Citation: Xiangqun Y, Ke G, Feng Y, Yalin Z (2014) Ultrastructure of antennal sensilla of four skipper butterflies in Parnara sp. and Pelopidas sp. (Lepidoptera, Hesperiidae). ZooKeys 399: 17–27. doi: 10.3897/zookeys.399.7063
Most species of Parnara and Pelopidas (Hesperiidae) are important pests of rice. In this study, the antennal morphology, types of sensilla, and their distribution of four skipper butterflies, including Parnara guttata (Bremer & Grey), Pa. bada (Moore), Pelopidas mathias (Fabricius) and Pe. agna (Moore), were observed using a scanning electron microscope. Six distinct morphological types of sensilla were found on the antennae of all of these species: sensilla squamiformia, sensilla trichodea, sensilla chaetica, sensilla auricillica, sensilla coeloconica, and Böhm sensilla. The sensilla trichodea are the most abundant sensilla among the four skipper butterflies, and the sensilla auricillica are confirmed on the antennae of butterflies for the second time. In addition, the possible functions of these sensilla are discussed in the light of previously reported lepidopteran insects, which may provide useful information for further study of the function of these antennal sensilla and for related pests control by applying sex pheromones.
Lepidoptera, Hesperiidae, morphology, fine structure
The antennae of insects have various types of sensilla that play important roles in insect behaviors, including host location, feeding, mate attraction and oviposition (
Parnara guttata (Bremer & Grey), Parnara bada (Moore), Pelopidas mathias (Fabricius) and Pelopidas agna (Moore) are among the most important pests of rice in China. The larvae of these four species feed on the leaves of rice, causing considerable damage and great loss of rice production. So far, the control of rice plant skippers chiefly relies on the use of chemical insecticides, which in turn causes many negative consequences. Biological controls, including the application of sex pheromones, have become increasingly important. Consequently, research of pest antennae has immediate application to the suppression of pests (
All insects studied are specimens in the entomological museum of Northwest A&F University. More specific information is provided in Table 1.
Material localities and collection dates.
| Species | Collection location | Collection date |
|---|---|---|
| Parnara guttata (Bremer & Grey) | Huxian County, Shaanxi Province | 2009.08.15 |
| Lantian County, Shaanxi Province | 2012.08.15 | |
| Fuzhou City, Fujian Province | 2006.07.01 | |
| Zhenkang County, Yunnan Province | 2007.07.09 | |
| Parnara bada (Moore) | Ding’an County, Hainan Province | 2002.08.08 |
| Fuzhou City, Fujian Province | 2005.11.19 | |
| Jinghong City, Yunnan Province | 2007.07.21 | |
| Pelopidas mathias (Fabricius) | Hanzhong City, Shaanxi Province | 1993.07.23 |
| Fuzhou City, Fujian Province | 2003.12.28 | |
| Minqing County, Fujian Province | 2005.10.21 | |
| Pelopidas agna (Moore) | Wuzhi Mountain, Hainan Province | 2007.05.20 |
| Luxi County, Yunnan Province | 2005.08.19 |
The antennae of 10 adults of each of the four species were removed under a microscope (Nikon SMZ1500) by using sharp blades. The antennae were washed for 20 s (four times, each for 5 s) in 70% ethanol solution in an ultrasonic cleaner (KH-250DB; 15°C, 50HZ). After critical point drying, the specimens were attached to a holder using electric adhesive tape, sputter-coated with gold, examined and photographed with a S-4800 SEM (at 10 kV~15 kV).
The antennae of the four studied species of skipper butterflies are located between the compound eyes, and each consists of three components: a basal scape, pedicel, and an elongated flagellum. The first two components consist of a single short segment each one of them (Fig. 1a). The third component, the flagellum, consists of many subsegments. The typical flagellum is thin basally and becomes gradually thicker and curved, covered with scales (Fig. 1c). More types of sensilla are observed on the curved hook (Fig. 1d).
