(C) 2013 Hiroshi Kajihara. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Ovicides paralithodis sp. n. is described from the egg mass of the red king crab Paralithodes camtschaticus (Tilesius, 1815) from the Sea of Okhotsk, off Hokkaido, Japan, and Alaska, USA. Among four congeners, Ovicides paralithodis can be distinguished from Ovicides julieae Shields, 2001 and Ovicides davidi Shields and Segonzac, 2007 by having no eyes; from Ovicides jonesi Shields and Segonzac, 2007 by the presence of basophilic, vacuolated glandular lobes in the precerebral region; and from Ovicides jasoni Shields and Segonzac, 2007 by the arrangement of the acidophilic submuscular glands, which are not arranged in a row. Ovicides paralithodis represents the third described species of egg-predatory nemertean from Paralithodes camtschaticus, the second described carcinonemertid species from Japan, and the 21st described species in the family. The intensity of infestations may exceed 24, 000 worms per a single egg-bearing pleopod of Paralithodes camtschaticus. A preliminary molecular phylogenetic analysis based on sequences of 28S rRNA and cytochrome c oxidase subunit I genes among selected monostiliferous hoplonemertean species supported the monophyly of Carcinonemertidae, suggesting that within the lineage of the family, evolution of the unique vas deferens, Takakura’s duct, preceded loss of accessory stylets and accessory-stylet pouches.
Nemertini, Crustacea, Paralithodes camtschatica, symbiont, egg predator
Nemerteans in the monostiliferous hoplonemertean family Carcinonemertidae are ectosymbiont egg predators of decapod crustacean hosts (
The red king crab, Paralithodes camtschaticus (Tilesius, 1815), is a commercially important anomuran decapod, native to the Bering Sea, the Sea of Japan, the Sea of Okhotsk, and the North Pacific from the Kamchatka Peninsula to Alaska.
A survey of egg masses of Paralithodes camtschaticus in Hokkaido, northern Japan, yielded specimens that correspond to Form 4 of
Twenty female specimens of the red king crab Paralithodes camtschaticus were obtained in the Sea of Okhotsk, off Abashiri, Hokkaido, Japan, at 44°06'N, 144°32'E, from 215 m in depth, by crab cages set from 28 November 2011 to 15 December 2011. Of these female crabs, 16 were ovigerous, from three of which we procured a single nemertean specimen. The worms were anaesthetized in MgCl2 solution isotonic to seawater. The anterior halves of the worms were fixed in Bouin’s solution for histological preparation; the posterior halves were preserved in 99% ethanol for DNA extraction. Histological preparation follows that of
DNA extraction, PCR amplification, and sequencing of the nuclear 28S rRNA gene and mitochondrial cytochrome c oxidase subunit I gene (COI) largely follow those of
A preliminary analysis was carried out to assess the phylogenetic affinities of the new species, including 16 species of Distromatonemertea, in addition to two outgroup species, for which 28S rRNA and COI sequences were available in GenBank (Table 1). Alignment of the sequences was carried out by MUSCLE (
List of species included in the phylogenetic analysis, with GenBank accession numbers.
| Species | 28S rRNA | COI | Sources |
|---|---|---|---|
| Amphiporus imparispinosus Griffin, 1898 | HQ856878 | HQ848612 |
|
| Amphiporus lactifloreus (Johnston, 1828) | HQ856876 | HQ848611 |
|
| Antarctonemertes varvarae Chernyshev, 1999 | AJ436845 | AJ436900 |
|
| Argonemertes australiensis (Dendy, 1892) | HQ856892 | HQ848601 |
|
| Carcinonemertes carcinophila (Kölliker, 1845) | HQ856893 | HQ848619 |
|
| Carcinonemertes cf. carcinophila imminuta Humes, 1942 | AJ436846 | AJ436901 |
|
| Emplectonema gracile (Johnston, 1837) | HQ856883 | HQ848620 |
|
| Gononemertes parasita Bergendal, 1900 | HQ856889 | HQ848607 |
|
| Leptonemertes chalicophora (Graff, 1879) | HQ856898 | HQ848596 |
|
| Nemertellina yamaokai Kajihara et al., 2000 | AJ436852 | AJ436907 |
|
| Oerstedia dorsalis (Abildgaard, 1806) | AY210465 | AY791971 |
|
| Oerstedia venusta Iwata, 1954 | AJ436856 | AJ436911 |
|
| Ovicides paralithodis sp. n. | AB704416 | AB704417 | Present study |
| Paranemertes peregrina Coe, 1901 | AJ436860 | AJ436915 |
|
| Paranemertes sanjuanensis Stricker, 1982 | AJ436862 | AJ436917 |
|
| Zygonemertes simoneae Corrêa, 1961 | AJ436867 | AJ436922 |
|
| Zygonemertes virescens (Verrill, 1879) | AJ436868 | AJ436923 |
|
| Outgroups | |||
| Nipponnemertes punctatula (Coe, 1905) | AJ436855 | AJ436910 |
|
| Paradrepanophorus crassus (Quatrefages, 1846) | HQ856867 | HQ848603 |
|
Observations on abundance and geographic distribution in Alaska were conducted from 1983 to 1985, as described in
urn:lsid:zoobank.org:act:1E52DC7A-C52F-4502-AEAC-7A3EB0244F4D
http://species-id.net/wiki/Ovicides_paralithodis
Figs 1–5Holotype: female, ZIHU 4271, serial transverse sections (8 µm thick) of anterior body fragment, stained with Mallory’s trichrome method, 5 slides. Allotype: male, ZIHU 4272, serial transverse sections (8 µm thick) of anterior body fragment, stained with Mallory’s trichrome method, 3 slides. The other specimen obtained (female) was destroyed and lost during preparation.
