(C) 2013 James E. O’Hara. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: O’Hara JE, Raper CM, Pont AC, Whitmore D (2013) Reassessment of Paleotachina Townsend and Electrotachina Townsend and their removal from the Tachinidae (Diptera). ZooKeys 361: 27–36. doi: 10.3897/zookeys.361.6448
The monotypic genera Paleotachina Townsend, 1921 and Electrotachina Townsend, 1938 were originally described as fossils in amber but were later discovered to be inclusions in copal. Both taxa were originally assigned to the Tachinidae (Diptera) and this placement has continued to the present day. The holotypes of the two type species, P. smithii Townsend and E. smithii Townsend, were examined and the following taxonomic and nomenclatural changes are proposed: Paleotachina is transferred to the Muscidae and placed in synonymy with Aethiopomyia Malloch, 1921, syn. n.; P. smithii Townsend, type species of Paleotachina, is synonymized with Aethiopomyia gigas (Stein, 1906), syn. n.; Electrotachina is transferred to the Sarcophagidae and placed in synonymy with Dolichotachina Villeneuve, 1913, syn. n.; E. smithii Townsend, type species of Electrotachina, is recognized as a valid species of Dolichotachina comb. n. Images of the holotypes of P. smithii and E. smithii are provided and features that have helped place these copal inclusions in their new combinations are discussed.
Tachinidae, Muscidae, Sarcophagidae, amber, copal, inclusions
For such a large family of Diptera, the Tachinidae have a very meager fossil record. There are about 8500 valid species in the family (
A preliminary investigation into the authenticity of the presumed oldest tachinid fossils by
Treated in this paper are the monotypic genera Paleotachina and Electrotachina. Both were described by
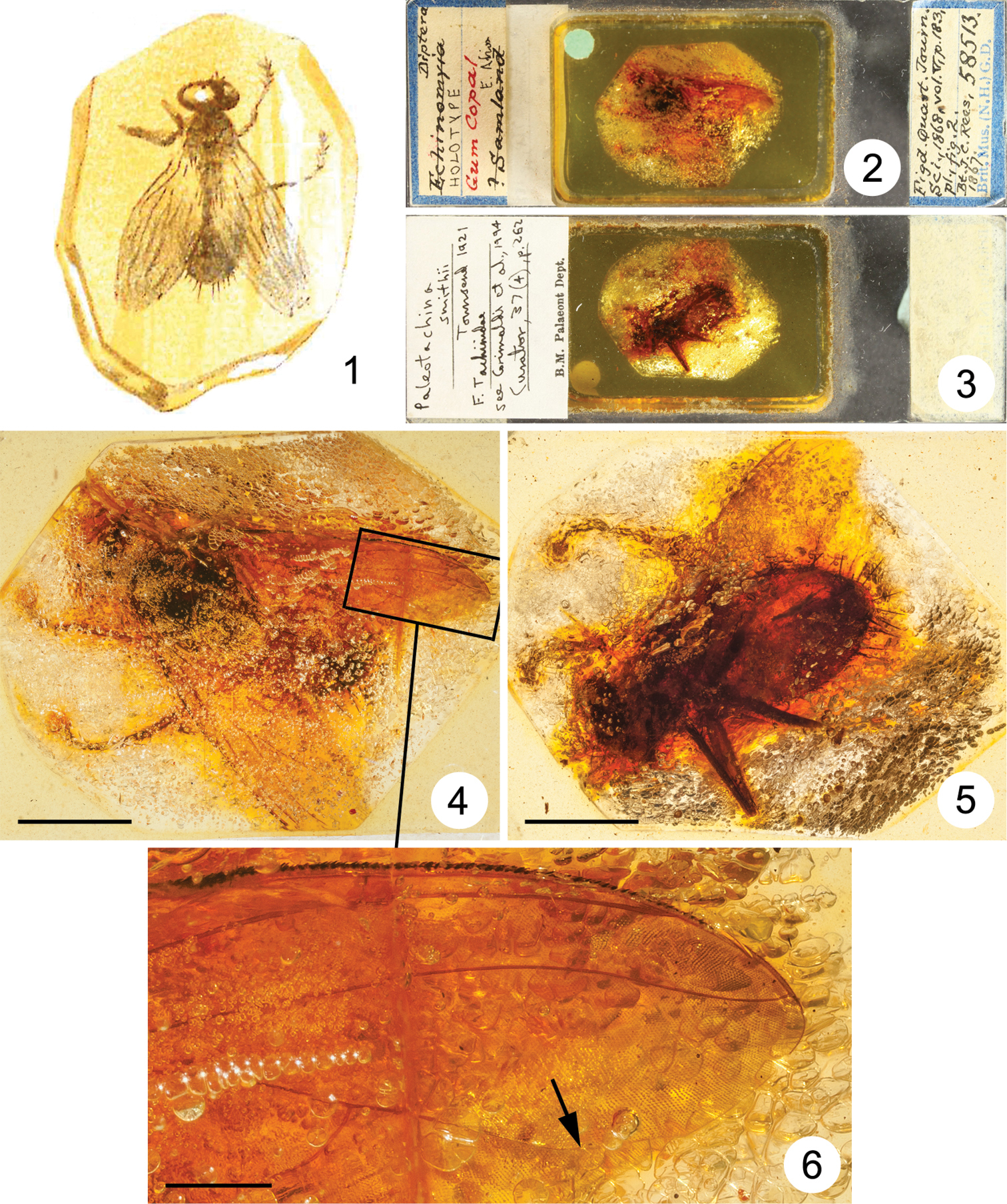
