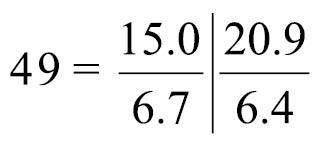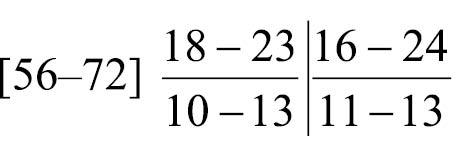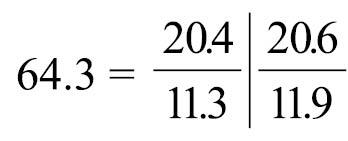Citation: Hirose M, Fukiage R, Katoh T, Kajihara H (2014) Description and molecular phylogeny of a new species of Phoronis (Phoronida) from Japan, with a redescription of topotypes of P. ijimai Oka, 1897. ZooKeys 398: 1–31. doi: 10.3897/zookeys.398.5176
We describe Phoronis emigi sp. n. as the eighth member of the genus based on specimens collected from a sandy bottom at 33.2 m depth in Tomioka Bay, Amakusa, Japan. The new species is morphologically similar to P. psammophila Cori, 1889, but can be distinguished from the latter by the number of longitudinal muscle bundles in the body wall (56–72 vs. 25–50 in P. psammophila) and the position of the nephridiopores (situated level with the anus vs. lower than the anus in P. psammophila). Using sequences of the nuclear 18S and 28S rRNA genes and the mitochondrial cytochrome c oxidase subunit I (COI) gene, we inferred the relationship of P. emigi to other phoronids by the maximum likelihood method and Bayesian analysis. The analyses showed that P. emigi is closely related to P. hippocrepia Wright, 1856 and P. psammophila Cori, 1889. We describe the morphology of the topotypes and additional material for P. ijimai Oka, 1897. Neither our morphological observations of P. ijimai, nor the phylogenetic analyses based on 18S and COI sequences, contradicts that P. vancouverensis Pixell, 1912 is conspecific with P. ijimai, a synonymy that has long been disputed.
Lophophorata, 3D reconstruction, cladistic analyses, Japan, Misaki, Kyushu
Phoronids, or horseshoe worms, are exclusively marine, sedentary, vermiform animals with a crown of ciliated tentacles, the lophophore, used in suspension feeding. They comprise the small phylum Phoronida, which currently contains two genera, Phoronis Wright, 1856 and Phoronopsis Gilchrist, 1907, with seven and three species, respectively (
For over the last half century, no new species of phoronids have been established, although the current species diversity is likely to have been underestimated (
One of the unsettled taxonomic issues in phoronid systematics is whether or not Phoronis ijimai Oka, 1897 (type locality: Misaki, Japan) is conspecific with Phoronis vancouverensis Pixell, 1912 (type locality: Vancouver, Canada).
In this paper, we 1) describe a new phoronid species from Japan, which differs from all the previously known species in adult morphology; 2) reconstruct the phylogeny of representative phoronids, including the new species, based on DNA sequences of the nuclear 18S and 28S rRNA genes (hereafter, 18S and 28S, respectively), and the mitochondrial cytochrome c oxidase subunit I gene (COI); 3) describe topotypes of Phoronis ijimai from Misaki, Sagami Bay, and discuss the synonymy with Phoronis vancouverensis in the context of adult morphology and the molecular phylogeny; and 4) provide a key to the Japanese phoronid species.
A sediment sample was obtained with a Smith-McIntyre grab having an aperture of 25 cm × 25 cm, from a sandy bottom at 33.2 m depth (32°32'27"N, 130°03'17"E) in Tomioka Bay, Amakusa, Kumamoto, Japan (Fig. 1A, 1B) on 26 November 2009 by Keiichi Kakui, Hiroshi Yamasaki, and Shushi Abukawa on board the research and training vessel Seriola of the Amakusa Marine Biological Laboratory (AMBL), Kyushu University. The sediment was agitated and stirred in a bucket with seawater and the supernatant was decanted; specimens suspended in the supernatant were collected with a sieve having a 0.3-mm mesh size. Of the 560 specimens obtained, most were fixed in 10% formalin seawater, and the rest were placed directly in 99% EtOH.
Topotypes of Phoronis ijimai were collected in Moroiso Bay, from a pier (≈35°09'28"N, 139°36'44"E) in front of the Misaki Marine Biological Station (MMBS), The University of Tokyo, Kanagawa, Japan (Fig. 1C) on 10 May 2012 by Hisanori Koutsuka, and from a rocky shore (≈35°09'32"N, 139°36'40"E) beside Arai Beach, Sagami Bay, near MMBS on 7 May 2012 by Mayumi Masuda. Additional specimens of Phoronis ijimai were collected at Irukabana (≈34°13'42"N, 132°23'03"E), Etajima Island, Hiroshima, Japan (Fig. 1D) on 13 February 2011 by Daisuke Ueno.
Maps showing the locations of collecting sites. A Map of Japan showing the collecting localities and the locations of Lake Hamana and Akkeshi B enlargement of west-central Kyushu, with the solid circle indicating the collecting site at Amakusa C enlargement of the southwestern part of the Miura Peninsula, with solid circles indicating the topotype collecting sites (type localities) of Phoronis ijimai Oka, 1897 at Misaki, Sagami Bay D enlargement of Hiroshima Bay, with the solid circle indicating an additional collecting site for Phoronis ijimai at Etajima.
Measurements of the lophophore and body size were taken from digital photographs with ImageJ 1.37v software (
Total genomic DNA was extracted from one of the ethanol-fixed specimens of the new species, as well as one of the topotypes of Phoronis ijimai (NSMT-Te 881), using a DNeasy Blood and Tissue Kit (Qiagen), following the manufacturer’s protocol. The 18S gene was amplified with three primer sets: 1F/4R, 3F/18sbi, and 18Sa2.0/9R (
Taxa included in the phylogenetic analyses and GenBank accession numbers for sequences. Sequences obtained in this study are in bold.
| Species | COI | 18S | 28S | Reference |
|---|---|---|---|---|
| Phoronis emigi sp. n. | AB621915 | AB621913 | AB621914 | this study |
| Phoronis architecta | AY368231.1 | AF025946 | EY334109 | a (COI), b (18S), c (28S) |
| Phoronis australis (New Caledonia) | EU484457 | AF202111 | EU334110 | c (COI, 28S), d (18S) |
| Phoronis australis (Japan) | EU484458 | EU334122 | EU334111 | c |
| Phoronis australis (Australia) | — | EU334123 | EU334112 | c |
| Phoronis australis (Spain) | — | AF119079 | — | e |
| Phoronis hippocrepia | EU484459 | AF202112 | AY839251 | c (COI), d (18S), f (28S) |
| Phoronis ijimai | AB752304 | AB752305 | — | this study |
| Phoronis muelleri | EU484460 | EU334125 | EU334114 | c |
| Phoronis ovalis | EU484461 | EU334126 | EU334115 | c |
| Phoronis pallida | — | EU334127 | EU334116 | c |
| Phoronis vancouverensis/ijimai | EU484462 | AF202113 | AF342797 | c (COI), d (18S), g (28S) |
| Phoronopsis californica | EU484463 | EU334129 | EU334118 | c |
| Phoronopsis harmeri | EU484464 | EU334130 | EU334119 | c |
| Phoronopsis viridis | EU484465 | AF123308 | EU334120 | c |
| Novocrania anomala | — | AY842018 | AY839245 | f |
| Discinisca cf. tenuis | — | AY842020 | AY839248 | f |
| Glottidia pyramidata | — | U12647 | AY839249 | f (28S), h (18S) |
a
From the literature (
We checked validity of the yielded COI sequences to prevent the isolation of nuclear encoded mitochondrial psuedogenes (NUMTS) instead of true mitochondrial sequences before phylogenetic analyses. We regarded the consistently yielded fine single peaks for all the analysed sites in chromatograms and including neither indel nor stop codon as the criteria for judging the safely rejection of the possibility for the contamination of NUMTS.
The COI, 18S, and 28S sequences obtained for the new species were aligned with those from other phoronids deposited in GenBank (Table 1) using Clustal W (
Maximum likelihood (ML) analyses was performed with MEGA 5.05. For ML, the best-fit model for all data sets determined by the AICc implemented in MEGA 5.1 was GTR+G+I (general time reversible [
Bayesian analyses were performed by using MrBayes 3.1.2 (
The 18S and 28S trees were rooted with three brachiopods (Novocrania anomala, Discinisca cf. tenuis, and Glottidia pyramidata) as outgroup taxa (
Since most of the sequences used in this study were obtained from GenBank, we used the original specific names in GenBank given by the previous authors (
http://species-id.net/wiki/Phoronis_ijimai
Figures 2–7Five series of transverse sections and 34 whole specimens. NSMT-Te 878, several specimens, fixed and preserved in 10% formalin, collected at Etajima Island; NSMT-Te 879, several individuals, fixed and preserved in 10% formalin, collected in Moroiso Bay, attached to the pier in front of MMBS; NSMT-Te 880, several individuals on a living shell of Barbatia sp. (Mollusca: Bivalvia), collected in Sagami Bay; NSMT-Te 881, same data as NSMT-Te 879; NSMT-Te 882, same data as NSMT-Te 880; NSMT-Te 883, 6-μm transverse section stained with HE, collected at Etajima Island; NSMT-Te 884, same data as NSMT-Te 883; NSMT-Te 885, 6-μm transverse sections stained with HE, collected in Moroiso Bay; NSMT-Te 886, same data as NSMT-Te 885; NSMT-Te 887, 6-μm transverse sections stained with HE, collected in Sagami Bay.
