(C) 2013 D.J. Williams. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Williams DJ, Hodgson CJ (2013) Are some prepupae and pupae of male mealybugs and root mealybugs (Hemiptera, Coccoidea, Pseudococcidae and Rhizoecidae) mobile? ZooKeys 364: 19–28. doi: 10.3897/zookeys.364.6459
It is hypothesised here that some mealybug (Pseudococcidae) and root mealybug (Rhizoecidae) prepupae and pupae are mobile. The prepupa and pupa of the mealybug Promyrmococcus dilli Williams and the prepupa of the root mealybug Ripersiella malschae (Williams) are described and illustrated and their probable mobility is discussed. It is also suggested that the prepupae and pupae of the mealybug Macrocepicoccus loranthi Morrison can move rapidly on the leaves when disturbed.
Coccoidea, Pseudococcidae, Rhizoecidae
Most male scale insects (Hemiptera: Coccoidea) only feed in their first and second instars and then pass through non-feeding prepupal and pupal instars before emerging as an adult that can be either wingless or alate. During development of what are now usually termed the archaeococcoid scale insects (
Because most male prepupae and pupae develop beneath this waxy cover, the legs are usually non-functional with the claw either showing no signs of development or reduced to a mere point. However, among male mealybugs (Pseudococcidae), there are some taxa in which the prepupa and pupa have relatively well-developed legs, including claws and digitules, that would appear to allow mobility of these insects (Figs 1–3).
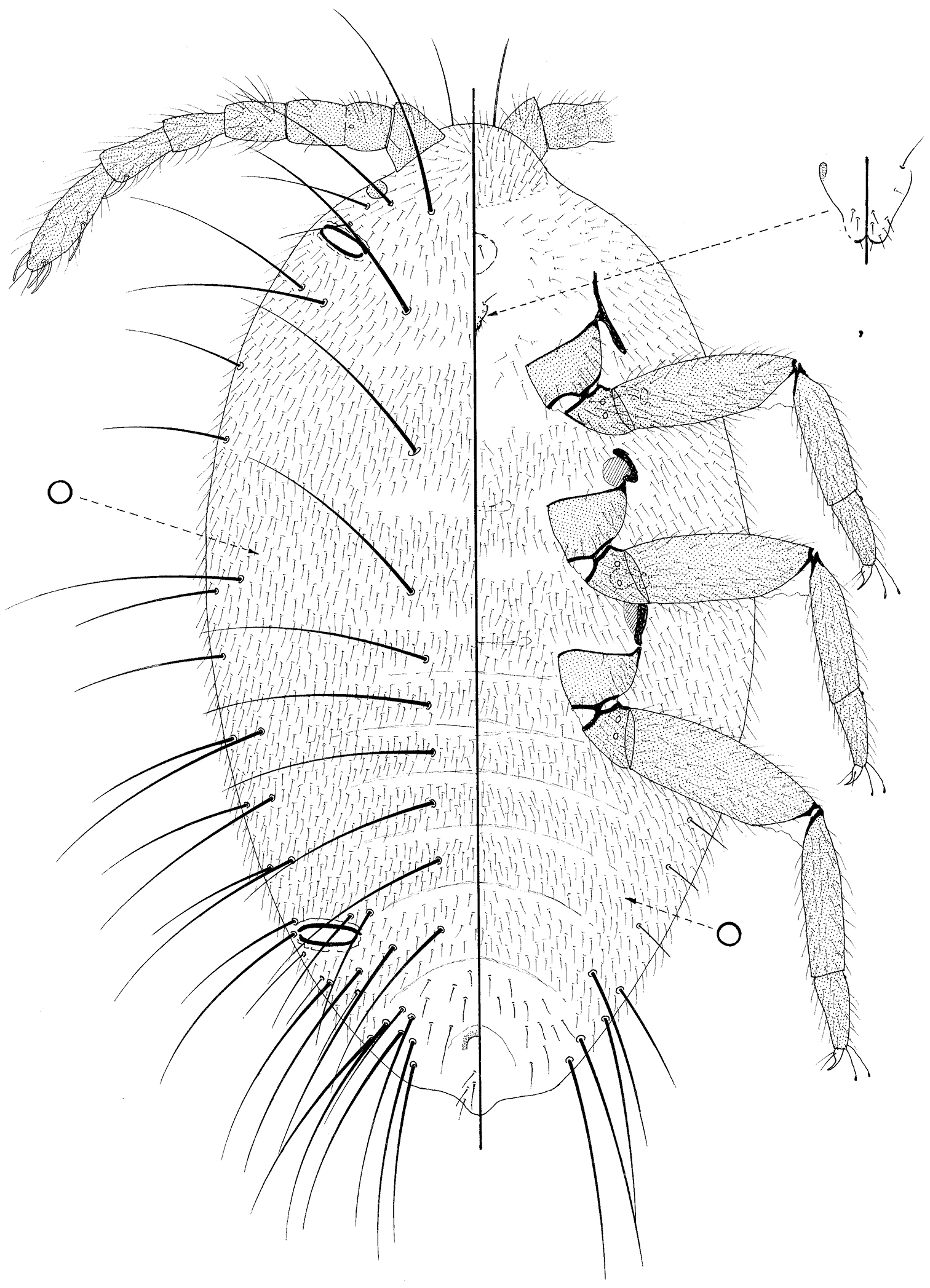
Prepupa of Promyrmococcus dilli Williams.
