adioristidius chilensis: Mulinum spp. (Apiaceae); Adioristidius tuberculatus: Solanum tuberosum L. (Solanaceae) (
South American Transition Zone (Puna biogeographical province) and Central Chilean and Subantarctic subregions (Andean region), from Peru to Central Chile (
Adioristidius anchonoideus (CMNC, DEI, MLP, MZFC), Adioristidius chilensis (MHNS), Adioristidius costulatus (DEI), Adioristidius crassirostris (DEI), Adioristidius granulatus (DEI), Adioristidius hirsutus (MHNS, MLP, MZFC), Adioristidius hydanius (DEI), Adioristidius jorgei (MHNS, MLP, MZFC), Adioristidius lidiae (CMNC), Adioristidius manu (CMNC, FMNH), Adioristidius morio (CWOB, MLP, MZFC), Adioristidius nivalis (MHNS, NZAC), Adioristidius puncticollis (DEI, MZFC), Adioristidius similaris (DEI), Adioristidius sulcicollis (DEI), Adioristidius tuberculatus (CWOB, MZFC, USNM), Adioristidius variegatus (DEI).
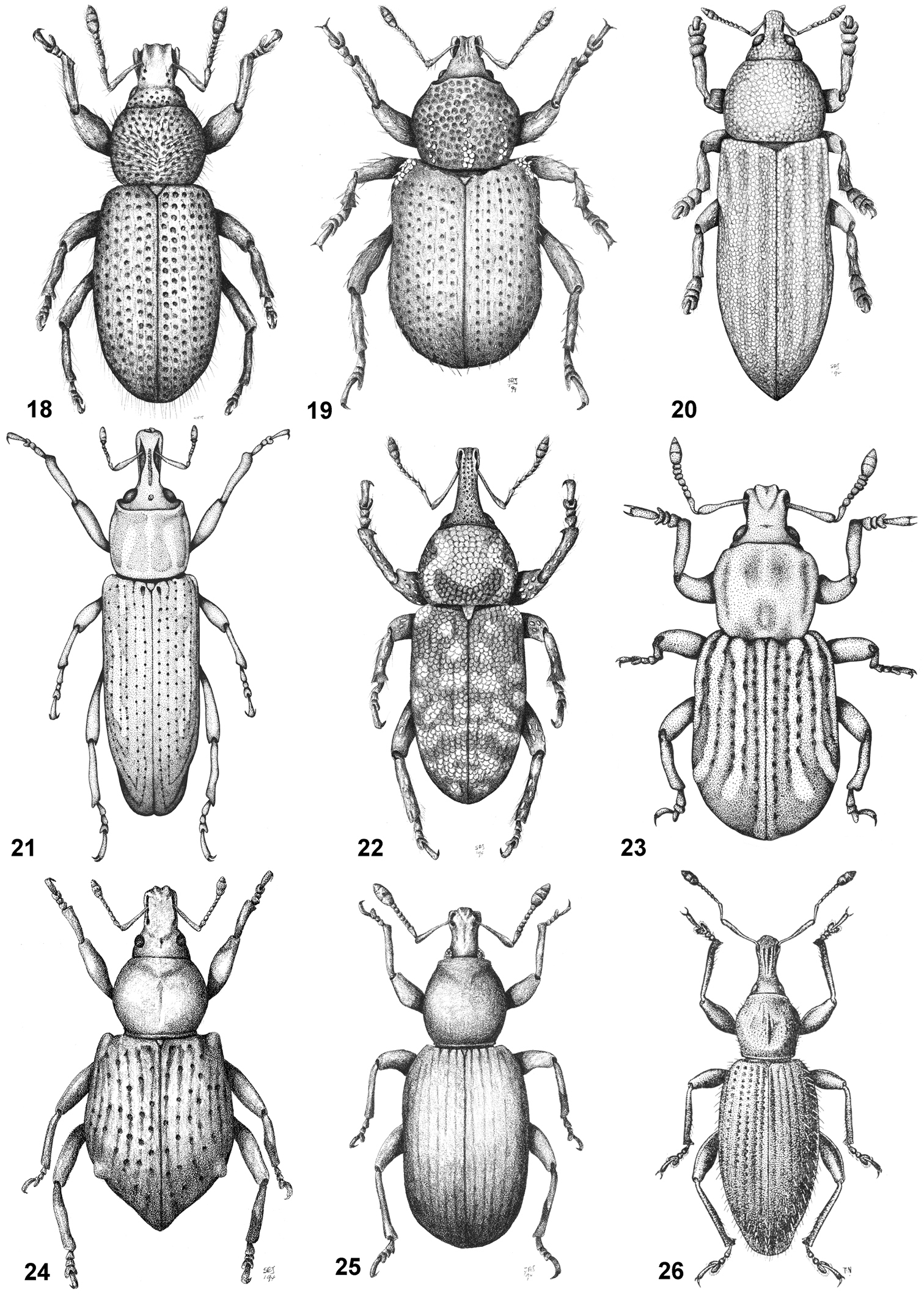
Habitus of representative Listroderini. 18 Adioristidius hirsutus 19 Puranius nigrinus 20 Haversiella albolimbata 21 Listronotus bosqi 22 Neopachytychius squamosus 23 Falklandiellus suffodens 24 Falklandiopsis magellanica 25 Falklandius antarcticus 26 Gromilus veneris.
http://species-id.net/wiki/Amathynetoides
Amathynetes appendiculatus Kuschel, 1949.
Small to very small (3.0–6.6 mm); vestiture consisting of seta-like scales and setae; pronotum subcircular with subparallel flanks, disc smooth, polished; metanepisternal sutures present, complete; elytral intervals flat.
Amathynetoides is the sister genus of Nacodius.
Amathynetoides appendiculatus (Kuschel, 1949); Amathynetoides ebeninus (Hustache, 1938); Amathynetoides intemperatus Morrone, 1994; Amathynetoides longulus (Kuschel, 1949); Amathynetoides morbeamus Morrone, 1994; Amathynetoides nitidiventris (Hustache, 1938); Amathynetoides normae Morrone, 1994; Amathynetoides palustris (Kuschel, 1949); Amathynetoides sparsesetosus (Hustache, 1938); Amathynetoides sundrianus Morrone, 1994.
Amathynetoides nitidiventris: Ullucus tuberosus Caldas (Basellaceae) (
South American Transition Zone (Puna and Coastal Peruvian Desert biogeographical provinces), from Peru to northern Chile (
Amathynetoides appendiculatus (CWOB, CMNC, MHNS, MZFC, NZAC, USNM), Amathynetoides ebeninus (BPBM, CWOB, DEI, MZFC), Amathynetoides intemperatus (AMNH, CWOB, MLP, MZFC), Amathynetoides longulus (CWOB, MHNS, NZAC, MZFC, USNM), Amathynetoides morbeamus (FIML), Amathynetoides nitidiventris (DEI), Amathynetoides normae (CMNC, MLP, MZFC), Amathynetoides palustris (CWOB, FIML, MHNS, MZFC, NZAC, USNM), Amathynetoides sparsesetosus (CWOB, DEI, CMNC, MZFC), Amathynetoides sundrianus (BMNH, CWOB, FIML, MLP, MZFC).
http://species-id.net/wiki/Andesianellus
Andesianellus microphthalmicus Anderson & Morrone, 1996.
Very small (1.9–3.3 mm); vestiture consisting of setae only; eyes very small, (8 or fewer facets), flat; postocular lobes absent; basal elytral margin raised, subcarinate.
Andesianellus is the sister genus of Macrostyphlus, ashypothesizedin previous analyses (
Species of this genus have been reported as leaf-litter inhabitants (
Andesianellus carltoni Anderson & Morrone, 1996; Andesianellus cotopaxi Anderson & Morrone, 1996; Andesianellus fulgidus Anderson & Morrone, 1996; Andesianellus hermani Anderson & Morrone, 1996; Andesianellus masneri Anderson & Morrone, 1996; Andesianellus microphthalmicus Anderson & Morrone, 1996; Andesianellus minutus Anderson & Morrone, 1996; Andesianellus planirostris Anderson & Morrone, 1996; Andesianellus tricarinatus Anderson & Morrone, 1996.
South American Transition Zone (North Andean Paramo biogeographical province), in Colombia, Ecuador and Peru (
Andesianellus carltoni (CMNC), Andesianellus cotopaxi (AMNH), Andesianellus fulgidus (CMNC), Andesianellus hermani (AMNH), Andesianellus masneri (CMNC), Andesianellus microphthalmicus (CMNC, MLP), Andesianellus minutus (CMNC, FMNH), Andesianellus planirostris (AMNH, BMNH, CMNC, CWOB, FMNH, MLP, USNM), Andesianellus tricarinatus (CMNC, FMNH).
Macrostyphlus gualcalae Kirsch, 1889 (by indication, monotypy).
Very small (1.9–3.5 mm); vestiture consisting of subcircular scales and setae; pronotum subclyndrical; metanepisternal sutures posteriorly fused or obliterated; elytra with intervals convex.
Macrostyphlus is the sister genus of Andesianellus, ashypothesized in a previous analysis (
Macrostyphlus bilbo Morrone, 1994; Macrostyphlus coelorum (Olliff, 1891); Macrostyphlus frodo Morrone, 1994; Macrostyphlus gandalf Morrone, 1994; Macrostyphlus gualcalae Kirsch, 1889; Macrostyphlus howdenorum Morrone, 1994; Macrostyphlus peruvianus Morrone, 1994; Macrostyphlus sturmi Morrone, 1994; Macrostyphlus transatlanticus (Kirsch, 1889); Macrostyphlus venezolanus Morrone, 1994.