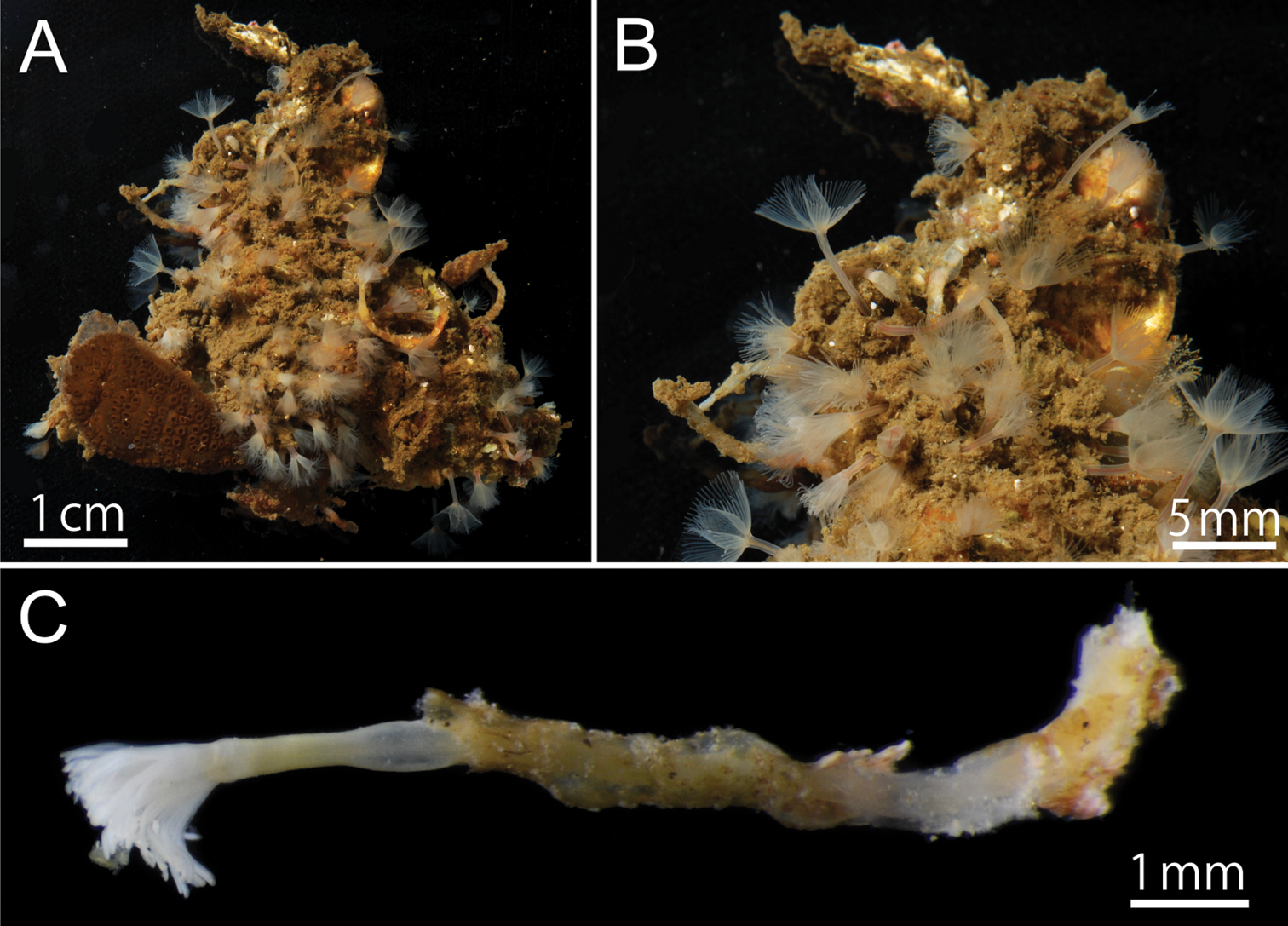
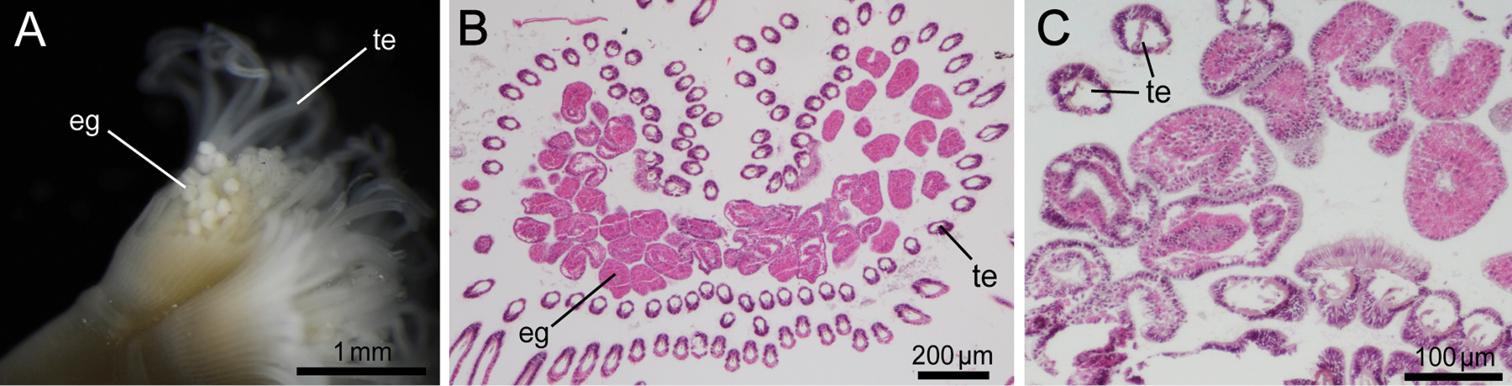
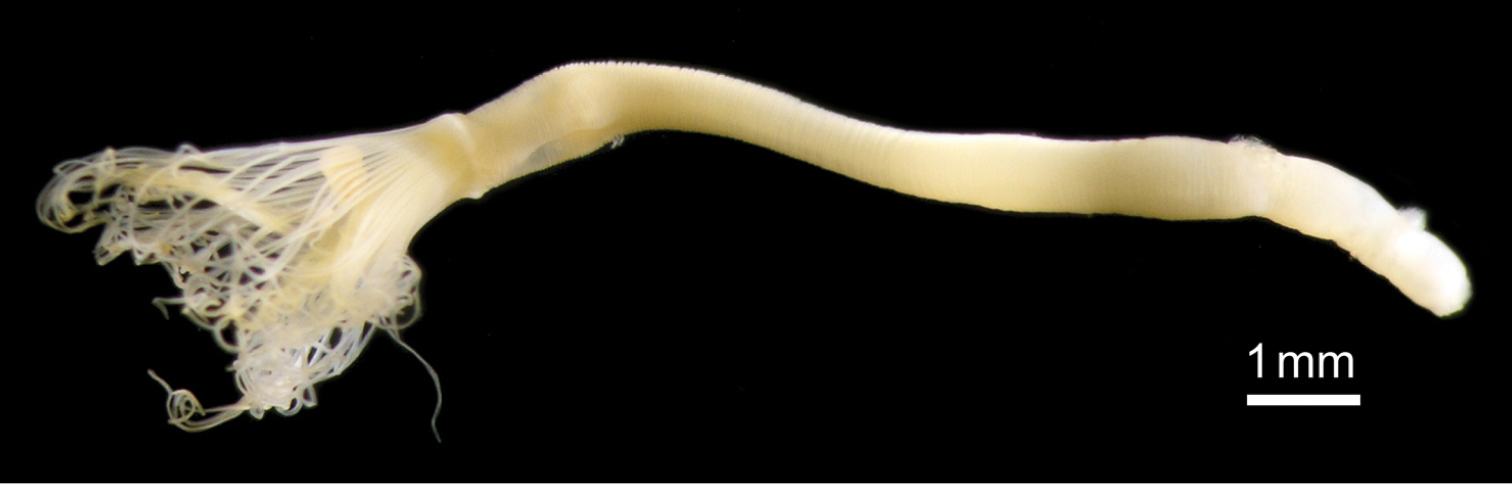
Body except lophophore 2.40–16.83 mm in length (avg. 5.87±4.04 mm, n = 34; average of topotypes 9.55±4.78 mm, n = 12); 0.49–0.90 mm in diameter at ampula (avg. 0.64±0.11 mm, n = 34; average of topotypes 0.59±0.12 mm, n = 12); white and translucent in living state (Figs 2A, 2B, 3), yellowish white after fixation (Fig. 2C). Lophophore horseshoe-shaped, without significant coiling (Fig. 4); 0.87–3.11 mm in length (avg. 2.17±0.55 mm, n = 34; average of topotypes 1.66±0.49 mm, n = 12), 0.27–0.99 mm in diameter at its base (avg. 0.61±0.17 mm, n = 34; avg. of topotypes 0.43±0.09 mm, n = 12); tentacles 106–151 in number (avg. 129±18, n = 7; avg. of topotypes 110±5, n = 3). Inhabits a transparent cylindrical tube either encrusting or burrowing in hard substrates (Fig. 2C).
Phoronis ijimai Oka, 1897, NSMT-Te 879. A Living individuals collected from the pier of Misaki Marine Biological Station B enlargement of living individuals C preserved individual (10% formalin seawater) with a transparent cylindrical tube.
Phoronis ijimai Oka, 1897, NSMT-Te 880. A Living bivalve (Barbatia sp.) with various sessile organisms B Living Phoronis ijimai on the shell (arrowheads).
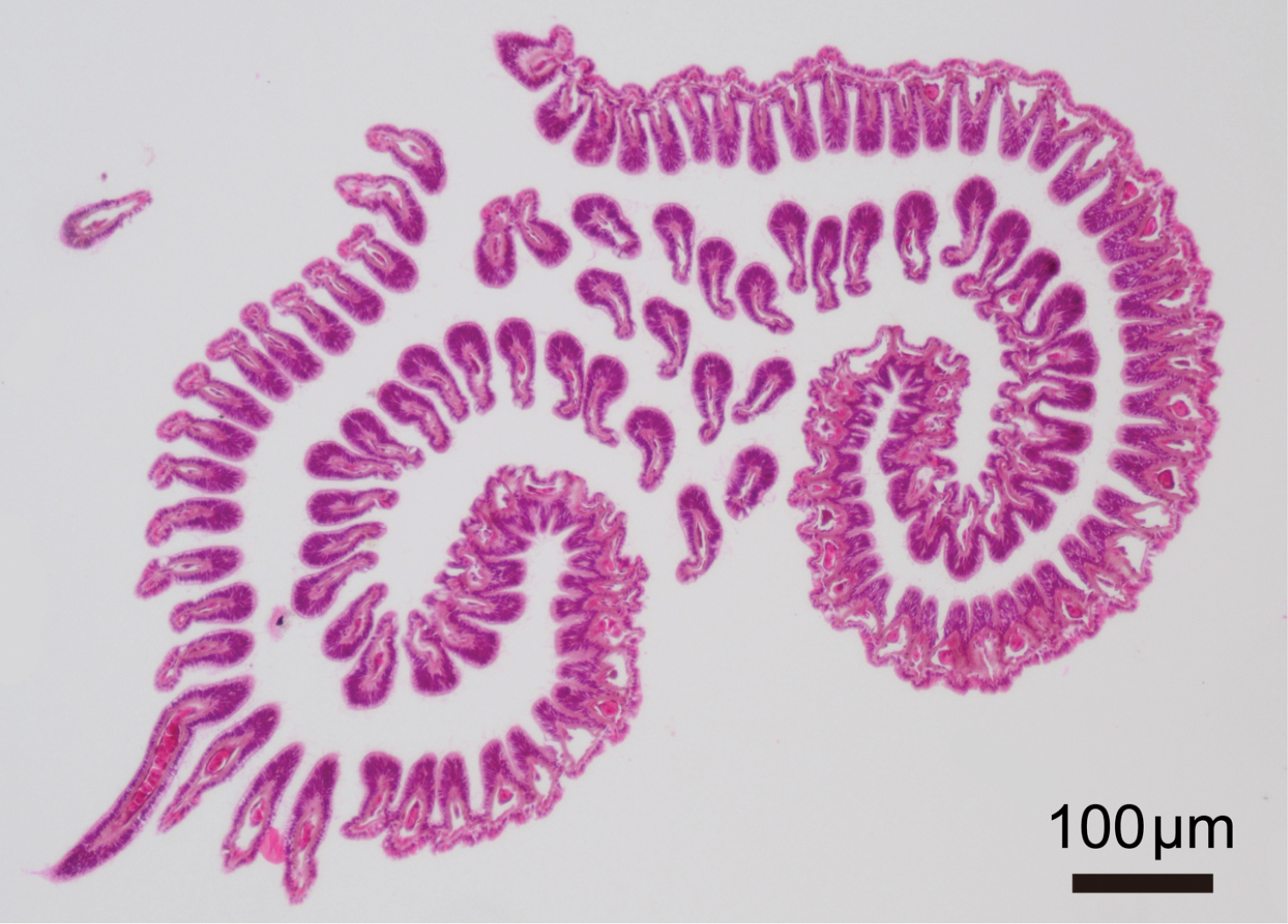
Phoronis ijimai Oka, 1897, NSMT-Te 885, transverse section through basal part of lophophore.
