(C) 2013 Chi-Feng Lee. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
New records of four species (Lema lacertosa Lacordaire, 1845, Lema diversipes Pic, 1921, Lema cyanella (Linnaeus, 1758), Lema trivittata trivittata Say, 1824 and additional information on one recently recorded species (Lema solani Fabricius, 1798) are reported for Taiwan. Lema diversipes Pic, 1921 is removed from synonymy with Lema lacertosa Lacordaire, 1845; both species are redescribed. A lectotype is designated for Lema phungi Pic, 1924. The synonymies of Lema phungi Pic, 1924 and Lema jeanvoinei Pic, 1932 with Lema lacertosa Lacordaire, 1845 are supported. A revised key to the known species in Taiwan is provided.
Lema diversipes, Lema lacertosa, flagellum, genitalia, Insecta, Taiwan
Lema Fabricius is the largest genus of the subfamily Criocerinae and is distributed worldwide (
The Taiwanese islands, the focus of this study, are located in eastern-south Asia and are a subtropical to tropical region. Although taxonomic and/or faunal studies of Taiwan were done by
Most of Taiwanese populations were collected by the Taiwan Chrysomelid Research Team. These specimens are kept in Matsumura’s private collection (Jena, Germany) temporarily, and these will be deposited in the laboratory of Systematic Entomology of Hokkaido University (Japan) at a future date. In addition to these specimens, we used specimens which were borrowed from Muséum National d’Histoire Naturelle (MNHN, Paris, France), the laboratory of Systematic Entomology of Hokkaido University (SEHU, Sapporo, Japan), and the Taiwan Agricultural Research Institute (TARI, Taichung, Taiwan). In the results section, the following symbols are used to describe information on data labels exactly: a slash (/) indicates that words were written on one line of a data label, and double slashes (//) indicates that they were written on another label.
Scanning electronic microscopy (LSM-6510, JEOL) images were captured to observe fine structures in detail. To observe male and female genitalia, firstly we softened dried specimens by warming them in distilled water over night (50–60°C). Then, the abdomen was removed from the body, and softened in KOH solution (ca. 5-10%) for two days. Then we observed the genitalia under the microscope (Olympus SZX12) and drew them.
Terminology. The terminology for the exoskeleton is based mainly on
http://species-id.net/wiki/Lema_diversipes
Holotype ♀: “Pe Yen Tsing/ Yunnan// Lema // voir Mouhoti Baly // diversipes/ n sp// type // MUSEUM PARIS / 1958/ coll. M. Pic // HOLOTYPE (red label) // MNHN/ EC2232”.
Taiwan: 22 exs.: Taipei: Shihting, 11.VIII.2007, leg. M.-H. Tsao (6 exs. in SEHU; 16 exs. in TARI).
Lema diversipes can be separated from Lema lacertosa by the following combination of characters: body is distinctly larger and relatively stout in shape; anterior margin of the clypeus is waved and protruded slightly; posterior lines of the vertex grove slightly curved; almost all of the ventral surface is black; sternites covered by pubescence, except the posterior margins of the sterna 1-4 glabrous.
Body coloration (Figs 1–2). Dorsum (holotype): Head reddish-brown except for black apex of mandible, antenna yellowish-brown; pronotum and elytra reddish-brown, scutellum brown; prolegs orange except for coxae which are brownish-orange, mesolegs blackish-brown, metalegs black. Dorsum (Taiwanese individuals): Antennomeres 1–4 orange, 5–11 blackish-brown; profemur and tibia orange, procoxae, trochanter, tarsi and claws blackish-brown. Venter: prosternum orange; meso- and metasterna black; abdomen blackish-brown. Pubescence white.
Habitus. 1–2 Lema diversipes 1 dorsal view 2 ventral view 3–4 Lema lacertosa 3 dorsal view 4 ventral view. (all at the same scale)
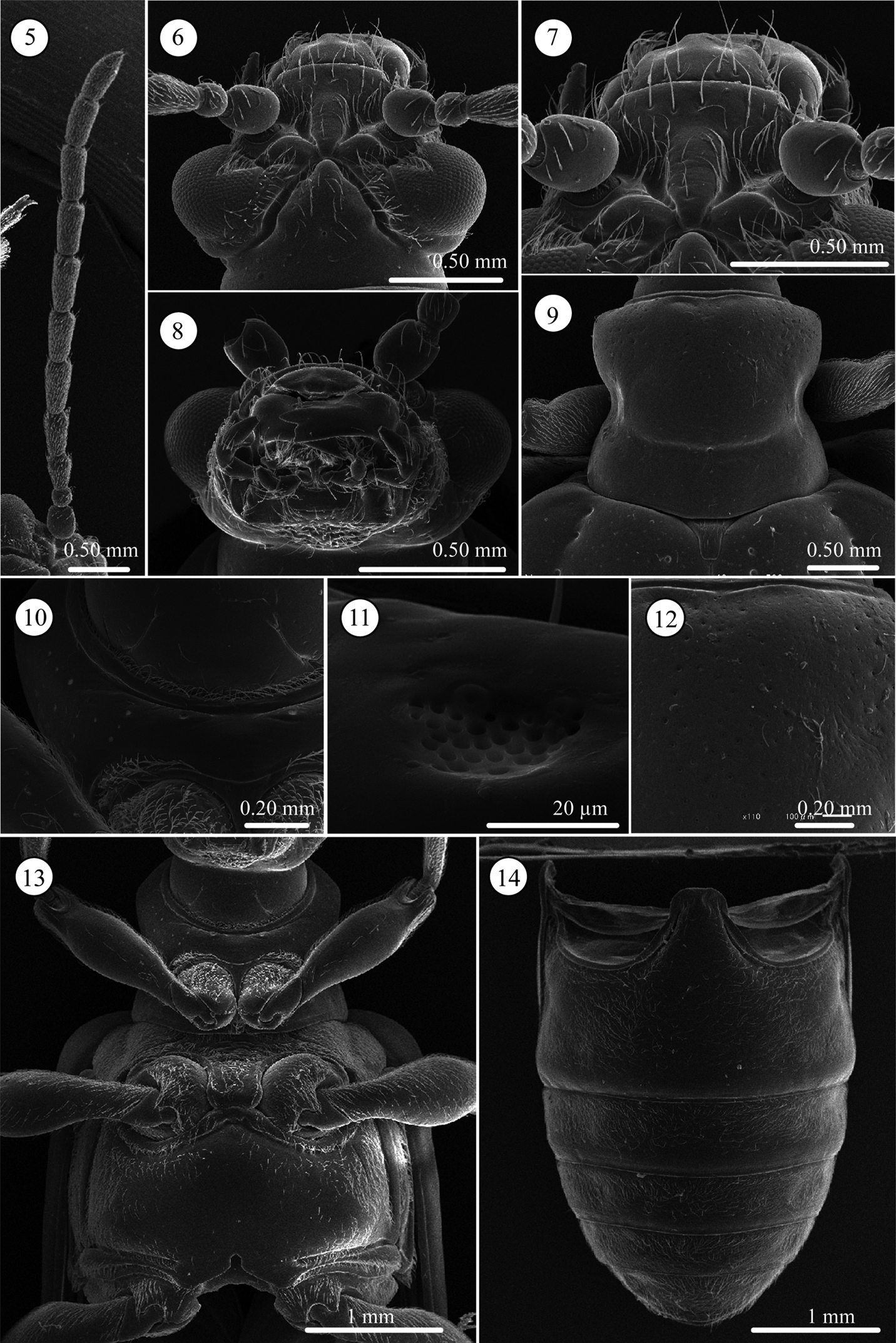
Head (Figs 5–7). Slightly longer than wide; vertex not so raised, coarsely covered with relatively long setae, its surface smooth, with shallow fovea on top, in some cases, fovea longitudinally elongate; area between X-shaped vertex groove and compound eye bearing relatively long setae; orbital area triangular, very densely covered with pubescence; frontal tubercle glabrous; frontoclypeus triangular, bearing relatively long setae, central region glabrous; labrum with ca. Eight relatively long setae, anterior margin corrugated, medial part anteriorly projected; antenna filiform, ca. 0.6 times as long as body length, antennomeres 1 and 2 subglobular and almost glabrous with a few setae, antennomeres 3–11 bearing velutinous setae, antennomeres 5–11 each ringed along apex by a few long setae, antennomere 3 slightly shorter than 4, antennomere 5 slightly longer than 6, antennomeres 6-10 subequal in length, antennomeres 3–10 elongate but slightly thickening apically, apex of antennomere 11 conically prominent.