South American Transition Zone (North Andean Paramo and Puna biogeographical provinces), from eastern Venezuela to southern Peru (
Macrostyphlus bilbo (CNCI), Macrostyphlus coelorum (CWOB), Macrostyphlus frodo (ICNB, USNM), Macrostyphlus gandalf (CMNC, CNCI, MLP, MZFC), Macrostyphlus gualcalae (SMTD), Macrostyphlus howdenorum (CMNC), Macrostyphlus peruvianus (FMNH), Macrostyphlus sturmi (ICNB), Macrostyphlus transatlanticus (SMTD), Macrostyphlus venezolanus (MZFC).
Nacodius martitae Morrone, 1994.
Small (4.6–6.9 mm); vestiture of seta-like scales and setae; eyes large, slightly convex; pronotum lacking postocular lobes, with disc smooth, polished; elytra with intervals flat.
Nacodius is the sister genus to Amathynetoides, and both are placed in Macrostyphlina. In a previous analysis (
Nacodius alectrus Morrone, 1994; Nacodius brevirostris (Voss, 1954); Nacodius martitae Morrone, 1994; Nacodius omissus (Kuschel, 1952).
South American Transition Zone (North Andean Paramo and Puna biogeographical provinces), in Ecuador and Peru (
Nacodius alectrus (CWOB), Nacodius brevirostris (SMTD), Nacodius martitae (AMNH, CWOB, MLP, MZFC) and Nacodius omissus (BMNH).
http://species-id.net/wiki/Puranius
Fig. 19Puranius inaequalis Germain, 1896 (subsequent designation by Morrone, 1994c).
Puranius is the sister genus to Amathynetoides-Nacodius.
Small to very small (1.9–6.5 mm); vestiture of subcircular scales and setae; pronotum transverse to strongly transverse; metanepisternal suture present, complete; elytra oblong-oval, with small, rounded tubercles.
Puranius argentinensis Morrone, 1994; Puranius australis Germain, 1896; Puranius championi (Kuschel, 1952); Puranius dubius (Germain, 1896); Puranius elguetai Morrone, 1994; Puranius exsculpticollis (Enderlein, 1907); Puranius fasciculiger (Blanchard, 1851); Puranius hispidus (Germain, 1896); Puranius inaequalis Germain, 1896; Puranius midas Morrone, 1994; Puranius nigrinus (Fairmaire, 1884); Puranius obrienorum Morrone, 1994; Puranius pusillus Morrone, 1994; Puranius scaber (Enderlein, 1907); Puranius sylvanius Morrone, 1994; Puranius torosus Morrone, 1994; Puranius tothus Morrone, 1994; Puranius tuberosus Germain, 1896; Puranius verrucosus (Germain, 1896); Puranius vulgaris Morrone, 1994.
Puranius argentinensis:Mulinum sp. (Apiaceae); Puranius championi: Poa flabellata (Lam.) Raspail (Poaceae); Puranius fasciculiger: Senecio smithii DC (Asteraceae); Puranius nigrinus: Taraxacum officinale Weber ex F. H. Wigg. (Asteraceae) and Nothofagus sp. (Nothofagaceae); Puranius vulgaris: Mulinum sp. (Apiaceae); Puranius scaber: Baccharis sp. (Asteraceae) and Ephedra sp. (Ephedraceae) (Morrone, 1994c).
Andean region (Subantarctic and Central Chilean subregions) and South American Transition Zone, from southern Argentina, including the Falkland Islands (Islas Malvinas), to Peru (
Puranius argentinensis (AMNH, BMNH, MLP, MZFC), Puranius australis (AMNH, CWOB, MHNS, NZAC), Puranius championi (BMNH, CWOB, NZAC), Puranius dubius (CWOB, MHNS, NZAC), Puranius elguetai (AMNH, MHNS, MLP, MZFC), Puranius exsculpticollis (BMNH), Puranius fasciculiger (CWOB, MHNS, NZAC, USNM), Puranius hispidus (CWOB, MHNS, NZAC), Puranius inaequalis (CMNC, CWOB, MHNS, MZFC, NZAC), Puranius midas (AMNH), Puranius nigrinus (ARPC, BMNH, CADIC, CBPC, CMNC, CNCI, CWOB, DEI, FIML, IPUM, MCZ, MHNS, MZFC, NZAC, USNM), Puranius obrienorum (AMNH, CMNC, CWOB, MLP, MZFC), Puranius pusillus (MHNS, MLP, MZFC), Puranius scaber (AMPC, BMNH, CWOB, NZAC), Puranius sylvanius (AMNH, BMNH, CMNC, MLP, MZFC), Puranius torosus (MHNS, MLP, MZFC), Puranius tothus (MHNS), Puranius tuberosus (CWOB, MHNS, NZAC), Puranius verrucosus (CMNC, CWOB, MHNS, MZFC, NZAC) and Puranius vulgaris (AMNH, BMNH, CMNC, MHNS, MLP, MZFC).
Palaechthus C. O. Waterhouse, 1884 (by original designation, as Palaechtus, incorrect subsequent spelling).
Rostrum slender, as long as or longer than pronotum (except for some species of Listronotus where the rostrum is shorter than pronotum); scrobes long, deep, sharply bordered, reaching eyes; scape usually short (not reaching anterior margin of eye when resting in scrobe); pronotum usually subclyndrical or subcircular; elytra oblong-oval to elongate-oval.
Most of the species of Palaechthina are associated to aquatic or semiaquatic plants, being found in wet or damp conditions (
This subtribe includes the genera Anorthorhinus, Gunodes, Haversiella, Inaccodes, Listronotus, Neopachytychius, Palaechthus, Palaechtodes, Steriphus and Tristanodes. Anorthorhinus and Steriphus are Australian; Gunodes, Inaccodes, Palaechthus, Palaechtodes and Tristanodes are distributed in the Tristan da Cunha-Gough islands; and the remaining three genera are found in the Americas: Haversiella and Neopachytychius in South America and Listronotus has a disjunct distribution in South and North America.
| 1 | Funicular segment 1 subequal to or shorter than 2 | 2 |
| – | Funicular segment 1 longer than 2 | 6 |
| 2 | Elytra with intervals convex; North and South America | Listronotus (Fig. 21) |
| – | Elytra with intervals flat; Tristan da Cunha-Gough islands | 3 |
| 3 | Small to very small (3.7–6.5 mm) | Tristanodes |
| – | Medium-sized to large (7.0–12.0 mm) | 4 |
| 4 | Vestiture of subcircular scales and setae; pronotum subcircular; elytra oblong-oval | Gunodes |
| – | Vestiture of seta-like scales and setae; pronotum subtrapezoidal or subclyndrical; elytra elongate-oval | 5 |
| 5 | Large (11.0–12.0 mm); rostral dorsal carinae absent; pronotum subtrapezoidal | Palaechthus |
| – | Medium-sized (7.0–7.5 mm); rostral dorsal carinae present; pronotum subclyndrical | Palaechtodes |
| 6 | Scape long (surpassing posterior margin of eye when resting in scrobe); elytra with anteapical tubercle | Steriphus |
| – | Scape short (not reaching anterior margin of eye when resting in scrobe); elytra lacking anteapical tubercle | 7 |
| 7 | Vestiture of seta-like scales and setae; Australia and Tristan da Cunha-Gough islands | 8 |
| – | Vestiture of subcircular scales ans setae; South America | 9 |
| 8 | Funicular segments 3-6 elongate; club fusiform; pronotum subclyndrical; elytra with intervals convex; Australia | Anorthorhinus |
| – | Funicular segments 3-6 globose; club inflated; pronotum subcircular; elytra with intervals flat; Tristan da Cunha-Gough islands | Inaccodes |
| 9 | Vestiture of subcircular scales and setae; rostral dorsal carinae present; mandibles long and narrow; antennal insertion distal; postocular lobes slightly developed; elytra oblong-oval; tibiae with spurs | Neopachytychius (Fig. 22) |
| – | Vestiture of subcircular scales only; rostral dorsal carinae absent; mandibles robust; antennal insertion at the middle of the rostrum; postocular lobes absent; elytra elongate-oval; tibiae lacking spurs | Haversiella (Fig. 20) |
http://species-id.net/wiki/Anorthorhinus
Anorthorhinus pictipes Blackburn, 1890 (by indication, monotypy).
Small to very small (2.5–6.0 mm); vestiture of seta-like scales and setae; funicular segments 3-6 elongate; club fusiform; pronotum subclyndrical; elytra with intervals convex.
Anorthorhinus is the sister genus to the clade comprising Haversiella, Neopachytychius and the five genera from the Tristan da Cunha-Gough islands.
Anorthorhinus apicalis Lea, 1899; Anorthorhinus brevicornis Lea, 1899; Anorthorhinus pictipes Blackburn, 1890.
Australia (
Anorthorhinus apicalis (MZFC) and Anorthorhinus pictipes (MZFC).
Gunodes major Brinck, 1948.
Medium-sized (7.5 mm); vestiture of subcircular scales and setae; pronotum subcircular; elytra oblong-oval.
Gunodes is the sister genus to Palaechthus-Paleachtodes-Tristanodes.
Gunodes major Brinck, 1948.
Tristan da Cunha-Gough islands (
http://species-id.net/wiki/Haversiella
Fig. 20Haversiella albolimbata Champion, 1918 (by original designation).
Haversiella is the sister genus to Neopachytychius, andboth constitute the sister group to the five genera from the Tristan da Cunha-Gough islands.
Very small (3.0–3.9 mm); vestiture of subcircular scales only; maxillary mala lacking teeth; antennal insertion at the middle of the rostrum; pronotum subcircular; elytra elongate-oval; tibiae lacking spurs; plate of female sternum 8 reduced.
Haversiella albolimbata (Champion, 1918).
