Citation: Zhang M, Chen J-l, Gao X-z, Pape T, Zhang D (2014) First description of the female of Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975) (Diptera, Sarcophagidae). ZooKeys 396: 43–53. doi: 10.3897/zookeys.396.6752
Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975) is documented from specimens collected in Hubei Province, China, using morphological characters and wing interference patterns (WIPs). The female of S. (S.) gracilior is described for the first time, the male is redescribed, and both sexes are photographed. The distribution of the species is updated.
Sarcophagidae, Sarcophaga (Sarcorohdendorfia) gracilior, female, wing interference patterns, morphology, taxonomy
The Sarcophagidae (flesh flies) is a medium-sized family of Diptera with about 2600 known species worldwide, which includes various life history strategies ranging from inhabitants of pitcher plants to bat coprophages, crab saprophages, wasp nest inquilines, and insect parasitoids (
Sarcorohdendorfia Baranov is a large subgenus of Sarcophaga Meigen (sensu lato), and it currently comprises 61 species known mainly from the Oriental and Australasian/Oceanian Regions (
Wing interference patterns (WIPs) were recently introduced as a potential new character system of extremely thin insect wings (
The primary aims of this article are: 1) to provide the first description of the female of Sarcophaga (Sarcorohdendorfia) gracilior and a redescription of the male, and 2) to provide the first data on WIPs for flesh flies as a potential tool in associating conspecific males and females.
