Citation: Cosín DJD, Novo M, Fernández R, Marchán DF, Gutiérrez M (2014) A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data. ZooKeys 399: 17–33. doi: 10.3897/zookeys.399.7273
The morphological and anatomical simplicity of soil dwelling animals, such as earthworms, has limited the establishment of a robust taxonomy making it sometimes subjective to authors’ criteria. Within this context, integrative approaches including molecular information are becoming more popular to solve the phylogenetic positioning of conflictive taxa. Here we present the description of a new lumbricid species from the region of Extremadura (Spain), Eiseniona gerardoi sp. n. The assignment to this genus is based on both a morphological and a phylogenetic study. The validity of the genus Eiseniona, one of the most controversial within Lumbricidae, is discussed. A synopsis of the differences between the type species and the west-European members of the genus is provided.
Earthworms, lumbricids, Eiseniona, species description
Earthworm fauna is still poorly known within vast areas of the Iberian Peninsula. The available data indicate the common presence of cosmopolitan species such as Aporrectodea trapezoides (Dugès, 1828) or Aporrectodea rosea (Savigny, 1826). In contrast, other species show more restricted distributions but are locally abundant (
An intensive earthworm sampling campaign was accomplished between 2009 and 2012 in the surroundings of Plasencia (North of Cáceres, Extremadura, Spain) within the European Project “BioBio, Biodiversity Indicators for European Farming Systems, Indicators for Biodiversity in Organic and Low Input Farming Systems”. The Spanish team within this project studied the potential use of soil fauna as bioindicators in dehesas (i.e., Mediterranean grazed open woodlands of Quercus ilex Linné and olive groves under different types of land management. Among the several thousands of earthworm specimens collected during this sampling campaign, nineteen individuals sampled close to El Bronco (Cáceres, Spain) are of special taxonomical interest as they represent a new species as described in the present study.
The taxonomical assignment to a genus level in earthworm lumbricid taxonomy is confusing and varies regarding the criteria used by the different authors. In addition, it lacks robustness because it is not necessarily based on phylogenetic relationships. The number of genera proposed for the family Lumbricidae varies from five when reviewed by
Soil dwelling animals are subject to a series of limitations in their corporal design. This is reflected in earthworms that present a very simple body externally without many differential morphological characters. The position of clitellum and tubercula pubertatis, type of prostomium, pigmentation, chaetal arrangement, number and position of spermathecae, seminal vesicles, Morren’s glands, nephridia or typhosole are some of the most widely-used morphological characters in earthworm systematics. Nevertheless, these characters may probably have evolved as adaptations to a particular soil environment or independently in several phylogenetic lineages, therefore hindering establishment of a robust taxonomical system based on morphology. The solution to this taxonomical chaos would be the phylogenetic resolution of earthworms based on molecular and morphological studies. This would allow the generation of stable and robust phylogenies in which systematic classifications are properly defined. Unlike earthworms from the family Hormogastridae (e.g.,
In the context of this controversial classification of genera in lumbricid earthworms, one of the most conflictive ones is Eiseniona (Omodeo, 1956). This genus was established by
Despite the extended use of morphological and anatomical characters in earthworm taxonomy, during the last years the concept of integrative taxonomy as a tool to describe and delimit species has become more popular. This concept, consisting of a multidisciplinary approach including the morphological, molecular, ecological and geographical available data, has been applied to earthworms (e.g.,
In this context, this manuscript aims to describe a new lumbricid species (Eiseniona gerardoi sp. n.) based on morphological, molecular and ecological data.
Nineteen individuals were collected at four different but geographically-close sampling points nearby El Bronco (Cáceres, Extremadura, Spain). Soil was a sandy-loam on underlying slate (Figure 1); collectors G. Moreno, E. Juárez.
Map showing the position of sampling points.
D4 Le1: 2 ex. (1 adult, 1 subadult) (40°12'42.76"N, 6°19'0.68"W). Altitude 430 m. Grazed dehesa with Quercus ilex. Mean precipitation 876 mm. Present plant species: Eleocharis palustris, Pulicaria paludosa. Other earthworm species: Allolobophora molleri 1 ex. (0.75 g).