The holotypes of Paleotachina smithii and Electrotachina smithii are deposited in the Natural History Museum, London, United Kingdom (NHM). One of us (AP) studied the holotype of Paleotachina smithii and another (DW) studied the holotype of Electrotachina smithii, thus allowing these inclusions to be placed with some confidence to the species or genus level within the Muscidae and Sarcophagidae, respectively. Each specimen is preserved within a small piece of copal, which is in turn embedded in Canada balsam within a square open-topped glass case glued to a slide. The glass case containing Electrotachina smithii was covered with a cover slip following the recent restoration of the Canada balsam surface, which was scratched. Images for Figs 2–3 and 8–9 were taken with a Canon EOS 550D camera fitted with a Canon MP-E 65 mm lens; images for Figs 4–6 and 10–11 were taken with a Canon EOS 5D Mark II camera fitted with a Canon MP-E 65 mm lens; images for Figs 12–13 were taken with a Canon EOS 650D camera fitted with a 0.63x adaptor mounted on a Leica MZ125 stereomicroscope. Images for Figs 4–6 and 10–13 were stacked using Helicon Focus (version 5.3) software. Figures 1 and 7 were scanned from a plate in
Paleotachina smithii Townsend, 1921 (junior synonym of Aethiopomyia gigas (Stein, 1906), syn. n.), Muscidae 1 reproduction of illustration in Smith (1868, fig. 2) showing inclusion originally identified as “Echinomyia” sp. (i.e., Echinomya Latreille, 1805, junior synonym of Tachina Meigen, 1803, Tachinidae) 2–6 holotype male 2–3 entire slide 2 dorsal view 3 ventral view 4–6 inclusion 4 dorsal view (scale bar = 5.0 mm) 5 ventral view (scale bar = 5.0 mm) 6 enlarged portion of wing circumscribed in Fig. 4 (arrow indicates bend of vein M) (scale bar = 1.0 mm).
Electrotachina smithii Townsend, 1938 (now Dolichotachina smithii (Townsend, 1938), comb. n.), Sarcophagidae 7 reproduction of illustration in Smith (1868, fig. 5) showing inclusion originally identified as a new genus near “Tachinus” (i.e., Tachina Meigen, 1803, Tachinidae) 8–13 holotype female 8–9 entire slide 8 dorsal view 9 ventral view 10–13 inclusion 10 dorsal view (scale bar = 2.0 mm) 11 ventral view (scale bar = 2.0 mm) 12 head, right lateral view (scale bar = 0.5 mm) 13 body, left lateral view (scale bar = 1.0 mm).