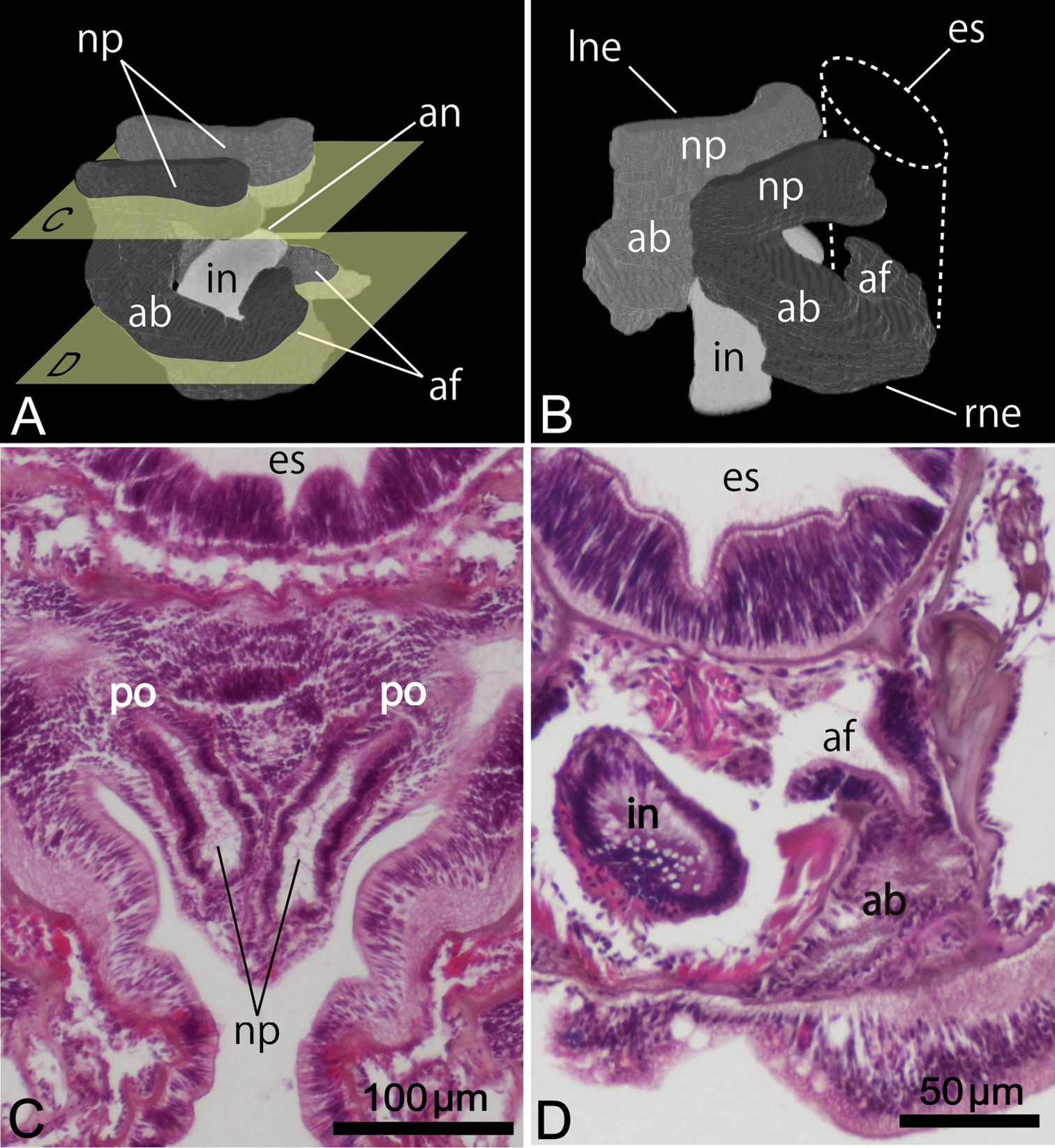
Nephridium 162.00–204.00 μm in height (avg. 183.00±29.70 μm, n = 2), with straight nephridial papilla and curved ascending branch (Fig. 5A, 5B). Descending branch absent. Ascending branch with single chamber. Nephridial papilla situated beside anus, 294.24–324.91 μm in length (avg. 309.57±21.69 μm, n = 2); nephridiopore situated on nephridial papilla opening above (in living orientation) anus level (Fig. 5A, 5C). Ascending branch offset along body axis near intestine, with its lower end extending toward esophagus (Fig. 5B, 5D); 277.55–323.49 μm in length (avg. 300.52±32.49 μm, n = 2). Two nephridial funnels present; anal funnel larger than oral funnel. Anal funnel large (avg. 69.00±4.24 μm in height, 45.77±3.15 μm in width at base, 111.94±16.48 μm in maximum width at tip; n = 2), its aperture located at lower end of ascending branch. Oral funnel small (avg. 20.01±1.40 μm in diameter, n = 2), its aperture opening on lateral surface of ascending branch, situated slightly lower than anal funnel.
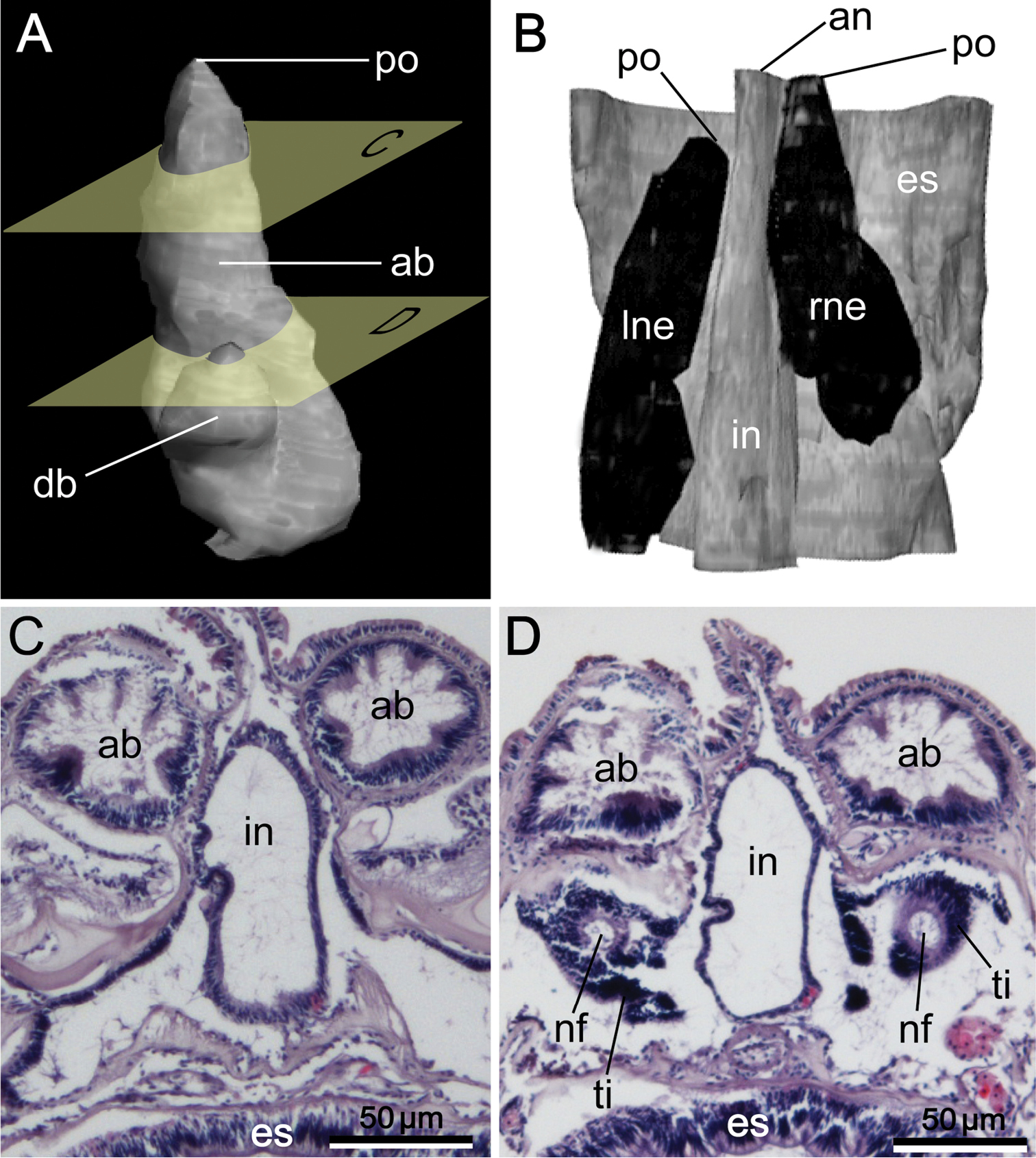
Reconstructed three-dimensional images and transverse sections of the nephridium of Phoronis ijimai Oka, 1897, from NSMT-Te 886 (A, B, D) and NSMT-Te 884 (C). A Lateral view, showing the long nephridial papillae above the anus B dorsolateral view, showing the offset arrangement of the nephridia, with the curved ascending branch and large anal funnel extending toward the esophagus C transverse section through the nephridial papilla, showing the nephridiopore D transverse section through the ascending branch, showing the large anal funnel opening toward the esophagus, Abbreviations: ab ascending branch; af anal funnel; an anus; es esophagus; in intestine; lne left nephridium; np nephridial papilla; p nephridiopore; rne right nephridium. Planes C and D in panel A indicate the positions of the transverse sections in C and D.