D4 R2: 2 ex. (2 adults) (40°12'45.22"N, 6°18'39.22"W). Altitude 414 m. Grazed dehesa with Quercus ilex. Mean precipitation 876 mm. Present plant species: Anthoxanthum aristatum, Isoetes hystris. Other earthworm species: Allolobophora molleri 8 ex. (6.72 g), Aporrectodea trapezoides 16 ex. (4.96 g).
D4 S1: 2 ex. (2 subadults) (40°12'41.51"N, 6°19'1.20"W). Altitude 430 m. Grazed dehesa with Quercus ilex. Mean precipitation 879 mm. Present plant species: Festuca ampla, Trifolium dubium. Other earthworm species: Allolobophora molleri 2 ex. (2.02 g), Aporrectodea trapezoides 3 ex. (2.01 g).
D5 P2: 13 ex. (5 adults, 8 subadults) (40°13'38.80"N, 6°18'36.04"W). Altitude 428 m. Grazed dehesa with Quercus ilex. Mean precipitation 923 mm. Present plant species: Juncus bufonius, Conyza sp. Other earthworm species: Allolobophora molleri 6 ex. (5, 32 g), Aporrectodea rosea 4 ex (1.05 g), Aporrectodea trapezoides 32 ex (18, 91 g).
The following molecular regions were amplified by the methods described in
In order to have an evaluation of the selection of species to include in the molecular analyses, M. Pérez-Losada and J. Domínguez (Universidad de Vigo) kindly compared the sequences of 16S and 28S rRNA from the specimens included in this study with an unpublished database that includes most lumbricid genera. This comparison provided the first evidence indicating that the new species was closely related to Eiseniona albolineata and Eiseniona oliveirae. As a second method, we collected some individuals belonging to Eiseniona albolineata and sequenced the mitochondrial gene COI. In addition, we retrieved from GenBank all available COI sequences from as many different lumbricid species as possible to date (Table 2), although many of these have their identities unconfirmed. We excluded from the analyses the sequenced genes in the public databases for which information is scarce and biased. Bayesian phylogenetic inference was then explored with the COI sequences as described in