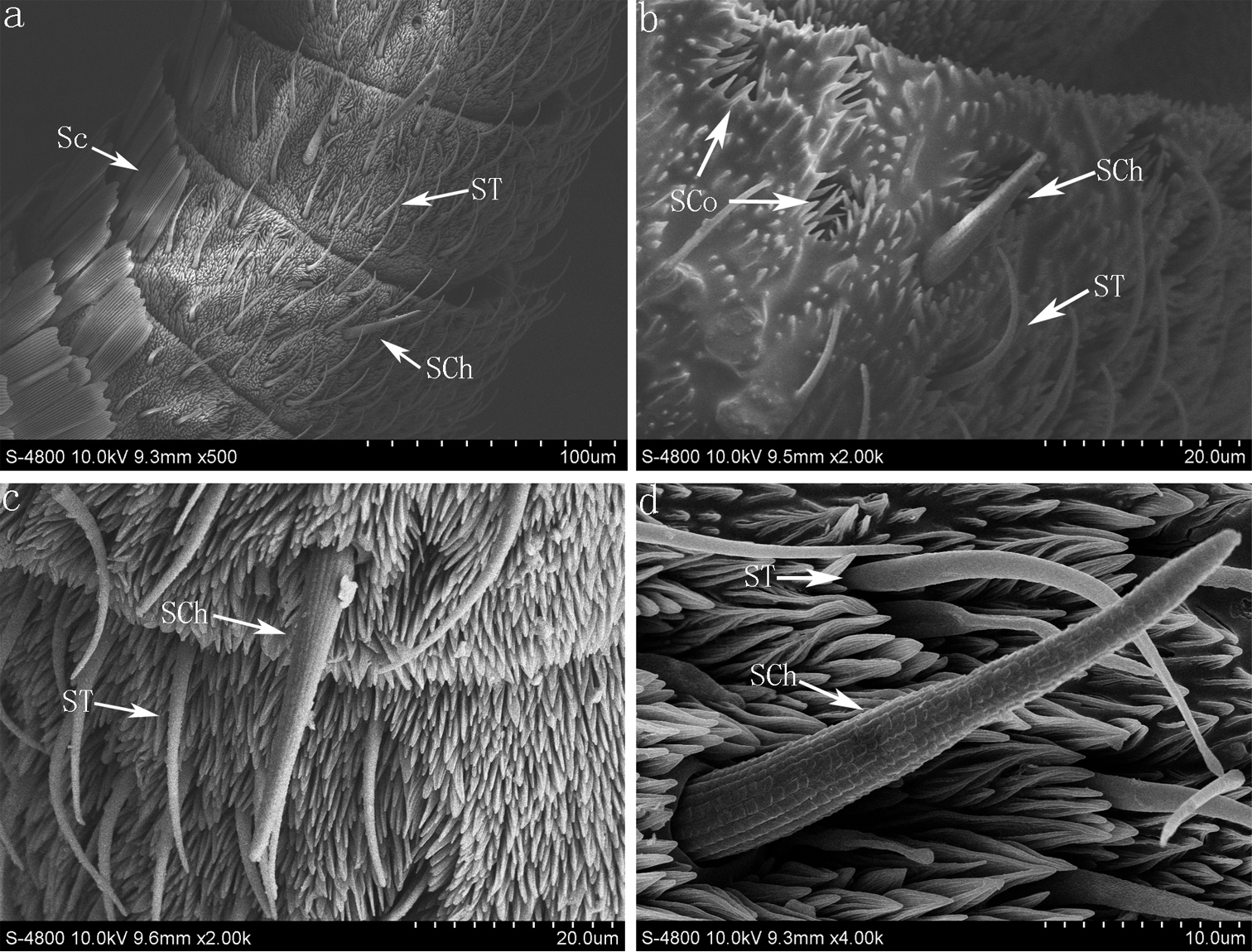
SEM photomicrographs of Parnara guttata. (a) the scape and pedicel of Parnara guttata and location of the Böhm sensilla (b) the Böhm sensilla on the scape of antenna (c) profile of the flagellum with scales (d) profile of last flagellar subsegment of the antenna and the sensilla chaetica, sensilla trichodea and sensilla coeloconica. S Scape; P Pedicel; BS Böhm sensilla; Sc Scales; SCh sensilla chaetica; ST sensilla trichodea; SCo sensilla coeloconica.
In total, six types of sensilla were observed on the antennae of these four skippers: sensilla squamiformia, sensilla trichodea, sensilla chaetica, sensilla auricillica, sensilla coeloconica, and Böhm sensilla.