Flies inhabiting forested areas in the mountainous region of the Hubei Province, China, were attracted by the viscera of grass carps (Ctenopharyngodon idellus) obtained from the local market. Viscera were kept frozen until needed, thawed and left to decompose for about two days before being deployed separately in traps consisting of open plastic containers (5.0 cm high, 10.0 cm in diameter). Flies that visited the bait during 1–2 hours from the time of deployment, were collected. Specimens were deposited in the Museum of Beijing Forestry University (MBFU), Beijing. Photographs were taken with a Canon 550D camera mounted on an Olympus SZX16 stereomicroscope. The methods applied to view and document interference colour patterns in the flies’ wings followed
http://species-id.net/wiki/Sarcophaga_gracilior
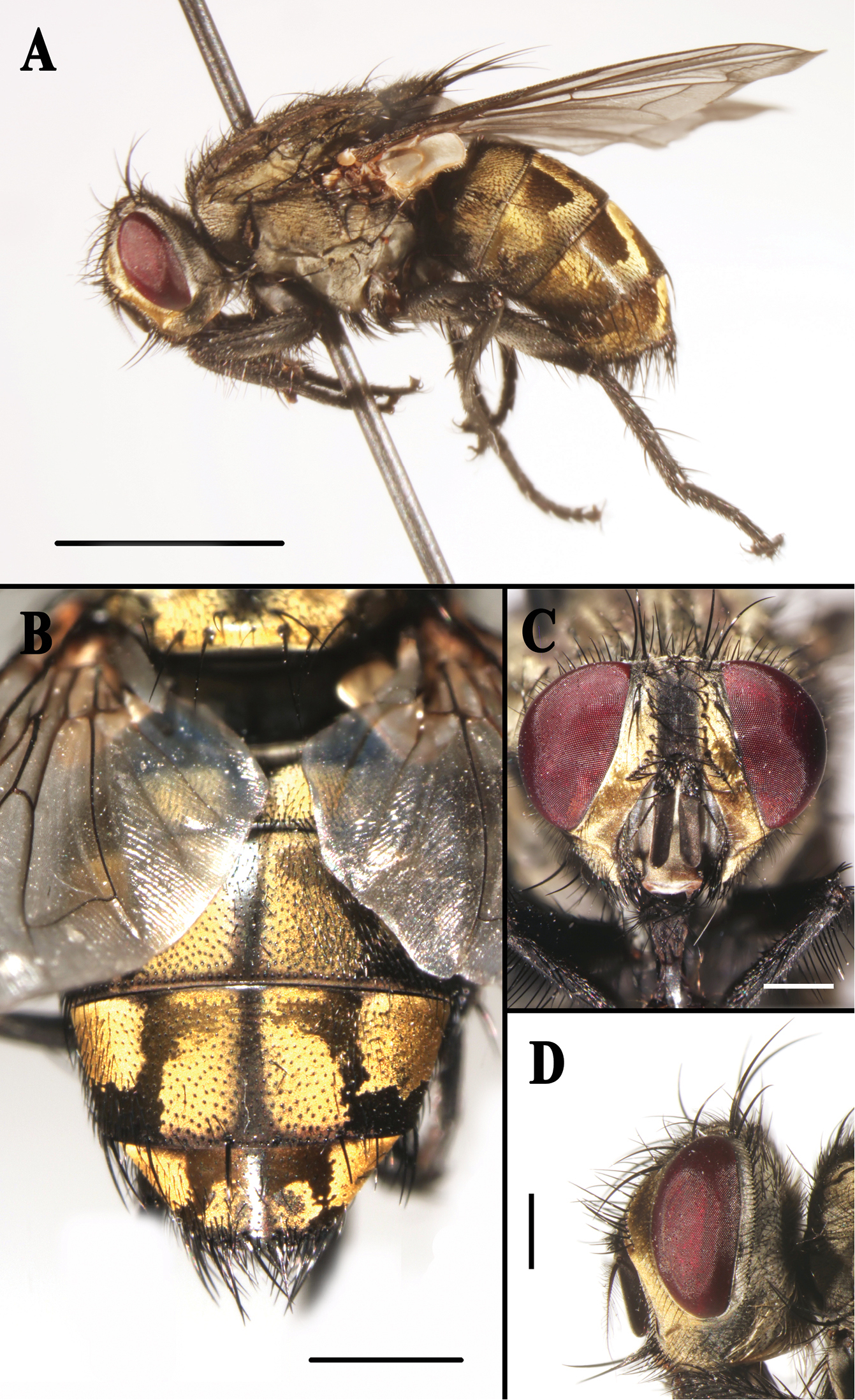
Description. Body length about 13.0 mm. Eyes bare. Fronto-orbital and parafacial plates black with golden yellow pollinosity, postocular strip black with silvery pollinosity; parafacial bristles in one row, fronto-orbital plate with rows of fine setulae. Frontal vitta black, about as broad as fronto-orbital plate at the narrowest point; frons at vertex 0.3 × head width; frontal row of 9–14 strong bristles; outer vertical bristle differentiated from postocular bristles, one reclinate and two proclinate orbital bristles. One pair of strong ocellar bristles, directed antero-laterally. Gena ground colour black, with black setulae in anterior 2/3, white setulae in posterior 1/3; height 0.3 × eye height in lateral view, postgena with white setulae. Antennal first flagellomere brown, not reaching the level of vibrissal insertion, 3.4 × as long as wide and 2.3 × as long as pedicel, pedicel black; arista long plumose in basal 2/3. Palpus black, expanded in distal part.