Bryophytes (
Southern Argentina, including the Falkland Islands (Islas Malvinas), and southern Chile (
Haversiella albolimbata (BMNH, MHNS, MZFC, USNM).
Inaccodes oblongus Brinck, 1948.
Small (4.5 mm); vestiture of seta-like scales and setae; funicular segments 3-6 globose; club inflated; pronotum subcircular; elytra with intervals flat.
Inaccodes is the sister genus to the clade comprising the four remaining genera from the Tristan da Cunha-Gough islands.
Inaccodes oblongus Brinck, 1948.
Tristan da Cunha-Gough islands (
http://species-id.net/wiki/Listronotus
Fig. 21Rhynchaenus caudatus Say, 1824 (subsequent designation by
Very small to medium-sized (1.0–14.0 mm); vestiture of subcircular scales and setae; antennal insertion distal; funicular segment 1 subequal to or shorter than 2; postocular lobes present, well-developed; elytra oblong-oval to elongate-oval, with intervals convex.
Listronotus is the sister genus to Steriphus (Australia). In a previous analysis based only on American taxa (
Listronotus alternatus (Dietz, 1889); Listronotus americanus LeConte, 1876; Listronotus angustatus (Champion, 1902); Listronotus annulipes (Blatchley, 1925); Listronotus anthracinus (Dietz, 1889); Listronotus apicalis (Hustache, 1926); Listronotus appendiculatus (Boheman, 1842); Listronotus argentinensis (Hustache, 1926); Listronotus arizonicus O’Brien, 1981; Listronotus blandus Henderson, 1940; Listronotus blatchleyi Henderson, 1940; Listronotus bonariensis (Kuschel, 1955); Listronotus borrichiae O’Brien, 1981; Listronotus bosqi (Hustache, 1926); Listronotus breyeri (Brèthes, 1910); Listronotus burkei O’Brien, 1981; Listronotus californicus (Dietz, 1889); Listronotus callosus LeConte, 1876; Listronotus carinatus (Blatchley, 1928); Listronotus carinicollis (Hustache, 1926); Listronotus caudatus (Say, 1824); Listronotus cinnamoneus (Hustache, 1926); Listronotus conabilis O’Brien, 1981; Listronotus crypticus O’Brien, 1981; Listronotus cryptops (Dietz, 1889); Listronotus cyrticus (Desbrochers des Loges, 1898); Listronotus dauci (Brèthes, 1926); Listronotus debilis Blatchley, 1916; Listronotus deceptus (Blatchley, 1916); Listronotus delumbis (Gyllenhal, 1834); Listronotus dietrichi (Stockton, 1963); Listronotus dietzi O’Brien, 1979; Listronotus distinctus Henderson, 1940; Listronotus dorsalis (Dietz, 1889); Listronotus dorytomoides (Hustache, 1926); Listronotus durangoensis O’Brien, 1977; Listronotus echinatus (Dietz, 1889); Listronotus echinodori O’Brien, 1977; Listronotus elegans Van Dyke, 1929; Listronotus elegantulus O’Brien, 1981; Listronotus elongatus (Hustache, 1939); Listronotus fasciatus O’Brien, 1981; Listronotus filiformis (LeConte, 1876); Listronotus frontalis LeConte, 1876; Listronotus geminatus (Hustache, 1926); Listronotus griseus (Hustache, 1926); Listronotus grypidioides (Dietz, 1889); Listronotus haldemani (Burke, 1963); Listronotus hirtellus (Dietz, 1889); Listronotus hoodi (Stockton, 1963); Listronotus hornii (Dietz, 1889); Listronotus hubbardi (LeConte, 1876); Listronotus humilis (Gyllenhal, 1834); Listronotus hyperodes (Dietz, 1889); Listronotus incompletus (Hatch, 1971); Listronotus ingens Henderson, 1940; Listronotus insignis Henderson, 1940; Listronotus laevis (Hustache, 1926); Listronotus laramiensis (Angell, 1893); Listronotus latinasus (Blatchley, 1922); Listronotus lineolaticollis (Blanchard, 1851); Listronotus lodingi (Blatchley, 1920); Listronotus lucens (Hustache, 1926); Listronotus lutulentus (Boheman, 1843); Listronotus maculatus (Hatch, 1971); Listronotus maculicollis (Kirby, 1837); Listronotus manifestus Henderson, 1940; Listronotus marginalis O’Brien, 1977; Listronotus marginicollis (Hustache, 1926); Listronotus marshalli O’Brien, 1981; Listronotus meridionalis O’Brien, 1977; Listronotus minutus (Blanchard, 1851); Listronotus montanus (Dietz, 1889); Listronotus nebulosus LeConte, 1876; Listronotus neocallosus O’Brien, 1981; Listronotus nevadicus LeConte, 1876; Listronotus nigropunctatus (Suffrian, 1871); Listronotus novellus (Blatchley, 1916); Listronotus obscurellus (Dietz, 1889); Listronotus obtectus (Dietz, 1889); Listronotus oregonensis (LeConte, 1876); Listronotus ornatipennis (Blanchard, 1851); Listronotus pallidus O’Brien, 1981; Listronotus palustris Blatchley, 1916; Listronotus pampaensis (Voss, 1954); Listronotus peninsularis (Blatchley, 1916); Listronotus plumosiventris O’Brien, 1977; Listronotus porcellus (Say, 1831); Listronotus poseyensis (Blatchley, 1916); Listronotus pseudosetosus O’Brien, 1981; Listronotus puncticollis (Hustache, 1926); Listronotus punctiger LeConte, 1876; Listronotus pusillus (Hustache, 1926); Listronotus rotundicollis LeConte, 1876; Listronotus rubtzoffi O’Brien, 1981; Listronotus rufomarginatus (Hustache, 1939); Listronotus salicorniae O’Brien, 1981; Listronotus scapularis Casey, 1895; Listronotus setosipennis (Hustache, 1926); Listronotus setosus LeConte, 1876; Listronotus similis Henderson, 1940; Listronotus sondondoanus (Voss, 1954); Listronotus sordidus (Gyllenhal, 1834); Listronotus sparsus (Say, 1831); Listronotus squamiger (Say, 1831); Listronotus sulcipennis (Boheman, 1834); Listronotus suturalis O’Brien, 1981; Listronotus teretirostris (LeConte, 1857); Listronotus testaceipes (Champion, 1902); Listronotus texanus (Stockton, 1963); Listronotus truncatus (Hatch, 1971); Listronotus tuberosus LeConte, 1876; Listronotus turbatus O’Brien, 1981; Listronotus vitticollis (Kirby, 1837); Listronotus vulgaris (Hustache, 1926); Listronotus wallacei (Stockton, 1963); Listronotus weiseri (Hustache, 1926).
Listronotus appendiculatus: Sagittaria latifolia Willdenow (Alismataceae); Listronotus argentinensis: Triticum aestivum L. (Poaceae); Listronotus blandus : Polygonum hydropiperoides Michx. (Polygonaceae); Listronotus bonariensis: Dactylis glomerata L., Festuca arundinacea Schreber, Hordeum vulgare L., Lolium multiflorum L., Lolium perenne L., Poa annua L., Triticum aestivum L., Zea mays L. (Poaceae) and Trifolium repens L. (Fabaceae); Listronotus borrichiae: Borrichia frutescens (L.) DC (Asteraceae) and Salvinia sp. (Salviniaceae); Listronotus caudatus: Polygonum bicorne Raf. (Polygonaceae); Listronotus cinnamoneus: Limnobium stoloniferum (G. F. W. Meyer) Griseb. (Hydrocharitaceae); Listronotus cryptops: Sagittaria lancifolia L. (Alismataceae); Listronotus dauci: Daucus carota L. (Apiaceae); Listronotus dietrichi: Dahlia sp. (Asteraceae), Gossypium sp. (Malvaceae), Persus sp. (Lauraceae), Phaseolus sp. (Fabaceae), Cenchrus sp., Chloris sp., Cynodon sp., Eleusine sp., Zea sp. (Poaceae), Coffea sp. (Rubiaceae), Lycopersicum sp. (Solanaceae) and Menta sp. (Lamiaceae); Listronotus echinodori: Echinodorus cordifolius (L.) Griseb. and Sagittaria latifolia Willdenow (Alismataceae); Listronotus elongatus: Hydrocotyle ranunculoides L. f. (Apiaceae); Listronotus haldemani: Juncus nodatus Coville in N. L. Britton and A. Brown (Juncaceae); Listronotus maculicollis: Agrostis palustris Huds. and Poa annua L. (Poaceae); Listronotus manifestus: Sagittaria longiloba Engelm. ex J. G. Sm. (Alismataceae); Listronotus marginicollis: Myriophyllum aquaticum (Velloso) Verde (Haloragaceae); Listronotus montanus: Triticum aestivum L. (Poaceae); Listronotus neocallosus: Sagittaria engelmanniana J. G. Smith, Sagittaria graminea Michaux and Sagittaria stagnorum Small (Alismataceae); Daucus carota L. and Petroselinum crispum (Miller) A. W. Hill. (Apiaceae) (Listronotus oregonensis); Listronotus plumosiventris: Sagittaria latifolia Willdenow (Alismataceae); Listronotus rotundicollis: Crinum sp. (Amaryllidaceae); Listronotus rubtzoffi: Sagittaria cuneata Sheldon (Alismataceae); Listronotus salicorniae: Salicornia virginica L. (Amaranthaceae); Listronotus scapularis: Sagittaria longiloba Engelm. ex J. G. Sm. and Sagittaria sp. (Alismataceae); Listronotus setosipennis: Parthenium hysterophorus L. (Asteraceae); Listronotus similis: Paspalum distichum L. (Poaceae) and Polygonum bicorne Raf. (Polygonaceae); Listronotus teretirostris: Eleocharis macrostachya Britton (Cyperaceae); Listronotus texanus: Daucus carota L. (Apiaceae); Listronotus turbatus: Sagittaria sp. (Alismataceae) (
Listronotus bonariensis(May, 1977, 1993, 1994; Marvaldi, 1998).