An Ovicides without eyes; vacuolated, basophilic glandular lobes extending pre- and post-cerebrally; acidophilic submuscular glands scattered among basophilic lobes, not arranged in row; sexes separate; female and male about 1 cm and 5 mm in length, respectively.
Paralithodes camtschaticus (Tilesius, 1815) (Decapoda, Anomura).
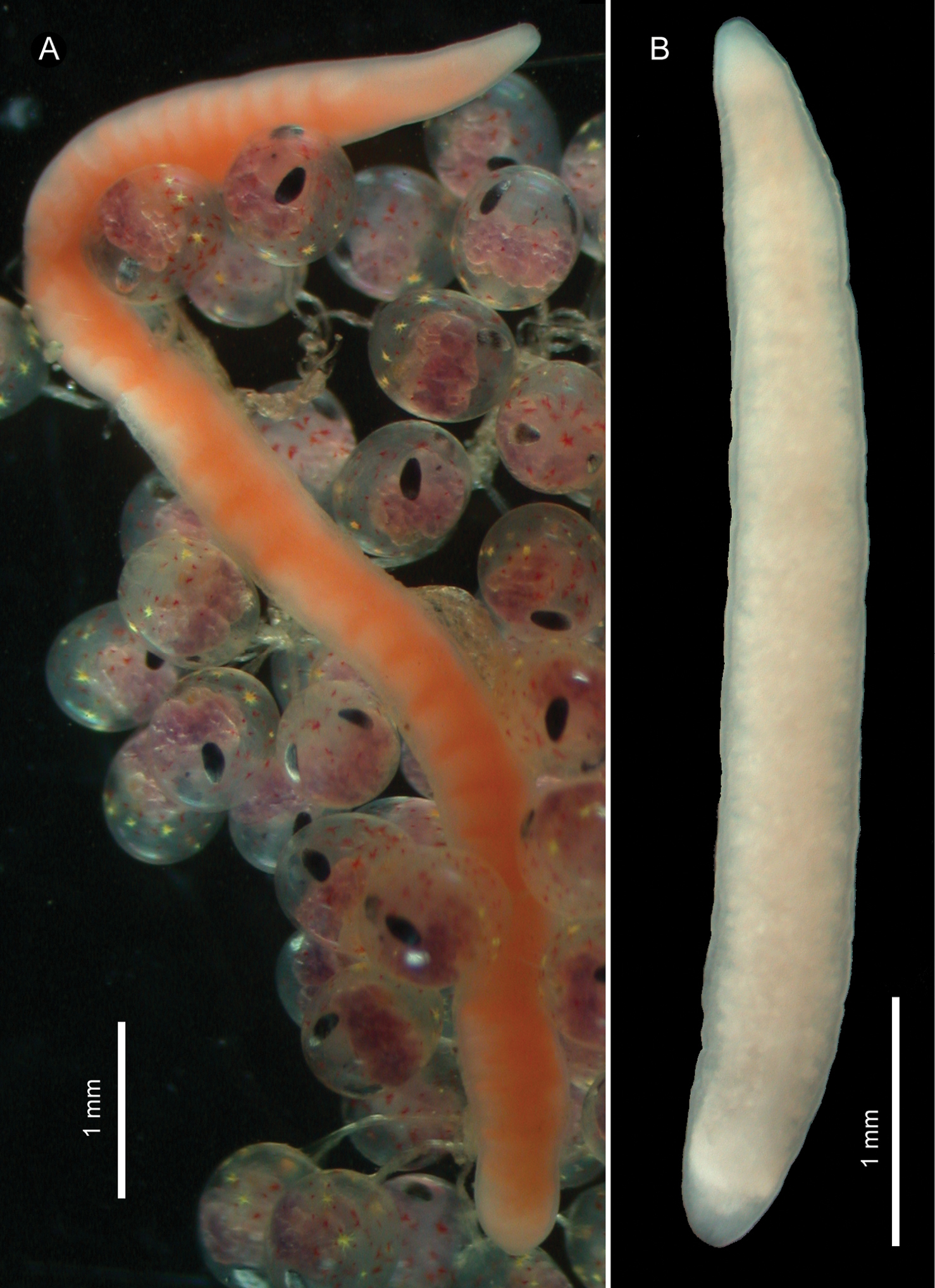
External features. In life, holotype (female) about 1 cm long, 0.9 mm wide; pale orange in colour (largely due to alimentary canal), except whitish tip of head (Fig. 1A). Allotype (male) about 5 mm in length, 0.3 mm in width; cream white in colour (Fig. 1B). Living in thin, transparent mucous tube.