The paper by
The editors of the Quarterly Journal of Science followed
Neither
http://species-id.net/wiki/Aethiopomyia
The genus-group names Aethiopomyia and Paleotachina were both made available in 1921. The paper by
http://species-id.net/wiki/Aethiopomyia_gigas
Figs 1–6The “Larvaevorini” of
A considerable amount of artistic liberty was taken in the depiction of NHM specimen #58513 (holotype of Paleotachina smithii) in fig. 2 in
The holotype of Paleotachina smithii is a large fly in the family Muscidae, with a body length of about 14 mm and a wing length of about 14 mm. It is well preserved, but large parts of it are obscured by masses of small air bubbles (see Figs 2–5). The conformation of the abdominal tip suggests that it is a male, but nothing can be seen of the head and associated features. Because of its size, coloration and habitus, the presence of very long stout setae on abdominal tergites 4 and 5, and a vein M that is weakly curved forward towards vein R4+5 in its apical part (Fig. 6), leaving a wide open cell r4+5, the species can be readily assigned to either Aethiopomyia Malloch or Alluaudinella Giglio-Tos, two genera confined to the Afrotropical Region. It is possible to see several small setulae on the node at the base of vein R4+5, and such setulae are present in Aethiopomyia but not in Alluaudinella. Other characters used to differentiate these genera (proepisternal depression setulose or bare, katatergite with fine setulae or bare) cannot be seen in the holotype.
The scutum, scutellum and at least abdominal tergites 4 and 5 are black; the remainder of the body (the head excepted) is yellow. The femora and tibiae are yellow, and the tarsi black. This coloration is most similar to that of Aethiopomyia gigas (Stein), described from Cameroon and widespread though never common across western, eastern and southern Africa. Paleotachina smithii Townsend, 1921 is accordingly synonymized with Aethiopomyia gigas (Stein, 1906), syn. n.
http://species-id.net/wiki/Dolichotachina_smithii
Figs 7–13As with Paleotachina smithii, certain liberties were taken in the depiction of NHM specimen #58551 (holotype of Electrotachina smithii) in fig. 5 in
Electrotachina smithii belongs to the family Sarcophagidae, subfamily Miltogramminae. The holotype female has a body length of about 7 mm and is preserved in a small block of copal (approx. 15 × 10 × 7 mm) together with two other adult dipterans: a small female Agromyzidae and a Cecidomyiidae. The specimen is in very good condition except for the lack of its right fore tarsus. Antennae, wings and chaetotaxy are all in excellent condition. The specimen can be confidently assigned to the genus Dolichotachina based on the following combination of external character states: arista short pubescent, thickened on approximately basal 1/5; eye bare; parafacial with an uneven row of setae anteriorly; proepisternum bare; katepisternum with two, widely separated, setae; mid tibia with one anterodorsal seta; wing cell r4+5 open at wing margin.
In addition to the above features, Dolichotachina smithii is characterized by an elongated postpedicel (about 3 times length of pedicel), relatively short vibrissa, and short proboscis (about twice as long as wide).
Dolichotachina is a mainly Afrotropical genus with 12 species previously known from this region (
Fossils are the most reliable indicators of the minimum age of the lineage to which they belong, but they provide false information if they are incorrectly identified or dated. As explained above, the minimum age of the Tachinidae is no longer the Eocene based on fossil evidence. Instead, the oldest fossils date the family to the Oligocene (
The merging of phylogenetic data with data from fossils of known age and identity to create chronograms is becoming more common in evolutionary studies. The results are generally speculative but provide an estimated evolutionary timeline that can be further refined and tested by future research. Two recent studies on the Diptera have suggested different ages for the origin of the Tachinidae. One, a large study by
We are indebted to staff of the Natural History Museum (London) for their assistance during the preparation of this paper: Claire Mellish (Department of Palaeontology) facilitated our access to the holotypes discussed herein and brought to our attention the paper in which their true age was reported; Harry Taylor (Image Resources) provided the stacked images for Figs 4–6, 10–11; Vladimir Blagoderov (Sackler Biodiversity Imaging Laboratory) kindly assisted in the preparation of Figs 12–13; Lu Allington-Jones (Conservation Centre) restored the upper surface of the Electrotachina smithii slide; and Nigel Wyatt (Department of Life Sciences) took part in a valuable discussion of the Paleotachina inclusion. Thomas Pape (Natural History Museum of Denmark, Copenhagen) and an anonymous reviewer are thanked for their helpful comments on the submitted manuscript.