Lema diversipes. 5 left antenna 6 head, dorsal view 7 frontoclypeus, dorsal view 8 mouth parts 9 prothorax, dorsal view 10 frontal area of prothorax with lotus-seeds-like structure, ventral view 11 lotus-pod like structure, enlarged 12 prothorax with fine punctures, dorsal view 13 thorax, ventral view 14 abdomen, ventral view.
Pronotum (Figs 9 and 12). Subequal in width and length, laterally constricted in middle; surface sparsely, coarsely punctate also micropunctate between larger punctures; transverse groove present near base with fovea medially; anterior and basal margins narrowly margined, basal margin densely pubescent; a long seta present in anterior and posterior angles.
Scutellum (Fig. 9). Trapezoidal and relatively longitudinally elongated, posterior angles round, in some specimens posterior margin completely rounded; sparsely pubescent.
Elytra (Figs 1–2). 1.7 times longer than wide; very slightly depressed on anterior region in Taiwanese individuals but not depressed in the holotype. Lateral margin parallel; punctures slightly weakening posteriorly, interspaces smooth and slightly raised on apical ⅓.
Pygidium. Anterior ⅓ densely covered with short hair-like projections except for stridulatory organ in anterior middle; posterior ⅔ with dense, stout setae.
Palpi of mouth parts (Fig. 8). Apical palpomere of maxillary palpi relatively slender, conico-cylindrical; other palpomeres elongate, tapering basally. Labial palpi with four palpomeres, apical three palopmeres relatively stout, apical palpomere conico-cylindrical.
Prothorax(lateral and ventral, Figs 10, 11, and 13). Anterior part of prosternum transversely oblong, posterior margin covered with pubescence, anterior region glabrous with very fine lotus-pod like structures, some specimens with transverse wrinkles. Prosternal process very narrow and higher than anterior part, with pubescence on ridgeline, widened posteriorly. Surface of pronotal hypomeron smooth. Posterior arms of pronotal hypomeron not closed and forming arms, but prosternal process bridges them. Anterior and posterior margins of prothorax with pubescent fringe; anterior margin fringed with two rows of setae; anterior margin with curved and straight setae and posterior margin with one straight seta.
Mesothorax (Fig. 13). Surface of mesosternum with deep transverse wrinkles, posterior ½ with pubescence; posterior process with small ridge along posterior margin, its surface covered with relatively long setae. Mesepisternum and mesepimeron entirely covered with dense pubescence.
Metathorax (Fig. 13). Metasternum oblong; almost all margins with ridge; surface of median ½ glabrous except margin sparsely covered with relatively long setae; lateral ½ densely pubescent; ridgeline of posterior ridge with dense pubescence. Metepisternum with dense pubescence, lateral ⅓ with glabrous area.
Legs (Fig. 13). Procoxae conico-cylindrical, with dense and relatively long pubescence, protrochanters glabrous except with relatively long setae on anterior ridgeline; profemora almost glabrous except lateral apex with dense pubescence ventrally, in dorsal view with relatively dense pubescence except for basal ½ of inner margin glabrous. Mesocoxae spherical, with dense, relatively long pubescence on lower anterior ½; mesotrochanters glabrous except with a few long setae on posterior ridgeline; meso- and metafemora with dense pubescence in ventral view, glabrous except for lateral 1⁄5 with dense pubescence in dorsal view; metatrochanters glabrous except with a few short setae on posterior ridgeline; meso- and metatibiae slender and uniform in shape, covered with dense pubescence, basal ½ with slightly curved pubescence, apical ½ with transparent straight, stiff pubescence, lateral margin of its apex bordered with translucent brown spines, and armed with two subequal black-brown very short spines on ventral margin.
Abdominal sterna (Fig. 14). Surface almost entirely densely covered by short pubescence except posterior margin of sterna 1-4 glabrous; lateral area near base of sternite one with more or less glabrous patch, middle of lateral region more or less depressed.
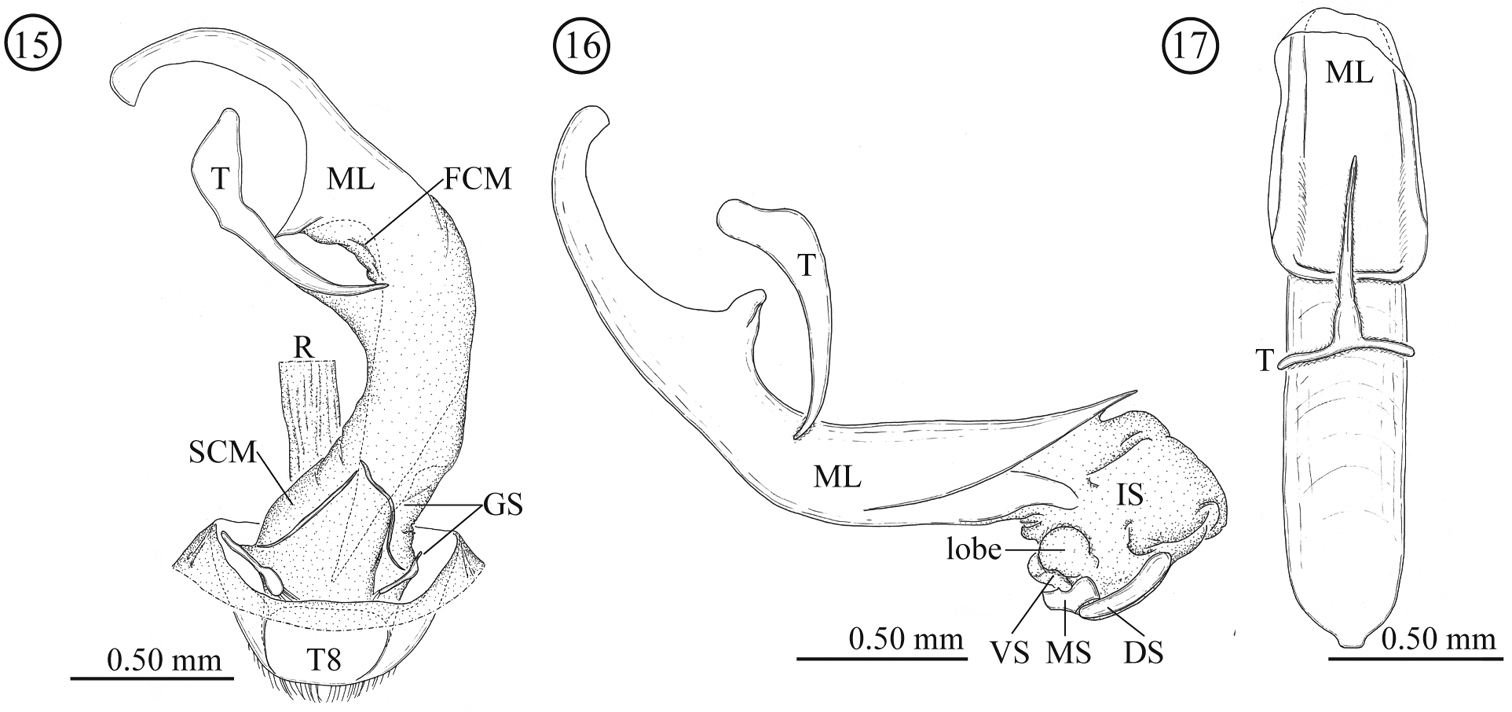
Male genitalia (Figs 15–17). Consisting of five parts: tergite 8, gastral spiculum, tegmen, median lobe and internal sac. Tergite 8 similar to that of the female as described below. Gastral spiculum consisting of two pairs of twig-like sclerites, one pair longer than the other, shorter pair asymmetrical and spoon-like in ventral view. Basal piece of tegmen triangular with rounded corner in lateral view, tapering toward base. Median lobe relatively slender, median foramen expanding and occupying ⅓ of ventral surface in lateral view, ventral end of median orifice with rectangular protrusion which has rounded corner. Internal sac without flagellum and pocket as observed in Lema lacertosa; having dorsal-, median-, and ventral sclerites; dorsal, median and ventral sclerites block-like; ventral sclerite covered with a pair of rounded lobes formed by a membrane.
