(C) 2013 Kyu-Tek Park. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two species of Lecithoceridae (Lepidoptera, Gelechioidea), Caveana senuri sp. n. and Lecithocera dondavisi sp. n., are described from Taiwan. The monotypic Caveana Park was described from Thailand, based on Caveana diemseoki Park, 2011. Lecithocera Herrich-Schäffer, 1853 is the most diverse genus of the family, comprising more than 300 species worldwide. Lecithocera dondavisi sp. n. is the largest species of the genus so far, and closely resembles the Indian species, Lecithocera praeses Meyrick, 1919. A revised check list of the family in Taiwan is provided.
Caveana, Lecithocera, Lepidoptera, Lecithoceridae, new species, Taiwan, taxonomy
The family Lecithoceridae (Lepidoptera, Gelechioidea) is a relatively poorly known group of microlepidoptera that comprises more than 1, 200 extant known species worldwide (van
In Taiwan, a total of 63 species of Lecithoceridae have been reported (
The monotypic Caveana Park, 2010 was described from Thailand, based on Caveana diemseoki Park, 2010. The genus is placed in the subfamily Torodorinae because it shares the presence of spinose zones on the abdominal tergites and the male genitalia lacks costal bars that connects the tegumen and valva. The genus isallied to Nosphistica Meyrick, 1911 and Philoptilia Meyrick, 1918by having a similar the venation, with M3, CuA1 and CuA2 on a common stalk in the forewing and M2 absent from the hindwing, but Caveana differs from them by the brightly colored forewing and the lack of rough scale projections of the hindwing costa and strongly sinuate termen. Philoptilia is distinguished by the forewing R5, which is absent in Nosphistica.
A revised check list of the family Lecithoceridae in Taiwan, with 74 known species, is provided in Table 1.
Check list of Lecithoceridae in Taiwan.
| Species | Type locality | Type depository |
|---|---|---|
| Homaloxestis Meyrick, 1910 | ||
| Homaloxestis baibaraensis Park, 1999 | Taiwan | USNM |
| Homaloxestis cholopis (Meyrick, 1906) (Lecithocera) | Myanmar | BMNH |
| Homaloxestis hilaris Gozmány, 1978 | Zhejiang, China | ZFMK |
| Homaloxestis myeloxesta Meyrick, 1932 | Taiwan | BMNH |
| Lecithocera Herrich-Schäffer, 1853 | ||
| Lecithocera angustiella Park, 1999 | Taiwan | KNA |
| Lecithocera altusana Park, 1999 | Taiwan | KNA |
| Lecithocera atricastana Park, 1999 | Taiwan | USNM |
| Lecithocera aulias Meyrick, 1910 | Khasi Hills, India | BMNH |
| Lecithocera bimaculata Park, 1999 | Taiwan | BMNH |
| Lecithocera chartaca Wu & Liu, 1993 | Jiangxi, China | IZAS |
