(C) 2013 Dana Carrison-Stone. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of Conopea (Say 1822) are described from the Gulf of Guinea: Conopea saotomensis sp. n.and Conopea fidelis sp. n. These two new species were collected from the historically isolated volcanic islands of São Tomé and Príncipe. The relationship between Conopea saotomensis sp. n., Conopea fidelis sp. n.and two other Atlantic barnacle species, Conopea calceola (Ellis 1758) and Conopea galeata (Linnaeus 1771), is examined. The methods employed are the construction of a molecular phylogeny using mitochondrial COI and nuclear H3 gene sequence data along with morphological comparisons of calcareous and cuticular body parts. It is found that Conopea saotomensis sp. n., Conopea fidelis sp. n.and Conopea calceola are most closely related to each other but the relationship among them is unresolved. Gorgonian hosts are identified. Preliminary observations show species level host specificity for Conopea fidelis sp. n.
Barnacle, Cirripedia, Conopea calceola, Conopea fidelis sp. n. , Conopea galeata, Conopea saotomensis sp. n. , COI, endemic, gorgonian, Gulf of Guinea, H3, host specificity, octocoral, phylogeny
The Gulf of Guinea island chain consists of Bioko, São Tomé, Príncipe, and Annobón. This study focuses on São Tomé and Príncipe, which are approximately 140 km apart and 274 km west of northern Gabon. They are the products of large shield volcanoes originating 3, 000 m below the ocean’s surface along the Cameroon line. São Tomé and Príncipe are old islands, 13 and 30 myo, respectively, and have never been connected to the African mainland.
Conopea is a widespread genus that is found in temperate and tropical oceans around the world. Currently, there are 21 described species of Conopea. In general, Conopea is not a well documented group. There is very little data on host associations, species ranges are not well defined, published descriptions are often incomplete and occasionally contain questionable information.
There are three known species of Conopea found in the Atlantic Ocean and Caribbean Sea: Conopea calceola, Conopea galeata, and Conopea merrilli. Conopea calceola was originally described from the Strait of Gibraltar, by
Morphologically Conopea merrilli and Conopea galeata are clearly different and easily distinguishable from Conopea calceola, Conopea saotomensis sp. n., and Conopea fidelis sp. n.. Conopea calceola is morphologically similar to the Gulf of Guinea species and is therefore compared in detail to aid in future identifications. Conopea galeata was chosen over Conopea merrilli as an outgroup for molecular analysis because of its larger distribution range and greater availability of specimens.
Approximately 40 individuals of Conopea saotomensis sp. n. and 20 individuals of Conopea fidelis sp. n. were collected from São Tomé and Príncipe by Carrison-Stone, Van Syoc, and Williams in 2006 and 2009. Barnacles were collected from three different localities on São Tomé: Diogo Vaz (0°18.89'N, 6°29.39"E), Ponta Baleia (0°2.13'N, 6°33.51'E), and Ilheu Santana (0°16'N, 6°45.48'E) and two different localities on Príncipe: Ilheu BomBom (1°42'8.8"N, 7°24'14"E) and Pedra de Galé (1°43'30.1"N, 7°22'51.5"E). Collections were done via SCUBA at depths of 9–33m. Seven individuals of Conopea calceola were collected from 3 separate sites at Porto Covo, Portugal, by Van Syoc in 2008. Samples of the associated gorgonian were also collected. All specimens were preserved in 95% EtOH.
Conopea galeata from St. Catherine Is., Georgia (USA) were borrowed from the California Academy of Sciences Invertebrate Zoology Department (CASIZ). Conopea galeata from South Padre Is., Texas and Mexico Beach, Florida were collected by Mary Wicksten. Conopea galeata from Port Aransas, Texas were collected by Carol Cox.
Barnacle cirri, mouthparts and opercular plates from São Tomé, Príncipe, and Portugal specimens were dissected for morphological comparisons. These physical traits, along with shell shape, in particular basis shape and presence/absence of longitudinal tubes in shell wall plates, are traditionally used for identification. The cirri and mouthparts were mounted on microslides and photographed at 100x with a Leitz microscope imaging system. Images of the opercular plates were taken with a scanning electron microscope (SEM, LEO/Zeiss 1450VP).
Identification of host gorgonians was based on external and sclerite morphology. Branching patterns, polyp shape, color and sclerite types were examined. Sclerites were isolated by dissolving small amounts of gorgonian tissue in sodium hypochlorite solution, followed by rinsing with water and then 75% ethanol. Images of the sclerites were taken with SEM and Leitz optical microscope imaging systems. All gorgonians harboring barnacles were identified using
Genomic DNA was extracted from adductor muscle tissue using the Qiagen DNeasy Blood and Tissue kit (Valencia, CA). The cytochrome c oxidase subunit I (COI) primers COI-N: TGAGAAATTATTCCGAAGGCTGG (
Molecular phylogeny was determined by Bayesian and likelihood analyses. Semibalanus balanoides (GenBank accession AF242660.1), another archaeobalanid, was used as an outgroup. Bayesian analyses were run in Mr. Bayes (
Data associated with the specimens used in this study.
| Barnacle taxon | Gorgonian host | CASIZ Catalog | GenBank accession’s | Collection Locality |
|---|---|---|---|---|
| Conopea calceola | Eunicella verrucosa | 175916 | HQ290142, HQ290155 | Porto Covo, Portugal |
| Conopea calceola | Eunicella verrucosa | 175917 | HQ290143, HQ290156 | Porto Covo, Portugal |
| Conopea calceola | Eunicella verrucosa | 180065 | HQ290135, KC349910 | Porto Covo, Portugal |
| Conopea galeata | unknown | 106216 | HQ290146, HQ290147 | St.Catherine Is., Georgia |
| Conopea galeata | unknown | 184331 | JQ966287, JQ966283 | South Padre Is., Texas |
| Conopea galeata | Leptogorgia setacea | 183496 | JQ966288, JQ966284 | Port Aransas, Texas |
| Conopea galeata* | unknown | 184416A 184416B | JQ966289, JQ966285, JQ966290, JQ966286 | Mexico Beach, Florida |
| Conopea saotomensis sp. n. | Leptogorgia viminalis | 173189 | HQ290134, HQ290149 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Eunicella kochi | 173190 | HQ290136, KC349911 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia ruberrima | 174321 | KC349913, KC349922 | Ilheu Santana, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia dakarensis | 174804 | KC349904, KC349916 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia varians | 174805 | KC349906, KC349917 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia gaini | 174806 | HQ290152, KC349918 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia ruberrima | 175525 | KC349907, KC349919 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia dichotoma | 175526 | KC349908, KC349920 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | unknown | 178662 | KC349909, KC349925 | Diogo Vaz, São Tomé |
| Conopea saotomensis sp. n. | Leptogorgia dakarensis | 178655 | HQ290137, HQ290159 | Bom Bom Is., Príncipe |
| Conopea saotomensis sp. n. | unknown | 178656 | HQ290160, KC349924 | Bom Bom Is., Príncipe |
| Conopea saotomensis sp. n. | Leptogorgia sp. | 180025 | JQ966291 | Pedra de Galé, Príncipe |
| Conopea saotomensis sp. n. | Leptogorgia dichotoma | 185253 | Diogo Vaz, São Tomé | |
| Conopea fidelis sp. n.* | Muriceopsis tuberculata | 174803A 174803B | HQ290140, HQ290151, KC349905, KC349915 | Diogo Vaz, São Tomé |
| Conopea fidelis sp. n. | Muriceopsis tuberculata | 174320 | KC349912, KC349921 | Ponta Baleia, São Tomé |
| Conopea fidelis sp. n. | Muriceopsis tuberculata | 174322A 174322B | HQ290140, HQ290150, KC349914, KC349923 | Ponta Baleia, São Tomé |
| Conopea fidelis sp. n.* | Muriceopsis tuberculata | 178651A 178651B | HQ290138, HQ290139, HQ290157, HQ290158 | Pedra de Galé, Príncipe |
| Conopea fidelis sp. n. | Muriceopsis tuberculata | 185252 | Ponta Baleia, São Tomé |
* Two barnacles were used from this lot.