Widespread in the Americas, from Canada to Argentina and Chile (
Listronotus americanus (BMNH), Listronotus apicalis (MLP), Listronotus appendiculatus (AMNH, BMNH), Listronotus argentinensis (AMNH, MACN, MLP9, Listronotus blandus (AMNH, BMNH), Listronotus bonariensis (BMNH, MHNS), Listronotus bosqi (BMNH, MLP, MZFC), Listronotus breyeri (MACN, MZFC), Listronotus californicus (AMNH), Listronotus callosus (BMNH, AMNH), Listronotus caudatus (BMNH, AMNH), Listronotus cinnamoneus (MLP), Listronotus cryptops (BMNH, AMNH), Listronotus cyrticus (AMNH, MACN, MLP), Listronotus dauci (MACN), Listronotus debilis (AMNH), Listronotus delumbis (BMNH, AMNH), Listronotus dietzi (AMNH), Listronotus distinctus (BMNH), Listronotus durangoensis (AMNH, BMNH), Listronotus echinatus (AMNH), Listronotus echinodori (AMNH, BMNH), Listronotus elongatus (MLP, MZFC), Listronotus filiformis (BMNH, AMNH), Listronotus frontalis (AMNH, BMNH), Listronotus geminatus (MACN, MLP), Listronotus griseus (AMNH, MACN, MLP), Listronotus grypidioides (AMNH), Listronotus haldemani (BMNH), Listronotus hornii (AMNH), Listronotus hubbardi (BMNH), Listronotus humilis (AMNH), Listronotus hyperodes (AMNH), Listronotus incompletus (AMNH), Listronotus ingens (AMNH), Listronotus lineolaticollis (MLP), Listronotus lutulentus (BMNH), Listronotus maculicollis (AMNH), Listronotus manifestus(AMNH, BMNH), Listronotus marginalis (BMNH), Listronotus marginicollis (MACN, MLP), Listronotus meridionalis (BMNH), Listronotus minutus (AMNH), Listronotus nebulosus (AMNH), Listronotus novellus (AMNH), Listronotus oregonensis (AMNH, BMNH, MZFC), Listronotus ornatipennis (MHNS), Listronotus palustris (AMNH, BMNH), Listronotus plumosiventris (BMNH), Listronotus porcellus (AMNH), Listronotus puncticollis (MLP), Listronotus punctiger (AMNH, BMNH), Listronotus pusillus (MLP, MZFC), Listronotus rotundicollis (AMNH, BMNH), Listronotus rubtzoffi (AMNH), Listronotus rufomarginatus (MLP), Listronotus scapularis (AMNH), Listronotus setosipennis (MLP), Listronotus setosus (AMNH), Listronotus similis (AMNH, BMNH), Listronotus sordidus (AMNH, BMNH), Listronotus sparsus (AMNH, BMNH), Listronotus squamiger (AMNH, BMNH), Listronotus teretirostris (AMNH, BMNH), Listronotus texanus (AMNH), Listronotus truncatus (AMNH), Listronotus tuberosus (AMNH, BMNH), Listronotus vitticollis (AMNH) and Listronotus vulgaris (MLP).
http://species-id.net/wiki/Neopachytychius
Fig. 22Neopachytychius squamosus Hustache, 1939.
Small (3.8–6.5 mm); vestiture of subcircular scales and setae; mandible and pharyngeal process long and narrow; rostral dorsal carinae present; antennal insertion distal; postocular lobes slightly developed; elytra oblong-oval.
Neopachytychius is the sister genus to Haversiella, andboth constitute the sister group to the five genera from the Tristan da Cunha-Gough islands. In a previous analysis based only on American genera (
Neopachytychius squamosus Hustache, 1939.
Neotropical region, in Argentina, Bolivia, Chile and Uruguay (
Neopachytychius squamosus (FIML, IADIZA, MACN, MHNS, MLP, MZFC).
http://species-id.net/wiki/Palaechthus
Palaechthus glabratus Waterhouse, 1884 (subsequent designation by
Medium-sized (11.0–12.0 mm); vestiture of seta-like scales and setae; rostral dorsal carinae absent; pronotum subtrapezoidal.
Palaechthus is the sister genus to both Paleachtodes and Tristanodes.
Palaechthus glabratus C. O. Waterhouse, 1884.
Tristan da Cunha-Gough islands (
Palaechthus glabratus (BMNH).
Palaechthus cossonoides C. O. Waterhouse, 1884 (by original designation).
Medium-sized (7.0–7.5 mm); vestiture of seta-like scales and setae; rostral dorsal carinae present; pronotum subclyndrical.
Palaechtodes is the sister genus to both Paleachthus and Tristanodes.
Palaechtodes cossonoides (C. O. Waterhouse, 1884).
Tristan da Cunha-Gough islands (
Palaechtodes cossonoides (BMNH).
http://species-id.net/wiki/Steriphus
Steriphus solidus Erichson, 1842 (by indication, monotypy).
Small to very small (3.0–6.5 mm); vestiture of subcircular scales and setae; scape long (surpassing posterior margin of eye when resting in scrobe); elytra with anteapical tubercle.
Steriphus is the sister genus to the American genus Listronotus.
Steriphus albidoparsus (Lea, 1928); Steriphus alpinus (Lea, 1928); Steriphus angusticollis (Pascoe, 1870); Steriphus ascitus (Pascoe, 1876); Steriphus binodulus Broun, 1903; Steriphus caudatus (Pascoe, 1870); Steriphus curvisetosus (Lea, 1928); Steriphus diversipes (Pascoe, 1870); Steriphus humeralis (Lea, 1928); Steriphus incotaminatus (Lea, 1899); Steriphus inermis (Lea, 1928); Steriphus irrasus (Lea, 1899); Steriphus longus (Lea, 1928); Steriphus major (Blackburn, 1890); Steriphus mecaspis (Lea, 1899); Steriphus metallicus (Lea, 1928); Steriphus mucronatus (Lea, 1928); Steriphus murinus (Pascoe, 1870); Steriphus parvicornis (Lea, 1928); Steriphus parvonigrus (Lea, 1928); Steriphus parvus (Blackburn, 1890); Steriphus pullus (Broun, 1910); Steriphus sericeus (Blackburn, 1890); Steriphus solidus Erichson, 1842; Steriphus stenoderes (Lea, 1928); Steriphus variabilis (Broun, 1885); Steriphus vittatus (Blackburn, 1893).
Steriphus ascitus: Baumea articulata (R. Br.) Blake, Baumea rubiginosa (Spreng.) Boeck., Scirpus fluviatilis (Torr.) Sojak (Cyperaceae) and Typha orientalis C. B. Presl. (Typhaceae); Steriphus diversipes: Medicago sativa L. (Fabaceae) and Rumex acetosella L. (Polygonaceae); Steriphus variabilis: Cotula spp. (Asteraceae), Dichondra sp. (Convolvulaceae) and Myriophyllum sp. (Haloragaceae) (May, 1977; Kuschel, 1990).
Steriphus ascitus, Steriphus caudatus, Steriphus diversipes and Steriphus variabilis (May, 1970, 1977, 1993, 1994).
Australia and New Zealand (
Steriphus ascitus (MZFC) and Steriphus variabilis (MZFC).
Tristanodes craterophilus Brinck, 1948.
Small to very small (3.7–6.5 mm); vestiture of seta-like scales and setae; pronotum subcylindrical.
Tristanodes is the sister genus to both Palaechthus and Palaechtodes.
Tristanodes attai Brinck, 1948; Tristanodes conicus Brinck, 1948; Tristanodes craterophilus Brinck, 1948; Tristanodes echinatus Brinck, 1948; Tristanodes insolidus Brinck, 1948; Tristanodes integer Brinck, 1948; Tristanodes medius Brinck, 1948; Tristanodes minor Brinck, 1948; Tristanodes reppetonis Brinck, 1948; Tristanodes scirpophilus Brinck, 1948; Tristanodes sivertseni Brinck, 1948.
Tristanodes scirpophilus (Kuschel, 1962).
Tristan da Cunha-Gough islands (
Tristanodes attai (BMNH) and Tristanodes spp. (BMNH).
Falklandius Enderlein, 1907.
Small to very small (except Liparogetus and some species of Gromilus, which are medium-sized); rostrum stout, shorter than pronotum (except Gromilus and Nestrius, with relatively stout, medium-sized rostrum); pterygiae auriculate, exposed (Fig. 5); scrobes short, ill-defined, broad; eyes usually flat; postocular lobes usually absent (except Gromilus and Falklandiopsis); pronotum usually subcircular or subcylindrical; metanepisternal suture usually posteriorly fused or obliterated; elytra oblong-oval.