Male genitalia of Lema diversipes. 15 entire genitalia in ventral view 16 aedeagus in lateral view, right side corresponds to posterior end 17 median lobe and tegmen in ventral view; DS: dorsal sclerite; FCM: first connecting membrane; GS: gastral spiculum; IS: internal sac; ML: medial lobe; MS: median sclerite; R: rectum; SCM: second connecting membrane; T: tegmen; T8: tergite 8; VS: ventral sclerite.
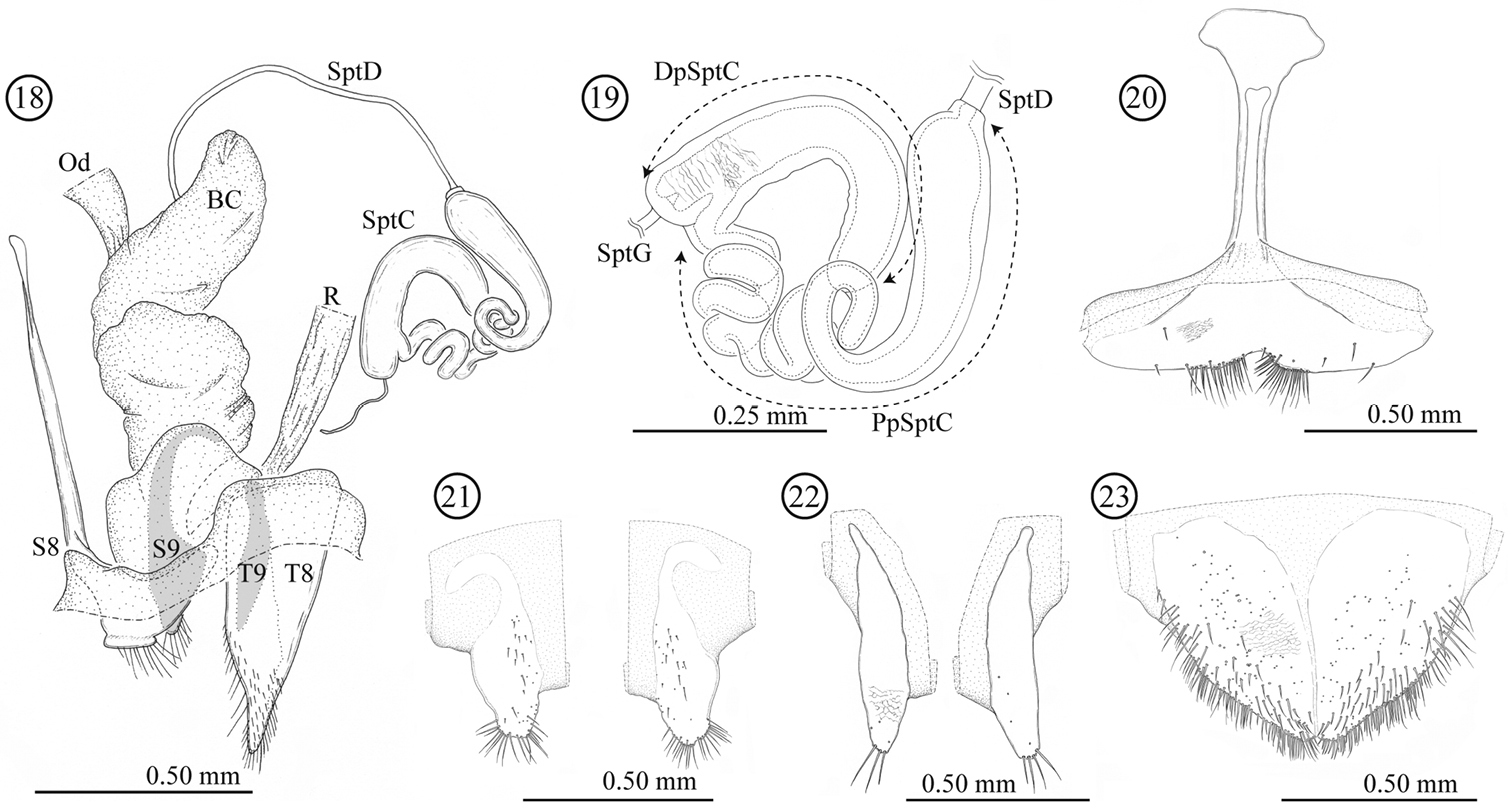
Female genitalia and a part of reproductive systems (Figs 18–23). Bursa copulatrix balloon-like with its wall thickened but soft. Spermatheca with relatively long duct (0.79 mm, N = 1), opening to ventral side of bursa copulatrix. Wall of spermathecal capsule well sclerotized and thick; distal part of spermathecal capsule hook-shaped, proximal part strongly coiled; inner surface completely covered by very fine winkle-like sculpture. Genitalia consisting of four parts: tergites 8 and 9, and sternites 8 and 9; sclerotization of tergite 8 gradually weakened toward midline; sternite 8 with stick-like apodeme; tergite and sternite 9 consisting of a pair of sclerites; tergites 8 and 9 largely covered by scale- to winkle-like sculpture, marginal region of tergite 8 covered by relatively stout setae; both sides of the sternite 8 covered by scale-like sculpture; posterior area of sternite 9 weakly wrinkled.
Female genitalia of Lema diversipes. 18 entire female genitalia and a part of reproductive system in lateral view 19 spermathecal capsule 20 sternite 8 21 sternite 9 22 tergite 9 23 tergite 8; BC: bursa copulatrix; Od: oviduct; R: rectum; SptD: spermathecal duct; SptG: spermathecal gland; SptC: spermathecal capsule; PpSptC: proximal part of spermathecal capsule; DpSptC: distal part of spermathecal capsule; S8: sternite 8; S9: sternite 9; T8: tergite 8; T9: tergite 9.