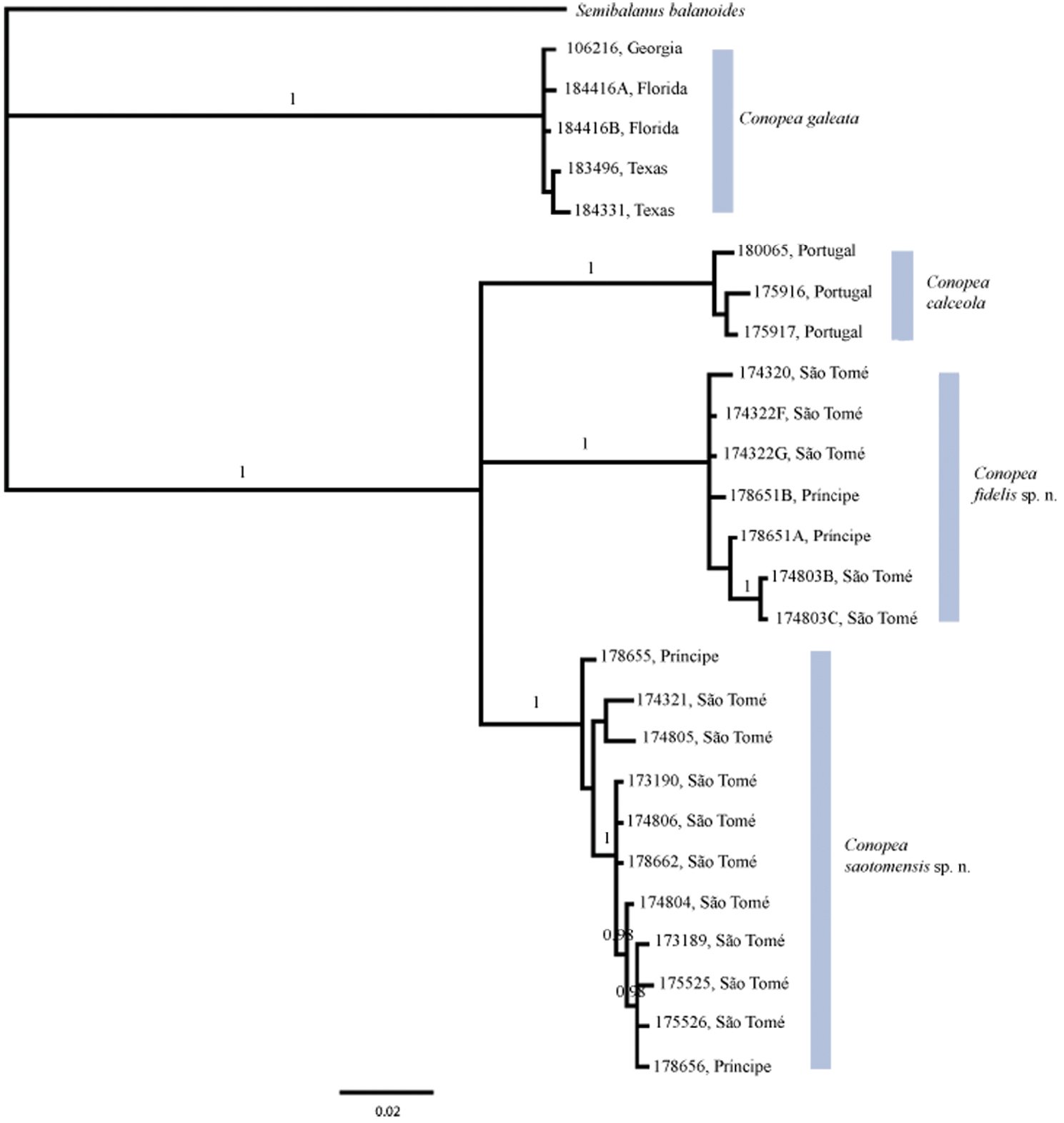
Two major clades resulted from molecular analysis. One clade contains Conopea calceola, Conopea saotomensis sp. n.and Conopea fidelis sp. n, and the other contains Conopea galeata. Unfortunately, the gene data used was not sufficient to completely resolve the relationship among the three eastern Atlantic species. We do know that they are each other’s closest relatives but we do not know which two of the three are most closely related. Bayesian (Fig. 1) and likelihood phylogenies, based on concatenated COI and H3 datasets, between Conopea saotomensis sp. n., Conopea fidelis sp. n., and Conopea calceola are unresolved. Bayesian phylogeny generated solely on COI data shows the two Gulf of Guinea species as being more closely related to each other than to Conopea calceola. However the likelihood phylogeny generated with solely COI data again showed an unresolved relationship among Conopea saotomensis sp. n., Conopea fidelis sp. n., and Conopea calceola. Separate Bayesian and likelihood analyses of H3 sequence data showed similar unresolved topologies among the three eastern Atlantic species.
Pairwise uncorrected p-distances (Table 2) of COI and H3 also could not resolve the relationship. Distances for COI indicate that Conopea saotomensis sp. n.and Conopea fidelis sp. n. are more closely related to each other (8.2%) than to Conopea calceola (8.8% and 10.4%, respectively) whereas H3 distances indicate that Conopea saotomensis sp. n.and Conopea fidelis sp. n. are more closely related to Conopea calceola (1.4% and 1.3%, respectively) than to each other (2.2%). Pairwise uncorrected p-distances within groups is as follows: Conopea saotomensis sp. n. = 0.8%/0.0%, Conopea fidelis sp. n. = 0.7%/0.2%, Conopea calceola = 0.7%/0.0%, Conopea galeata = 0.3%/0.1% (COI/H3 respectively).
Uncorrected pairwise distances among groups, COI (lower half of matrix) and H3 (upper half of matrix).
| Barnacle taxon | Conopea saotomensis sp. n. | Conopea fidelis sp. n. | Conopea calceola | Conopea galeata |
| Conopea saotomensis sp. n. | 0.022 | 0.014 | 0.110 | |
| Conopea fidelis sp. n. | 0.082 | 0.013 | 0.106 | |
| Conopea calceola | 0.088 | 0.104 | 0.102 | |
| Conopea galeata | 0.148 | 0.165 | 0.166 |
Bayesian phylogeny based on concatenated H3 nDNA and COI mtDNA sequences. Posterior probabilities of 0.95 or greater are shown. The relationship among the Gulf of Guinea species and Conopea calceola is unresolved.
urn:lsid:zoobank.org:act:1164CCD8-C9F3-46E2-BBB9-1F6411123DA7
http://species-id.net/wiki/Conopea_saotomensis
Figures 2–5, Table 3Holotype: CASIZ185253, separated from CASIZ175526, 95% EtOH. Diogo Vaz, São Tomé, Gulf of Guinea, 0°18.89'N, 6°29.39'E, collected by hand/SCUBA, 12–27 m, attached to Leptogorgia c.f. dichotoma, G. Williams, 29 May 2006. Original label: “S-3”, California Academy of Sciences, San Francisco.
