(C) 2013 Leen P. van Ofwegen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Abstract
Sinularia leptoclados (Ehrenberg, 1834) is re-described. Sinularia leptoclados var. gonatodes Kolonko, 1926 is synonymized with Sinularia maxima Verseveldt, 1977. Two new species of Sinularia with digitiform lobules, leptoclados-type surface clubs and unbranched interior spindles, are described. An updated maximum likelihood tree of Sinularia species with leptoclados-type clubs (clade 5C) based on two mitochondrial genes (mtMutS, COI) and a nuclear gene (28S rDNA) is presented.
Alcyonacea, re-description, new species, Indo-Pacific, Red Sea, taxonomy, phylogeny
In his revision of the soft coral genus Sinularia,
The first two authors have based their identifications of Sinularia leptoclados on the microscope slides of Verseveldt at their disposal, and following the Sinularia revision of
While collecting new material of Sinularia leptoclados at Eilat, northern Gulf of Aqaba, Red Sea, we unexpectedly found two other species with leptoclados-type clubs and leptoclados-like colony shape: Sinularia verseveldti Ofwegen, 1996 (Fig. 5f), so far only known from the Pacific, and a yet undescribed species which is described here.
In order to identify the material, sclerites from different parts of the colony were obtained by dissolving the tissues in 10% sodium hypochlorite, followed by rinsing in fresh water. When appropriate, they were prepared for scanning electron microscopy as follows: the sclerites were carefully rinsed with double-distilled water, dried at room temperature, coated with gold and examined with a Jeol 6480LV electron microscope, operated at 10 kV.
Material studied is deposited in the Naturalis Biodiversity Center (formerly Rijks-museum van Natuurlijke Historie, Leiden, the Netherlands (RMNH)), Zoological Museum, Department of Zoology, Tel Aviv University, Israel (ZMTAU), Museum für Naturkunde der Humboldt-Universität, Berlin, Germany (ZMB), Zoological Reference Collection (ZRC) of the Raffles Museum of Biodiversity Research, Singapore, and the Museum and Art Gallery of the Northern Territory, Darwin, Australia (NTM).
Extraction of DNA from ethanol-preserved tissue samples, PCR amplification, and sequencing of the mtMutS (msh1), COI and 28S rDNA genes followed the protocols published in McFadden et al. (2011) and
Specimens of Sinularia included in the molecular phylogenetic analyses. NTM = Museum and Art Gallery of the Northern Territory; RMNH = Naturalis Biodiversity Center; ZMTAU = Zoological Museum, Tel Aviv University. Bold = new GenBank accessions; NA = no sequence obtained.
| GenBank Acc. No. | ||||
|---|---|---|---|---|
| Species | Museum Acc. No. | COI | mtMutS | 28S rDNA |
| Sinularia abrupta | NTM C14012 | KC542862 | KC542849 | NA |
| Sinularia abrupta | ZMTAU Co 33623 | JX991256 | JX991168 | KC542822 |
| Sinularia acuta | RMNH Coel. 38721 | KC542863 | FJ621376 | NA |
| Sinularia acuta | ZMTAU Co 33617 | JX991257 | JX991169 | KC542823 |
| Sinularia australiensis sp. n. | NTM C14492 | KC542864 | FJ621437 | KC542824 |
| Sinularia australiensis sp. n. | NTM C14519 | KC542865 | FJ621438 | KC542825 |
| Sinularia bisulca | RMNH Coel. 38724 | KC542866 | FJ621378 | KC542826 |
| Sinularia corpulentissima | RMNH Coel. 40839 | KC542867 | KC542850 | KC542827 |
| Sinularia daii | ZMTAU Co 34665 | JX991258 | JX991170 | KC542828 |
| Sinularia densa | RMNH Coel. 40840 | KC542868 | KC542851 | KC542829 |
| Sinularia digitata | RMNH Coel. 40841 | KC542869 | KC542852 | KC542830 |
| Sinularia eilatensis sp. n. | ZMTAU Co 35260 | KC542870 | KC542853 | KC542831 |
| Sinularia eilatensis sp. n. | ZMTAU Co 35305 | KC542873 | KC542856 | KC542834 |
| Sinularia ?eilatensis sp. n. | ZMTAU Co 35303 | KC542871 | KC542854 | KC542832 |
| Sinularia ?eilatensis sp. n. | ZMTAU Co 35304 | KC542872 | KC542855 | KC542833 |
| Sinularia erecta | ZMTAU Co 34144 | GU355981 | FJ621404 | KC542835 |
| Sinularia gardineri (5A) | ZMTAU Co 34097 | GU355982 | FJ621414 | KC542819 |
| Sinularia hirta (5B) | ZMTAU Co 34100 | GU355983 | FJ621428 | KC542820 |
| Sinularia leptoclados | ZMTAU Co 35308 | KC542874 | KC542857 | KC542836 |
| Sinularia leptoclados | ZMTAU Co 34095 | GU355980 | FJ621439 | KC542837 |
| Sinularia longula | RMNH Coel. 38439 | KC542875 | FJ621441 | KC542838 |
| Sinularia maxima | NTM C14512 | KC542876 | FJ621448 | KC542839 |
| Sinularia molesta | RMNH Coel. 38440 | KC542877 | FJ621449 | NA |
| Sinularia penghuensis | ZMTAU Co 34659 | JX991273 | JX991183 | KC542840 |
| Sinularia penghuensis | ZMTAU Co 34681 | JX991274 | JX991184 | KC542841 |
| Sinularia penghuensis | ZMTAU Co 34739 | JX991276 | JX991186 | KC542842 |
| Sinularia robusta | NTM C14518 | KC542878 | FJ621473 | KC542843 |
| Sinularia slieringsi | ZMTAU Co 34654 | JX991277 | JX991187 | NA |
| Sinularia terspilli (5B) | ZMTAU Co 34156 | GU355984 | FJ621481 | KC542821 |
| Sinularia verseveldti | ZMTAU Co 35309 | KC542879 | KC542858 | KC542844 |
| Sinularia verseveldti | RMNH Coel. 40842 | KC542880 | KC542859 | KC542845 |
| Sinularia verseveldti | RMNH Coel. 40843 | KC542881 | KC542860 | KC542846 |
| Sinularia verseveldti | RMNH Coel.40844 | KC542882 | KC542861 | KC542847 |
| Sinularia wanannensis | ZMTAU Co 34704 | JX991281 | JX991190 | KC542848 |
urn:lsid:zoobank.org:act:C0EC77D7-A9DF-49A6-8BC4-C93AC3AFE8AF
http://species-id.net/wiki/Sinularia_australiensis
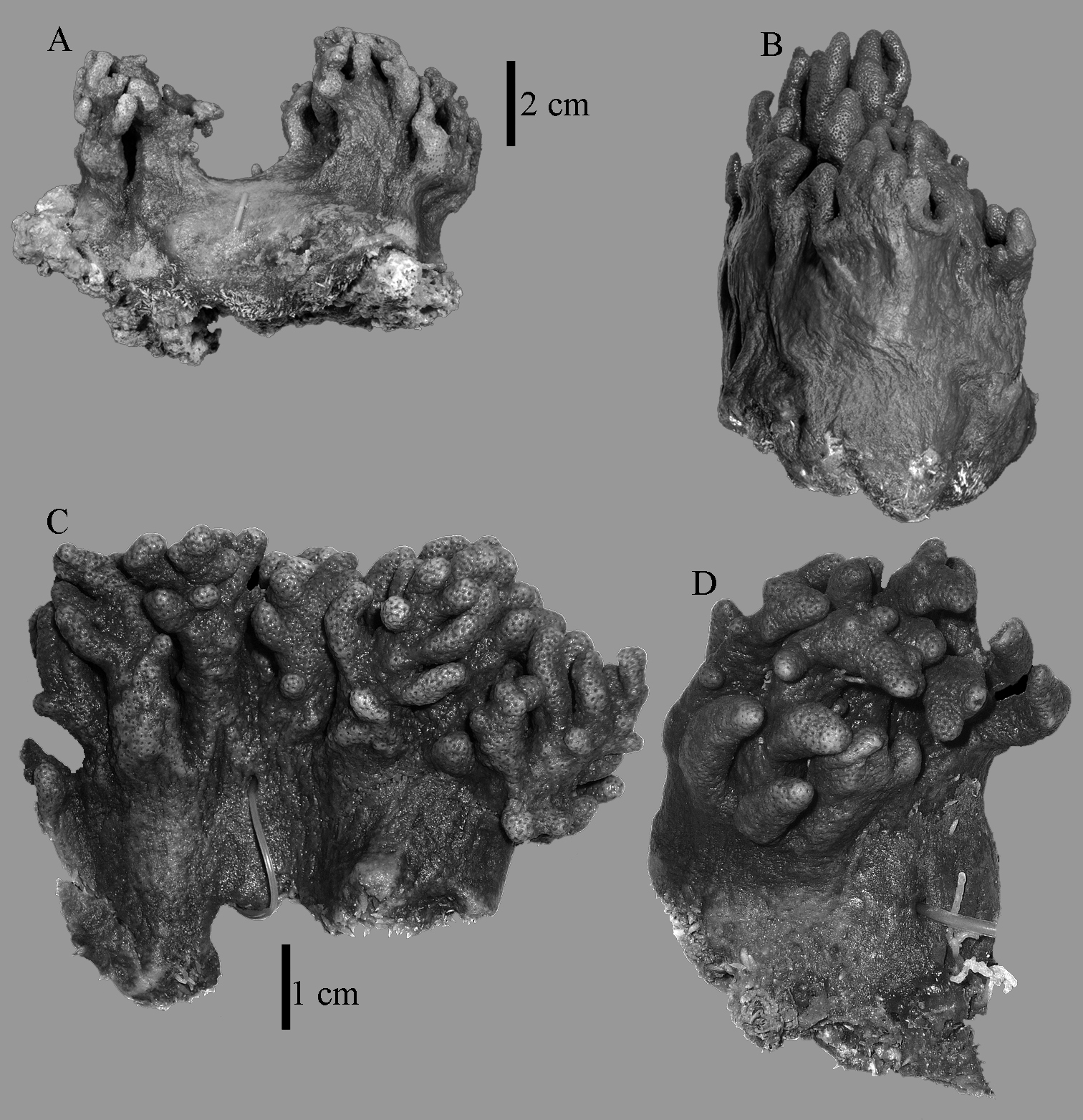
Figs 1–4Holotype: NTM C14519, Australia, Northern Territory, Gulf of Carpentaria, West of Bremer island, 12°05.660'S, 136°47.754'E, depth 1–3 m, coll. P. Alderslade & party, 17 December 2003. Paratypes: NTM C14492, C14520, C14521, same data as holotype.
