Citation: Salles FF, Gattolliat J-L, Angeli KB, De-Souza MR, Gonçalves IC, Nessimian JL, Sartori M (2014) Discovery of an alien species of mayfly in South America (Ephemeroptera). ZooKeys 399: 1–16. doi: 10.3897/zookeys.399.6680
Despite its wide, almost worldwide distribution, the mayfly genus Cloeon Leach, 1815 (Ephemeroptera: Baetidae) is restricted in the Western hemisphere to North America, where a single species is reported. In the Neotropics, except for some species wrongly attributed to the genus in the past, there are no records of Cloeon. Recently, however, specimens of true Cloeon were collected along the coast of Espírito Santo, Southeastern Brazil. In order to verify the hypothesis that this species was recently introduced to Brazil, our aim was to identify the species based on morphological and molecular characters and to confirm the presence of true representatives of the genus in the Neotropics. Our results revealed that the specimens found in Brazil belong to the Afrotropical species C. smaeleni Lestage, 1924. The identity of the species, its distribution, along with its previous absence in regularly sampled sites, is a clear sign that the specimens of C. smaeleni found in Espírito Santo are introduced, well established, and that the colonization took place very recently.
Aquatic insects, Ephemeroptera, invasive species, Baetidae, Cloeon, Neotropics
Aquatic insects, despite their dominance in terms of diversity in most freshwater ecosystems, show a disproportional low number of invasive species when compared to other freshwater macroinvertebrates (
Due to their diversity, abundance and role in nutrient cycling, mayflies are a critical component in freshwater ecosystems throughout the world, and most species are good bioindicators of the water quality (
The genus Cloeon Leach, 1815 (Ephemeroptera) is one of the most common and most diversified genera of mayflies. It encompasses 75 species with 24 of them in the Palaearctic realm, 23 in the Afrotropical and 20 in the Oriental. It is also present, though less diversified, in the other realms, except the Neotropics where true Cloeon have never been reported. Because of its long imaginal stage in females, ca 14 days for mated (
Cloeon colonizes all kind of still and standing waters. It can be collected in the riparian vegetation of streams, in ponds and lakes as well as artificial habitats. Larvae feed on detritus and small algae. They swim rather rapidly and actively move their gills. They support water of low quality, i.e. α–β mesosaprobic (
The genus Cloeon is characterized at the larval stage by double rounded gills on segments I to VI and gills VII simple; sclerotized spines on the lateral margins of the abdomen; segment III of labial palp apically tapered or falcate; legs long and slender, tarsal claws elongated with two rows of numerous denticles; median caudal filament equal to the cerci. In the imaginal stage: forewing with single intercalary veins; hindwings absent; female forewing with costal and subcostal fields coloured in some species; male with 3-segmented gonopods without lateral extensions, segment III reduced, genital plate rounded or conical.
Historically, several species of Cloeon were described from the Western Hemisphere, but all of them have been transferred to other genera. While the species from the Neartic realm were transferred to Centroptilum Eaton, 1869 or Procloeon Bengtsson, 1915 (
The case of Cloeon dipterum (mentioned as Cloeon cognatum Stephens, 1835 in some papers) is of great interest; this widespread European species was first reported from the U.S.A. based on a single female from Illinois (
Recently, unexpected adults and larvae of Cloeon were found in the State of Espírito Santo, Southeastern Brazil. During the last seven years this state was one of the most sampled and studied areas in Brazil regarding mayflies (e.g.,
Larvae were collected with the usual techniques for aquatic insects, such as surber samples and other net sampling methods. Cloeon female adults were gathered mostly inside houses, while male adults were obtained by rearing larvae in the laboratory. The examined material is housed in the Coleção Zoológica Norte Capixaba, Universidade Federal do Espírito Santo, São Mateus, Brazil; Coleção Entomológica Prof. José Alfredo Dutra, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; and Musée cantonal de zoologie, Lausanne, Switzerland. The geographic records of the species were mapped with DIVA-GIS (version 7.17.2, http://www.diva-gis.org/) and then edited with Adobe Illustrator and Adobe Photoshop CS6.