This new subtribe, which basically corresponds to the Falklandius generic group of
| 1 | Scrobes lateral | 2 |
| – | Scrobes dorsolateral to dorsal (Fig. 5) | 6 |
| 2 | Eyes transverse (Fig. 8); female elytral apex not produced; female ventrites 3 and 4 combined shorter than 5 | 3 |
| – | Eyes subcircular (Fig. 5); female elytral apex produced; female ventrites 3 and 4 combined longer than 5 | Telurus(Fig. 24) |
| 3 | Eyes slightly convex; postocular lobes absent | 4 |
| – | Eyes flat; postocular lobes slightly developed | 5 |
| 4 | Vestiture of setae only; rostrum very short, stout; rostral dorsal carinae absent; scrobes short, ill-defined; eyes dorsal; scape medium-sized (reaching eye when resting in scrobe); funicular segments 3-6 globose; pronotum subcircular; elytra with humeral tubercles; femora subcylindrical, markedly clavate; southern South America | Falklandiopsis(Fig. 24) |
| – | Vestiture of seta-like scales and setae; rostrum medium-sized, relatively stout; rostral dorsal carinae present; scrobes long, deep, sharply bordered, reaching eyes; eyes lateral; scape long (surpassing posterior margin of eye when resting in scrobe); funicular segments 3-6 elongate; pronotum subcylindrical; elytra lacking humeral tubercles; femora subcylindrical, clavate; New Zealand | Gromilus(Fig. 26) |
| 5 | Very small (2.6–3.5 mm); vestiture of subcircular scales and setae; rostrum lacking dorsal carinae; antennal insertion distal; club fusiform; pronotum transverse; metanepisternal suture posteriorly fused or obliterated; elytra with series of declivital tubercles; tibiae with spurs; southern South America | Falklandiellus(Fig. 23) |
| – | Medium-sized (6.0–10.0 mm); vestiture of seta-like scales and setae; rostrum with dorsal carinae; antennal insertion at the middle of the rostrum; club inflated; pronotum subquadrate; metanepisternal suture complete; elytra lacking series of declivital tubercles; tibiae lacking spurs; New Zealand | Liparogetus |
| 6 | Rostrum relatively stout, medium-sized, with dorsal carinae; eyes lateral; funicular segment 2 elongate; pronotum subcylindrical; scutellum not visible; New Zealand | Nestrius |
| – | Rostrum very short, stout, lacking dorsal carinae; eyes dorsal; funicular segment 2 globose; pronotum subcircular; scutellum visible; southern South America | 7 |
| 7 | Vestiture of seta-like scales and setae; eyes small; club inflated; pronotum with disc rugose; elytra with intervals convex; femora subcylindrical; tibiae subcylindrical | Falklandius(Fig. 25) |
| – | Vestiture of setae only; eyes very small, microphthalmic; club fusiform; pronotum with disc smooth, polished; elytra with intervals flat; femora dorsoventrally compressed; tibiae apically expanded | Lanteriella(Fig. 27) |
http://species-id.net/wiki/Falklandiellus
Fig. 23Falklandius suffodens Enderlein, 1907 (by original designation).
Very small (2.6–3.5 mm); vestiture of subcircular scales and setae; rostrum lacking dorsal carinae; antennal insertion distal; club fusiform; pronotum transverse; metanepisternal suture posteriorly fused or obliterated; elytra with series of declivital tubercles; tibiae with spurs.
Falklandiellus is the sister genus to Telurus-Nestrius-Falklandius-Lanteriella.
Falklandiellus suffodens (Enderlein, 1907).
Bryophytes (
Andean region (Subantarctic subregion), in southern Argentina, including the Falkland Islands (Islas Malvinas), and southern Chile (
Falklandiellus suffodens (BMNH, CADIC, MACN, MLP, MZFC, USNM, ZMHU).
http://species-id.net/wiki/Falklandiopsis
Fig. 24Falklandius magellanicus Morrone, 1992.
Very small (3.5–4.0 mm); vestiture of setae only; rostrum very short, stout; rostral dorsal carinae absent; scrobes short, ill-defined; eyes dorsal; scape medium-sized (reaching eye when resting in scrobe); funicular segments 3-6 globose; pronotum subcircular; elytra with humeral tubercles; femora subcylindrical, markedly clavate.
Falklandiopsis is the sister genus to both Liparogetus and the clade Falklandiellus-Telurus-Nestrius-Falklandius-Lanteriella.
Falklandiopsis magellanica (Morrone, 1992).
Nothofagus betuloides (Mirb.) Oerst. (Nothofagacae) (
Andean region (Subantarctic subregion), in southern Chile (
Falklandiopsis magellanica (MLP, MZFC, NZAC, ZMHU).
http://species-id.net/wiki/Falklandius
Fig. 25Falklandius brachyomma Enderlein, 1907 (= Otiorhynchus antarcticus Stierlin, 1903) (by original designation).
Small to very small (1.9–6.1 mm); vestiture of seta-like scales and setae; eyes small; club inflated; pronotal disc rugose; elytra with intervals convex.
Falklandius is the sister genus to Lanteriella, asfound in a previous analysis (Morrone, 1997a).
Falklandius antarcticus (Stierlin, 1903); Falklandius chilensis Morrone and Anderson, 1995; Falklandius goliath Morrone, 1992; Falklandius kuscheli Morrone, 1992; Falklandius peckorum Morrone and Anderson, 1995; Falklandius turbificatus Enderlein, 1907.
Falklandius antarcticus: Callitriche sp. (Callitrichaceae), Myrteola nummularia (Poir.) O. Berg (Myrtaceae), Nothofagus antarctica (G. Forster) Oerst. (Nothofagacae) and Poa flabellata (Lam.) Raspail (Poaceae) (Morrone, 1992b); Falklandius turbificatus: Myrteola nummularia (Poir.) O. Berg (Myrtaceae) (Morrone, 1992b).
Andean region (Subantarctic subregion), in southern Argentina, including the Falkland Islands (Islas Malvinas) and southern Chile (
Falklandius antarcticus (AMPC, BMNH, CADIC, CMNC, CWOB, MACN, MHNS, MZFC, USNM), Falklandius chilensis (AMNH, BMNH, CMNC, CWOB, FMNH, MLP, MZFC, USNM), Falklandius goliath (BMNH), Falklandius kuscheli (BMNH), Falklandius peckorum (AMNH, BMNH, CMNC, CWOB, FMNH, MLP, MZFC, USNM), Falklandius turbificatus (BMNH) and Falklandius spp. (MZFC).
http://species-id.net/wiki/Gromilus
Fig. 26Gromilus insularis Blanchard, 1853 (by indication, monotypy).
Small to medium-sized (3.5–7.5 mm); vestiture of seta-like scales and setae; rostrum medium-sized, relatively stout; rostral dorsal carinae present; scrobes long, deep, sharply bordered, reaching eyes; eyes lateral; scape long (surpassing posterior margin of eye when resting in scrobe); funicular segments 3-6 elongate; pronotum subcylindrical; elytra lacking humeral tubercles.
Gromilus is the sister genus to the remaining genera of Falklandiina.
Gromilus anthracinus (Broun, 1921); Gromilus aucklandicus Kuschel, 1971; Gromilus bicarinatus (Broun, 1921); Gromilus bifoveatus (Broun, 1923); Gromilus brevicornis (Broun, 1893); Gromilus brounii Morrone, 2011; Gromilus calvulus (Broun, 1913); Gromilus caudatus (Broun, 1913); Gromilus clarulus (Broun, 1917); Gromilus cockaynei (Broun, 1905); Gromilus cordipennis (Broun, 1893); Gromilus cristatus (Broun, 1893); Gromilus dorsalis (Broun, 1921); Gromilus exiguus (Brookes, 1951); Gromilus fallai (Brookes, 1951); Gromilus foveirostris (Broun, 1913); Gromilus furvus (Broun, 1921); Gromilus gracilipes (Sharp, 1883); Gromilus granissimus (Broun, 1917); Gromilus halli (Broun, 1917); Gromilus impressus (Broun, 1893); Gromilus inophloeoides (Broun, 1904); Gromilus insularis Blanchard, 1853; Gromilus kuschelii Morrone, 2011; Gromilus laqueorum Kuschel, 1964; Gromilus majusculus (Broun, 1915); Gromilus merus (Broun, 1917); Gromilus narinosus Kuschel, 1971; Gromilus nitidellus (Broun, 1917); Gromilus nitidulus (Broun, 1915); Gromilus nodiceps (Broun, 1914); Gromilus philpotti (Broun, 1917); Gromilus setosus (Broun, 1893); Gromilus sparsus (Broun, 1921); Gromilus striatus (Broun, 1915); Gromilus sulcicollis (Broun, 1913); Gromilus sulcipennis (Broun, 1917); Gromilus tenuiculus (Broun, 1921); Gromilus thoracicus (Broun, 1893); Gromilus variegatus (Broun, 1893); Gromilus veneris (Kirsch, 1877).
Gromilus fallai: Blechnum capense Burm. f. (Blechnaceae); Gromilus insularis: Colobanthus sp. (Caryophyllaceae), Pleurophyllum sp. (Asteraceae), Poa litorosa Cheeseman (Poaceae), Polystichum vestitum (G. Forst.) C. Presl. (Dryopteridaceae), Pleurophyllum criniferum Hook. f. (Asteraceae), Stilbocarpa polaris (Homb. and Jacq.) Gray (Araliaceae) and Tillaea moschata DC (Crassulaceae); Gromilus setosus: Blechnum sp. (Blechnaceae) and Gahnia sp. (Cyperaceae); Gromilus veneris: Blechnum capense (L.) Schlecht. (Blechnaceae), Polystichum sp. (Dryopteridaceae) and Pteris sp. (Pteridaceae); Gromilus thoracicus: Anisotome latifolia Hook. f. (Apiaceae), Bulbinella sp. (Liliaceae), Cotula plumosa Hook. f. and Pleurophyllum criniferum Hook. f. (Asteraceae) and Poa litorosa Cheeseman (Poaceae) (
Immature stages. Gromilus exiguus, Gromilus insularis, Gromilus thoracicus and Gromilus veneris (May, 1971).
New Zealand (
Gromilus gracilipes (MZFC), Gromilus insularis (MZFC), Gromilus laqueorum (MZFC). Gromilus merus (MZFC), Gromilus nitidellus (MZFC) and Gromilus veneris (MZFC).
http://species-id.net/wiki/Lanteriella
Fig. 27Lanteriella microphtalma Morrone, 1992.
Very small (3.4–3.8 mm); vestiture of setae only; eyes very small, microphthalmic; pronotal disc smooth, polished; femora dorsoventrally compressed; tibiae apically expanded.