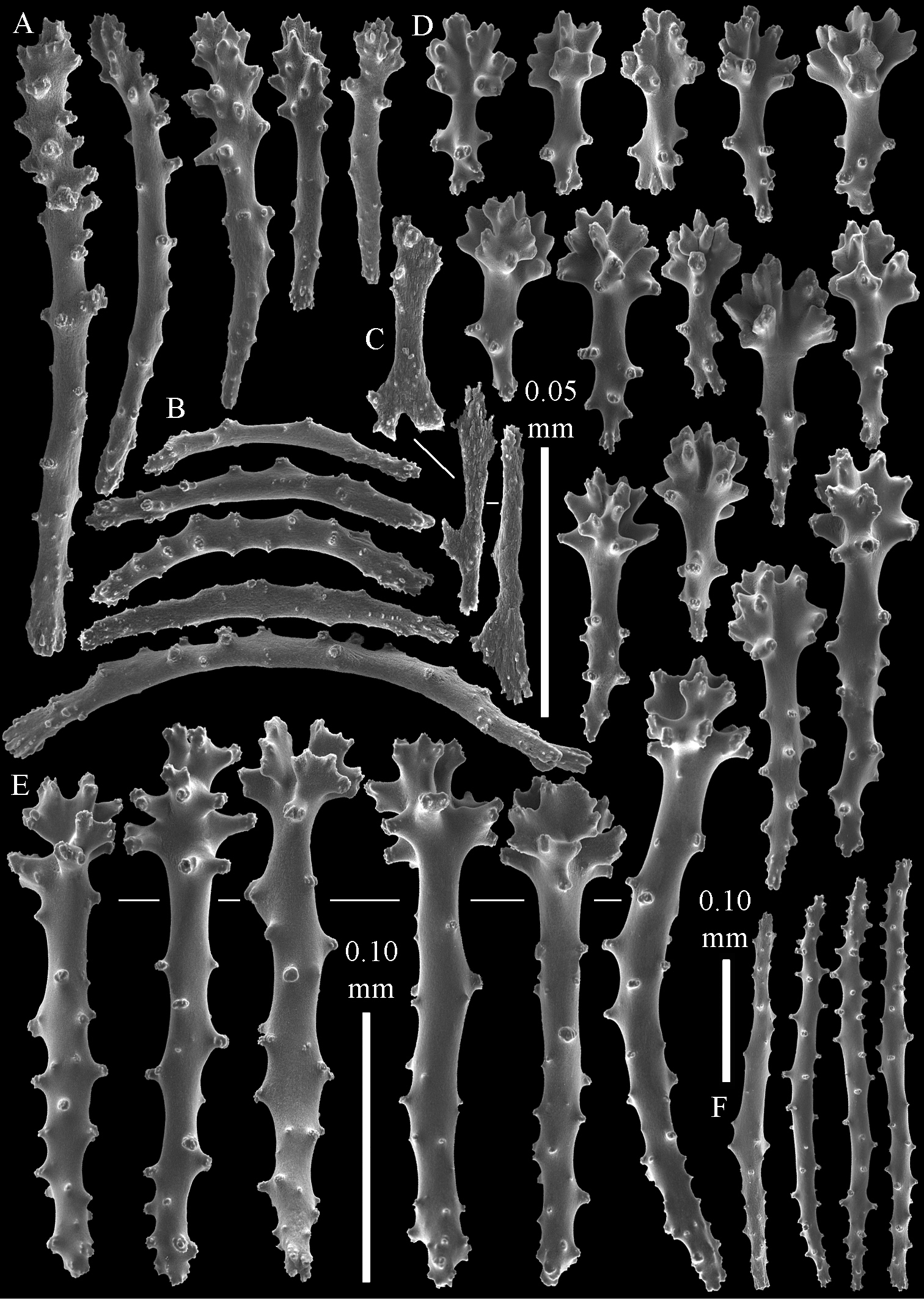
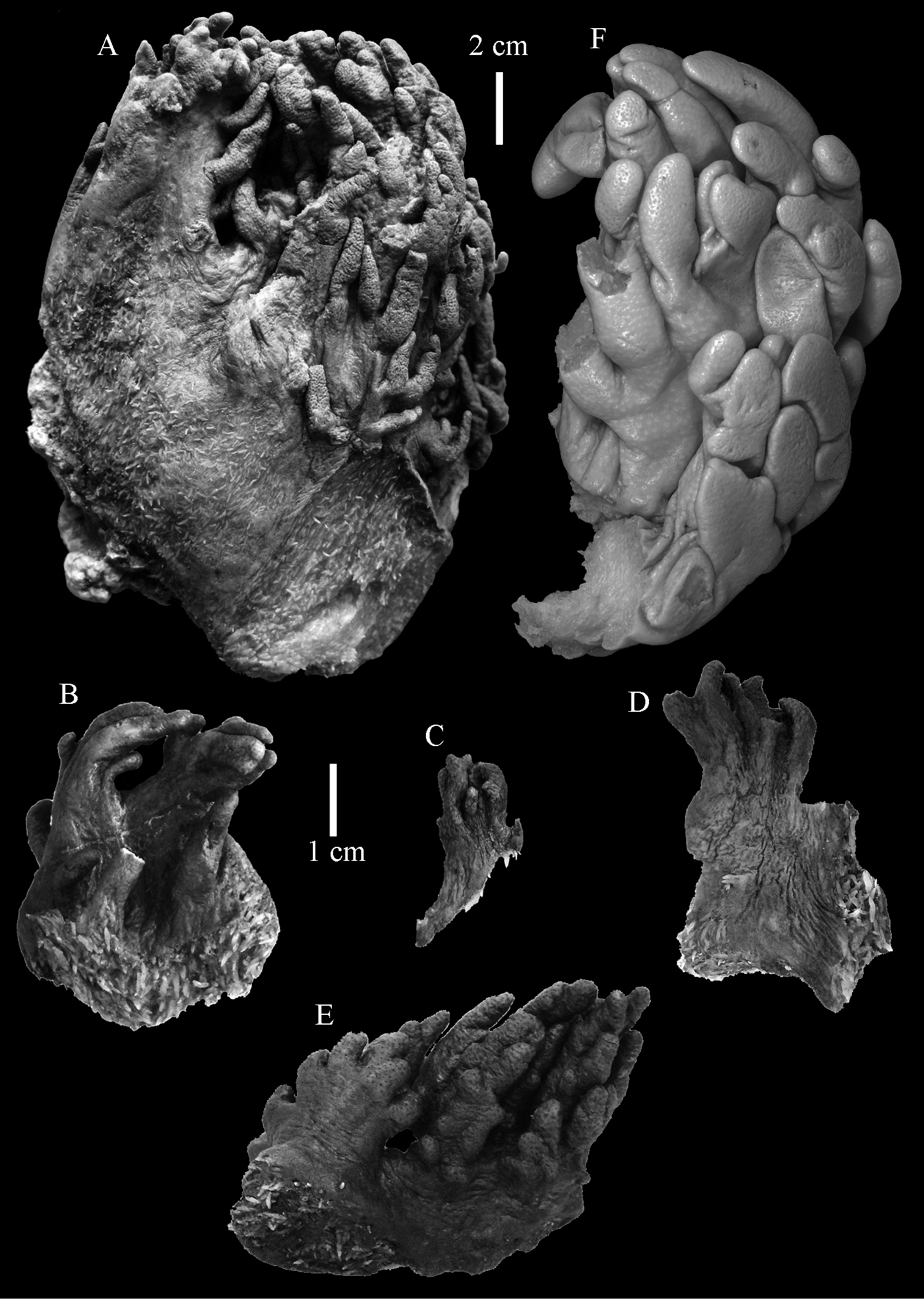
The holotype is 6 cm high and 9.5 cm wide, attached to a piece of rock (Fig. 1A). The middle part of the colony is devoid of lobes, possibly a colony in the process of colony fission. The primary lobes branch off once or twice, lobules knob- to finger-shaped, up to 4 mm wide and 1 cm long.