For the specific identification, the most important morphological characters of the genus were examined (e.g.
For the molecular analyses DNA was extracted from specimens stored in pure alcohol using a Qiagen Extraction Kit. The 658 pb of a mitochondrial protein-coding gene fragment (cytochrome oxydase subunit I, or CO1) were amplified using primers LCO 1490 (GGTCAACAAATCATAAAGATATTGG) and HCO 2198 (TAAACTTCAGGGTGACCA A AAAATCA) (
Brazil
Conceição da Barra, Parque Estadual de Itaúnas, Alagado, 17/v/2012, 18°24'34.66"S, 39°41'59.84"W, 54 larvae, 1 female subimago, 1 female imago, 3 male subimagos and 2 male imagos. Same data, 18/v/2012, 9 larvae (GenBank accession numbers: HG935106 and HG935107). São Mateus: Meleira, 17/vi/2011, 18°43'14.8692"S, 39°46'9.1302"W, 2 female imagos; Bairro Colina, 09/x/2012, 1 male imago; Chácara do Cricaré, Rua Traíra, 17 female imagos, 07/vi/2013, 18°42'57.9162"S, 39°50'29.5902"W, 4 female imagos; Rio Preto, 31/x/2012, 18°44'8.16"S, 39°47'47.0394"W, 4 larvae; Rio Preto, 25/ix/2012, 18°44'8.16"S, 39°47'47.03"W, 3 female imagos. Jaguaré: Córrego Água Limpa, 04/viii/2012, 18°55'40.3962"S, 39°59'9.8988"W, 7 larvae; Santa Maria, Cachoeira do Bereco, 22/ix/2011, 18°53'4.45"S, 40°12'23.14"W, 2 larvae. Vitória, Port Complex of Tubarão: impoundment on Carapina stream, 20°15'23.09"S, 40°14'57.95"W, 15.x.2009, 108 larvae. Same data, 16–17.xii.2009, 5 larvae. Impoundment on Carapina Stream, 20°15'45.89"S, 40°15'1.93"W, 15.x.2009, 7 larvae. Same data, 16–17.xii.2009, 9 larvae. Impoundment on Carapina Stream, 20°15'32.71"S, 40°15'34.29"W, 14.x.2009, 4 larvae. Same data, 16–17.xii.2009, 54 larvae. Guarapari, Parque Estadual Paulo César Vinha: Lagoa Feia, 03/v/2012, 19°26'33.71"S, 40°24'7.2"W, 8 larvae; Lagoa Manilha, 02/v/2012, 19°23'40.92"S, 40°25'20.27"W, 3 larvae. Bom Jesus do Norte, Ilha do Vicente, Rio Itabapoana, 31/vii/2012, 21°6'53.59"S, 41°41'30.90"W, 2 larvae.
Madagascar
Antananarivo, Atanandrano, 23/v/2003, 25 larvae. (GenBank accession numbers: HG935104 and HG935105).
South Africa
S2125, Limpopo Prov., Louis Trichardt, Bass. Limpopo, Riv. Luvuvhu, Alt. 700m, 24/v/2003, 23°05'11"S, 30°10'29"E, 15 larvae.
Saudi Arabia
AR47, Al-Itnayn, dam, Alt. 2300m, 14/xi/2012, 18°01'21"N, 42°45'50"E, 10 larvae, 5 female imagos and subimagos. (GenBank accession numbers: HG935108 and HG935109).
Switzerland
Zurich, Kleinandelfingen, Räubrichsee, 15/v/2012, 47°36'46"N, 8°40'35"E, 2 larvae. (Unpublished sequences from Sereina Rutschmann, IGB, Berlin).
Korea
(GenBank accession number: KC135930).
Canari Islands
GC01: Gran Canaria, Telde, Barranco de los Cernicalos, 25/i/2009, 27°57'54"N, 15°29'46"W, 6 larvae. (GenBank accession numbers: KF438141 and KF438144).
TF03: Tenerife, Igueste de St Andrés, Alt. 100m, 18/iii/2007, 28°32'21"N, 16°09'26"W, 20 larvae. (GenBank accession numbers: KF438163 and KF438120).
