Citation: Roxo FF, Zawadzki CH, Troy WP (2014) Description of two new species of Hisonotus (Ostariophysi, Loricariidae) from the rio Paraná-Paraguay basin, Brazil. ZooKeys 395: 57–78. doi: 10.3897/zookeys.395.6910
Two new species of Hisonotus are described from the rio Paraná-Paraguay basin in Brazil. The most remarkable features of the new species are the odontodes forming longitudinally aligned rows (one odontode after the other, but not necessarily forming parallel series) on the head and trunk (vs. odontodes not forming longitudinally aligned rows), a pair of rostral plates at the tip of the snout (vs. a single rostral plate), the functional v-shaped spinelet (vs. spinelet non-functional, square-shaped, or absent). These features suggest close phylogenetic relationships with Hisonotus bockmanni, H. insperatus, H. luteofrenatus and H. piracanjuba. Additionally, both new species are distinguished from their congeners by characters related to head length and depth, orbital diameter, suborbital depth, caudal peduncle depth, pectoral-fin spine length, snout length and counts of teeth. Hisonotus paresi sp. n. further differs from its congeners by having contrasting dark geometric spots on the anterodorsal region of the body, a character lacking in H. oliveirai sp. n. The variation in number and shape of the rostral plate, posterior rostrum plates, infraorbitals and the preopercle in both new species and in H. insperatus are discussed.
Cascudinhos, fresh water, head plates, Hypoptopomatinae, Neotropical fish
Hypoptopomatinae is composed of 19 genera and about 135 valid species (
The genus Hisonotus currently contains 31 valid species (
All measurements and counts were taken from the left side of the fish. Measurements were made from point to point to the nearest 0.1 mm with a digital caliper. Body plate and osteology nomenclature follows
Morphometrics and meristics of Hisonotus oliveirai and Hisonotus paresi. SD = standard deviation.
| Hisonotus oliveirai n = 27 | Hisonotus paresi n = 15 | |||||||
|---|---|---|---|---|---|---|---|---|
| Holotype | Range | Mean | SD | Holotype | Range | Mean | SD | |
| SL | 26.4 | 22.8−28.4 | 24.4 | 1.43 | 26.2 | 18.0−26.2 | 22.7 | 2.99 |
| Percents of SL | ||||||||
| Head length | 36.5 | 35.6−41.1 | 37.7 | 1.41 | 39.2 | 36.1−41.7 | 39.4 | 1.44 |
| Predorsal length | 46.8 | 45.3−52.1 | 48.3 | 1.51 | 47.9 | 46.9−51.8 | 49.0 | 1.54 |
| Dorsal-fin spine length | 22.4 | 22.4−28.3 | 24.5 | 1.62 | 25.4 | 25.2−27.0 | 26.2 | 0.50 |
| Anal-fin unbranched ray length | 18.7 | 16.3−21.3 | 19.2 | 1.34 | 18.2 | 17.4−21.4 | 19.8 | 0.87 |
| Pectoral-fin spine length | 23.6 | 21.6−27.6 | 24.7 | 1.57 | 27.5 | 27.0−30.1 | 28.2 | 0.53 |
| Pelvic-fin unbranched ray length | 18.4 | 16.8−23.2 | 20.6 | 1.45 | 18.7 | 18.0−21.1 | 19.7 | 0.98 |
| Cleithral width | 24.6 | 23.8−26.8 | 25.3 | 0.89 | 23.5 | 22.2−24.3 | 23.3 | 0.49 |
| Thoracic length | 18.4 | 17.6−21.6 | 19.0 | 0.80 | 18.8 | 16.1−19.8 | 17.8 | 1.12 |
| Abdominal length | 21.9 | 17.9−22.3 | 20.5 | 1.24 | 21.5 | 16.2−21.6 | 19.0 | 1.82 |
| Body depth at dorsal-fin origin | 21.1 | 18.6−23.9 | 21.6 | 1.25 | 18.8 | 16.9−20.7 | 18.1 | 1.30 |
| Caudal-peduncle length | 28.3 | 26.3−31.5 | 29.3 | 1.18 | 27.5 | 25.3−29.8 | 27.7 | 1.61 |
| Caudal-peduncle depth | 10.5 | 10.8−12.5 | 11.4 | 0.64 | 10.6 | 10.2−11.3 | 10.7 | 0.27 |
| Percents of HL | ||||||||
| Snout length | 50.7 | 46.9−52.2 | 49.6 | 1.49 | 51.5 | 50.7−57.1 | 53.7 | 1.50 |
| Orbital diameter | 15.9 | 13.9−17.6 | 15.6 | 0.93 | 12.8 | 11.0−14.1 | 12.5 | 0.88 |
| Interorbital width | 35.2 | 32.1−37.1 | 34.9 | 1.52 | 32.8 | 32.4−36.0 | 34.2 | 1.21 |
| Head depth | 54.7 | 51.6−59.2 | 55.4 | 2.17 | 45.3 | 42.4−47.7 | 44.8 | 1.99 |
| Suborbital depth | 24.7 | 20.9−25.5 | 24.1 | 1.26 | 20.8 | 17.4−22.0 | 20.0 | 0.85 |
| Mandibular ramus | 11.2 | 6.8−12.9 | 10.7 | 1.12 | 6.0 | 6.0−8.0 | 6.8 | 0.57 |
| Meristics | Holotype | Low−High | Mode | SD | Holotype | Low−High | Mode | SD |
| Left premaxillary teeth | 13 | 11−18 | 14 | 2.0 | 10 | 6−10 | 8 | 1.37 |
| Left dentary teeth | 14 | 11−15 | 13 | 1.22 | 6 | 4−7 | 6 | 0.42 |
| Left lateral scutes | 24 | 24−25 | 24 | 0.64 | 24 | 24−25 | 24 | 0.48 |
http://zoobank.org/2D0CE389-F31D-48AE-8C62-E1C6531410DF
http://species-id.net/wiki/Hisonotus_oliveirai
Figure 1; Table 1MZUSP 115061, 26.4 mm SL, female, Brazil, Paraná State, boundary between municipalities of Cambira and Apucarana, ribeirão Cambira, affluent of rio Ivaí, upper rio Paraná basin, 23°38'54"S, 51°29'58"W, coll. Zawadzki CH, de Paiva S, 29 October 2007.
Hisonotus oliveirai, holotype, MZUSP 115061, female, 26.4 mm SL, from ribeirão Cambira, affluent rio Ivaí, upper rio Paraná basin, boundary between municipalities of Cambira and Apucarana, Paraná State, Brazil.