Body-wall longitudinal muscles of generally bushy type (Fig. 6A, 6B) but sometimes feathery in lower part of body; 45–50 in number, arranged in following formula (
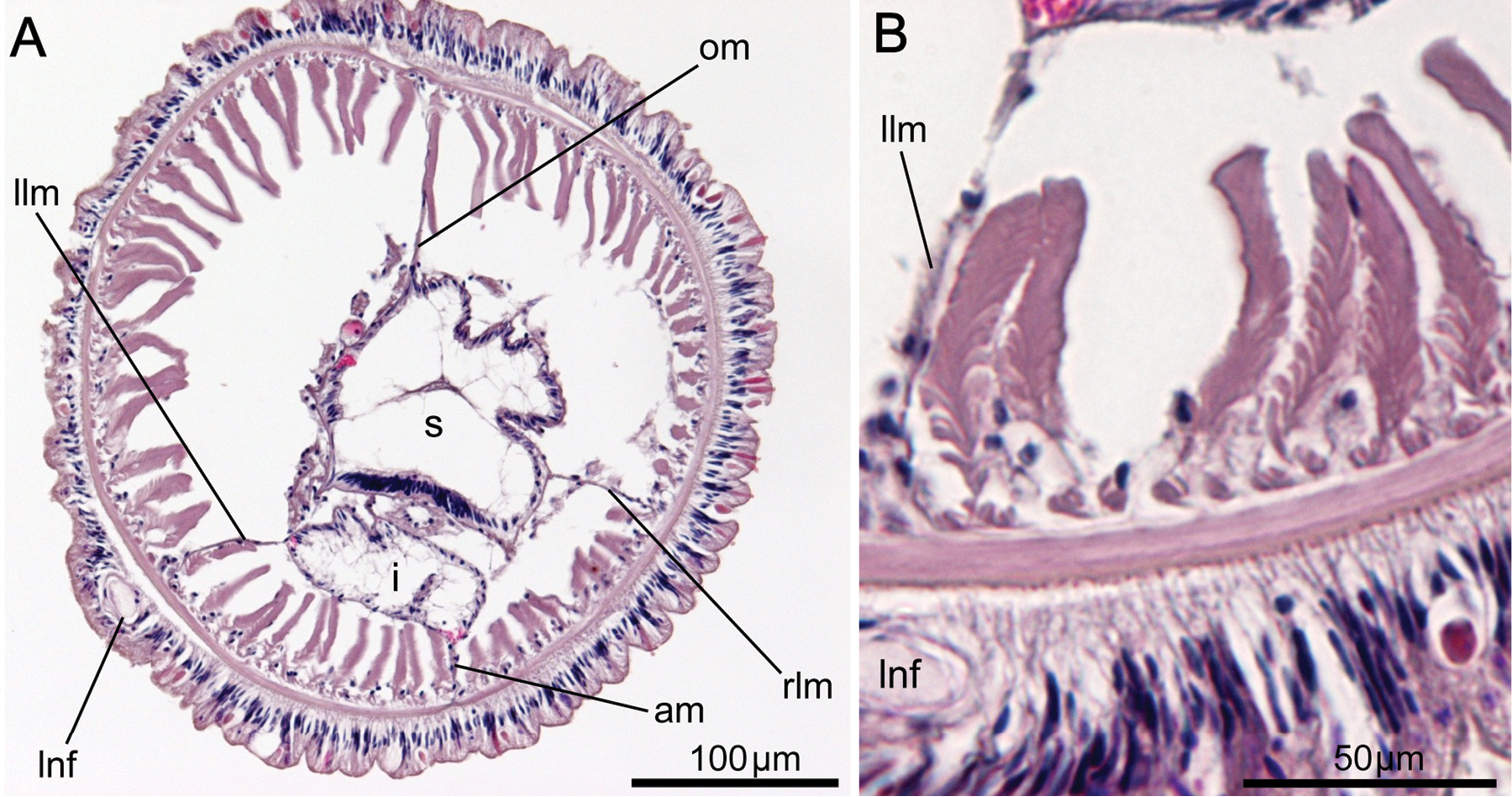
Left and right lateral mesenteries present (Fig. 6A). Two giant nerve fibers present; left giant nerve fiber 3.16–10.61 μm in diameter (avg. 6.72±3.27 μm, based on eight sections from different parts of the body, from two individuals), situated at base of left lateral mesentery (Fig. 6C); right giant nerve fiber 2.47–7.81 μm in diameter (avg. 4.55±2.15 μm, based on nine sections of different parts of the body from two individuals), situated at base of right lateral mesentery. Esophageal valve absent.
Phoronis ijimai. A NSMT-Te 886, transverse section through the posterior part of the body, showing four mesenteries and the position of the giant nerve fibers B NSMT-Te 885, enlargement showing longitudinal muscles of the bushy type C NSMT-Te 885, enlargement of the left giant nerve fiber situated at the base of the left lateral mesentery. Abbreviations: am anal mesentery; i intestine; llm left lateral mesentery; lnf left giant nerve fiber; m longitudinal muscle; om oral mesentery; rlm right lateral mesentery; rnf right giant nerve fiber; s stomach.
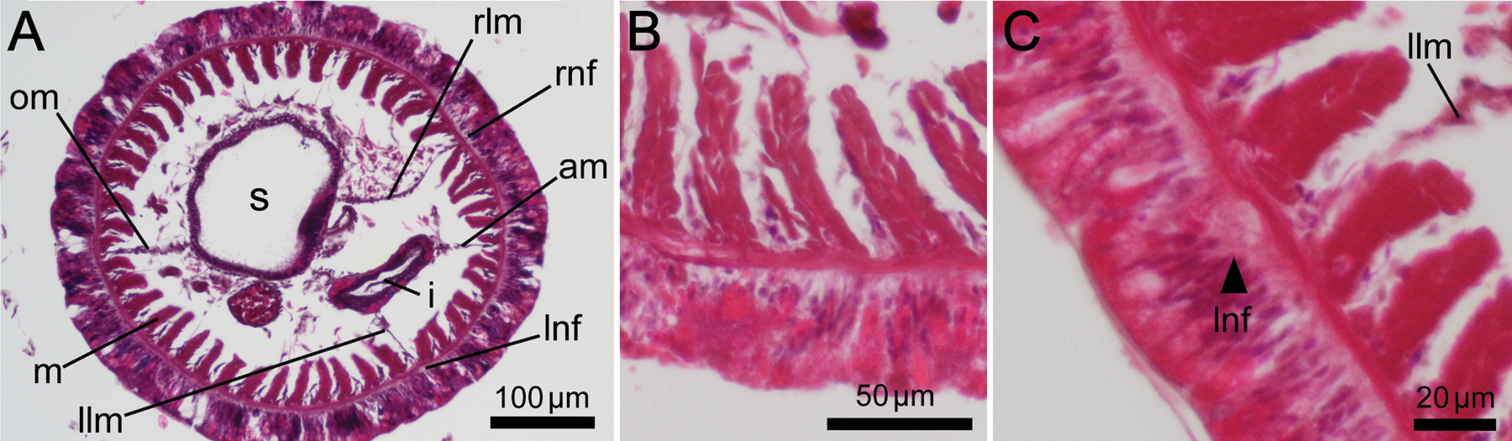
Hermaphroditic; early-stage ova and spermatocytes found beside lateral blood vessel. Brooded eggs observed in specimens from Hiroshima (Fig. 7A, 7B, 7C); embryos of various developmental stages brooded on basal nidamental glands on lophophore (Fig. 7C).
Phoronis ijimai. A NSMT-Te 878, eggs brooded in the lophophore (some tentacles have been removed) B NSMT-Te 884, transverse section through the basal part of the lophophore, showing mature eggs on the basal nidamental glands C NSMT-Te 883, enlargement of brooded eggs, showing various developmental stages. Abbreviations: eg egg; te tentacle.
Phoronis ijimai is widely distributed in the North Pacific, along the coasts of North America, Canada, Japan, and Russia, including the Sea of Japan (
Our topotype material of Phoronis ijimai collected from Misaki perfectly agrees with previous morphological accounts of this species (
http://zoobank.org/51F10DA8-DE79-4537-86E7-DE2F1CBC1B56
http://species-id.net/wiki/Phoronis_emigi
Figures 8–11Eleven series of transverse sections and two series of longitudinal sections, and nine whole specimens. Holotype: NSMT-Te 714, 5-μm transverse sections stained with HE. Paratypes: NSMT-Te 703–708, seven intact specimens, fixed and preserved in 10% formalin seawater; NSMT-Te 711–713, 715–721, 5-μm transverse sections stained with HE; and NSMT-Te 722, 723, 5-μm longitudinal sections stained with HE. Other material examined: NSMT-Te 709, 710, two intact specimens.
The specific name, a masculine noun in the genitive case, is in honor of the French researcher Dr. Christian C. Emig for his remarkable contributions to lophophorate systematics.
Body except lophophore 4.42–20.06 mm in length (holotype 9.67 mm; avg. 10.87±4.70 mm, n = 10); 0.34–0.66 mm in diameter at ampula (holotype 0.39 mm; avg. 0.47±0.10 mm, n = 9); reddish in living state, yellowish white after fixation (Fig. 8). Lophophore horseshoe-shaped, without significant coiling (Fig. 9); 2.00–3.51 mm in length (holotype 3.18 mm; avg. 2.77±0.52 mm, n = 10), 0.54–0.76 mm in diameter at base (holotype 0.68 mm; avg. 0.67±0.07 mm, n = 10); tentacles 136–170 in number (holotype 137; avg. 147±13.17, n = 6).
Phoronis emigi sp. n., NSMT-Te 714 (holotype), photographed in the preserved state (10% formalin seawater) before sectioning.
Phoronis emigi sp. n., NSMT-Te 713 (paratype), transverse section through the basal part of the lophophore.
Nephridium 205.00–324.00 μm in length (holotype 310 μm; avg. 276.78±38.69 μm, n = 5), with straight ascending branch (ab) and short descending branch (db) (Fig. 10A), ab/db length ratio 3.5 (n = 5). Ascending branch with single chamber (Fig. 10C). Nephridiopore situated on anal papilla. Tip of ascending branch (i.e., nephridiopore) lying against intestine. Nephridia slightly offset along body axis (Fig. 10B); left nephridiopore lower (in living orientation) than anus, right nephridiopore same level as anus. Single nephridial funnel present, with aperture at tip of descending branch (Fig. 10D).
Reconstructed three-dimensional images and transverse sections of the nephridium of Phoronis emigi sp. n., based on NSMT-Te 721 (paratype). A Lateral view, showing the different lengths of the ascending and descending branches B dorsal view, showing the offset arrangement of the nephridia, with the nephridiopores at different levels along the body axis C transverse section through the ascending branch D transverse section through the tip of the descending branch, showing the nephridial funnels. Abbreviations: ab ascending branch; an anus; db descending branch; es esophagus; in intestine; lne left nephridium; nf nephridial funnel; p nephridiopore; rne right nephridium; ti funnel tissue. Planes C and D in panel A indicate the positions of the transverse sections in C and D.
Body-wall longitudinal muscles of feathery type (Fig. 11A, 11B); 56–72 (holotype 67) in number, arranged in following formula (
Left and right lateral mesenteries present (Fig. 11A). Single giant nerve fiber, 15.98–36.03 μm in diameter (holotype avg. 27.40±6.29 μm, based on 5 sections from different parts of the body; avg. 25.93±6.05, based on 11 sections from different parts of the body, from five individuals [5 sections from holotype and 6 sections from 4 paratypes]), situated at base of left lateral mesentery (Fig. 11A, 11B). Esophageal valve absent.
Phoronis emigi sp. n., NSMT-719 (paratype). A Transverse section through the posterior part of the body, showing four mesenteries and the position of the giant nerve fiber B enlargement of longitudinal muscles of the long feathery type. Abbreviations: am anal mesentery; lnf left giant nerve fiber; i intestine; llm left lateral mesentery; om oral mesentery; rlm right lateral mesentery; s stomach.