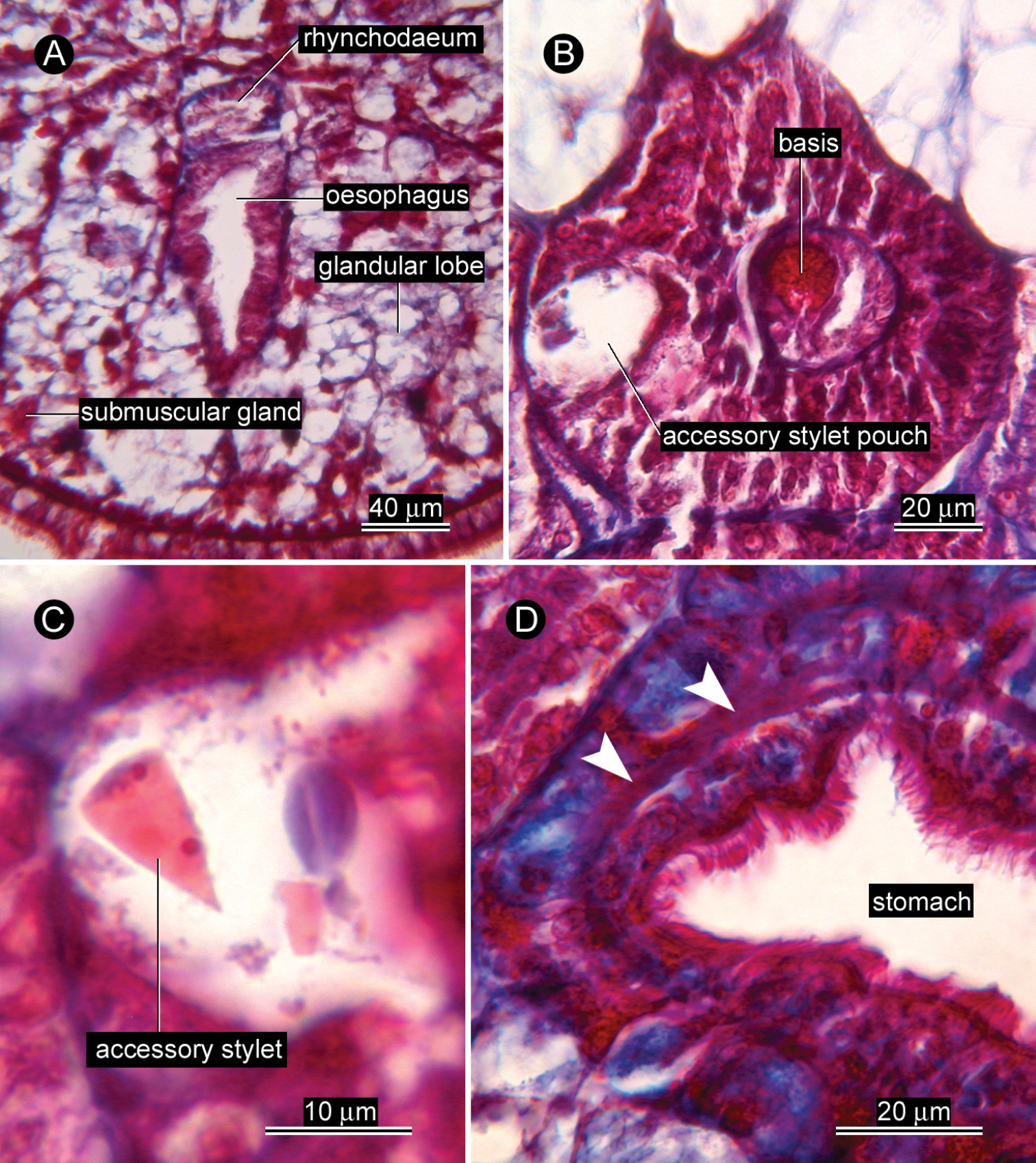
Proboscis apparatus. Rhynchodaeum opening to dorsal wall of oesophagus (Fig. 2A). Anterior proboscis chamber 136 µm (unknown in allotype) long by 100 µm (82 µm in allotype) diameter; central stylet basis 48 µm (56 µm in allotype) long by 20 µm (20 µm in allotype) diameter (Figs 2B, 3); central stylet 16 µm (12 µm in allotype) in length (all measured from transverse sections); stylet to basis ratio 0.21–0.33; two accessory stylet pouches each containing two accessory stylets (Fig. 2C). Middle proboscis chamber 80 µm (54 µm in allotype) in diameter. Posterior proboscis chamber 240 µm (unknown in allotype) long by 130 µm (94 µm in allotype) wide. Proboscis almost same length as rhynchocoel, extending posteriorly behind pylorus-intestine junction; musculature of rhynchocoel wall uncertain in light microscopy.
