(C) 2013 K. M. Shameem. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Callispa keram sp. n. infesting coconut palm (Cocos nucifera L.) in Kerala, India is described and illustrated. Livistona chinensis R.Br. and Syagrus romanzoffiana (Cham.) Glassman are reported as additional host plants.
Chrysomelidae, coconut, Callispa, new species, host plants, insect pest, India
The coconut palm, Cocos nucifera L. is an important source of food and vegetable oil and is intimately associated with the social and cultural heritage of the people in Asia and Oceania (
The genus Callispa comprises about 165 species distributed in the Oriental and Afrotropical Regions (
Coconut is raised on a large scale both in homesteads as well as plantations in coastal and midland regions of Kerala. The presence of adults and larvae of the coconut Callispa is indicated by the characteristic feeding damage and could be easily collected from the abaxial side of leaflets. Specimens were collected from the plains of southern, central and northern Kerala. Attempts were also made to check its presence in the high range regions of Kerala as well as the dry coconut growing tracts of Tamil Nadu, adjoining Kerala. Besides coconut, other palms were also searched for the feeding damage and life stages of the species. Observations on the biology were carried out in the field as well as in the laboratory at the Vellayani campus of the Kerala Agricultural University.
Descriptive terminology follows
Adults are oblong ovate, neither spiny nor tuberculate, 3–10 mm long, flat to moderately convex beetles. Other salient features of the genus are head narrowly produced between antennae; pronotum quadrate, broader than long, anterolateral angles rounded, anterior trichobothrium absent, disc shallowly depressed on either side; elytron with ten rows of punctures and a short scutellar row; claw tarsomere small, hardly extending beyond setae on ventral side of bilobed third tarsomere; upper border of mouth cavity in close proximity to antennal sockets; and scutellum quadrate with rounded posterior margin.
urn:lsid:zoobank.org:act:2AD413D6-160B-4911-81CE-8F9A8B6FB863
http://species-id.net/wiki/Callispa_keram
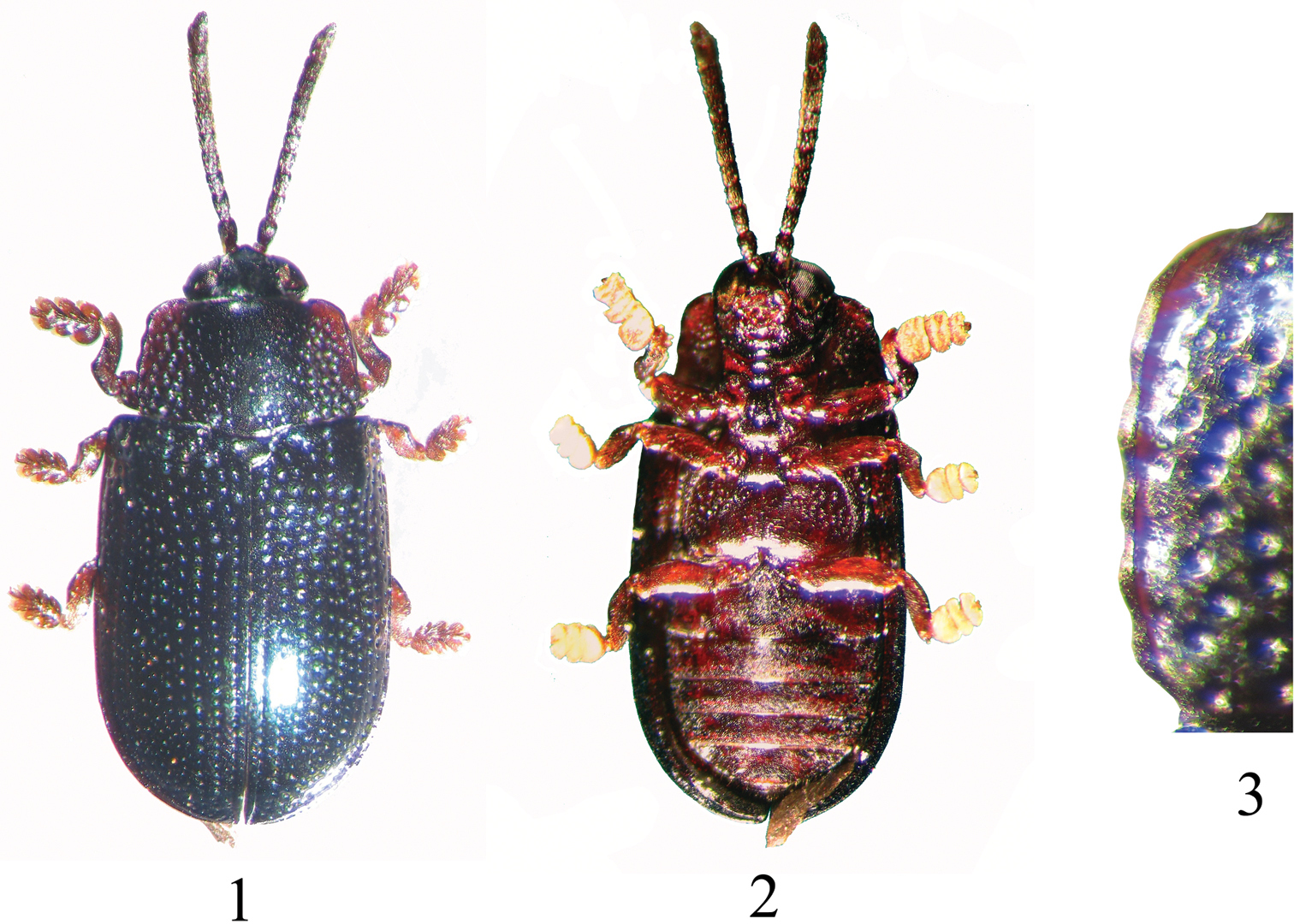