Paratypes: CASIZ173189 (4 specimens) and CASIZ174804, Diogo Vaz, São Tomé, Gulf of Guinea (0°18.89'N, 6°29.39'E), collected by hand/SCUBA, 9–26 m, R. Van Syoc, 29 May 2006; CASIZ 173190 (3 specimens), 174805 (4 specimens), 174806 (2 specimens), and 175526 (7 specimens), Diogo Vaz, São Tomé, Gulf of Guinea (0°18.89'N, 6°29.39'E), collected by hand/SCUBA, 12–27 m, G. Williams, 29 May 2006; CASIZ178655 (2 specimens) and CASIZ178656 (2 specimens), Ilheu BomBom, Príncipe, Gulf of Guinea (1°42'8.8"N, 7°24'14"E), collected by hand/SCUBA, 11 m, R. Van Syoc, 24 Jan 2009.
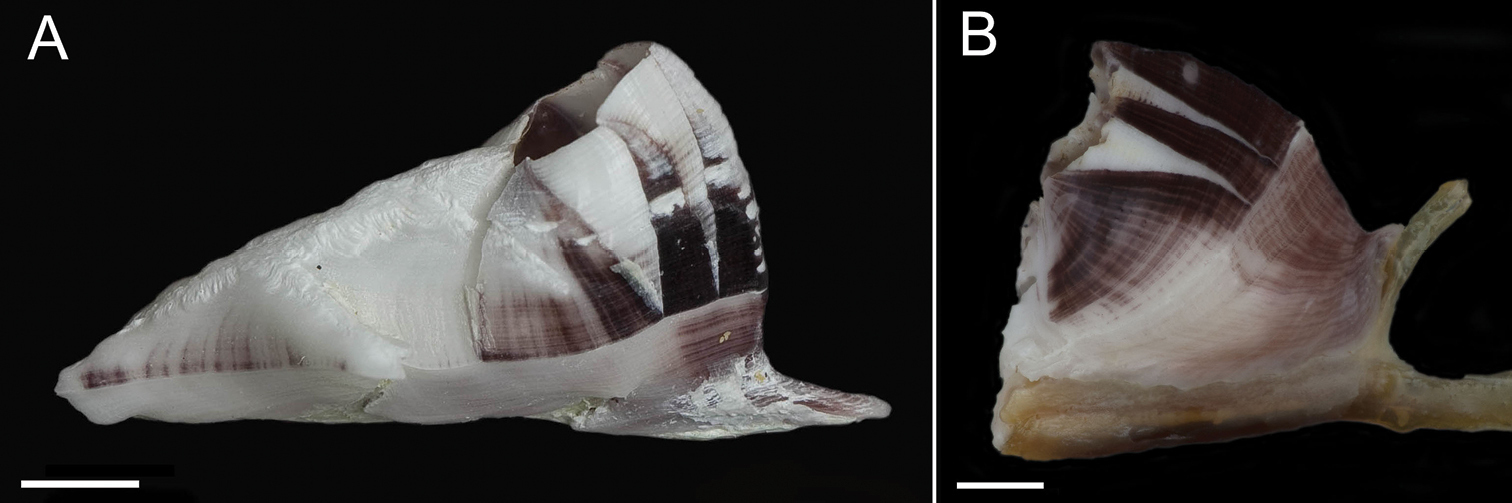
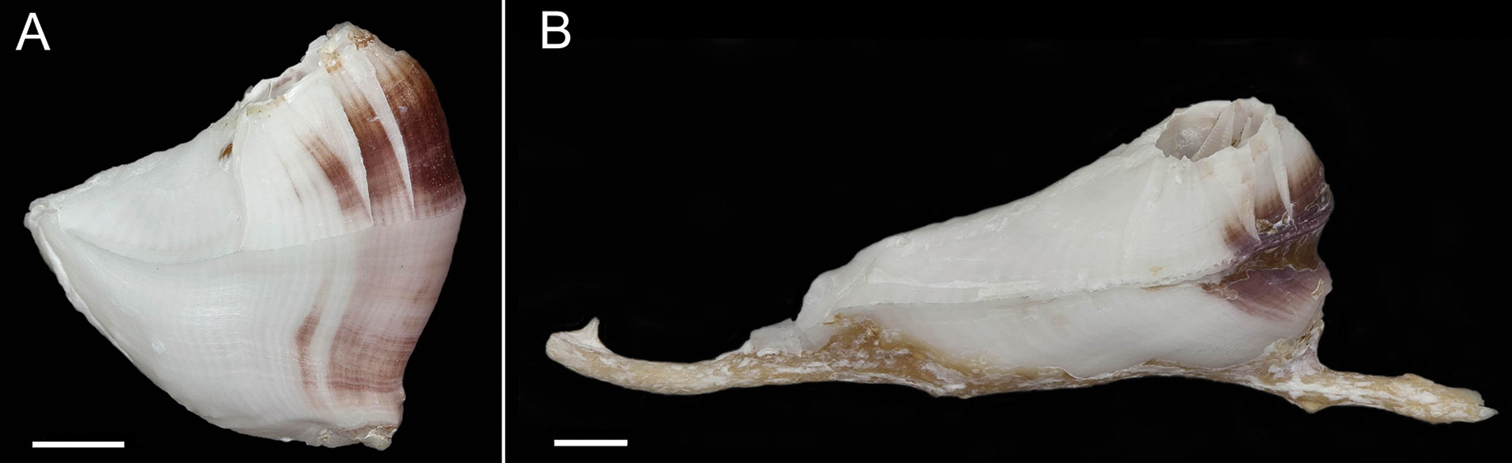
Exterior of shell with minute bumps, most prominent on parieties. Color variable, white with varying shades of purple concentrated on parietes and basis often at carina side of shell. Radii usually white but can be colored, basis lighter shade of purple to light purplish-red (Fig. 2A–B). Opercular opening round to diamond shaped, small in comparison to shell. Mantle tissue purple near opercular opening. Basis boat shaped (Fig. 2A–B) highly variable depth and length. Basis length of the paratypes 9–21mm. Basis elongated in rostro-carina axis, often deeply indented and/or warped from growing around axis of gorgonian. Carina convex. Rostrum often elongate. Basis and parieties with longitudinal tubes, alae and radii solid. Tubes of basis hollow near wall plate suture where outgrowths from wall plates articulate, otherwise secondarily filled. Wall plates with small, hollow tubes close to external plate surface. Sutural margins denticulated. Shell strong, not disarticulating in sodium hypochlorite solution.
