(C) 2013 Andrey I. Khalaim. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Khalaim AI, Ruíz-Cancino E (2013) Mexican species of the genus Stethantyx Townes (Hymenoptera, Ichneumonidae, Tersilochinae). ZooKeys 360: 83–94. doi: 10.3897/zookeys.360.6362
Six species of the genus Stethantyx Townes are found to occur in Mexico. One species, S. mexicana sp. n., is described as new, and four recently described Neotropical species, S. alajuela Khalaim & Broad, S. heredia Khalaim & Broad, S. osa Khalaim & Broad and S. sanjosea Khalaim & Broad, are new records from Mexico. A key to species of Stethantyx occurring in Mexico is provided.
En México se han encontrado seis especies del género Stethantyx Townes. Se describe la especie S. mexicana sp. n. Cuatro especies descritas recientemente para la región Neotropical son nuevos registros para México: S. alajuela Khalaim & Broad, S. heredia Khalaim & Broad, S. osa Khalaim & Broad y S. sanjosea Khalaim & Broad. Se elaboró la clave de especies de Stethantyx que ocurren en México.
Key, Neotropical region, new records, new species, North America, taxonomy
Stethantyx is a large predominantly Neotropical genus with 42 described and many undescribed species (
Only two native species, Stethantyx crassa Horstmann and Stethantyx nearctica Townes, and Stethantyx parkeri (Blanchard), introduced from South America, are known to occur in the Nearctic region (
Some species of Stethantyx were reared from the beetle families Nitidulidae and Curculionidae (Coleoptera). In the Nearctic region, Stethantyx crassa was reared from Cryptarcha sp. and/or Lobiopa undulata Say (Nitidulidae) from sap spots on oak (
Only one species, Stethantyx nearctica, was previously known from northern Mexico (
The ichneumonid collection of the Universidad Autónoma de Tamaulipas, Cd. Victoria, Mexico (further UAT) was studied. One specimen of Stethantyx was borrowed from the Essig Museum of Entomology, University of California, Berkeley, U.S.A. (further EMEC). Some specimens were deposited in the Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia (further ZISP).
Morphological terminology predominantly follows
Stethantyx nearctica Townes, 1971.
| 1 | Mesosoma entirely black (Figs 20, 23). Ovipositor sheath twice as long as first tergite | Stethantyx nearctica Townes |
| – | Mesosoma entirely or predominantly reddish orange (Figs 8, 17, 25). Ovipositor sheath 1.2–1.8 times as long as first tergite (unknown for Stethantyx mexicana sp. n.) | 2 |
| 2 | Head reddish orange, frons sometimes infuscate centrally (Figs 28, 29). Propodeum with narrow basal area (Fig. 30) | Stethantyx sanjosea Khalaim & Broad |
| – | Head black, face and clypeus sometimes reddish brown or yellow (Figs 2, 6, 7, 10, 11, 24, 25). Propodeum not as above: with basal keel (Figs 9, 18), broad basal area (Fig. 26) or longitudinal wrinkles mediodorsally (Fig. 5) | 3 |
| 3 | Flagellum with conspicuous pale band (Fig. 1). Face yellow (Fig. 2), with strong prominence centrally. Notaulus strongly impressed, distant from anterolateral margin of mesoscutum (Fig. 3). Propodeum usually without distinct basal area, with longitudinal wrinkles dorsally (Fig. 5) | Stethantyx alajuela Khalaim & Broad |
| – | Flagellum black, without pale band (as in Fig. 27). Face yellow or black, with weak prominence centrally. Notaulus not impressed, as wrinkle or small tubercle distant from anterolateral margin of mesoscutum (Figs 6, 10), or completely absent. Propodeum with distinct basal area (Fig. 26) or basal keel (Figs 9, 18) | 4 |
| 4 | Propodeum with broad basal area (Fig. 26). Propodeal spiracle separated from pleural carina by 1.5–2.0 times diameter of spiracle (Fig. 25). Face black (Fig. 24) | Stethantyx osa Khalaim & Broad |
| – | Propodeum with basal keel (Figs 9, 18). Distance between propodeal spiracle and pleural carina less than one diameter of spiracle (Figs 8, 17). Face usually reddish brown or yellowish (Figs 7, 11) | 5 |
| 5 | Mesoscutum, scutellum and propodeum black, strongly contrasting with remaining mesosoma (Fig. 17) | Stethantyx mexicana sp. n. |
| – | Mesosoma uniformly reddish orange (Fig. 8) | Stethantyx heredia Khalaim & Broad |
Mexico: Veracruz, Estación Biológica Los Tuxtlas, 480 m, 2.VI.1986, coll. P. Sinaca, 1 female (UAT).