Measurements.Elytral length: male: 4.53 ± 0.22 mm (mean ± SD, N = 5), female: 5.14 ± 0.03 mm (N = 2). Elytral width: male: 2.68 ± 0.06 mm, female: 2.92 ± 0.06 mm. Pronotum length: male: 1.44 ± 0.04 mm, female: 1.46 ± 0.07 mm. Pronotum width: male: 1.43 ± 0.05 mm, female: 1.58 ± 0.08 mm.
(Figs 43–44) Fabaceae: Pueraria lobata (Willd.) subsp. thomsonii (Benth.) Ohashi .
China, Taiwan (new record).
Condition of holotype. Right side of the head and abdomen in dorsal view with wormholes. Almost all pubescence of the body surface is lost. However punctures remain, which enable us to know setal arrangement. Comparing the arrangement of the punctures and setae in newly collected Taiwanese specimens, we identified the specimens collected in Taiwan as Lema diversipes and described the characteristic of setae based on the Taiwanese specimens.
Justification of resurrection of Lema diversipes and removing it from synonymy of from Lema lacertosa. Lema diversipes was synonymized under Lema lacertosa by
The original descriptions (
http://species-id.net/wiki/Lema_lacertosa
Lema phungi: lectotype 1 ♀, here designated, labeled: Hoa Binh/ Tonkin// Phungi/ Pic// Muséum Paris/ 1958/ Coll. Pic// SYNTYPE// MNHN/ EC3057//; paralectotype: 1 ♀// Hoa Binh/ / Tonkin// Phungi/ Pic// Museum Paris/ 1958/ Coll. Pic// SYNTYPE// MNHN/ EC3058//.
Lema jeanvoinei. 1 ♀// Tonkin/ Hanoi/ 7. IV. 1918/ JEANVOINE// Dessous/ et/ membres/ largement/ noirs// jeanvoinei/ n sp// Museum Paris/ 1958/ Coll. Pic// HOLOTYPE// MNHN/ EC3059//.
Taiwan: 4 exs.: Chiayi, Minhsiung, 29.IV.2010, leg. W.-T. Liu (TARI); 2 exs.: Chiayi, Niutoulun, 30.III.2010, leg. W.-T. Liu (TARI); 2 exs.: Kaoshiung, Niaosung, 2.VI.2008, leg. C.-H. Liu (TARI); 1 ex.: Kaoshiung, Taiao, 8.IX.2008, leg. W.-T. Liu (TARI); 1 ex.: Nantou, Chichi, 19.VIII.2010, leg. W.-T. Liu (TARI); 5 exs.: Pingtung: Chaochou, 5.XI.2009, leg. J.-C. Chen (TARI); 3 exs., same locality, 16.III.2010, leg. M.-H. Tsou (TARI); 11 exs., same locality, 2.VI.2010, leg. M.-H. Tsou (2 exs. in SEHU; 9 exs. in TARI); 3 exs.: Pingtung, Hengchun, 9.VII.2011, leg. J.-C. Chen (TARI); 1 ex.: Pingtung, Kaoshih, 8.V.2012, leg. J.-C. Chen (TARI); 1 ex.: Pingtung, Nanjen Lake, 3.I.2011, leg. J.-C. Chen (TARI); 1 ex., same locality, 29.IV.2011, leg. J.-C. Chen (TARI); 5 exs.: Pingtung, Wukoushui, 21.VIII.2010, leg. J.-C. Chen (TARI); 1 ex.: Taipei, Kuantu, 15.IX.2010, leg. S.-F. Yu (SEHU); 2 exs., same locality, 8.VI.2011, leg. S.-F. Yu (TARI); 1 ex.: Taitung, Anshuo, 7.XI.2011, leg. J.-C. Chen (TARI); 3 exs.: Taoyuan, Meihuali, 14.VII.2010, leg. H. Lee (SEHU); 5 exs.: Taoyuan, Chungli, 19.X.2010, leg. H. Lee (TARI); 4 exs.: Taoyuan, Kuanyin, 16.VI.2010, leg. M.-H. Tsou (TARI). Malaysia: 3 exs.: Negeri Selangor, Ulu Gomback, (Univ. Malaysia Field Studies centre. 220 m alt.), 10.III.2009, leg. Y. Matsumura (SEHU); 1ex.: Negeri Selangor, Ulu Gomback (Univ. Malaysia Field Studies/ centre. 220 m alt.) 8.XI.2009, leg. Y. Matsumura (SEHU); 1ex: Jalan Pahang Perk, Batu 19 (570m alt.), 8.XI.2009, leg. Y. Matsumura et al. (SEHU). India: 2 exs.: Calcutta, 14-19.X.1978, leg. JAP-IND CO TR (SEHU).
Lema lacertosa can be separated from Lema diversipes by the following combination of characters: body is distinctly smaller; anterior margin of the clypeus is curved inward and slightly concave; posterior lines of the vertex grove nearly straight; anterior region of the ventral surface is nearly black and posterior ⅓ (sterna 2-5) orange to brown; sterna almost entirely covered by pubescence, except around midline of the sternum 1 glabrous.
Body coloration (Figs 3–4). Dorsum: Labrum and anterior ½ of frontoclypeus black, antenna brownish-black except antennomeres 1 and 2 which are orange to brown; remaining part of head, pronotum, scutellum and elytra brownish to reddish-orange. Procoxae black, protrochanters brown, profemora, protibiae, and protarsi orange with diffuse brown to blackish line; meso- and metatrochanters brown, femora, tibiae, and tarsi of meso- and metalegs black. Venter: anterior ⅓-½ of prothorax orange, remaining area black to brownish-black; meso- and metathorax black; first abdominal sternite black to blackish-brown, other sterna orange to brown. Pubescence white. Antenna lighter colored than other parts, protrochanter and apical section of procoxae orange; proleg black basally. Basal ½ of first abdominal sternite black; especially in Malaysian populations with brighter orange color.