Figs 1–14Length 3.36–4.32 mm, width 1.73–2.35 mm. Vertex metallic black with blue tint; frontoclypeus black; gena, gula piceous to dark rufous brown; mouth parts dark rufous brown with labrum distinctly darker. Antenna piceous to dark rufous brown, often with proximal antennomeres darker than distal. Pronotal disc metallic black with blue tint, and turning rufous brown towards lateral margin in many specimens. Scutellum metallic black with blue tint. Elytra entirely dark metallic blue (Fig. 1). Venter and legs entirely dark rufous brown (Fig. 2).
Vertex minutely punctate, surface finely reticulate; midfrontal sulcus absent. Midcranial sulcus present as shallow indistinct groove evident anteriorly and posteriorly. Post-callinal transverse depression deeply impressed. Last maxillary palpomere as long as or longer than preceding two combined. Scape a little longer than half of pedicel; length ratio of antennomeres 2–11 equals 1.00 : 0.93-1.07 : 0.73-0.78 : 0.73-0.78 : 0.60-0.71 : 0.67-0.71 : 0.64-0.67 : 0.67-0.78 : 0.71-0.73 : 1.35-1.50.
Pronotum 1.53–1.67 times wider than long; posteriorly 1.14–1.20 times wider than anteriorly. Disc distinctly raised along middle 1/3, with transverse depression near posterior margin in front of scutellum. Disc impunctate anteriorly in middle as well as along a narrow mid-line; rest of raised middle portion with scattered small and minute punctures. Disc on either side of raised middle area moderately depressed with deep, large, circular punctures; distance between punctures less than diameter of individual puncture. Lateral margin anteriorly as broad as posteriorly, prominently scalloped with four to six emarginations (Fig. 3). Scutellum broader than long, convex on top, very minutely punctate and reticulate.
Elytron with shallow post-basal transverse depression deeper laterally; elytral apex convex. Scutellar row short, with three to five punctures; additional sixth row of punctures arises from middle of elytron, forming eleven rows of punctures, excluding scutellar row, present just behind middle. Distance between adjacent rows more than diameter of one puncture in middle of disc; distance between adjacent punctures in one row variable. Interstices flat, extremely minutely punctate. Punctures large, deep, round to oval. Hypomeron of pronotum with large, shallow punctures, punctures absent towards lateral margin, denser towards tergosternal suture. Prosternal intercoxal process channelled near margins on four sides, convexly raised along middle on top. Metasternum with granulate area bearing number of large, round, deep punctures anteriorly on either side, posterior to mesocoxal cavity.