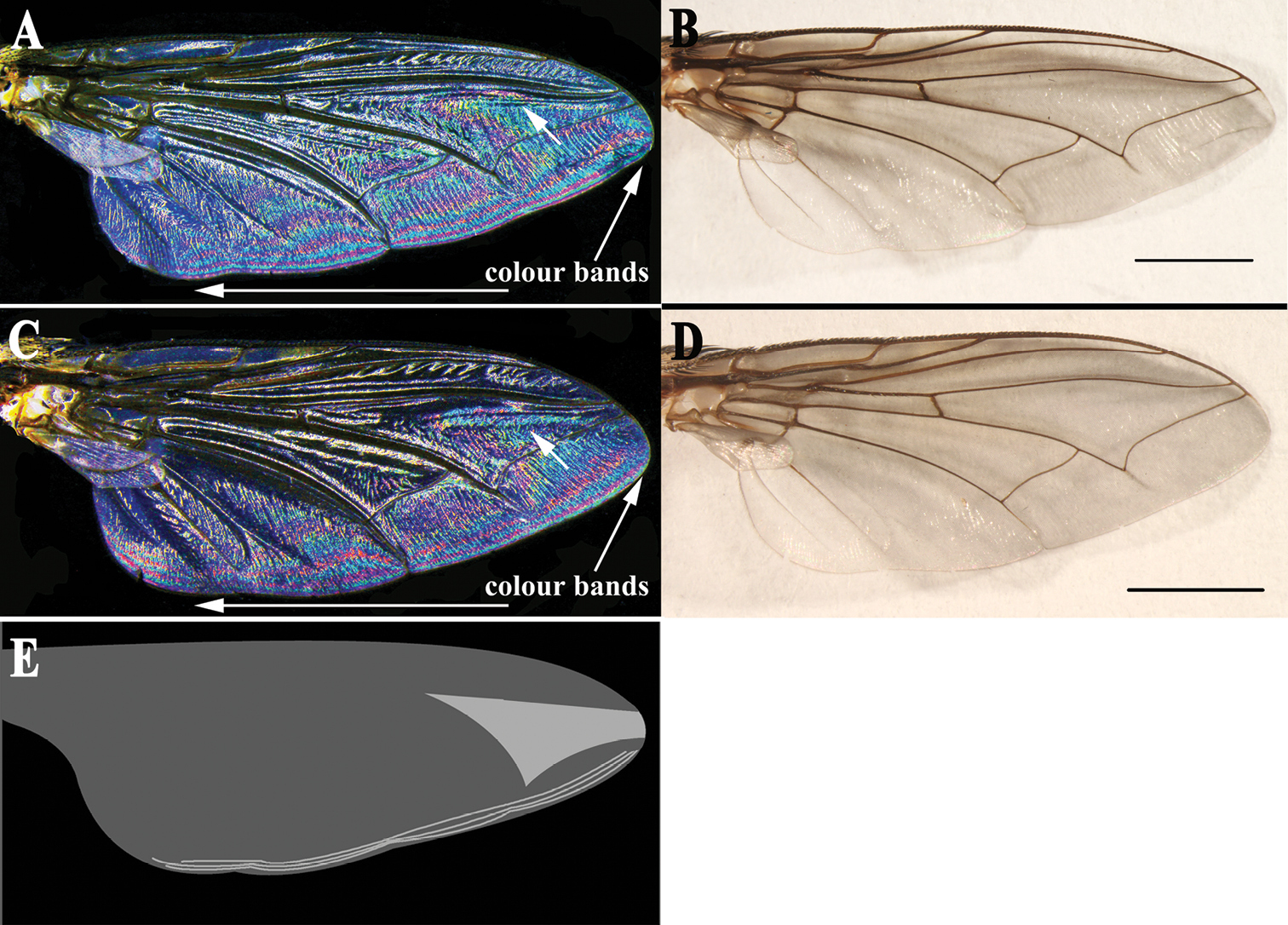
Thorax ground colour black, with yellow pollinosity; scutum with three black dorsal vittae. Chaetotaxy: acrostichals 5(6) + 1, dorsocentrals 4 + 4, intra-alars 1 + 2 (3), supra-alars 3 or 4, postpronotals 3, scutellum with 1 discal and 4 marginal bristles. Meropleurals 10 or 11, katepisternal bristles 1:1:1, prosternum, metasternum, proepisternum and postalar wall with dense black fine setulae. Wing hyaline; subcostal sclerite yellowish brown, bare; tegula black, with black setulae; basicosta light yellow, bare; costal spine not differentiated; vein R1 bare, three ventral setulae at node of R4+5-R2+3, vein R4+5 setulose dorsally from junction of R2+3 halfway to crossvein r-m; wing WIP (Fig. 5C) with clearly demarcated magenta and blue bands, and one large and almost triangular blue area on the apical part (shown with an arrow in Fig. 5C).
Legs dark, with grayish black pollinosity; fore femur with one row of dorsal bristles, one row of posteroventral bristles and one row of posterodorsal bristles, fore tibia with four anterodorsal and one posterior bristles; mid femur with four median anterior, one apical posterior and one apical posterodorsal bristles, mid tibia with two anterodorsal, one ventral and one subapical posterior bristles, and with one row of posterodorsal bristles (one strong); hind femur with one row of anterodorsal bristles, and with one apical posterodorsal and two apical posterior bristles, hind tibia with one row of anterodorsal bristles (among them three strong), and with one anteroventral and four posterodorsal bristles.
