(C) 2013 Jans Morffe. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Morffe J, García N (2013) Batwanema gen. n. and Chokwenema gen. n. (Oxyurida, Hystrignathidae), new nematode genera as parasites of Passalidae (Coleoptera) from the Democratic Republic of Congo. ZooKeys 361: 1–13. doi: 10.3897/zookeys.361.6351
Two new genera and species parasitizing passalid beetles from the Democratic Republic of Congo are described. Batwanema congo gen. n. et sp. n. is characterized by having females with the cervical cuticle armed with scale-like projections, arranged initially in rows of eight elements that gradually divide and form pointed spines toward the end of the spiny region, two cephalic annuli, clavate procorpus and genital tract monodelphic-prodelphic. Two Malagasian species of Artigasia Christie, 1934 were placed in this genus as B. latum (Van Waerebeke, 1973) comb. n. and B. annulatum (Van Waerebeke, 1973) comb. n. Chokwenema lepidophorum gen. n. et sp. n. is characterized by having females with the cervical cuticle armed with scale-like projections, arranged initially in rows of eight elements (similar to Batwanema) that divide gradually, forming spines; a single cephalic annule cone-like, truncated, moderately inflated; procorpus sub-cylindrical and genital tract didelphic-amphidelphic.
Nematoda, Hystrignathidae, Batwanema, Chokwenema, Passalidae, Democratic Republic of Congo
The nematode family Hystrignathidae Travassos, 1920 is well known as a parasite group restricted to the gut of passalid beetles. At present, 29 genera have been described with more than 100 species.
One of these genera: Artigasia Christie, 1934 was diagnosed on the basis of presenting spines on the cervical cuticle, a clavate procorpus and a genital system monodelphic-prodelphic (
The African fauna of Hystrignathidae is poorly known with a few species belonging to the genera Artigasia (with the larger number of species), Hystrignathus Leidy, 1850; Passalidophila Van Waerebeke, 1973 and Xyo Cobb, 1898 (
As a continuation of the studies on Congolese hystrignathids, the present paper deals with two new genera. One of these is created in order to separate two peculiar species of Artigasia.
Several specimens of passalid beetles from the Democratic Republic of Congo were examined during a research visit to the Royal Museum of Central Africa, Tervuren, Belgium. Six specimens of Pentalobus barbatus (Fabricius, 1801), three of Didimoides cf. parastictus (Imhoff, 1843) (all the latter from Mongwalu, Ituri province, Democratic Republic of Congo) and four specimens of Pentalobus sp. from Bambesa, Uele region, Democratic Republic of Congo were revised for parasitological studies. All of these passalids were collected during the Belgian expeditions to the Congo in the 1930’s and stored in 70% ethanol.
The hosts were dissected by making incisions in both pleural membranes and the last abdominal sternites. Intestines were extracted and kept in Petri dishes with 70% ethanol. The guts were excised and the parasites removed. Nematodes were transferred to anhydrous glycerine via the slow evaporation method and mounted in the same medium. The edges of the coverslips were sealed using nail polish. Measurements were made with a calibrated eyepiece micrometer attached to a compound microscope. De Man’s ratios a, b, c and V% were calculated. Each variable is shown as the range followed by the mean plus standard deviation in parentheses; the number of measurements is also given. Micrographs were taken with an AxioCam digital camera attached to a Carl Zeiss AxioScop 2 Plus compound microscope. Line drawings were made with the softwares CorelDRAW X3 and Adobe Photoshop CS2 using the micrographs as masters. Scale bars of all plates are given in millimeters.
Some specimens were processed for SEM as follows: they were dehydrated in a graded ethanol series, critical point-dried, mounted in aluminum stubs and coated in gold. SEM micrographs were taken at an acceleration voltage of 22–25 kV.
The type material and vouchers are deposited in the Colección Helmintológica de las Colecciones Zoológicas (CZACC), Instituto de Ecología y Sistemática, Havana, Cuba; the Collection of the Royal Museum of Central Africa (RMCA), Tervuren, Belgium; the Royal Belgian Institute of Natural Sciences (RIT), Brussels, Belgium and the Coleçao Helmintologica do Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, Brazil.