Madeira
Funchal, Alt. 270m., 17/iv/2006, 32°39'43"N, 16°53'44"O, 27 larvae
Funchal, Alt. 70m., 02/xii/2005. 32°38'30"N, 16°55'36"O, 2 female imagos.
Saudi Arabia
AR44, Wadi Shahadan, Alt. 190m, 13/ii/2012, 17°27'7"N, 42°42'49"E, 20 larvae. (GenBank accession number: KF438120).
Saudi Arabia
AR39, Wadi Damad, Alt. 260m, 11/ii/2012, 17°27'7"N, 42°42'49"E, 35 larvae. (GenBank accession number: HG935111).
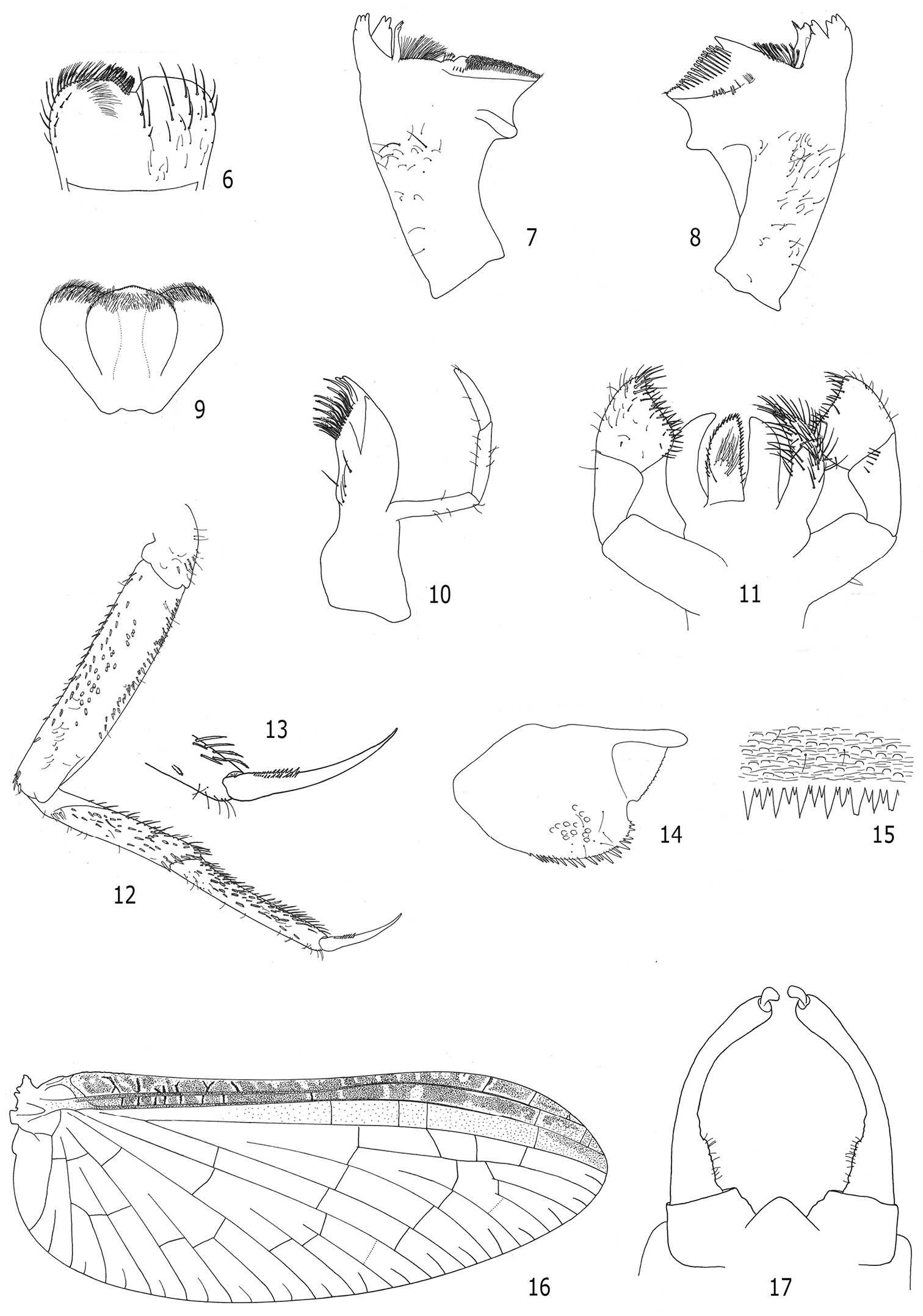
Morphological, as well as molecular analyses revealed that the specimens found in Brazil belong to the Afrotropical species Cloeon smaeleni. The main diagnostic characteristics were, as usual among Cloeon species, the color pattern of adults, especially the fore wing (Figs 1, 2, 3 and 16) and abdominal sterna (Figs 1, 2 and 3), along with the color of the fore legs (Figs 1, 2 and 3). In addition, the maxillary palp three-segmented (Fig. 10), the labial palp segment III clavate (Fig. 11), the lateral spines restricted to segments VIII and IX (Figs 4 and 5), the teeth and shape of the tarsal claws (Figs 12 and 13), the spines on posterior margin of abdominal terga alternating one long one short (Fig. 15), and the male genitalia with a conical genital plate (Fig. 17) were crucial for the specific identification. Other morphological features such as labrum (Fig. 6), mandibles (Figs 7 and 8), hypopharynx (Fig. 9), and paraproct (Fig. 14) are also illustrated as they may be useful to separate Cloeon from other Neotropical genera especially Callibaetis Eaton, 1881 and Callibaetoides Cruz, Salles & Hamada, 2013.
