(C) 2013 Christopher K. Taylor. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Mangatangi parvum gen. n. and sp. and Forsteropsalis pureroa sp. n. are described from the North Island of New Zealand. Pantopsalis listeri (White 1849) and Pantopsalis cheliferoides (Colenso 1882) are redescribed and no longer regarded as nomina dubia; Pantopsalis luna (Forster 1944) is identified as a junior synonym of Pantopsalis listeri. A key to Pantopsalis species is provided.
Palpatores, Phalangioidea, taxonomy
The Enantiobuninae (sensu
Both genera are represented in museum collections by material from throughout both of the main islands of New Zealand (personal observations). However, accidents of history have resulted in the fauna of the South Island being more extensively investigated than that of the North Island, with the greater number of described species coming from the former. Only one species of Pantopsalis and four species of Forsteropsalis have been described to date from the North Island. In examining North Island material held in the collection of Te Papa Tongarewa, Wellington (MONZ), a further species of Forsteropsalis was recognised, as well as specimens of the previously inadequately described Pantopsalis cheliferoides. Examination of these specimens, as well as of specimens attributed to Pantopsalis listeri (
A third novel species from the North Island is of particular interest as it does not accord with either Pantopsalis or Forsteropsalis, and may represent an outgroup to both. It is here described as representing a new genus.
Specimens were sourced from the collections of Te Papa Tongarewa, Wellington, New Zealand (MONZ) and the Muséum national d’Histoire naturelle, Paris, France (MNHP). Photographs and measurements were taken using a Nikon SMZ1500 stereo microscope and the NIS-Elements D 4.00.03 programme, and a Leica DM2500 compound microscope. Coloration is described as in alcohol. Measurements are given in millimetres.
urn:lsid:zoobank.org:act:1812C6B3-9428-42CB-8855-3609F113E9D7
Mangatangi parvum new species.
Neuter, named for the type locality of the type species.
As for type and only species.
urn:lsid:zoobank.org:act:F3982336-8FDC-49EF-B17D-E899B32F312D
http://species-id.net/wiki/Mangatangi_parvum
Figure 1♂. New Zealand, AK. Mangatangi, Hunua Ra., 8 Feb–8 Mar 1977, I. Barton, ARA Kauri Seed Project, pit trap.
1 ♀, as for holotype; 1 ♂, Cuvier Is, July, R. Forster.
From the Latin parvus, small, in reference to its small size compared to other New Zealand Enantiobuninae.
Mangatangi parvum can be distinguished from all other New Zealand Enantiobuninae by the presence of a well developed tooth comb on the pedipalpal tarsal claw. It can be distinguished from Monoscutum titirangiense, Acihasta salebrosa and Templar incongruens by its relatively long legs and unsclerotised dorsum. It differs from ‘Megalopsalis’ triascuta, all Pantopsalis and most Forsteropsalis species in the absence of either a mediodistal apophysis or hypersetose region on the pedipalpal patella, from Pantopsalis species by its relatively bowed cheliceral fingers, and from Forsteropsalis species by the absence of denticles on the medial side of the pedipalp coxa. Mangatangi parvum can also be distinguished from all other New Zealand species, so far as is known, by its genital morphology: all other New Zealand species investigated to date have a relatively long glans that is either narrow in lateral view (most species) or possesses a distinct dorsal keel (Pantopsalis). The deep and short glans of Mangatangi parvum is also distinct from that of Enantiobuninae elsewhere: Neopantopsalis species have a very elongate and relatively flat glans, and Megalopsalis and Spinicrus species have a short but also distally flattened glans. The only other Enantiobuninae in Australasia to possess comparatively deep glans are Australiscutum and Tercentenarium linnaei; Mangatangi parvum differs from Australiscutum in possessing relatively long legs and retaining an anterior grill of spines over the spiracle, and from Tercentenarium linnaei in lacking a large dorsolateral flange at the junction between shaft and glans. The glans of Thrasychirus has never been illustrated in lateral view, but Mangatangi parvum is clearly distinguished from that genus by possessing paired bristle groups at the junction between shaft and glans rather than single bristles.