Uncorrected pairwise differences were calculated between these species with Arlequin 3.5 (
The data underpinning the analysis reported in this paper are deposited in the Dryad Data Repository at https://doi.org/10.5061/dryad.5k76c
The specimen with voucher number UCMLT 60000 is the designated holotype. The paratypes bear the numbers UCMLT 60001 to 60018.
The specimens were sketched using an Olympus binocular microscope with digital camera, dissected, and described.
Type-species. Allolobophora handlirschi Rosa, 1897 by original designation.
Holotype. Adult (Catalog # UCMLT 60000), 40°13'38.80"N, 6°18'36.04"W (“spanish dehesa” mediterranean grazed open woodlands of Quercus ilex), near El Bronco (Cáceres, Spain), leg. G. Moreno, E. Juárez, April 2010.
Paratypes. 18 specimens (Catalog # UCMLT 60001 to 60018), leg G. Moreno, E. Juárez, April 2010.
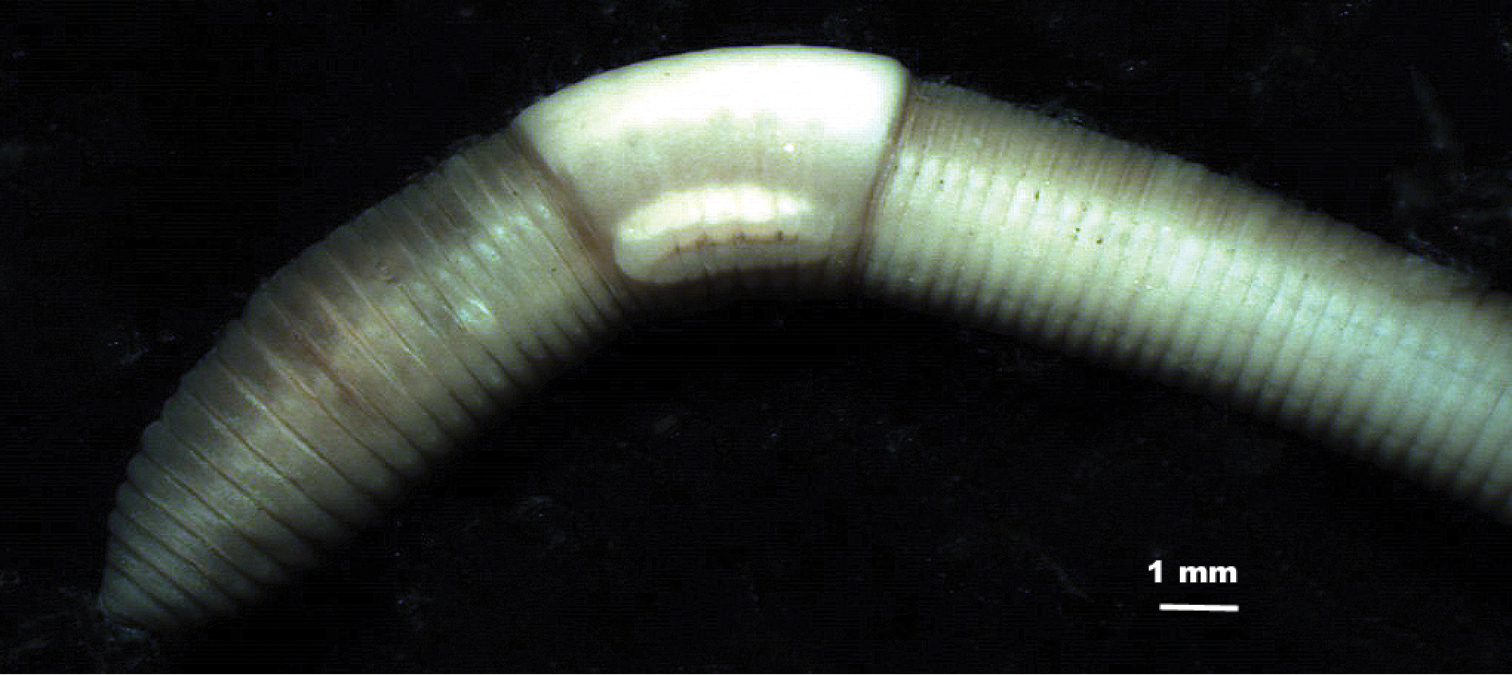
External morphology (Figures 2, 3). Length of mature specimens: 21–40 mm, x: 28 mm, SD: 5.6 mm, holotype: 31 mm. Diameter: clitellar x: 2.5 mm, SD: 0.4 mm, holotype: 2.5 mm, postclitellar x: 1.8 mm, SD: 0.2 mm, holotype: 1.7 mm. Body cylindrical in the anterior part, wider at clitellum and trapezoidal or rectangular in postclitellar region, with chaetae in the corners. Number of segments: 89 to 124, x: 109.5, SD: 10.7, holotype: 117. Weight (fixed specimens): 38 to 64 mg, x: 52 mg, SD: 13 mg, holotype 62 mg.
External view of the anterior part of the body of Eiseniona gerardoi.
Colour: When alive, the anterior part is red-brownish showing noticeable antero-posterior and dorso-ventral gradients. Cream-coloured or whitish clitellum. After a long period within alcohol the red pigment is gradually lost and transformed into brown of different intensities (Figure 2).