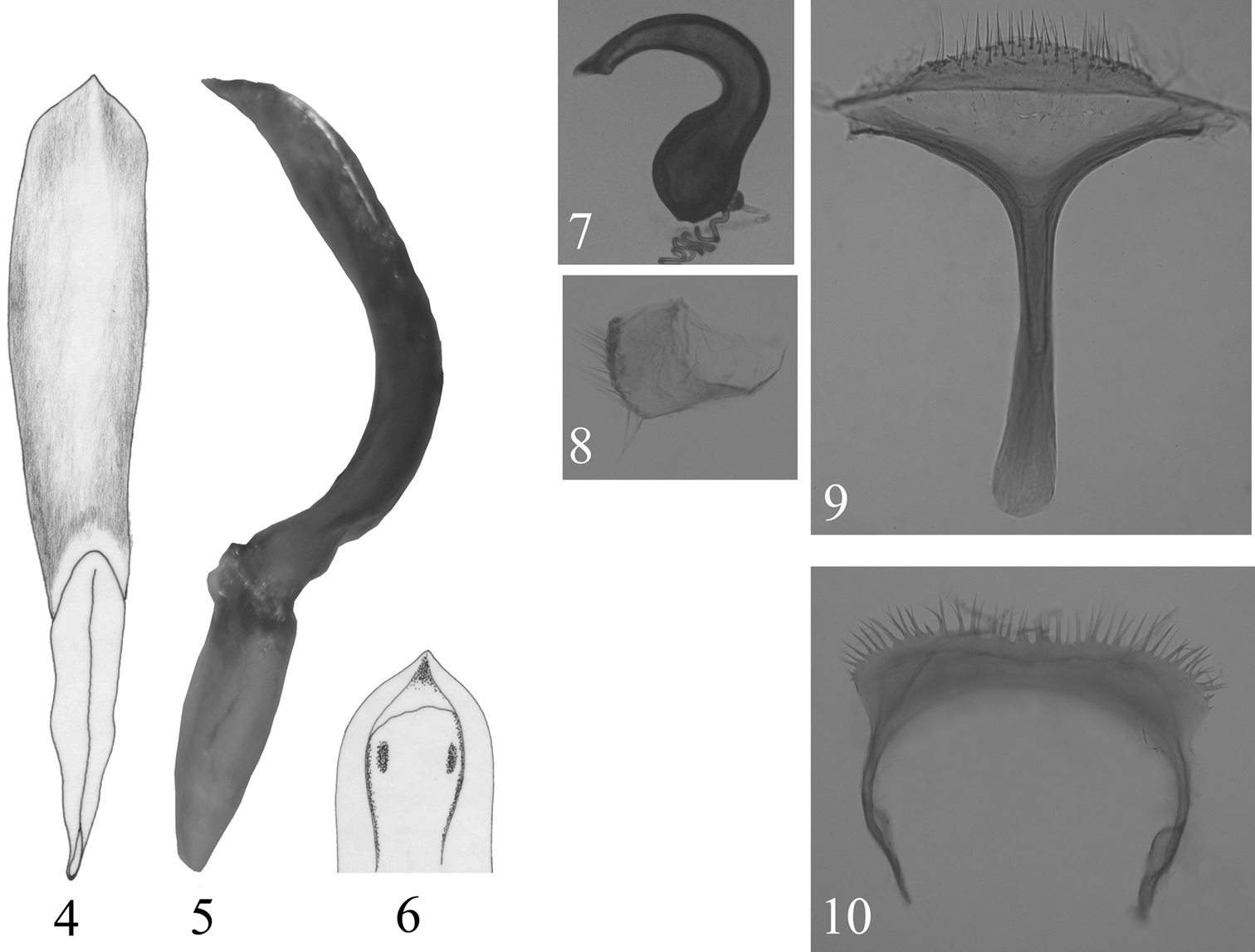
Aedeagus with basal piece poorly sclerotized. In lateral view, strongly curved near middle, apical 1/3 almost straight, apex acutely pointed (Fig. 5). Ventral side convex, with a sharply raised ridge along middle of distal region, with shallow depression on either side of ridge (Fig. 4). Apical foramen partially covered with a lamina bearing sclerotized plate on either side (Fig. 6). Arms of tegmen subequal to stem.
