(C) 2013 Jans Morffe. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new genera and species parasitizing passalid beetles from the Democratic Republic of Congo are described. Kongonema meyeri gen. n. sp. n. is characterized by having females with the cervical cuticle unarmed, first cephalic annule cone-like and truncate, sub-cylindrical procorpus and genital tract didelphic-amphidelphic. The males of Kongonema meyeri gen. n. sp. n. have the procorpus sub-cylindrical, the dorsal cuticle of the tail end thickened, a single large, median mammiform pre-cloacal papilla and a pair of small, pre-cloacal, sub-lateral papillae at a short distance before the level of the cloaca. Lubanema decraemerae gen. n. sp. n. is characterized by the body markedly fusiform, cuticle unarmed and strongly annulated, procorpus sub-cylindrical, isthmus as a constriction between procorpus and basal bulb, genital tract monodelphic-prodelphic and the posterior end rounded with a very short tail appendage.
Nematoda, Hystrignathidae, Kongonema, Lubanema, Passalidae, Democratic Republic of Congo
The family Hystrignathidae comprises 27 nominal genera with more than 100 species of monoxenous nematodes specific of the hind gut of passalid beetles. The family shows a mostly Gondwanian distribution, with taxa from North, Central and South America, West Indies, Africa and Australasia (
In Africa, the group still remains neglected with most of the species restricted to their type localities.
This paper retakes the study on African hystrignathids, describing two new genera and species parasitizing passalid beetles from the Democratic Republic of Congo.
Several specimens of passalid beetles from the Democratic Republic of Congo (formerly Zaire) were examined in a parasitological survey during a research visit to the Royal Museum of Central Africa, Tervuren, Belgium. Eight specimens of Didimus sp. and two specimens of Erionomus pilosus Aurivillus, 1896 from Katale, Kivu region were included in this study, all collected during the Belgian expeditions to the Congo in the 1930´s and stored in 70% ethanol.
The hosts were dissected by practicing incisions in both pleural membranes and the intestines were extracted and kept in Petri dishes with 70% ethanol. The guts were excised and the parasites removed.
Nematodes were transferred and cleared in glycerine via slow evaporation method (
Some specimens were prepared for SEM as follows: they were dehydrated in a graded ethanol series, critical point-dried, mounted in aluminum stubs and coated in gold. SEM micrographs were taken at an acceleration voltage of 22–25 kV.
Classification at generic level was followed after
Female. Body comparatively robust. Cervical cuticle unarmed. Lateral alae present, from the oesophageal region to a short distance beyond the level of the anus. Posterior ends of the lateral alae rounded, forming lobes. Head bearing eight paired papillae. First cephalic annule cone-like, truncate, barely inflated, about two head-lengths long. Oesophagus consisting of a muscular sub-cylindrical procorpus, its base well set-off from the isthmus. Nerve ring encircling procorpus at its midpoint. Excretory pore post-bulbar. Reproductive system didelphic-amphidelphic. Eggs ovoid, ridged-shelled. Tail filiform and subulate.
Male. Body shorter and more slender than female. Cervical cuticle unarmed. Lateral alae present, from the oesophageal region to the level of the single median mammiform papilla. First cephalic annule inconspicuous. Stoma scarcely developed. Oesophagus with a sub-cylindrical procorpus, well set-off from the short isthmus. Nerve ring encircling procorpus at its posterior half. Excretory pore post-bulbar. Monorchic. Testis outstretched. Spicule absent. Posterior end ventrally curved, tapering abruptly, forming a very short, rounded tail appendage. Dorsal cuticle of the tail end thickened. A single large, median mammiform pre-cloacal papilla present. A pair of small, pre-cloacal, sub-lateral papillae located at a short distance before the level of the cloaca.
Kongonema meyeri Morffe & García gen. n. sp. n. (monotypic genus).
Democratic Republic of Congo.
The generic name (neuter) is a combination of Kongo, after the main ethnic group in the country of this taxon, and the suffix –nema.
urn:lsid:zoobank.org:act:0E02D195-40DE-4D6A-A752-D9FEAF8910E7
http://species-id.net/wiki/Kongonema_meyeri
Figs 1 A–G , 2 A–D , 3 A–E♀ holotype, Democratic Republic of Congo, Kivu Region, Katale, 1°19'S, 29°22'E; in Didimus sp.; 4.V.1939; Hautmann coll.; CZACC 11.4653. Paratypes: 10♀♀, same data as holotype, CZACC 11.4654-11.4663; 10♀♀, same data as holotype, RMCA; 4♀♀, same data as holotype, CHIOC; ♂, same data as holotype, CZACC 11.4664; ♂, same data as holotype, RMCA.
Vouchers: 2♀♀, Democratic Republic of Congo, Kivu Region, Katale, 1°19'S, 29°22'E; in Didimus sp.; 4.V.1939; Hautmann coll., RBINS. 2♀♀, Democratic Republic of Congo, Kivu Region, Katale, 1°19'S, 29°22'E; in Erionomus pilosus; 4.V.1939; Hautmann coll.;CZACC 11.4665-11.4666; 2♀♀, same data as the latter, RMCA;
Holotype (female) a = 12.15, b = 5.06, c = 7.26, V% = 58.08, total length = 1.670, maximum body width = 0.138, first cephalic annule (length×width) = 0.013×0.038, stoma length = 0.050, procorpus length = 0.260, isthmus length = 0.020, diameter of basal bulb = 0.058, total length of oesophagus = 0.330, nerve ring to anterior end = 0.185, excretory pore to anterior end = 0.440, vulva to posterior end = 0.700, anus to posterior end = 0.230, eggs = 0.123×0.050 (n = 1).