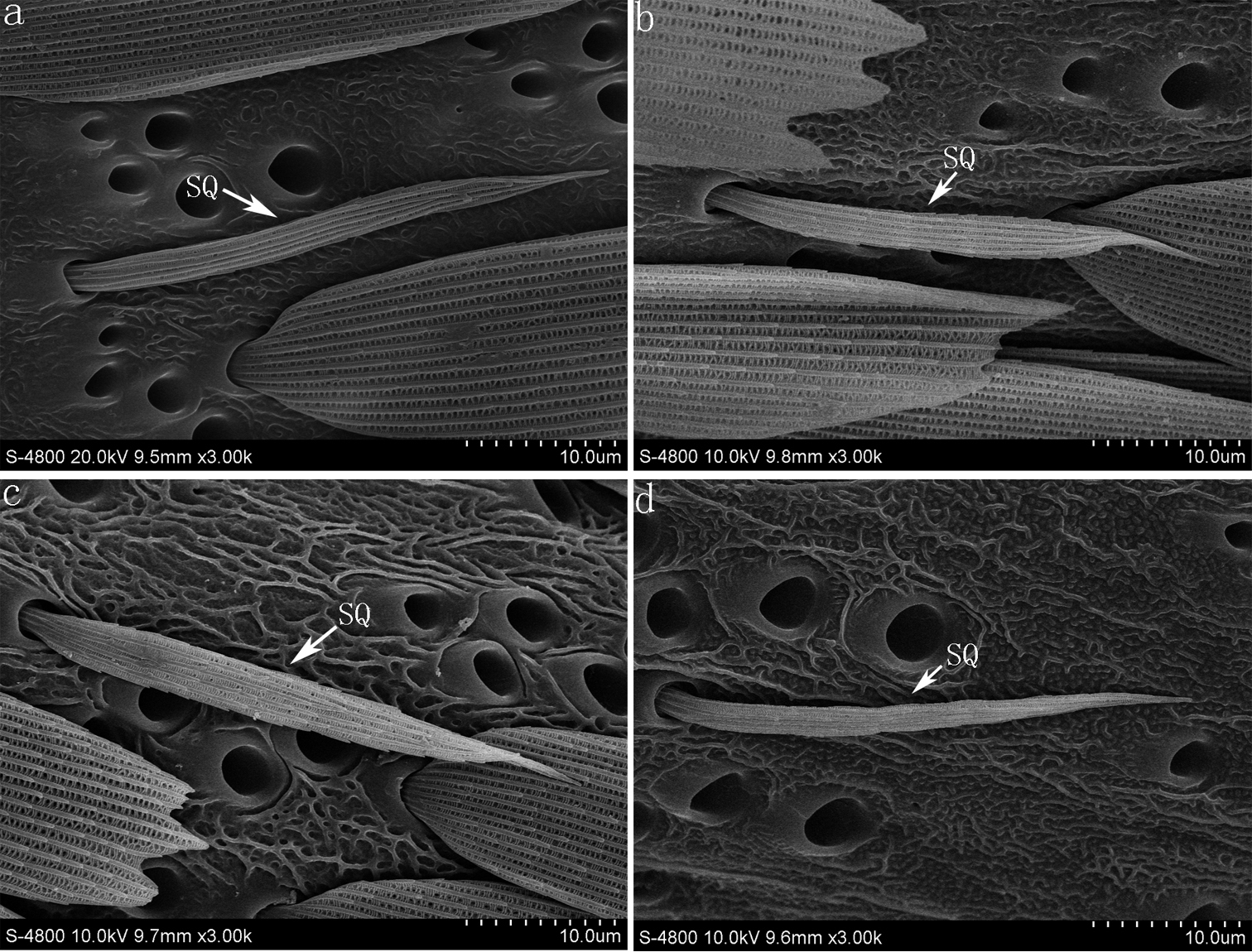
This type of sensillum is scale-like and elongated with a distal end tapering, found along the base or center flagellum among the scales (Fig. 2a–d). The length of the sensilla squamiformia is 43.5±4.0 μm (Parnara guttata), 48.5±6.7 μm (Parnara bada), 47.5±5.8 μm (Pelopidas mathias), 46.3±3.8 μm (Pelopidas agna). The number of sensilla is 1–4 per flagellomere, with the terminal flagellomeres without any among the four skipper butterflies.
(a) sensilla squamiformia on the flagellum of Parnara guttata (b) sensilla squamiformia of Parnara bada (c) sensilla squamiformia of Pelopidas mathias (d) sensilla squamiformia of Pelopidas agna. SQ sensilla squamiformia.