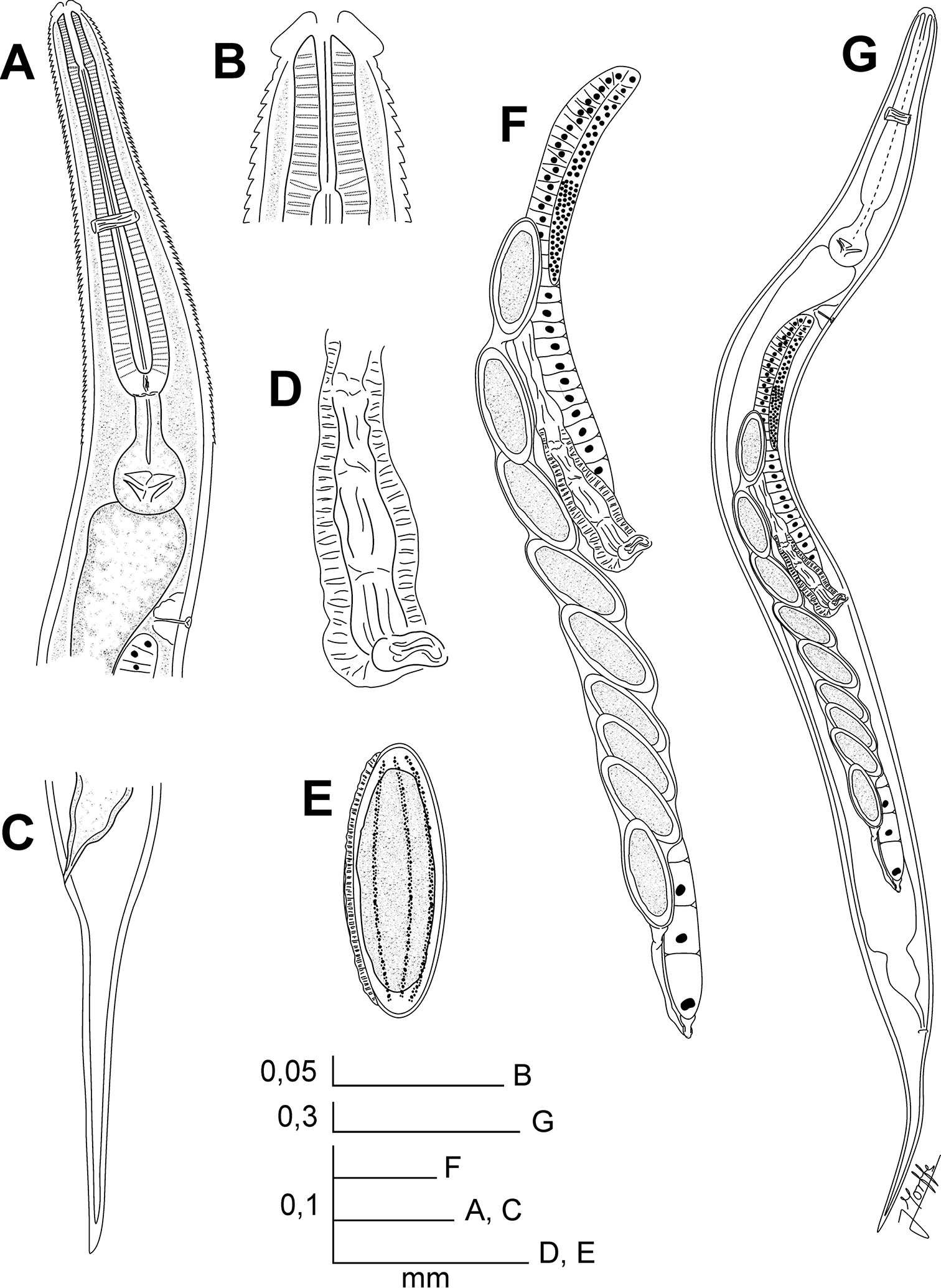
Female. Cervical cuticle armed with wide, scale-like projections, arranged initially in rows of eight elements. Scales divide gradually, forming pointed spines toward the end of the spiny region. Lateral alae present. Head bearing eight rounded, paired papillae. Two short, not prominent cephalic annuli next to head, the second slightly longer and wider than the first. Oesophagus with procorpus clavate, its base set-off from the isthmus. Excretory pore post-bulbar. Genital tract monodelphic-prodelphic. Eggs ovoid. Tail subulate.
Batwanema congo Morffe & García, gen. n. et sp. n.
Batwanema latum (Van Waerebeke, 1973) comb. n.; Artigasia lata Van Waerebeke, 1973: pag. 13, fig. 90–101.; Batwanema annulatum (Van Waerebeke, 1973) comb. n.; Artigasia annulata Van Waerebeke, 1973: pag. 13, fig. 84–89.
Democratic Republic of Congo, Madagascar.
The generic epithet (neuter in gender), is a combination of Batwa, after the pygmy ethnic group that inhabits the D. R. of Congo, and the suffix–nema.
http://zoobank.org/303612B9-C74C-4296-9BC9-8C291145AFD1
http://species-id.net/wiki/Batwanema_congo
Figure 1A–G, 2A–E♀ holotype, Democratic Republic of Congo, Ituri province, Mongwalu; in Pentalobus barbatus; 5.VI.1939; Lepersonne coll.; CZACC 11.4700. Paratypes: 4 ♀♀, same data as holotype, CZACC 11.4701-11.4704; 3 ♀♀, same data as holotype, RMCA; 1 ♀♀, same data as holotype, RIT820; 1 ♀♀, same data as holotype, CHIOC.
Vouchers: 3♀♀, Democratic Republic of Congo, Uele region, Bambesa, 3°28'N, 25°43'E; in Pentalobus sp.; 15.V.1937; J. Vrijdagh coll.; CZACC 11.4705-11.4707. 2♀♀, same data as the latter, RMCA.