All from Brazil, Paraná State. DZSJRP 18244, 3 males, 26.3−26.8 mm SL, ribeirão Salto Grande, rio Ivaí basin, municipality of Maria Helena, 23°37'08"S, 53°12'18"W, coll. Graça WJ, 30 December 2004. LBP 7358, 1 female, 28.4 mm SL, 1 unsexed, 12.4 mm SL, ribeirão Keller, rio Ivaí basin, boundary between municipalities of Marialva and Bom Sucesso, 23°38'30"S, 51°51'33"W, coll. Devidé R, 15 October, 2002. LBP 13332, 1 male, 23.2 mm SL, 1 unsexed c&s, 23.7 mm SL, rio Mourão, rio Ivaí basin, municipality of Campo Mourão, 24°02'23"S, 52°16'22"W, coll. Zawadzki CH, November 2010. LBP 13333, 1 male, 23.6 mm SL, 1 female, 25.4 mm SL, rio Mourão, rio Ivaí basin, municipality of Campo Mourão, 24°02'23"S, 52°16'22"W, coll. Pavanelli CS, 4 December 2006. LBP 13334, 1 male, 24.9 mm SL, ribeirão Keller, rio Ivaí basin, boundary between municipalities of Marialva and Bom Sucesso, 23°38'30"S, 51°51'32"W, coll. Zawadzki CH, November 2010. LBP 13335, 1 male, 26.0 mm SL, ribeirão Salto Grande, rio Ivaí basin, municipality of Maria Helena, 23°37'08"S, 53°12'18"W, coll. Graça WJ, 30 December 2004. LBP 14917, 4 females, 28.8−29.6 mm SL, 2 males, 26.6−27.4 mm SL, ribeirão Cambira, rio Ivaí basin, boundary between municipalities of Cambira and Apucarana, 23°58'54"S, 51°29'58"W, coll. Zawadzki CH, de Paiva S, 29 November 2007. LBP 17578, 3 females, 27.7−30.4 mm SL, 2 males, 25.4−26.1 mm SL, rio Mourão, rio Ivaí basin, boundary between municipalities of Engenheiro Beltrão and Quinta do Sol, 23°49'41"S, 52°11'43"W, coll. Zawadzki CH, Ruiz HB, Vieira RS, 01 April 2013. MCP 47860, 1 male, 25.6 mm SL, 1 female, 25.9 mm SL, ribeirão Salto Grande, rio Ivaí basin, municipality of Maria Helena, 23°37'08"S, 53°12'18"W, coll. Graça WJ, 30 December 2004. NUP 3578, 7 females, 27.8−28.1 mm SL, 8 males, 24.7−26.8 mm SL, 1 female c&s, 27.6 mm SL, 1 male c&s, 25.5 mm SL, ribeirão Salto Grande, rio Ivaí basin, municipality of Maria Helena, 23°37'08"S, 53°12'18"W, coll. Graça WJ, 30 December 2004. NUP 7065, 1 male, 23.3 mm SL, 1 female, 25.4 mm SL, 1 c&s unsexed, 24.5 mm SL, rio Mourão, rio Ivaí basin, municipality of Campo Mourão, 24°02'23"S, 52°16'22"W, coll. Zawadzki CH, 7 April 2009. NUP 9839, 1 male, 25.3 mm SL, 1 female, 25.8 mm SL, 1 female c&s, 25.0 mm SL, collected with holotype. NUP 15614, 10, 3 males, 25.9−26.5 mm SL, 7 females, 27.2−29.9 mm SL, rio Mourão, rio Ivaí basin, municipality of Engenheiro Beltrão, 23°37'41"S, 52°03'38"W, coll. Zawadzki CH, Ruiz HB, Silva HP, 22 October 2012. ZUEC 8006, 2, unsexed, 25.0−27.9 mm SL, rio Mourão, rio Ivaí basin, municipality of Engenheiro Beltrão, 23°37'41"S, 52°03'38"W, coll. Zawadzki CH, Ruiz HB, Silva HP, 22 October 2012. ZMA 250.056, 2, 1 male, 26.1 mm SL, 1 female, 25.6 mm SL, rio Mourão, rio Ivaí basin, municipality of Engenheiro Beltrão, 23°37'41"S, 52°03'38"W, coll. Zawadzki CH, Ruiz HB, Silva HP, 22 October 2012.
Hisonotus oliveirai can be distinguished from all congeners, except Hisonotus insperatus Britski & Garavello, 2003, Hisonotus luteofrenatus and Hisonotus paresi, by having odontodes forming longitudinally aligned rows (one odontode after the other, but not necessarily forming parallel series) on head and trunk, Fig. 2(A), (B) (vs. odontodes not forming longitudinally aligned rows). Additionally, the new species can be distinguished from all congeners except Hisonotus insperatus, Hisonotus luteofrenatus, Hisonotus paresi, and Hisonotus piracanjuba by having a pair of rostral plates at the tip of the snout (vs. a single rostral plate). Moreover, Hisonotus oliveirai can be further distinguished from all congeners except Hisonotus bockmanni, Hisonotus chromodontus, Hisonotus insperatus, Hisonotus luteofrenatus, and Hisonotus paresi by having a functional v-shaped spinelet (vs. spinelet non-functional, square-shaped, or absent). The new species can be distinguished from Hisonotus bockmanni and Hisonotus paresi by lacking contrasting dark geometric spots on the anterodorsal region of the body (vs. presence); from Hisonotus insperatus by having small, inconspicuous odontodes forming rows on the head and trunk (Fig. 2A, B; vs. large, conspicuous odontodes forming rows on the head and the trunk, Fig. 2E, F), a deeper head 51.6−59.2% HL (vs. 44.3−48.7% HL) and higher suborbital depth 20.9−25.5% HL (vs. 16.6−20.1% HL); from Hisonotus luteofrenatus by having a deeper caudal peduncle 10.8−12.5% SL (vs. 8.9−10.2% SL) and shorter snout 46.9−52.2% HL (vs. 67.0−75.3% HL); from Hisonotus paresi by a having deeper head 51.6−59.2% HL (vs. 42.4−47.7% HL), more premaxillary teeth 11−18 (vs. 6−10), and more dentary teeth 11−15 (vs. 4−7); fromHisonotus piracanjuba by having a deeper caudal peduncle 10.8−12.5% SL (vs. 8.3−9.5% SL), and shorter snout 46.9−52.2% HL (vs. 67.7−72.7% HL).
Variation in hypertrophied series of anterolateral (A, C, E) and anterodorsal (B, D, F) odontodes across three species. A Hisonotus oliveirai, paratype, NUP 9839, female, 25.8 mm SL, small odontodes B Hisonotus oliveirai, paratype, NUP 9839, female, 25.8 mm SL, small odontodes C Hisonotus paresi, paratype, NUP 10928, male, 24.2 mm SL, small odontodes D Hisonotus paresi, paratype, NUP 10928, male, 24.2 mm SL, small odontodes E Hisonotus insperatus, LBP 1316, 24.7 mm SL, large and conspicuous odontodes F Hisonotus insperatus, LBP 1316, 24.7 mm SL, large and conspicuous odontodes.
Morphometric data presented in Table 1. Maximum body length 28.4 mm SL. Dorsal profile of head slightly convex to straight from upper part of rostrum to posterior margin of nares, convex from eyes to posterior margin of parieto-supraoccipital, and straight to dorsal-fin origin. Dorsal profile of trunk slightly concave and descending from dorsal-fin origin to end of dorsal-fin base, straight to caudal peduncle. Ventral profile strongly concave from snout tip to opercular region; convex from opercular region to anal-fin origin; concave to caudal-fin insertion. Greatest body depth at dorsal-fin origin (18.6−23.9% SL). Greatest body width at opercular region, gradually decreasing towards snout and caudal fin. Cross-section of caudal peduncle almost ellipsoid; rounded laterally and almost flat dorsally and ventrally.