Lanteriella is the sister genus to Falklandius, asfound in a previous analysis (Morrone, 1997a).
The only species of this genus was hypothesized to live in litter or soil (
Lanteriella microphtalma Morrone, 1992.
Andean region (Subantarctic subregion), in the Falkland Islands (Islas Malvinas) (
Lanteriella microphtalma (BMNH).
Habitus of representative Listroderini. 27 Lanteriella microphtalma 28 Telurus caudiculatus 29 Acrorius papallacta 30 Acrostomus bruchi 31 Antarctobius lacunosus 32 Germainiellus dentipennis 33 Lamiarhinus aelficus 34 Listroderes annulipes 35 Philippius superbus.
Taxon-area cladogram of the tribe Listroderini, with the geographical distribution of the subtribes represented on maps. AUS, Australia; CEN, Central Chilean subregion; MTZ, Mexican Transition Zone; NEA, Nearctic region; NEO, Neotropical region; NZ, New Zealand; PAT, Patagonian subregion; SATZ, South American Transition Zone; SUB, Subantarctic subregion; TCF, Tristan da Cunha-Gough islands.
Liparogetus sulcatissimus Broun, 1915 (by indication, monotypy).
Diagnosis. Small to medium-sized (6.0–10.0 mm); vestiture of seta-like scales and setae; rostrum with dorsal carinae; antennal insertion at the middle of the rostrum; club inflated; pronotum subquadrate; metanepisternal suture complete; tibiae lacking spurs.
Liparogetus is the sister genus to both Falklandiopsis and the clade Falklandiellus-Telurus-Nestrius-Falklandius-Lanteriella.
Liparogetus sulcatissimus Broun, 1915.
New Zealand (
Liparogetus sulcatissimus (MZFC).
http://species-id.net/wiki/Nestrius
Nestrius serripes Broun, 1893a (by indication, monotypy).
Small to very small (2.8–5.0 mm); vestiture of seta-like scales and setae; rostrum relatively stout, medium-sized, with dorsal carinae; eyes lateral; funicular segment 2 elongate; pronotum subcylindrical; scutellum not visible.
Nestrius is the sister genus to Falklandius-Lanteriella, confirming Kuschel’s (
Nestrius bifurcus Kuschel, 1964; Nestrius cilipes Broun, 1909; Nestrius crassicornis Broun, 1915; Nestrius foveatus (Broun, 1893); Nestrius hudsoni Marshall, 1953; Nestrius irregularis (Broun, 1910); Nestrius laqueorum Kuschel, 1964; Nestrius ovithorax (Broun, 1893); Nestrius prolixus Broun, 1917; Nestrius serripes Broun, 1893; Nestrius sculpturatus (Broun, 1909); Nestrius simmondsi Broun, 1921; Nestrius sulcirostris Broun, 1917; Nestrius zenoscelis Broun, 1921.
New Zealand (
Nestrius foveatus (MZFC) and Nestrius sculpturatus (MZFC).
http://species-id.net/wiki/Telurus
Fig. 28Antarctobius laticauda Champion, 1918 (= Telurus dissimilis [Fairmaire, 1885]) (by original designation).
Small (3.9–6.5 mm); vestiture of setae only; eyes subcircular, slightly convex; female elytral apex produced; female ventrites 3 and 4 combined longer than 5.
Telurus is closely related to Falklandius-Lanteriella, as found in a previous analysis (
Telurus caudiculatus Morrone and Anderson, 1995; Telurus dissimilis (Fairmaire, 1885).
Andean region (Subantarctic subregion), in southern Chile (
Telurus caudiculatus (AMNH, BMNH, CMNC, CNCI, CWOB, MCZ, MHNS, MLP, MZFC, USNM, ZMC) and Telurus dissimilis (BMNH, IPUM, MHNS, MZFC, NZAC).
Listroderes Schönherr, 1826.
Rostrum relatively stout, medium-sized, shorter than pronotum; scrobes short, ill-defined, broad; funicular segmen 1 longer than 2; elytra usually oblong-oval (subrectangular in Lamiarhinus and Philippius), with intervals convex and with anteapical tubercle (except for Rupanius).
This subtribe, representing the listroderines in the strictest sense, includes the genera Acroriellus, Acrorius, Acrostomus, Antarctobius, Germainiellus, Hyperoides, Lamiarhinus, Listroderes, Methypora, Philippius, Rupanius and Trachodema. In a previous analysis restricted to American taxa (
| 1 | Elytral disc slightly convex to flat | 2 |
| – | Elytral disc convex | 6 |
| 2 | Elytra oblong-oval (Fig. 10) | 3 |
| – | Elytra subrectangular (Fig. 9) | 4 |
| 3 | Vestiture of seta-like scales and setae; scape medium-sized (reaching eye when resting in scrobe) | Acrorius (Fig. 29) |
| – | Vestiture of scales with finger-like processes and setae; scape long (surpassing posterior margin of eye when resting in scrobe) | Trachodema |
| 4 | Pronotum transverse; elytra with carina on apical declivity, disc slightly convex, lacking anteapical tubercle | Rupanius |
| – | Pronotum subcircular or subcylindrical; elytra lacking carina on apical declivity, disc flat, with anteapical tubercle | 5 |
| 5 | Large (17.5–22.8 mm); mandibles with 3-4 setae; pronotum subcircular, wider than elytra, with tubercles; scutellum not visible; elytra fused along interelytral suture, with series of declivital tubercles; female elytral apex not produced; tibiae lacking spurs; tarsomeres 3 subcylindrical (Fig. 12); southern South America | Philippius (Fig. 35) |
| – | Small (4.0–7.0 mm); mandibles with 2 setae; pronotum subcylindrical, narrower than elytra, lacking tubercles; scutellum visible; elytra not fused along interelytral suture, lacking series of declivital tubercles; female elytral apex produced; tibiae with spurs; tarsomeres 3 bilobed (Fig. 11); Australia | Methypora |
| 6 | Funicular segments 3-6 globose; pronotum lacking tubercles; elytra oblong-oval, not fused along interelytral suture | 7 |
| – | Funicular segments 3-6 elongate; pronotum with tubercles; elytra subrectangular, fused along interelytral suture | Lamiarhinus (Fig. 33) |
| 7 | Pronotum transverse or subquadrate; postocular lobes present | 8 |
| – | Pronotum subcircular; postocular lobes absent | Antarctobius (Fig. 31) |
| 8 | Integument reddish brown; epistome not raised; pronotum transverse | 9 |
| – | Integument black; epistome raised; pronotum subquadrate | Acrostomus (Fig. 30) |
| 9 | Vestiture of seta-like or lanceolate scales and setae; scrobal ventral tooth absent | 10 |
| – | Vestiture of subcircular scales and setae; scrobal ventral tooth usually present | Listroderes (Fig. 34) |
| 10 | Vestiture of seta-like scales and setae | 11 |
| – | Vestiture of lanceolate scales and setae | Hyperoides |
| 11 | Elytral interval 3 lacking series of three declivital tubercles | Germaniellus (Fig. 32) |
| – | Elytral interval 3 with series of three declivital tubercles | Acroriellus |
http://species-id.net/wiki/Acroriellus
Acroriellus viridisquamosus Morrone and Ocampo, 1995.
Very small (2.5–3.8 mm); vestiture of seta-like scales and setae; elytra with small, rounded tubercles and series of three tubercles on interval 3.
Acroriellus is the sister genus to Acrostomus-Hyperoides. Originally, it was suggested that it was close to Acrorius (
Acroriellus bobi Morrone and Ocampo, 1995; Acroriellus carinatus Morrone and Ocampo, 1995; Acroriellus similaris Morrone and Ocampo, 1995; Acroriellus tuberculosus Morrone and Ocampo, 1995; Acroriellus viridisquamosus Morrone and Ocampo, 1995; Acroriellus vittetae Morrone and Ocampo, 1995.
South American Transition Zone (North Andean Paramo and Puna biogeographical provinces), in Colombia, Ecuador and Peru (
Acroriellus bobi (AMNH, CMNC), Acroriellus carinatus (CMNC), Acroriellus similaris (CMNC), Acroriellus tuberculosus (CMNC), Acroriellus viridisquamosus (CMNC, FMNH) and Acroriellus vittetae (AMNH, USNM).
http://species-id.net/wiki/Acrorius
Fig. 29Acrorius puncticollis Kirsch, 1889 (by indication, monotypy).
Small (4.0–6.8 mm); vestiture of seta-like scales and setae; scape medium-sized (reaching eye when resting in scrobe); elytra with small, rounded tubercles.
Acrorius is the sister genus to Trachodema-Lamiarhinus-Philippius, taxa that in a previous analysis (
Acrorius andersoni Morrone, 1994; Acrorius bolivianus Ocampo and Morrone, 1996; Acrorius cuprinus Morrone, 1994; Acrorius nymphalis Morrone, 1994; Acrorius otramas Ocampo and Morrone, 1996; Acrorius papallacta Morrone, 1994; Acrorius pillahuata Morrone, 1994; Acrorius plicatifrons Morrone, 1994; Acrorius puncticollis Kirsch, 1889; Acrorius sisyphus Morrone, 1994.
Bolivia, Ecuador and Peru (
Acrorius andersoni (CMNC), Acrorius bolivianus (CMNC, MZFC), Acrorius cuprinus (CMNC), Acrorius nymphalis (CMNC), Acrorius otramas (CMNC), Acrorius papallacta (CMNC, MZFC), Acrorius pillahuata (CMNC, FMNH), Acrorius plicatifrons (FMNH) and Acrorius sisyphus (CNCI, CMNC).
http://species-id.net/wiki/Acrostomus
Fig. 30Adioristus bruchi Hustache, 1926 (by original designation).