Holotype (female) a = 14.81, b = 6.24, c = 7.90, V% = 50.63, total length = 2.370, maximum body width = 0.160, stoma length = 0.045, procorpus length = 0.290, isthmus length = 0.033, diameter of basal bulb = 0.070, total length of oesophagus = 0.380, nerve ring to anterior end = 0.200, excretory pore to anterior end = 0.550, anus to posterior end = 0.300, eggs = 0.100–0.110×0.045–0.050 (0.106 ± 0.005×0.048 ± 0.003 n = 3).
Paratypes (females) (n = 9) a = 13.20–17.50 (14.72 ± 1.50 n = 9), b = 4.92–5.56 (5.33 ± 0.24 n = 9), c = 6.28–7.48 (6.87 ± 0.40 n = 9), V% = 51.83–54.29 (52.76 ± 0.92 n = 7), total length = 1.820–2.170 (2.013 ± 0.138 n = 9), maximum body width = 0.120–0.160 (0.138 ± 0.015 n = 9), stoma length = 0.040–0.050 (0.045 ± 0.003 n = 9), procorpus length = 0.270–0.320 (0.283 ± 0.016 n = 9), isthmus length = 0.025–0.035 (0.031 ± 0.004 n = 9), diameter of basal bulb = 0.060–0.075 (0.067 ± 0.004 n = 9), total length of oesophagus = 0.350–0.420 (0.378 ± 0.020 n = 9), nerve ring to anterior end = 0.170–0.210 (0.191 ± 0.013 n = 9), excretory pore to anterior end = 0.490–0.550 (0.515 ± 0.026 n = 4), anus to posterior end = 0.260–0.310 (0.293 ± 0.015 n = 9), eggs = 0.098–0.123×0.030–0.053 (0.108 ± 0.006×0.041 ± 0.006 n = 16).
Females (n = 5) a = 15.91–21.00 (18.40 ± 2.22 n = 5), b = 5.11–6.10 (5.60 ± 0.40 n = 5), c = 5.39–6.13 (5.68 ± 0.30 n = 5), V% = 49.74–54.30 (51.84 ± 1.65 n = 5), total length = 1.510–1.890 (1.716 ± 0.162 n = 5), maximum body width = 0.080–0.110 (0.094 ± 0.011 n = 5), stoma length = 0.040–0.050 (0.043 ± 0.004 n = 5), procorpus length = 0.185–0.270 (0.227 ± 0.030 n = 5), isthmus length = 0.025–0.038 (0.032 ± 0.005 n = 5), diameter of basal bulb = 0.033–0.058 (0.049 ± 0.010 n = 5), total length of oesophagus = 0.258–0.360 (0.308 ± 0.036 n = 5), nerve ring to anterior end = 0.135–0.180 (0.155 ± 0.019 n = 4), excretory pore to anterior end = 0.380–0.430 (0.397 ± 0.029 n = 3), anus to posterior end = 0.280–0.340 (0.302 ± 0.023 n = 5), eggs = 0.095–0.118×0.033–0.045 (0.110 ± 0.008×0.039 ± 0.005 n = 10).
Body comparatively slender, widening from the base of the second cephalic annule, maximum body diameter at level of the vulva, tapering towards anus. Cuticle markedly annulated in the spiny region, annuli (ca. 2 µm) less marked in the rest of body. Cervical region armed with rows of cuticular projections from the end of the second cephalic annule to the base of isthmus. First row consisting of eight wide, rectangular, scale-like cuticular projections. At level of row 3-4, a shallow cleavage at midpoint of the scales becoming deeper and wider towards the posterior region of body, until reaching row 8–9, where each scale is divided in two or three spines, their tips rounded. Next to it, spines become pointed gradually and increase their number: ca. 22 elements in the median rows and ca. 34 in the last rows. Lateral alae commencing at level of the isthmus, within the spiny region and extending to about three body-widths posterior to the vulva. Sub-cuticular longitudinal striae present. Head set-off from body by a deep groove, bearing eight rounded, less prominent, paired papillae. Amphids lateral, pore-like. Next to head, two short, not prominent cephalic annule; the second slightly wider and longer than the first. Cephalic annuli poorly differentiated one from the other, only by a shallow groove. Mouth circular. Stoma about four head-lengths long, surrounded by an oesophageal collar. Lumen of anterior region of stoma triangular, with a ridge in each side. Oesophagus consisting of a muscular clavate procorpus, well set-off from the isthmus. Basal bulb rounded, valve plate well developed. Intestine simple, sub-rectilinear. Rectum short, anus not prominent. Nerve ring encircling procorpus at about its midpoint. Excretory pore situated at about half of body width posterior to basal bulb. Vulva a median transverse slit near midbody, lips slightly prominent. Vagina muscular, forwardly directed. Genital tract monodelphic-prodelphic. Ovary reflexed behind the excretory pore, distal flexure ca. 1.5 body-widths long. Eggs ovoid, shell with eight rough, longitudinal, hardly prominent ridges. Tail conical, subulate, ending in a sharp point. Male unknown.