Head rounded in dorsal view, snout round to slightly pointed. Dorsal and ventral series of odontodes along anterior margin of snout completely covering its tip; odontodes larger than remaining ones on head. Odontodes on head and trunk hypertrophied and arranged in longitudinal rows (most prominent on head). Eyes moderately small (13.9−17.6% in HL), dorsolaterally positioned. Lips roundish with papillae uniformly distributed on base of dentary and premaxilla and slightly decreasing distally. Lower lip larger than upper lip; its border fringed. Maxillary barbel present; joined to lower lip by membrane for half its length. Teeth slender and bicuspid; mesial cusp larger than lateral cusp. Premaxillary teeth 11−18. Dentary teeth 11−15.
Dorsal-fin ii, 7; dorsal-fin spinelet short and V-shaped; dorsal-fin lock functional; dorsal-fin origin slightly posterior to pelvic-fin origin. Tip of adpressed dorsal fin almost reaching end of anal-fin base. Pectoral-fin i, 6; its tip almost reaching middle of pelvic-fin unbranched ray length when depressed. Pectoral axillary slit present between pectoral-fin insertion and lateral process of cleithrum. Pectoral spine supporting odontodes on ventral, anterior and dorsal surfaces. Pelvic-fin i, 5; tip of pelvic-fin longest ray almost reaching anal-fin origin when depressed in females and reaching anal-fin origin in males. Pelvic-fin unbranched ray with dermal flap along its dorsal surface in males. Anal-fin i, 5; its tip reaching 7th or 8th plate from its origin. Caudal-fin i, 14, i; distal margin forked. Adipose-fin absent. Total vertebrae 27.
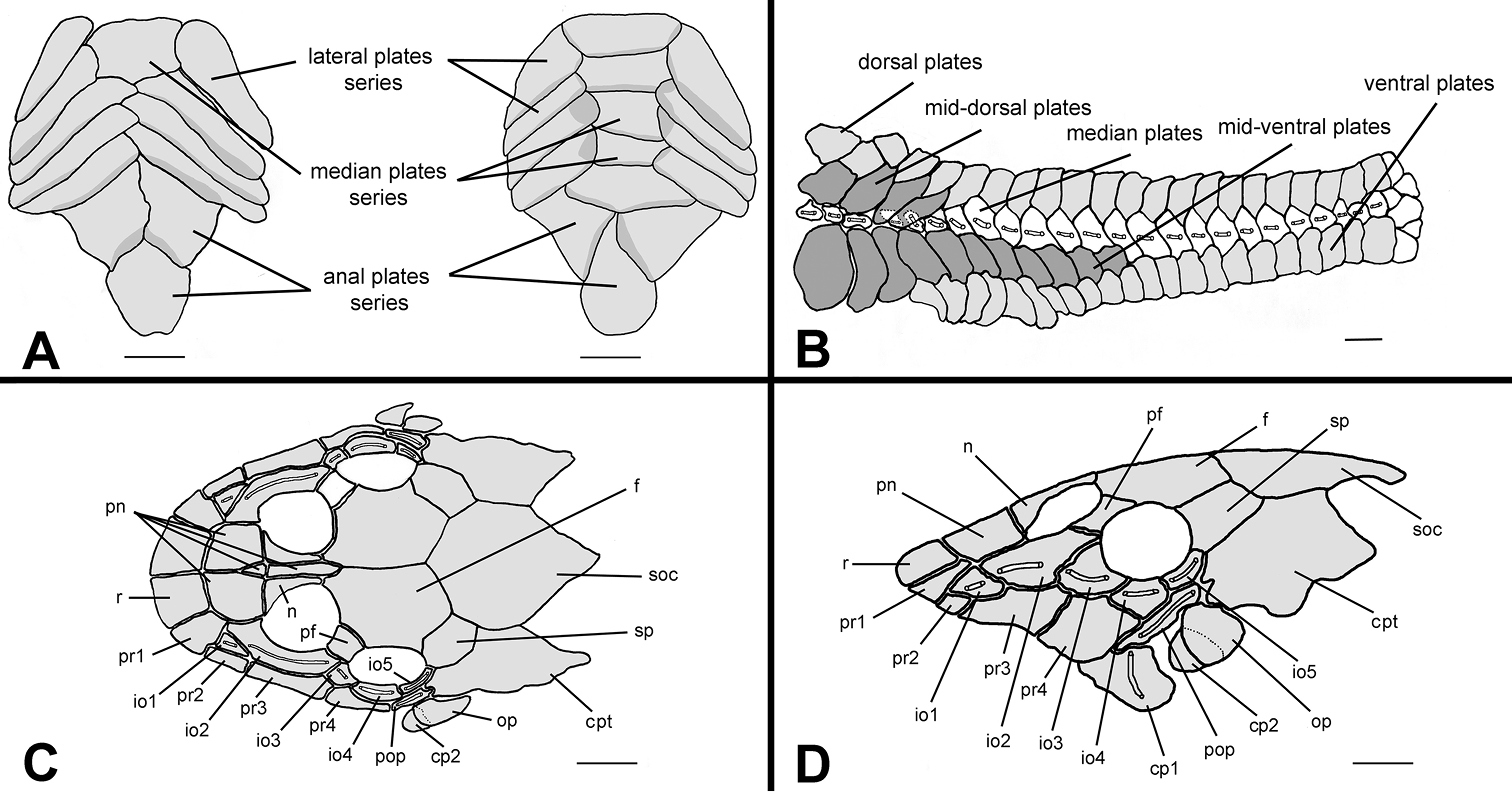
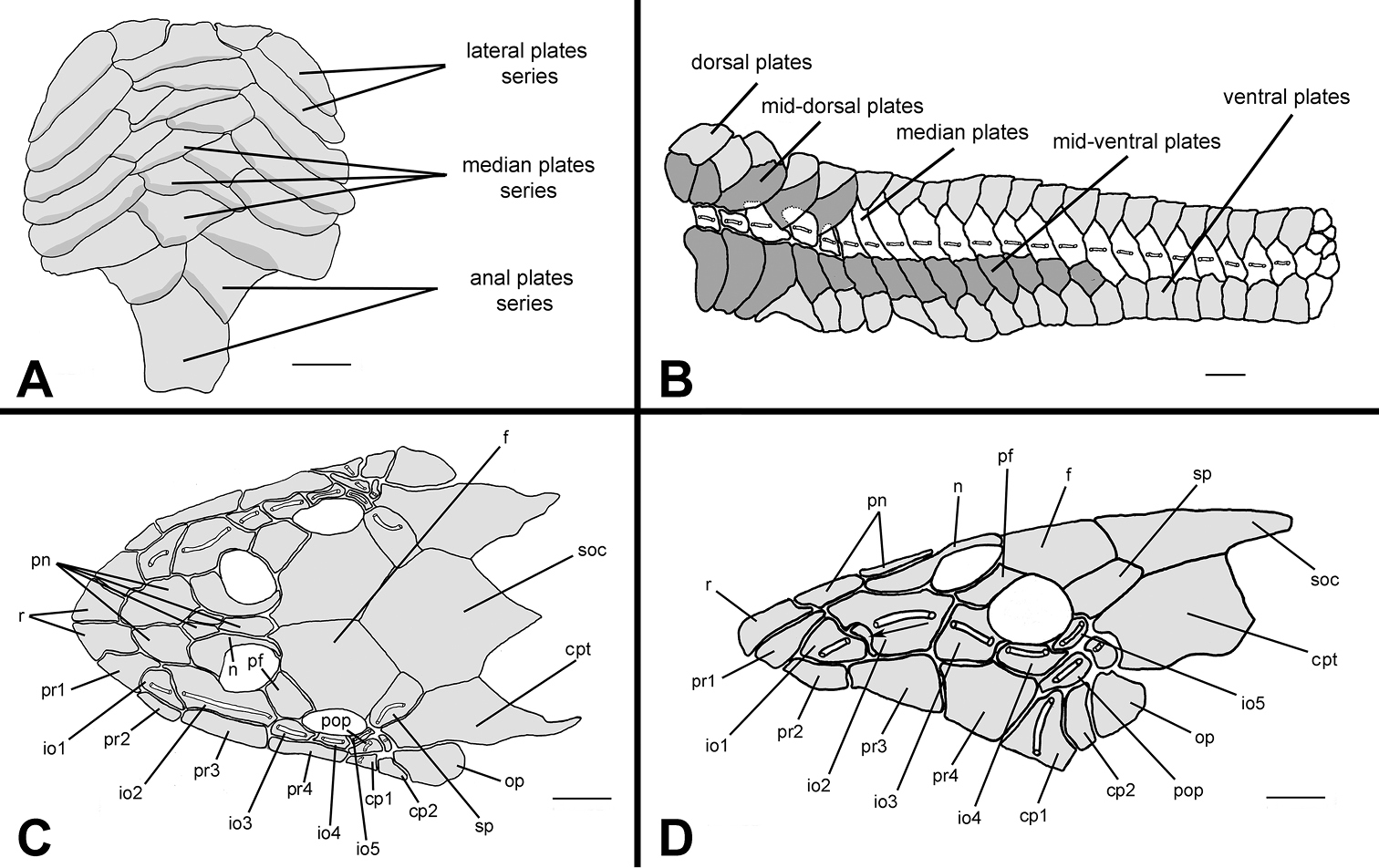
Body covered with bony plates except above lower lip, around pectoral and pelvic-fin origins and on dorsal-fin base. Cleithrum and coracoid totally exposed. Arrector fossae partially to completely enclosed by ventral lamina of coracoids. Abdomen entirely covered by plates (Fig. 3A); abdomen covered by large, elongate lateral plate series, formed by two lateral rows, approximately of same size; median plates formed by two patterns of plate distributions; first, median plate series not