Alimentary canal. Oesophagus opening ventrally at tip of head. Stomach wall containing circular muscle fibres (Fig. 2D).
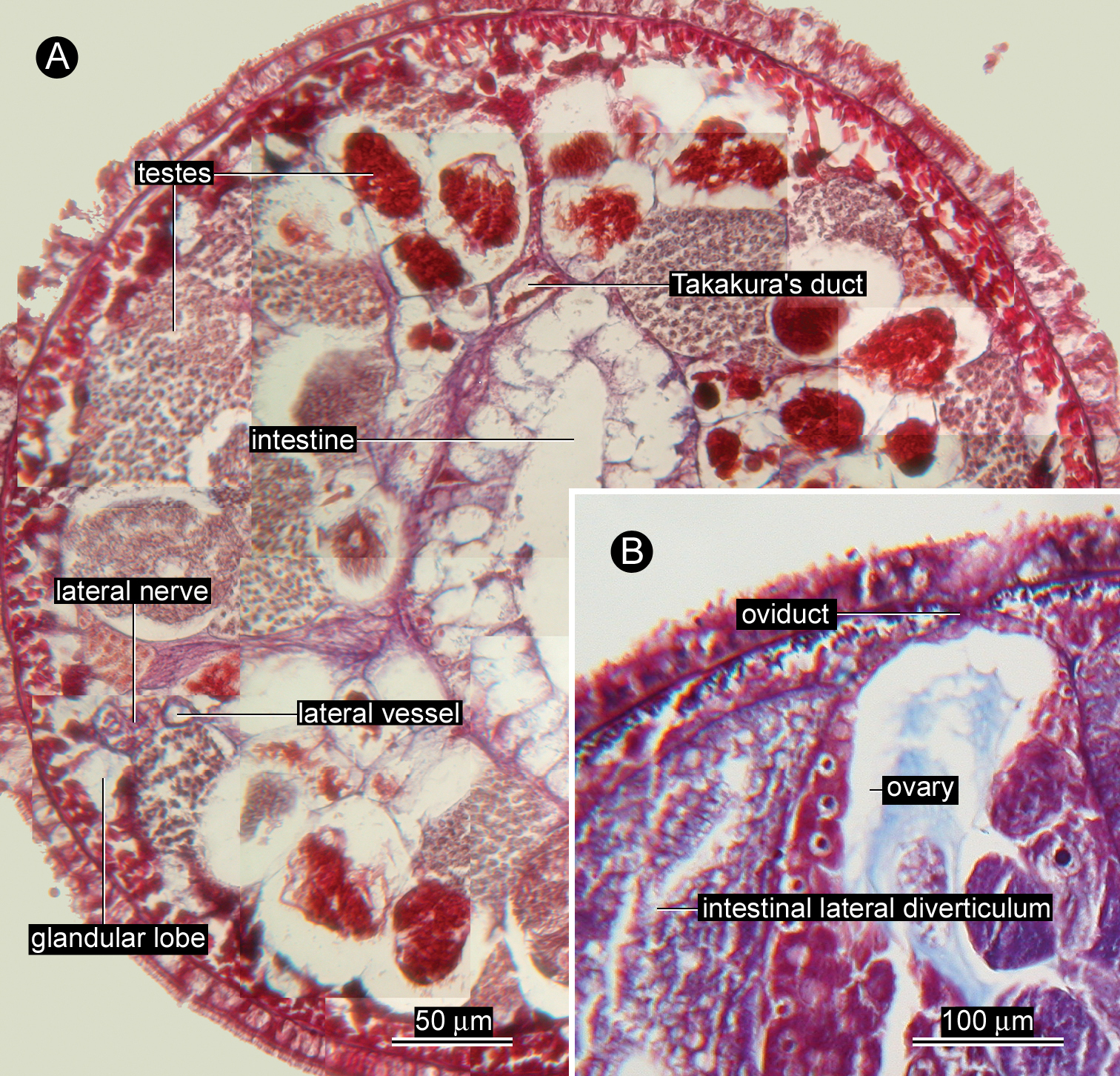
Glandular system. Vacuolated, basophilic glandular lobes filling much space of precerebral region between body-wall musculature and oesophagus (Fig. 2A), extending post-cerebrally in intestinal region, but gradually less distinct posteriorly (Fig. 4A). Acidophilic submuscular glands scattered among basophilic lobes (Fig. 2A), not arranged in row beneath body-wall musculature.