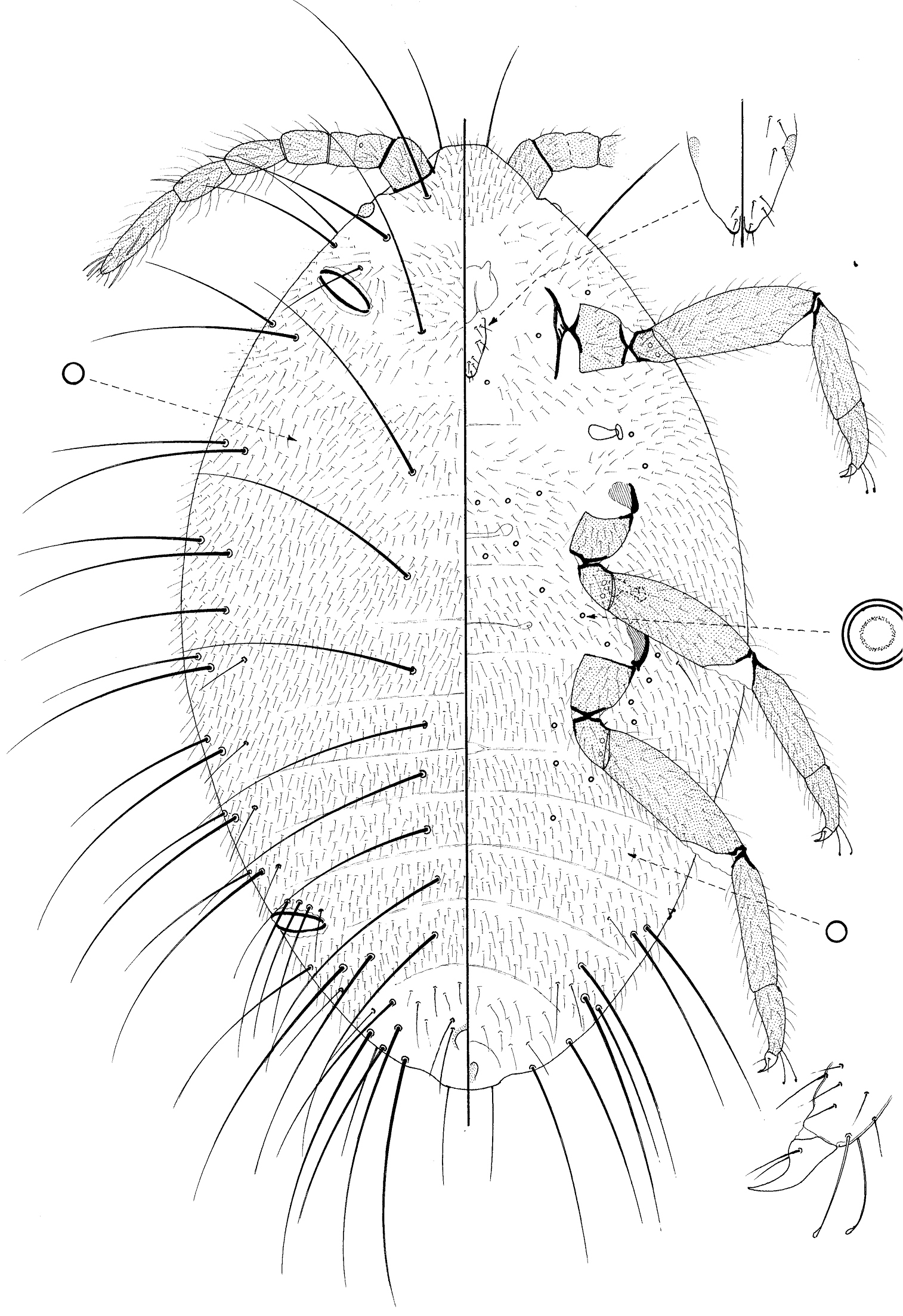
Pupa of Promyrmococcus dilli Williams.