reaching anal shield plates with lateral plate series beginning to contact each other at middle of abdomen; second, median plate series reaching anal shield and lateral plate series remaining separate; anal plates series covered by large square or triangular plates. Body entirely covered laterally by plates (Fig. 3B); mid-dorsal plates poorly developed and reaching middle of dorsal-fin base; median plates series continuous in median portion of body; mid-ventral plates reaching vertical through end of dorsal-fin base.
Hisonotus oliveirai, paratype, NUP 7065, sex unknown, 24.5 mm SL A Ventral view of abdominal region showing intraspecific variation in abdominal dermal plate patterns B lateral trunk plates; cranial bones and dermal plates of the head in dorsal C and lateral D view. Scale bars: 1 mm.
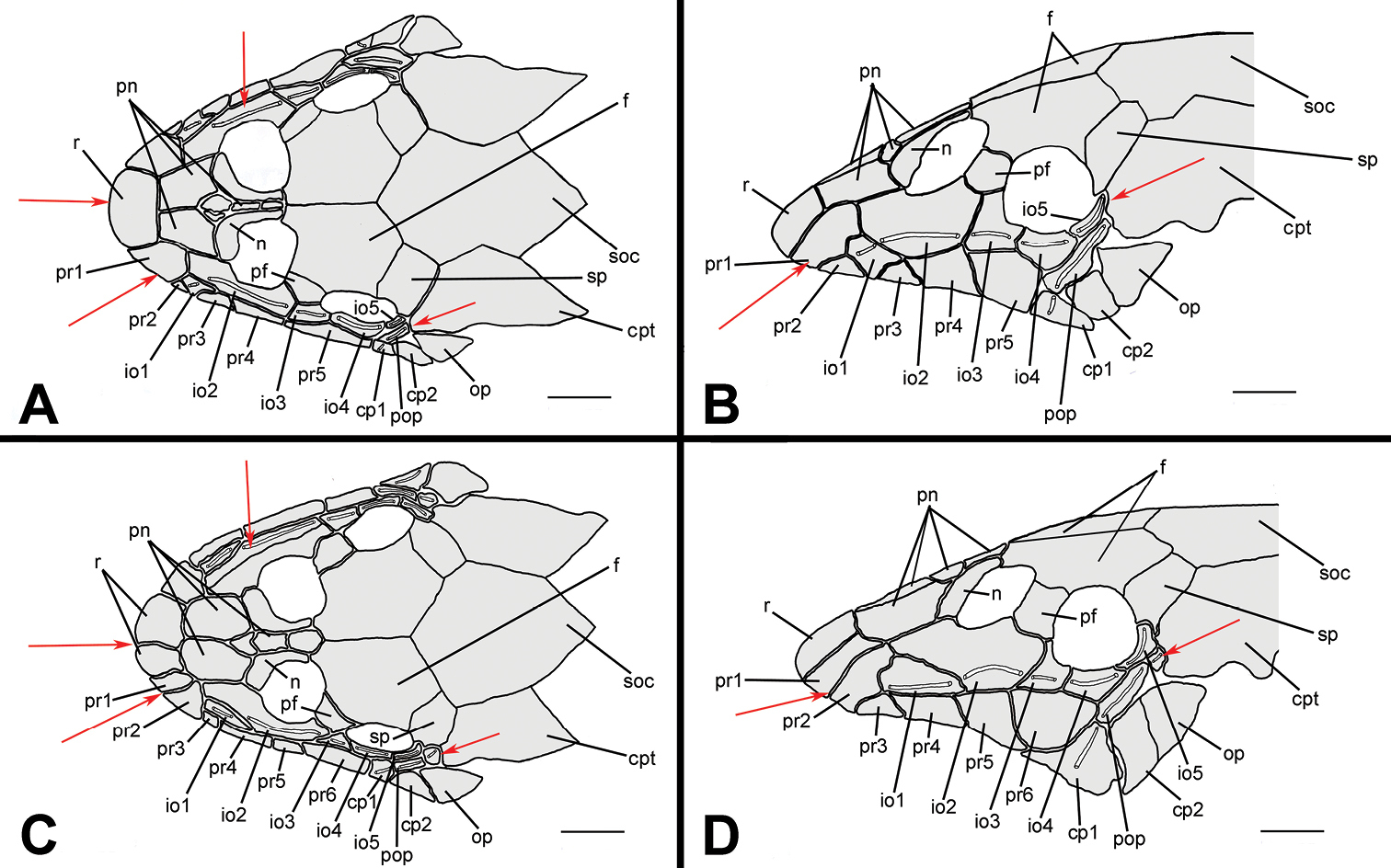
Parts of dorsal head bone plates presented in Fig. 3C. Snout tip formed by one pair of square rostral plates (r). Nasal (n) rectangular, forming anterior medial nostril margin, posterior nasal margin contacting frontals (f), anterior and lateral margins contacting pre-nasals (pn). Pre-nasals (pn) positioned posterior to rostral plates (r); formed by two large square-shaped plates, one small and triangular and one elongated and rectangular between nares. Posterodorsal head plates consist of compound pterotic (cpt), parieto-supraoccipital (soc) and frontal (f; largest bones of head), prefrontal (pf) and sphenotic (sp). Compound pterotic (cpt) covered with few and small, unclustered fenestra. Lateral surface of head illustrated in Fig. 3D. Posterior rostrum plates pr1-pr2 smallest, rectangular shaped; pr4-pr3 largest, first rectangular and second square. Complete infraorbital plate series (io1-io5), present just above posterior rostrum series, all covered by laterosensory canal system; io2 largest and io5 smallest; io3, io4 and io5 forming inferior orbital margin of eyes. Preopercle (pop) elongate and rectangular, covered by laterosensory canal; preopercle present under pr4, io4 and io5, and upper cp1, cp2 and op. Subocular cheek plates (cp1-cp2) and opercle (op) form posterior lateral margin of head.