The sensilla trichodea are hair-like, tapering apically. They occur along the distal segments on the ventral surface (Figs 1d and 3a and b). The surface of the cuticular wall of sensilla trichodea is smooth and the wall pores are not seen with scanning electron microscope (Fig. 3c and d). These sensilla (range 27.1±3.2 μm–28±1.5 μm) are the most abundant with about 32–69 per flagellomere in the four species.
(a) The sensilla chaetica, sensilla trichode and scales on the flagellum of Parnara guttata (b) the sensilla chaetica, sensilla trichode and sensilla coeloconica on the flagellum of Parnara bada (c) the sensilla chaetica and sensilla trichode on the flagellum of Pelopidas mathias (d) the sensilla chaetica and sensilla trichode on the flagellum of Pelopidas agna. Sc Scales; SCh sensilla chaetica; ST sensilla trichodea; SCo sensilla coeloconica.
The sensilla chaetica have a straight needle-like appearance with a grooved surface (Figs 1d and 3a–c). Each sensilla arise from a round socket, is wide at the base and sharp at the distal end (Fig. 3d). These sensilla (range from 29.5±4.1 μm to 39.5±7.5 μm) are distributed evenly (1–3 per flagellomere) among the scales at the base and center of the flagellomere and among the sensilla trichodea along the flagellum. 4–7 larger sensilla chaetica (80.3±5.8 μm) are distinct and can be found on the terminal segment of flagellum.
The sensilla auricillica are short and ear-shaped with a blunt and rounded tip. The surface of the cuticular wall of ear-shaped sensilla is covered with small pores (Fig. 4a–d). These sensilla are only scattered along the distal end of the flagellum. These sensilla (about 6–14 per flagellomere) are very similar and the length varies from 12.8±3.4 μm to 15.5±0.3 μm among all four skipper butterflies.
The sensilla auricillica of Parnara guttata (a) Parnara bada (b) Pelopidas mathias (c) and Pelopidas agna (d).
The sensilla coeloconica consist of a submerged central peg with a grooved surface and blunt tip surrounded by a ring of cuticular spines (Figs 3b, 5a–d). They are found on the distal end of the flagellum (about 6–12 per flagellomere) in the four species (Fig. 1d). In Pelopidas mathias and Pelopidas agna, these sensilla are also found occasionally on the base or center of the flagellomere as they are difficult to discern since the scales will conceal them (Fig. 5c and d).
The sensilla coeloconica of Parnara guttata (a) Parnara bada (b) Pelopidas mathias (c) and Pelopidas agna (d). SCo sensilla coeloconica
Böhm sensilla are spine-like structures with smooth cuticles. Böhm sensilla, in clusters, are inserted to the base of scape and pedicel segments only (Fig. 1a and b). Each cluster has approximately 56, 59, 34 and 32 sensilla respectively among Parnara guttata, Parnara bada, Pelopidas mathias and Pelopidas agna.
Sensilla squamiformia are commonly present in lepidopteran insects (
We identified only one type of sensilla trichodea among the four skipper butterflies. However, studies of other moth species have shown those sensilla can be divided into more subtypes according to their size and pore density (
Sensilla chaetica found in this study are similar in structure to those reported for the Lycaenidae: Chilades pandava (Horsfield) and Heliophorus phoenicoparyphus (Jian et al. 2011;
Although the sensilla auricillica have been easily observed in the months, these sensilla on the antenna of butterfly was described for the first time in Pieris rapae L. (
In this study, the multiporous sensilla coeloconica closely resemble those observed in many other Lepidoptera. This type of sensilla is considered to have a humidity and temperature sensitive function (
Böhm sensilla observed here are morphologically similar to those presented in other families of Lepidoptera, e.g., Pyralidae, Tortricidae, Sesiidae (
In summary, we identified six different types of sensilla on the antennae of Parnara guttata, Parnara bada, Pelopidas mathias and Pelopidas agna. The external morphology and distribution of these sensilla among Parnara and Pelopidas, is very similar and also somewhat similar to other reported Lepidoptera. However, documents on morphology of antennal sensilla in butterfly species are still very limited yet. Further exploration on antennal sensilla of these group need merits to be conducted, which may provide useful information for taxonomy and phylogeny of Lepidoptera, and for further studies on the function of antennal sensilla and related pests control by application of sex pheromones.
We are grateful to Dr. J. R. Schrock, Emporia State University, Kansas, USA for reviewing the manuscript. This study is supported by the National Natural Science Foundation of China (No. 31272345; No. 31071693) and the Fundamental Research Funds for the Central Universities (No. 2011K01-35).