Paratypes (females) (n = 24) a = 8.65-13.08 (10.68 ± 0.90 n = 23), b = 4.30-5.03 (4.71 ± 0.71 n = 21), c = 5.65-7.45 (6.43 ± 0.37 n = 23), V% = 53.02-58.82 (55.91 ± 1.43 n = 23), total length = 1.400-1.670 (1.530 ± 0.075 n = 23), maximum body width = 0.120-0.170 (0.144 ± 0.012 n = 24), first cephalic annule (length×width) = 0.013-0.025×0.038-0.043 (0.016 ± 0.003×0.041 ± 0.002 n = 19), stoma length = 0.033-0.050 (0.045 ± 0.005 n = 19), procorpus length = 0.210-0.270 (0.244 ± 0.013 n = 20), isthmus length = 0.020-0.033 (0.024 ± 0.003 n = 22), diameter of basal bulb = 0.053-0.070 (0.061 ± 0.004 n = 24), total length of oesophagus = 0.283-0.350 (0.324 ± 0.014 n = 21), nerve ring to anterior end = 0.148-0.190 (0.172 ± 0.011 n = 21), excretory pore to anterior end = 0.320-0.490 (0.402 ± 0.046 n = 23), vulva to posterior end = 0.620-0.750 (0.674 ± 0.038 n = 23), anus to posterior end = 0.200-0.280 (0.239 ± 0.019 n = 23), eggs = 0.120-0.133×0.043-0.063 (0.125 ± 0.004×0.051 ± 0.006 n = 26).
Paratypes (males) (n = 2) a = 15.67-17.33 (16.50 ± 1.18 n = 2), b = 3.47-3.58 (3.52 ± 0.08 n = 2), c = 121.33-125.33 (123.33 ± 2.83 n = 2), total length = 0.910-0.940 (0.925 ± 0.021 n = 2), maximum body width = 0.053-0.060 (0.056 ± 0.005 n = 2), procorpus length = 0.250 (n = 2), isthmus length = 0.018-0.020 (0.019 ± 0.002 n = 2), diameter of basal bulb = 0.035 (n = 2), total length of oesophagus = 0.263 (n = 2), nerve ring to anterior end = 0.138-0.148 (0.143 ± 0.007 n = 2), excretory pore to anterior end = 0.290-0.330 (0.310 ± 0.028 n = 2), cloacae to posterior end = 0.008 (n = 2).
Females (n = 4) a = 9.46-10.75 (10.06 ± 0.68 n = 4), b = 4.75-4.97 (4.87 ± 0.09 n = 4), c = 6.58-7.13 (6.82 ± 0.23 n = 4), V% = 55.56-61.59 (57.75 ± 2.72 n = 4), total length = 1.640-1.750 (1.703 ± 0.046 n = 4), maximum body width = 0.153-0.185 (0.170 ± 0.015 n = 4), first cephalic annule (length×width) = 0.018-0.020×0.043-0.048 (0.019 ± 0.001×0.045 ± 0.002 n = 4), stoma length = 0.048-0.053 (0.050 ± 0.003 n = 4), procorpus length = 0.255-0.275 (0.267 ± 0.009 n = 4), isthmus length = 0.020-0.025 (0.023 ± 0.002 n = 4), diameter of basal bulb = 0.068-0.075 (0.071 ± 0.004 n = 4), total length of oesophagus = 0.330-0.360 (0.350 ± 0.014 n = 4), nerve ring to anterior end = 0.185-0.195 (0.189 ± 0.004 n = 4), excretory pore to anterior end = 0.420-0.510 (0.475 ± 0.040 n = 4), vulva to posterior end = 0.630-0.760 (0.720 ± 0.61 n = 4), anus to posterior end = 0.230-0.260 (0.250 ± 0.014 n = 4), eggs = 0.120-0.130×0.048-0.065 (0.126 ± 0.004×0.058 ± 0.007 n = 7).