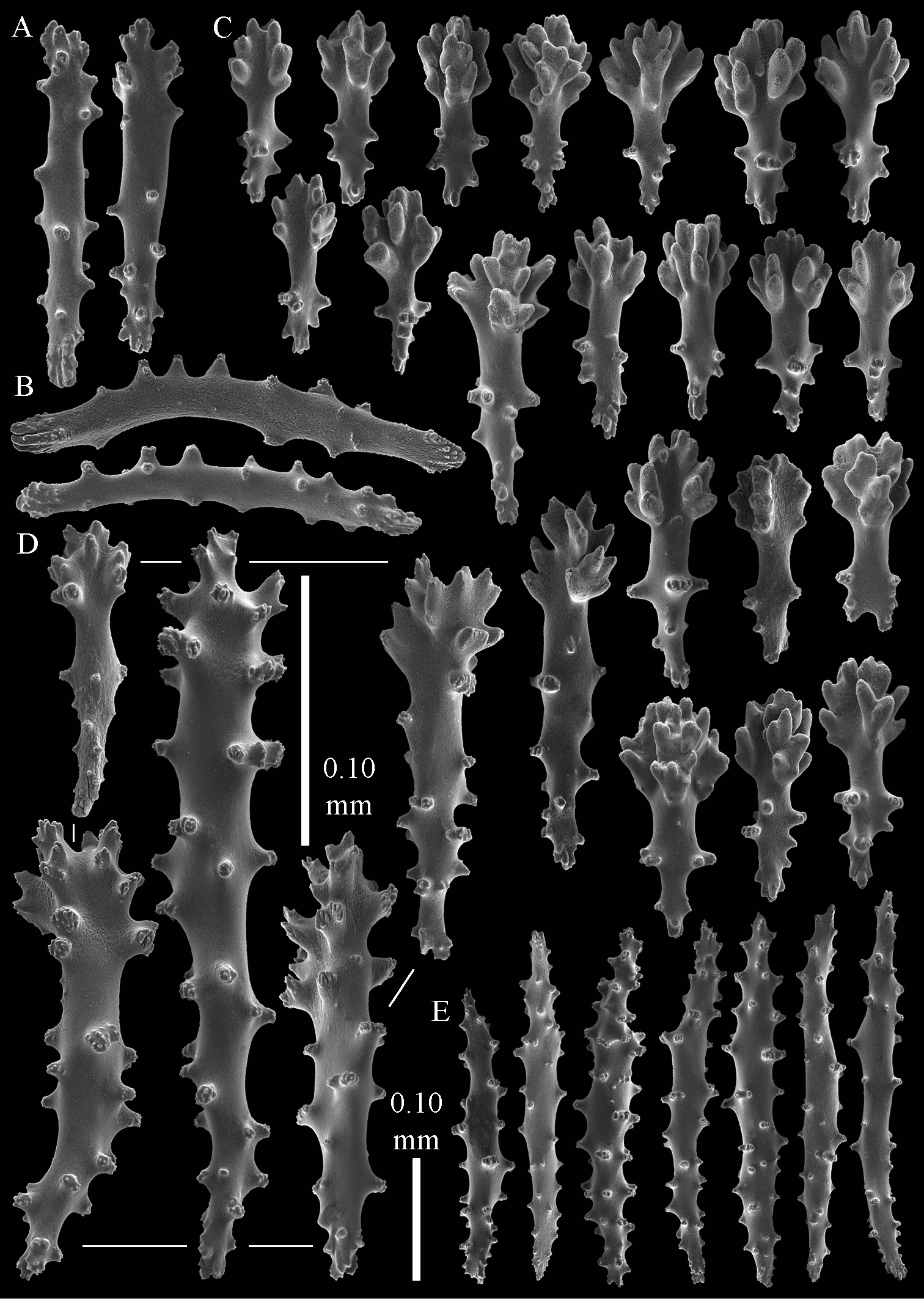
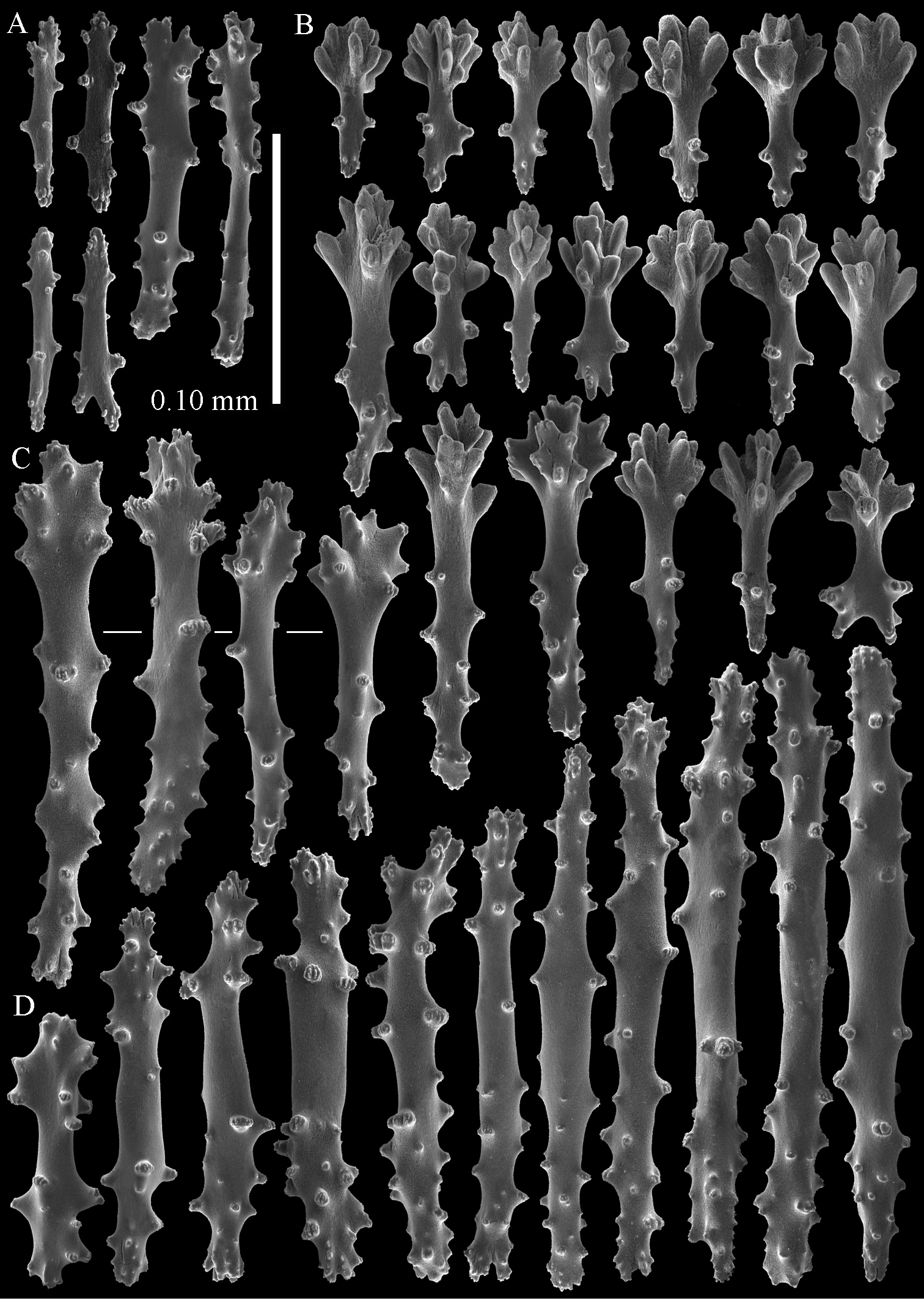
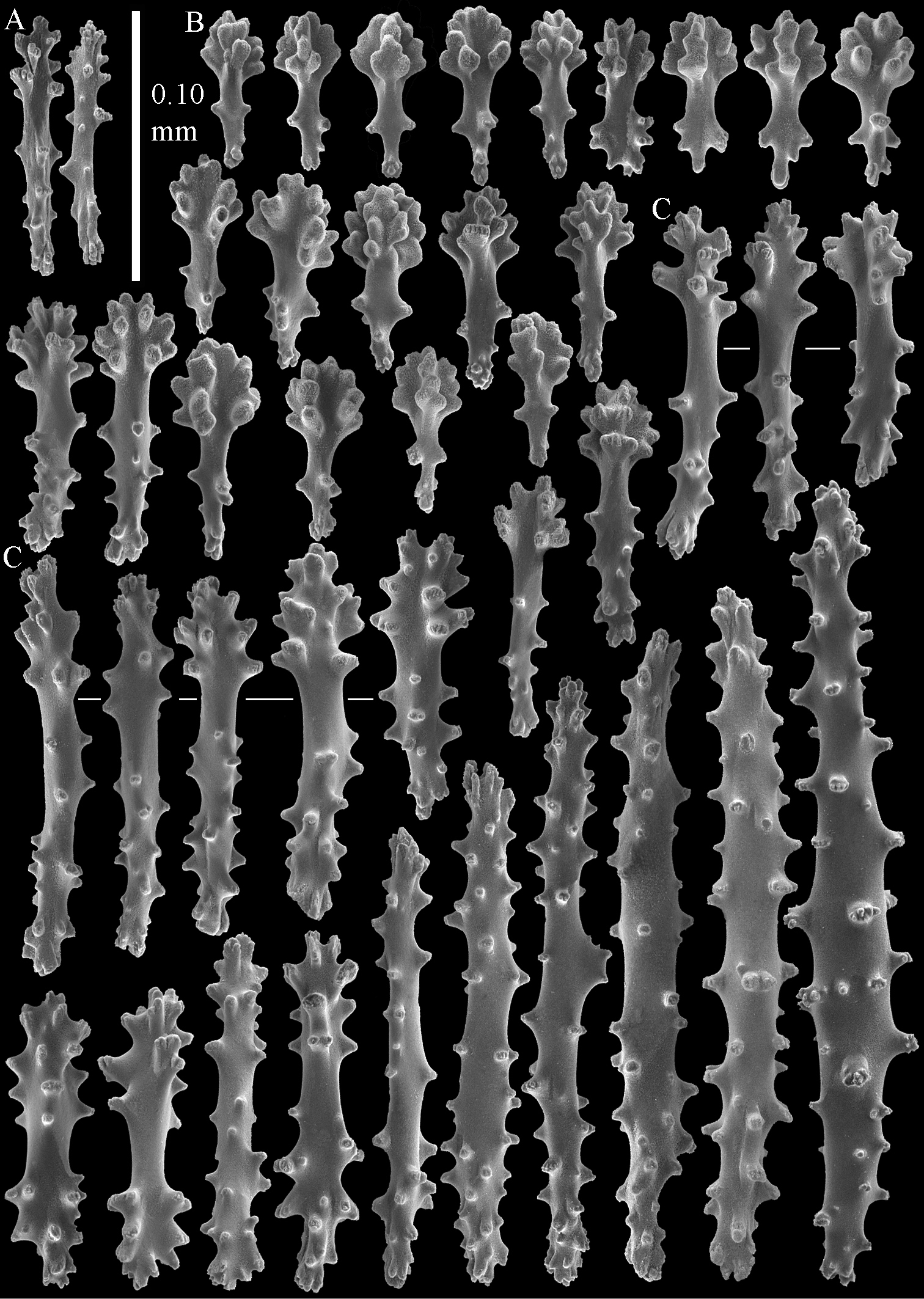
The polyps have a collaret and eight points. Points with poorly developed clubs, up to 0.15 mm long (Fig. 2A). Collaret has bent spindles, up to 0.20 mm long (Fig. 2B). Tentacle sclerites were not present.
The surface layer of the lobules has leptoclados-type clubs, the smallest are 0.07 mm long, most are around 0.10 mm, but some even reach a length of 0.15 mm (Fig. 2C); in addition, longer wart clubs are present, up to 0.25 mm long (Fig. 2D). Furthermore, the surface layer of the lobules has spindles, up to 0.40 mm long, with simple tubercles (Fig. 2E).
The sclerites of the surface layer of the base of the colony resemble those of the surface layer of the lobules but the clubs have wider handles and the spindles are wider (Fig. 3).
The interior of the colony has mostly unbranched spindles; a few have one or two side branches. In the lobules the spindles are up to 2.5 mm long (Fig. 4A), almost all having simple tubercles (Fig. 4B). In the base of the colony they are up to 3 mm long (Fig. 4C), with more complex tubercles (Fig. 4D).
Sinularia australiensis sp. n., A holotype NTM C14519 B paratype NTM C14492 C paratype NTM C14520 D paratype NTM C14521. Scale at A also applies to B, scale at C also to D.
Sinularia australiensis sp. n., holotype NTM C14519. A point clubs B collaret spindles C leptoclados-type clubs of surface layer of lobule D wart clubs of surface layer of lobule E spindles of surface layer of lobule. Scale of 0.10 mm at E only applies to E.