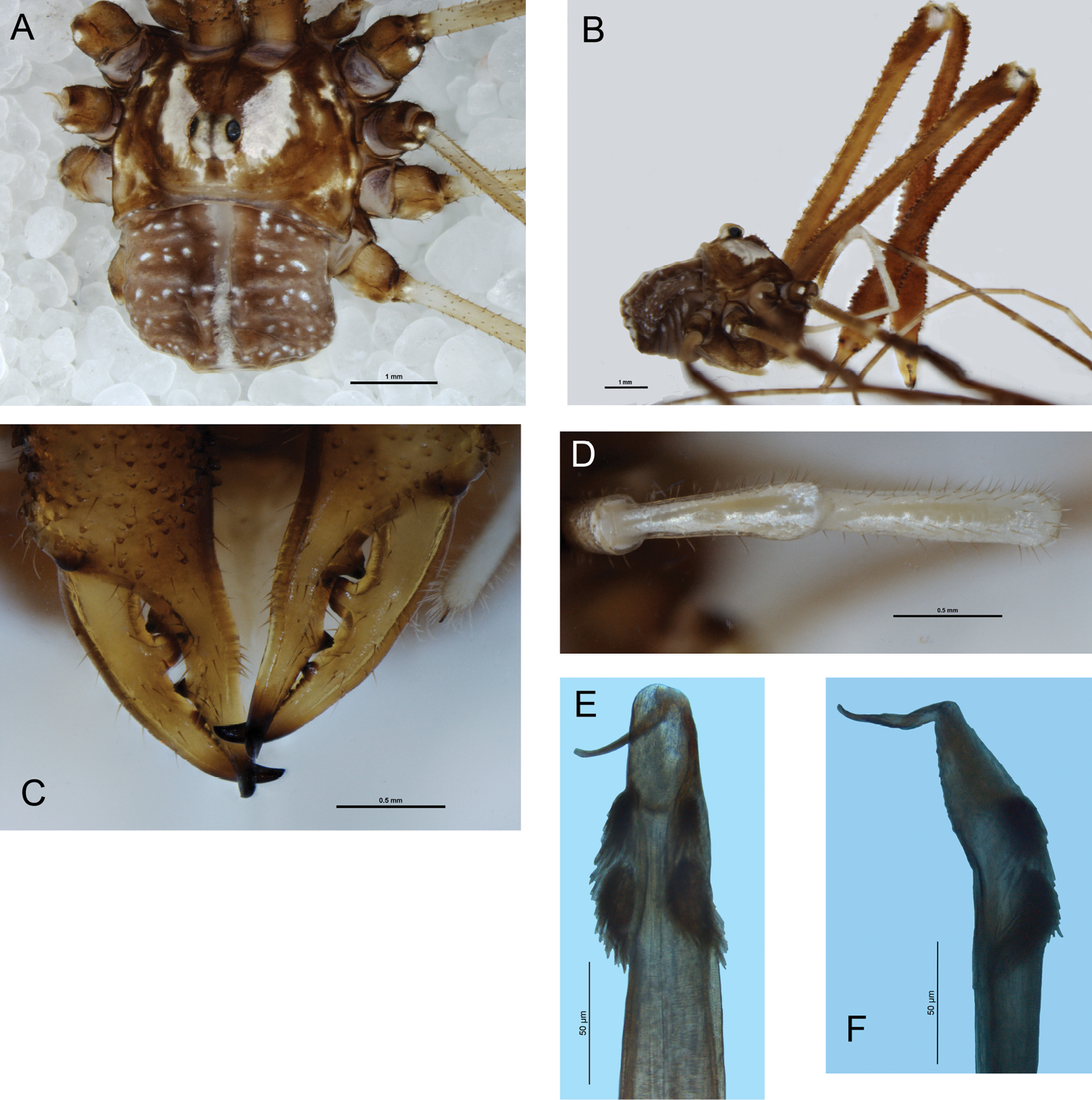
Male (Figs 1a–b, d–e, g–i, l): Total body length 2.06–2.74 (larger value in all measurements represents holotype), prosoma length 0.97–1.19, prosoma width 1.76–2.01. Dorsal prosomal plate mostly light orange-yellow, unarmed except short, spinose black setae scattered over entire body; anterior propeltidium lighter yellow-cream, supracheliceral groove extending roughly halfway between anterior margin of carapace and ocularium; median propeltidium with diffuse purple stripes along border with anterior propeltidium with diffuse silver-white markings behind purple stripes, dark brown markings on lateral edge of dorsal prosomal plate; ocularium silver with black stripes margining eyes, unarmed; postocularium not distinguished from remainder of posterior propeltidium. Mesopeltidium forming raised ridge, medially pale yellow, laterally dark brown. Ozopores on raised lateral lobes, anterior lobes of prosoma and ozopore lobes dark brown, posterior of ozopore lobes silver-white, remainder of lateral shelves mostly yellow with dark brown lateral margins broadening to diffuse dark brown patch at about three-quarters of distance from front of prosoma. Metapeltidium and dorsum of opisthosoma with background colour of purple broken by pale yellow mottling, particularly along segment boundaries, longitudinal mediolateral broken stripes of silver-white present as well as longitudinal medial rows of silver-white spots, sides of opisthosoma with purple background heavily broken by pale yellow punctations. Mouthparts white; coxae proximally pale yellow; coxae I and II distally with purple mottling, coxae III and IV with dark yellow-brown mottling laterally; genital operculum pale yellow; venter of opisthosoma mottled light purple with pale yellow stripes along segment boundaries.
Chelicerae: Segment I 2.85–3.51, segment II 3.82–4.62. Segment I ventrally cream, dorsally orange-yellow, sparsely denticulate dorsally; segment II inflated, orange-yellow, densely dorsally and sparsely ventrally denticulate. Cheliceral fingers (Fig. 1d) long, bowed, movable finger with setae close to median tooth.