Ovicides paralithodis sp. n., photographs taken in life. A holotype, female, ZIHU 4271 B allotype, male, ZIHU 4272.
Ovicides paralithodis sp. n., photomicrographs of transverse sections. A precerebral region, showing rhynchodaeum just after branched off from oesophagus B anterior proboscis chamber showing stylet basis and one of the two accessory stylet pouches C accessory stylet D stomach, showing circular muscle fibres (indicated by arrowheads). A, C, D, allotype, male, ZIHU 4272; B, holotype, female, ZIHU 4271.
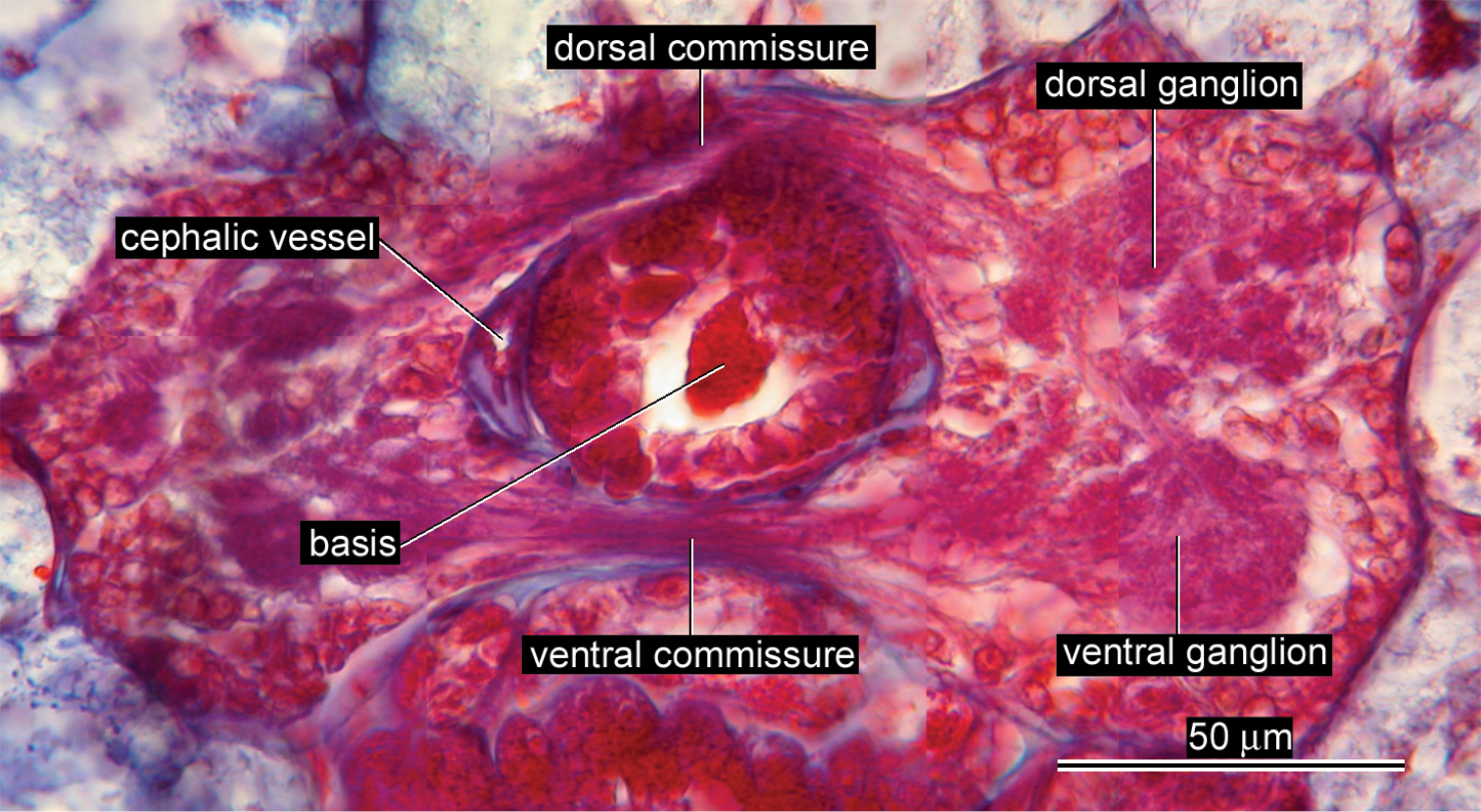
Ovicides paralithodis sp. n., photomicrograph of transverse section through brain ring, allotype, male, ZIHU 4272.