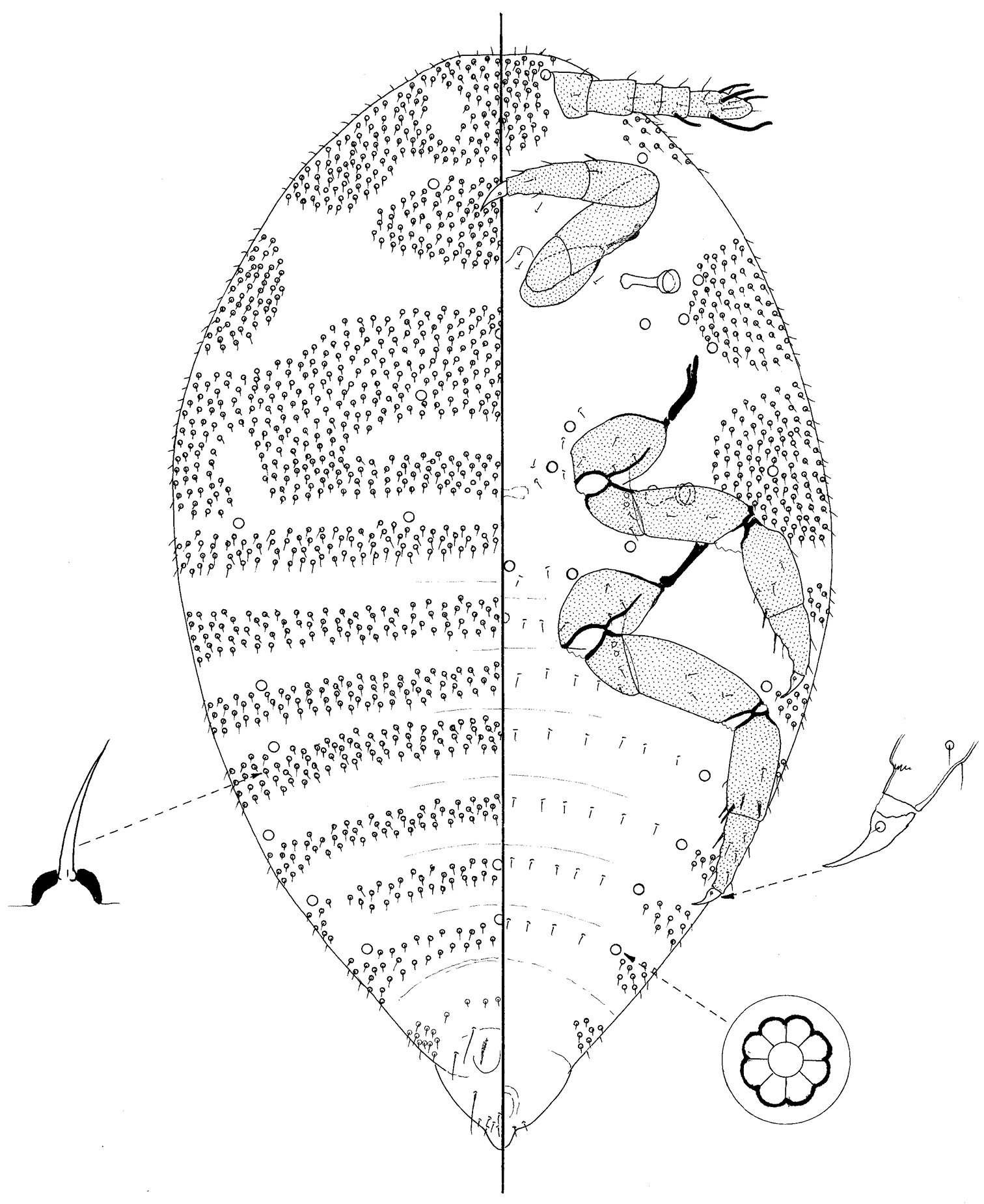
Prepupa of Ripersiella malschae (Williams).