Pedipalps: Femur 1.53–2.13, patella 0.65–0.77, tibia 0.71–1.03, tarsus 1.80–2.47. Coxae unarmed. Femur to tarsus long, slender, unarmed, femur to tibia cream with paler distal ends to each segment, tarsus off-white with yellow-brown shading at distal end. Patella and tibia (Fig. 1e) straight, patella without distal prolateral apophysis or hypersetose region. Plumose setae absent. Microtrichia on distal half of tarsus only. Claw with ventral tooth-comb.
Legs: Leg I femur 2.99–3.80, patella 0.71–0.91, tibia 2.93–3.87; leg II not preserved; leg III 2.56–3.38, patella 0.77–0.93, tibia 2.70–3.45; leg IV femur 4.05–5.01, patella and tibia not preserved. All segments unarmed. Trochanters pale yellow, trochanters III and IV with dark yellow-brown mottling laterally. Femora to tarsi pale yellow, patellae and distal ends of femora and tibiae darkening to orange-yellow. Leg II not preserved; tibia IV with three pseudosegments.
Penis (Figs 1g–i): Glans noticeably short and deep, sides parabolic in ventral view. Bristle groups of medium length. Tendon short, not extending far behind bristle groups.
Spiracle (Fig. 1l): Curtain of distally anastomosing spines extending over entire spiracle; shortening to cluster of tubercles (possibly lace tubercles) at medial corner.
Female (Figs 1c, f, j–k): Coloration similar to that of male. Other features as for male except for following: Chelicerae not enlarged, unarmed, segment I without ventral spine. Pedipalp (Fig. 1f) with microtrichia over entire patella, tibia and tarsus except glabrous dorsal line on patella and tibia. Ovipositor (Figs 1j–k) with single pair of seminal receptacles.
Mangatangi parvum. A Holotype, dorsal view B holotype, lateral view C female, dorsal view D holotype, cheliceral fingers, anterior view E holotype, patella and tibia of left pedipalp, dorsal view F female, patella and tibia of right pedipalp, dorsal view G penis, right lateral view H glans, ventral view I glans, left lateral view J ovipositor K close-up of seminal receptacles L left spiracle of female.