Medium-sized (7.3–13.8 mm); integument black; vestiture of seta-like scales and setae; epistome raised; scrobal ventral tooth usually present; pronotum subquadrate.
Acrostomus is the sister genus to Hyperoides.
Acrostomus bruchi (Hustache, 1926); Acrostomus cruralis Kuschel, 1958; Acrostomus foveicollis Kuschel, 1958; Acrostomus griseus (Guérin-Ménéville, 1839); Acrostomus magellanicus Kuschel, 1958; Acrostomus mordor Morrone, 1994; Acrostomus vianai Kuschel, 1958.
Acrostomus magellanicus and Acrostomus vianai: Azorella trifurcata (Gaertner) Pers., Bolax gummifera (Lam.) Spreng. and Mulinum spinosum (Cav.) Pers. (Apiaceae) (Morrone, 1994b).
Andean region (Patagonian subregion), in southern Argentina and southern Chile (
Acrostomus bruchi (CWOB, IPCN, MACN, MLP, MZFC), Acrostomus cruralis (MACN, USNM), Acrostomus foveicollis (CBPC, CWOB, MHNS, MZFC), Acrostomus griseus (CWOB, FIML, IPUM, MHNS, MLP, MZFC), Acrostomus magellanicus (BMNH, MHNS, USNM), Acrostomus mordor (AMNH, MACN, MLP, MZFC) and Acrostomus vianai (BMNH, MHNS).
http://species-id.net/wiki/Antarctobius
Fig. 31Antarctobius lacunosus Fairmaire, 1885 (subsequent designation by Morrone, 1992a).
Small to medium-sized (3.7–9.5 mm); vestiture of seta-like or subcircular scales and setae; pronotum subcircular; postocular lobes absent.
Antarctobius is closely related to Germainiellus, Listroderes and the clade Methypora-Rupanius-Acrorius-Trachodema-Lamiarhinus-Philippius. The distinction between Antarctobius, Germainiellus and Listroderes is not without doubt (see Morrone and Marvaldi, 1998), and future analyses may determine if they are merged into a single genus.
Antarctobius abditus (Enderlein, 1907); Antarctobius bidentatus (Champion, 1918); Antarctobius falklandicus (Enderlein, 1907); Antarctobius germaini (Kolbe, 1907); Antarctobius hyadesii Fairmaire, 1885; Antarctobius lacunosus Fairmaire, 1885; Antarctobius malvinensis Posadas and Morrone, 2004; Antarctobius rugirostris Champion, 1918; Antarctobius vulsus (Enderlein, 1907); Antarctobius yefacel Morrone, 1992.
Antarctobius abditus: Senecio candidans DC (Asteraceae); Antarctobius hyadesii: Senecio alloeophyllus O. Hoffm. and Senecio candidans DC (Asteraceae) (Morrone, 1992a; Marvaldi, 1998).
Antarctobius abditus and Antarctobius falklandicus (Marvaldi, 1998).
Andean region (Subantarctic subregion), in southern Chile and southern Argentina, including the Falkland Islands (Islas Malvinas) (
Antarctobius abditus (BMNH), Antarctobius bidentatus (BMNH), Antarctobius falklandicus (AMPC, BMNH, MZFC), Antarctobius germaini (AMNH, BMNH, CADIC, CMNC, CWOB, IPUM, MHNS, MLP, MZFC), Antarctobius hyadesii (BPBM, CMNC, MHNS, MZFC), Antarctobius lacunosus (BMNH, MCZ, MHNS), Antarctobius rugirostris (BMNH), Antarctobius vulsus (BMNH, USNM) and Antarctobius yefacel (AMNH).
http://species-id.net/wiki/Germainiellus
Fig. 32Listroderes dentipennis Germain, 1895 (by original designation).
Small to medium-sized (6.0-8.4 mm); vestiture of seta-like scales and setae; pronotum transverse; postocular lobes present.
Germainiellus is closely related to Antarctobius, Listroderes and the clade Methypora-Rupanius-Acrorius-Trachodema-Lamiarhinus-Philippius. It was originally described as intermediate between Antarctobius and Listroderes (Morrone, 1993a). The distinction between Antarctobius, Germainiellus and Listroderes is not without doubt (see Morrone and Marvaldi, 1998), and future analyses may determine if they are merged into a single genus.
Germainiellus angulipennis (Germain, 1895); Germainiellus attenuatus (Germain, 1895); Germainiellus dentipennis (Germain, 1895); Germainiellus fulvicornis (Germain, 1895); Germainiellus laevirostris (Germain, 1895); Germainiellus lugens (Germain, 1895); Germainiellus ovatus (Boheman, 1842); Germainiellus philippii (Germain, 1896); Germainiellus planipennis (Blanchard, 1851); Germainiellus punctiventris (Germain, 1895); Germainiellus rugipennis (Blanchard, 1851); Germainiellus salebrosus (Enderlein, 1907); Germainiellus spp. (MZFC).
Germainiellus dentipennis and Germainiellus fulvicornis: Nothofagus sp. (Nothofagaceae); Germainiellus laevirostris: Senecio smithii DC (Asteraceae); Germainiellus planipennis: Nothofagus dombeyi (Mirb.) Oerst. (Nothofagaceae) and Peumus boldus Mol. (Monimiaceae); Germainiellus salebrosus: Empetrum rubrum Vahl ex Willd. (Empetraceae) (Morrone, 1993a).
Andean region (Subantarctic subregion), in southern Chile and southern Argentina, including the Falkland Islands (Islas Malvinas) (
Germainiellus angulipennis (MHNS), Germainiellus attenuatus (ARPC, MHNS), Germainiellus dentipennis (CBPC, CMNC, CWOB, MHNS, USNM), Germainiellus fulvicornis (AMNH, BPBM, CBCP, CMNC, CWOB, FIML, MCZ, MHNS, MLP, MZFC, USNM), Germainiellus laevirostris (BPBM, IPUM, MCZ, MHNS, MLP, USNM), Germainiellus lugens (CMNC, CWOB, IPUM, MACN, MCZ, MHNS, MZFC), Germainiellus ovatus (BMNH, MHNS, USNM), Germainiellus philippii (CMNC, DEI, MHNS, MZFC), Germainiellus planipennis (BMNH, CWOB, MHNS), Germainiellus punctiventris (MHNS), Germainiellus rugipennis (AMNH, BPBM, CADIC, CBCP, CMNC, MHNS, MLP, MZFC, USNM) and Germainiellus salebrosus (BMNH).
Hyperoides fragariae Marshall, 1914 (by indication, monotypy).
Small to medium-sized (5.1–7.5 mm); vestiture of lanceolate scales and setae; postocular lobes present; elytra lacking anteapical tubercle.
Hyperoides is the sister genus to Acrostomus, contrasting with its more isolated position in a previous analysis (Morrone, 1997a).
Hyperoides balfourbrownei (Kuschel, 1952); Hyperoides fragariae Marshall, 1914; Hyperoides murinus (Germain, 1896); Hyperoides subcinctus (Boheman, 1842); Hyperoides victus (Germain, 1896).
Hyperoides fragariae: Fragaria vesca L. (Rosaceae); Hyperoides subcinctus: Senecio sp. (Asteraceae); Hyperoides murinus: Citrulus vulgaris Schrad. (Cucurbitaceae), Phaseolus sp. (Fabaceae) and Solanum tuberosum L. (Solanaceae); Hyperoides victus: Senecio bahioides Hook. et Arn. (Asteraceae) (
Neotropical region and Andean region (Central Chilean subregion), in Argentina, Chile and Uruguay, and introduced into South Africa (
Hyperoides balfourbrownei (MLP, MZFC), Hyperoides fragariae (BMNH, CBPC, MNHN), Hyperoides murinus (BMNH, CWOB, MHNS, MZFC), Hyperoides subcinctus (AMNH, BMNH, CBPC, CMNC, CWOB, IADIZA, MACN, MHNS, MNHN, MZFC) and Hyperoides victus (BMNH, CMNC, CWOB, MHNS).
http://species-id.net/wiki/Lamiarhinus
Fig. 33Lamiarhinus aelficus Morrone, 1992.
Small to medium-sized (5.7–6.8 mm); vestiture of seta-like scales and setae; funicular segments 3-6 elongate; pronotum with tubercles; elytra subrectangular, fused along interelytral suture.
Lamiarhinus is the sister genus to Philippius. In a previous analysis (
Lamiarhinus aelficus Morrone, 1992; Lamiarhinus horridus (Germain, 1896).
Lamiarhinus aelficus: Podanthus ovatifolius Lag. (Asteraceae) (
Andean region (Central Chilean subregion) (
Lamiarhinus aelficus (CMNC, CWOB, MLP, MZFC) and Lamiarhinus horridus (MHNS).
http://species-id.net/wiki/Listroderes
Fig. 34Listroderes costirostris Schönherr, 1826 (by original designation, combined description).
Small to medium-sized (3.9–12.5 mm); vestiture of subcircular scales and setae; scrobal ventral tooth usually present.