Sinularia australiensis sp. n., holotype NTM C14519. Sclerites of the surface layer of the base of the colony A leptoclados-type clubs B wart clubs C–D spindles. Scale of 0.10 mm at D only applies to D.
Sinularia australiensis sp. n., holotype NTM C14519. sclerites of the interior A spindles from the lobules B tuberculation of one of the lobule spindles C spindles from the base D tuberculation of one of the base spindles. Scale of 1 mm at C also applies to A.
The preserved specimen is brown.
Named after Australia, where the type was collected.
NTM C14492 (Fig. 1B) and NTM C14521 (Fig. 1D) have stouter lobules, up to 1 cm wide.
The species resembles Sinularia leptoclados regarding clubs and colony shape. It differs in having small surface lobule spindles with uniformly placed tubercles and many internal lobule spindles with simple tubercles. Other species resembling Sinularia australiensis are Sinularia acuta Manuputty & Ofwegen, 2007, S. corpulentissima Manuputty & Ofwegen, 2007 and Sinularia longula Manuputty & Ofwegen, 2007, all three described from Ambon. Sinularia acuta and Sinularia longula have more slender spindles and wart clubs in the surface layer of the lobules (
urn:lsid:zoobank.org:act:2DE6BD04-F415-48CB-AFB5-ABF3EF9BA63D
http://species-id.net/wiki/Sinularia_eilatensis
Figs 5A–E, 6 –9holotype ZMTAU Co 35260, Israel, Red Sea, northern Gulf of Aqaba, Eilat, IUI (the Interuniversity Institute for Marine Sciences in Eilat) reef, depth 6 m, coll. Y. Benayahu, 10 January 2011; paratypes: ZMTAU Co 35261, same data as holotype; ZMTAU Co 35305, same data as holotype, 30 May 2011.
Other material examined: ZMTAU Co 35303-04, Israel, Red Sea, northern Gulf of Aqaba, Eilat, IUI reef, depth 5 m, coll. Y. Benayahu, 30 May 2011.
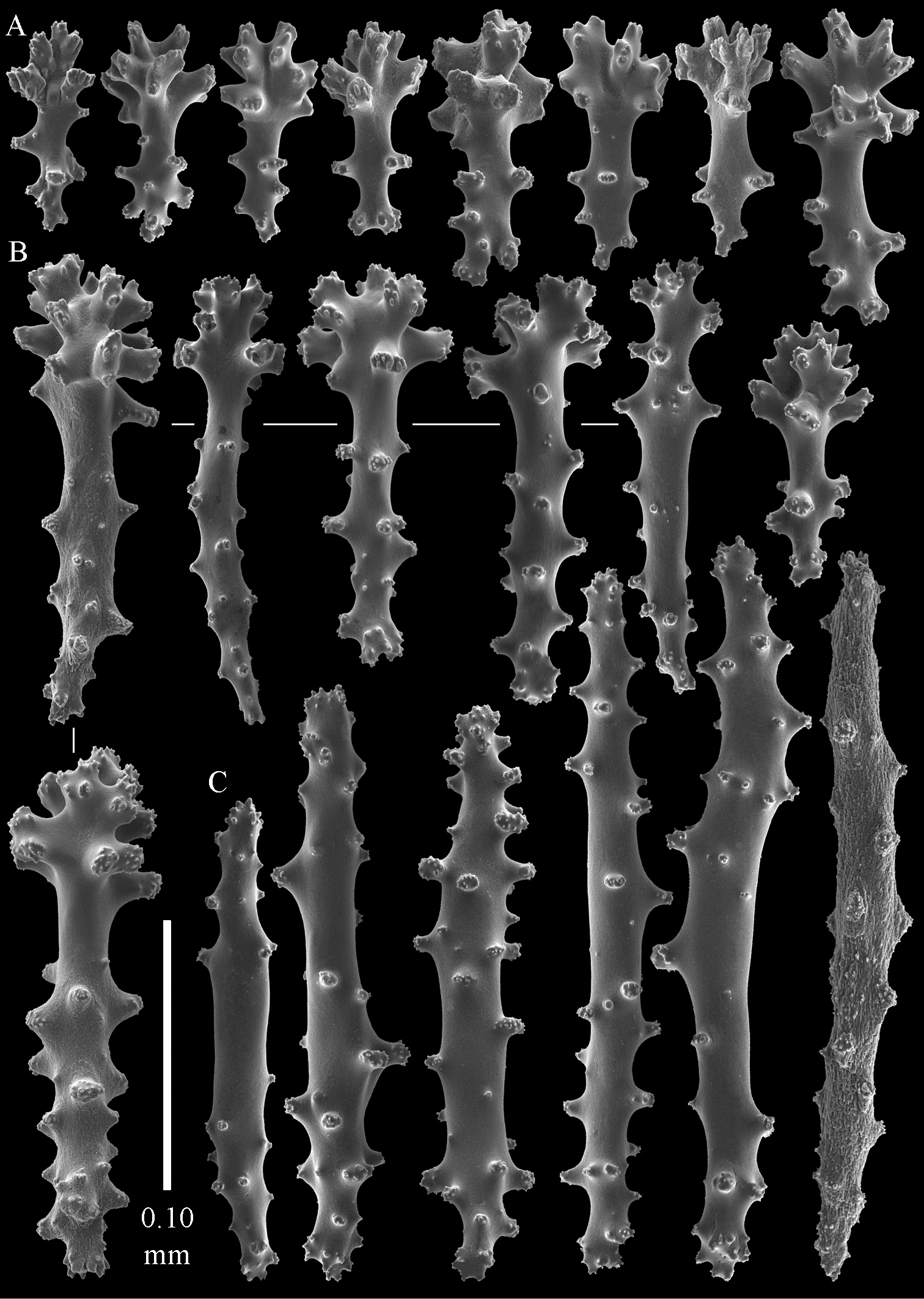
The holotype is 3.4 cm high and wide (Fig. 5A). The primary lobes branch off once or twice, lobules finger-shaped, up to 2 mm wide and 1 cm long.
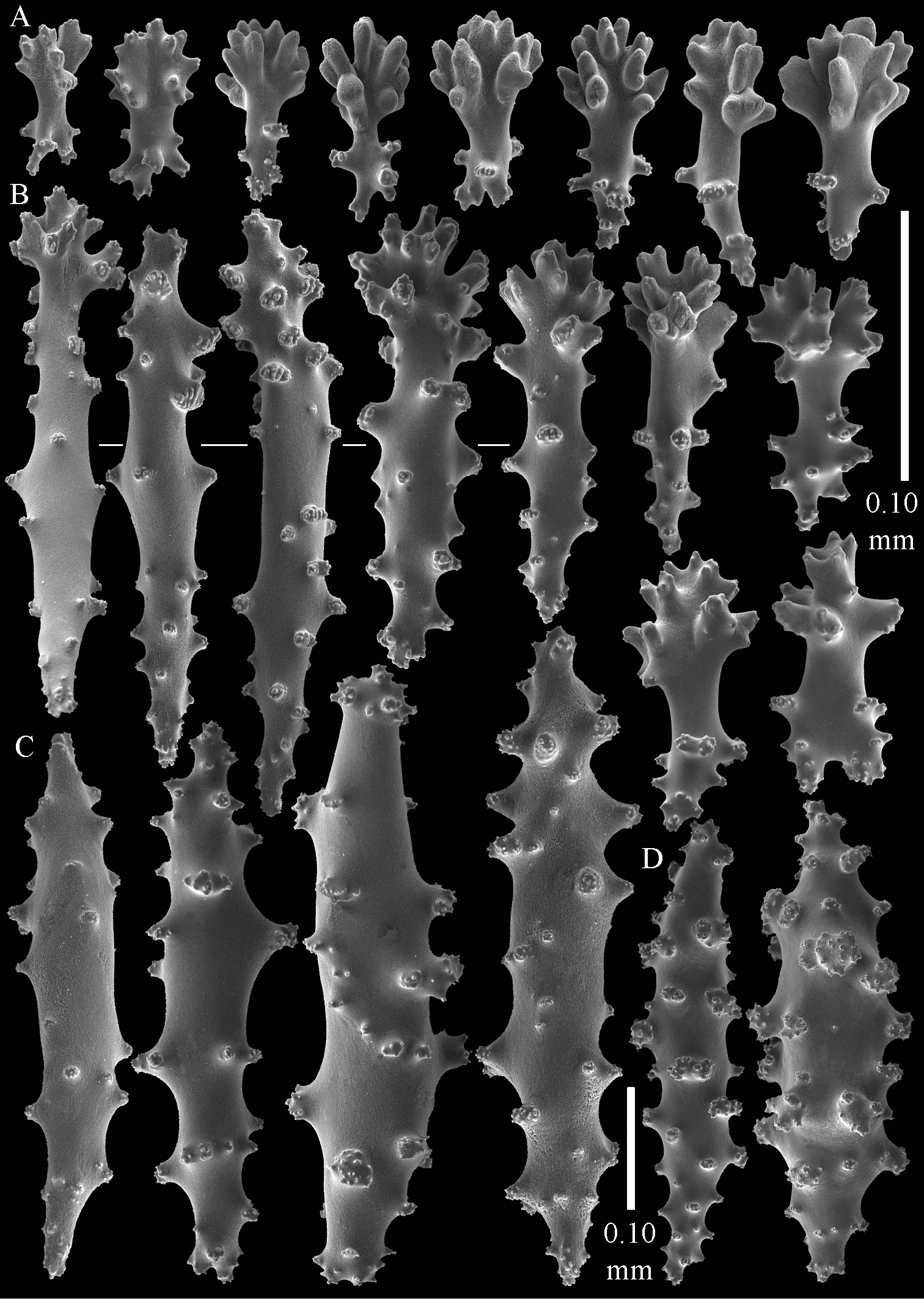
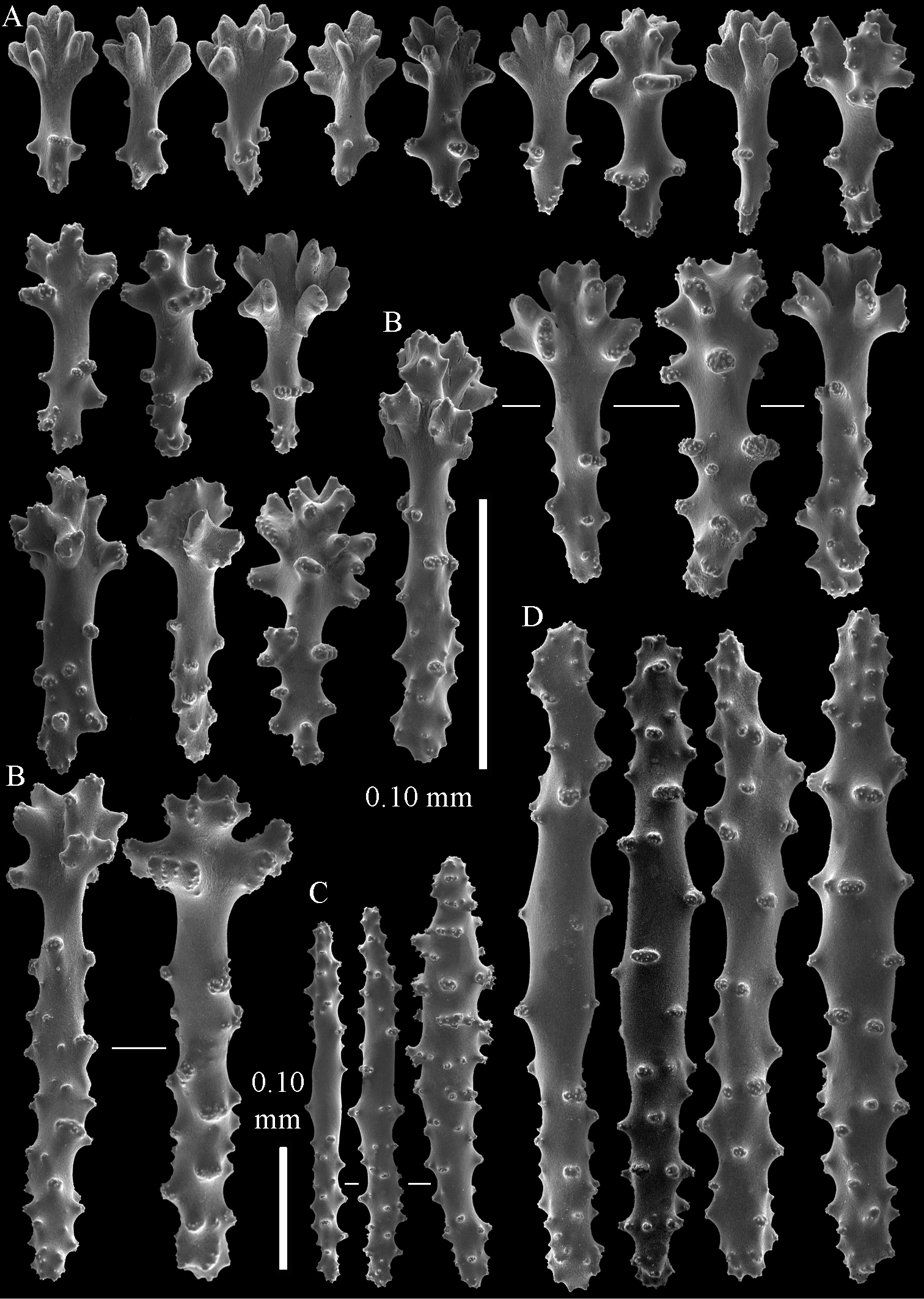
The polyps have a collaret and eight points. Points with poorly developed clubs, up to 0.25 mm long (Fig. 6A), collaret with bent spindles, up to 0.25 mm long (Fig. 6B) Tentacles with rods, about 0.05 mm long (Fig. 6C).
The surface layer of the lobules has leptoclados-type clubs, the smallest are 0.07 mm long, most are around 0.10 mm, but some reach a length of 0.15 mm (Fig. 6D); in addition longer wart clubs are present, up to 0.25 mm long (Fig. 6E). Furthermore, the surface layer of the lobules has spindles, up to 0.35 mm long, with simple tubercles (Fig. 6F).
The sclerites of the surface layer of the base of the colony resemble those of the surface layer of the lobules but they are wider (Fig. 7).
The interior of the colony has mostly unbranched spindles, a few have one or two side branches. In the lobules they are up to 2.5 mm long (Fig. 8A), with simple or complex tubercles (Fig. 8B). In the base of the colony the spindles are up to 2 mm long (Fig. 8C–D), with more complex tubercles (Fig. 8E).
Sinularia eilatensis sp. n., colonies. A ZMTAU Co 35260, holotype B ZMTAU Co 35261, paratype C ZMTAU Co 35305, paratype D ZMTAU Co 35303 E ZMTAU Co 35304 F Sinularia verseveldti, ZMTAU Co 35309.
Sinularia eilatensis sp. n., holotype ZMTAU Co 35260. A point clubs B collaret spindles C tentacle rods D leptoclados-type clubs of surface layer of lobule E wart clubs of surface layer of lobule F spindles of surface layer of lobule. Scale of 0.10 mm at F only applies to F.
Sinularia eilatensis sp. n., holotype ZMTAU Co 35260. Sclerites of the surface layer of the base of the colony. A leptoclados-type clubs B wart clubs C spindles.