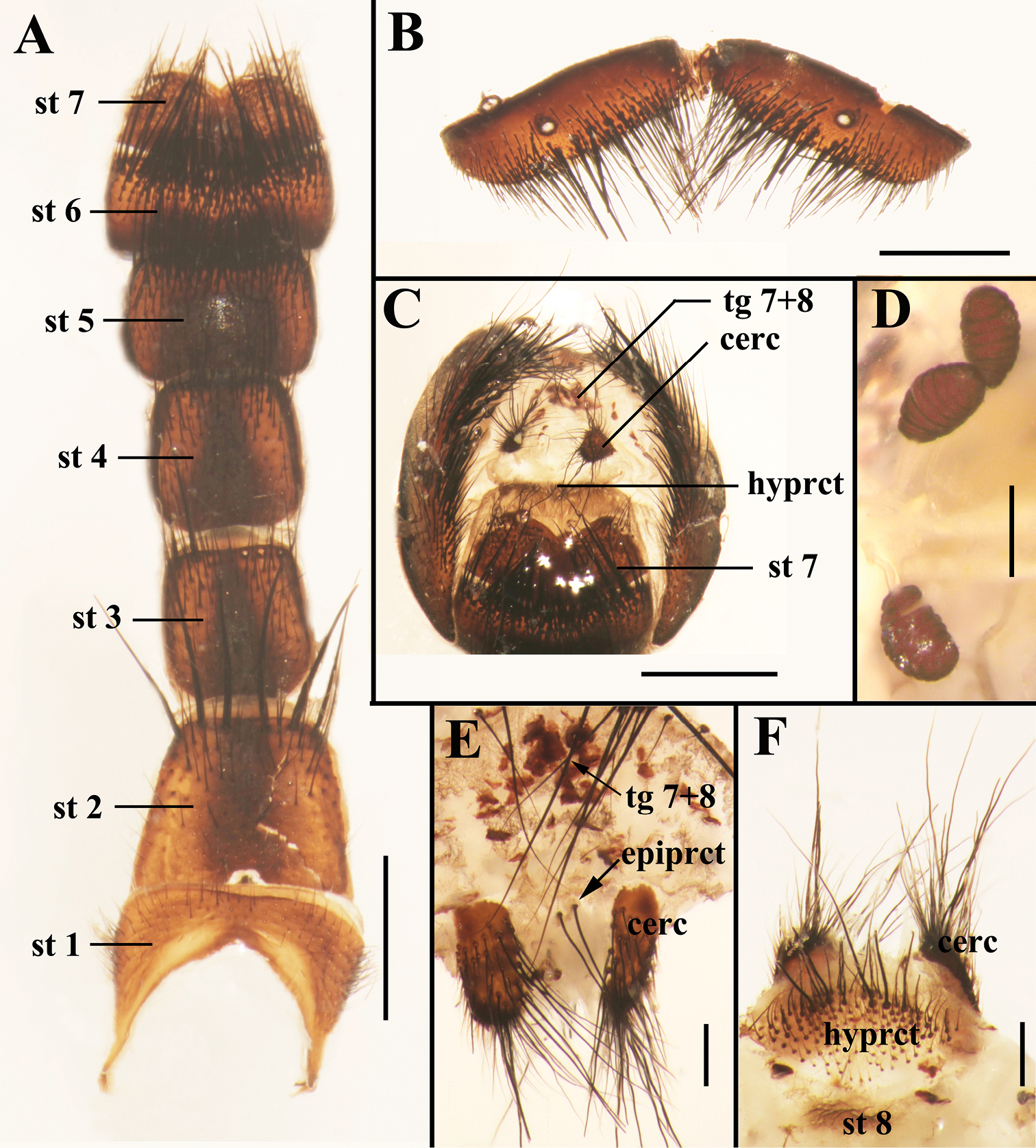
Abdomen oval with yellow pollinosity; tergite 3 without median marginal bristles, tergite 4 with one pair of median marginal bristles, tergite 5 with strong marginal bristles; sternite 2 with six long bristles along posterior margin. Terminalia: tergite 6 undivided (Fig. 2B), tergites 7+8 fused together (Figs 2C, 2E), sternite 2 with a small isolated sclerite on the posterior margin, sternites 5 and 6 rectangular in ventral view (Fig. 2A), sternite 8 represented by a membranous fold, hypoproct well developed but not particularly sclerotized and with numerous setulae (Fig. 2F), epiproct with only two strong bristles (Fig. 2E).
Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975). Female. A Habitus, left lateral view B Abdomen, dorsal view C Head, anterior view D Head, left lateral view. Scale bars: A = 5.00 mm; B = 2.00 mm; C and D = 1.00 mm.
Photomicrographs of the female terminalia of Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975). A Sternites 1−7, ventral view B Tergite 6, dorsal view C Terminalia, posterior view D Spermathecae E Terminalia, tergites 7+8, cerci and epiproct, dorsal view F Terminalia, cercus, hypoproct and sternite 8, ventral view. Scale bars: A–C = 1.00 mm; D–F = 0.25 mm. Abbreviations: cercus (cerc); epiproct (epiprct); hypoproct (hyprct); sternite (st); tergite (tg).
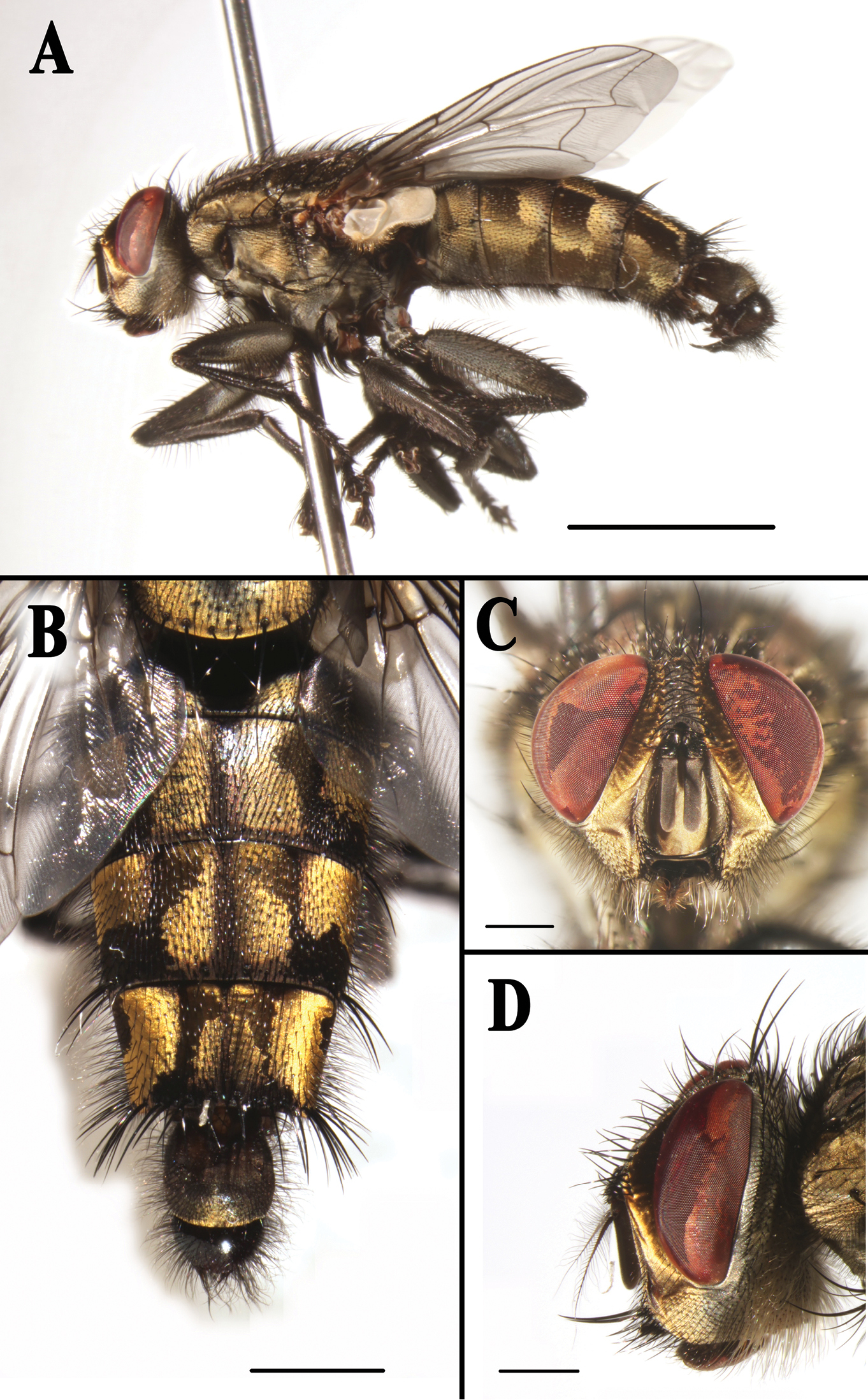
Redescription. Body length 16.0–17.0 mm. Frontal vitta 1.6 × as broad as fronto-orbital plate at the narrowest point; frons at vertex 0.22 × head width; frontal row of 11–13 bristles; outer vertical bristle not differentiated from postocular bristles, one reclinate orbital bristle. Antennal first flagellomere 4.1 × as long as wide and 3.1 × as long as pedicel.