| Lecithocera dondavisi Park, sp. n. | Taiwan | MCUF |
| Lecithocera erecta Meyrick, 1935 | Zheijang, China | BMNH |
| Lecithocera fascicula Park, 1999 | Taiwan | KNA |
| Lecithocera fascinatrix Meyrick, 1935 | Taiwan | BMNH |
| Lecithocera fuscosa Park, 1999 | Taiwan | KNA |
| Lecithocera glabrata (Wu & Liu, 1992) (Quassitagma) | Jiangxi, China | IZAS |
| Lecithocera indigens (Meyrick, 1914) (Frisilia) | Taiwan | DEI |
| Lecithocera latiola Park, 1999 | Taiwan | KNA |
| Lecithocera megalopis Meyrick, 1916 | Philippines | BMNH |
| Lecithocera metacausta Meyrick, 1910 | Khasi Hills, India | BMNH |
| Lecithocera palingensis Park, 1999 | Taiwan | KNA |
| Lecithocera paralevirota Park, 1999 | Taiwan | USNM |
| Lecithocera pelomorpha Meyrick, 1931 | Sichuan, China | BMNH |
| Lecithocera pulchella Park, 1999 | Taiwan | KNA |
| Lecithocera rotundata Gozmány, 1978 | Zhejiang, China | ZFMK |
| Lecithocera serena Gozmány, 1978 (Sarisophora) | Shaanxi, China | ZFMK |
| Lecithocera shanpinensis Park, 1999 | Taiwan | KNA |
| Lecithocera thaiheisana Park, 1999 | Taiwan | USNM |
| Lecithocera tienchiensis Park, 1999 | Taiwan | KNA |
| Lecitholaxa Gozmány, 1978 | ||
| Lecitholaxa thiodora (Meyrick, 1914) (Lecithocera) | Taiwan | HNHM |
| Frisilia Walker, 1864 | ||
| Frisilia chinensis Gozmány, 1978 | Sichuan, China | BMNH |
| Frisilia cornualis Park, 2008 | Taiwan, Vietnam | KNA |
| Frisilia homalistis Meyrick, 1935 | Taiwan | BMNH |
| Spatulignatha Gozmány, 1978 | ||
| Spatulignatha idiogena Wu, 1994 | Fujian, China | IZAS |
| Spatulignatha olaxana W, 1994 | Zhejiang, China | IZAS |
| Synersaga Gozmány, 1978 | ||
| Synersaga bleszynskii (Gozmány, 1978) (Anaminmnesis) | Zhejiang, China | ZFMK |
| Synersaga caradjai Gozmány, 1978 | Taiwan | MGAB |
| Carodista Meyrick, 1925 | ||
| Carodista cultrata Park, 2000 | Taiwan | MCUF |
| Carodista montana Park, 2000 | Taiwan | KNA |
| Carodista notolychna (Meyrick, 1936) (Homaloxestis) | Taiwan | BMNH |
| Dinochares Meyrick, 1925 | ||
| Dinochares notolepis Park, 2000 | Taiwan | USNM |
| Issikiopteryx Moriuti, 1973 | ||
| Issikiopteryx zonophaera (Meyrick, 1935) (Olbothrepta) | Zhejiang, China | BMNH |
| Issikiopteryx taipingensis Park, 2003 | Taiwan | KNA |
| Tisis Walker, 1864 | ||
| Tisis mesozosta Meyrick, 1914 | Taiwan | DEI |
| Nosphistica Meyrick, 1911 | ||
| Nosphistica bisinuata Park, 2002 | Taiwan | KNA |
| Nosphistica fenestrata (Gozmány, 1978) (Philoptila) | Fujian, China | HMNH |
| Nosphistica fuscolepis Park, 2002 | Taiwan | USNM |
| Nosphistica parameocola (Wu, 1996) (Athymoris) | Hainan, China | IZAS |