Sinularia eilatensis sp. n., holotype ZMTAU Co 35260. Sclerites of the interior A spindles from the lobules B tuberculation of one of the lobule spindles C–D spindles from the base E tuberculation of one of the base spindles. Scale at D only applies to D.
Sinularia eilatensis sp. n., ZMTAU Co 35304. A point clubs B collaret spindles C leptoclados-type clubs of surface layer of lobule D wart clubs of surface layer of lobule E spindles of surface layer of lobule F interior spindles of lobule G tuberculation of one of the lobule spindles. Scale of 0.10 mm at E only applies to E, 1 mm scale at F only to F.
The preserved holotype is dark brown.
Named after Eilat, the type locality.
ZMTAU Co 35305 (Fig. 5C) has distinctly longer lobules, up to 2 cm long.
The speciesis unique among Sinularia species with leptoclados-type clubs by its very long point and collaret sclerites.
We excluded ZMTAU Co 35303-04 (Fig. 5D–E) from the type series. Morphologically we could not find a difference between these two specimens and the types, but their mitochondrial gene haplotypes differ by 0.5%. For comparison, we also present sclerites of ZMTAU Co 35304 (Fig. 9).
http://species-id.net/wiki/Sinularia_leptoclados
Figs 10A–E, 11–14ZMB 304, holotype of Lobularia leptoclados Ehrenberg; 1834, Rotes Meer, leg. Hemprich. Additional material: Red Sea; ZMTAU Co 25763, Egypt, Sinai, Tiran Strait, Thomas W., depth 3 m, coll. Y. Benayahu, 25 June 1985; ZMTAU Co 25940, Egypt, Gulf of Suez, Jubal Island, Bluf Point, depth 16 m, coll. Y. Benayahu, 24 March 1988; ZMTAU Co 34093-95, Israel, Gulf of Aqaba, Eilat, Nature Reserve, 29°30.6'N, 34°55.35'E, depth 2.4–5.5 m, coll. Y. Benayahu, 24 July 2007; ZMTAU Co 35308, Israel, Gulf of Aqaba, Eilat, Nature Reserve, depth 3 m, coll. Y. Benayahu, 31 May 2011; Kenya; ZMTAU Co 30354, off Mombasa, Shelly Reef, 04°07'S, 39°40'E, depth 12–13 m, coll. Y. Benayahu & S. Perkol, 20 January 2000; ZMTAU Co 32549, Shimoni, Wasini Is., opposite the building, depth 5 m, coll. Y. Benayahu, 2 February 2003; Tanzania; RMNH Coel. 18953, off Dar es Salaam, Pangavinne Island, seaward slope (P02), 6°50'S, 39°17'E, depth 6 m, coll. J.N. Nyanda; RMNH Coel. 18954, off Dar es Salaam, Pangavinne Island, seaward slope (P18), 6°50'S, 39°17'E, depth 8 m, coll. J.N. Nyanda; RMNH Coel. 18955, off Dar es Salaam, Mbudya Island, seaward slope (P35), 6°50'S, 39°17'E, depth 5 m, coll. J.N. Nyanda; ZMTAU Co 26314, Pangavinne Is., depth 6 m, coll. J.N. Nyanda, 1991; ZMTAU Co 26316, Mbudya Is., depth 5 m, coll. J.N. Nyanda, 1991; Mozambique; ZMTAU Co 28796, Bazaruto Is., Manta Reef, depth 15 m, coll. M. Schleyer, 7 October 1994; Madagascar; RMNH Coel. 6653, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 22 July 1967, coll. A.G. Humes (1183); RMNH Coel. 6654, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 11 August 1967, coll. A.G. Humes (1250); RMNH Coel. 6655, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 23 August 1967, coll. A.G. Humes (1320); RMNH Coel. 6659, Nosy Iranja, SW Nosy Bé, depth 15 m, 9 August 1967, coll. A.G. Humes (1239); RMNH Coel. 6660, W of Andilana, 13°18'S, 48°07'E, 20 m deep, 24 August 1967, coll. A.G. Humes (1331); RMNH Coel. 6656, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 23 August 1967, coll. A.G. Humes (1321); RMNH Coel. 6657, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 23 August 1967, coll. A.G. Humes (1322); RMNH Coel. 6658, Ankify, on mainland of Madagascar, opposite Nosy Komba, depth 1 m, 23 August 1967, coll. A.G. Humes (1323); RMNH Coel. 6661, Pass at Pte Lokobe, Nosy Bé, Madagascar, depth 15 m, 19 June 1967, coll. A.G. Humes (A28).