Gonads not observed in any of our specimens; sex could thus not be determined.
Phoronis emigi is known only from a sandy bottom in northern Tomioka Bay, Amakusa, Japan, where we detected densities of up to about 90 individuals per 100 cm2. We observed no chitinous tubes after agitation and decantation during sampling, but the tubes would be fragile and might have been lost.
Phoronis emigi sp. n. is morphologically most similar to Phoronis psammophila Cori, 1889, with which it has in common 1) a long ascending branch of nephridium that is more than three times the length of the descending branch, 2) a single nephridial funnel, with the aperture situated at the tip of the descending branch, 3) a single giant nerve fiber situated on the left side, and 4) two lateral mesenteries. Phoronis emigi differs from Phoronis psammophila in the number of longitudinal muscle bundles in the body wall (56–72 vs. 25–50 in Phoronis psammophila) and the position of the right nephridiopores (at the same level as the anus vs. lower than the anus in Phoronis psammophila) (cf.
Naturally, Phoronis emigi is morphologically similar to, but distinct from, the nominal Phoronis architecta Andrews, 1890, which is regarded as a junior synonym of Phoronis psammophila (
The lack of gonads in our specimens was probably due to breeding seasonality. The breeding period of phoronid species previously studied is generally from spring to autumn (
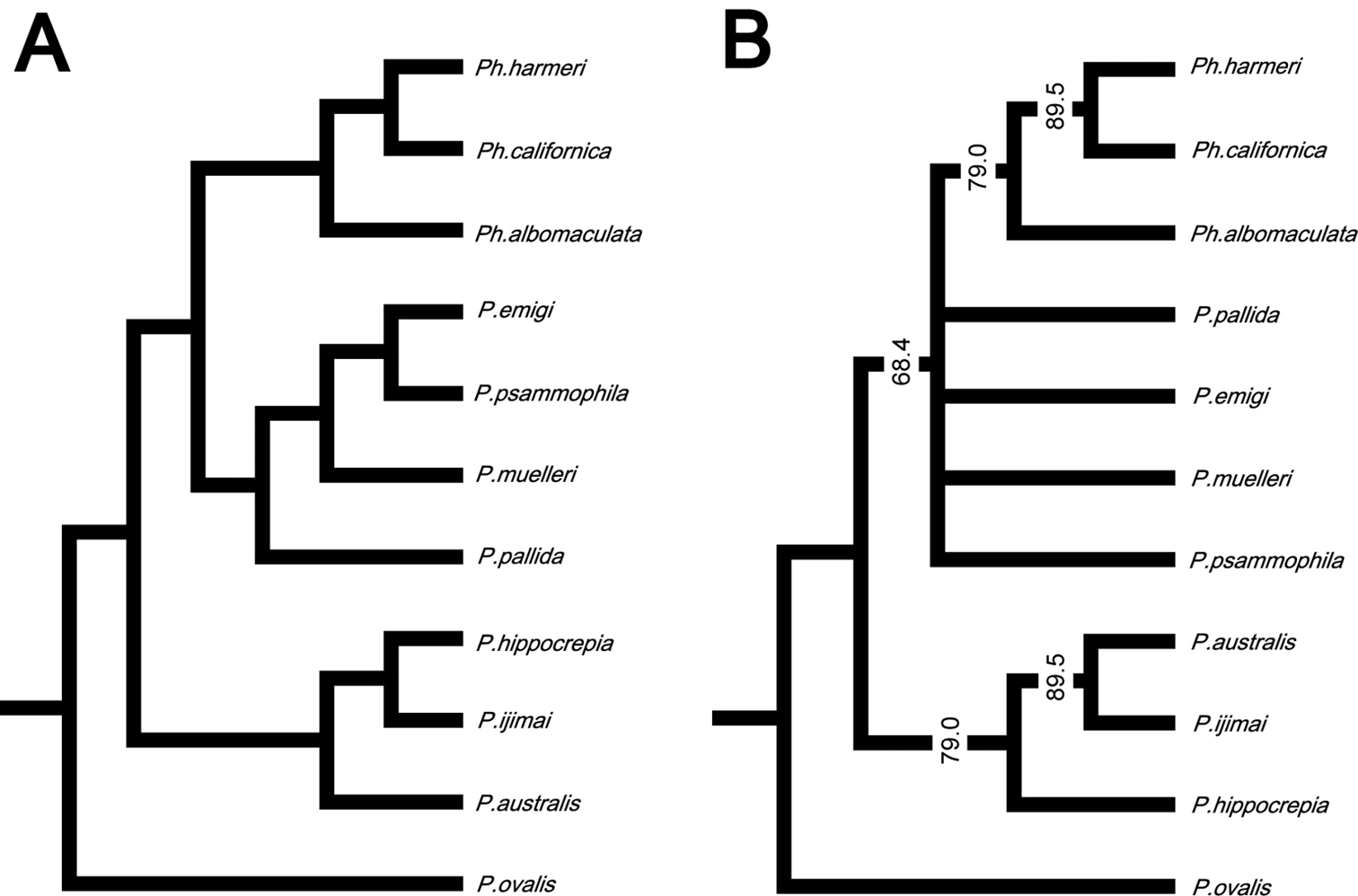
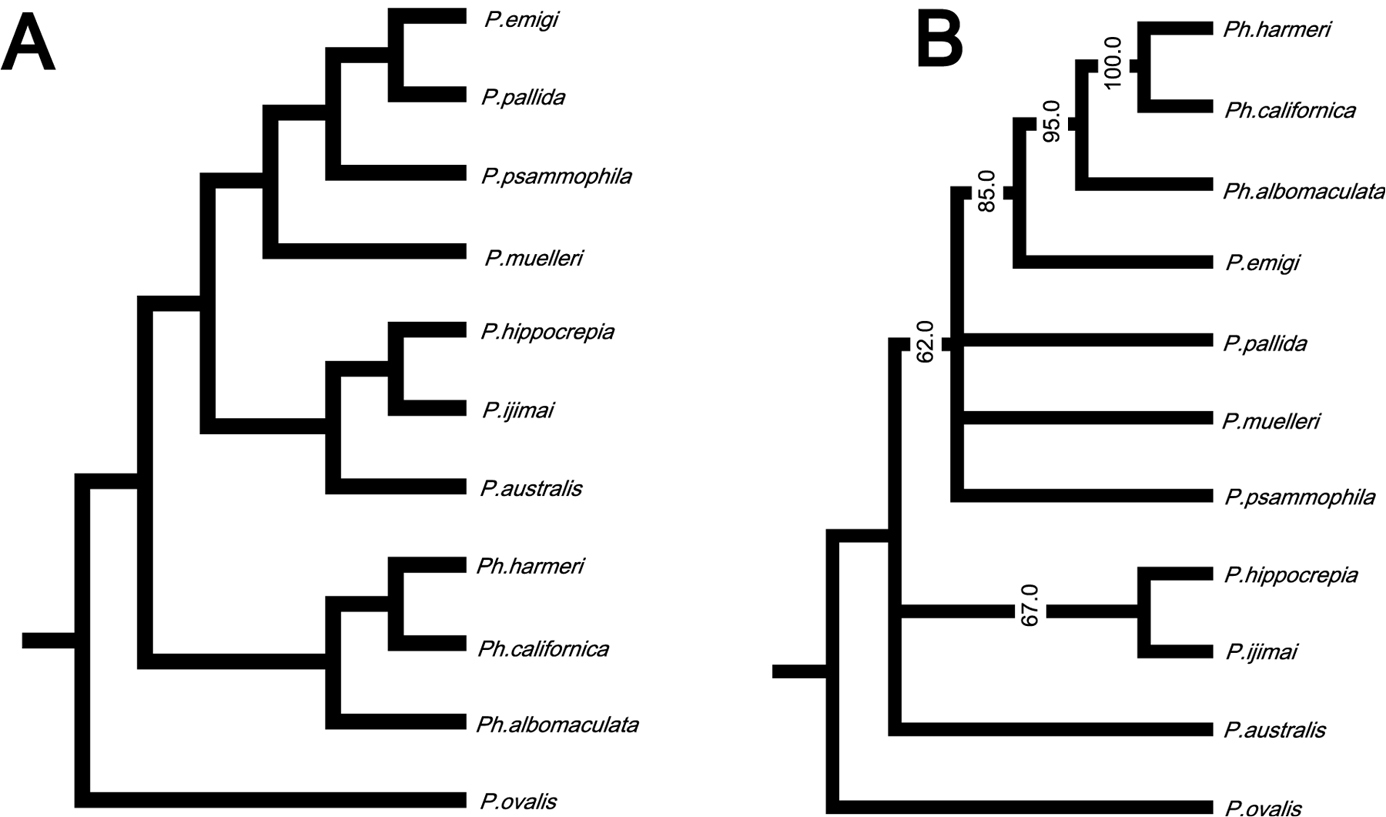
In the resulting cladogram from the cluster analysis (Fig. 12A), three major clades were retrieved: 1) Phoronopsis harmeri + Phoronopsis californica + Phoronopsis albomaculata; 2) Phoronis emigi + Phoronis psammophila + Phoronis muelleri + Phoronis pallida; and 3) Phoronis hippocrepia + Phoronis ijimai + Phoronis australis. It shows the morphological similarity of the new species Phoronis emigi with Phoronis psammophila, sharing 16 adult morphological characters. Phoronis emigi also resembles Phoronis muelleri and Phoronis pallida, with which it shares 15 and 12 characters, respectively (Fig. 12A; Suppl. material 1). We conducted another cluster analysis without nephridial characters (eliminating character 6–14 in Suppl. material 1) to test the influence of the large amount of nephridial characters. In the resulting cladogram (Appendix 1 - Supplementary Fig. S1A), the same three major clades mentioned above were also obtained, although the topology between/within the three clades changed.
A Cladogram of single-linkage cluster analysis among 11 phoronid species based on 32 morphological characters B majority-rule consensus tree of 57 equally parsimonious tree obtained by cladistic analysis among 11 phoronid species based on 32 morphological characters. Numerals on nodes indicate frequency values.
Our cladistic analysis yielded 57 equally parsimonious trees. The majority-rule consensus tree of those (Fig. 12B) did not resolve the relationship between Phoronis emigi, Phoronis psammophila, Phoronis muelleri, and Phoronis pallida; these four species formed a large clade together with Phoronopsis spp., with low consensus frequency value (68.4%). Another clade including three species (Phoronis australis + Phoronis ijimai + Phoronis hippocrepia) appeared as a sister group to this large clade; Phoronis australis formed a clade with Phoronis ijimai (89.5% in consensus frequency), to which Phoronis hippocrepia was the sister taxon (79.0% in consensus frequency). A parsimony tree without nephridial characters (Appendix 1 - Supplementary Fig. S1B) was almost identical to the tree including nephridial characters, except that Phoronis emigi appeared as sister to Phoronopsis (85.0% in consensus frequency), and Phoronis ijimai formed a clade with Phoronis hippocrepia (67.0% in consensus frequency).