| Nosphistica tarokoensis Park, 2002 | Taiwan | KNA |
| Subfamily TORODORINAE | ||
| Torodora Meyrick, 1894 | ||
| Torodora albicruris Park & Heppner, 2000 | Taiwan | USNM |
| Torodora capillaries Park & Heppner, 2000 | Taiwan | USNM |
| Torodora chianensis Park, 2003 | Taiwan | USNM |
| Torodora manoconta Wu & Liu, 1994 | Jiangxi, China | IZAS |
| Torodora octavana (Meyrick, 1911)(Brachmia) | Khasi Hills, India | BMNH |
| Torodora parthenopis (Meyrick, 1932) (Lecithocera) | Taiwan | BMNH |
| Torodora pseudogalera Park, 2003 | Taiwan | USNM |
| Torodora rectilinea Park, 2003 | Taiwan | MNHU |
| Torodora sciadosa Wu & Liu, 1994 | Sichuan, China | IZAS |
| Torodora ortilege (Meyrick, 1911) | Khasi Hills, India | BMNH |
| Deltoplastis Meyrick, 1925 | ||
| Deltoplastis commatopa Meyrick, 1932 | Taiwan | BMNH |
| Deltoplastis lobigera Gozmany, 1978 | Zhejiang, China | ZFMK |
| Deltoplastis ovatella Park, 2001 | Taiwan | MCUF |
| Thubana Walker, 1864 | ||
| Thubana albisignis (Meyrick, 1914) (Lecithocera) | Taiwan | DEI |
| Thubana deltaspis Meyrick, 1935 | Taiwan | BMNH |
| Caveana Park, 2010 | ||
| Caveana senuri Park, sp. n. | Taiwan | MCFU |
| Athymoris Meyrick, 1935 | ||
| Athymoris aurantiella Park, 2000 | Taiwan | MCUF |
| Athymoris liukueiensis Park, 2000 | Taiwan | MCUF |
| Athymoris martialis Meyrick, 1935 | Taiwan | BMNH |
| Athymoris phreatosa (Wu, 1994) | Sichuan, China | IZAS |
| Athymoris subtrigona Park, 2000 | Taiwan | MCFU |
| Halolaguna Gozmány, 1978 | ||
| Halolaguna oncopteryx (Wu, 1994) | Sichuan, China | IZAS |
| Halolaguna palinensis Park, 2000 | Taiwan | KNA |
| Halolaguna sublaxata Gozmány, 1978 | Kiangsu, China | HNMH |
| Philharmonia Gozmány, 1978 | ||
| Philharmonia adusta Park 2000 | Taiwan | MCFU |
BMNH- The Natural History Museum, London, UK; HMNH- Hungarian Museum of Natural History, Budapest, Hungary; IZAS- Institute of Zoology, Academia Sinica, Beijing, China; DEI- Deutsches Entomologisches Institut, Eberswald, Germany; KNA- Korea national Arboretum, Pocheon, Korea; MCUF- McGuire Center for Lepidoptera and Biodiversity, University of Florida, Gainesville, USA; MNHU- Museum für Naturkunde, Zentralinstitu Hummboldt-Universität, Berlin, Germany; USNM- U. S. National Museum of Natural History, Washington, USA.
Most specimens examined were collected in 1980 and 1989 by the second author and H. Wang, researcher in the National Taiwan Museum, Taipei, Taiwan, and Donald R. Davis, US National Museum of Natural History, Smithsonian Institution (USNM), Washington D.C., USA. The material is preserved in the collections of USNM and the McGuire Center for Lepidoptera and Biodiversity, Florida Museum of the Natural History, University of Florida (MCUF), Gainesville, FL, USA. The holotypes of the new species are deposited in MCUF and paratypes are in both museums, on indefinite loan from Taiwan.