Mexico (Veracruz), Costa Rica, Ecuador, Peru, Paraguay. First record from Mexico.
Stethantyx alajuela Khalaim & Broad, ♀: 1 antennae, lateral view 2 head, frontal view 3 head and anterior part of mesosoma, lateral view 4 mesoscutum and scutellum, dorsal view 5 propodeum, dorsolateral view.
Mexico: Chiapas, Jaltenango, Reserva El Triunfo, Malaise trap, 19–22.VII.1997, coll. A. González Hernández, 1 female (UAT). Quintana Roo, Rancho #3, Valle Hermoso, 21.VII.1993, coll. H. Delein, 2 females (UAT).
http://zoobank.org/8FD563AE-170A-4D0E-A09E-F38CD0B5B343
http://species-id.net/wiki/Stethantyx_mexicana
Figs 10–19Differs from its North and Central American congeners by the combination of brownish orange mesosoma with black mesoscutum, scutellum and propodeum (Fig. 17), and propodeum with basal keel (Fig. 18). In the key to Costa Rican species of Stethantyx (
Male. Body length 4.8 mm. Fore wing length 3.45 mm.
Head: Roundly narrowed behind eyes in dorsal view (Fig. 10); temple almost 0.6 times as long as eye width. Mandible with upper tooth much longer than lower tooth (Fig. 12). Clypeus lenticular, flat in lateral view, about 2.5 times as broad as long, smooth, punctate on upper 0.3 (Fig. 11). Malar space 0.2 times as long as basal width of mandible (Fig. 13). Flagellum of antenna with over 28 segments (tips of all antennae absent), distinctly tapered towards apex; all flagellomeres, except the basal one, 1.3–1.4 times as long as broad (Fig. 14); flagellomeres 4 to 14 bear finger-shaped subapical structures on outer surface. Face and frons finely and densely punctate on finely granulate dull background. Vertex finely and densely punctate (punctures distinct medially and indistinct laterally) on very finely granulate, weakly shining background. Temple with very fine, mostly indistinct punctures, weakly shining. Face with weak prominence centrally. Occipital carina complete.
Mesosoma: Notaulus as small tubercle (in both paratypes) or short wrinkle (in holotype) distant from anterolateral margin of mesoscutum (Figs 10, 13). Mesoscutum very finely (sometimes indistinctly) punctate on very finely granulate, dull background. Scutellum with lateral longitudinal carinae extending from its base to posterior 0.6–0.7. Mesopleuron finely but distinctly punctate on smooth and shining background (area just above foveate groove impunctate), peripherally finely granulate. Foveate groove situated more or less in centre of mesopleuron, strongly oblique, deep and crenulate (Fig. 17). Propodeum with all carinae strong, transverse carina without adjacent wrinkles (Fig. 18). Dorsolateral area very shallowly granulate, dull, with sparse indistinct punctures. Basal keel of propodeum almost 0.4 times as long as apical area (Fig. 18). Propodeal spiracle big, adjacent to pleural carina (Fig. 17). Apical area flat, pointed anteriorly (Fig. 18).
Wings: Fore wing (Fig. 16) with first and second sections of radius angled about 130°. Intercubitus 1.5–2.0 times as long as abscissa of cubitus between intercubitus and second recurrent vein. Metacarp almost reaching apex of fore wing. Hind wing (Fig. 16) with nervellus distinctly inclivous.
Legs: Slender (Fig. 15). Hind femur slightly clavate, 4.7 times as long as broad and 0.8 times as long as tibia. Hind spurs slightly curved at apex. Tarsal claws rather long, not pectinate.
Metasoma: First tergite slender, 4.6 times as long as posteriorly broad, entirely smooth. Glymma situated somewhat behind centre of tergite, moderately large, groove between glymma and ventral part of postpetiole weak but distinct in two paratypes and absent in the holotype. Second tergite almost 2.6 times as long as anteriorly broad (Fig. 19). Thyridial depression very long, more than 5.0 times as long as broad (Fig. 19).