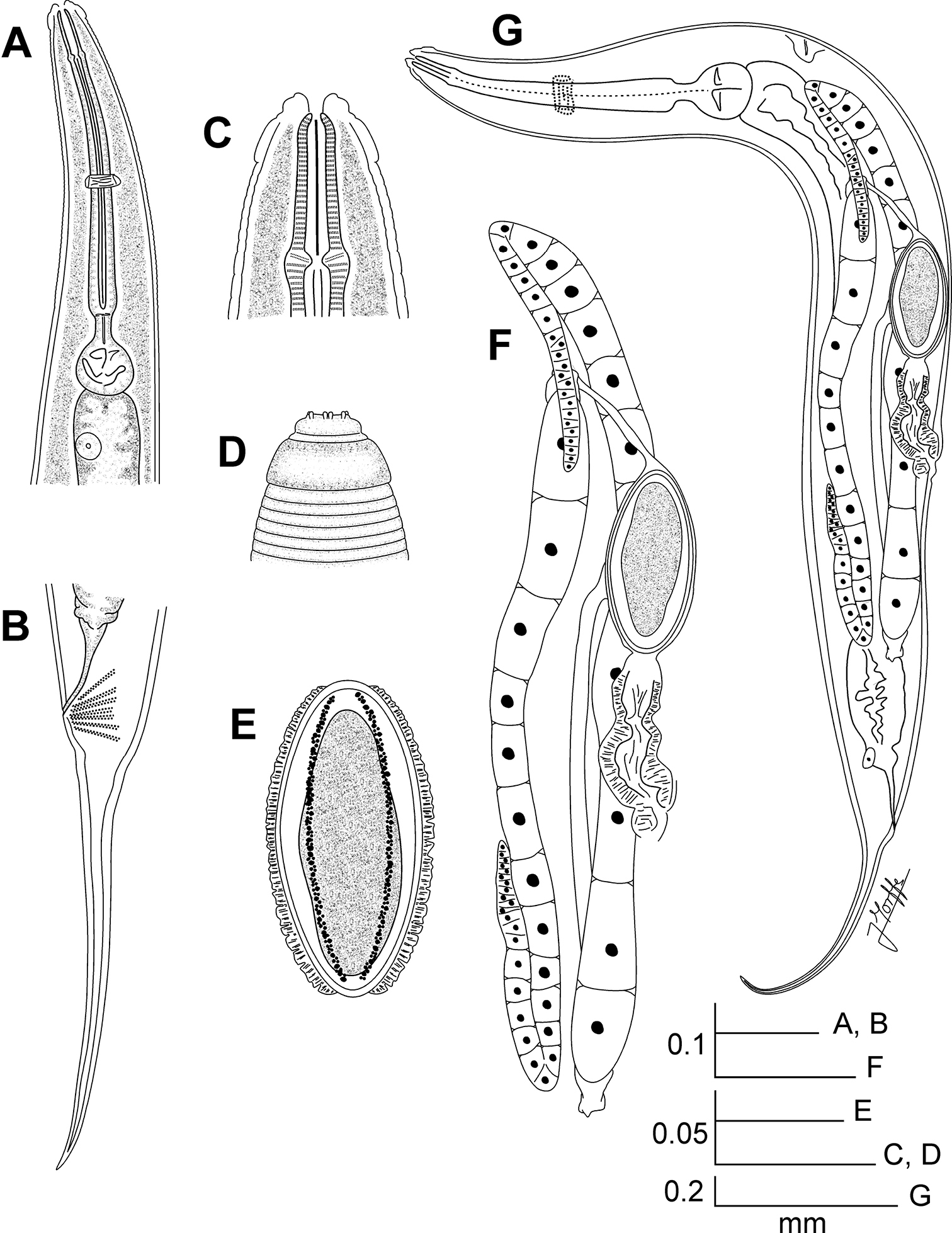
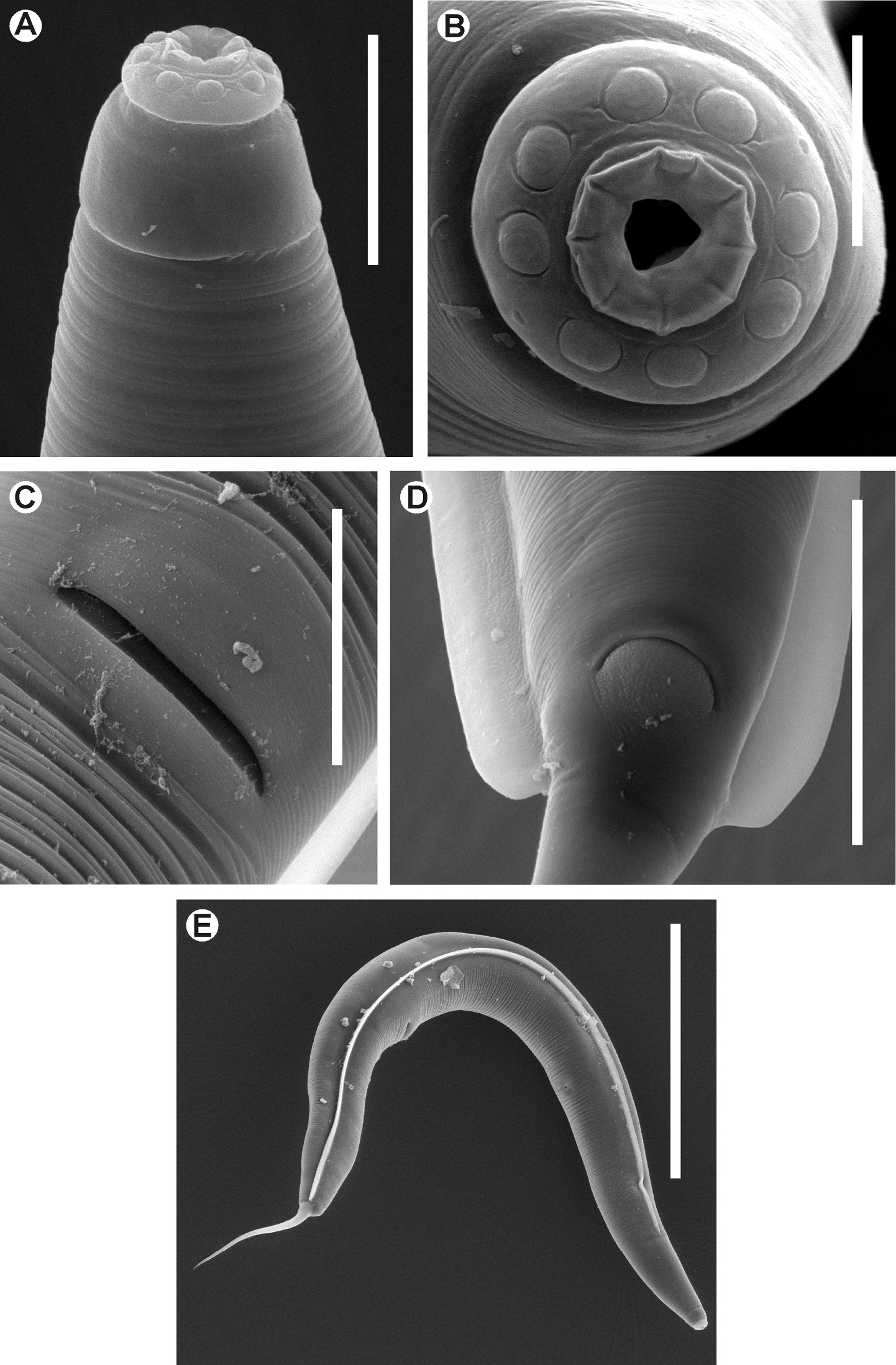
Female.Body comparatively robust, widening from the base of the first cephalic annule, maximum body diameter at level of the vulva, then tapering towards anus. Cervical cuticle unarmed, markedly annulated (annuli ca. 5-7 µm). Rest of the body with marked annuli decreasing their width towards the level of the anus. Sub-cuticular longitudinal striae present. Lateral alae ca. 9 µm wide, from the oesophageal region (ca. 30 µm before the level of the nerve ring) to a very short distance beyond the level of the anus. Posterior ends of the lateral alae rounded, forming short lobes. Head well developed, set-off from body by a single, deep groove and bearing eight rounded, paired papillae. Amphids pore-like, laterally situated. Mouth sub-triangular in shape. First cephalic annule cone-like, truncate, barely inflated, about two head-lengths long. Stoma comparatively long, about three first cephalic annule lengths long, surrounded by an oesophageal collar. Oesophagus consisting of a muscular, sub-cylindrical procorpus, its base slightly wider and well set-off from the short isthmus. Basal bulb sub-spherical, valve plate well developed. Intestine simple, sub-rectilinear, anterior portion dilated. Rectum short, anus not prominent, as a crescent-like slit. Nerve ring encircling procorpus at about its midpoint. Excretory pore situated at about half of a body width posterior to basal bulb. Genital tract didelphic-amphidelphic, both ovaries reflexed. Anterior ovary reflexed behind the excretory pore, posterior ovary reflexed at about a body width before the anus. Distal flexures of ovaries about one body width-length long. Oöcytes in single rows. Vulva a median transverse slit slightly displaced to the posterior half of body, lips prominent. Vagina muscular, forwardly directed. Eggs ovoid, bearing eight longitudinal, rough, ridges on the shell. Tail comparatively long, filiform, subulate, ending in a sharp point.