Excretory system. Flame cells, nephridioducts, and nephridiopores not found.
Nervous system. Dorsal and ventral brain commissures 13 µm (9 µm in allotype) and 10 µm (7 µm in allotype) in thickness, respectively (Fig. 3).
Vascular system. Pair of cephalic vessels meeting above rhynchodaeum, posteriorly passing through cerebral ring (Fig. 3), extending further backward as lateral vessel on each side, situated near lateral nerve cord (Fig. 4A).
Sensory system. No eyes. No cerebral organs. No frontal organ.
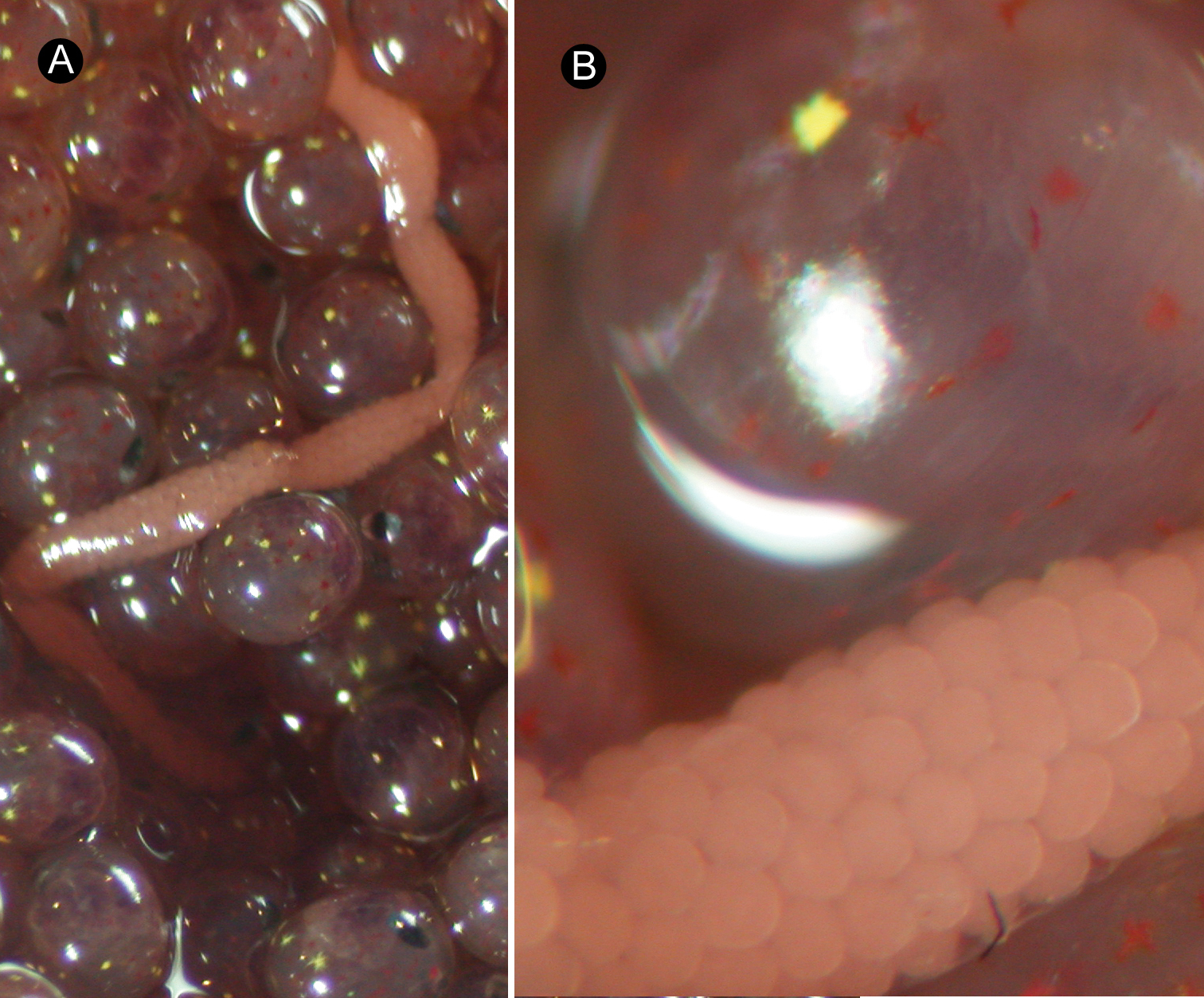
Reproductive system. Ovaries more or less regularly interspersed with intestinal lateral diverticula, arranged in row on each side of body; single oviduct from each ovary extending dorsally (Fig. 4B). Single egg string found in the same crab egg mass about 1 cm in length, containing pink eggs (Fig. 5A, B). Takakura’s duct present in male, about 40 µm in diameter (Fig. 4A).