Coloration: Head black with lower part of face slightly reddish brown, clypeus and malar space yellow. Palpi and mandible (teeth reddish black) yellow. Scape and pedicel of antenna dark brown or brownish black; flagellum black, probably without pale band. Mesosoma brownish orange; propleuron and lower part of pronotum yellowish; mesoscutum, scutellum and propodeum dark brown or black, strongly contrasting with remaining mesosoma. Tegula fuscous. Pterostigma dark brown. Legs yellow to brownish yellow; hind leg with coxa with brown mark on extero-outer surface, femur brown to dark brown, and tibia and tarsus blackish. First tergite black. Metasoma behind first tergite predominantly dark brown (almost black dorsally), ventrally yellowish, second and following tergites with pale band posteriorly.
Female unknown.
Stethantyx mexicana sp. n., ♀, holotype (except Figs 10 and 12): 10 head and anterior part of mesosoma, dorsal view 11 head, frontal view 12 head, ventral view 13 head and anterior part of mesosoma, lateral view 14 antenna (apex absent), lateral view 15 posterior part of mesosoma and hind leg, lateral view.
Stethantyx mexicana sp. n., ♀, holotype (except Fig. 16): 16 wings 17 head and mesosoma, lateral view 18 propodeum, dorsolateral view 19 second tergite, dorsal view.
The three specimens are very uniform, with minor variation in structure and coloration. One paratype has the basal keel of the propodeum centrally indistinct.
From the type locality, Mexico.
Holotype male, Mexico, Chiapas, Reserva El Triunfo, Jaltenango, Red de golpeo, 15°39'22"N, 92°48'31"E, 1400 m, 21.VII.1997, coll. A. González Hernández, CIB 97-063a (UAT).
Mexico, Chiapas, Palo Gordo, screen sweeping, 21.VI.1997, coll. A. González Hernández, 2 males (UAT, ZISP).
Mexico (Chiapas).
Mexico: Tamaulipas, Cd. Victoria, 20.X.2007, coll. E. Ruíz-Cancino, 1 female (UAT). U.S.A.: Florida, Jefferson Co., Monticella, University of Florida, Malaise trap, 5–19.I, 5.X–30.XI.2001, coll. R. Mitzel, 2 females (UAT, ZISP).
U.S.A. (Arizona, District of Columbia, Florida, Iowa, Maryland, North Carolina, South Carolina, Texas, Virginia), Mexico (Nuevo León, Tamaulipas).
Reared in Virginia, U.S.A., from Balaninus sp. (Curculionidae) on Quercus alba L. (Fagaceae), probably from acorns (