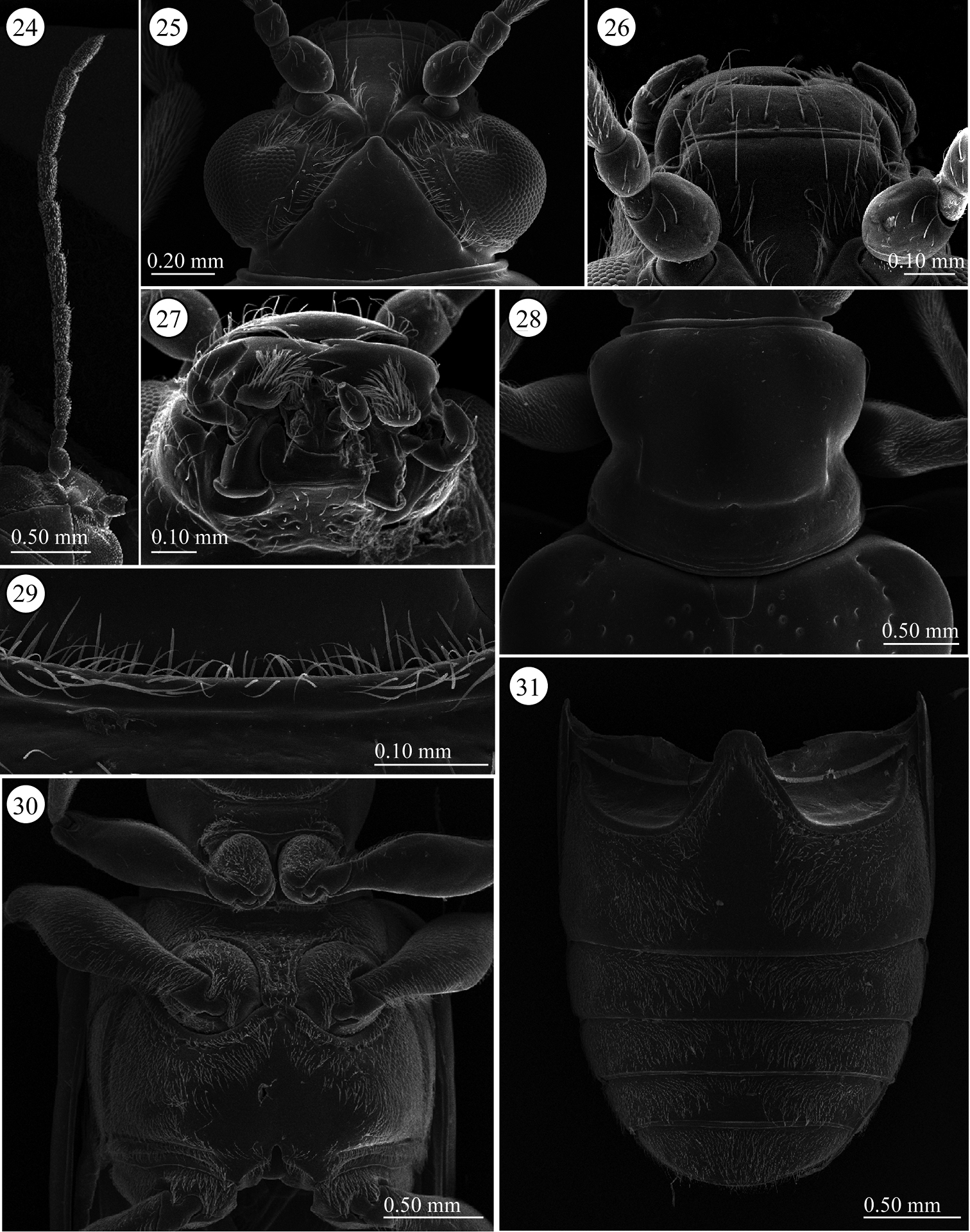
Head (Figs 24–26). Width and length almost equal; vertex not raised, glabrous, surface smooth; area between X-shaped vertex groove and compound eye with relatively long setae, covered with fine sculpture; orbital area triangular, densely covered with pubescence; frontal tubercle indistinct, glabrous; frontoclypeus triangular, covered with setae, setae relatively dense on posterior ½, medial line region glabrous; labrum with ca. Seven relatively long setae, anterior margin curved inward and slightly concave; antenna filiform, ca. 0.7 times as long as body length, antennomeres 1–2 subglobular and almost glabrous with a few setae, antennomeres 3-11 bearing velutinous pubescence, apex of antennomeres 5–11 ringed with a few long setae, antennomere 3 subequal in length to 4, antennomeres 3+4 slightly longer than 5, antennomere 4 or 5 longest depending on individuals, antennomeres 6-10 subequal in length, antennomeres 3-10 cylindrical slightly thickening apically, apex of antennomere 11 conically prominent.
Lema lacertosa. 24 left antenna 25 head, dorsal view 26 frontoclypeus, dorsal view 27 mouth parts 28 prothorax, dorsal view 29 frontal fringe of prothorax, ventral view 30 thorax, ventral view 31 abdomen, ventral view.
Pronotum (Fig. 28). Slightly wider than long to almost equal, laterally constricted at middle; surface with a few small punctures around midline and anterior angles, rest with very fine punctures, transverse groove present near base with fovea in middle, anterior and posterior margins narrowly margined, posterior ridge internally with dense short setae. A long seta present in each anterior and posterior angle.
Scutellum (Fig. 28). Trapezoidal and relatively wide, posterior margin concave, indistinct in some specimens. Surface glabrous, but in three of five Taiwanese specimens covered with a few setae.
Elytra (Figs 3–4). 1.7 times longer than wide; one of six Taiwanese specimens very slightly depressed anteriorly but not depressed or indistinctly impressed in the other specimens. Lateral margins parallel; punctures slightly weakening posteriorly.
Pygidium. Anterior ⅓ densely covered with short hair-like projections except for stridulatory organ in anterior middle, size of stridulatory organ relatively small; posterior ⅔ with dense, stout setae.
Palpi of mouth parts (Fig. 27). Apical maxillary palpomere relatively stout and conico-cylindrical but not enlarged; other palpomeres cylindrical, narrowing basally; one of two Indian specimens examined with relatively slender apical palpomere. Labial palpi with four palpomeres, apical three palpomeres relatively stout but not enlarged, apical palpomere conico-cylindrical.
Prothorax(lateral and ventral, Figs 29–30). Anterior area of prosternum transversely oblong anteriorly, with pubescent patch posteriorly, glabrous anteriorly, some specimens with very weak transverse wrinkles. Prosternal process very narrow and not raised, widened posteriorly. Surface of pronotal hypomeron smooth. Posterior arms of pronotal hypomeron normally not closed in most specimens, but closed in one Malaysian specimen and fused in one Indian specimen; prosternal process with bridge arms, bridge relatively short and not completely covering arms. With pubescent fringe anteriorly and posteriorly; anterior margin fringed with two rows of setae.
Mesothorax (Fig. 30). Surface of mesosternum with fine sculpture and pubescence; posterior process with ridge along margin, pubescence on posterior ridge relatively long. Mesepisternum and mesepimeron with dense pubescence.
Metathorax (Fig. 30). Metasternum oblong; almost entire margin with ridge; surface of medial area glabrous and other areas covered with pubescence; medial part of anterior ridge with relatively long pubescence; posterior margin between metacoxae with curved pubescence. Metepisternum with dense pubescence, lateral ⅓ with glabrous area overlapping elytra.
Legs. Procoxae conico-cylindrical, densely covered with pubescence, protrochanters glabrous, with relatively long setae on anterior ridgeline; profemora nearly glabrous except apex laterally with pubescence ventrally, dorsum with relatively dense pubescence except for glabrous base. Mesocoxae spherical, densely pubescent on lower anterior ½; mesotrochanters glabrous with very long pubescence on posterior ridgeline; meso- and metafemora with dense pubescence ventrally, glabrous dorsally except for dense pubescence apically. Metacoxae pubescent; metatrochanters glabrous except with long pubescence on posterior ridgeline; tibiae slender and only slightly tapering apically, covered with dense pubescence, basally ⅓ to ½ with slightly curved pubescence, apically with straight, transparent setae, almost glabrous dorsally; tibiae with lateral margin bordered with translucent brown spines apically, and armed with pair of very short, subequal, black-brown spines ventrally.
Abdominal sterna (Fig. 31). Surface almost entirely densely covered by short pubescence; only around midline of sternite one glabrous, some specimens more or less depressed laterally.