Receptacle of spermatheca longer than wide with inner side strongly convex, outer side gently concave; pump strongly curved, not differentiated from receptacle, about twice as long as receptacle, apical appendix acute, well developed; duct and gland inserted separately (Fig. 7). Sternite VIII with convex distal margin bearing numerous setae (Fig. 9). Spiculum gastrale anteriorly as wide as posteriorly. Coxite broader than long with setae along posterior margin (Fig. 8). Tergum IX represented by horseshoe shaped single sclerite with bristles along posterior margin (Fig. 10).
No apparent sexual dimorphism, except for slightly larger females (3.69–4.32 mm) compared to males (3.36–3.79 mm).
Callispa keram sp. n. 1 dorsal habitus 2 ventral habitus 3 lateral margin of pronotum.
Callispa keram sp. n. 4 median lobe of aedeagus, ventral view 5 median lobe of aedeagus, lateral view 6 median lobe of aedeagus, distal opening 7 spermatheca 8 coxite 9 sternite VIII 10 tergum IX.
The specific epithet keram, literally means coconut in Malayalam, the language of Kerala, the southern Indian state where the insect occurs. It refers to the host plant as well as the type locality, Keralam, the land of coconut.
Holotype ♂, with labels as follows: 1) India: Kerala / Vellayani / 08°25'47.5"N, 76°59'8.3"E/ 9.ii.2012 18 m / Shameem K. Coll. / Ex Coconut (white label). 2) HOLOTYPE / Callispa keram sp. n. / des. Shameem & Prathapan, 2012 (red label) (BMNH).
Paratypes (99 specimens, all specimens with a white locality label as given below, besides a second pink label: PARATYPE / Callispa keram sp. n. / des. Shameem & Prathapan, 2012): 5 unsexed. the same labels as for holotype; 1 ♀, 1 unsexed. same data as for holotype except date 8.i.2012; 1 unsexed. same data except date 19.i.2012; 6 unsexed. same data except date 24.i.2012; 1 ♀. same data except date 27.i.2012; 8 unsexed. same data except date 2.ii.2012; 2 unsexed. same data except date 9.ii.2012; 4 unsexed. same data except date 16.ii.2012; 3 unsexed. same data except date 21.ii.2012; 1 ♀, 1 unsexed. same data except date 22.ii.2012; 8 unsexed. India: Kerala / Vellayani / 08°25'47.5"N, 76°59'8.3"E/ 12.xii.2011 18 m / Shameem K. Coll. / Ex Livistona; 1 ♀, 1 unsexed. same data except date 22.xii.2011; 2 ♂, 1 ♀, 1 unsexed. same data except date 2.ii.2012; 1 unsexed. same data except date 9.ii.2012; 1 unsexed. India: Kerala / Vellayani / 08°25'47.5"N, 76°59'8.3"E/ 9.i.2012 18 m / Shameem K. Coll. / Ex Syagrus; 1 ♀. same data except date 21.i.2012; 2 ♂, 1 ♀, 9 unsexed. India: Kerala / Vellayani / 08°25'47.5"N, 76°59'8.3"E/ 6.i.2012 18 m / Shameem K. Coll.; 3 ♂, 6 unsexed. same data except date 7.i.2012; 1 ♂, 1 ♀. same data except date 8.i.2012; 2 unsexed. same data except date 9.i.2012; 1 ♀, 1 unsexed. same data except date 11.i.2012; 2 ♂, 3 unsexed. same data except date 12.i.2012; 2 unsexed. same data except date 17.i.2012; 1 ♂. same data except date 19.i.2012; 1 unsexed. same data except date 24.i.2012; 1 unsexed . India: Kerala / Vallamkulam / 25.xii.2011 / Prathapan KD Coll. / Ex Coconut; 1 ♀. same data except locality Pandanad; 1 ♀, 1 unsexed. India: Kerala / Tirurangadi / 25.xii.2011 / Shameem K. Coll. / Ex Coconut; 2 unsexed. India: Kerala / Calicut University / 26.xii.2011 / Shameem K. Coll. / Ex Coconut; 1 unsexed. India: Kerala / Tirurangadi / 25.xii.2011 / Shameem K. Coll. / Ex Livistona; 1 unsexed. same data except date 6.ii.2012.