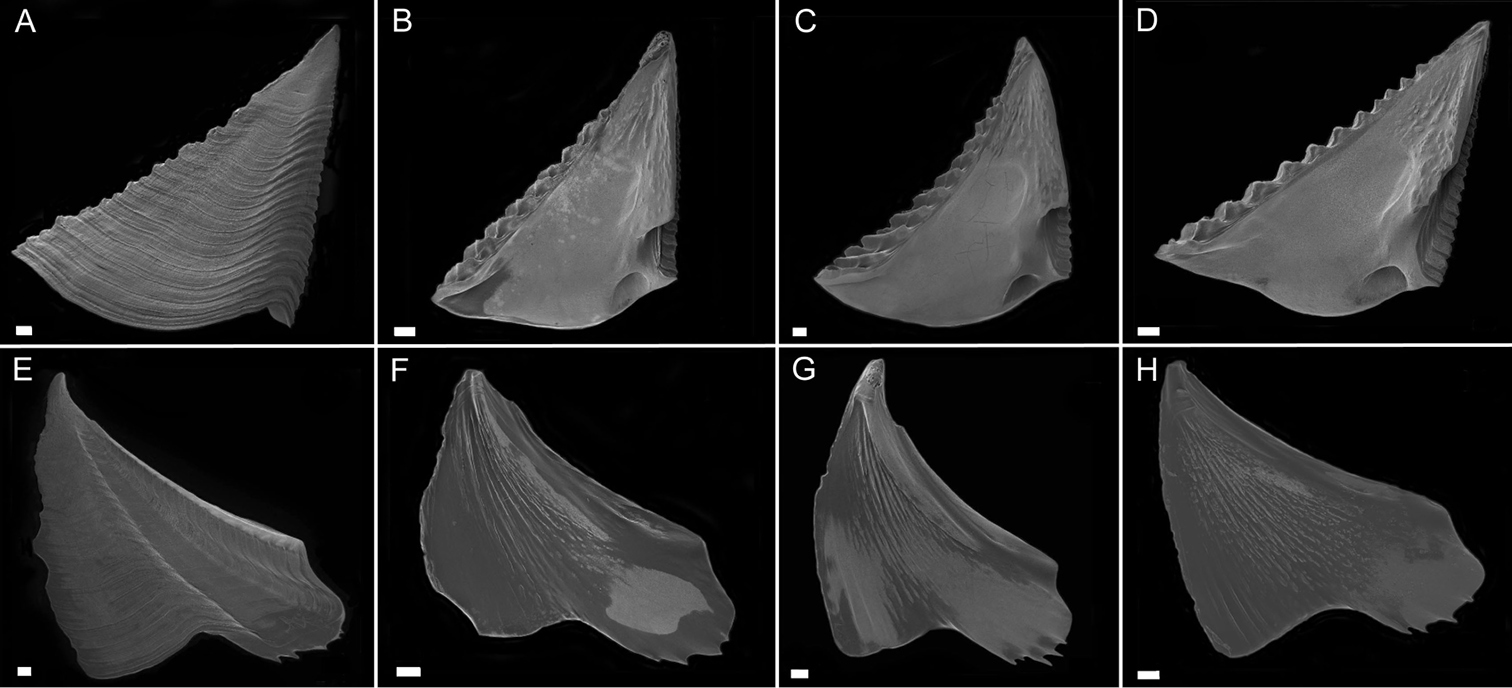
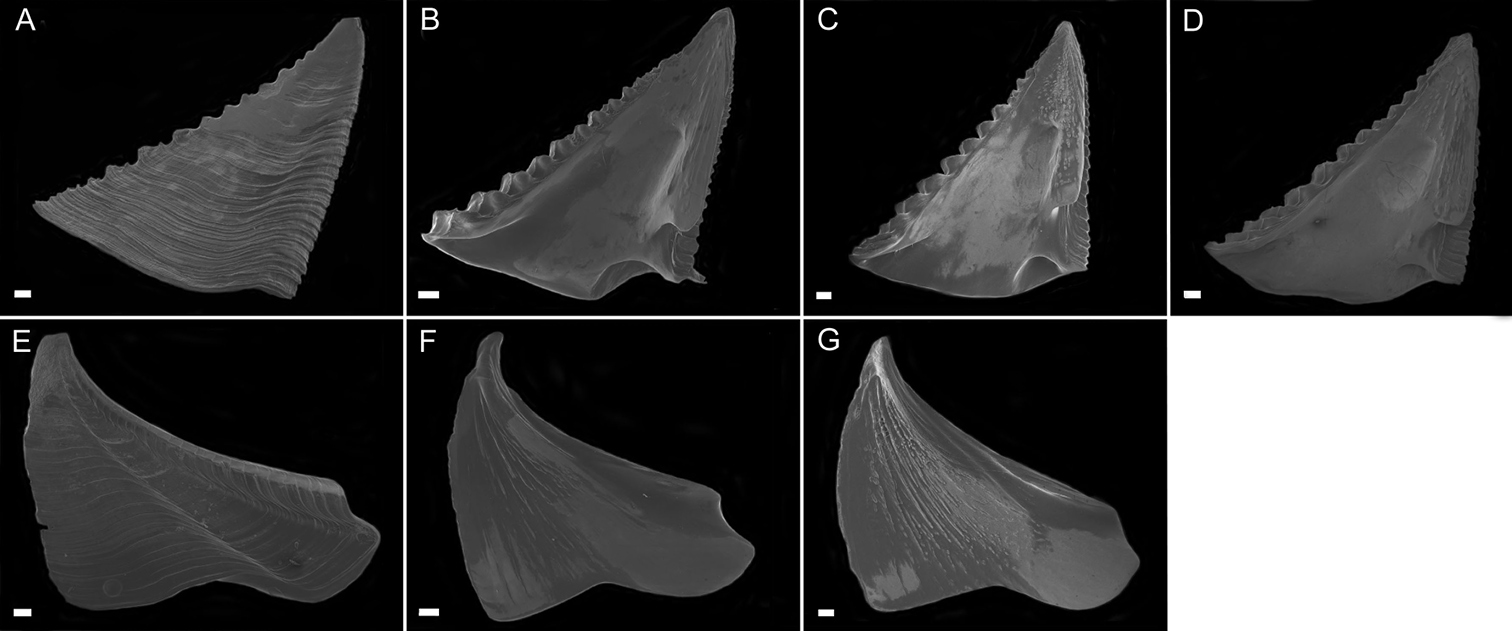
Scutum (Fig. 3A–D) with fairly straight tergal and occludent margins, occludent margin may be concave. Basal margin curved. Apex acute. Articular ridge about ⅔ length of tergal margin. Articular furrow present. Adductor ridge absent. Depressor muscle pit deep, medium to large in diameter. Adductor muscle pit shallow. Interior surface of articular ridge and above adductor muscle pit rough with small flat ridges, remainder of interior surface smooth. Interior and exterior of tergum white with varying degrees of purple coloration, most often dark purple, concentrated at apex.
Tergum (Fig. 3E–H) with concave scutal and convex carinal margins, basal margin slightly convex or straight. Apex acute. Basiscutal angle shallow upper corner recessed. Spur smooth, broad, corners rounded approximately ½ to ⅓ width of tergum. Spur margin bearing 3–5 small teeth. Length of spur teeth variable. Spur furrow open. Articular ridge low ⅓ to ½ length of scutal margin. Articular furrow shallow. Depressor muscle crests faint. Interior surface rough with multiple low longitudinal ridges. Coloration matches that of scutum.