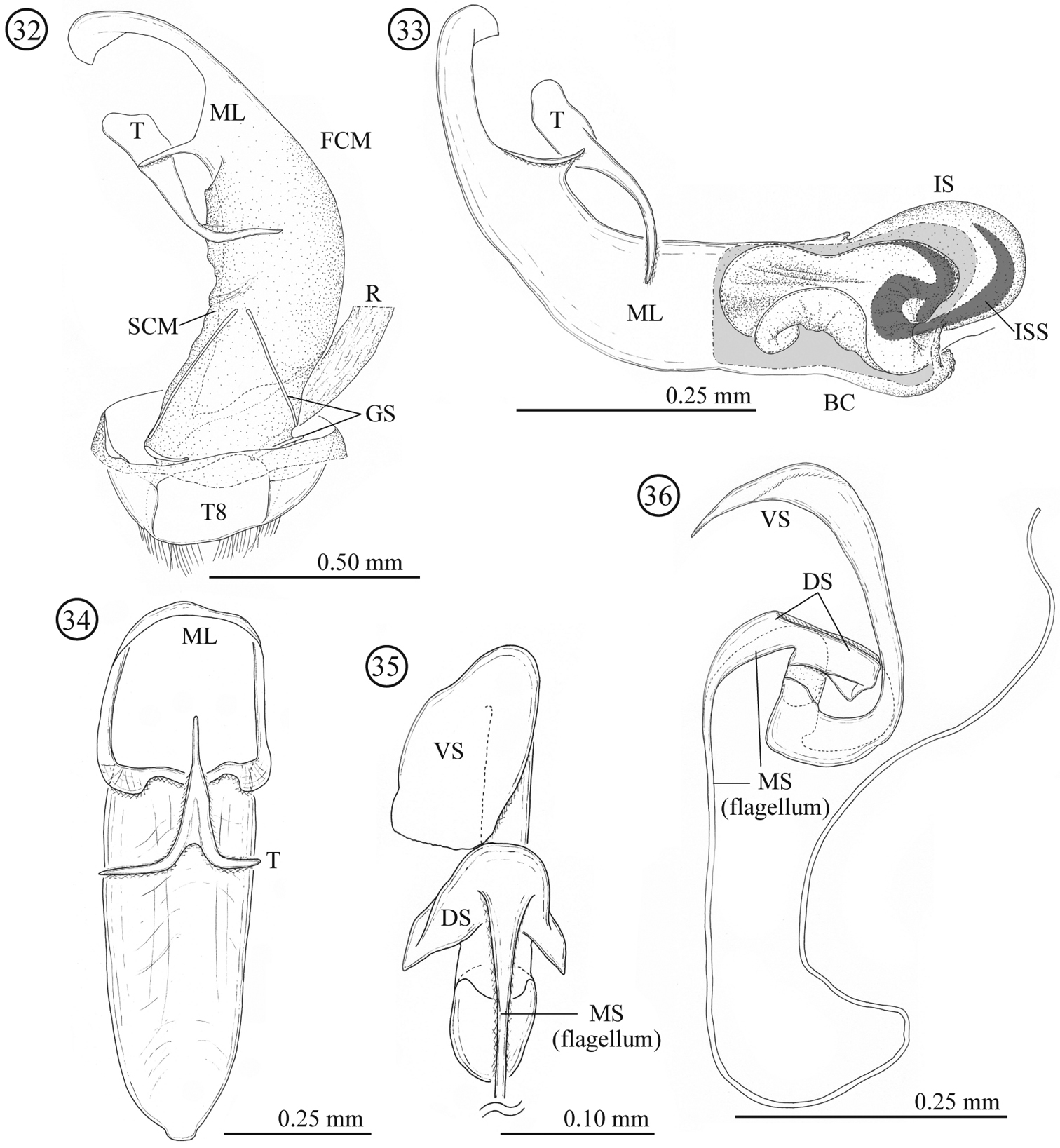
Male genitalia (Figs 32–36). Consisting of five parts: tergite 8, gastral spiculum, tegmen, median lobe and internal sac. Tergite 8 similar to that of female as described below. Gastral spiculum consisting of two pairs of twig-like sclerites, one pair longer than the other. Basal piece of tegmen rectangular in lateral view, tapering toward base. Median lobe stout, median foramen expanding and occupying ⅓ of ventral surface in lateral view, ventral end of median orifice round with rectangular and rounded protrusion. Internal sac with specialized state as in many members of the subgenus Lema, i.e. having pocket for storing elongated flagellum; median and ventral sclerites forming flagellum (1.58 mm, N=1); dorsal sclerite not separated.
Male genitalia of Lema lacertosa. 32 entire genitalia in ventral view 33 aedeagus in lateral view; outer membrane of internal sac was partly removed. Right side corresponds to posterior end 34 median lobe and tegmen in ventral view 35 internal-sac sclerites in dorsal view, basal part is enlarged 36 internal-sac sclerites in lateral view; Bc: body cavity; DS: dorsal sclerite; FCM: first connecting membrane; GS: gastral spiculum; IS: internal sac; ISS: internal-sac sclerites; ML: median lobe; MS: median sclerite; R: rectum; SCM: second connecting membrane; T: tegmen; T8: tergite 8; VS: ventral sclerite.
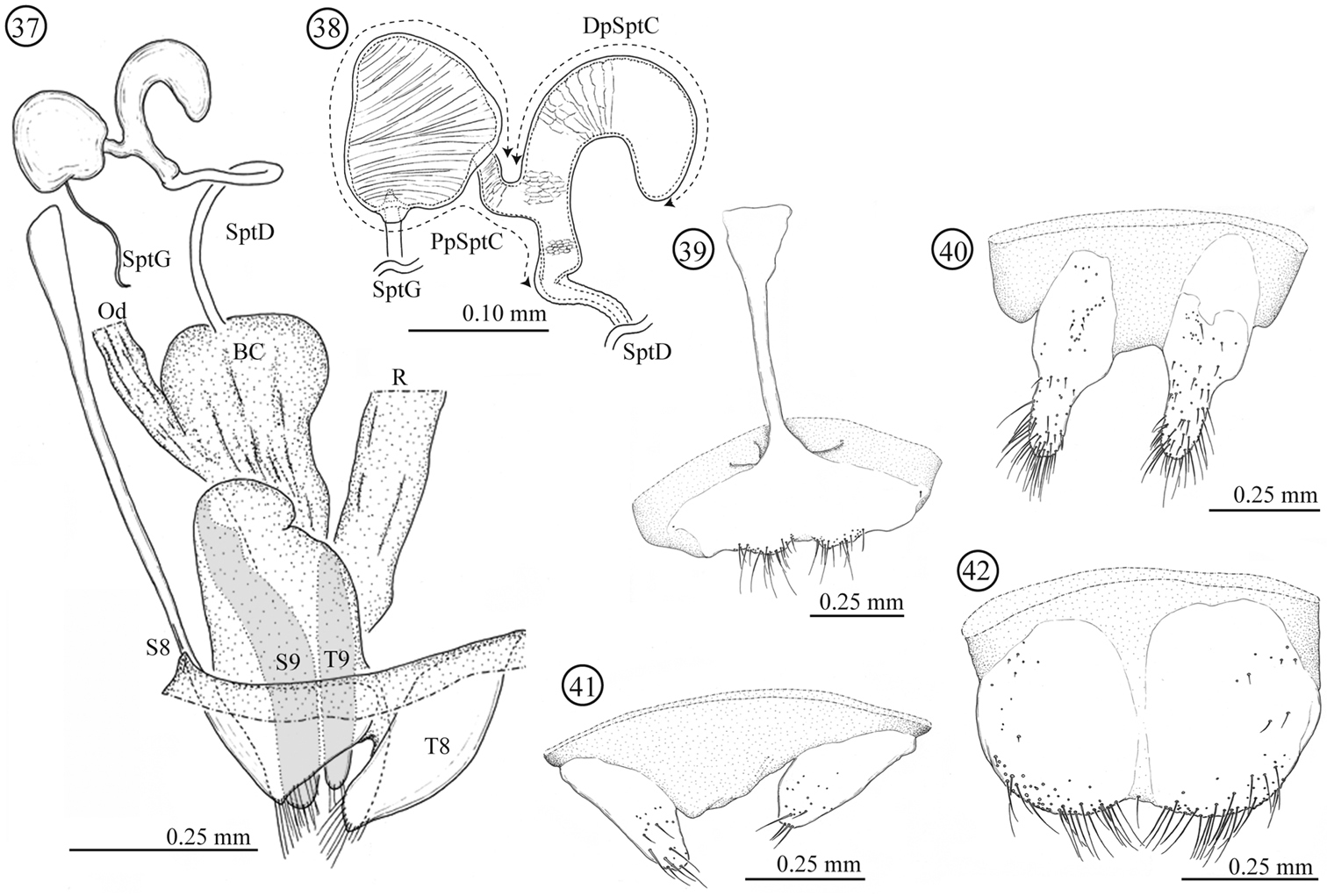
Female genitalia and a part of reproductive systems (Figs 37–42). Spermathecal duct relatively long (0.36–0.49mm, N = 2) with no specialized structure in opening to bursa copulatrix. Spermathecal capsule well sclerotized, its wall relatively thick; distal part hook-shaped, inner surface covered by winkle-like sculpture, junction area to spermathecal duct covered by scale-like sculpture; proximal part with a large potato-like structure, inner surface covered by transverse winkles. Spermathecal gland opening on a light-bulb like structure. Genitalia of four parts: tergites 8 and 9, and sternites 8 and 9; tergites 9 and sternite 9 consisting of a pair of sclerites; sclerotization of tergite 8 gradually weakened toward midline; sternite 8 with stick-like apodeme; posterior area of sternite 8 covered by scale-like sculpture; upper area of tergite 8 weakly covered by scale-like sculpture and lower area with fine pointed projections.