(4 specimens with the following labels, besides a pink label: PARATYPE / Callispa keram sp. n. / des. Shameem & Prathapan, 2012) 1 unsexed. 1) On Coconut / Vellayani / 26-8-56 / M.R.G.K.N. 2) 2. 3) Z.S.I. / Lot No. 47 / 1956; 1 unsexed. 1) 2. 2) Z.S.I. / Lot No. 47 / 1956; 1 unsexed. 1) 2. 2) Z.S.I. / Lot No. 47 / 1956. 3) Callispa sp / nr. minima / gestro / S.P. Shukla det ’57; 1 unsexed. 1) 5. 2) ? Callispa sp. / R. N. Mathur det. (10 BMNH, 5 MCSN, 10 NBAII, 48 NPC, 3 PKDC, 13 UASB, 10 USNM).
India (Kerala).
Callispa keram sp. n. can be differentiated from the other metallic black or blue species of Callispa in southern India, by the shape of the distinctly scalloped lateral margin of the pronotum (Fig. 3). Other southern Indian species with metallic black or blue dorsum, namely, Callispa coerulodorsata Maulik, Callispa minima Gestro and Callispa violaceicornis Pic have straight or evenly curved lateral pronotal margin. Callispa keram closely resembles Callispa minima in having shiny blue black dorsum and brown venter, besides being more or less similar in size. However, they can be separated based on the shape of the lateral margin of the pronotum as well as the finely rugose interstices on the basal portion of the elytron (elytral interstices are smooth in Callispa keram). In Callispa coerulodorsata, the ventral side is black and the scutellum bears three characteristic deep notches radiating from the centre, however, the ventral side in Callispa keram is rufous brown and the radiating notches on the scutellum are absent. The pronotum is strongly narrowed anteriorly in Callispa violaceicornis, while it is weakly narrowed towards front in the new species. Metallic blue-black species of southern Indian Callispa can be separated using the key given below.
Arecaceae: Cocos nucifera L., Livistona chinensis R.Br. (Chinese fan palm or fountain palm) and Syagrus romanzoffiana (Cham.) Glassman (Queen palm).
Callispa keram sp. n. 11 ootheca 12 larva 13 pupa 14 adult 15 adult feeding trough 16 larval feeding trough.
| 1 | Scutellum with three deep radiating notches from the centre: one to the apex and others to the basal angles; abdominal ventrites black | Callispa coerulodorsata Maulik |
| – | Scutellum without deep radiating notches; abdominal ventrites rufous brown to dark brown | 2 |
| 2(1) | Pronotum strongly narrowed anteriorly, posteriorly 1.5 times wider than anteriorly; length 5.0 mm | Callispa violaceicornis Pic |
| – | Pronotum weakly narrowed anteriorly, posteriorly 1.1–1.2 times wider than anteriorly; length 3.3–4.4 mm | 3 |
| 3(2) | Lateral pronotal margin prominently scalloped with four to six emarginations; elytral interstices smooth throughout | Callispa keram sp. n. |
| – | Lateral pronotal margin straight, not scalloped, without emarginations; elytral interstices rugose basally | Callispa minima Gestro |
We are indebted to A. K. Pradeep, University of Calicut, Reji Jacob, Central Plantation Crops Research Institute, Kayamkulam and V. B. Sreekumar, Kerala Forest Research Institute, Peechi for identification of Syagrus romanzoffiana. Identity of Livistona chinensis was confirmed by A. K. Pradeep. Images of the types of Callispa coerulodorsata, Callispa minima and Callispa violaceicornis were kindly provided by Drs M. Barclay, Natural History Museum, London, United Kingdom, R. Poggi, Museo Civico di Storia Naturale, Genova, Italy and A. Mantilleri, Muséum National d’Histoire Naturelle, Paris, France respectively. Drs. R. Ajith, J. Bezděk, C. S. Chaboo, C. A. M. Reid, M. K. Sindhu, C. L. Staines and R. Sujith provided essential literature. Critical reviews by Drs C. A. Viraktamath, J. Poorani, C. S. Chaboo, C. Nandakumar and C. L. Staines greatly improved the manuscript. Our work on leaf beetles is supported by the Kerala State Council for Science, Technology and Environment, Trivandrum as well as the Indian Council of Agricultural Research through the Network Project on Insect Biosystematics.