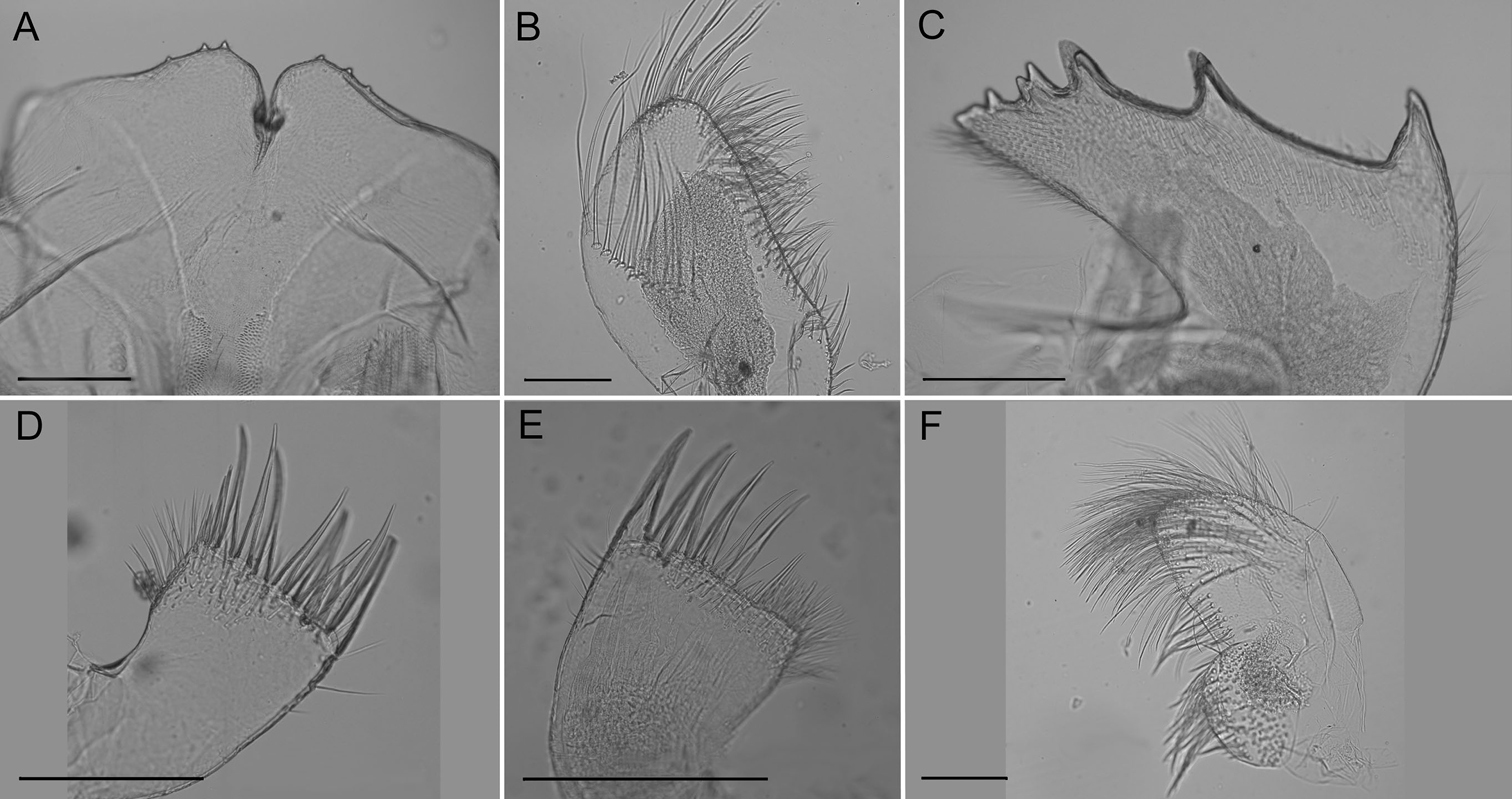
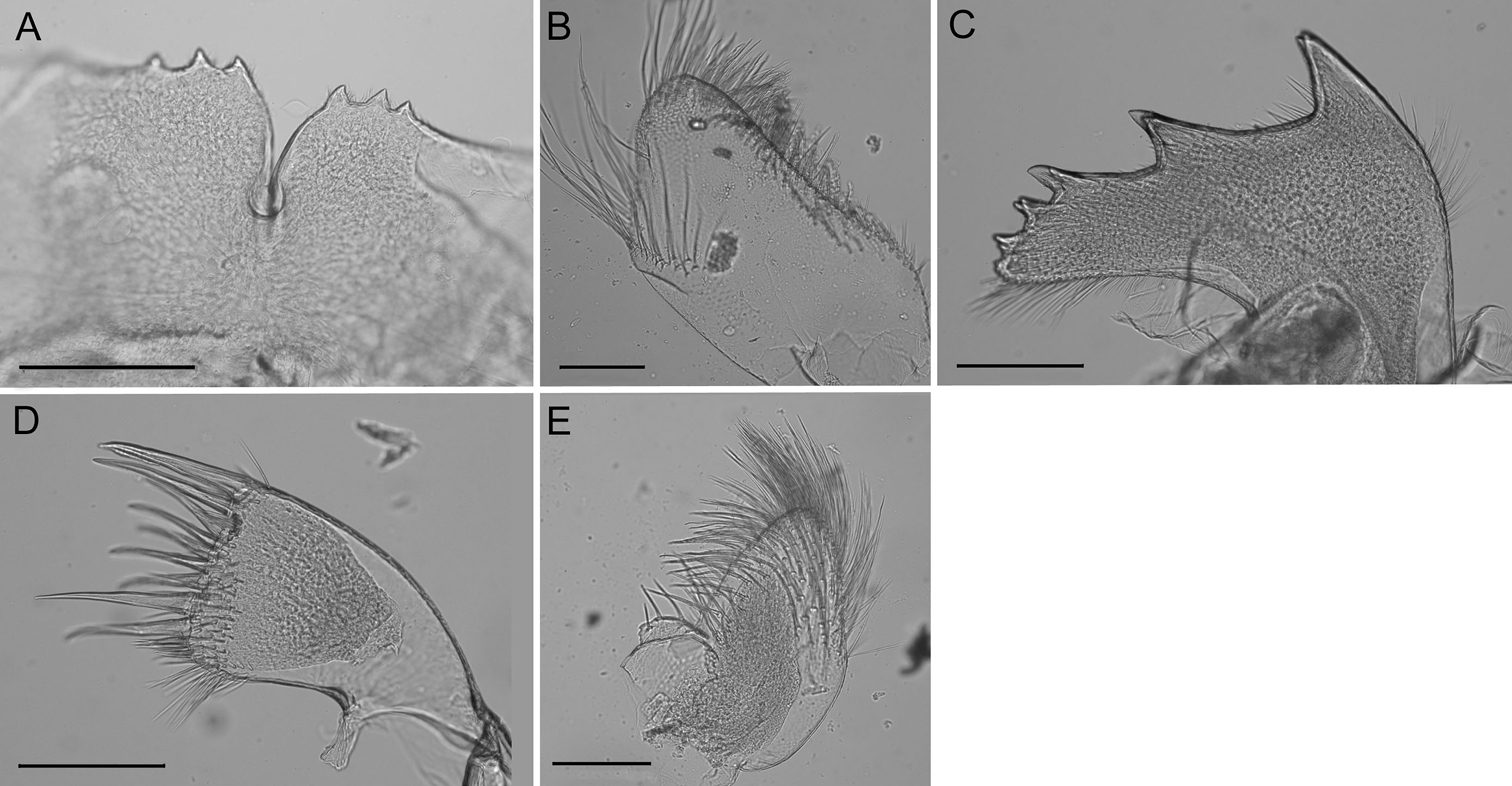
Labrum (Fig. 4A) with deep medial notch, 0–3 teeth on both or one side of notch.
Mandibular palp (Fig. 4B) elongate; superior margin convex, partially covered with long setae; apex with long setae; inferior margin with many shorter setae (Fig. 3).
Mandible (Fig. 4C) with 4–5 teeth excluding inferior angle, decreasing in size, tooth 1 largest, well separated from tooth 2, 2 separated from 3 by smaller distance, teeth 3–5 smallest and closest together, teeth 2–5 may be bidentate. Inferior margin densely setose near angle, superior margin and cutting margin below teeth sparsely setose.