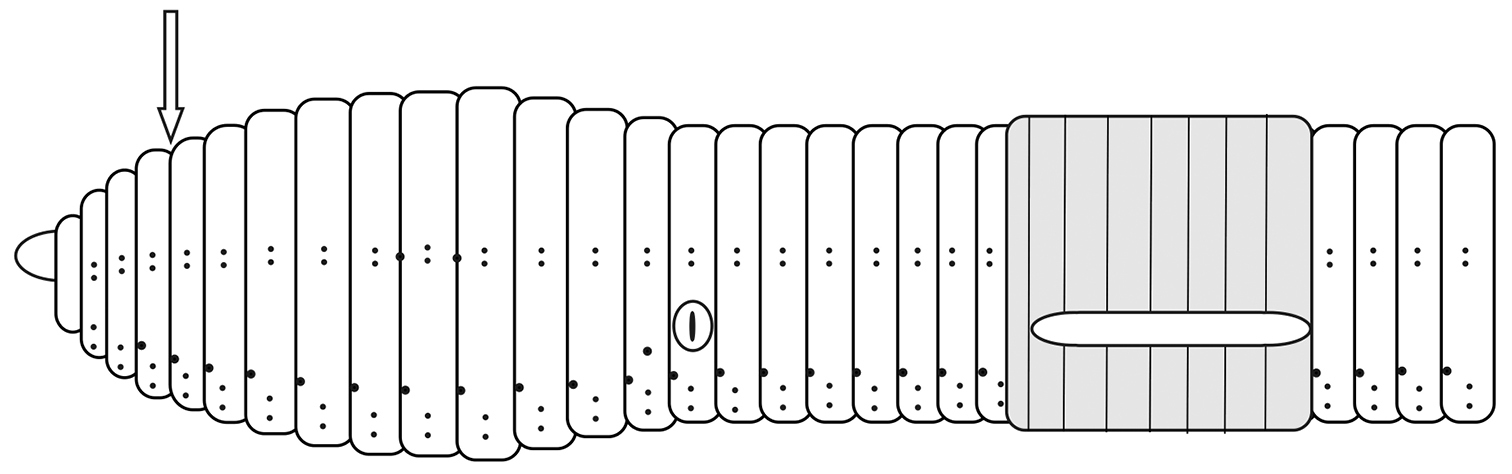
Prostomium epilobic ±1/3. No longitudinal lines are noticeable in segments 1 and 2. First dorsal pore in (3/4) 4/5. Nephridial pores inconspicuous in a row slightly above b. Spermathecal pores at intersegments 9/10 and 10/11, at the level of chaetae cd (Figure 3).
Schematic view of the external morphology of Eiseniona gerardoi.
Male pores as vertical grooves in the segment 15 between chaetae b and c showing small porophores with whitish areolae shape. Female pores in 14 slightly above b. Chaetae paired, interchaetal ratio at segment 40, aa: 16, ab: 1.4, bc: 7, cd: 1, dd: 24. Chaetae are simple with a wider base and a sharp and bent distal end. (Figure 4).
Chaetae ab from segment 40–41 (DIC Nomarski).
Clitellum white or cream-coloured, saddle-shaped extending over 22, 23–29, 30, in the holotype 1/n 22, 23–29. When well developed it invades the ventral area and the intersegmental lines are hard to distinguish. Tubercula pubertatis extended as a belt in 23-(27)28, 29, in the holotype in 23–29. Occasionally they appear folded or wrinkled. No noticeable papillae are present in any of the specimens.
Internal anatomy. Slightly thickened anterior septa. Last pair of oesophageal hearts in 11. Morren’s glands with small diverticula in 10 and little lamellae in 11 and 12. Crop in 15, 16, gizzard in (17)18, 19. First section of the intestine is not dilated. Simple typhlosole pleated, which begins in 20, 21 and ends near the anus leaving only 10–15 atyphlosolate segments.
Fraying testes and iridescent and very large seminal funnels in 10 and 11. Three pairs of seminal vesicles in 9, 11 and 12. The last pair is very large pushing back the septum 12/13. Large ovaries and female funnels in 13, ovarian receptacles (ovisacs) in 14. Two pairs of very large and iridescent spermathecae in segments 10 and 11.
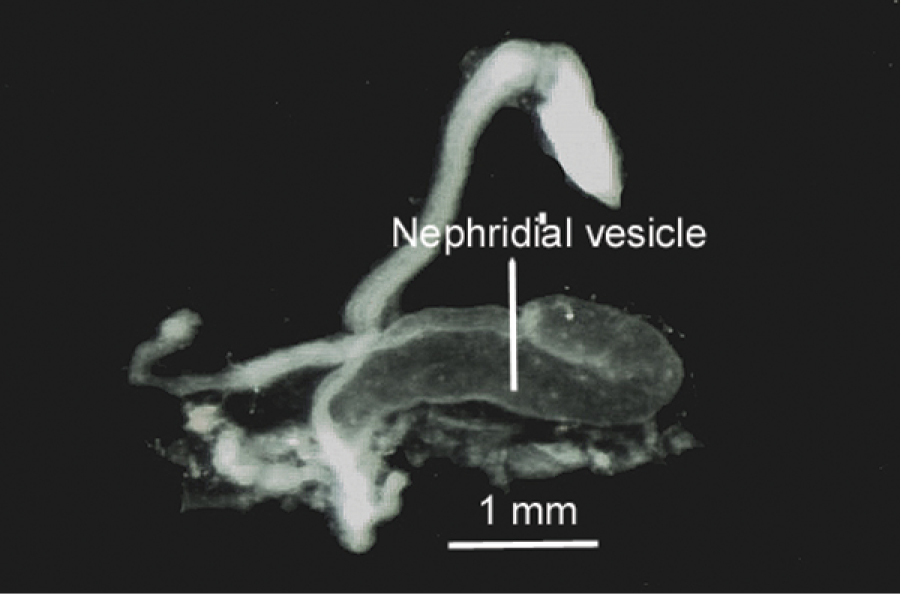
In the posterior region of the body the nephridia are much enlarged, the nephridial bladders are curved and J-shaped with curved section 1/3 of total length. (Figure 5).