Body shorter than female, comparatively slender, posterior region ventrally curved. Cervical cuticle unarmed. Sub-cuticular longitudinal striae present. Lateral alae from the oesophageal region (about three body-widths posterior to the cephalic end) to the level of the single mammiform papilla (about a body-width before the level of anus). Head not set-off from body. First cephalic annule not developed. Stoma not defined. Oesophagus consisting of a sub-cylindrical procorpus, well set-off from the short isthmus. Basal bulb rounded, valve plate well developed. Intestine simple, anterior portion slightly dilated. Nerve ring encircling procorpus at its posterior half, about 65% of its length. Excretory pore situated at about 1.5 body-widths posterior to basal bulb. Monorchic. Testis outstretched, arising at a short distance behind the excretory pore. Spicule absent. Dorsal cuticle of the tail region thickened. A single, large, pre-cloacal ventromedian mammiform papilla situated at about a body width before the posterior end. A pair of small, pre-cloacal, sub-lateral papillae situated at a short distance before the level of the cloaca. Tail region becoming sharp visibly from the beginning of the cuticular thickening, until forming a very short tail appendage, its tip rounded.
Kongonema meyeri gen. n. sp. n. Female. A Oesophageal region, ventral view B Tail, lateral view C Cephalic end, internal view D Cephalic end, external view E Egg F Reproductive system, lateral view G Entire nematode, lateral view.
Kongonema meyeri gen. n. sp. n. Male. A Oesophageal region, lateral view B Cephalic end, internal view C Posterior end, lateral view D Entire nematode, lateral view.
