(C) 2013 Dmitry E. Shcherbakov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Shcherbakov DE (2013) Permian ancestors of Hymenoptera and Raphidioptera. ZooKeys 358: 45–67. doi: 10.3897/zookeys.358.6289
The origin of Hymenoptera remains controversial. Currently accepted hypotheses consider Hymenoptera as the first side branch of Holometabola or sister-group to Mecopteroidea. In contrast, fossils confirm the idea of Martynov that Hymenoptera are related to Megaloptera and Raphidioptera. Hymenoptera have descended along with Raphidioptera from the earliest Megaloptera, the Permian Parasialidae. A related new family, minute Nanosialidae from the Permian of Russia is supposedly ancestral to Raphidioptera. The fusion of the third ovipositor valvulae is shown to be not a synapomorphy of Neuropteroidea. Parasialids and nanosialids bridge the gap between megalopterans and snakeflies; all can be classified into a single order, Panmegaloptera nom. n., including a new suborder Siarapha for Nanosialidae. The earliest megalopterans and their descendants, Raphidioptera and Hymenoptera, have passed through a “miniaturization bottleneck, ” likely a common macroevolutionary mechanism.
Holometabola, Neuropteroidea, Hymenoptera, Raphidioptera, Permian, miniaturization
Wings are the very books in which the identities of many insect groups are written
(Conrad 2013)
The earliest and most primitive Hymenoptera are sawflies (Symphyta) from the Triassic subfamily Archexyelinae of the extant family Xyelidae (
This latter hypothesis was formulated as follows: “…Hymenoptera evolved from ancestors, somewhat intermediate between Megaloptera, Raphidioptera and Mecoptera” (
The Permian fossils discussed below partly bridge the gaps between megalopterans, snakeflies, and hymenopterans and confirm the neuropteroid nature of the latter.
Like many authors, especially those tracing taxa transforming through time, I follow traditional phylogenetics rather than cladistics and accept both ancestral and terminal taxa (in cladistics, paraphyletic and holophyletic)—these are just two stages in the taxon history, all paraphyletic taxa have once been holophyletic, and vice versa, the now holophyletic taxa may eventually turn paraphyletic. As
The material on the new taxa described herein is deposited at the Borissiak Paleontological Institute, Russian Academy of Sciences (PIN). The fossils were photographed using a Leica MZ9.5 stereomicroscope and Leica DFC420 camera, and imaged without coating with secondary electron (SE) and backscattered electron (BSE) detectors of a Tescan Vega XMU scanning electron microscope. Images were adjusted with Adobe Photoshop CS3. Line drawings were prepared with Inkscape 0.48.
The only Megaloptera known from the Palaeozoic are Permian Parasialidae (
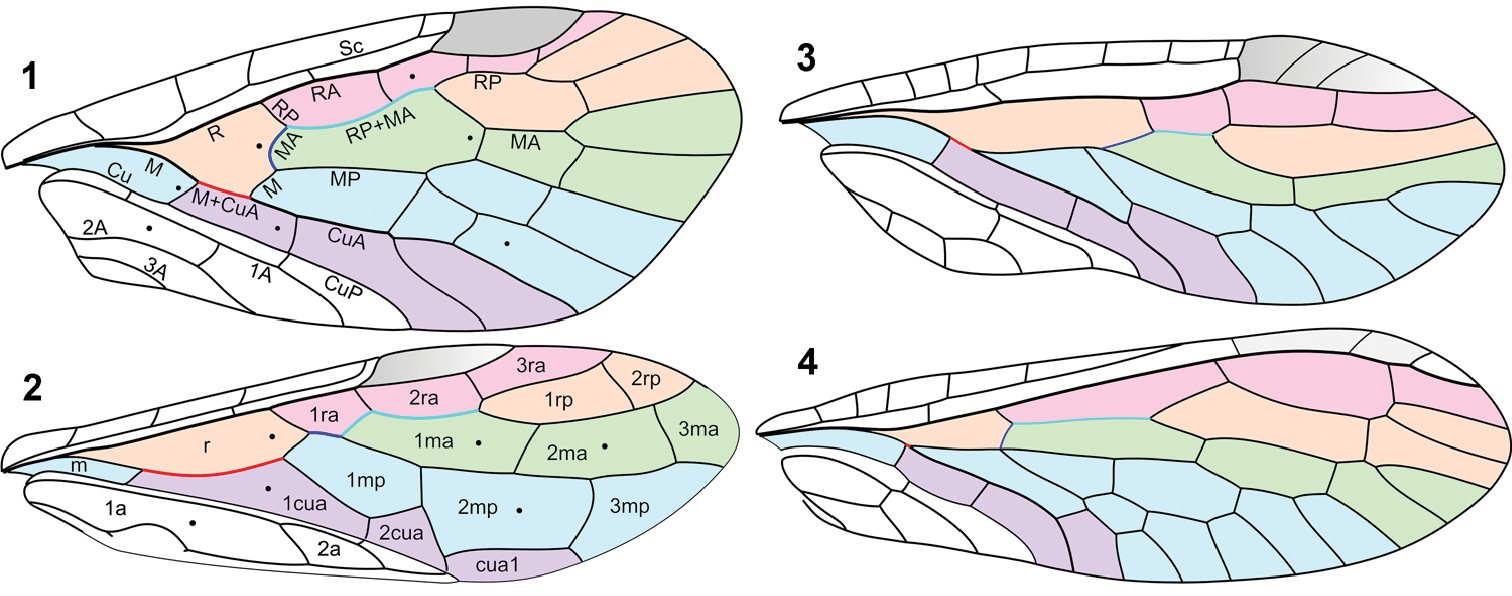
Forewing venation: 1 Parasialis latipennis (Parasialidae; veins named) 2 Xyelidae (based mainly on Triassic Asioxyela paurura and Madygenius primitivus; cells named) 3 Nanosialisponomarenkoi gen. et sp. n. (Nanosialidae) 4 Grimaldiraphidia cf. parvula (Mesoraphidiidae). Black dots, nygmata. Not to scale.