Mangatangi parvum is probably related to the clade formed by Forsteropsalis and Pantopsalis, with which it shares the presence of sharp papillae on the glans, and of setae close to the major tooth of the mobile finger of the chelicera (this last feature is also present in Neopantopsalis). The retention in Mangatangi parvum of a plesiomorphic tooth-comb on the pedipalpal tarsal claw, together with Mangatangi parvum’s distinctly short glans, could suggest a sister relationship between Mangatangi parvum and the Pantopsalis + Forsteropsalis clade, but this should perhaps be treated with caution. Pantopsalis rennelli and Pantopsalis cheliferoides each retain reduced teeth arrays (a single tooth in the latter species) on the tarsal claw, and that of Pantopsalis albipalpis has a ventral rugose area that may correspond to the remains of the tooth-row. The loss of the tooth-row in Pantopsalis and Forsteropsalis has therefore happened at least partially in parallel. As regards the short glans of Mangatangi parvum compared to the long glans of Pantopsalis and Forsteropsalis, our understanding of enantiobunine phylogeny is not yet robustly resolved (
urn:lsid:zoobank.org:act:F97E7775-669A-42CB-81ED-5578B8997191
http://species-id.net/wiki/Forsteropsalis_pureora
Figure 2♂. New Zealand, TO. Pureora, Waipapa Reserve, 570 m, 15 December 1983, J. Hutchinson, malaise trap in shrublands.
Named for the type locality.
The genus Forsteropsalis was established and revised by
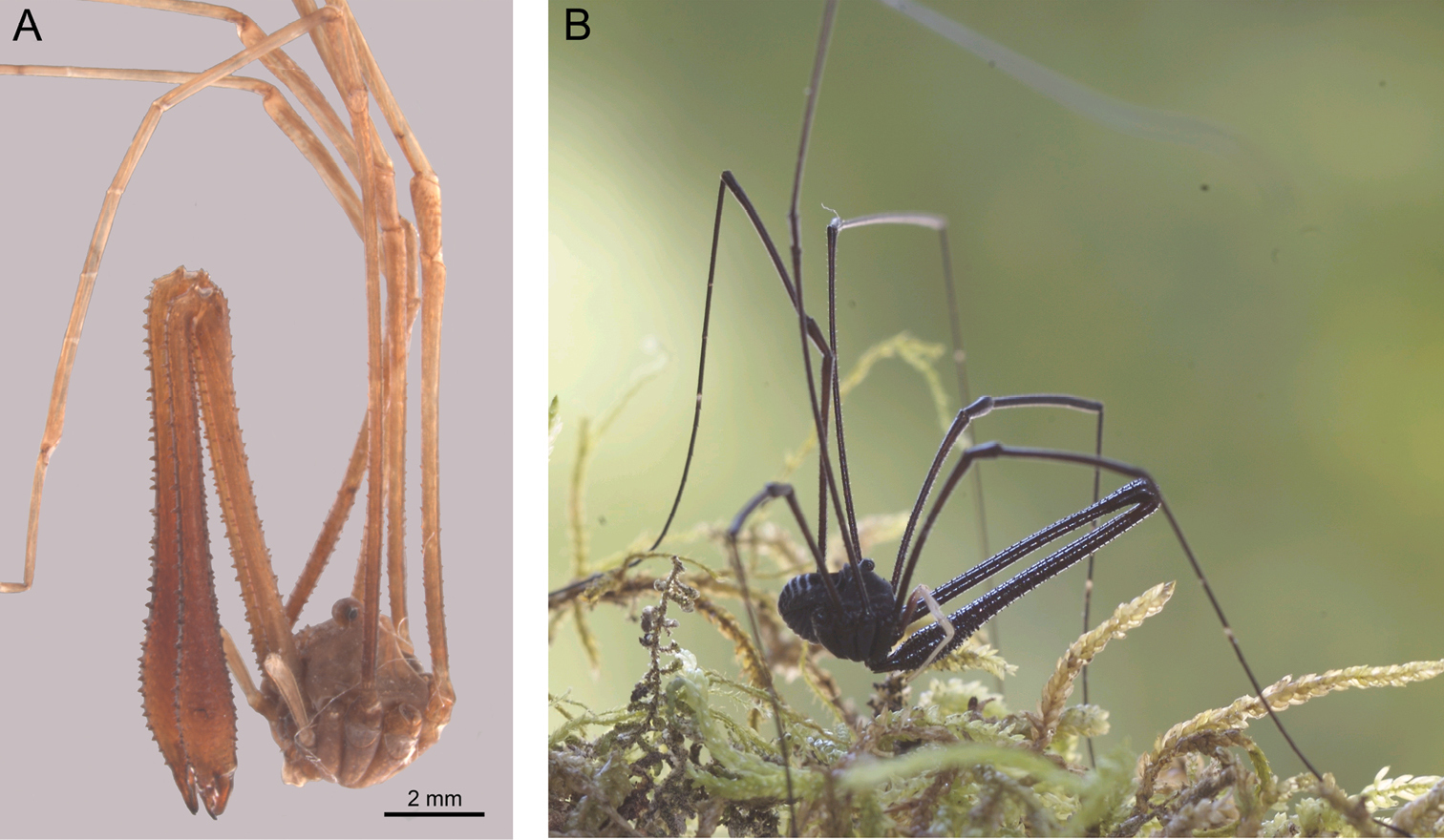
Male: Total body length 3.73, prosoma length 1.77, prosoma width 2.83. Dorsal prosomal plate honey-brown with large white patches covering much of median propeltidium on either side of ocularium and becoming diffuse behind ocularium, postocularial area yellow-grey; anterior propeltidium with sharp denticles, remainder of dorsum unarmed but with scattered black setae; ocularium white. Mesopeltidium forming raised ridge, medially pale yellow-grey, darkening