Batwanema congo gen. n. et sp. n. Female. A Oesophageal region, lateral view B Cephalic end, internal view C Tail, lateral view D Vulva, ventro-lateral view E Egg F Genital tract, ventro-lateral view G Habitus, ventro-lateral view.
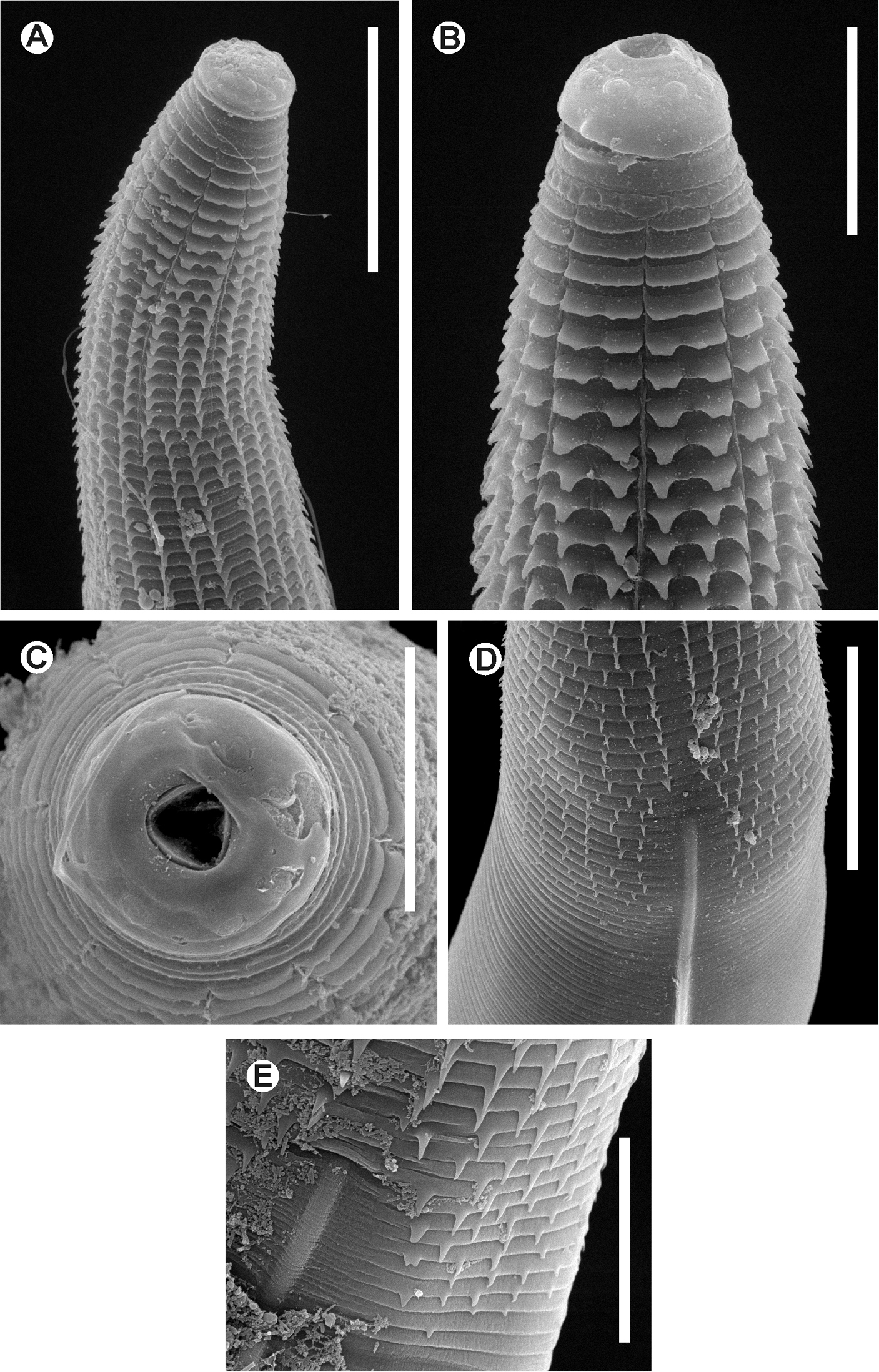
Batwanema congo gen. n. et sp. n. Female. SEM images A Cervical region B Cephalic end, lateralview C Cephalic end, en face view D End of the spiny region and beginning of a lateral ala E Detail of the beginning of a lateral ala. Scale lines: A, D 0.05 mm, B 0.025mm, C, E 0.02 mm.
Batwanema gen. n. presents a similar arrangement of the cervical cuticular projections to Chokwenema gen. n., consisting of a first row of eight rectangular scales that gradually bifurcate, becoming pointed spines. It can be differentiated by its reproductive system monodelphic-prodelphic contrary to didelphic-amphidelphic. The genus has two cephalic annuli barely expanded vs. the unique truncate, more expanded first cephalic annule of Chokwenema gen. n. In addition, the procorpus of Batwanema gen. n. is clavate vs. sub-cylindrical.