Pale yellowish ground color. Dorsal surface of head dark brown, except for pale yellowish areas on snout tip, lateral margin of head and tip of parieto-supraoccipital. Three dark-brown saddles crossing dorsum, reaching longitudinal dark stripe on side of trunk: first below dorsal-fin origin, second typically at adipose-fin region, and third at end of caudal peduncle. Ventral region of anal-fin origin with small single-chromatophore spots. Caudal fin hyaline with two black bars; first at caudal-fin origin, second at middle of caudal fin (Fig. 1).
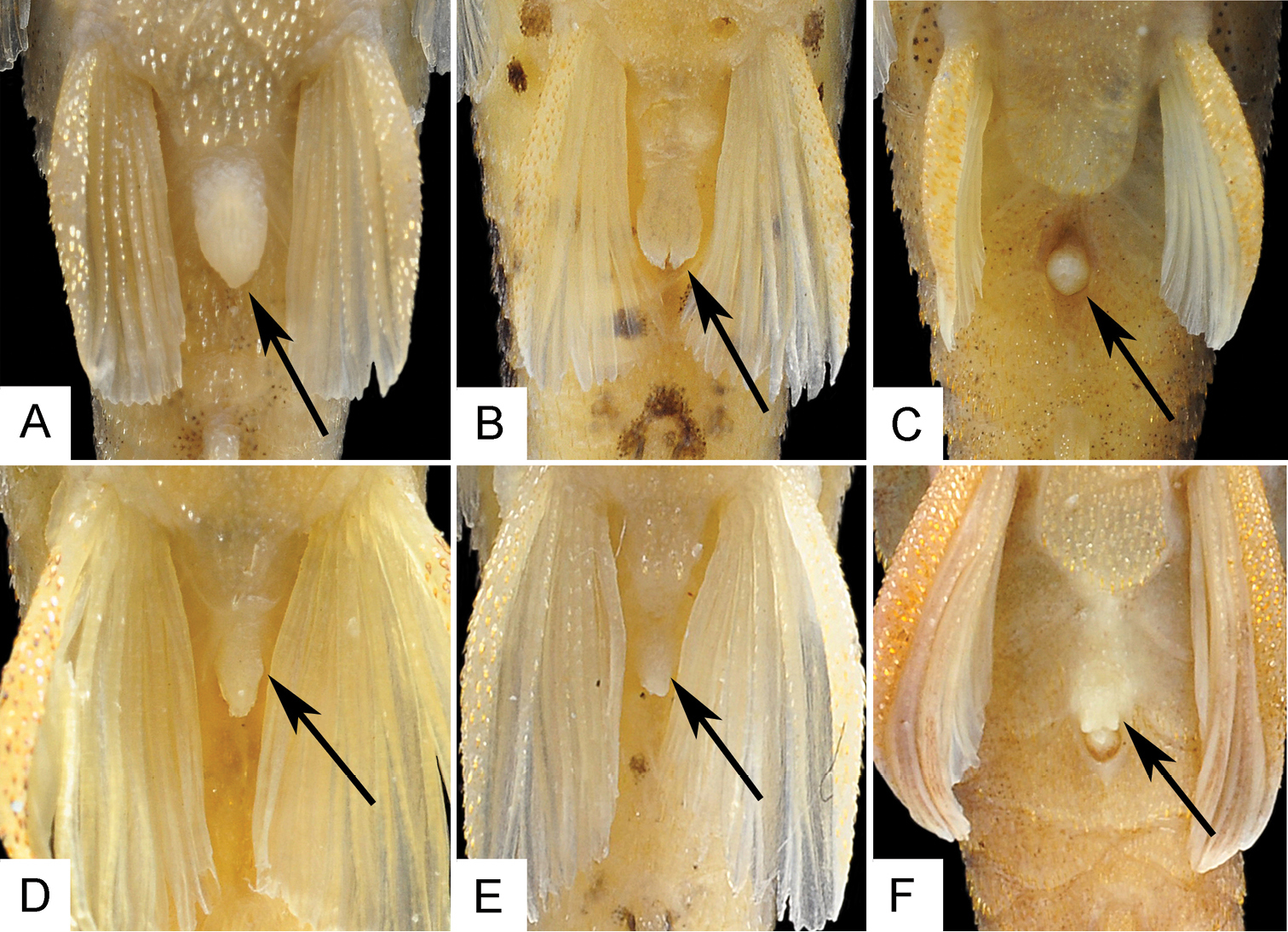
Adult males are distinguished by having a papilla at the urogenital opening (vs. papilla absent in females); a pelvic fin that extends beyond anal-fin origin (vs. pelvic fin not reaching anal-fin origin in females); and an unbranched pectoral- and pelvic-fin ray supporting a dermal flap on their proximal dorsal surface in males. Both sexes have a membrane at anal opening; however, the membrane is longer and large in females (Fig. 4A) than in males (Fig. 4D), covering almost the entire urogenital opening.
Ventral view of abdominal region of three species of Hisonotus, arrows indicate anal membrane in Hisonotus oliveirai (A, D) and Hisonotus paresi (B, E) contrasting with the lack of the anal membrane in Hisonotus chromodontus (C, F). A Hisonotus oliveirai, MZUSP 115061, holotype, female, 26.4 mm SL B Hisonotus paresi, MZUSP 115062, holotype, female, 26.2 mm SL C Hisonotus chromodontus, LBP 7964, female, 28.1 mm SL D Hisonotus oliveirai, NUP 3578, male, 27.1 mm SL E Hisonotus paresi, NUP 10928, male, 24.2 mm SL F Hisonotus chromodontus, LBP 12278, male, 26.7 mm SL.