Digitules in scale insects are modified setae. The term is usually used for a pair of setae at the base of the claw and a pair at the outer distal end of the tibia of all female instars and the male feeding stages of most taxa. Although sometimes these digitules are setose, many are so-called knobbed or actually spoon-shaped. The adult females of Steingelia Nasonov (Steingeliidae) have 12 such digitules on the claw (
Digitules reach their fullest development in mealybugs of the tribe Allomyrmococcini (Pseudococcidae) as they are much larger than the claw and are widely expanded distally, sometimes wider than the width of the claw (
Allomyrmococcine mealybugs associated with herdsmen ants are either in the ant trails or in the ants’ nests. It has been shown by
Female mealybugs of the genus Leptococcus Reyne and Macrocepicoccus Morrison have unusually long legs and long slender claws. These mealybugs live on leaf surfaces and are probably parenchyma feeders (
Among the group of root mealybugs based on the genus Rhizoecus Künckel d’Herculais, elevated recently to family level, the Rhizoecidae (
It is clear that prepupae and pupae of some Pseudococcidae and probably of the Rhizoecidae can show mobility. Observations on other species of mealybugs and root mealybugs in which the prepupae and pupae have claw and tarsal digitules also may show that these stages can move.
We are taking the opportunity to describe and illustrate the prepupa and pupa of Promyrmococcus dilli and the prepupa of Ripersiella malschae.
http://species-id.net/wiki/Promyrmococcus_dilli
Sabah, Kinabalu, Poring, in nest of Dolichodorus, 18.vii.1991, M. Dill (BMNH): 7/2 prepupa (one pharate) + 5 pupae (3 pharate) (good – descriptions taken from non-pharate individuals, with details checked on others).
(Fig. 1)
Moderate sized, body 1.23–1.38 mm long, 0.7–0.84 mm widest; oval. Body with numerous very long flagellate setae. Legs and antennae well developed; mouthparts present but lacking stylets; wing buds absent. Ostioles present both anteriorly and posteriorly.
Dorsum membranous, segmentation obvious, particularly on abdomen. Each segment densely covered in fine flagellate setae, each 40–50 μm long; also with frequent, extremely long setae with very flagellate apices, each up to about 350 μm long, distributed approximately as follows: with medial pairs on pro-, meso- and metathorax and on abdominal segments I–VI; with 1 long and 1 slightly shorter seta on each side of each segment but with more on abdominal segments VI and VII; with 1–4 slightly shorter setae anterior to each ostiole, and with intermediate fine flagellate setae (each about 100 or so μm long) sparsely throughout. Loculate pores absent but small simple pores frequent throughout, each about 2 μm wide. Ostioles each 90–95 μm wide. Anus about 45 μm wide, with two setae of intermediate length on either side and a pair on posterior body margin.
Margin not demarcated; without wing buds. Eyespot 33–35 μm wide.
Venter membranous. Circulus present medially between abdominal segments II and III. Fine, flagellate setae similar to those on dorsum, covering most of venter; extremely long flagellate setae only present submarginally on abdominal segments V and VI, and perhaps only marginally on VII; setae of intermediate length infrequent, but present sparsely on VII. Loculate pores, each 6–7 μm wide with an uncertain number of loculi, mainly present medially and submedially on thorax and anterior abdominal segments; simple pores frequent throughout.
Antennae about 515 μm long, 6 segmented but with segment II clearly partially divided and with a campaniform pore present distally on more proximal half of this segment; each segment with many flagellate setae similar to those covering most of body, but with fewest on distal half of segment II; subapical segment with 1 fleshy seta and apical segment with 3 or 4 fleshy setae. Mouthparts clearly present; tentorium barely sclerotized but quite large; labium perhaps 3 segmented, 50–65 μm long, with (on ventral surface) 2 pairs of setae on basal segment, 2 pairs on medial segment and 5 pairs on apical segment; also with 2 pairs on dorsal surface. Spiracles each with peritreme 20–24 μm wide. Legs particularly well developed, lengths (in μm for metathoracic leg): coxa about 120–135; trochanter+ femur 275–310; tibia 190–210; tarsus 95–112; claw 35–38; each trochanter with 2 roundish campaniform pores on each side; each tibia and tarsus without tibial spurs; tarsi one-segmented; tarsi each with a tarsal campaniform pore; tarsal digitules long, extending as long as claw and capitate; claw digitules setose and barely reaching claw apex; claws without a denticle.