The other genera with scales in the cervical cuticle are Lepidonema Cobb, 1898 and Salesia Travassos & Kloss, 1958, both having genital tracts didelphic-amphidelphic and with more elements in the first row of spines: 16 vs. 8 and a single, large cephalic annuli vs. the two shorter of Batwanema gen. n. Also, Lepidonema has a sub-cylindrical procorpus vs. the clavate of Batwanema gen. n.
Batwanema congo gen. n. et sp. n. can be segregated from Batwanema latum comb. n. by the extension of the cervical spines and lateral alae. In the new species, the spines end at level of the basal bulb vs. the level of the nerve ring in Batwanema latum comb. n. On the other hand, the lateral alae of Batwanema congo gen. n. et sp. n. arise within the spiny region and extend to a distance beyond the vulva, whereas Batwanema latum comb. n. presents lateral alae from the beginning of the isthmus to the level of anus. Batwanema congo gen. n. et sp. n. has a larger body (1.820–2.370 vs. 1.360–1.472) but the oesophagus is comparatively shorter (b = 4.92–6.24 vs. 4.10–4.60).
The eggs of both taxa are similar in size (Batwanema congo gen. n. et sp. n. = 0.098–0.123×0.030–0.053; Batwanema latum = 0.112–0.116×0.039–0.042), but are ridged-shelled in Batwanema congo gen. n. et sp. n. vs. the smooth-shelled eggs of Batwanema latum comb. n. The tail of Batwanema congo gen. n. et sp. n. is comparatively longer (c = 6.28–7.90 vs. 11.00–13.00).
Batwanema congo gen. n. et sp. n. differs from Batwanema annulatum comb. n. by the cervical spines extending further down the body, whereas Batwanema annulatum comb. n. has spines ending before the level of the basal bulb. Lateral alae of Batwanema annulatum comb. n. extend from the level of the isthmus to the level of the anus in opposition to Batwanema congo gen. n. et sp. n. where the lateral alae clearly finish before the anus. Batwanema congo gen. n. et sp. n. is also longer (1.820–2.370 vs. 1.439–1.509), with the oesophagus comparatively shorter (b = 4.92–6.24 vs. 4.50–4.60). Moreover, the eggs of Batwanema annulatum comb. n. are smooth-shelled vs. the ridged-shelled ones of Batwanema congo gen. n. et sp. n.
Pentalobus barbatus (Fabricius, 1801) (Coleoptera: Passalidae).
Pentalobus sp. (Coleoptera: Passalidae).
Gut caeca.
Mongwalu, Ituri province, Democratic Republic of Congo.
Bambesa, Uele region, Democratic Republic of Congo.
Specific epithet in apposition refers to the country of the new taxon.
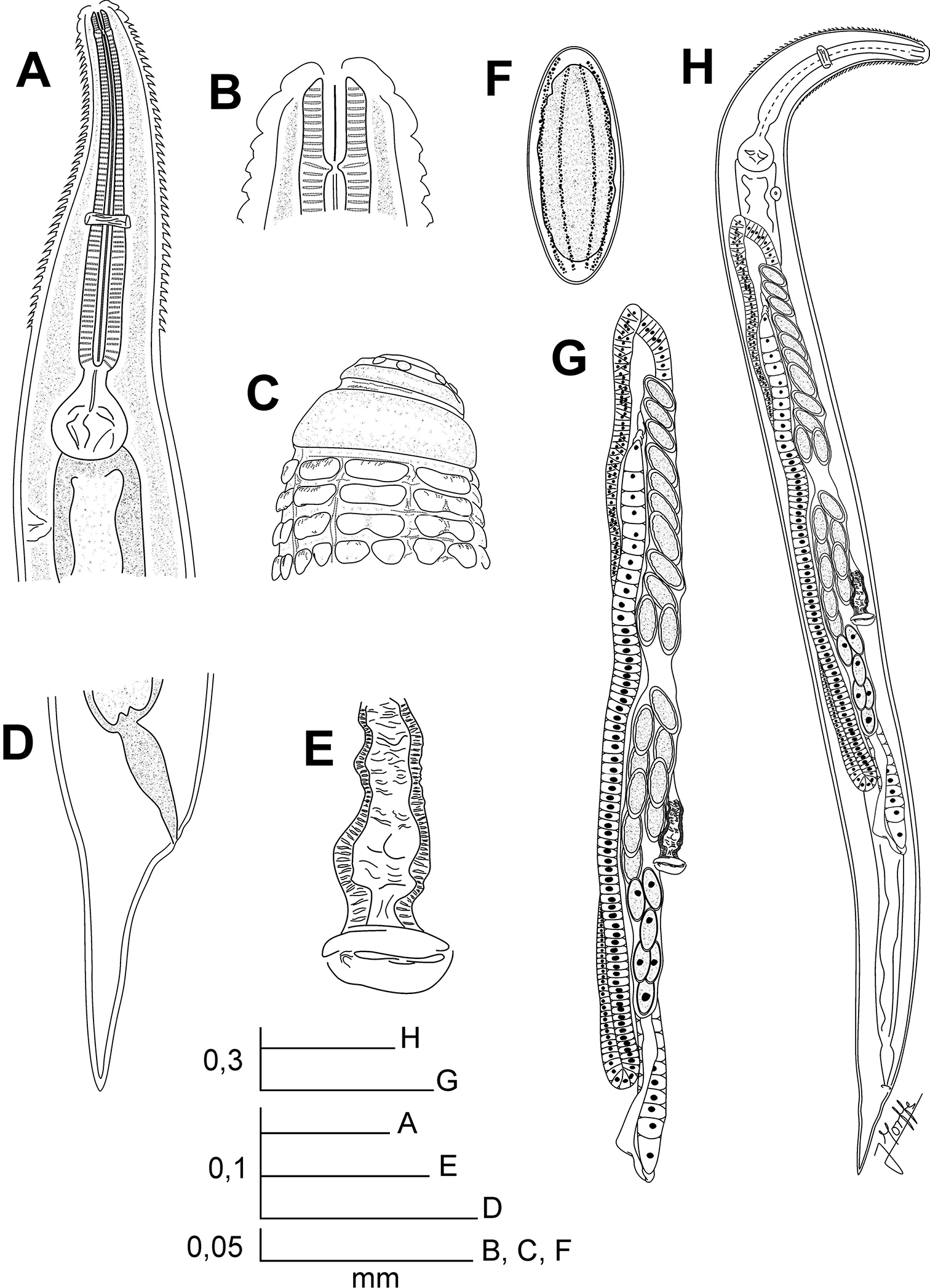