to honey-brown laterally. Ozopores on raised lateral lobes, anterior ozopore lobe with distinct white patch, remainder of lateral shelves honey-brown with smaller white patches on posterior ozopore lobe and near posterior end of shelf. Metapeltidium and dorsum of opisthosoma light purple with median white stripe and transverse rows of white spots across segments. Mouthparts white; coxae mottled honey-brown proximally, darker brown distally; genital operculum honey-brown; venter of opisthosoma light purple.
Chelicerae: Segment I 7.06, segment II 8.94. Segment I darker yellow-brown with white patches at distal end; segment II orange-brown; both segments evenly denticulate; segment II not inflated. Cheliceral fingers (Fig. 2c) long, only slightly bowed, movable finger with numerous setae close to median tooth.
Pedipalps: Femur 2.00, patella 0.98, tibia 1.21, tarsus 2.75. Coxa with numerous sturdy denticles on prolateral margin. Pedipalps long, slender, femur with dorsal and ventral rows of denticles; femur proximally honey-brown except cream-coloured heel; distal end of femur, patella and tibia white mottled with cream; tarsus cream-coloured. Patella and tibia (Fig. 2d) straight, patella with concentration of strong setae at prolateral distal end but without distinct apophysis. Microtrichia on distal two-thirds of tarsus. Tarsal claw ventrally rugose.
Legs: Leg I femur 7.03, patella 1.48, tibia 6.04; leg II femur 11.70, patella 1.74, tibia 11.64; leg III femur 7.00, patella 1.51, tibia 5.44; leg IV femur 8.32, patella 1.38, tibia 7.83. Femora with relatively few small denticles dorsally; other segments unarmed. Trochanters honey-brown with white distal retrolateral spot on each trochanter. Femora proximally dull medium yellow, distal ends of femora to tibiae honey-brown mottled with white spots; tibiae lighter orange-brown banded with dull yellow. Tibia I with two pseudosegments; tibia II with ten pseudosegments; tibia IV with three pseudosegments.