Ovicides paralithodis sp. n., photomicrographs of transverse sections through intestinal region. A testes and Takakura’s duct, allotype, male, ZIHU 4272 B gonopore opening dorsally, holotype, female, ZIHU 4271.
Ovicides paralithodis sp. n. A egg strand laid by holotype B magnification of A.
Fed on Paralithodes camtschaticus eggs in vitro, piercing the egg membrane with its stylet and consuming the contents of the ruptured eggs. In vivo feeding confirmed by frequent observations of gut contents containing crab egg yolk and eye placodes. Juvenile worms were recovered from two of 30 male and non-ovigerous female crabs collected at Juneau and Seward, Alaska. The presence of juvenile worms on hosts lacking eggs suggests that the life cycle of Ovicides paralithodis may be more similar to carcinonemertids such as Carcinonemertes errans Wickham, 1978 where worms can transfer from males to females, and from premoult to postmoult cuticles of non-ovigerous crabs (
The proportion of infested crabs exceeded 50 percent at 13 localities in Alaska, reaching 100 percent at five localities. At six localities the intensity of infestations exceeded 1, 000 worms per pleopod (red king crabs have six egg-bearing pleopods), with the highest reported intensity at Terror Bay, Kodiak Island, >24, 000 worms per pleopod (
The specific name, paralithodis, is a noun in the genitive case, derived from the generic name of the host crustacean, Paralithodes camtschaticus.
In addition to the type locality, the Sea of Okhotsk, off Abashiri, Hokkaido, Japan, Ovicides paralithodis has been reported from Adak, Dutch Harbor, Morshovoi Bay, Pavlof Bay, Kodiak Island, Resurrection Bay, Seward, Cook Inlet and Southeastern Alaska (Barlow Cove, Deadman’s Reach, Gambier Cove, and Pybus Cove, Juneau) by
Of the four currently recognised congeners in Ovicides, Ovicides paralithodis is distinguished from Ovicides julieae and Ovicides davidi by the absence of eyes. Ovicides jasoni and Ovicides jonesi are eye-less as is the new species. Ovicides jasoni can be distinguished from Ovicides paralithodis in having densely arranged submuscular glands (
Ovicides paralithodis has only been confirmed from Paralithodes camtschaticus. However, a similar eyeless form with accessory stylet pouches is common on tanner crab, Chionoecetes bairdi Rathbun, 1924 and has also been found on the Dungeness crab, Cancer magister Dana, 1852 in Alaskan waters (AMK, unpublished observations).
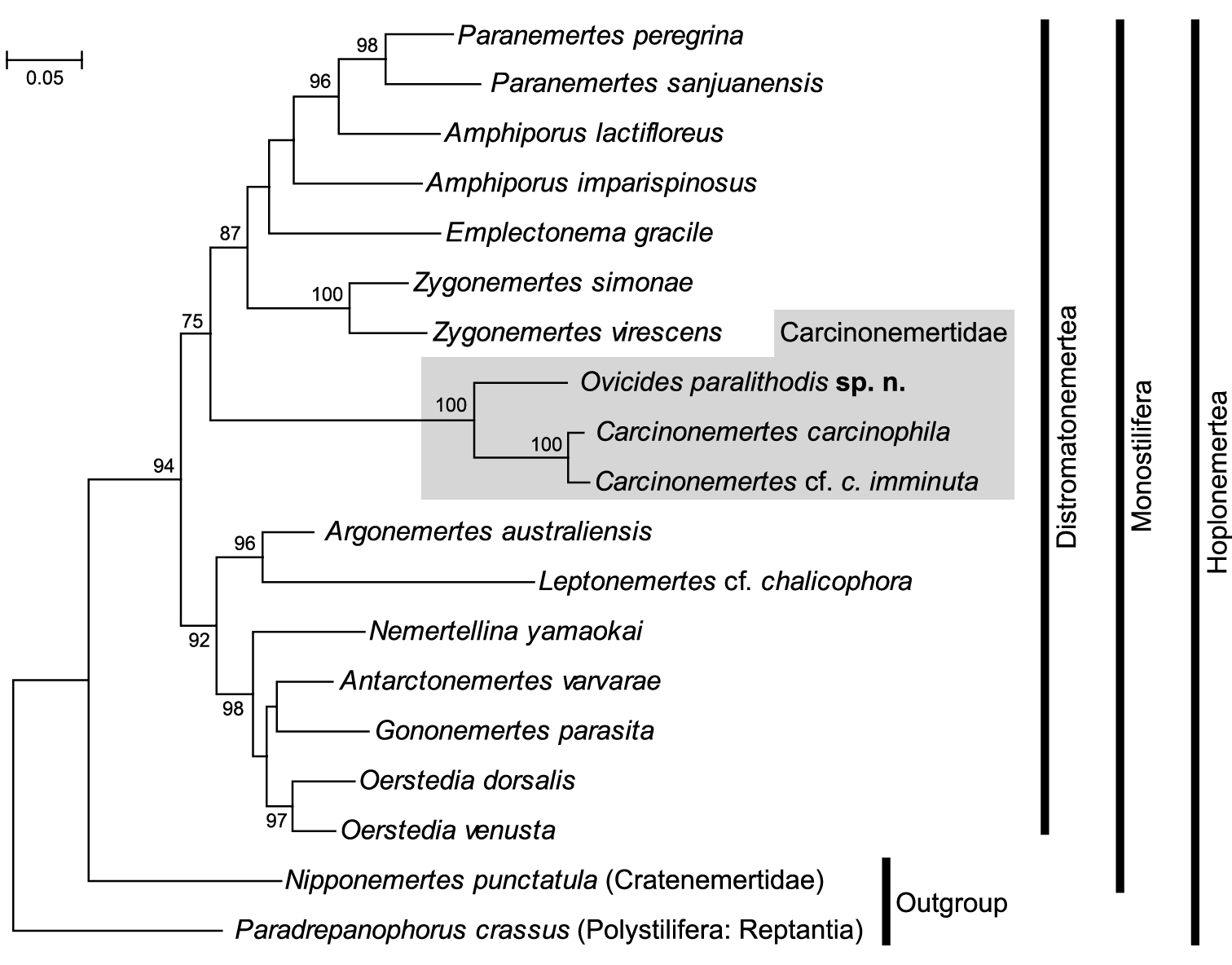
In the maximum-likelihood tree (ln L = –9804.30) (Fig. 6), Ovicides paralithodis appeared as a sister taxon to the clade comprised of Carcinonemertes carcinophila (Kölliker, 1845) of