Female. Cervical cuticle armed with wide, scale-like projections, arranged initially in rows of eight elements. Scales divide gradually, forming spines. Head bearing eight rounded, paired papillae. First cephalic annule cone-like, truncated, comparatively long, moderately inflated. Oesophagus with procorpus sub-cylindrical, its base set-off from the short isthmus. Excretory pore post-bulbar. Genital tract didelphic-amphidelphic. Eggs ovoid. Tail subulate.
Chokwenema lepidophorum Morffe & García gen. n. et sp. n.
Democratic Republic of Congo.
The generic name (neuter in gender) is derived of Chokwe, after an ethnic group from Central Africa (including the D. R. of Congo) and the suffix–nema.
http://zoobank.org/124AD063-4948-4F9C-8BD2-F796C14DAA59
http://species-id.net/wiki/Chokwenema_lepidophorum
Figure 3A–H, 4A–C♀ holotype, Democratic Republic of Congo, Ituri province, Mongwalu; in Didimoides cf. parastictus.; 5.VI.1939; Lepersonne coll.; CZACC 11.4708. Paratypes: 3 ♀♀, same data as holotype, CZACC 11.4709-11.4711; 4 ♀♀, same data as holotype, RMCA; 1 ♀♀, same data as holotype, CHIOC.
Vouchers: 3♀♀, Democratic Republic of Congo, Ituri province, Mongwalu; in Pentalobus barbatus; 5.VI.1939; Lepersonne coll.; CZACC 11.4712-11.4714; 2♀♀, same data as the latter, RMCA; ♀, same data as the latter, RIT821.
Holotype (female) a = 11.65, b = 5.25, c = 13.40, V% = 55.97, total length = 2.680, maximum body width = 0.230, first cephalic annule (length×width) = 0.018×0.063, stoma length = 0.038, procorpus length = 0.400, isthmus length = 0.025, diameter of basal bulb = 0.090, total length of oesophagus = 0.510, nerve ring to anterior end = 0.260, excretory pore to anterior end = 0.720, anus to posterior end = 0.200, eggs = 0.093–0.098×0.038–0.040 (0.095 ± 0.003×0.039 ± 0.001 n = 3).
Paratypes (females) (n = 8) a = 9.09–15.63 (10.87 ± 2.40 n = 6), b = 4.26–4.90 (4.62 ± 0.28 n = 5), c = 11.68–12.53 (12.04 ± 0.36 n = 4), V% = 54.40–57.75 (55.76 ± 1.24 n = 6), total length = 2.000–2.500 (2.192 ± 0.181 n = 6), maximum body width = 0.160–0.225 (0.209 ± 0.021 n = 8), first cephalic annule (length×width) = 0.015–0.020×0.058–0.063 (0.018 ± 0.002×0.060 ± 0.002 n = 6), stoma length = 0.030–0.040 (0.035 ± 0.004 n = 7), procorpus length = 0.330–0.420 (0.376 ± 0.032 n = 7), isthmus length = 0.023–0.038 (0.027 ± 0.006 n = 5), diameter of basal bulb = 0.088–0.100 (0.093 ± 0.005 n = 8), total length of oesophagus = 0.450–0.520 (0.487 ± 0.026 n = 6), nerve ring to anterior end = 0.210–0.260 (0.235 ± 0.021 n = 4), excretory pore to anterior end = 0.660, anus to posterior end = 0.170–0.210 (0.184 ± 0.019 n = 4), eggs = 0.090–0.100×0.038–0.050 (0.097 ± 0.004×0.043 ± 0.004 n = 11).