urn:lsid:zoobank.org:act:BB330AA1-77FD-4D94-B7AE-D02781BAD8A9
http://species-id.net/wiki/Caveana_senuri
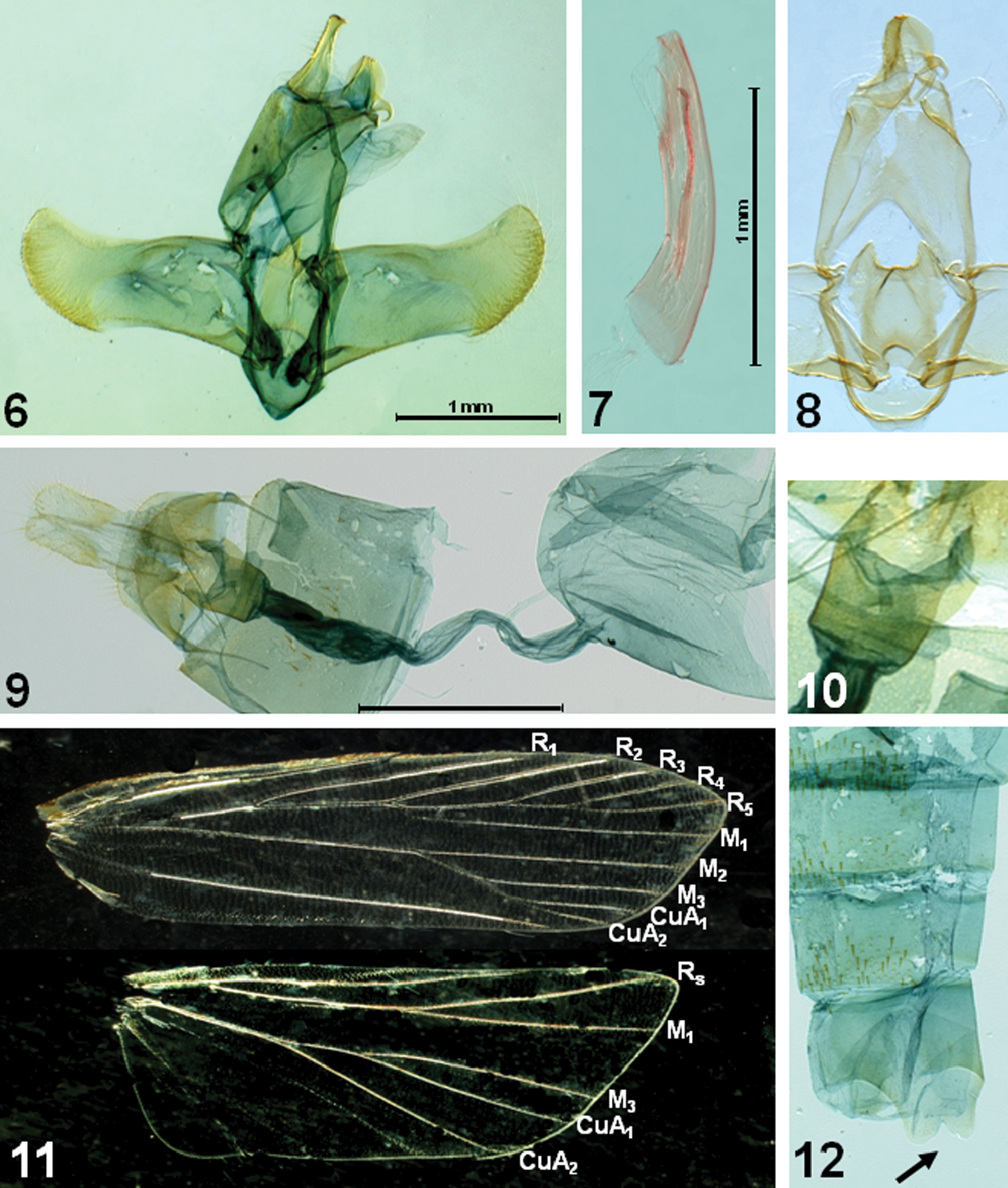
Figs 1–12The light-orange color pattern of the forewing is unique, with dark-brown streaks between veins. The pattern is more or less similar to that of Timyra aulonitis Meyrick, 1908 which was described from Sri Lanka, but the species can be distinguished by the venation of both wings, and by the absence of the scale projection in the basal segment of antenna and the scale-tuft in the hind tibia which are unique to Timyra Walker, 1864. The male genitalia are also different from those of the Timyra aulonitis.
Male and female (Figs 1–5, 11): Wingspan, 17–18 mm. Head light orange. Basal segment of antenna (Fig. 5) elongate, light orange, speckled with brownish scales dorsally; flagellum dark brown, sometimes paler from near half to before 7th. Second segment of labial palpus (Fig. 3) gently arched, shiny pale orange; 3rd segment slender, as long as 2nd segment, pale orange speckled with dark-brown scales, with acute apex. Thorax and tegula light orange. Forewing elongate; ground color light orange, clothed with dark-brown scales between veins; costa nearly straight, then gently arched beyond ¾, blackish along anterior margin; apex obtuse; 5-6 large, blackish spots from apex to tornus along termen; termen oblique, not sinuate; fringe light orange in basal 1/3, then dark brown; venation (Fig. 11) with R1 arising from before middle, R2 arising near upper corner of cell, R3 and R4 stalked near 2/3 length of R3+4+5, R4 and R5 stalked about 3/5 length; R5 reach before apex, M1 close to R3 at base, M2 straightly extended from lower margin of discal cell, M3 on common stalk with CuA1+2, CuA1 and CuA2 stalked beyond middle. Hindwing evenly clothed with dark-brown scales, except on veins; light orange along veins; distinct blackish line well-developed from prior to apex to tornus along margin; venation with Rs and M1 nearly connate, M2 absent, M3 and CuA1 stalked. Fore and mid tibia with black scales at apex. Hind tibia (Fig. 11) with rough, dark-brown scales above, denser near apex; tarsi with black scales at apex on each segment. Abdomen clothed with dark-brown scales; abdominal tergites with dense spines; sternite VIII bilobed medially, as indicated in Fig. 12.