Thorax: fore femur with slender ventral setulae in basal 1/2, fore tibia with three anterodorsal bristles; mid tibia with one anterodorsal bristle; hind femur with one row of anterior bristles, and with one apical posterior and three apical posterodorsal bristles, hind tibia with two posterodorsal bristles, and with slender and dense setulae along anteroventral and posteroventral surfaces.
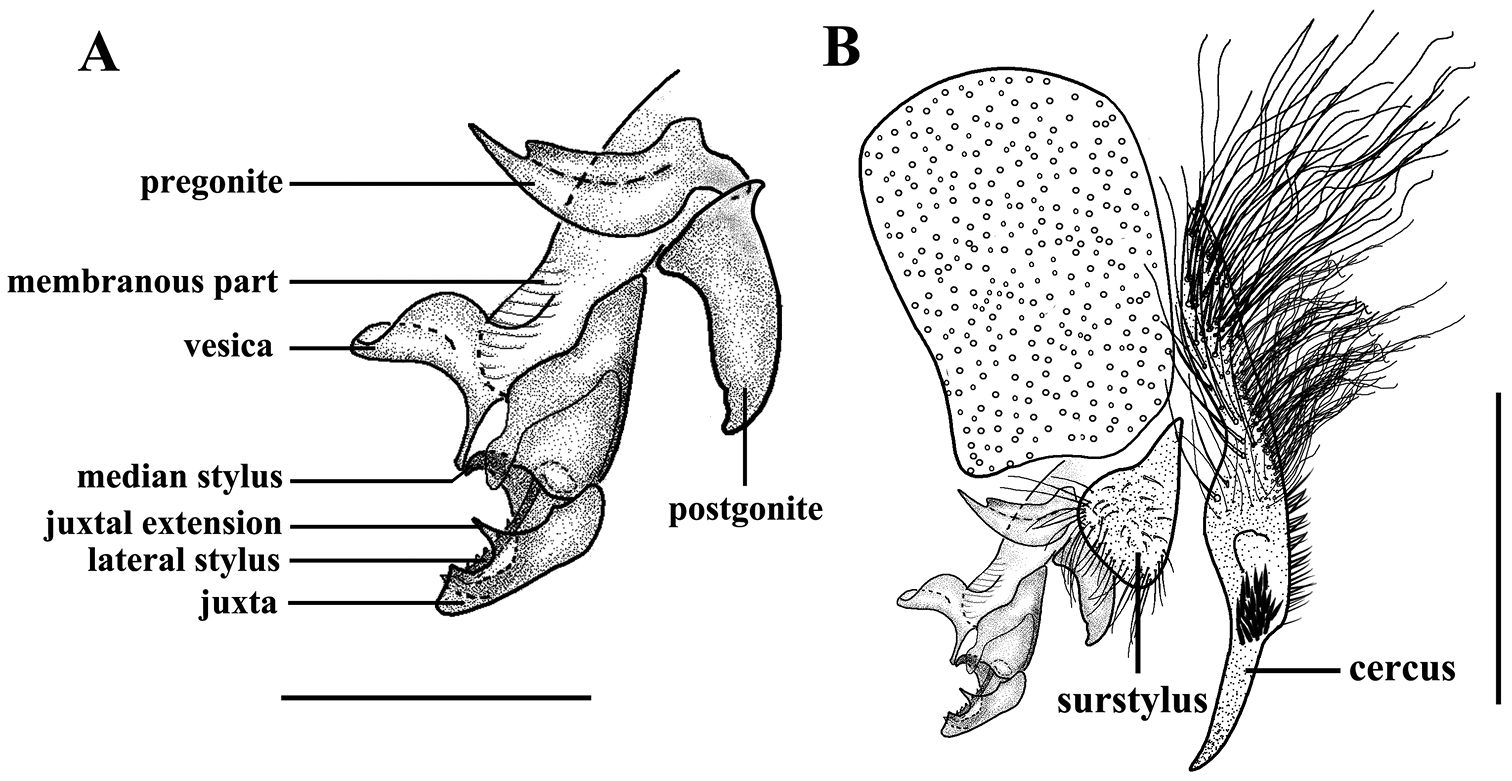
Abdomen long oval; epandrium black; sternites 1−4 with dense setulae, sternite 4 with a dark spot consisting of dense short setulae on posterior margin (see
Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975). Male. A Habitus, left lateral view B Abdomen, dorsal view C Head, anterior view D Head, left lateral view. Scale bars: A = 5.00 mm; B = 2.00 mm; C and D = 1.00 mm.
Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975). Male. A Phallus and gonites, lateral view B Terminalia, lateral view. Scale bar: A = 0.50 mm; B = 1.00 mm.
Sarcophaga (Sarcorohdendorfia) gracilior (Chen, 1975). A Male, right wing interference patterns, dorsal view B Male, right wing, dorsal view C Female, right wing interference patterns, dorsal view D Female, right wing, dorsal view. Scale bars = 2.00 mm E Schematic illustration of the distinctive clearly demarcated magenta and blue bands, and one large and almost triangular area on the apical part, which is blue in the WIP. Arrows in A & C show the most similar patterns and marginal colour bands of both sexes.
CHINA, Hubei, Yichang City, Dalaoling (31°5'00"N, 110°56'00"E): 1♀, Panlongling, 1600–1700 m, 17.VII.2013; 1♂, 1♀, Mt. Tianzhushan, 2000 m, 19.VII.2013; 1♀, Panlongling, 1600–1700 m, 22.VII.2013; all collected by Zhang D. & Zhang M.
The specimens of this species have been taken in traps baited with fish viscera, indicating that this species may be saprophagous like the majority members of the genus Sarcophaga.
China (Chongqing, Hubei [first record], Hunan, Guangdong, Guizhou, Sichuan, Taiwan, Xizang, Zhejiang), Nepal.
Females of most species of flesh flies are very similar in appearance and difficult to identify (
WIPs may arise in transparent insect wings due to their double layer of very thin cuticle (
We wish to extend our sincerest thanks to Drs. Jacek Szwedo and Adam Stroiński (Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland) for their assistance using the WIP method in this study, and an anonymous reviewer for helpful suggestions. This study was supported by the Program for New Century Excellent Talents in University (No. NCET-12-0783), National Science Foundation of China (No. 31201741), and Chinese Postdoctoral Science Foundation grants (No. 20100470009, No. SFG-201104059).