Female genitalia of Lema lacertosa. 37 entire female genitalia and a part of reproductive system in lateral view 38 spermathecal capsule 39 sternite 8 40 sternite 9 41 tergite 9 42 tergite 8; BC: bursa copulatrix; Od: oviduct; R: rectum; SptD: spermathecal duct SptG: spermathecal gland; SptC: spermathecal capsule; PpSptC: proximal part of spermathecal capsule; DpSptC: distal part of spermathecal capsule; S8: sternite 8; S9: sternite 9; T8: tergite 8; T9: tergite 9.
Specimens collected from India. Elytral length: male: 3.04 mm (N=1), female: 3.38 mm (N=1). Elytral width: male: 1.77 mm, female: 2.00 mm. Pronotum length: male: 1.00 mm, female: 1.15 mm. Pronotum width: male: 1.04 mm, female: 1.27 mm.
Specimens collected from Taiwan. Elytral length: male: 3.36 ± 0. 21 mm (mean ± SD, N=2), female: 3.56 ± 0.15 mm (N=4). Elytral width: male: 1.96 ± 0.11 mm, female: 2.05 ± 0.13 mm. Pronotum length: male: 1.08 ± 0.05 mm, female: 1.09 ± 0.05 mm. Pronotum width: male: 1.15 ± 0.02 mm, female: 1.20 ± 0.07 mm.
Specimens collected from Malaysia. Elytral length: male: 3.15 mm (N=1), female: 3.57 ± 0.21 mm (N=4). Elytral width: male: 1.81 mm, female: 2.13 ± 0.14 mm. Pronotum length: male: 0.96 mm, female: 1.10 ± 0.03 mm. Pronotum width: male: 1.13 mm, female: 1.21 ± 0.05 mm.
(Figs 45–46) Commelinaceae: Commelina communis L., 1753.
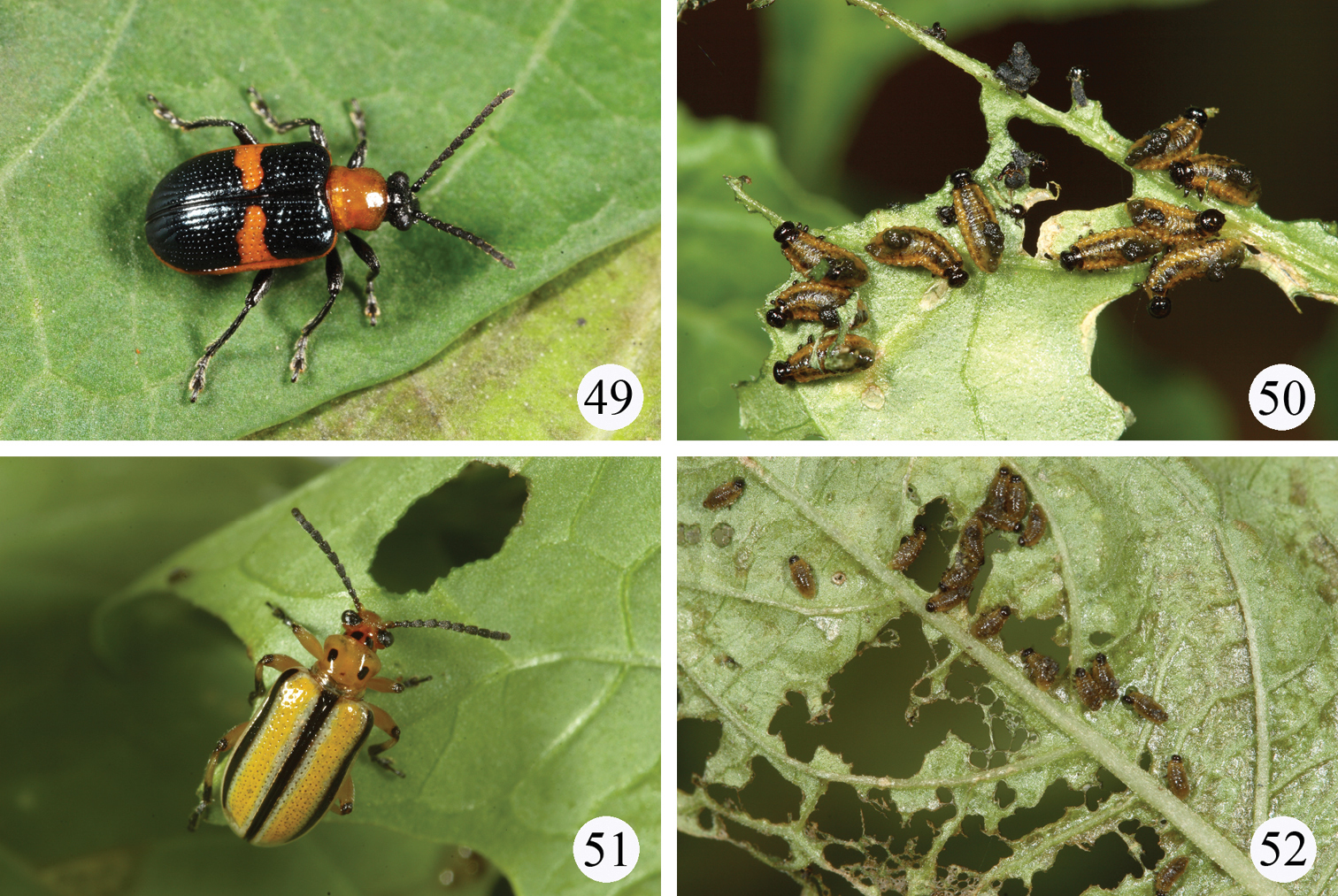
Live adults or larvae in the field. 43–44 Lema diversipes on Pueraria lobata (Willd.) subsp. thomsonii (Benth.) Ohashi 43 a pair of adults 44 a cluster of larvae 45–46 Lema lacertosa on Commelina communis L. 45 adult 46 larva 47–48 Lema cyanella on Cirsium japonicum 47 adult 48 larva.