In this study, most of the sites for both 18S and 28S were unambiguously aligned; therefore, we used the entire region excluding gap sites for our phylogenetic analyses. For the COI dataset, we used all the codon positions in our phylogenetic analyses.
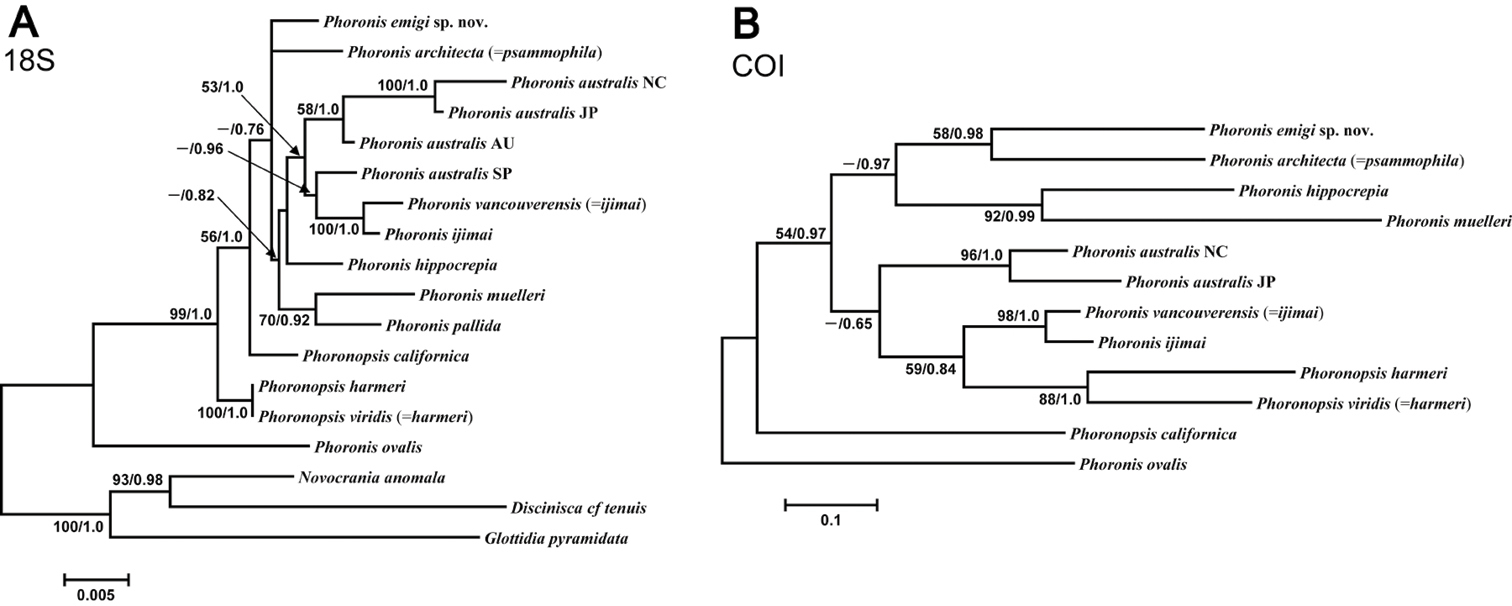
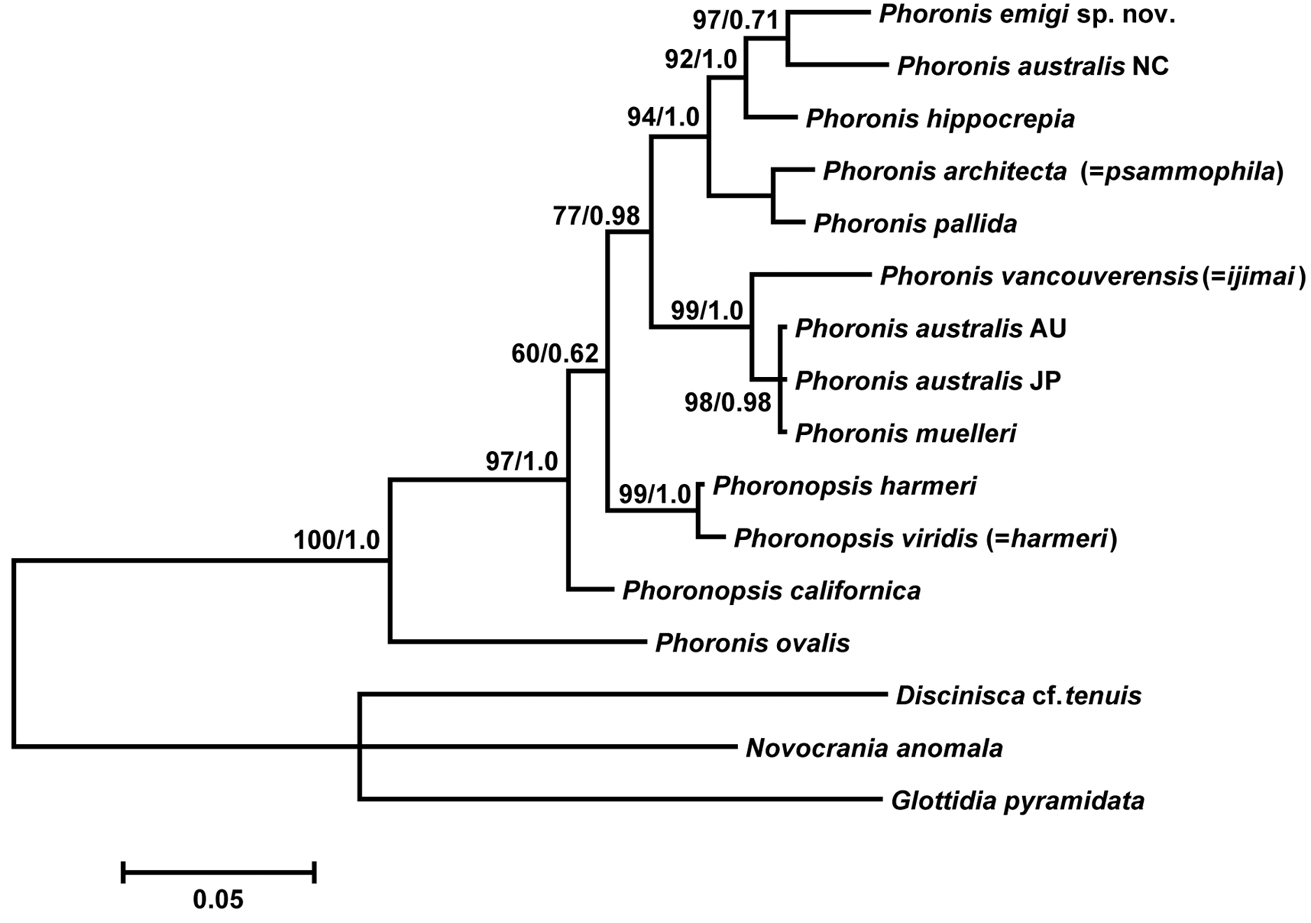
The 18S dataset comprised 1756 bp aligned sites, with 208 variable sites, for 15 ingroup taxa. In the resulting ML tree (Fig. 13A) (log L = −4104.32), not all nodes are resolved or well supported. Phoronis emigi appears in a polytomous clade along with Phoronis architecta (= psammophila) and a large, weakly supported clade that includes Phoronis ijimai and nominal “Phoronis vancouverensis” from California. Japanese Phoronis ijimai is the sister taxon to nominal “Phoronis vancouverensis” from California, with high nodal support (100/1.0). These species are embedded in a clade otherwise containing only Phoronis australis from various localities, with Spanish Phoronis australis the sister taxon to the ijimai/“vancouverensis” clade (nodal support, - /0.96). The Bayesian tree (log L = −4371.60) was identical in topology to the ML tree.
A Maximum-likelihood tree for 15 phoronid samples based on 18S data; three brachiopod species (Novocrania anomala, Discinisca cf. tenuis, and Glottidia pyramidata) are included as outgroup taxa B maximum-likelihood tree for 12 phoronid samples based on COI data; the tree is rooted with Phoronis ovalis. The scale bars indicate branch length in substitutions per site. Nodal support values are presented as the ML bootstrap value followed by the Bayesian posterior probability; only values >50% and 0.50, respectively, are shown.
The 28S dataset comprised 1065 bp aligned sites, with 333 variable sites, for 13 ingroup taxa. Most nodes in the ML tree (Appendix 1 - Supplementary Fig. S2) (log L = −3898.29) are resolved, and many have high nodal support. Phoronis emigi forms a clade with Phoronis australis from New Caledonia with moderate to high nodal support (97/0.71). Phoronis australis appears as polyphyletic, with nominal “Phoronis vancouverensis” comprising the sister taxon to a well-supported but polytomous clade containing Phoronis australis from Australia and Japan, and Phoronis muelleri. We did not obtain a 28S sequence for Phoronis ijimai, which is thus missing from this analysis. The resulting Bayesian tree (log L = −4601.76) is topologically identical with the ML tree, but the clade containing Phoronis emigi and New Caledonian Phoronis australis is supported by lower Bayesian posterior probability (0.71).
The COI dataset comprised 621 bp aligned sites, with 253 variable sites, for 12 ingroup taxa (the tree was rooted with Phoronis ovalis, which was the basal phoronid in all trees rooted with brachiopods). The resulting ML tree (Fig. 13B) (log L = −3633.85) is completely resolved, but with variable nodal support. The sister taxon to Phoronis emigi is Phoronis architecta (= psammophila) rather than New Caledonian Phoronis australis as in the 28S ML tree. The two Phoronis australis samples inlcuded in the analysis form a clade with high support (96/1). Phoronis ijimai and nominal “Phoronis vancouverensis” group together with high support (98/1), with this clade forming the sister group (nodal support, 59/0.84) to (Phoronopsis harmeri + Phoronopsis viridis). Phoronopsis appeared polyphyletic, with Phoronopsis californica the sister taxon to all other phoronids except Phoronis ovalis. The resulting Bayesian tree (log L = −3772.71) was identical in topology to the ML tree.