Penis (Figs 2e–f): Glans relatively long, sides parabolic in ventral view; triangular in lateral view. Bristle groups of medium length. Tendon long.
Forsteropsalis pureroa, holotype. A Dorsal view B lateral view C cheliceral fingers, anterior view D patella and tibia of right pedipalp, dorsal view E glans, ventral view F glans, right lateral view.
Another feature of Forsteropsalis pureora that may deserve further investigation is the unusually high number of pseudosegments in the leg tibiae. Not only does the holotype have a higher number of pseudosegments in tibia II than recorded for any other Forsteropsalis species, even the particularly large species Forsteropsalis fabulosa and Forsteropsalis tumida, it represents the first recorded instance in this genus of pseudosegmentation in tibia I. At our present level of knowledge, this cannot be considered a reliable distinguishing character for the species as tibial pseudosegment number has been found in other species to vary between individuals. However, pseudosegment number has been suggested to distinguish Forsteropsalis chiltoni and Forsteropsalis marplesi in which, so far as is known, males of each species have varying but non-overlapping ranges of pseudosegment number for tibia II (
http://species-id.net/wiki/Pantopsalis_listeri
Figure 31 ♂, ‘Ile du Milieu, Filhol, 1875-75’ (MNHP no. 134).
1 ♂, same data as neotype; 3 ♂, New Zealand, WD. Waiho Gorge, Sth Westland, 21 July 1927 (MONZ); photographs of live males provided by Simon Pollard (Canterbury Museum, Christchurch).
As described by
Pantopsalis listeri. A Neotype, lateral view B live specimen, photographed by Simon Pollard.
The original type specimen(s) of Phalangium listeri are lost; they have not been located in the collection of the Museum of Natural History, London (J. Beccaloni, pers. com.) or of the Muséum national d’Histoire naturelle, Paris (M. Judson, pers. com.) It was therefore treated as a nomen dubium by
Since the publication of
White’s (1849) original description of Pantopsalis listeri does not provide a more detailed type locality than ‘New Zealand’, but
http://species-id.net/wiki/Pantopsalis_cheliferoides
Figure 41 ♂, New Zealand, GB. Lake Waikaremoana, 19 November 1975, G. W. Ransay, beating; 1 ♂, New Zealand, GB. Te Urewera National Park, Lake Waikaremoana, Kaitawa, 38°46'S, 177°83'E, 18 November 2004, D. King, on outside of house.
Male: Total body length 3.55–4.03 (former measurement refers to 2004 specimen), prosoma length 1.62–2.06, prosoma width 2.55–3.70. Dorsal prosomal plate medium brown, with some yellowish patches laterally; anterior propeltidium and ocularium heavily denticulate, remainder of prosoma unarmed. Dorsum of opisthosoma dark purplish brown with few white spots medially in one specimen, longitudinal purple medial stripe in other; larger white spots in present in central part of opisthosomal dorsum, comparable to lateral ‘arms’ of median stripe in females of other Pantopsalis species. Coxae medium brown mottled with honey brown; venter of opisthosoma medium purplish brown mottled with lighter purple.
Chelicerae: Segment I 4.73–6.23, segment II 6.36–8.47. Segment I medium brown with cream patches at distal end; segment II orange-brown; both segments heavily denticulate. Segment II inflated in larger specimen, slender in smaller. Cheliceral fingers (Fig. 4c) short, mobile finger crescent-shaped.
Pedipalps: Femur 1.81–2.32, patella 0.97–1.15, tibia 1.01–1.18, tarsus 2.10–3.02. Femur light purple at base, remainder of pedipalp shining white. Patella and tibia (Fig. 4d) prolaterally hypersetose, patella bulging prodistally but without distinct apophysis. Microtrichia on distalmost end of tarsus only. Tarsal claw with single ventral tooth.