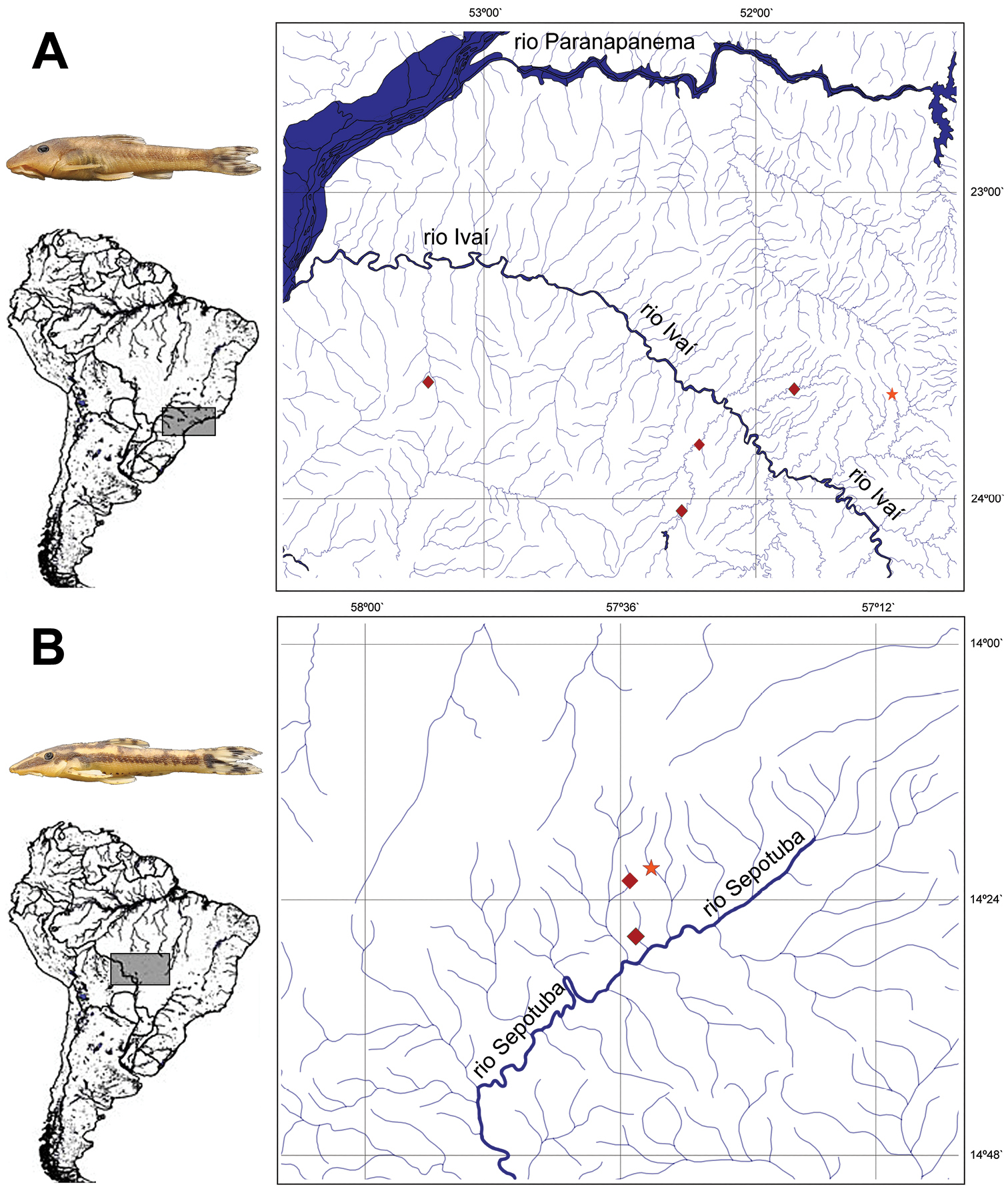
Hisonotus oliveirai is only known from four small to medium-sized streams, the ribeirão Salto Grande, ribeirão Keller, rio Mourão, and the ribeirão Cambira, all tributaries of the rio Ivaí in the upper rio Paraná basin (Fig. 5A).
Map of the distribution of A Hisonotus oliveirai. Star = holotype locality, ribeirão Cambira. Diamonds = paratype localities B Hisonotus paresi. Star = holotype locality, riacho Águas Claras. Diamonds = paratype localities.
The specific epithet oliveirai (a noun in the genitive case) is a patronym honoring professor Claudio Oliveira from the Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP), Botucatu, São Paulo State, in recognition of his dedication and contributions to the studies of Neotropical freshwater fishes.
http://zoobank.org/FBC435D8-A305-4027-A3C5-5556971CFF8E
http://species-id.net/wiki/Hisonotus_paresi
Figure 6; Table 1MZUSP 115062, 26.2 mm SL, female, Brazil, Mato Grosso State, municipality of Santo Afonso, riacho Águas Claras, affluent rio Sepotuba, rio Paraguay basin, 14°21'03"S, 57°33'07"W, coll. Troy WP, 14 September 2010.
Hisonotus paresi, holotype, MZUSP 115062, female, 26.2 mm SL, riacho Águas Claras, affluent rio Sepotuba, rio Paraguay basin, municipality of Santo Afonso, Mato Grosso State.
All from Brazil, Mato Grosso State, rio Sepotuba basin. DZSJRP 18245, 2 females, 19.9−24.3 mm SL, collected with holotype. LBP 13347, 2 females, 18.9−19.6 mm SL, collected with holotype. LBP 13351, 9, 14.7−24.3 mm SL, riacho Águas Claras, Santo Afonso, 14°21'03"S, 57°33'07"W, coll. Troy WP, April 2012. LBP 13352, 1, 23.7 mm SL, riacho Águas Claras, Santo Afonso, 14°21'03"S, 57°33'07"W, coll. Troy WP, April 2012. LBP 17532, 1 male 22.6 mm SL, 2 female 19.5−23.8 mm SL, 1 unsexed not measured, riacho Maracanã, boundary between municipalities of Santo Afonso and Nova Marilândia, 14°22'40"S, 57°35'11"W, coll. Troy WP, Paliga T, Silva VM, 3 April 2010. NUP 10928, 2 males, 23.2−24.2 mm SL, 2 c&s, 23.6−24.2 mm SL, 1 unsexed not measured, collected with holotype. NUP 10976, 3 unsexed, 16.7−20.5 mm SL, riacho São Jorge, municipality of Santo Afonso, 14°27'26"S, 57°34'34"W, coll. Zawadzki CH, Troy WP, 19 August 2010.