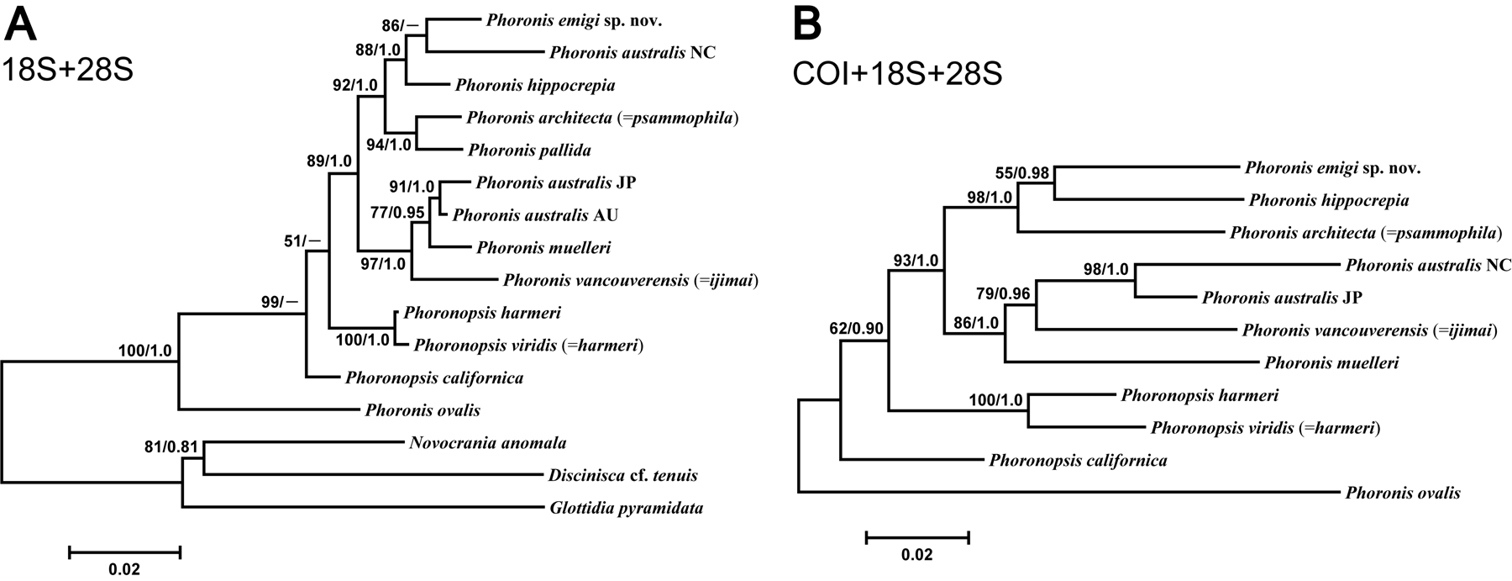
The concatenated 18S–28S dataset comprised 2819 bp aligned sites, with 537 variable sites, for 13 ingroup taxa. The ML tree (Fig. 14A) (log L = −8247.64) was identical in topology to the 28S ML tree (Appendix 1 - Supplementary Fig. S2), except the unresolved trichotomy of AU and JP Phoronis australis and Phoronis muelleri in the latter is resolved in the 18S-28S tree. The Bayesian tree (log L = −9181.86) differs from the ML tree in that Phoronis emigi forms a clade with Phoronis hippocrepia, with New Caledonian Phoronis australis the sister group to this clade.
A Maximum-likelihood tree for 13 phoronid samples based on the combined 18S + 28S data set; three brachiopod species (Novocrania anomala, Discinisca cf. tenuis, and Glottidia pyramidata) are included as outgroup taxa B maximum-likelihood tree for 11 phoronid samples based on the combined COI + 18S + 28S data set; the tree is rooted with Phoronis ovalis. Scale bars indicate branch length in substitutions per site. Nodal support values are presented as the ML bootstrap value followed by the Bayesian posterior probability; only values >50% and 0.50, respectively, are shown.
The concatenated 18S–28S–COI dataset comprised 3440 bp aligned sites, with 555 variable sites, for 11 ingroup taxa (the tree was rooted with Phoronis ovalis). The resulting ML tree (Fig. 14B) (log L = −10594.85) differs from the 28S and 18S–28S trees in several ways. The sister taxon to Phoronis emigi is Phoronis hippocrepia (nodal support, 55/0.98) rather than New Caledonian Phoronis australis. The positions of New Caledonian Phoronis australis and Phoronis architecta (= psammophila) are different in the 18S–28S–COI ML tree, but these changes in topology appear to some extent due to the omission of Phoronis pallida from the 18S–28S–COI dataset. The topology within the “Phoronis vancouverensis” / Phoronis australis / Phoronis muelleri clade also differs between 18S–28S–COI ML and the other trees that include 28S. The 18S–28S–COI Bayesian tree (log L = −10802.56), was identical to the ML tree in topology.
Before our study, three species of phoronids had been recorded from Japan: Phoronis ijimai, Phoronis australis, and Phoronis psammophila. The former two were reported from Misaki (
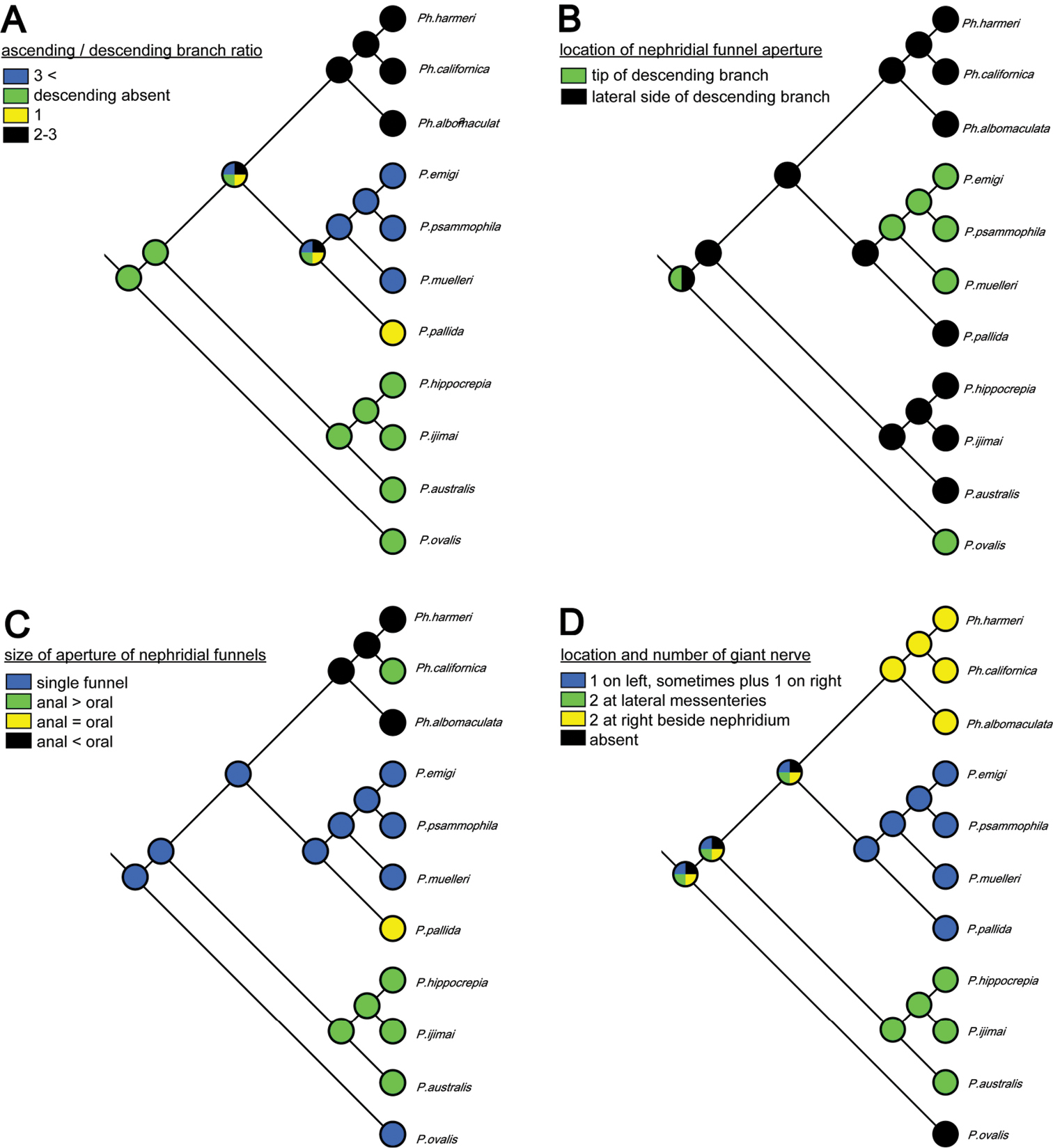
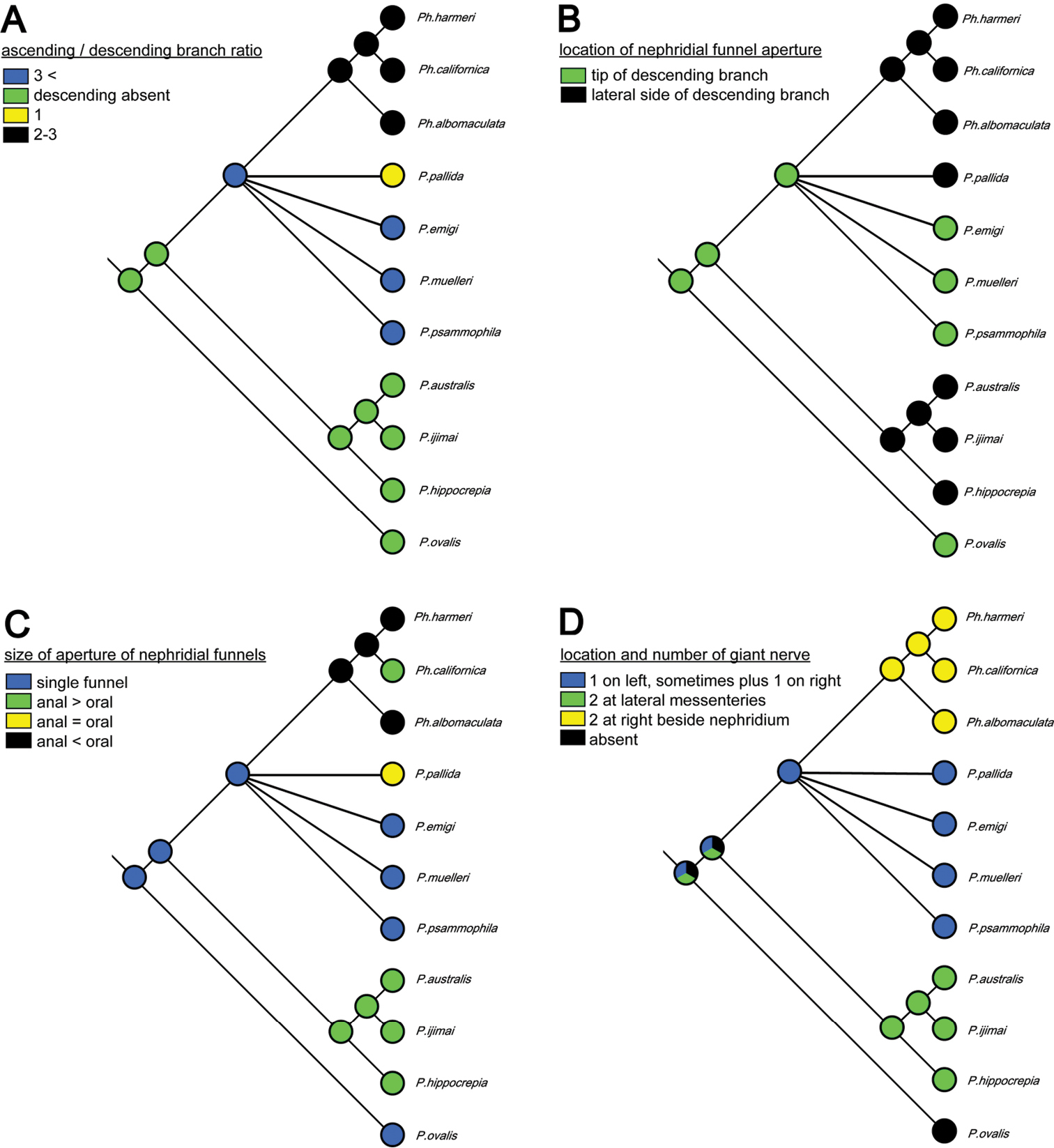
Although the molecular phylogenetic trees (Figs 13A, 13B, 14A, 14B; Appendix 1 - Supplementary Fig. S2) produced by the various datasets differed in topology, our phylogenetic reconstructions suggest that most of the adult morphological characters used to date in phoronid taxonomy are highly homoplastic (Fig. 15A–D), and thus phylogenetically less informative than the molecular data. According to the character matrix and the cladogram based on 32 morphological and reproductive characters among 11 phoronid species (Suppl. material 1; Fig. 12A, 12B; Appendix 1 - Supplementary Figs S1A, S1B, S3A–D, S4A–D), Phoronis emigi comprise a group with Phoronis psammophila, Phoronis muelleri, and Phoronis pallida. In none of our molecular trees (Figs 13A, 13B, 14A, 14B), however, did these four species alone comprise a clade. In the COI tree (Fig. 13B), Phoronis architecta (= psammophila), Phoronis muelleri, and Phoronis emigi comprise a clade that also includes Phoronis hippocrepia. In the COI–18S–28S tree (Fig. 14B), Phoronis emigi and Phoronis architecta (= psammophila) group with Phoronis hippocrepia, to the exclusion of Phoronis muelleri, but no morphological or reproductive characters (Suppl. material 1; Fig. 15) appear to be synapomorphic for this clade, though character 19 (ratio of number of longitudinal muscles in oral coelom / anal coelom) in these three species is smaller than in other species of the genus except for Phoronis ovalis, which lacks lateral mesenteries (Suppl. material 1).