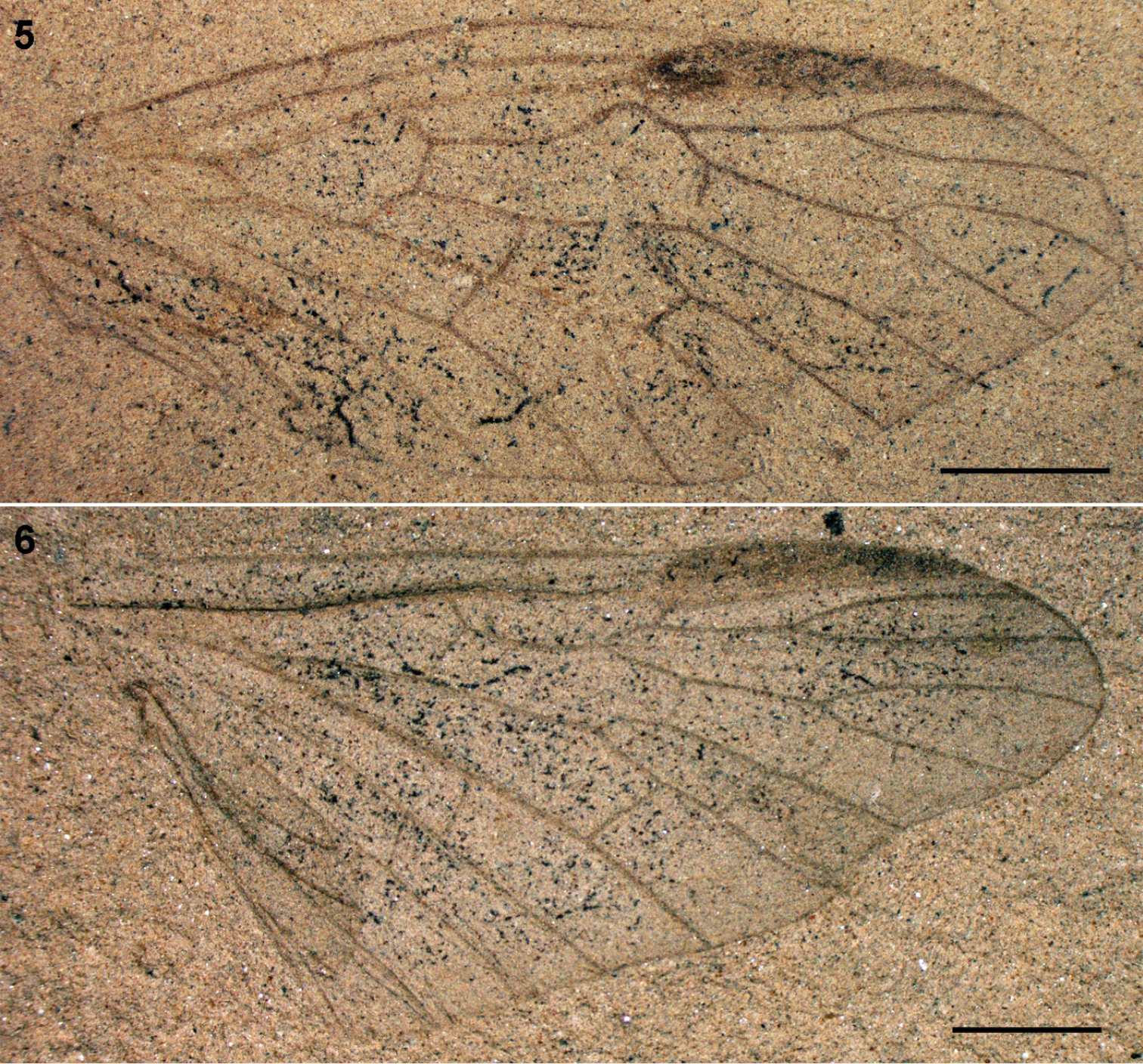
Parasialidae: 5 Parasialis dissedens, holotype forewing (mirror image) 6 gen. indet., hind wing PIN 3353/1073. Scale bars, 2 mm.