Legs: Leg I femur 7.91–8.56, patella 1.36–1.58, tibia 6.03–6.14; leg II femur 13.25–13.69, patella 1.82–1.80, tibia 11.18–11.47; leg III femur 6.91–7.38, patella 1.32–1.45, tibia 5.22–5.48; leg IV femur 9.88–10.81, patella 1.57–1.59, tibia7.53–7.38. Femora evenly but irregularly denticulate, except distal third of femur II unarmed; remaining segments unarmed. Legs medium brown mottled with yellowish, tibiae and tarsi tinged with purple, tibiae spotted with white; tarsi with white band at base of telotarsi. Tibia II with five pseudosegments; tibia IV undivided in larger specimen, with two pseudosegments in smaller.
Penis (Fig. 4e–f): Glans medium length, sides parabolic in ventral view; subtriangular in lateral view but not markedly flattened, slight dorsomedial bulge but keel essentially absent. Bristle groups short. Tendon long.
Pantopsalis cheliferoides, specimen collected in 1975. A Dorsal view B lateral view C fingers of left chelicera, anterior view D patella and tibia of right pedipalp, dorsal view E glans, ventral view F glans, left lateral view.
There is some variation in coloration between the two specimens available, most notably the presence of a medial stripe on the opisthosoma of one but not the other, with the former specimen also being overall lighter in coloration than the latter. It is possible that this difference may reflect differences in maturity between the two specimens, similar to what has been recorded for other Opiliones species (
Long regarded as something of a mystery after its initial description by
The absence of a distinct dorsal keel on the glans of the penis clearly distinguishes Pantopsalis cheliferoides from all other Pantopsalis species except Pantopsalis luna and possibly Pantopsalis pococki (for which the genital morphology remains unknown). Pantopsalis pococki has a very distinct colour pattern, with transverse light coloured stripes on the dorsum of the opisthosoma (
The presence of dimorphic males as described for other Pantopsalis species by
The last author to provide a key to species of Pantopsalis was
| 1 | Lateral parts of opisthosoma with extensive light-coloured markings, either broadly light-coloured or with broad transverse stripes, contrasting with darker median; light coloured transverse stripe often covering most of metapeltidium and/or first opisthosomal segment | 2 |
| – | Lateral parts of opisthosoma largely dark (longitudinal median stripe may be present; transverse stripes, if present, narrow and not covering most of lateral part of opisthosoma), no light transverse stripe over metapeltidium and first opisthosomal segment | 4 |
| 2 | Light-coloured lateral patches extending mediad as transverse stripes; articular membranes not brightly coloured | 3 |
| – | Light-coloured patches restricted to lateral part of opisthosoma, not extending mediad as transverse stripes; articular membranes brightly coloured (white in alcohol) |
Pantopsalis phocator |
| 3 | Dorsal prosomal plate with numerous well-developed denticles in both anterior and medial propeltidial areas |
Pantopsalis pococki |
| – | Dorsal prosomal plate with few denticles, and those low and rounded |
Pantopsalis coronata |
| 4 | Dorsal prosomal plate with denticles in anterior propeltidial area at least | 5 |
| – | Dorsal prosomal plate completely unarmed | 7 |
| 5 | Glans of penis with well-developed dorsal keel |
Pantopsalis albipalpis |
| – | Glans of penis without distinct dorsal keel | 6 |
| 6 | Ocularium unarmed; opisthosoma with narrow light-coloured transverse stripes |
Pantopsalis listeri ( |
| – | Ocularium denticulate; opisthosoma without transverse stripes |
Pantopsalis cheliferoides ( |
| 7 | Length of pedipalp femur less than half width of prosoma; femora of legs with few denticles |
Pantopsalis rennelli |
| – | Length of pedipalp femur more than half width of prosoma; femora of legs entirely smooth |
Pantopsalis snaresensis |
Thanks are due to Phil Sirvid (MONZ) and Mark Judson (MNHP) for arranging the loans of specimens used in this study, and to James Cokendolpher and Nobuo Tsurusaki for critical comments on the manuscript. I am also grateful to Simon Pollard (Canterbury Museum) for providing photographs of Pantopsalis listeri, and allowing one of them to be used in this publication.