India, Malaysia, and Taiwan (new record). This species is also recorded from Laos, Vietnam, S. China, and Singapore (
Justification of identification of Lema lacertosa. Although
Although we could not locate the holotype of Lema lacertosa, we have no evidence regarding the disappearance of the holotype. In addition, the identity of this species is relatively stable, so we do not designate a neotype for this species.
Taiwan: 4 exs.: Taipei, Juifang, 10.II.2010, leg. H. Lee (TARI); 1ex., same locality, 21.II.2010, leg. M.-H. Tsou (TARI); 2 exs., same locality, 6.III.2010, leg. M.-H. Tsou (TARI); 11 exs., same locality (= Nanya trail), 15.III.2010, leg. H. Lee (TARI); 2 exs., same locality, 19.III.2010, leg. H. Lee (TARI); 5 exs., same locality, 20.III.2010, leg. H. Lee (TARI); 8 exs., same locality, 1.IV.2010, leg. M.-H. Tsou (TARI); 1 ex., same localilty, 18.IV.2010, leg. M.-H. Tsou (TARI); 1 ex., same locality, 25.IV.2010, leg. M.-H. Tsou (TARI); 2 exs.: Taipei, Pinglin, 1.VII.2008, leg. H. Lee (TARI).
This species was redescribed by
Europe, China, Mongolia, Korea, Taiwan (new record), Japan.
Taiwan: 19 exs.: Chiayi, Chungpu, VIII.2007, leg. H.-T. Shih (TARI); 1 ex.: Hsinchu, Mamei, 4.V.2008, leg. S.-F. Yu (TARI); 1 ex.: Nantou, Wanfengtsun, 21.IV.2007, leg. W.-T. Liu (TARI); 1 ex.: Taichung, Pahsienshan, 5.VIII.2007, leg. W.-T. Liu (TARI); 4 exs.: Taichung, Tahsuehshan, 1.V.2012, leg. W.-T. Liu (TARI); 3 exs.: Tainan, Danei, 9.VII.2007, leg. W.-T. Liu (TARI); 1 ex.: Tainan, Meiling, 12.III.2011, leg. M.-L. Jeng (TARI); 2 exs.: Taipei, Sanchih, 7.VIII.2011, leg. C.-C. Cheng (TARI); 5 exs., same locality, 13.VIII.2011, leg. H. Lee (TARI); 2 exs.: Taoyuan, Luchu, 16.VI.2009, leg. W.-T. Liu (TARI).
Live adults and larvae in the field. 49–50 Lema solani on Solanum americanum 49 adult 50 larvae 51–52 Lema trivittata trivittata on Physalis angulata 51 adult 52 larvae.
Eastern United States to Texas, Taiwan.
Taiwan: 2 exs.: Taitung, Lanyu island, 5.V.2012, leg. S.-F. Yu (TARI); 16 exs.: Yunlin, Pichiao, 16.VI.2010, leg. H. Lee (TARI); 2 exs., same locality (= Tuku), 30.VI.2010, leg. H. Lee (TARI); 2 exs., same locality, 9.VII.2010, leg. H. Lee (TARI).
This species is also an introduced one for Taiwan because its original distribution is limited to the United States and Canada (
Beetles were found to feed on leaves of Physalis angulata L. (Figs 51–52) and Physalis peruviana L. in Taiwan. Both plants are introduced species for Taiwan originally from the United States.
United States and southern Canada, Taiwan (new record), and Japan (Ryukyu: Miyako and Iriomote islands).
A key to 11 known species of Lema from Taiwan was presented by
| 1 | Elytron without scutellar row of punctures | (subgenus Petauristes) 2 |
| – | Elytron with a short scutellar row of punctures | (subgenus Lema) 7 |
| 2 | Elytron blackish-blue | 3 |
| – | Elytron yellowish-brown | 5 |
| 3 | Entire elytron blackish-blue | 4 |
| – | Lateral margin of elytra yellow, with transverse yellow band at middle | Lema solani Fabricius |
| 4 | Generally reddish-brown except antenna black | Lema fortunei Bates |
| – | Generally black except head and prothorax reddish-brown | Lema honorata Baly |
| 5 | Entire elytra yellowish | Lema pectoralis Baly |
| – | Elytra with black spots or stripes | 6 |
| 6 | Elytra with three black, longitudinal stripes | Lema trivittata trivittata Say |
| – | Elytra with large, black spots at base and subapex; in some specimens entire elytra black except apex | Lema koshunensis Chûjô |
| 7 | Mesotibia with a distinct denticulation in middle | Lema coronata Baly |
| – | Mesotibia without distinct denticulation | 8 |
| 8 | Pronotum with two transverse furrows at basal ½ | Lema praeusta (Fabricius) |
| – | Pronotum with one transverse furrow at basal ½ | 9 |
| 9 | Generally blackish-blue | 10 |
| – | Generally yellowish-brown | 12 |
| 10 | Pronotum without punctures | Lema cyanea Fabricius |
| – | Pronotum with punctures | 11 |
| 11 | Abdominal ventrites III-V yellowish-brown, vertex relatively flat | Lema concinnipennis Baly |
| – | Abdominal ventrites blackish-blue, vertex swollen | Lema cyanella (Linneaus) |
| 12 | Middle and hind legs black except front femur reddish-brown | 13 |
| – | All legs yellowish-brown | 14 |
| 13 | Body longer (7.5–8.2 mm), abdominal ventrites I-V black (Fig. 2) | Lema diversipes Pic |
| – | Body shorter (4.5–5.5mm), abdominal ventrites II-V reddish-brown (Fig. 4) | Lema lacertosa Lacordaire |
| 14 | Elytron with basal and postmedian black spots | Lema esakii Chûjô |
| – | Entire elytron yellowish-brown | 15 |
| 15 | Elytron with transverse furrow behind humerus; vertex without pubescence | Lema rufotestacea Clark |
| – | Elytron without transverse furrow; vertex with dense pubescence | Lema coomani Pic |
We greatly thank P. Jolivet, M. Schmitt, M. Minami for giving us information on a repository of Lacordaire’s collection and M. Mantilleri, M. Ôhara, R. G. Beutel for type loans. P. Limbourg, P. Philip, N. E. Pierce kindly coped with our asking for the type repository. YM also thanks to H. Takizawa for valuable discussions on the taxonomic treatments and K. Yoshizawa, H. Yoshitomi, H. Suenaga, M. Maruyama, I. Yao, T. Kanbe, T. Shimozawa, and M. Shimomura for their cooperation. We thank the Taiwan Chrysomelid Research Team for assistance in collecting specimens, including J.-C. Chen, H. Lee, W.-T. Liu, M.-H. Tsou, and S.-F. Yu, and M.-H. Tsou and T.-H. Lee for taking photos of dead or live specimens. This study was supported by JSPS Research Fellowships for Young Scientists and for Research Abroad. We thank C. L. Staines for reviewing the draft.