Posterior nephridium isolated by dissection, showing the nephridial curved bladder.
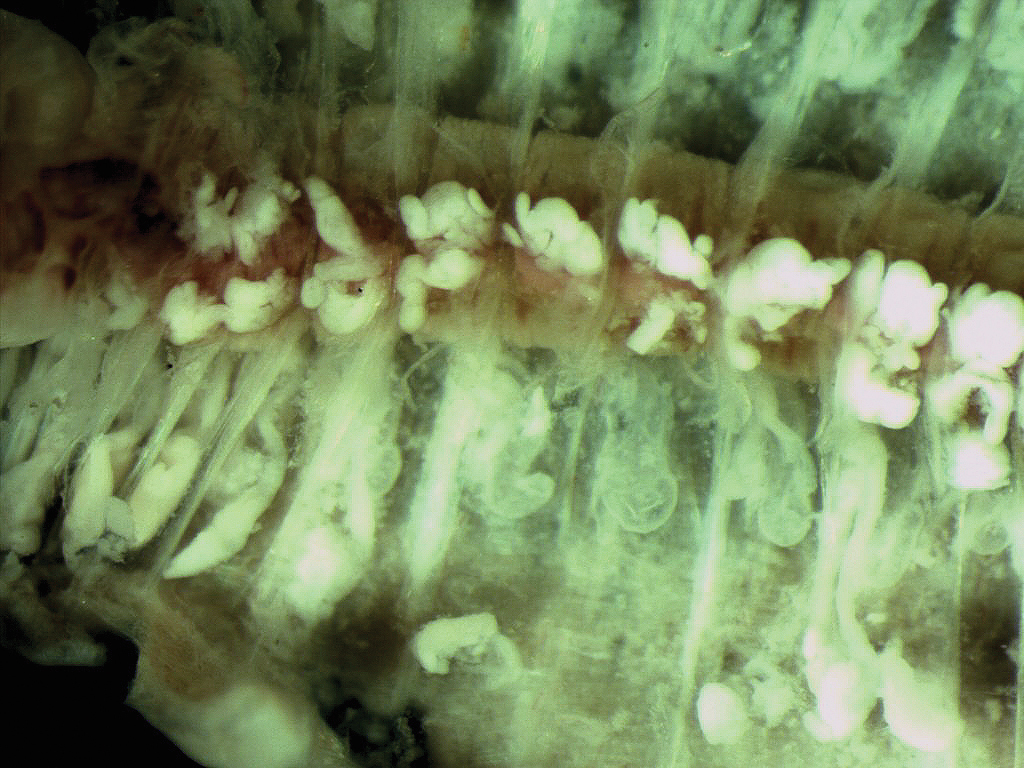
An important characteristic is the presence of dense white glands on top of the dorsal vessel initially around segment 20 and externally visible as a whitish line extending to the end of the body. (Figure 6).
White tissue associated with the dorsal vessel.
Known only from its type locality.
The species is dedicated to Prof. Gerardo Moreno from Centro Universitario de Plasencia, Universidad de Extremadura, Spain. He is the PI for the Bio-Bio program in Spain and collected the specimens described in this paper.
Sequences of the used genes have been deposited in GenBank (see Table 1). According to Drs. Pérez Losada and Domínguez (pers. comm.), the 16S and 28S sequences of Eiseniona gerardoi clustered with the two species classified as Eiseniona, Eiseniona albolineata and Eiseniona oliveirae.