Cloeon smaeleni. 1 Male imago (lateral view of living specimen) 2 Female imago (lateral view of living specimen) 3 Female imago (lateral view of living specimen) 4 Male larva (dorsal view) 5 Detail of male larval tergites VII to X.
Cloeon smaeleni: 6–15 larva morphology 6 Labrum 7 Right mandible 8 Left mandible 9 Hypopharynx 10 Maxilla 11 Labium 12 Fore leg 13 Fore tarsal claw 14 Paraproct 15 Posterior margin of abdominal tergum 16 and 17 adult morphology 16 Female fore wing 17 Male genitalia.
There is no genetic distance between the Brazilian specimens and they are highly supported as sister group to Afrotropical haplotypes of Cloeon smaeleni (Table 1). The genetic distance between Brazilian and Afrotropical haplotypes clearly corresponds to intraspecific variation (K2P distance = 0.02). We also sequenced material from Saudi Arabia morphologically similar to Cloeon smaeleni. These haplotypes appear as sister group to the Afrotropical + Brazilian clade, but present interspecific distance with this clade (K2P distance > 0.11). Brazilian haplotypes are not genetically related to any Palaearctic species including Cloeon dipterum s. l. (K2P distance > 0.20).
Sequences using the Kimura 2-parameter: Taxa: CS = Cloeon smaeleni or Cloeon cf. smaeleni; CD = Cloeon dipterum or Cloeon cf. dipterum; CL: Cloeon sp1; CP = Cloeon praetextum; CHS = Cheleocloeon soldani. Countries: MA = Madagascar; BR = Brazil; SA = Saudi Arabia; CH = Switzerland; KO = South Korea; TF = Tenerife (Canari Islands); GC = Gran Canaria (Canari Islands); NO = Norway.