Hisonotus paresi can be distinguished from all congeners, except Hisonotus bockmanni, by the presence of contrasting dark geometric spots on the anterodorsal region of body (vs. absence of geometric spots). Additionally, the new species can be distinguished from all congeners, except Hisonotus insperatus, Hisonotus luteofrenatus, Hisonotus oliveirai, Hisonotus piracanjuba) by having a pair of rostral plates at the tip of the snout (vs. a single rostral plate). Also Hisonotus paresi can be distinguished from all congeners, except Hisonotus insperatus, Hisonotus luteofrenatus and Hisonotus oliveirai by having odontodes forming longitudinally aligned rows on head and trunk, Fig. 2C, D (vs. odontodes not forming longitudinally aligned rows). The new species can be distinguished from Hisonotus bockmanni by having a continuous median series of perforated plate (vs. median plate series of perforated plates discontinuous, that is, with a gap of unperforated plates), by lacking unpaired plates between the contra-lateral dorsal series (vs. having two tiny unpaired plates between the contra-lateral dorsal series, placed eight plates posterior to dorsal fin – see fig. 4 in
Morphometric data presented in Table 1. Maximum body length 26.2 mm SL. Lateral profile of head convex; straight from upper part of rostrum to posterior margin of nares, slightly curved from eyes to posterior margin of parieto supraoccipital, almost straight to dorsal-fin origin. Dorsal profile of trunk slightly concave, descending from base of dorsal-fin origin to end of dorsal-fin base, straight to caudal peduncle. Ventral profile slightly concave from snout tip to pectoral-fin origin, convex to anal-fin origin, slightly concave to caudal peduncle. Greatest body depth at dorsal-fin origin (16.9−20.7% SL). Greatest body width at opercular region, gradually decreasing towards snout and caudal fin. Cross-section of caudal peduncle almost ellipsoid; rounded laterally and almost flat dorsally and ventrally.
Head rounded in dorsal view. Snout slightly pointed, its tip rounded, elongated (50.7−57.1% HL) and depressed in front of each nostril on dorsal surface. Dorsal and ventral series of odontodes completely covering anterior margin of snout; odontodes of snout similar in size to remaining ones found on head. Snout tip lacking band devoid of odontodes. Odontodes on head and trunk well defined and arranged into longitudinal rows (character more prominent in head). Eyes small (11−14.1% HL), dorsolaterally positioned. Lips roundish and papillose; uniformly distributed on base of dentary and premaxilla and slightly decreasing distally. Lower lip larger than upper lip; its border strongly fringed. Maxillary barbel present. Teeth slender and bicupid; mesial cusp larger than lateral cusp. Premaxillary teeth 6−10. Dentary teeth 4−7.
Dorsal-fin ii, 7; dorsal-fin spinelet short and V-shaped; dorsal-fin lock functional; its origin slightly anterior to pelvic-fin origin. Tip of adpressed dorsal-fin rays surpassing end of anal-fin base. Pectoral-fin i, 6; tip of longest pectoral-fin ray almost reaching half of pelvic-fin length, when depressed. Pectoral axillary slit present between pectoral-fin insertion and lateral process of cleithrum. Pectoral spine supporting odontodes anteroventrally. Pelvic-fin i, 5; its tip almost reaching anal-fin origin when depressed in females and reaching anal-fin origin in males. Pelvic-fin unbranched ray with dermal flap along its dorsal surface in males. Anal fin i, 5; its tip reaching 7th and 8th from its origin. Caudal-fin i, 14, i; distal margin emarginated. Adipose-fin absent. Total vertebrae 27.
Body covered with bony plates except on ventral part of head, around pectoral and pelvic-fin origin and on dorsal-fin base. Cleithrum and coracoid totally exposed. Arrector fossae partially enclosed by ventral lamina of coracoids. Abdomen entirely covered by plates (Fig. 7A), abdomen formed by lateral plate series with elongate and large plates, formed by two lateral plates series, similar in size; median plates formed by one to three plates series reaching anal shield. Lateral of body entirely covered by plates (Fig. 7B); mid-dorsal plates poor developed, reaching middle of dorsal-fin base; median plates not interrupted in median portion of body; mid-ventral plates reaching end of dorsal-fin base.
Hisonotus paresi, paratype, NUP 10928, male, 24.2 mm SL A Ventral view of abdominal plates B lateral trunk plates; cranial bones and dermal plates of the head in dorsal C and lateral D view. Black arrows (D) indicate an extra plate that is absent in the right side of the same specimen. Scale bars: 1 mm.
Parts of dorsal head bone plates presented in Fig. 7C. Snout tip formed by one pair of rostral square-shaped plates (r). Nasal (n) almost rectangular forming anterior medial nostril margin in contact posteriorly with frontals (f) and anteriorly and laterally with pre-nasals (pn). Pre-nasals (pn) positioned posteriorly of rostral plates (r), formed by two large and one small square-shaped plates, and one elongate rectangular shaped between nares. Top of head composed by compound pterotic (cpt), parieto supraoccipital (soc) and frontal (f), largest bones of head, and prefrontal (pf) and sphenotic (sp). Compound pterotic (cpt) fenestrated randomly distributed. Lateral surface of head presented in Fig. 7D. Posterior rostrum plates pr1-pr2 small, and rectangular shaped; pr4-pr3 largest, first rectangular and second square-shaped. Infraorbital plate series complete (io1-io5), present just above posterior rostrum series, all covered by latero-sensory canal system; io2 largest and io5 smallest; io3, io4 and io5 forming inferior orbital margin of eyes; preopercle (pop) elongated and rectangular, covered by latero-sensory canal; preopercle present under io4 and io5, and upper cp1, cp2 and op. Subocular cheek plates (cp1-cp2) and opercle (op) form posterior lateral margin of head.
Ground color of dorsal and ventral region of head and trunk pale yellowish. Conspicuous longitudinal dark stripe enlarging from rostral plates to anterior corner of eyes, straightening and bordering on ventral margin of eyes, enlarging again through compound pterotic and lateral series of plates to caudal-fin. Another conspicuous longitudinal dark stripe starting medially at pre-nasal plate region and enlarging on supraoccipital region. Unpigmented portion of snout appears as hyaline v-shaped mark from rostral plate passing through nares to orbital margins. Longitudinal dark stripe from superior portion of sphenotic through mid-dorsal plates to posterior margin of dorsal-fin base. Dark blotch on compound pterotic overlaps mid-dorsal longitudinal dark stripe. Dark saddle on middle portion of predorsal region reaches mid-dorsal longitudinal dark stripe. Overall, pigmentation pattern forms geometric spots on anterodorsal region of body. Three dark saddles usually cross posterodorsal region of body, reaching longitudinal stripe on side of trunk: first saddle at middle of dorsal fin, second at adipose-fin region, and third at end of caudal peduncle. Saddles inconspicuous in some specimens. Ventral region of body almost completely pale yellowish, except few dark spots on caudal peduncle and dark ring at anal-fin origin. Dorsal, pectoral, and pelvic fins with dark chromatophores forming irregular sets of bands: three on dorsal and pectoral fin, and one on pelvic fin. Anal fin with few scattered chromatophores, sometimes forming bands. Caudal fin hyaline, except for dark spot on origin of rays, and dark band on middle of rays (Fig. 6).