Paragenetypes (sensu
| Voucher | COI | 16S-tRNAs | 18S rRNA | 28S rRNA | H3 | H4 |
|---|---|---|---|---|---|---|
| UCMLT 60001 | KF737142 | KF737134 | KF737140 | KF737148 | KF737150 | HG780373 |
| UCMLT 60002 | KF737143 | KF737135 | KF737141 | KF737149 | KF737151 | HG780374 |
| UCMLT 60007 | KF737144 | KF737136 | KF737152 | HG780375 | ||
| UCMLT 60013 | KF737145 | KF737137 | ||||
| UCMLT 60015 | KF737146 | KF737138 | ||||
| UCMLT 60017 | KF737147 | KF737139 |
Taxa and specimens included in the molecular analysis. GenBank accession numbers are indicated. Species names were literally taken from GenBank. The correct name [after
| Species | COI GeneBank accession number |
|---|---|
| Allolobophora chlorotica | GU013806 |
| Aporrectodea longa | JN850544 |
| Aporrectodea rosea | FJ214232 |
| Aporrectodea trapezoides | JF313567 |
| Aporrectodea tuberculata | JN869877 |
| *Bimastus parvus | EF077605 |
| Dendrobaena attemsi | FJ214224 |
| Dendrobaena octaedra | GU013836 |
| Dendrobaena veneta | FJ214233 |
| Dendrodrilus rubidus | GU013839 |
| Eisenia andrei | DQ914619 |
| *Eisenia eiseni | AY874488 |
| Eisenia fetida | EF077596 |
| *Eiseniona albolineata | KF746384 |
| Helodrilus oculatus | FJ374775 |
| Hormogaster elisae | EF653905 |
| Lumbricus festivus | FJ937290 |
| Lumbricus rubellus | GU206189 |
| Lumbricus terrestris | JN869936 |
| Octodrilus juvyi | HE611693 |
| Octolasion cyaneum | JQ909144 |
| Octolasion lacteum | DQ092909 |
The phylogenetic tree presented here, based on the COI gene and including some of the available species in GenBank (Figure 7), shows that Eiseniona gerardoi specimens form a highly supported group (1.00 posterior probability, 0.99 bootstrap) with Eiseniona albolineata. The two species share the presence of whitish glands on top of the dorsal vessel. COI genetic divergence (uncorrected p-distances) between Eiseniona albolineata and Eiseniona gerardoi is 14.09%, and the intraspecific variability of the latter is 2.81% showing a very close relationship.
Bayesian inference tree based on COI sequences of Eiseniona gerardoi and other lumbricids represented in GeneBank. Eiseniona gerardoi (see UCMLT codes in Table 1) clusters with Eiseniona albolineata.
All the soils from sampling sites have been developed on slates and are sandy-loams. Precipitation corresponds to the typical values of intermediate semi-humid Spain. The associated species Allolobophora molleri is always present and this species is bound to terrains that are flooded during several months per year. Additionally, the presence of plants typical from wetlands, such as Eleocharis palustris, Pulicaria paludosa or Juncus bufonius indicates that in these sites there is enough humidity during most of the year, which supports hygrophile communities. Nevertheless they could be desiccated in the summer, which would force the earthworms to undergo aestivation in order to survive to these dry periods, resuming activity when humidity is restored. All these details are compatible with the diagnosis of the genus by
Eiseniona genus was created by
The species originally included in this genus were Eiseniona handlirschi (Rosa, 1897) [the designated type, now placed in Aporrectodea according to
Phylogenies recovered by molecular methods can aid to solve this problem by providing key information to support systematics and therefore approaching a natural system (
Some of the features of our specimens, such as the lack of papillae or the presence of porophores in segment 15, are different from the ones described for most Eiseniona. However male porophores of Eiseniona gerardoi are relatively small and
Considering all this data, we opt to include this new species, at least provisionally, within Eiseniona because it is the less troublesome position within the current genera system for Lumbricidae. This is suggested not only by morphological and ecological considerations but also by the molecular data placing it near Eiseniona albolineata and Eiseniona oliveirae.
The phylogeny of species historically included within Iberoscolex, Koinodrilus and Eiseniona will need to be thoroughly revised in the future, in order to clarify whether they represent good genera and to find a robust grouping of the species within genera, which does not seem possible exclusively with morphological tools. It is also noteworthy that within Eiseniona there is a group of species from Southern France and Iberian Peninsula and another one from Italy, Greece and Central and Eastern Europe. Future studies will unravel whether these two groups constitute independent phylogenetic units susceptible to be taxonomically divided.