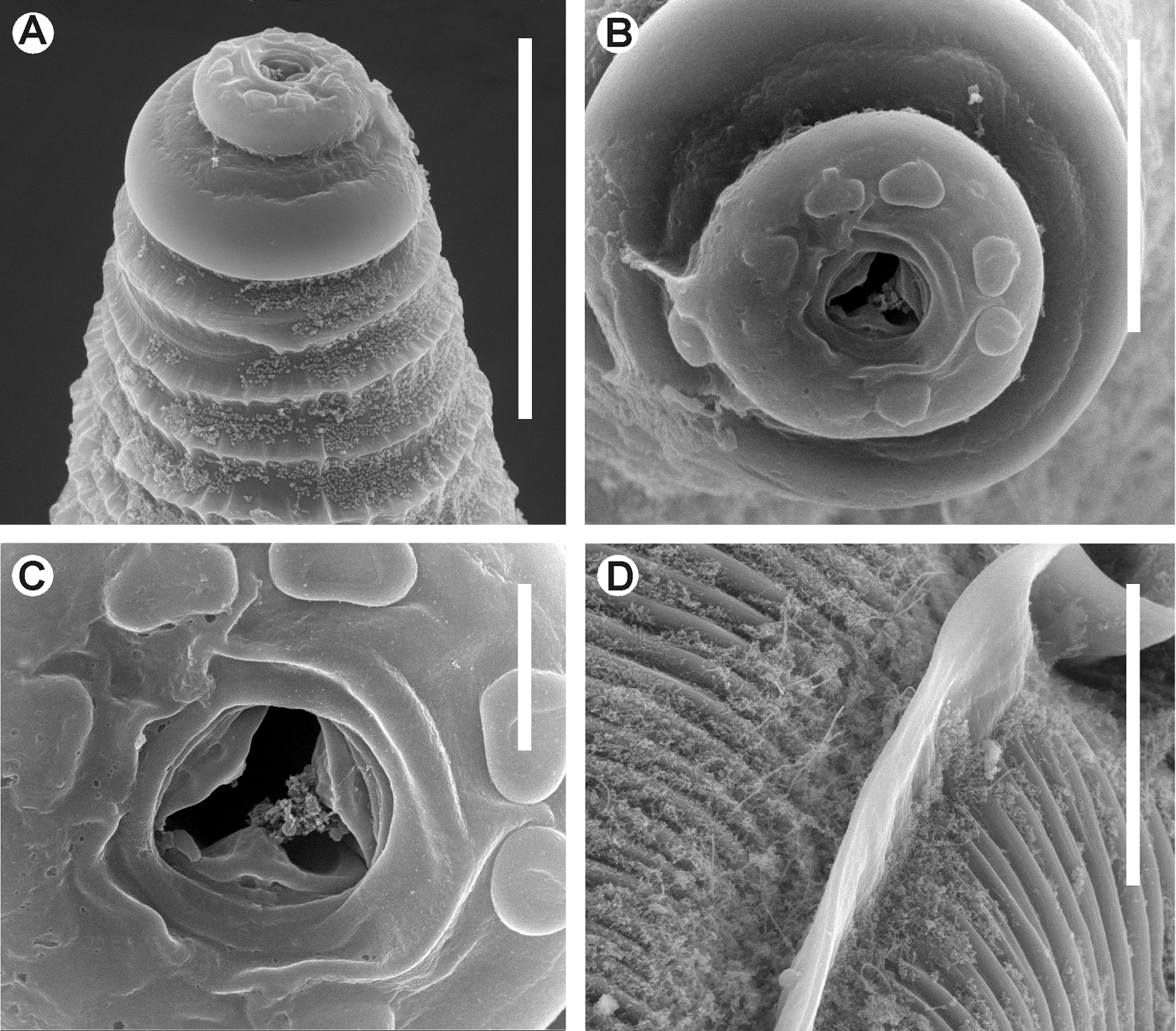
Kongonema meyeri gen. n. sp. n. Female. SEM images A Cephalic end B Cephalic end, en face view C Vulva D Anus and end of lateral alae E Habitus, lateral view. Scale lines: A 0.025 mm, B 0.01 mm, C, D 0.04 mm, E 0.3 mm.
There are three genera of hystrignathids the female of which present the cervical cuticle unarmed, procorpus sub-cylindrical and reproductive system digonant: Anomalostoma Cordeira, 1981; Coynema (Coy, García & Alvarez, 1993) Morffe & García, 2011 and Ventelia Travassos & Kloss, 1958. The first differs by having the anterior region of the procorpus strongly swollen, surrounding the stoma (
Females of Coynema can be segregated by the basal dilatation of its procorpus and the anterior region of the intestine notably inflated, forming a saccular structure (
The males of Kongonema gen. n. resemble their counterparts of Coynema (only close genus where the male is known) by lacking of spicule and by having a similar arrangement of the copulatory papillae: the ventromedian pre-cloacal papilla (typical of Hystrignathidae) and another pair of small sub-lateral pre-cloacal papillae. Kongonema gen. n. differs by having a sub-cylindrical procorpus, without the basal dilation and by lacking the saccular region of the intestine characteristic of Coynema. The posterior end of Kongonema gen. n. forms a short, rounded tail appendage vs. the sharp tail of Coynema.
On the other hand, Ventelia has the procorpus barely set-off from the isthmus, since the posterior third of the procorpus decreases its diameter. The hind procorpus of Kongonema gen. n. increases its diameter slightly and is well differentiated from the isthmus.
Didimus sp. (Coleoptera: Passalidae).
Erionomus pilosus Aurivillus, 1896 (Coleoptera: Passalidae).
Gut caeca.
Katale, Kivu region, Democratic Republic of Congo.
Specific epithet dedicated to Dr. Marc de Meyer, curator of the Entomological Collection of the Royal Museum of Central Africa, Tervuren, Belgium. In appreciation of his kind help by permitting access to the material assessed.