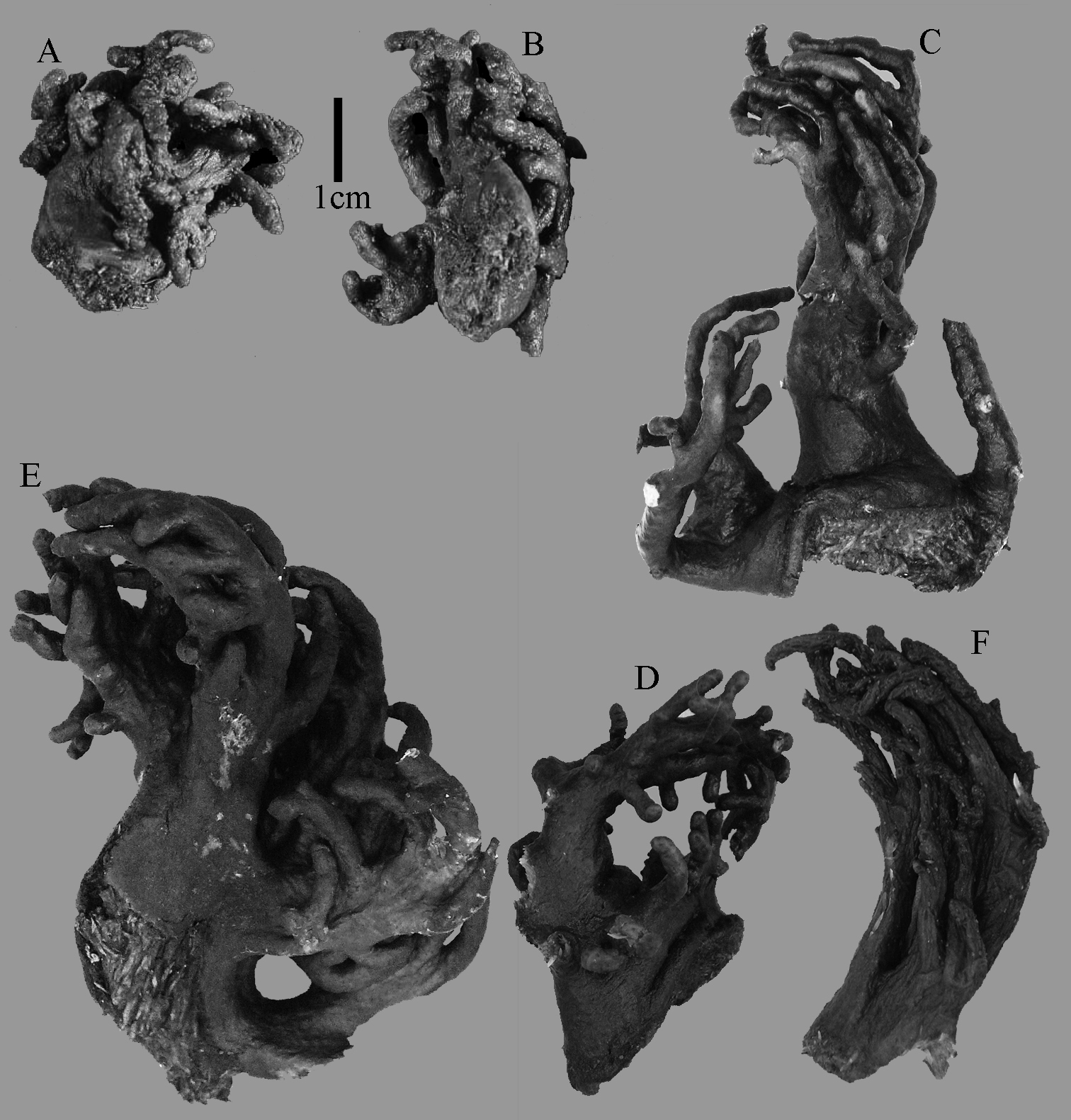
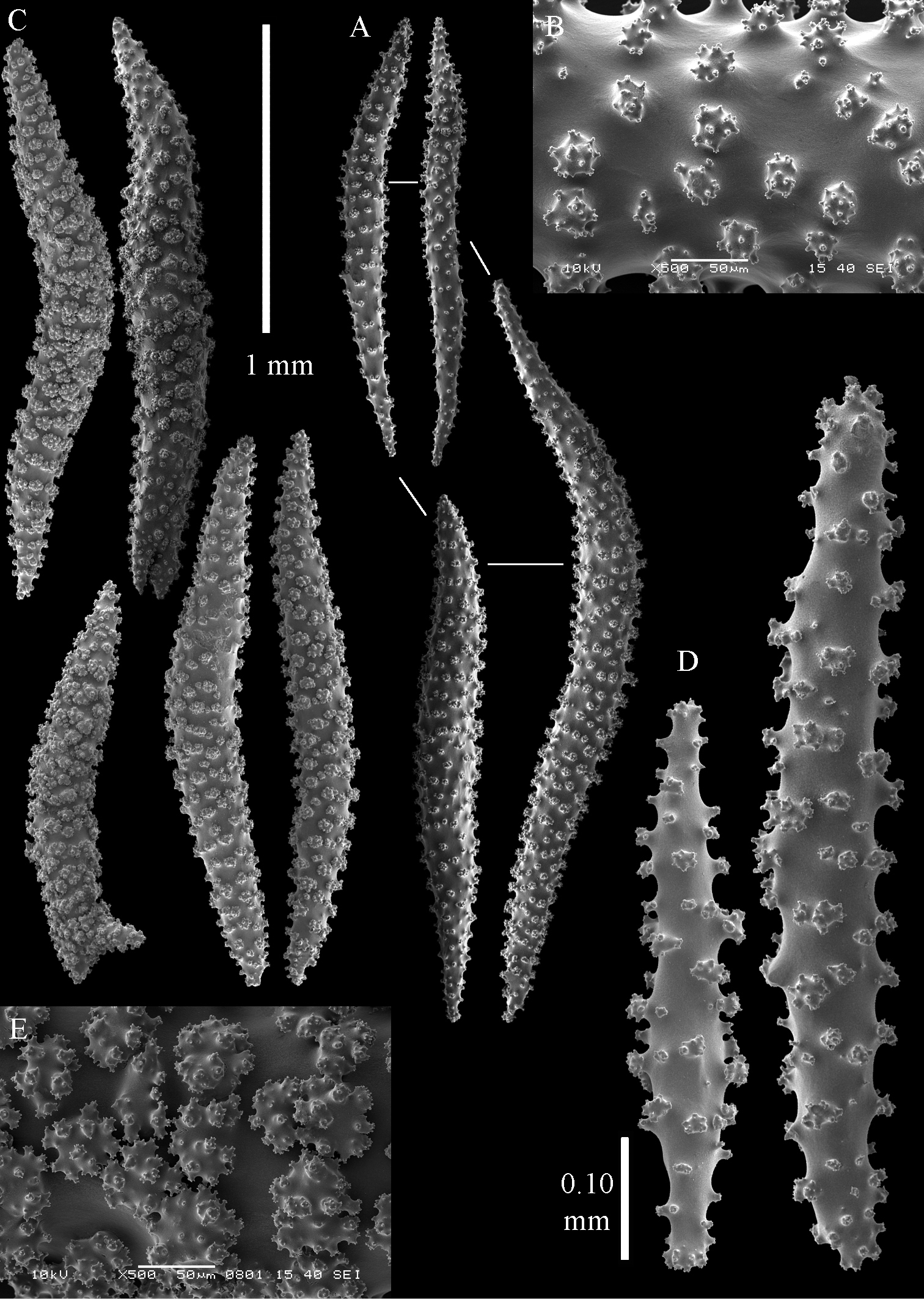
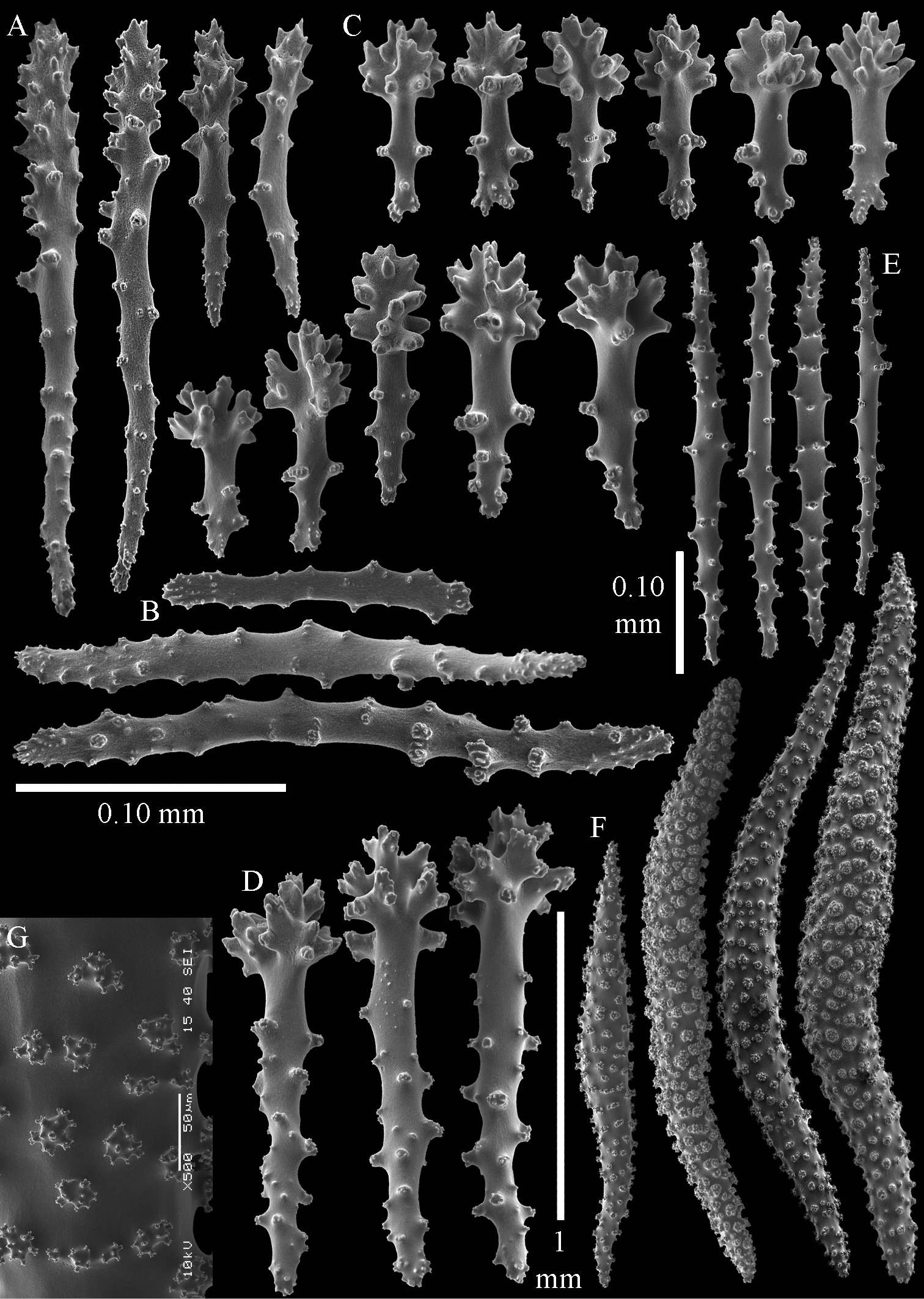
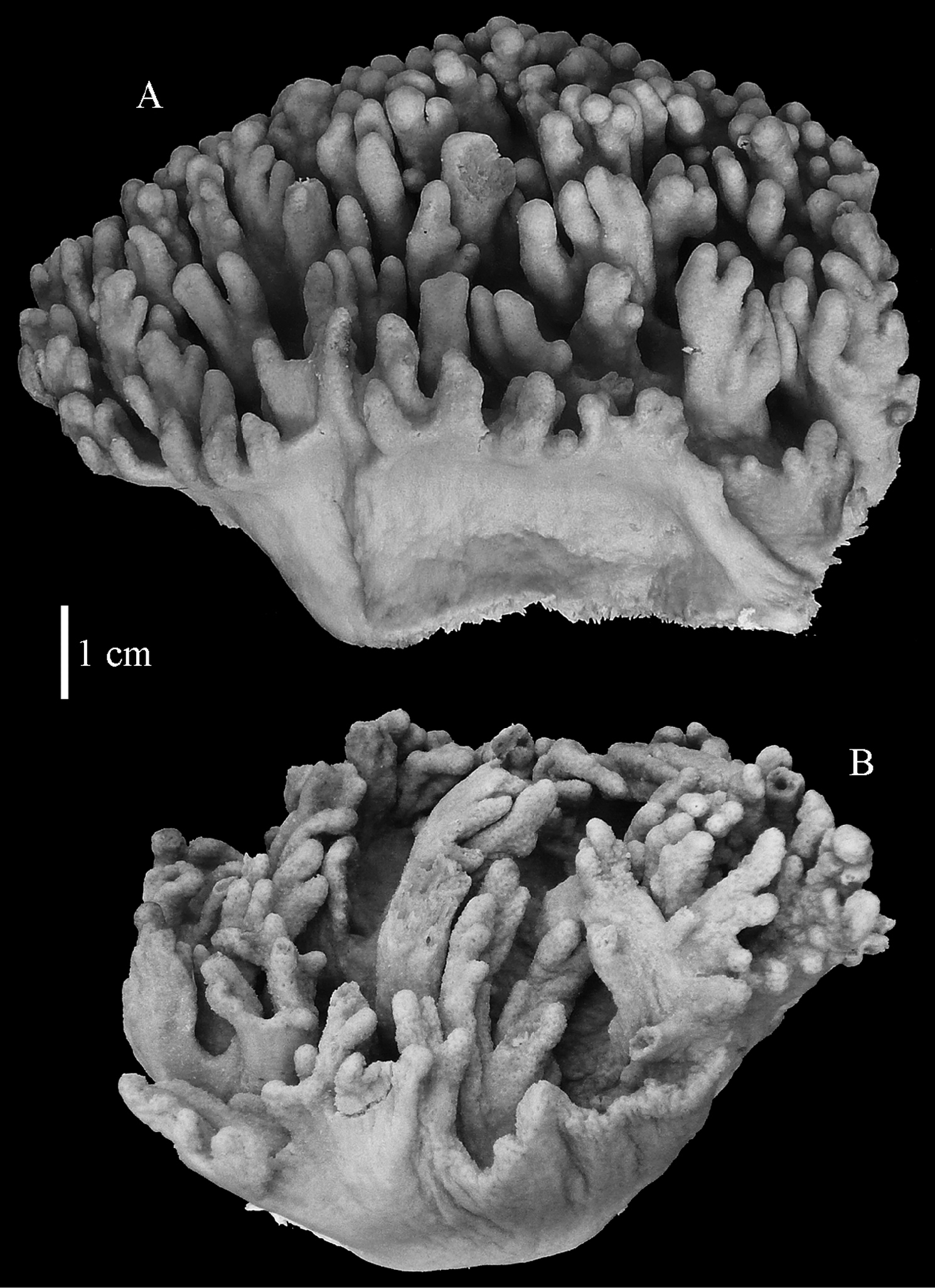
The holotype is 18 cm high and 13 cm wide (Fig. 10A). The primary lobes branch off once or twice, lobules finger-shaped, up to 1 cm wide and 3 cm long.
The polyps have a collaret and eight points. Points with poorly developed clubs, up to 0.13 mm long (Fig. 11A), collaret with bent spindles. Tentacle sclerites not observed.
The surface layer of the lobules has leptoclados-type clubs, the smallest are 0.05 mm long, most are around 0.10 mm, but some reach a length of 0.15 mm (Fig. 11B); in addition longer wart clubs are present, up to 0.20 mm long (Fig. 11C). Furthermore, the surface layer of the lobules has spindles, up to 0.45 mm long, with simple tubercles (Fig. 11D, 12A); the smaller ones with a distinct median waist.
The interior of the colony has unbranched spindles. In the lobules they are up to 2.5 mm long (Fig. 12B), with simple or complex tubercles (Fig. 12C). In the base of the colony the spindles are also up to 2 mm long (Fig. 12D), with more complex tubercles (Fig. 12E).
The sclerites of the surface layer of the base of the colony resemble those of the surface layer of the lobules but they are wider (Fig. 13).
Sinularia leptoclados colonies. A ZMB 304 holotype B ZMTAU Co 34093 C ZMTAU Co 34094 D ZMTAU Co 34095 E ZMTAU Co 35308 F Sinularia maxima, ZRC1999.1066. Scale of 2 cm only applies to A and F.
Sinularia leptoclados holotype ZMB 304. A point clubs B leptoclados-type clubs of surface layer of lobule C wart clubs of surface layer of lobule D spindles of surface layer of lobule.
Sinularia leptoclados holotype ZMB 304. A spindles of the surface layer of lobule B–D sclerites of the interior B spindles from the lobules C tuberculation of one of the lobule spindles D spindles from the base E tuberculation of two of the base spindles. Scale of 0.10 mm at A only applies to A.