Maxilla I (Fig. 4D–E) with 7–10 large thick spines, either evenly distributed or concentrated on ⅔ of the cutting margin near superior margin, remaining cutting margin covered in short fine setae. Many fine short setae along inferior margin near cutting margin and a few fine setae along superior margin near cutting margin.
Maxilla II (Fig. 4F) small, oblong, bi-lobed, covered in long fine setae.
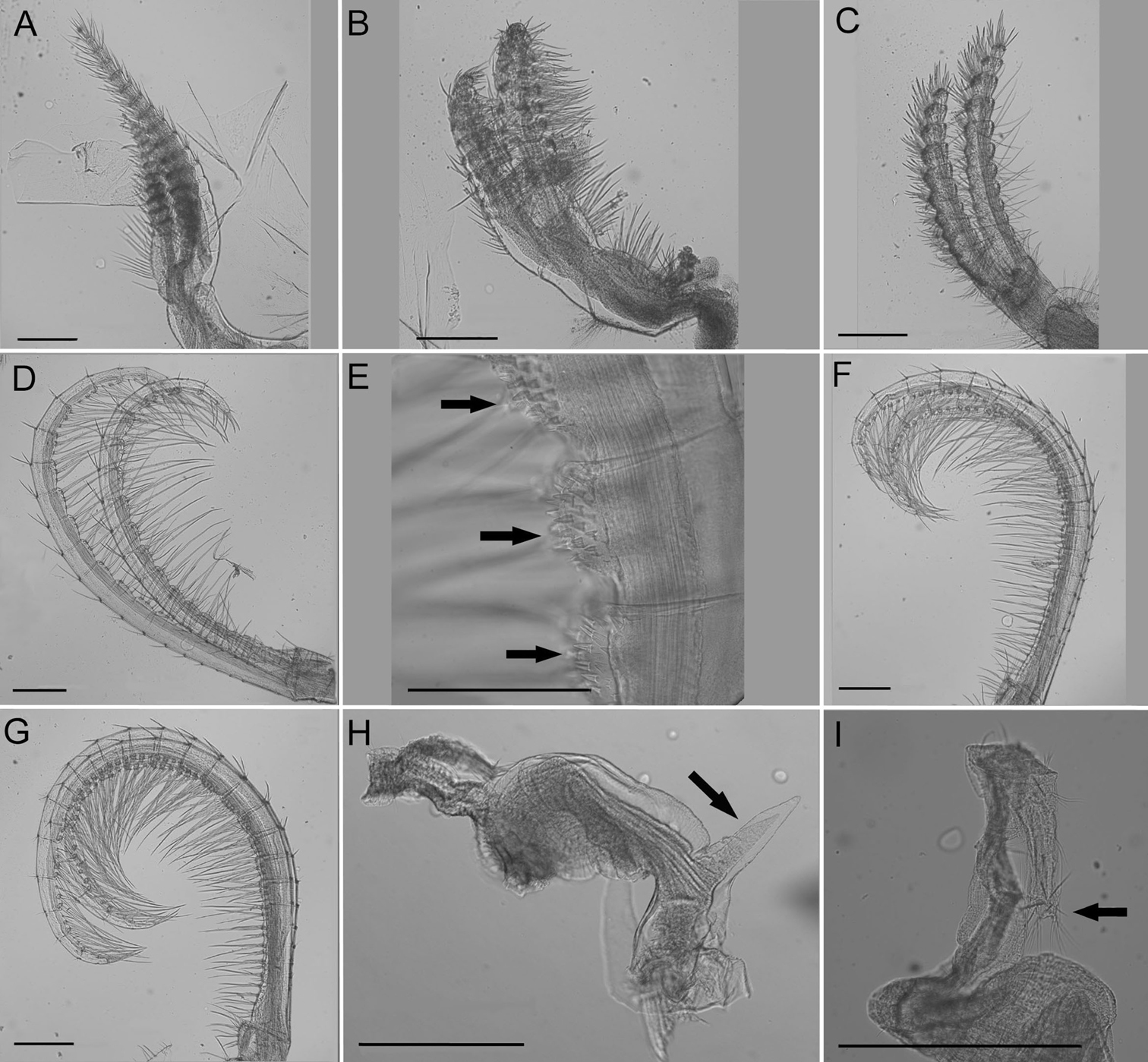
Cirrus I (Fig. 5A) with tapered rami, unequal in length, anterior ramus usually ⅓ longer, posterior ramus more distinct segmentation, setae fine, simple, moderately dense.
Cirrus II (Fig. 5B) rami thick, not tapered, unequal in length but less so than CI, anterior ramus longer, setae simple and dense, segmentation distinct, annulated.
Cirrus III (Fig. 5C) rami thick, slightly tapered, disparate in length, anterior ramus longer, setae simple, dense, thicker than CI or CII, segmentation distinct, annulated.
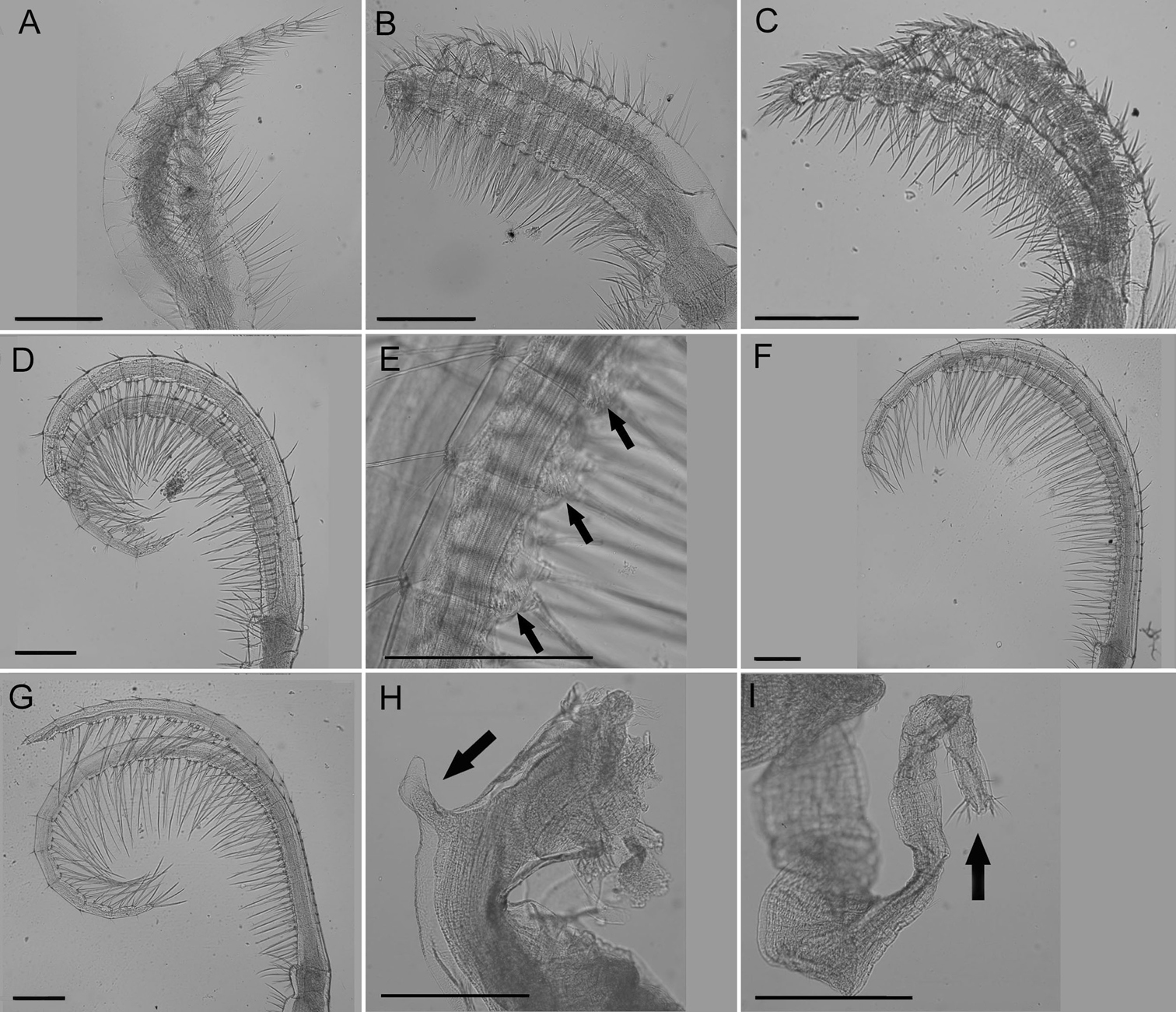
Cirrus IV (Fig. 5D) rami long, tapered, similar length, segments 1–20, end segment varies, with small spines at base of inferior setae, setae simple, superior setae short, sparse, inferior setae long, dense (Fig. 5E).