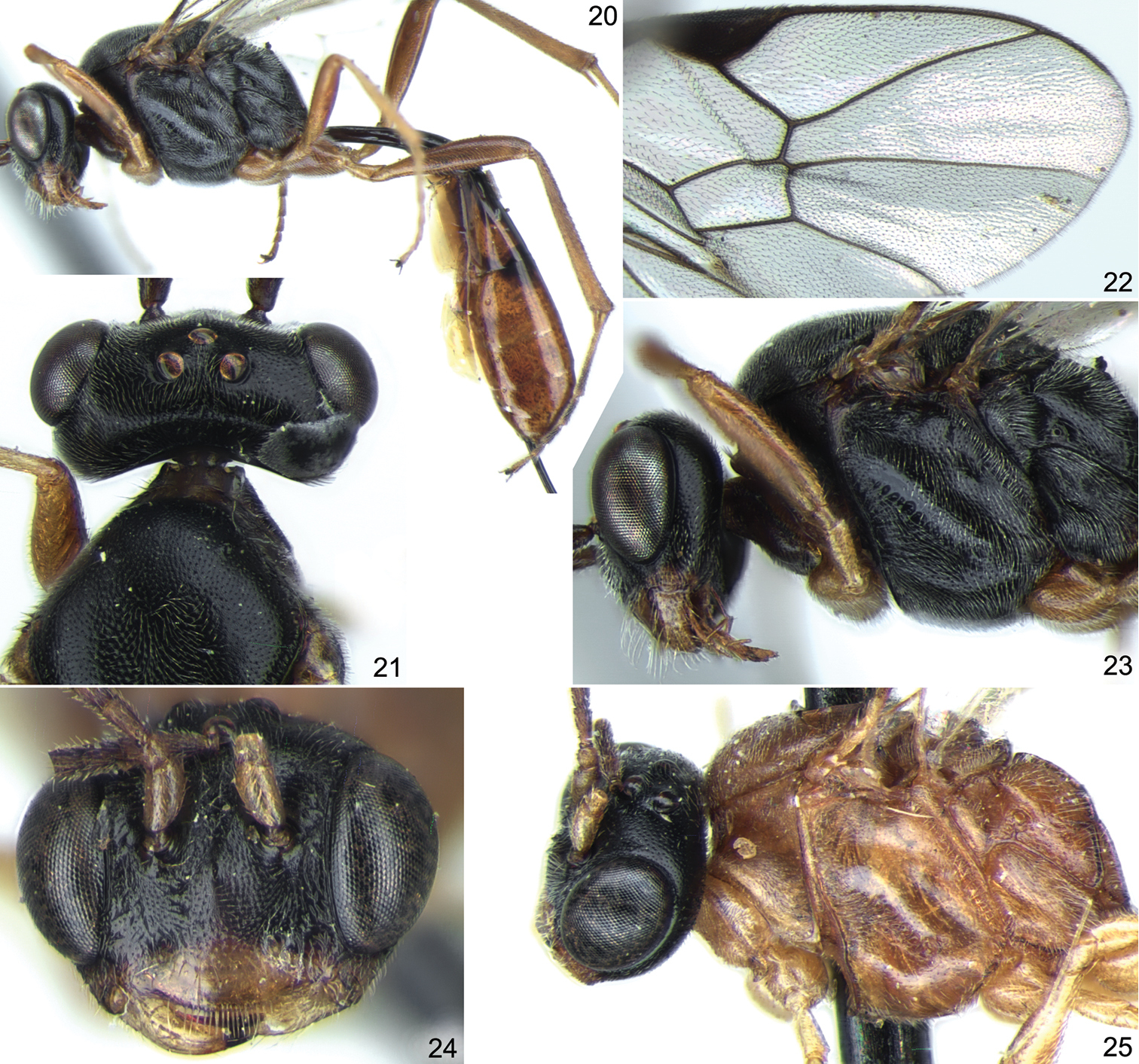
Stethantyx nearctica Townes, ♀: 20 habitus (without antennae and wings), lateral view 21 head and anterior part of mesosoma, dorsal view 22 apex of fore wing 23 head and mesosoma, lateral view. Stethantyx osa Khalaim & Broad, ♀: 24 head, frontal view 25 head and mesosoma, lateral view.
Mexico: Nayarit, Jesus Maria, 27.VII.1955, coll. B. Malkin, 1 female (EMEC).
Mexico (Nayarit), Costa Rica. First record from Mexico.
Stethantyx osa Khalaim & Broad, ♀: 26 propodeum, dorsal view. Stethantyx sanjosea Khalaim & Broad, ♀: 27 antennae, lateral view 28 head, frontal view 29 head and anterior part of mesosoma, dorsal view 30 propodeum, dorsal view 31 second tergite, dorsal view.
Mexico: Tamaulipas, Gómez Farías, Canindo, 1400 m, Malaise trap, 28–30.VII.1993, coll. J.B. Woolley, 3 females (UAT). Chiapas, Jaltenango, Reserva El Triunfo, Malaise trap, 19–22.vii.1997, coll. A. González Hernández, 1 female (UAT).
Mexico (Tamaulipas, Chiapas), Costa Rica, Ecuador, Peru. First record from Mexico.
Four species of Stethantyx, previously known from Costa Rica and South America, were recorded mostly from central and southern parts of Mexico and, along with the new species, belong to the Neotropical complex of species. Only one species, Stethantyx nearctica, occurring in northern Mexico and U.S.A., belongs to the Nearctic complex of species. All Mexican species of the Neotropical complex were collected in summer (June to August), while the flight period of Stethantyx nearctica in Mexico is from October to January. In comparison with Costa Rica, where many species of Stethantyx are rather abundant through the year, in Mexico this genus seems to be rather rare because all Mexican species are represented by single or few specimens in available material.
We are thankful to Dr. Peter T. Oboyski from EMEC for loan of valuable material, and to Dr. Gavin Broad (the Natural History Museum, London, UK) and anonymous referee for their important comments and corrections. This work was supported by the PROMEP Project “Taxonomía y ecología de fauna y micobiota en comunidades forestales y cultivos” and the Russian Foundation for Basic Research (grant no. 13-04-00026).