Adults males have a papilla in urogenital opening (vs. absent in females); have a longer pelvic fin that extends beyond anal-fin origin (vs. pelvic fin not reaching anal-fin origin in females); and have an unbranched pelvic-fin ray supporting a dermal flap along its dorsal surface. Both sex have a membrane on the anal opening; however, this membrane is more developed in females (Fig. 4B) than in males (Fig. 4E), covering almost the entire urogenital opening.
The species is known from three small tributaries the riacho Águas Claras, riacho Maracaña and riacho São Jorge, all draining to the rio Sepotuba, in the upper rio Paraguay basin (Fig. 5B).
The species name paresi (a noun in apposition), refers to the the Paresí Indians who speak Paresí, a branch of the Aruak language. The Paresí used to live throughout most of Mato Grosso State including the municipality of Santo Afonso. Paresí were also some of the main guides of Marechal Cândido Rondon, the famous Brazilian pioneer in this region of Brazil at the beginning of the 18th century.
Hisonotus oliveirai is externally similar to Hisonotus insperatus and Hisonotus piracanjuba both species from upper stretches of the rio upper rio Paraná basin, Hisonotus paresi resembles more closely to Hisonotus bockmanni from the rio Tapajós basin. Hisonotus insperatus, Hisonotus chromodontus, Hisonotus luteofrenatus, and Hisonotus oliveirai have conspicuous odontodes forming well defined and widely spaced rows on the head and trunk (the main character used to distinguish theses species), while Hisonotus paresi has smaller, less conspicuous odontodes that form closely spaced rows (Fig. 2). Additionally, Hisonotus insperatus, Hisonotus oliveirai and Hisonotus piracanjuba have a deep head with a snout tip that rises abruptly to the interorbital region in lateral view, resulting in a short-snouted head profile. In Hisonotus bockmanni, Hisonotus chromodontus, Hisonotus luteofrenatus and Hisonotus paresi, the snout tip rises gently to the interorbital region in lateral view, resulting in a more long-snouted profile. The two snout patterns fit existing geographic patterns since Hisonotus insperatus, Hisonotus oliveirai and Hisonotus piracanjuba inhabit the upper rio Paraná while Hisonotus paresi is from the upper rio Paraguay and Hisonotus bockmanni, Hisonotus chromodontus and Hisonotus luteofrenatus are from the upper rio Tapajós. Such patterns among apparently closely related but now allopatric species suggest that the latter three species may have once shared a more broadly distributed ancestor. Moenkhausia cosmops Lima, Britski & Machado 2007, Leporinus octomaculatus Britski & Garavello, 1993, Moenkhausia phaeonota Fink, 1979, Hyphessobrycon vilmae Géry, 1966, and Aequidens rondoni Miranda-Ribeiro, 1918, Parodon nasus Kner, 1859, Hemiodus semitaeniatus Kner, 1858, are other examples of fishes occurring in the upper rio Paraguay basin, as well as in the upper rio Tapajós basin. Also, Batrochoglanis melanurus Shibatta & Pavanelli, 2005, which occurs at the upper rio Paraguay, appears to have its sister-taxon in the rio Tapajós basin. According to
Hisonotus paresi has an unusual coloration pattern with contrasting dark stripes and bands converging to form geometric spots on the anterodorsal region of body, which is more similar in coloration to species of Otocinclus than to Hisonotus. However, Hisonotus paresi is morphologically similar to nominal species already assigned to Hisonotus, rather than to any other Hypoptopomatinae species. Additionally, Hisonotus paresi and Hisonotus oliveirai exhibit one of the diagnostic characters used to define Hisonotus in its resurrection by
Osteological characters are known to be conservative within Hypoptopomatinae species compared to external anatomy (
Cranial bones and dermal plates of the head of Hisonotus insperatus in dorsal (A, C) and lateral (B, D) view. Specimen illustrated in A and B: LBP 13336, female, 26.0 mm SL, from rio Capivara; specimen in C and D: LBP 13337, female, 28.6 mm SL, from rio Araquá, (both from Botucatu, São Paulo State). Red arrows indicate differences in osteology between the specimens. Scale bars: 1 mm.
All from Brazil, except when stated otherwise: Hisonotus aky Azpelicueta, Casciotta, Almirón & Koerber, 2004: MHNG 2643.039, 2, 33.1−34.2 mm SL, paratypes, arroio Fortaleza, Argentina; Hisonotus bocaiuva Roxo, Silva, Oliveira & Zawadzki, 2013: MZUSP 112204, male, 24.2 mm SL, holotype, córrego Cachoeira, Bocaiúva, Minas Gerais; LBP 9817, 9, 3 c&s, 18.3−23.2 mm SL, paratypes, córrego Cachoeira, Bocaiúva, Minas Gerais; Hisonotus carreiro Carvalho & Reis, 2011: MCP 40943, 3, 33.6−35.8 mm SL, arroio Guabiju, Guabiju, Rio Grande do Sul; Hisonotus charrua Almirón, Azpelicueta, Casciotta & Litz, 2006: LBP 4861, 1, 35.9 mm SL, arroio Guaviyú, Artigas, Uruguai; MHNG 2650.051, 1, 34.2 mm SL, paratype, arroio Aspinillar, Uruguay; Hisonotus chromodontus Britski & Garavello, 2007: LBP 7964, 25, 24.0−28.3 mm SL, 3 females c&s, 26.5−28.9 mm SL, 1 male c&s 24.9 mm SL, rio dos Patos, Nova Mutum, Mato Grosso; LBP 12278, 2, 26.7−28.7 mm SL, 1 unsexed c&s, 26.7 mm SL, rio Sumidouro, Tangará da Serra, Mato Grosso; MZUSP 45355, holotype, 25.9 mm SL, affluent rio Preto, Diamantino, Mato Grosso; Hisonotus depressicauda Miranda Ribeiro, 1918: MZUSP 5383, 24.4 mm SL, paralectotype (designated by Britski, 1969), Sorocaba; Hisonotus francirochai Ihering, 1928: LBP 13923, 22, 25.7−35.7 SL, córrego sem nome, Capitinga, Minas Gerais; MZUSP 3258, 29.4 mm SL, lectotype (designated by
We thank Guilherme J. Costa Silva, Gabriel S. Costa e Silva and Fernanda O. Martins for helping with the osteology analysis and with the measurements; Ricardo C. Benine for providing laboratorial support; Renato Devidé, Weferson da Graça, Claudio Oliveira and Carla S. Pavanelli for helping with field work; Mahmoud Mehanna for cleared and double stained specimens; Nathan K. Lujan, Marcelo Melo, Ricardo C. Benine, Bruno F. Melo, Fernanda O. Martins and Danilo Pinhal for reading the manuscript and giving valuable suggestions. Logistical support was provided by Nupelia (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Maringá, Paraná State, Brazil). This research was supported by the Brazilian agencies FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, proc. 2010/01610-9 to FFR), MCT/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (Edital Universal, proc. N. 484716-2006-9 and grants to CHZ - proc. N. 310733/2013-8) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).