Cirrus V (Fig. 5F) rami long, tapered, similar length, setae simple, superior setae short, sparse, inferior setae long, dense
Cirrus VI (Fig. 5G) rami long, tapered, similar length, setae simple, superior setae short, sparse, inferior setae long, dense.
All cirral setae simple.
Penis long, covered in sparse fine setae, large basidorsal point (Fig. 5H), tuft of setae distally (Fig. 5I).
Conopea saotomensis sp. n.is named after the island from which it was first collected, São Tomé.
Conopea saotomensis sp. n.is known from São Tomé and Príncipe at depths ranging from 5–34 m living on species of Leptogorgia and Eunicella.
Conopea saotomensis sp. n. differs from Conopea calceola by the following: distance between scutal depressor muscle pit and articular furrow is wider in Conopea saotomensis sp. n. than in Conopea calceola; angle between tergal spur and basal margin is smaller in Conopea saotomensis sp. n. than in Conopea calceola; in Conopea saotomensis sp. n. large spines on cutting edge of maxilla span ⅔ or entire length, span entire length in Conopea calceola.
Cirral formula for Conopea saotomensis sp. n. (CASIZ 175526; 174805; 178655)
| Cirrus | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Anterior ramus | 15–18 | 13–16 | 11–13 | 17–25 | 27 | 21–27 |
| Posterior ramus | 10–17 | 11–13 | 9–12 | 21–25 | 22–28 | 24–29 |
Conopea saotomensis sp. n., A whole shell (CASIZ174804) B whole shell attached to gorgonian axis (CASIZ174806). Scale bar = 2 mm.
Conopea saotomensis sp. n. Opercular plates. A scutum exterior (CASIZ175526) B scutum interior (CASIZ178655) C scutum interior (CASIZ175526) D scutum interior (CASIZ174804) E tergum exterior (CASIZ175526) F tergum interior (CASIZ178655) G tergum interior (CASIZ175526) H tergum interior (CASIZ174804). Scale bar = 200μm.
Conopea saotomensis sp. n. Mouth parts. A labrum (CASIZ174805) B mandibular palp (CASIZ174805) C mandible (CASIZ174805) D maxilla I (CASIZ173190) E maxilla I (CASIZ175526) F maxilla II (CASIZ178655). Scale bar = 200μm.
Conopea saotomensis sp. n. Cirri and penis. A CI (CASIZ174805) B CII (CASIZ174805) C CIII (CASIZ174805) D CIV (CASIZ174805) E CIV spines (CASIZ175526) F CV (CASIZ174805) G CVI (CASIZ174805) H penis basidorsal point (CASIZ175526) I penis tip (CASIZ175526). Scale bar = 200 μm.
urn:lsid:zoobank.org:act:252522DE-A3D4-4FBA-8EF4-5D4014FED8CD
http://species-id.net/wiki/Conopea_fidelis
Figures 6–9, Table 4Holotype: CASIZ185252, separated from CASIZ174322, 95% EtOH. Ponta Baleia, São Tomé, Gulf of Guinea, 0°2.13'N, 6°33.51'E, collected by hand/SCUBA, 24 m, attached to Muriceopsis tuberculata, R. Van Syoc, 30 May 2006. Original label: “RVS – 539, S-6, 30 May 2006, Sao Tome” [handwritten label], California Academy of Sciences, San Francisco.
Paratypes: CASIZ174803 (2 specimens), Diogo Vaz, São Tomé, Gulf of Guinea (0°18.89'N, 6°29.39'E), collected by hand/SCUBA, 9–26 m, R. Van Syoc, 29 May 2006; CASIZ174322 (14 specimens), Ponta Baleia, São Tomé, Gulf of Guinea (0°2.13'N, 6°33.51'E), collected by hand/SCUBA, 24 m, R.Van Syoc, 30 May 2006; CASIZ178651 (2 specimens), Pedra da Gale, Príncipe, Gulf of Guinea (1°43'30.1"N, 7°22'51.5"E), collected by hand/SCUBA, 30 m, R.Van Syoc, 20 Jan 2009.
Exterior of shell covered in very small bumps; color variable, white with pink or light purple on parietes and basis, radii usually white or lighter in color, rostrum often white (Fig. 6A–B). Opercular opening round to diamond shaped, small compared to shell size. Mantle tissue purple near opercular opening. Basis boat shaped (Fig. 6A–B), highly variable depth and length. Basis length of paratypes 14–32 mm. Basis elongated in rostro-carina axis, often deeply indented and/or warped from growing around axis of gorgonian. Carina convex. Rostrum often elongate. Basis with radiating longitudinal tubes, secondarily filled, hollow near wall plate suture. Wall plates with small longitudinal tubes near external surface of shell. Alae and radii lacking tubes. Sutural margins denticulated. Shell wall compartments strongly fused, not disarticulating in sodium hypochlorite solution.
Scutum (Fig. 7A–D) with straight to mildly convex tergal margin, occludent margin usually straight , occasionally with curve above basioccludent angle. Basal margin variable, sinuous. Apex subacute. Articular ridge prominent, extending ⅔– ¾ length of scutum. Articular furrow present. Adductor ridge absent. Adductor muscle pit shallow, fairly large. Depressor muscle pit large, deep, broad, may converge with basil margin. Majority of interior surface of scutum smooth, articular ridge and apex with low, flat ridges. Interior and exterior white with varying shades of purple coloration concentrated at apex.
Tergum (Fig. 7E–G) scutal and carinal margins curved. Basal margin straight or slightly curved. Apex acute. Basicutal angle shallow, upper corner recessed. Spur broad, bears no teeth, about ½ to ⅓ width of tergum, spur furrow open. Articular ridge ⅓ to ½ length of tergum. Articular furrow shallow. Depressor muscle crests faint. Interior rough with multiple small ridges. Coloration matches that of scutum.
Labrum (Fig. 8A) with deep notch, 0–3 teeth on both or one side of notch.