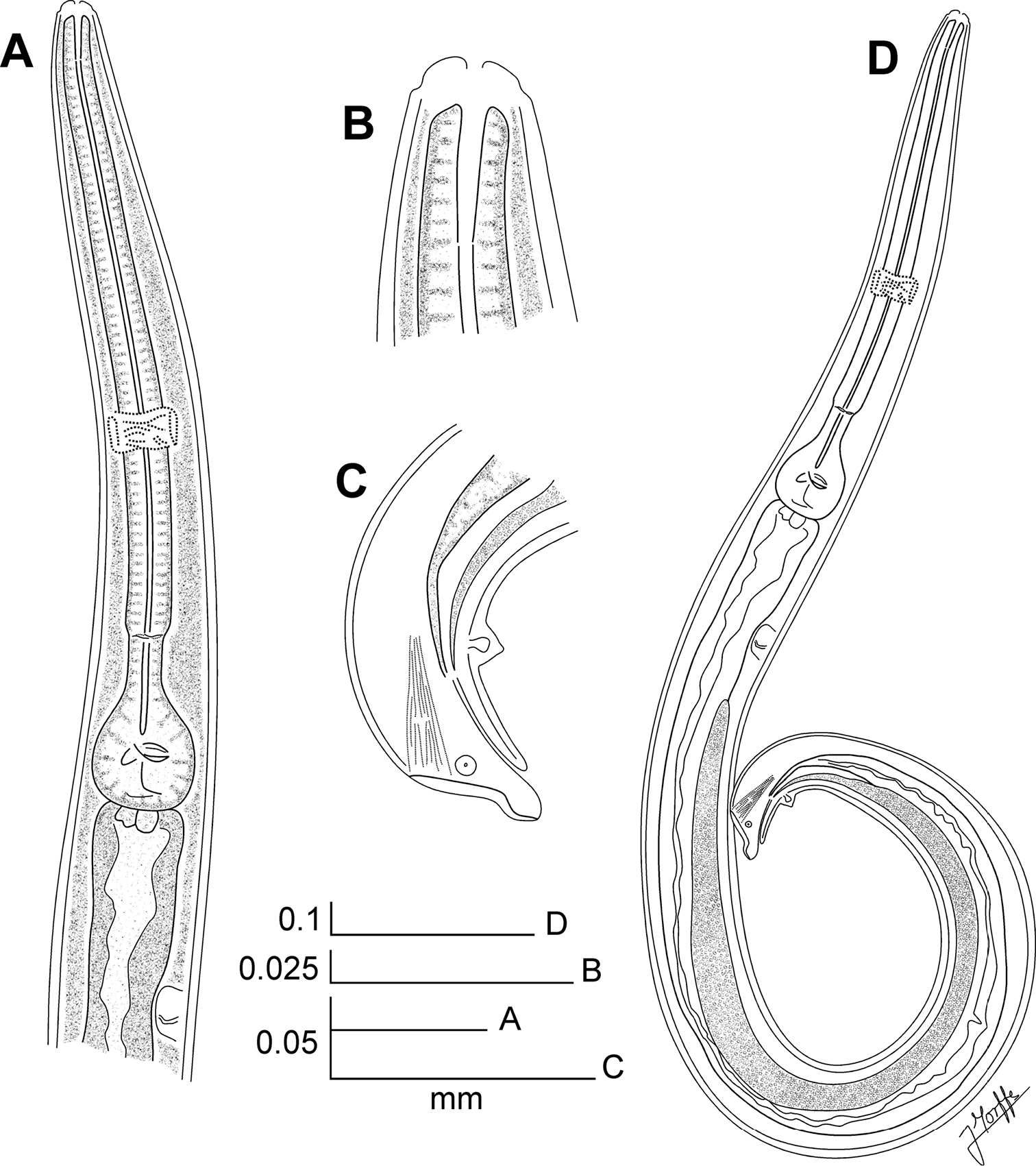
Female.Body notably robust and fusiform. Posterior end strongly rounded, bearing a terminal, very short, conical tail appendage. Cuticle unarmed, markedly annulated until the level of the anus. Lateral alae wide, from the oesophageal region to the level of the anus. Posterior ends of the alae almost forming a straight angle with the body axis, slightly convex and with short lobes in their margins. First cephalic annule cone-like, slightly inflated, its margins convex. Oesophagus with a sub-cylindrical, muscular procorpus. Isthmus as a constriction between the procorpus and basal bulb. Nerve ring encircling procorpus at its posterior half. Excretory pore post-bulbar. Reproductive system monodelphic-prodelphic. Ovary stout. Eggs markedly ovoid, smooth-shelled.
Lubanema decraemerae Morffe & García gen. n. (monotypic genus).
Democratic Republic of Congo.
The generic name (neuter) is a combination of Luba, after one of the ethnic groups in the country, and the suffix –nema.
urn:lsid:zoobank.org:act:D480D872-86F3-4920-B9A7-550925CCD97A
http://species-id.net/wiki/Lubanema_decraemerae
Figs 4 A–G , 5 A–D♀ holotype, Democratic Republic of Congo, Kivu Region, Katale, 1°19'S, 29°22'E; in Didimus sp.; 4.V.1939; Hautmann coll.; CZACC 11.4667. Paratypes: ♀, same data as holotype, CZACC 11.4668; ♀, same data as holotype, RMCA.
Holotype (female) a = 6.70, b = 5.97, c = 40.18, V% = 57.92, total length = 2.210, maximum body width = 0.330, first cephalic annule (length×width) = 0.020×0.070, stoma length = 0.048, procorpus length = 0.268, diameter of basal bulb = 0.108, total length of oesophagus = 0.370, excretory pore to anterior end = 0.520, vulva to posterior end = 0.930, anus to posterior end = 0.055, eggs = 0.168-0.173×0.075-0.080 (0.170 ± 0.004×0.078 ± 0.004 n = 2).