| CS-MA | CS-BR | CS-SA | CD-CH | CD-KO | CD-TF | CD-GC | CL-SA | CP-NO | |
|---|---|---|---|---|---|---|---|---|---|
| CS-BR | 0.02 | ||||||||
| CS-SA | 0.12 | 0.11 | |||||||
| CD-CH | 0.19 | 0.20 | 0.18 | ||||||
| CD-KO | 0.21 | 0.21 | 0.19 | 0.08 | |||||
| CD-TF | 0.21 | 0.22 | 0.20 | 0.10 | 0.09 | ||||
| CD-GC | 0.22 | 0.23 | 0.21 | 0.11 | 0.10 | 0.10 | |||
| CL-SA | 0.19 | 0.19 | 0.20 | 0.17 | 0.17 | 0.18 | 0.20 | ||
| CP-NO | 0.23 | 0.23 | 0.21 | 0.21 | 0.20 | 0.21 | 0.20 | 0.19 | |
| CHS-SA | 0.24 | 0.25 | 0.23 | 0.20 | 0.20 | 0.22 | 0.23 | 0.21 | 0.23 |
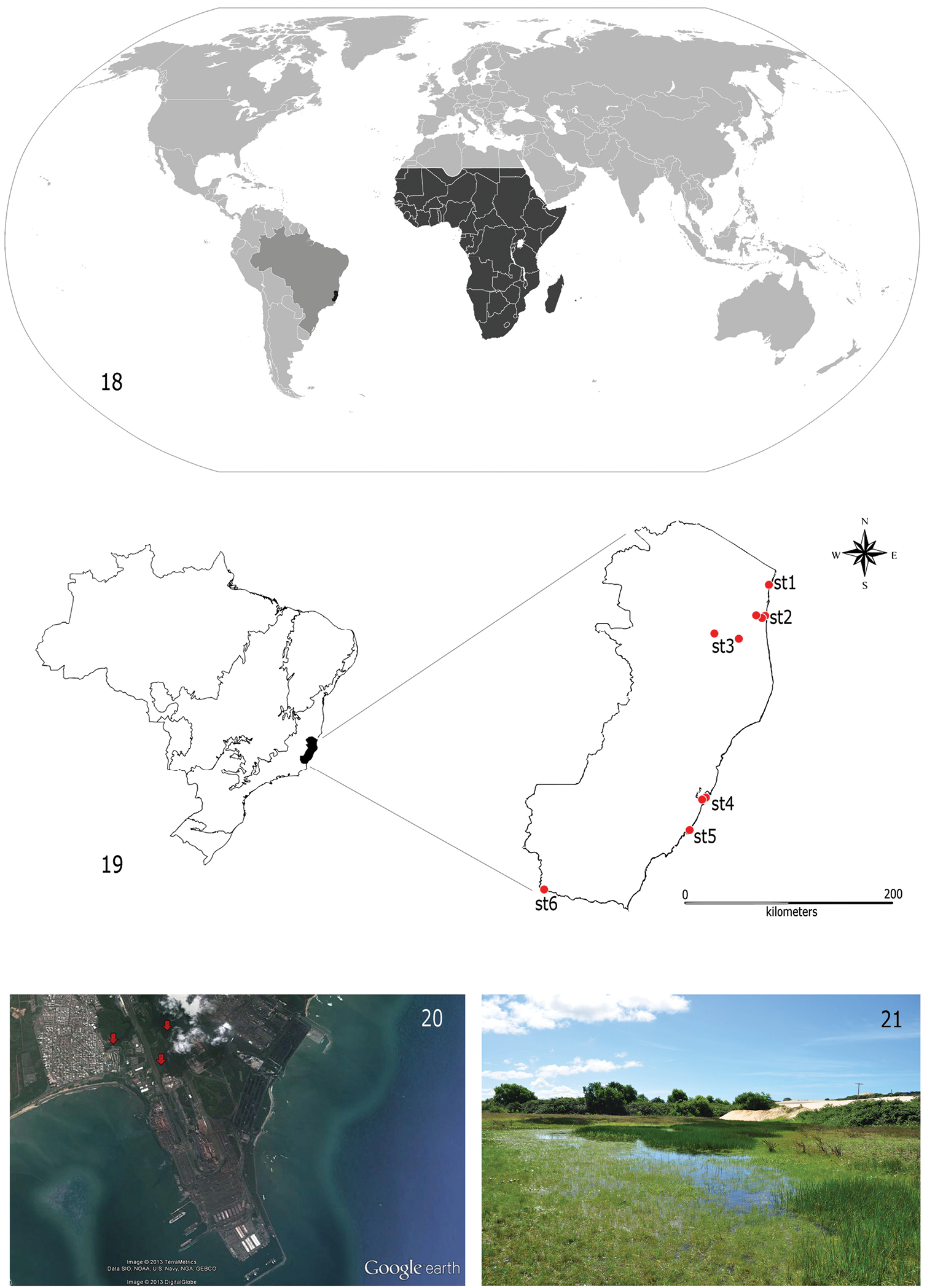
The specimens examined were found exclusively in the State of Espírito Santo, Southeastern Brazil. They have been reported from at least six localities, most of them along the coast of the state (Figs 18 and 19) and always in low altitude areas (from the sea level to 65 meters above it). In Vitória (st4), Guarapari (st5) and Conceição da Barra (st1) (Fig. 19), larvae were collected in ponds very close to the shoreline. In Vitoria (st4), larvae were gathered in artificial impoundments colonized by Pistia spp. or Typha spp. macrophytes on the final section of the Carapina stream. All impoundments are located inside the Port Complex of Tubarão (Fig. 20), the biggest iron ore export port in the world. In São Mateus (st2), larvae were found in Rio Preto, a small black water tributary (Fig. 19) of the main river of the region, the Rio São Mateus or Cricaré. Female adults were caught inside a house very close to a large tributary of the Cricaré, the Rio Mariricu, suggesting that the species might also be present there. Attempts to collect material at the Rio Cricaré, however, were unsuccessful. Jaguaré (st3) and Bom Jesus do Norte (st6) are located more distant from the Ocean coast (around 100 km); in these localities, specimens were collected in approximately five meter wide streams. In the streams or rivers, larvae were found exclusively in areas with slow or no current, among organic substrates, such as roots, macrophytes or leaf litter.
Distribution of Cloeon smaeleni. 18 World map (gray, Brazil; dark gray, species distribution) 19 Map of Brazil subdivided in biomes, with detail of the State of Espírito Santo and collection stations (red circles) (st1, Conceição da Barra; st2, São Mateus; st3, Jaguaré; st4, Vitória; st5, Guarapari; st6, Bom Jesus do Norte) 20 Satellite picture from the Tubarão Complex Port in Vitória (red arrows indicate collection stations) 21 General view of one of the stations at Parque Estadual de Itaúnas.