Mandibular palp (Fig. 8B) slightly convex oval shape, superior margin with curved ridge and sparse fine long setae, inferior margin with dense shorter setae.
Mandible (Fig. 8C) with 4–6 teeth excluding inferior angle, decreasing in size, tooth 1 largest, well separated from tooth 2, 2 separated from 3 by smaller distance, teeth 3-6 smallest and closest together, teeth 1and 2 may be bidentate, 4 and 5 may be bifurcated. Inferior margin densely setose near angle, superior margin and cutting margin below teeth sparsely setose.
Maxilla I (Fig. 8D) with 10–12 large thick spines, many smaller, thinner spines along cutting margin, short setae below margin, dense setae on anterior margin, posterior margin sparsely setose, may have shallow notch.
Maxilla II (Fig. 8E) small, oval shaped, bi-lobed, covered in long, fine setae.
Cirrus I (Fig. 9A) rami densely setose, tapered and unequal in length, anterior rami about ⅓ longer, posterior rami with more annulated segmentation.
Cirrus II (Fig. 9B) rami slightly unequal in length, width thick, segmentation distinct, annulated, thick dense setae.
Cirrus III (Fig. 9C) rami unequal in length but less so than CI, anterior ramus longer, width thick, dense thick setae, segmentation distinct, annulated.
Cirrus IV (Fig. 9D) rami long, tapered, anterior side with long dense setae and small spines at base of setae (Fig. 9E) extending from first to twentieth segment (end segment variable), posterior side with short sparse setae at segment divisions.
Cirrus V (Fig. 9F) rami long with long dense setae on anterior side and short sparse setae at segment divisions on posterior side, about equal in length.
Cirrus VI (Fig. 9G) rami long with long dense setae on anterior side, short sparse setae only at segment divisions of posterior side, similar length.
All cirral setae simple.
Penis long with large basidorsal point (Fig. 9H), covered in short very sparse setae, tuft of setae distally (Fig. 9I).
Conopea fidelis sp. n. is named so because it is found to be faithful to one host species of gorgonian, Muriceopsis tuberculata. From the Latin fidelis: faithful or true.
Conopea fidelis sp. n. is known from São Tomé and Príncipe at depths ranging from 5–34 m and is found living on the gorgonian Muriceopsis tuberculata.
Morphological differences between Conopea fidelis sp. n. and Conopea calceolaare as follows: Conopea fidelis sp. n. does not have tergal spur teeth, Conopea calceola does; scutal depressor muscle pit may converge with basal margin in Conopea fidelis sp. n., it does not in Conopea calceola; Conopea fidelis sp. n. maximum basis length is longer than that of Conopea calceola.
Morphological differences between Conopea saotomensis sp. n. and Conopea fidelis sp. n.are as follows: Conopea saotomensis sp. n. shell color ranges from dark purple to light purplish-red, Conopea fidelis sp. n. shell color ranges from light purple to pink; Conopea fidelis sp. n . basis length maximum is longer than that of Conopea saotomensis sp. n.; length of scutal articular furrow in Conopea saotomensis sp. n. is shorter than Conopea fidelis sp. n.; scutal depressor pit may converge with basal margin in Conopea fidelis sp. n. but not in Conopea saotomensis sp. n.; angle between tergal spur and basal margin is smaller in Conopea saotomensis sp. n. than Conopea fidelis sp. n.; tergal spur teeth present in Conopea saotomensis sp. n., absent in Conopea fidelis sp. n.; Conopea saotomensis sp. n. length of tergal articular ridge is equal or longer to that of Conopea fidelis sp. n.; cutting edge spines of maxilla I span entire margin or just ¾ in Conopea saotomensis sp. n, span entire margin in Conopea fidelis sp. n.; Conopea fidelis sp. n. maxilla I may have a notch, Conopea saotomensis sp. n. does not.
Cirral formula for Conopea fidelis sp. n.(CASIZ 178651; 174803A; 174322F)
| Cirrus | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Anterior ramus | 14–17 | 11–12 | 11–12 | 19–21 | 23–24 | 23–25 |
| Posterior ramus | 9–11 | 9–10 | 10–11 | 21–22 | 24–26 | 23–26 |
Conopea fidelis sp. n. A whole shell(CASIZ174322A) B whole shell attached to gorgonian axis (CASIZ174322B). Scale bar = 2 mm.
Conopea fidelis sp. n.Opercular plates. A scutum exterior (CASIZ174803) B scutum interior (CASIZ174322) C scutum interior (CASIZ178651) D scutum interior (CASIZ174803) E tergum exterior (CASIZ174803) F tergum interior (CASIZ174322) G tergum interior (CASIZ178651). Scale bar = 200μm.
Conopea fidelis sp. n. Mouth parts. A: labrum (CASIZ174322) B mandibular palp (CASIZ174803) C mandible (CASIZ174322) D maxilla I (CASIZ174322) E maxilla II (CASIZ174322). Scale bar = 200μm.
Conopea fidelis sp. n. Cirri and penis. A CI (CASIZ178651) B CII (CASIZ178651) C CIII (CASIZ178651) D CIV (CASIZ178651) E CIV spines (CASIZ178651) F CV (CASIZ178651) G VI (CASIZ178651) H penis basidorsal point (CASIZ174803) I penis tip (CASIZ174322). Scale bar = 200μm.
COI has been shown to be useful for delimiting species within the Crustacea (
The barnacles collected from the Gulf of Guinea for this study were originally identified as C. cf. calceola. The initial identifications were tentative because Conopea calceola is not well studied, has a reportedly large distribution, the original species description (
Attempts to obtain specimens of Conopea calceola from other locations/institutions were unsuccessful.
Barnacles are found permanently attached to many different types of living and non-living substrata. Locating a living substratum, especially one that is mobile or spatially rare, can be challenging for a small marine larva. For example; a gorgonian, a turtle, or a whale is harder to locate than a rock bed. When barnacle larvae locate and settle onto a gorgonian they may be recognizing the substratum, the presence of conspecifics, or both. It has been shown that barnacle larvae can determine where to settle by recognizing pheromone cues from their cohorts (
Although the details of the settling barnacle larvae and gorgonian interaction are not completely known, it appears, from our observations (specifically that Conopea fidelis sp. n. was found only on Muriceopsis tuberculata) that barnacle larvae may be capable of distinguishing between gorgonian species. Of course, more collections, identifications, and laboratory work testing settlement preference would be needed to answer this question.
The possibility that Conopea saotomensis sp. n. and Conopea fidelis sp. n. are endemic to the Gulf of Guinea Islands is likely for the following reasons: the islands’ distance (approx. 274 km), age (approx. 13 and 30 myo), and historic isolation from mainland Africa; they are not known from any previous locality; many endemic species, terrestrial and marine, are found on the Gulf of Guinea islands (
The authors would like to thank Robert Drewes (California Academy of Sciences) for initiating and organizing biodiversity research on the Gulf of Guinea Islands; Sarah Cohen (San Francisco State University/RTC) for academic support; Ned Seligman of the Step-Up Foundation for help with collecting permits and making local contacts on the islands; Africa’s Eden for lodging in São Tomé and Príncipe and dive operations in Príncipe; Mary Wicksten for specimens of Conopea galeata; Christina Piotrowski and Anna Sellas for their help in the Center for Comparative Genomics lab at the California Academy of Sciences; an anonymous referee and D. Jones for their participation as Zookeys reviewers, and editor Niel Bruce (Queensland museum). Molecular lab work was funded by a CCG student start-up grant.