Paratypes (females) (n = 2) a = 4.44-6.36 (5.40 ± 1.36 n = 2), b = 3.66-5.68 (4.67 ± 1.43 n = 2), c = 34.45-38.18 (36.31 ± 2.64 n = 2), V% = 56.67 (n = 1), total length = 2.100-2.400 (2.250 ± 0.212 n = 2), maximum body width = 0.330 (n = 2), first cephalic annule (length×width) = 0.020×0.063-0.065 (0.020×0.064 ± 0.002 n = 2), stoma length = 0.043-0.045 (0.044 ± 0.002 n = 2), procorpus length = 0.275-0.300 (0.288 ± 0.018 n = 2), diameter of basal bulb = 0.108 (n = 2), total length of oesophagus = 0.370-0.400 (0.385 ± 0.021 n = 2), nerve ring to anterior end = 0.223 (n = 1), excretory pore to anterior end = 0.410-0.600 (0.505 ± 0.134 n = 2), vulva to posterior end = 1.040 (n = 1), anus to posterior end = 0.043-0.055 (0.049 ± 0.009 n = 2), eggs = 0.183×0.078 (n = 1).
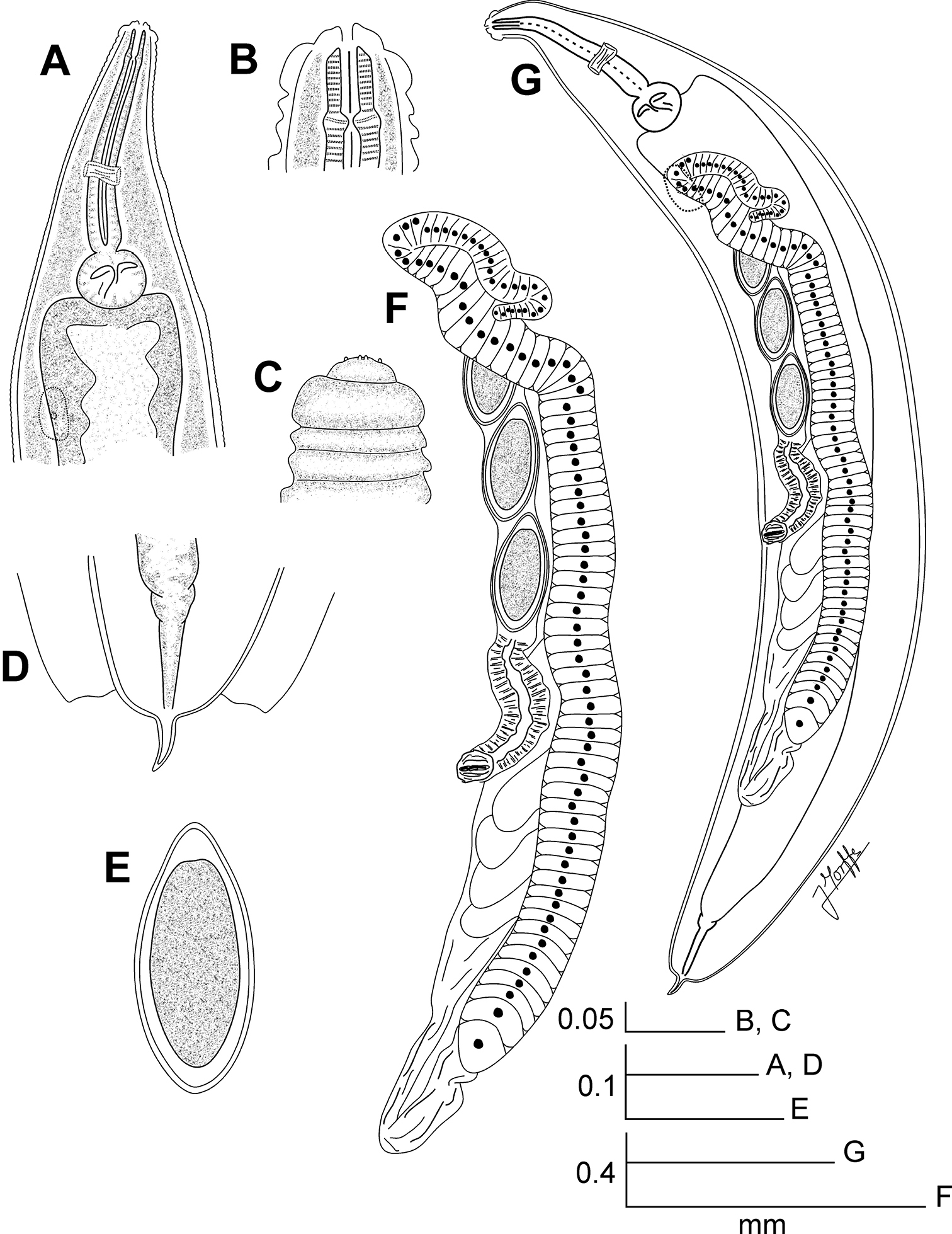
Female body large, notably robust and fusiform, widening gradually from the base of the first cephalic annule, reaching maximum width near mid-body, then tapering softly towards the posterior end that rounds off abruptly. A comparatively very short, conical tail appendage with its tip rounded arises terminally from the posterior end. Cervical cuticle unarmed, with marked annule (ca. 13 µm wide), extending to the rest of body, until level of the anus. Lateral alae thick, ca. 55 µm wide, extending from the hind third of the procorpus to the level of the anus. Posterior ends of lateral alae almost forming a straight angle with the body axis, slightly convex, their external margins forming a very short lobe. Head bearing eight rounded, paired papillae, set-off from body by a single, deep groove. Amphids pore-like, laterally situated. Mouth trirradiate. First cephalic annule cone-like, slightly inflated, its margins convex, about two head-lengths long. Stoma long, about 1.5 first cephalic annule lengths long, surrounded by an oesophageal collar. Oesophagus consisting of a muscular, sub-cylindrical procorpus, its diameter little increased at its base. Isthmus as a constriction between the procorpus and the large, rounded, basal bulb. Valve plate well developed. Intestine simple, sub-rectilinear, its fore region very inflated. Rectum short. Anus sub-terminal. Nerve ring encircling procorpus at its posterior half (ca. 60% of its length). Excretory pore located at about the half of a body width behind the basal bulb. Vulva a median trasverse slit, displaced to the posterior half of body, lips less prominent. Vagina muscular, forwardly directed. Genital tract monodelphic-prodelphic. Ovary stout, reflexed at about one third of the body width behind the basal bulb. Oöcytes in a single row, about four times wider than long (ca. 8×2 µm). Eggs large, markedly ovoid, smooth-shelled. Male unknown.