Sinularia leptoclados holotype ZMB 304. Sclerites of the surface layer of the base of the colony. A leptoclados-type clubs B wart clubs C–D spindles. Scale of 0.10 mm at C only applies to C.
Sinularia leptoclados colonies. A ZMTAU Co 25763 B ZMTAU Co 25940.
The holotype is brown.
Most of the colonies of Sinularia leptoclados are stalked and rarely feature an encrusting colony shape (Fig. 14).
One other species that can be confused with Sinularia leptoclados is Sinularia verseveldti Ofwegen, 1996. Its colony shape was described as being cup-shaped, but examination of many specimens from Indonesia showed that colony shape to be exceptional. Mostly the colonies resemble Sinularia leptoclados very closely.
Sinularia leptoclados var. gonatodes ZMB 6495 A point clubs B leptoclados-type clubs of surface layer of lobule C wart clubs of surface layer of lobule D spindles of surface layer of lobule.
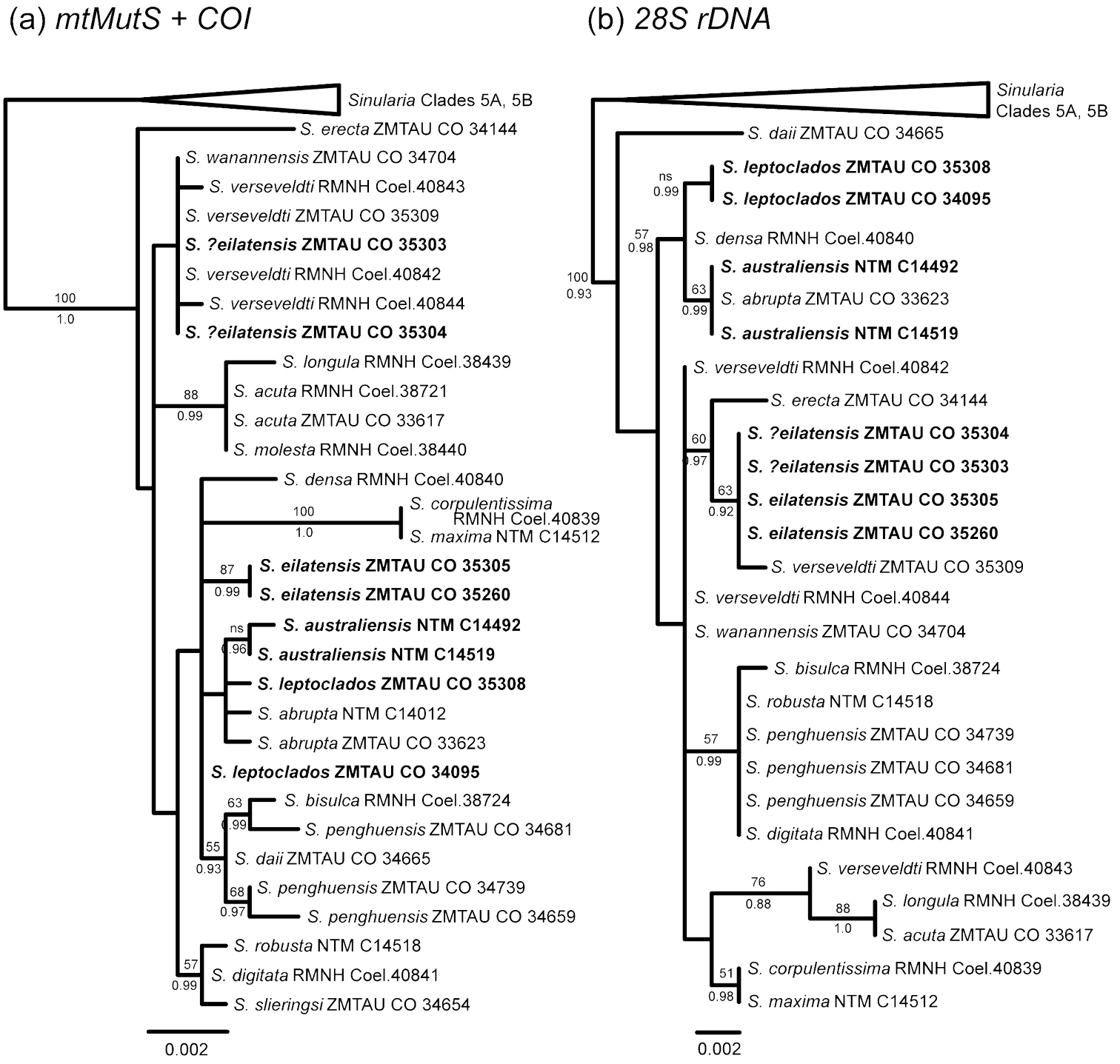
Sequences for mtMutS and COI (including igr1) were available or newly obtained for 31 specimens representing 19 morphospecies of Sinularia belonging to clade 5C; 28S rDNA sequences were obtained for all but four specimens (Table 1). mtMutS (735 nt) and COI (888 nt) sequences were concatenated for a total mitochondrial gene alignment of 1623 nt. 28S sequences ranged from 797–799 nt in length for a total alignment length of 801 nt. Maximum likelihood and Bayesian analyses resulted in identical tree topologies for all three data sets (mt genes only, 28S only, all three genes combined). Support values were generally somewhat stronger for Bayesian analyses, however, and several nodes that were not supported by maximum likelihood (bootstrap values <50%) nonetheless had Bayesian posterior probabilities >0.9 (Figs 16, 17). All alignments and trees have been submitted to TreeBASE (www.treebase.org ).