Parsimonious reconstruction of four adult morphological characters among nine phoronid species on the maximum-likelihood tree based on concatenated COI–18S–28S dataset.
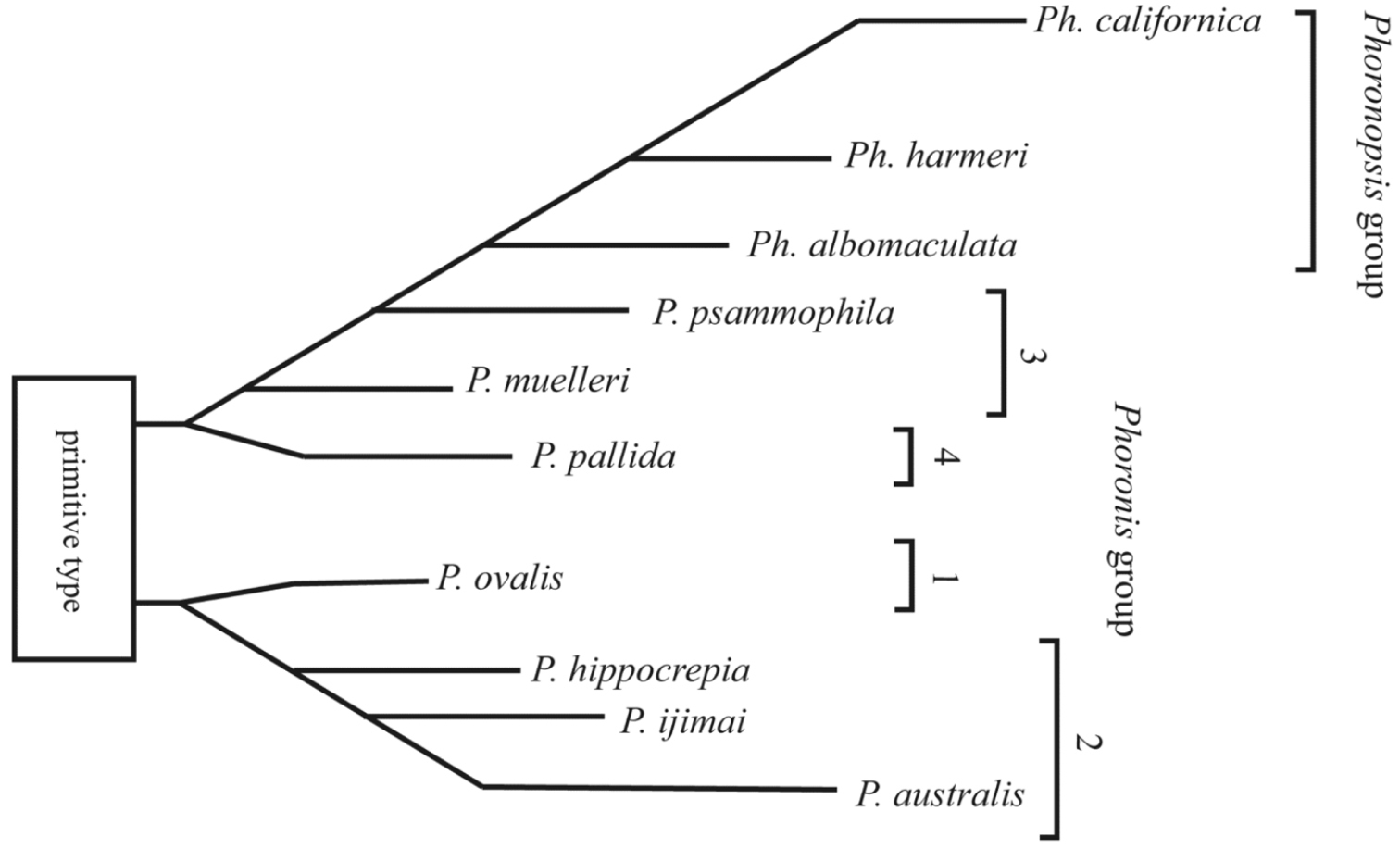
Our molecular trees do not correspond with any of the subdivisions of phoronids suggested by previous researchers solely based on morphological characters (
Our morphological and molecular results do not contradict that “Phoronis vancouverensis” is conspecific with Phoronis ijimai, as proposed by
| 1 | Inhabiting cerianthid tube-wall; lophophore multispiral; normally black in color | Phoronis australis Haswell, 1883 |
| – | Inhabiting cylindrical tube on hard substrate or soft sandy and muddy bottom; lophophore horseshoe-shaped without significant coiling; white or red in color | 2 |
| 2 | Cylindrical tube constructed of small sand grains; tentacles fewer than 100 in number, with white spots | Phoronis psammophila Cori, 1889 |
| – | Cylindrical tube obscure or not constructed of sand grains; tentacles more than 100 in number, without white spots | 3 |
| 3 | Left giant nerve fiber more than 15 µm in diameter, right giant nerve fiber absent; longitudinal muscles of feathery type, more than 10 in number on each side of anal coelom; nephridium with single funnel, nephridial papilla absent, descending branch present | Phoronis emigi sp. n. |
| – | Left giant nerve fiber less than 15 µm in diameter, right giant nerve fiber present; longitudinal muscles of bushy type, fewer than 10 in number on each side of anal coelom; nephridium with two funnels, nephridial papilla present, descending branch absent | Phoronis ijimai Oka, 1897 |
Pairwise genetic distances (K2P distances) based on 583 positions of COI sequences between Phoronis ijimai, Phoronis emigi, and the other species. The largest (Phoronis australis JP and Phoronis muelleri) and the lowest (Phoronis australis NC and Phoronis vancouverensis) interspecific distances are also listed. The analysis involved 12 phoronid sequences.
| Species 1 | Species 2 | K2P Distance |
|---|---|---|
| Phoronis australis JP | Phoronis muelleri | 0.287 |
| Phoronis australis NC | Phoronis vancouverensis | 0.164 |
| Phoronis australis NC | Phoronis australis JAPAN | 0.115 |
| Phoronis ijimai | Phoronis muelleri | 0.278 |
| Phoronis architecta | 0.258 | |
| Phoronopsis californica | 0.258 | |
| Phoronis ovalis | 0.239 | |
| Phoronis hippocrepia | 0.222 | |
| Phoronopsis viridis | 0.216 | |
| Phoronopsis harmeri | 0.215 | |
| Phoronis australis JAPAN | 0.206 | |
| Phoronis australis NC | 0.179 | |
| Phoronis vancouverensis | 0.070 | |
| Phoronis emigi sp. n. | Phoronis muelleri | 0.274 |
| Phoronopsis viridis | 0.259 | |
| Phoronopsis harmeri | 0.252 | |
| Phoronis ovalis | 0.240 | |
| Phoronis hippocrepia | 0.239 | |
| Phoronopsis californica | 0.238 | |
| Phoronis ijimai | 0.235 | |
| Phoronis vancouverensis | 0.218 | |
| Phoronis australis JAPAN | 0.208 | |
| Phoronis australis NC | 0.205 | |
| Phoronis architecta | 0.202 |
We thank Professor/Director Mutsunori Tokeshi, Dr. Keisuke Mori, Captain Teruo Samejima, and Mr. Kentarou Tanaka at AMBL for assistance in collecting at Amakusa; Keiichi Kakui, Hiroshi Yamasaki, and Shushi Abukawa (Hokkaido University) for specimens from Amakusa; Hisanori Koutsuka (MMBS), Yuji Ise (MMBS), Mayumi Masuda (The University of Tokyo), and Dr. Rei Ueshima (The University of Tokyo) for specimens from Misaki; Dr. Daisuke Ueno (University of Ryukyus) for specimens from Hiroshima; Dr. Toshihiko Fujita (NSMT) for providing research facilities; Dr. Hiroshi Namikawa (NSMT) for assistance in registering specimens; and Professor Matthew H. Dick (Hokkaido University) for reviewing the manuscript and editing the English. This study was financially supported in part by a Narishige Zoological Science Award to HK (FY 2009).
A Cladogram of single-linkage cluster analysis among 11 phoronid species based on 23 morphological characters excluding nephridial characters B majority-rule consensus tree of 100 equally parsimonious tree obtained by cladistics analysis among 11 phoronid species based on 23 morphological characters excluding nephridial characters. Numerals on nodes indicate frequency values.
Maximum-likelihood tree for 13 phoronid samples based on 28S data; three brachiopod species (Novocrania anomala, Discinisca cf. tenuis, and Glottidia pyramidata) are included as outgroup taxa. The scale bar indicates branch length in substitutions per site. Nodal support values are presented as the ML bootstrap value followed by the Bayesian posterior probability; only values >50% and 0.50, respectively, are shown.
Parsimonious reconstruction of four adult morphological characters among 11 phoronid species on the cladogram of the cluster analyses based on 32 morphological characters.
Parsimonious reconstruction of four adult morphological characters among 11 phoronid species on the parsimonious consensus tree based on 32 morphological characters.
Character matrix of 32 morphological and reproductive characters among 11 phoronid species considered in the Discussion.
Authors: Masato Hirose, Ryuma Fukiage, Toru Katoh, Hiroshi Kajihara
Data type: character matrix
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.