Lubanema decraemerae gen. n. sp. n. Female. A Oesophageal region, ventrolateral view B Cephalic end, internal view C Cephalic end, external view D Tail, ventral view E Egg F Reproductive system, ventrolateral view G Entire nematode, ventrolateral view.
Lubanema decraemerae gen. n. sp. n. Female. SEM images. A Cephalic end B Cephalic end, en face view C Mouth D Lateral ala, detail. Scale lines: A, D 0.05 mm, B 0.02 mm, C 0.005 mm
The Malagasian genus Passalidophila resembles Lubanema gen. n. by having both the body robust and fusiform, cervical cuticle unarmed and markedly annulated, a similar form of the cephalic end, the lateral alae extending from the level of the procorpus to the anus and the tail short (
Other monogonant hystrignathid genera with smooth cervical cuticle are Christiella Travassos & Kloss, 1957; Coronocephalus Cordeira, 1981; Glaber Travassos & Kloss, 1958; Longior Travassos & Kloss, 1958; and Vulcanonema Travassos & Kloss, 1958. All of these taxa can be differentiated from Lubanema gen. n. by having a well developed tail, from attenuate to subulate. Christiella and Longior females have a comparatively slender body vs. the notably more robust and fusiform body of Lubanema gen. n. Also, both genera present cylindrical procorpus more elongate than in Lubanema gen. n.
Coronocephalus bears prominent, digitiform oral papillae, instead of the shorter, less developed papillae of Lubanema gen. n. In the latter genus the procorpus meets directly the basal bulb, while Coronocephalus present an isthmus. Glaber differs from Lubanema gen. n. by having the base of the procorpus clavate, instead of the sub-cylindrical procorpus present in the new genus.
Vulcanonema presents the cephalic end consisting of a narrow cephalic annule separated of the head by a conical region. In opposition, Lubanema gen. n. have the first cephalic annule just after the head. Also, the procorpus of Vulcanonema is sub-cylindrical, with a basal dilation, absent in the present new genus.
Lubanema gen. n. shows morphological affinities with the Australian genera Anuronema Clark, 1978 and Sprentia Clark, 1978 by having the cuticle unarmed and strongly annulated, reduction of the isthmus and the tail. Moreover, the lateral alae of Anuronema extends from the oesophageal region to almost the level of the anus, similar to Lubanema gen. n. The new genus differs from both by its genital tract monodelphic-prodelphic vs. didelphic-amphidelphic, procorpus sub-cylindrical vs. claviform and development of the lateral alae, which in Lubanema gen. n. are very wide and with lobes in the margins at their terminal ends.The procorpus is widely amalgamated with the basal bulb in Anuronema and Sprentia, whereas Lubanema gen. n. presents a well defined constriction separating both structures. Anuronema has a total reduction of the tail appendage (
Carlosia Travassos & Kloss, 1957 also presents a reduction of the isthmus, a large, slightly inflated first cephalic annule and marked annule in the cervical region (
Didimus sp. (Coleoptera: Passalidae).
Hind gut, out of the caeca.
Katale, Kivu region, Democratic Republic of Congo.
Specific epithet dedicated to Prof. Dr. Wilfrieda Decraemer, from the Royal Belgian Institute of Natural Sciences. In appreciation for her help and support during the current research.
We are grateful to Dr. Marc de Meyer, curator of the Entomological Collection of the Royal Museum of Central Africa, Tervuren, Belgium for his kind permission to access material deposited in this collection. To Dr. Wilfrieda Decraemer, Dr. Yves Samyn, Dr. Marie-Lucie Susini and Dr. Alain Drumont for their assistance during the visit of the senior author to the Royal Belgian Institute of Natural Sciences (RBINS), Brussels. To Julian Cillis (RBINS) for technical help with SEM. To Dr. Coralie Martin for permission to access the nematode collection of the Museum of Natural History, Paris. To MSc. Yamir Arias, MSc. Eduardo Furrazola and Lic. Susett González (Instituto de Ecología y Sistemática) for their help with the micrographs. To Dr. Pedro Reyes-Castillo (Instituto de Ecología, Veracruz, México) for his accurate identification of the hosts. Our thanks to Dr. Luis F. de Armas and Dr. Pedro Herrera (Instituto de Ecología y Sistemática) for their review of the manuscript and the English language, respectively. The visit to collections in Belgium and France to access the material and SEM techniques was supported by the Belgian Development Cooperation through the Belgian Focal Point of the Global Taxonomy Initiative (GTI), 2010 and 2012 calls. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).