Male genitalia (Figs 6–8). Uncus elongate, heavily sclerotized, broadened basally; apex slightly bifurcate. Gnathos relatively short, small, strongly bent downward beyond 2/3. Tegumen long, relatively broad; anterior margin deeply concave. Valva broad; costa slightly concave beyond middle, nearly parallel to ventral margin; cucullus short; outer margin rounded, with dense setae along margin. Juxta shield-shaped, concave in U-shape on caudal margin, with triangular caudal lobes laterally; anterior margin deeply concave. Vinculum narrow, band-shaped. Saccus short, rounded. Aedeagus rather slender, as long as valva, slightly bent; cornutus long, narrow sclerite, as long as 2/3 length of aedeagus. Abdominal tergites with dense spinose zones; sternite VIII bifurcated medially (arrow indicated in Fig. 12).
Female genitalia (Figs 9, 10). Abdominal sternite VIII weakly sclerotized, deeply emarginated on caudal margin medially. Apophyses anteriores less than half length of apophyses posteriores. Antrum (Fig. 10) cup-shaped, weakly sclerotized, about 1/4 length of ductus bursae. Ductus bursae longer than corpus bursae, broadened in distal 2/5 length, then slightly narrowed; ductus seminalis arising from near conjunction with corpus bursae. Corpus bursae large, ovate; signum absent.
Adult of Caveana senuri Park, sp. n. 1 adult, paratype 2 ditto, lateral view 3 labial palpus 4 hind tibia 5 basal part of antenna.
Genitalia and wing venation of Caveana senuri Park, sp. n. 6 male genitalia 7 aedeagus 8 juxta and vinculum 9 female genitalia 10 ditto, close-up of antrum; 11 wing venation 12 abdominal segment (arrow indicates the bilobed sternite VIII). Scale bar: 1 mm.