Cloeon smaeleni is a widespread species (Fig. 18) originally described from a female imago from Katanga, Congo. Subsequently, all ontogenetic stages of Cloeon smaeleni were described (
Larvae of Cloeon smaeleni are found in many types of slow waters: temporary ponds, rice fields, reservoirs, slow moving streams and the margins of lakes (
The identity of the species, its distribution, along with its previous absence in regularly sampled sites, is a clear sign that the specimens of Cloeon smaeleni found in Espírito Santo are introduced, well established, and that the colonization took place very recently. It is not possible to ascertain where or how this event has occurred, or even if there was a single or multiple entrances. The presence of larvae of Cloeon smaeleni in a port, though highly speculative, suggests that they may have arrived in by ship traveling from Africa.
It is also difficult to predict the impact caused by the presence of Cloeon smaeleni in Brazil. In its original habitats, this species feeds on detritus contributing to the recycling of organic matter. It is often the eudominant species but generally co-occurs with other Cloeon species. As this species is not a predator and has no significant economic importance, the impact is probably more related to its competition with other native species at the same trophic level (or controphics species, according to
Our finding raises again the idea, put forward by earlier authors (e.g.
We are grateful to Instituto Estadual de Meio Ambiente e Recursos Hídricos do Espírito Santo (IEMA) and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for collection permissions. We are indebted to Sereina Rutschmann (Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin) for providing sequences of Palearctic Cloeon. Carolina Nieto, Eduardo Domínguez, Luke Jacobus and an anonymous reviewer for comments on the manuscript. Rafael Boldrini is acknowledged for collecting the first specimen and donating the material. This work was partially funded by FAPES (Fundação de Amparo a Pesquisa do Estado do Espírito Santo / processes number 54689627/2011, 511187434/2010) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico / processes number 558246/2009–5, 402939/2012–3, 245924/2012–4).