A considerable effort is still necessary to establish a robust genera system based on phylogeny within lumbricids. This system should integrate the study of mitochondrial and nuclear markers with morphological characters and include representatives from all the proposed genera and type species. Until the moment when such big picture is available controversy on lumbricids’ genera system will continue and different authors will apply subjective criteria.
The most similar species to Eiseniona gerardoi regarding clitellum position and tubercula pubertatis is Eiseniona intermedia, but the last has a much greater size, its tubercula pubertatis start in a more posterior segment and presents four pairs of seminal vesicles. In addition, it was only found in Bashkiria (Bashkortostan, Russia) (data from
Comparison of species living in the western part of the geographic range of Eiseniona. The type species Eiseniona handlirschi is included and the hemiandric Eiseniona paradoxa and Eiseniona gavarnica are excluded.
| Eiseniona albolineata | Eiseniona carpetana | Eiseniona oliveirae | Eiseniona gerardoi | Eiseniona handlirschi | |
|---|---|---|---|---|---|
| Length (mm) | 78–122 matures | 52–74 | 85–110 * 30–86** 45*** |
21–40 | 50–60* 50–170** 50–95*** |
| Segments | 138–172 | 129–150 | 167 * (77) 100–131** 125*** |
89–124 | 120–130* 115–163** 78–119*** |
| Colour | Grey, posterior white line | Rose violet | Light flesh tone* Brown or violet “in vivo”, greyish when fixed** Brown, red*** |
Red-brownish “in vivo”, posterior white line | Colourless* Colourless** Pale reddish*** |
| Chetae | Separate 2.5 - 1.2 - 2.2 - 1 - 5 |
Separate | Closely paired* 6.7 – 1.3 – 6.2 – 1 – 11.8** Closely paired 9 – 1.5 – 7.5 – 1 - 18*** |
Paired 16 - 1.4 - 7 - 1 - 24 |
Closely paired 8 – 1.15 – 6 – 1 – 20*** |
| First dorsal pore | (4/5) 5/6 | 4/5 | 4/5* (4/5) 5/6** 5/6*** |
(3/4) 4/5 | From 4/5, usually 19/20** 17/18 to 23/24*** |
| Spermathecae | 10, 11, pores 9/10, 10/11 near d | 10, 11, pores 9/10, 10/11 c | 10, 11, pores 9/10 10/11 near c | 10, 11, large, iridescent, pores 9/10, 10/11 cd | 9, 10, pores in 9/10 10/11 |
| Clitellum | (24)25 – 30(31) | Annular in (1/2 24)25 -1/2 31(31) | 24–30* (23)24–29(30)** 24–29*** |
22, 23–29, 30 | 26–33* (25, 26)27–32(33)** 25, 26(27)-(32)33*** |
| T. pubertatis | 1/n 26 – 28(1/2 29) | 25–30 | 24–30* 24–29, 30** 1/2 25–28*** |
23-(27)28, 29 | 29–32* (1/2 27, 28)29–30 (31, 32)** 1/n 28–31, 32*** |
| Gld. Morren | 10–12, diverticula in 10 | 11- 12, no diverticula | 10–13 diverticula in 10** 11–14, no diverticula*** |
10, 11, 12 small diverticula in 10 | Diverticula in 10 10–13*** |
| Nephridial vesicle | S - shaped | ? | Curved, reclined*** | J - shaped | Inverted J*** |
| Typhlosole | Bifid initially, later simple | ? | Simple | Simple, pleated | Circular, transversally pleated*** |
| Seminal vesicles | 9, 10, 11, 12 | 9, 10, 11, 12 | 9, 11, 12* 9, 10, 11, 12** 9, 11, 12*** |
9, 11, 12 | 9, 11, 12* 9, (10), 11, 12** 9, 11, 12*** |
| Others | White tissue on top of the dorsal vessel. | * ** Díaz Cosín et al. (1985) *** |
White tissue on top of the dorsal vessel | * ** *** |
Genetic divergence between Eiseniona gerardoi and Eiseniona albolineata (COI, uncorrected distances) is 14.09%, which is within the interval of uncertainty proposed by