Within Sinularia species with leptoclados-type clubs (clade 5C), genetic distances (uncorrected p) among recognized morphospecies range from only 0–1.7% for mtMutS, 0–0.8% for COI and 0–1.4% for 28S rDNA. Despite these relatively low levels of genetic differentiation among taxa, several moderately- to well-supported clades appear in both the mitochondrial and 28S gene trees (Fig. 16). Sinularia maxima and Sinularia corpulentissima share identical mt and 28S haplotypes with one another, but are well differentiated from all other species in clade 5C. Sinularia acuta, Sinularia longula and Sinularia molesta are also very similar to one another genetically (Sinularia molesta and Sinularia acuta share identical mt and 28S haplotypes), and form a well-supported clade in both trees. Finally, Sinularia erecta is genetically distinct, separated from all other species by genetic distances of >0.8% at mtMutS (28S was not available for Sinularia erecta).
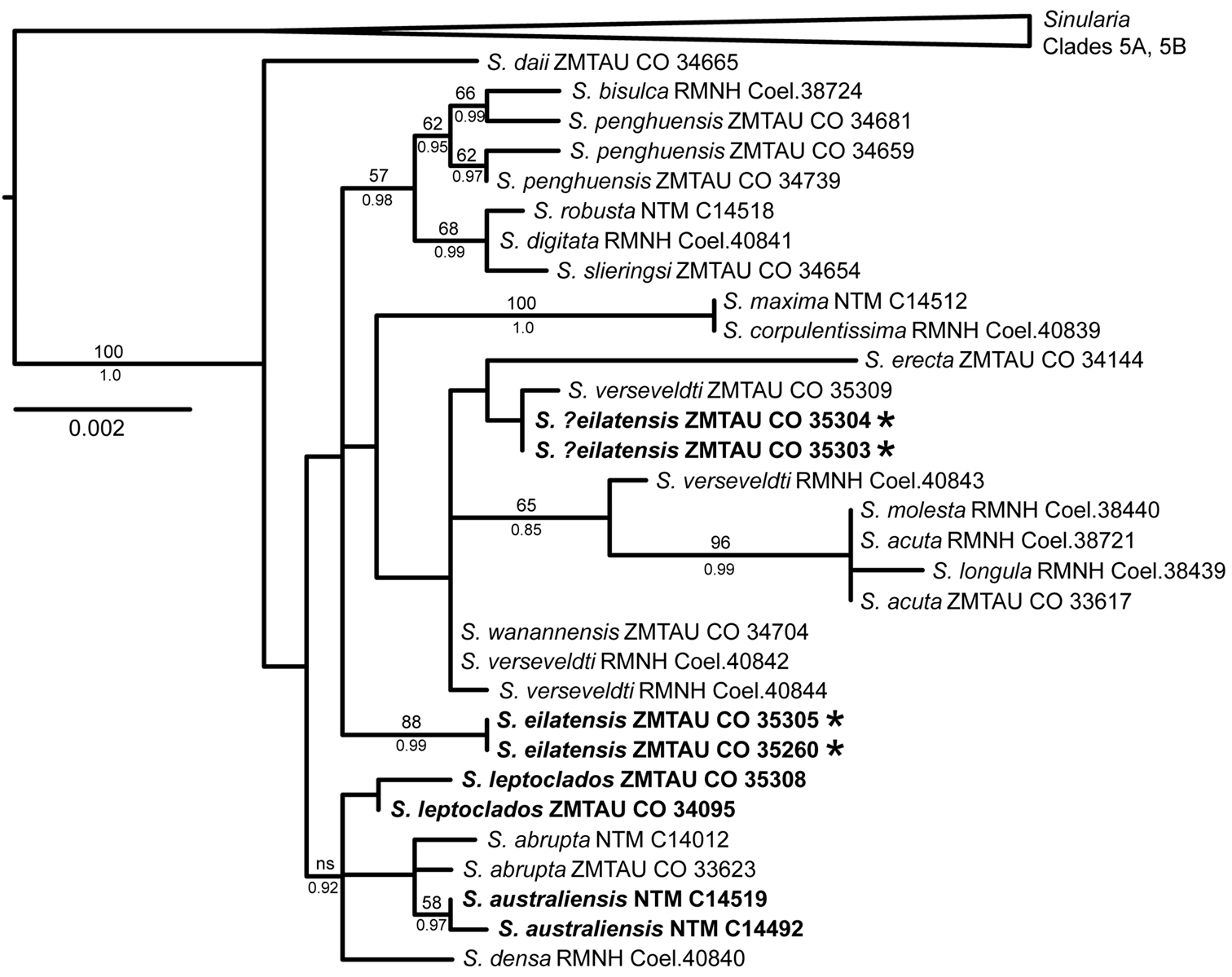
Two additional clades are moderately supported by the combined analysis of the mt and 28S genes (Fig. 17); the species in these clades also group together in the separate analyses, but with low bootstrap support (<50%) (Fig. 16). Sinularia penghuensis, Sinularia bisulca, Sinularia robusta, Sinularia digitata and Sinularia slieringsi comprise one of these moderately-supported clades (Fig. 17); these species share identical or nearly identical 28S sequences (28S was not available for Sinularia slieringsi) (Fig. 16b). Within the mt gene tree (Fig. 16a) they constitute two distinct clades, one comprised by Sinularia robusta, Sinularia digitata and Sinularia slieringsi and the other by Sinularia penghuensis, Sinularia bisulca and Sinularia daii. The latter is, however, distinct from all other species at 28S, and falls outside of this clade in the combined analysis (Fig. 17). Sinularia leptoclados, Sinularia abrupta, Sinularia australiensis sp. n. and Sinularia densa also form a moderately-supported clade in the 28S tree (Fig. 16b) and in the combined tree (supported by Bayesian but not maximum likelihood analyses; Fig. 17), but their relationship is unresolved in the mt tree (Fig. 16a). Sinularia australiensis sp. n. and Sinularia abrupta share identical 28S haplotypes, but differ from Sinularia leptoclados by 0.3%. Sinularia australiensis sp. n. differs from both Sinularia leptoclados and Sinularia abrupta by 0.1% and 0.1-0.2% at mtMutS and COI respectively.
The relationships among the remaining species in the clade — Sinularia verseveldti, Sinularia wanannensis and Sinularia eilatensis sp. n. — were poorly resolved and exhibited some incongruence between the mitochondrial and 28S gene trees. Sinularia wanannensis, all four specimens of Sinularia verseveldti, and two specimens (ZMTAU Co 35303, ZMTAU Co 35304) that were tentatively assigned to Sinularia eilatensis sp. n. share identical or nearly identical mtMutS and COI haplotypes, and cluster together within the mt tree (but with bootstrap values <50%). Two specimens of Sinularia eilatensis sp. n. (ZMTAU Co 35305, ZMTAU Co 35260) fall outside of that group, and differ from it by >0.5% at mtMutS (Fig. 16a). At 28S, however, ZMTAU Co 35303 and ZMTAU Co 35304 are genetically identical to both individuals of Sinularia eilatensis sp. n., and those four specimens form a moderately-supported clade together with Sinularia verseveldti ZMTAU Co 35309 (Fig. 16b). Two additional specimens of Sinularia verseveldti share identical 28S haplotypes with Sinularia wanannensis. The combined tree reflects the topology of the mt gene tree, and shows the separation of Sinularia eilatensis sp. n. (ZMTAU Co 35305, ZMTAU Co 35260) from ZMTAU Co 35303, ZMTAU Co 35304 and all other species (Fig. 17).
Our findings indicate that specimens of the same species generally shared identical or nearly identical sequences at all three loci. The only exceptions were the two distinct mitochondrial haplotypes of Sinularia eilatensis sp. n. discussed above, and the four specimens of Sinularia verseveldti. All Sinularia verseveldti shared identical or nearly identical mtMutS and COI sequences, but differed at 28S. Most of these differences, however, reflected polymorphic nucleotide positions at which one or more specimens exhibited heterozygosity. For example, at position 533 of the 28S alignment, ZMTAU Co 35309 and Coel. 40842 had C, Coel. 40843 had T, and Coel. 40844 had both C and T. A total of 8 such heterozygous nucleotide sites among the four Sinularia verseveldti specimens contribute to their disjunct distribution within the 28S and combined trees.
Maximum likelihood trees of Sinularia clade 5C (
Figure 17. Maximum likelihood tree of Sinularia clade 5C (
The two new species described here are supported both by morphological characters and by the molecular analysis. Although Sinularia australiensis sp. n. is similar genetically to Sinularia leptoclados and both belong to the same sub-clade within Sinularia clade 5C, they differ at all three of the loci sequenced here. Furthermore, the 28S and combined analyses suggest that Sinularia leptoclados and Sinularia australiensis sp. n. are not sister taxa, but that Sinularia australiensis sp. n. is closer to Sinularia abrupta, a species with which it shares a 28S haplotype. The disjunct geographical distribution between Sinularia leptoclados, which occurs in the Red Sea and western Indian Ocean, and Sinularia australiensis sp. n. from Australia, further supports their distinction.
Although sympatric with Sinularia leptoclados in the Red Sea, Sinularia eilatensis sp. n. is clearly distinct from that species, both morphologically and genetically. Within clade 5C, Sinularia eilatensis sp. n. is most similar genetically to the geographically widespread Sinularia verseveldti and to Sinularia wanannensis, a species recently described from Taiwan (
Previous molecular systematic work on Sinularia and other octocoral genera has highlighted the inadequacies of mitochondrial gene markers for species discrimination and species-level phylogenetic analyses in the group (
This research (Application DE-TAF-662) received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program. It also was in part supported by The Israel Cohen Chair in Environmental Zoology to Y.B. We would like to thank Dr. Carsten Lüter, Museum für Naturkunde der Humboldt Universität, Berlin, Germany, for entrusting us with the holotype of Sinularia leptoclados, and Anna Lee for assistance with DNA sequencing.We wish to thank Alex Shlagman for curatorial skills, Michal Weis for technical assistance and the Interuniversity Institute for Marine Sciences in Eilat (IUI) for facilities.