(Fig. 2)
Moderate sized, body 1.20–1.4 mm long, 0.63–0.73 mm widest; oval. Body with numerous very long flagellate setae. Legs and antennae well developed; mouthparts present but very reduced and without stylets; wing buds absent. Ostioles present both anteriorly and posteriorly.
Dorsum almost identical to that of the prepupa, and with 0–3 setae of intermediate length on either side of anus.
Margin not demarcated, without wing buds. Eyespot 33–35 μm wide.
Venter membranous. As for prepupa except loculate pores absent.
Antennae 6 segmented as in prepupa, length about 520–615 μm long. Mouthparts very reduced; tentorium a small, roundish membranous area and labium short, about 35 μm long, with a few setae both dorsally and ventrally. Spiracles each with peritreme 20–28 μm wide. Legs particularly well developed, lengths (in μm for metathoracic leg): coxa about 125–150; trochanter+ femur 295–330; tibia 200–225; tarsus 95–105; claw 35–38; legs otherwise as on prepupa.
The basic morphology of the prepupa and pupa of Promyrmococcus dilli is very similar to that of the adult male (
http://species-id.net/wiki/Ripersiella_malschae
(Fig. 3)
Paratype, Sabah, Kinabalu Park, Poring Hot Springs, with Pseudolasius, 28.iii.1998, A. Malsch (BMNH): 1/1 pharate prepupa (good, but distribution of pores and leg setae difficult to ascertain as pupa fairly-well developed).
Small, body 508 μm long, 286 μm widest, oval but rather pointed posteriorly. Legs and antennae well developed; mouthparts and wing buds absent.
Dorsum membranous, segmentation obvious, particularly on abdomen. Each segment with a dense band of short setae, each 5–7 μm long on a convex basal socket; bands narrowest on posterior segments. With 3 pairs of long setae on posterior-most segments, each 23–30 μm long; incipient penial sheath with a group of about 18 setae, similar to those on rest of dorsum. With loculate pores, each about 6 μm wide with mainly 8 loculi, near margins of abdominal segments II–VI and also on metathorax.
Margin not demarcated; without wing buds.
Venter membranous. Small setae, similar to those covering most of dorsum, present anteriorly and laterally on head, in large broad groups laterally on pro- and mesothorax, and in small groups laterally on metathorax and abdominal segments II–VII; somewhat similar setae also present very sparsely medially across all segments except perhaps prothorax. Loculate pores similar to those on dorsum present submarginally on abdominal segments and sparsely medially on all thoracic segments and head.
Antennae 6 segmented, about 100 μm long; pedicel very short, about 10 μm long; all segments with a few setose setae; subapical segment with a fleshy seta and apical segment with 3 or 4 fleshy setae. Mouthparts absent. Spiracles each with peritreme 16–18 μm wide. Legs particularly well developed, lengths (metathoracic leg in μm): coxa about 50; trochanter + femur 88; tibia + tarsus 85; claw 21; each trochanter with 2 roundish campaniform pores on each side; each tibia with 2 tibial spurs on distal ventral margin but also with perhaps 2 more laterally; tarsi with a spur-like seta on ventral margin near proximal end; tarsal campaniform pores present but tarsal digitules considered to be absent; claw digitules present but minute; claws without a denticle. Anus apparently on ventral surface.
The prepupa of Ripersiella malschae looks similar to the adult male (
We thank Penny Gullan, Research School of Biology, the Australian National University, Canberra, Australia, for reviewing the manuscript and are most grateful to Takumasa (Demian) Kondo, Entomology Laboratory, CORPOICA, Palmira, Colombia, and to Andrea Amalia Ramos Portilla, Instituto Colombiano Agropecuario, Bogotá, Colombia, for kindly giving us their observations on Macrocepicoccus loranthi.