Listroderes is closely related to Antarctobius, Germainiellus and the clade Methypora-Rupanius-Acrorius-Trachodema-Lamiarhinus-Philippius. The distinction between Antarctobius, Germainiellus and Listroderes is not without doubt (see Morrone and
Listroderes affinis Hustache, 1926; Listroderes angusticeps Blanchard, 1851; Listroderes annulipes Blanchard, 1851; Listroderes apicalis Waterhouse, 1841; Listroderes bimaculatus Boheman, 1842; Listroderes brevirostris Germain, 1895; Listroderes brevisetis Hustache, 1926; Listroderes bruchi Hustache, 1926; Listroderes charybdis Morrone, 1993; Listroderes cinerarius Blanchard, 1851; Listroderes confusus Hustache, 1926; Listroderes costirostris Schönherr, 1826; Listroderes curvipes Germain, 1895; Listroderes delaiguei Germain, 1895; Listroderes desertorum Germain, 1895; Listroderes difficilis Germain, 1895; Listroderes elegans Hustache, 1926; Listroderes erinaceus Germain, 1895; Listroderes fallax Germain, 1895; Listroderes foveatus (Lea, 1928); Listroderes hoffmanni Germain, 1895; Listroderes howdenae Morrone, 1993; Listroderes leviculus Kuschel, 1952; Listroderes montanus Germain, 1895; Listroderes nodifer Boheman, 1842; Listroderes obliquus Klug, 1829; Listroderes obrieni Morrone, 1993; Listroderes paranensis Hustache, 1926; Listroderes punicola Kuschel, 1949; Listroderes pusillus Hustache, 1926; Listroderes robustior Schenkling and Marshall, 1931; Listroderes robustus Waterhouse, 1841; Listroderes scylla Morrone, 1993; Listroderes trivialis Germain, 1895; Listroderes tuberculifer Blanchard, 1851; Listroderes uruguayensis Kuschel, 1952; Listroderes wagneri Hustache, 1926; Listroderes wittei Hustache, 1926.
Listroderes apicalis: Betavulgaris L. (Chenopodiaceae), Helianthus annus L. (Asteraceae) and Triticum aestivum L. (Poaceae); Listroderes bimaculatus: Baccharis linearis (Ruiz and Pav.) Pers. (Asteraceae) and Puya chilensis Molina (Bromeliaceae); Listroderes bruchi: Baccharis salicifolia (Ruiz and Pavón) Pers. and Senecio subulatus Don Hooker et Arnott (Asteraceae); Listroderes cinerarius: Atriplex sp. (Chenopodiaceae); Listroderes costirostris, Listroderes difficilis and Listroderes obliquus: Apium graveolens L. and Daucus carota L. (Apiaceae), Brassica rapa L., Listroderes oleracea L. and Coronopus didymus (L.) Smith (Brassicaceae), Rumex altissimus Wood (Polygonaceae), Nicotiana tabacum L. and Solanum tuberosum L. (Solanaceae) and Stellaria spp. (Caryophyllaceae); Listroderes robustus: Atriplex semibaccata R. Br. (Chenopodiaceae); Listroderes uruguayensis: Hydrocotyle bonariensis Lam. (Apiaceae) (
Listroderes bruchi, Listroderes delaiguei and Listroderes difficilis (
Andean region (Subantarctic, Central Chilean and Patagonian subregions), South American Transition Zone and Neotropical region, in Argentina, Brazil, Chile, Paraguay, Peru and Uruguay, and introduced into Australia, Easter Island, Israel, Japan, New Zealand, South Africa, Spain and USA (
Listroderes affinis (CBPC, IPCN, IPUM, MACN, MNHN), Listroderes angusticeps (MHNS, MNHN, MZFC), Listroderes annulipes (CBPC, CWOB, MHNS, MNHN, MZFC), Listroderes apicalis (AMNH, BMNH, CMNC, MACN, MHNS, MLP, MZFC), Listroderes bimaculatus (AMNH, BMNH, CMNC, CWOB, MACN, MHNS), Listroderes brevirostris (MHNS), Listroderes brevisetis (CBCP, DZUP, IPCN, MACN, MLP, MNHN), Listroderes bruchi (CMNC, DZUP, FIML, IADIZA, MACN, MLP, MZFC), Listroderes charybdis (MACN, MLP), Listroderes cinerarius (BMNH, CMNC, CWOB, IADIZA, MHNS, MNHN, MZFC), Listroderes confusus (DZUP, FIML, MACN, MLP, MNHN), Listroderes costirostris complex (AMNH, BMNH, CBPC, CMNC, CWOB, DZUP, FIML, GJWC, MACN, MHNS, MLP, MNHN, MZFC, MZSP, USNM), Listroderes curvipes (BMNH, CWOB, MHNS), Listroderes delaiguei (BMNH, CADIC, CWOB, IPUM, MHNS, MZFC), Listroderes desertorum (BMNH, CMNC, CWOB, MHNS, MZFC), Listroderes elegans (GJWC, MACN, MLP, MNHN), Listroderes erinaceus (MHNS), Listroderes fallax (CWOB, MHNS, MZFC), Listroderes foveatus (BMNH, CMNC, DZUP, FIML, GJWC, MACN, MZSP), Listroderes hoffmanni (BMNH, CWOB, MHNS, MZFC), Listroderes howdenae (CMNC, MLP, MZFC), Listroderes leviculus (BMNH), Listroderes montanus (MHNS, MZFC), Listroderes nodifer (BMNH, CWOB, MACN, MHNS), Listroderes obrieni (MHNS, MLP, MZFC), Listroderes paranensis (DZUP, MNHN), Listroderes punicola (CMNC, MHNS, MZFC), Listroderes pusillus (CBPC, MLP, MNHN, MZFC), Listroderes robustior (BMNH, CMNC, CWOB, MHNS, MLP, MZFC), Listroderes robustus (CMNC, CWOB, MHNS), Listroderes scylla (FIML, MLP), Listroderes trivialis (MHNS), Listroderes tuberculifer (CMNC, MHNS, MZFC), Listroderes uruguayensis (BMNH, CMNC), Listroderes wagneri (BMNH, MNHN) and Listroderes wittei (MACN, MNHN).
Methypora postica Pascoe, 1865 (by indication, monotypy).
Small (4.0–7.0 mm); vestiture of subcircular scales and setae; pronotum subcylindrical, lacking tubercles; scutellum visible; elytra not fused along interelytral suture, lacking series of declivital tubercles; female elytral apex produced; tibiae with spurs.
Methypora is the sister genus to Rupanius.
Methypora postica Pascoe, 1865 and Methypora tibialis Lea, 1911.
Australia (
Methypora postica (BMNH).
http://species-id.net/wiki/Philippius
Fig. 35Listroderes superbus Reed, 1872 (subsequent designation by
Large to very large (17.5–22.8 mm); vestiture of scales with finger-like processes and setae; mandible with 3-4 setae; pronotum wider than elytra; scutellum not visible; elytra subrectangular, fused along interelytral suture; tibiae lacking spurs; tarsomeres 3 subcylindrical.
Philippius is the sister genus to Lamiarhinus.
Philippius superbus (Reed, 1872).
Andean region (Subantarctic subregion), in southern Argentina and southern Chile (
Philippius superbus (IADIZA, MACN, MHNS, MLP, USNM).
Rupanius carinatus Morrone, 1995c.
Small (5.3–6.6 mm); vestiture of seta-like scales and setae; pronotum transverse; elytra subrectangular, with carina on apical declivity, disc slightly convex, lacking anteapical tubercle.
Rupanius is the sister genus to Methypora (Australia), and both are placed in Listroderina. In a previous analysis (
Rupanius carinatus Morrone, 1995.
South American Transition Zone (North Andean Paramo biogeographical province), in Colombia (
Rupanius carinatus (CMNC).
http://species-id.net/wiki/Trachodema
Trachodema tuberculosa Blanchard, 1849 (by indication, monotypy).
Small to very small (2.5–5.3 mm); vestiture of scales with finger-like processes and setae; scape long (surpassing posterior margin of eye when resting in scrobe); pronotum transverse.
Trachodema is the sister genus to Lamiarhinus-Philippius.
Trachodema paolae Alonso-Zarazaga, 2012 and Trachodema tuberculosa Blanchard, 1849.
Trachodema tuberculosa: Atriplex semibaccata R. Br. (Chenopodiaceae) (
Andean region (Central Chilean subregion) (
Trachodema paolae (MHNS) and Trachodema tuberculosa (CMNC, CWOB, DZUP, FIML, MHNS, MZFC, USNM).
Ecuador and Peru (
Chile (
The geographical distribution of the genera analysed indicates that Listroderini are basically a Gondwanan taxon, with Listronotus being the only genus distributed in North America. All the subtribes have Andean representatives (especially in the Subantarctic subregion), each showing a different pattern:
1 Macrostyphlina: exclusively Andean, in both the Andean region (Subantarctic and Central Chilean subregions) and the South American Transition Zone.
2 Falklandiina: distributed in the Andean region (Subantarctic subregion) and New Zealand.
3 Listroderina: distributed in the Andean region (Subantarctic, Patagonian and Central Chilean subregions), the South American and Mexican Transition Zones and the Neotropical and Australian Temperate regions.
4 Palaechthina: distributed in the Andean (Subantarctic and Central Chilean subregions), Neotropical and Nearctic regions, the South American Transition Zone, the Tristan da Cunha-Gough islands, New Zealand and the Australian Temperate region.
By replacing the genera for the areas where they are distributed, a taxon-area cladogram was obtained (Fig. 36). The paralogy-free subtrees that can be obtained from this taxon-area cladogram are mostly uninformative, and the informative ones cannot be combined into a general area cladogram. Geographical paralogy is particularly evident in the Subantarctic subregion, where representatives of the four subtribes are represented, suggesting that Listroderini are an ancient Gondwanan group. Several extinction events might have obscured the relationships among the areas.
I thank Rolf Oberprieler for stimulating discussions on weevil systematics over the years and for donating specimens of Anorthorhinus, Samuel Brown for communicating some omissions and corrections to my 2011 checklist, Silvia Espinosa Matías for preparing the Scanning Electron Microscope images at the Facultad de Ciencias, UNAM, and Sergio Roig-Juñent for the habitus illustrations. Rolf Oberprieler and one anonymous reviewer provided very useful comments that helped improve the manuscript. The image of Gromilus veneris is used with permission of the Bishop Museum, Honolulu.