Pentalobus barbatus. Females (n = 6) a = 10.36–15.00 (13.25 ± 1.85 n = 6), b = 4.94–5.80 (5.41 ± 0.31 n = 6), c = 13.35–15.73 (14.21 ± 0.95 n = 6), V% = 51.31–56.99 (53.49 ± 2.40 n = 5), total length = 2.175–2.720 (2.453 ± 0.228 n = 6), maximum body width = 0.170–0.210 (0.187 ± 0.016 n = 6), first cephalic annule (length×width) = 0.015–0.018×0.048–0.058 (0.016 ± 0.001×0.053 ± 0.004 n = 6), stoma length = 0.033–0.038 (0.036 ± 0.002 n = 6), procorpus length = 0.310–0.390 (0.353 ± 0.027 n = 6), isthmus length = 0.018–0.033 (0.025 ± 0.006 n = 5), diameter of basal bulb = 0.083–0.103 (0.091 ± 0.007 n = 6), total length of oesophagus = 0.410–0.490 (0.453 ± 0.029 n = 6), nerve ring to anterior end = 0.210–0.250 (0.228 ± 0.015 n = 5), anus to posterior end = 0.150–0.200 (0.173 ± 0.022 n = 6), eggs = 0.088–0.100×0.035–0.050 (0.095 ± 0.004×0.042 ± 0.004 n = 10).
Female body robust, widening from the base of the first cephalic annule, maximum body diameter at level of the vulva, then tapering towards anus. Cuticle markedly annulated in the spiny region, annuli less marked in the rest of the body (ca. 3–5µm). Cervical cuticle armed initially by opposite rows of rectangular scales, arranged in number of eight. Scales bifurcate gradually at the third row by a cleavage. Division is total at level of the fifth row, with 16 shorter scales, their tips rounded. Scales becoming pointed towards the end of the spiny region. Last rows of spines (a total of 35–36) end at about one seventh of the body-width before the base of the procorpus. Sub-cuticular longitudinal striae present. Lateral alae absent. Head bearing eight rounded, paired papillae. Amphids lateral, pore-like. First cephalic annule similar in length to head, cone-like, truncated, slightly inflated. Stoma short, about two first cephalic annule-lengths long, surrounded by an oesophageal collar. Oesophagus consisting of a muscular, sub-cylindrical procorpus, its base well set-off from the short isthmus. Basal bulb pyriform, valve-plate well developed. Intestine simple, sub-rectilinear. Rectum short, anus not prominent. Nerve ring encircling the procorpus at ca. 55% of its length. Excretory pore located at ca. three fourths of the body-width posterior to the basal bulb. Vulva a median transverse slit, displaced to the posterior half of body, its lips slightly prominent. Vagina muscular, directed forwardly. Genital tract didelphic-amphidelphic, both ovaries reflexed. Oocytes in single rows. Eggs comparatively small, ovoid, with eight rough longitudinal ridges in the shell. Tail short, conical, subulate, ending in a sharp tip. Male unknown.
Chokwenema lepidophorum gen. n. et sp. n. Female. A Oesophageal region, lateral view B Cephalic end, internal view C Cephalic end, external view D Tail, lateral view E Vulva, ventral view F Egg G Genital tract, ventro-lateral view H Habitus, ventro-lateral view.