♂, Taiwan, Kaohsiung County, Lukuei Forest Station., 750 m, 29 iv- 3 v 1989 (J. Heppner & H. Wang), deposited in MCUF.
1♂, 1♀, same data as the holotype, genitalia slide no. CIS-6138/Park (♂), -6139/Park(♀); 1♀, Taiwan, Nantou Co., Lu-shan, 30 km E Wushe, 1000 m, 27–31 v 1980 (D.R. Davis), gen. slide no. USNM-92404; 1 gen. slide no. CIS-6138/Park; 1♂, Taiwan, Nantou Co., 15 km E of Puli, 700 m, 6 v 1989 (J. Heppner & H. Wang).
Taiwan.
The specific epithet is a Korean term, senuri, meaning “a new country”.
urn:lsid:zoobank.org:act:6D63A8D5-E85E-4D8E-9F14-95ED992901E6
http://species-id.net/wiki/Lecithocera_dondavisi
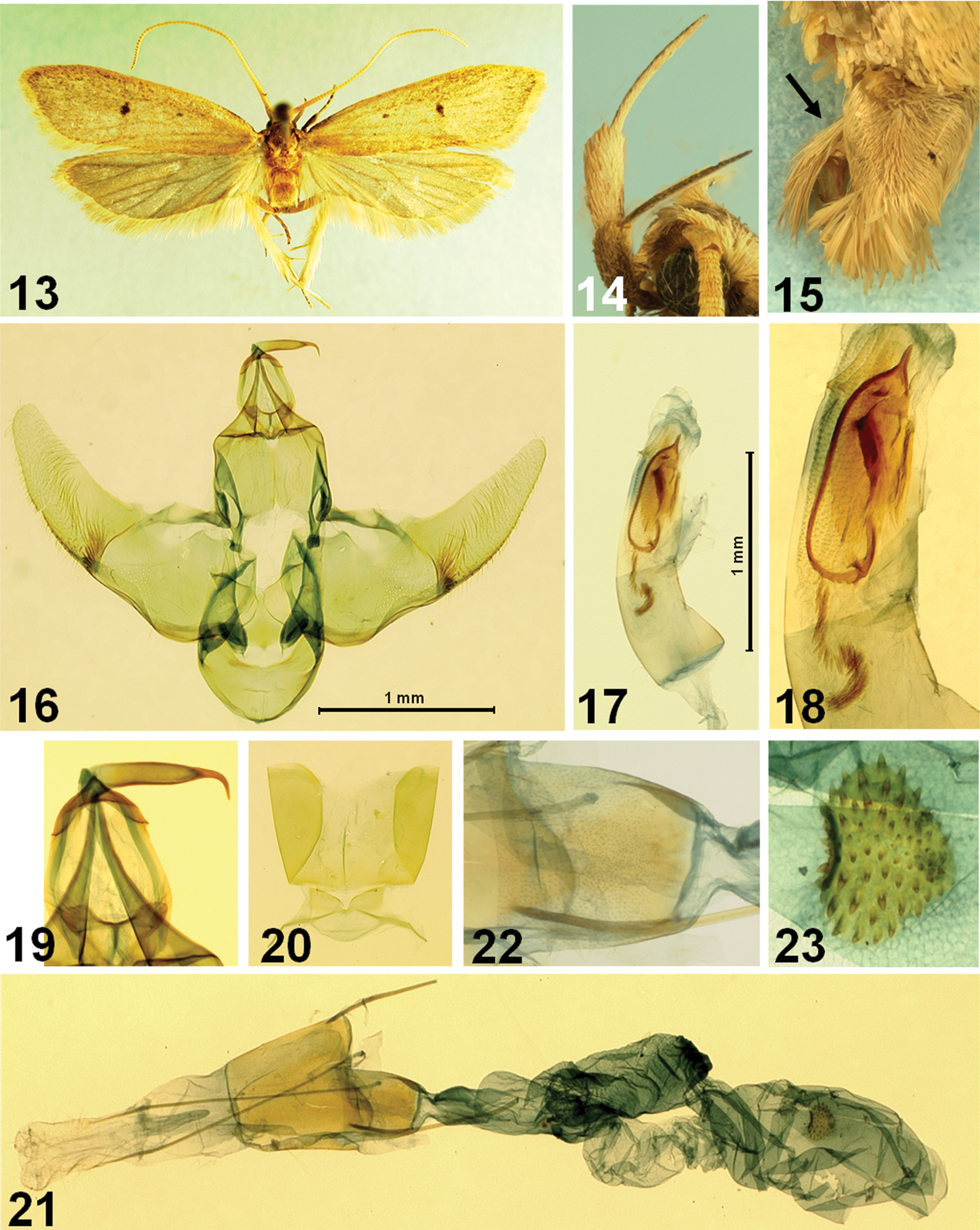
Figs 13–23This species is one of the largest species of Lecithocera. It is externally similar to Lecithocera praeses Meyrick, 1919 from North India, but can be distinguished by different following genital features: male genitalia with uniquely specialized cornuti of aedeagus, consisting of a heavily sclerotized ellipticity with an acute spine apically, a heavily sclerotized, elongate trapezoidal plate, and a series of spines, as in Figs 17, 18; and also cucullus with more gently arched ventral margin and juxta not so much produced latero-caudally. Female genitalia with cup-shaped antrum, instead of the elongate, more or less triangular antrum in Lecithocera praeses, and the signum strawberry-shaped, located medially, whereas it is transverse elongated and located posteriorly in the latter.