Phylogenetic tree resulting from maximum likelihood analysis of combined 28S rRNA and COI (ln L = –9804.30). Numbers above/below nodes indicate bootstrap support values >50%.
One may infer from the present tree topology that the acquisition of Takakura’s duct and the loss of cerebral organs occurred in the common ancestor of the family, prior to the loss of accessory stylet pouches or stylets, which happened only in the lineage leading to Carcinonemertes, but not in Ovicides. We conclude so because 1) Takakura’s duct is possessed by all carcinonemertids, and is otherwise unique in the phylum, 2) with a few exceptions, monostiliferans generally possess cerebral organs, and 3) accessory stylets and their pouches are widespread features among Hoplonemertea, including Ovicides, but are absent in Carcinonemertes. An implication of this character-evolution scenario is that the genus Ovicides, currently diagnosed as a nemertean egg predator having accessory stylets (a plesiomorphy for Carcinonemertidae), may not be monophyletic.
This study supports monophyly of Carcinonemertidae, in agreement with the views of
The sister-taxon relationship of Carcinonemertidae among Monostilifera remains uncertain, although the search for it would have a fundamental significance in divergence-time estimates within the phylum. So far, all the carcinonemertids are symbiotic egg predators of Achelata, Anomura, and Brachyura (
The position of Carcinonemertidae is likely to be susceptible to long-branch attraction. Carcinonemertes cf. carcinophila imminuta appeared as sister to all the rest of Distromatonemertea included in the analysis by
We thank Yuji Yoshida (Abashiri Fisheries Cooperative), Taka-aki Watanabe (Department of Fisheries, Ports, and Harbours, Abashiri City), and Ryoichi Tamura (Mariculture Fisheries Research Institute, Fisheries Research Department, Hokkaido Research Organization) for their kind arrangement for the collection of crab specimens. HK also thanks the staffs at the Abashiri Fisheries Science Centre for providing laboratory facilities and Hiroshi Yamasaki (Hokkaido University) for his help in molecular analyses. This study was financially supported in part by Grants-in-Aid for Regional R&D Proposal-Based Program from Northern Advancement Centre for Science & Technology of Hokkaido, Japan (#T-3-22) and Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (#23370038) for HK, and by the University of California Sea Grant Program under grant NA80AA-D-00120 for AMK.