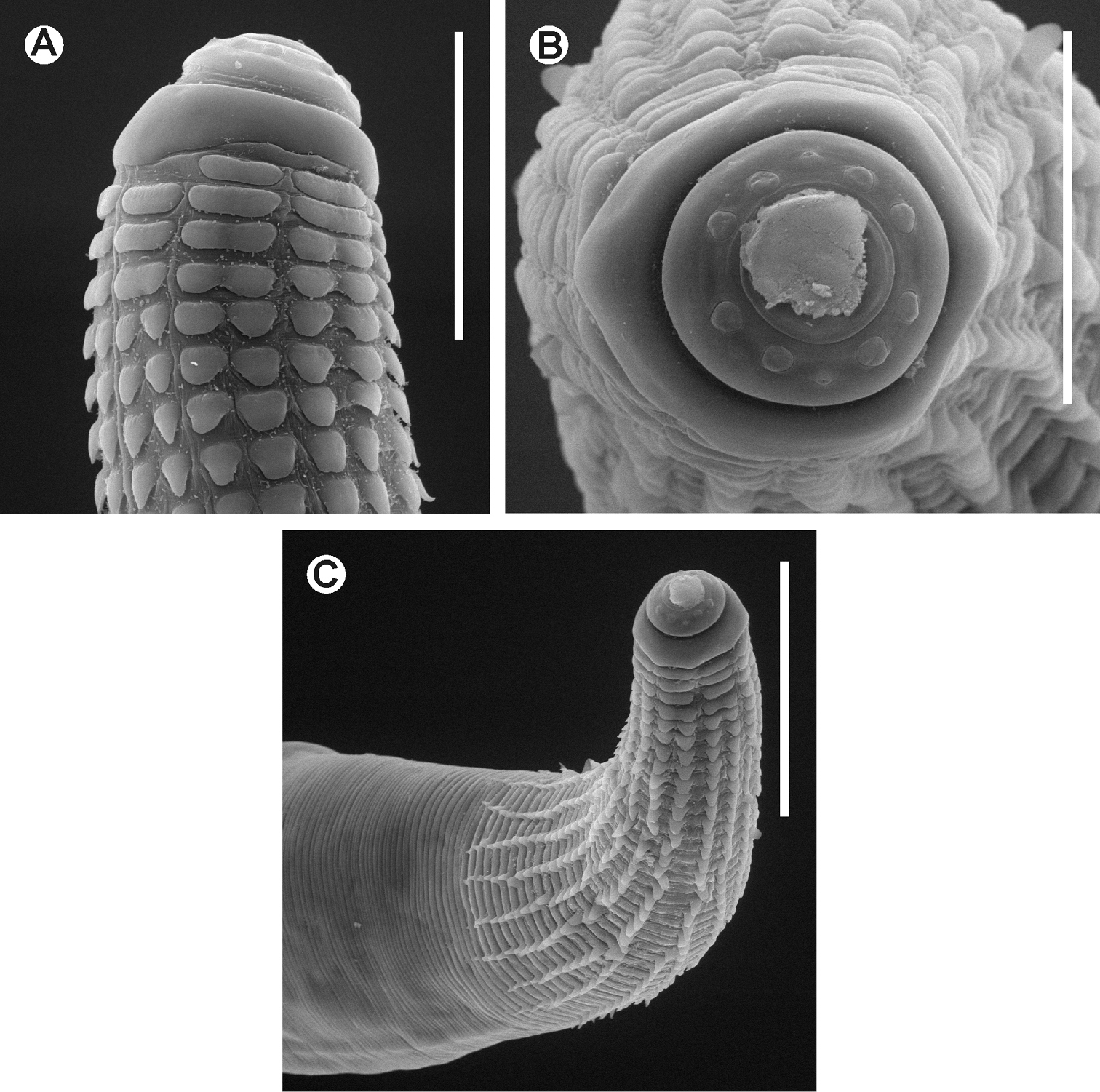
Chokwenema lepidophorum gen. n. et sp. n. Female. SEM images A Cephalic end B Cephalic end, en face view C Cervical region. Scale lines: A 0.05 mm, B 0.04mm, C 0.1 mm.
Chokwenema gen. n. resembles the African genus Batwanema gen. n. by having a similar arrangement of the cervical spines: first row of eight rectangular scales gradually bifurcating, turning into pointed spines. The genus differs by its genital tract didelphic-amphidelphic vs. monodelphic-prodelphic in Batwanema gen. n. The procorpus is sub-cylindrical in Chokwenema gen. n. in opposition to the clavate procorpus of Batwanema gen. n. Moreover, Chokwenema gen. n. posses a single, evident truncate first cephalic annule, slightly inflated instead of the two hardly marked annuli of Batwanema gen. n., barely expanded.
Lepidonema and Salesia also bear scale-like projections in the cervical cuticle and shows a didelphic-amphidelphic genital system (
The other digonant genus with spines in the cuticle and sub-cylindrical procorpus is Soaresnema Travassos & Kloss, 1958, which can be segregated from Chokwenema gen. n. by lacking scales in the cervical cuticle, by the spines forming transverse rows vs. opposite rows and by the larger number of elements in the first row (16) vs. eight in Chokwenema gen. n.
Didimoides cf. parastictus (Imhoff, 1843) (Coleoptera: Passalidae).
Pentalobus barbatus (Fabricius, 1801) (Coleoptera: Passalidae).
gut caeca.
Mongwalu, Ituri province, Democratic Republic of Congo.
Specific epithet derived from the Greek lepidos: scale and phoreus: to bear, after the scale-like projections of the cervical cuticle.
We thank Dr. Marc de Meyer, curator of the Entomological Collection of the Royal Museum of Central Africa, Tervuren, Belgium for his kind permission to access material deposited in this collection. To Dr. Wilfrida Decraemer, Dr. Yves Samyn, Dr. Marie-Lucie Susini and Dr. Alain Drumont for their assistance during the visit of the senior author to the Royal Belgian Institute of Natural Sciences (RBINS), Brussels. To Julien Cillis (RBINS) for technical help with SEM. To MSc. Yamir Arias, MSc. Eduardo Furrazola and Lic. Susett González (Instituto de Ecología y Sistemática) for their help with the micrographs. To Dr. Pedro Reyes-Castillo (Instituto de Ecología, Veracruz, México) and Dr. Stéphane Boucher (Museum of Natural History, Paris, France) for the identification of the hosts. We are indebted to Dr. Pedro Herrera (Instituto de Ecología y Sistemática) for his review of the English language. The visit to collections in Belgium to access the material and SEM techniques was supported by the Belgian Development Cooperation through the Belgian Focal Point of the Global Taxonomy Initiative (GTI), 2010 and 2012 calls. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).