Male and female (Figs 13–15). Wingspan, 23–26 mm. Head yellowish brown medially on vertex, with pale grayish-orange erect scales laterally; frons pale grayish-orange. Basal segment of antenna rather short, pale grayish orange; flagellum orange white to pale grayish–orange, with distinct brownish annulations in apical third. Second segment of labial palpus (Fig. 14) thickened with appressed scales, grayish orange on outer surface, speckled with dark-brown scales in basal 2/3, orange white to pale grayish orange on inner surface; 3rd segment slender, shorter than 2nd segment, dark brown on ventral surface, with acute apex. Thorax and tegula yellowish brown. Forewing elongate; ground color pale grayish orange, speckled with fine dark-brown scales, more dense posteriorly; first discal stigma small, dark brown at middle of cell; second stigma larger, dark brown, at end of cell; basal blackish streak running along costa in ¼ length; costa nearly straight, then gently arched beyond ¾; apex obtuse; termen oblique, not sinuate, dark-brown scales along margin; fringe orange white in basal 1/3, then brownish; venation with R1 arising from before middle, R2 arising near upper corner of cell, distance between R1 and R2 about 2.5 times of distance between R2 and R3; R2 free; R3 and R4 stalked near middle; R5 reach apex; M1 at middle between R3 and M2, M2 nearly parallel with M1; M3 at middle between M2 and CuA1+2; CuA1 and CuA2 very short-stalked. Hindwing pale gray, broader than forewing; apex obtuse; termen oblique, slightly sinuate; fringe grayish, with orange white basal line; venation with, M2 well developed, connected to M3 with cross vein; M3 and CuA1 short-stalked; cell nearly closed with an oblique cross vein. Hind tibia with orange-white rough scales above. Abdomen with pale grayish-orange scales dorsally, with a well-developed scales-tuft dorsally in terminal segment, as indicated in Fig. 15; sternite VIII bilobed medially, as indicated in the Fig. 12.
Male genitalia (Figs 16–22). Basal lobes of uncus more or less semiovate, gently concave on caudal margin. Gnathos (Fig. 19) relatively slender; apical part heavily sclerotized, strongly bent downward. Tegumen weakly sclerotized with anterior margin incised medially. Valva broad at base, width as wide as length of tegumen; costal bar connecting with tegumen strong, angled medially; ventral margin gently concave before cucullus; cucullus elongate, narrowed towards apex, dense setose, with bundle of setae at lower corner at base, apex rounded; sacculus sclerotized, slender. Juxta shield-shaped, with small projection at middle on anterior margin; caudal margin slightly emarginated, with crescent extension laterally. Vinculum broad, with round apex. Saccus round. Aedeagus (Figs 17, 18) with uniquely specialized cornuti of aedeagus, consisting as heavily sclerotized ellipticity with acute spine at apex, about half length of aedeagus, and a row of short spines. Abdominal tergites without spines; sternite VII-VIII as figured in Fig. 20.
Female genitalia (Figs 21–23). Abdominal sternite VIII weakly sclerotized, nearly straight anterior margin. Apophyses anteriores thick, short, nearly 1/5 length of apophyses posteriors. Antrum (Fig. 22) cup-shaped, weakly sclerotized, about 2/3 length of abdominal sternite VIII. Ductus bursae slightly longer than corpus bursae, shortly necked between antrum and ductus bursae, then broadened; ductus seminalis as broad as ductus bursae, arising from middle. Corpus bursae large, elongate; signum strawberry-shaped, with dense conic spines.
Lecithocera dondavisi Park, sp. n. 13 adult, paratype 14 labial palpus 15 terminal segments of abdomen (arrow indicates the dorsal scale-tuft) 16 male genitalia 17 aedeagus 18 close-up of cornuti 19 close-up of signum 20 abdominal sternite VIII 21 female genitalia 22 close-up of antrum 23 close-up of signum. Scale bar: 1 mm.
. ♂, Taiwan, Hsinchu County., Kuangwu, 24-25 vi 1985 (J. Heppner & H. Wang), gen. slide no. CIS-6168/Park, deposited in MCUF.
4 ♂, 1♀, same data as the holotype, gen. slide no. CIS- 6192/Park(♀); 1♂, Taiwan, Nantou Co., Meifeng 30 km S Tayuling 2200 m, 1-8 vi 1980 (D. R. Davis), gen. slide no. USNM-92499/Park.
Taiwan.
The species is named after Dr. Donald R. Davis, Curator of Lepidoptera, US National Museum Natural History, Smithsonian Institution, USA, an authority on the microlepidoptera of the world.
We are grateful to H. Wang (Taiwan National Museum, Taipei, Taiwan), and Dr. R. Davis (Smithsonian Institution, Washington D.C., USA), for providing us the valuable material collected from Taiwan for this study. Special thanks to Akito Kawahara (McGuire Center for Lepidoptera and Biodiversity, Gainesville, USA), for helpful comments on the manuscript. The present study was supported by the University of Incheon Research Grant in 2010, Korea.