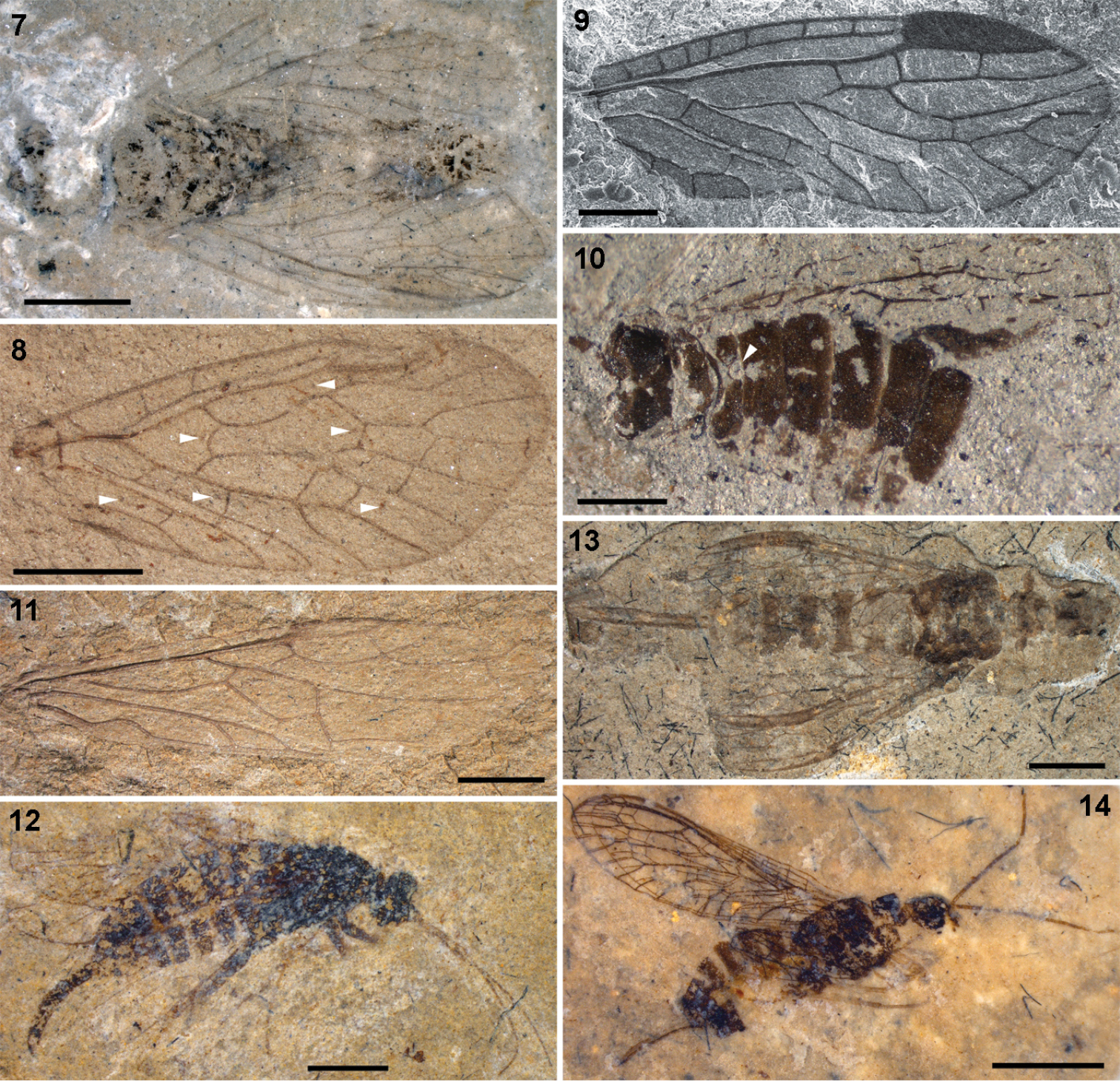
7–8 Parasialidae: 7 Parasialis rozhkovi, holotype male 8 Parasialis latipennis, holotype forewing (arrowheads, nygmata) 9–10 Nanosialidae: 9 Nanosialis ponomarenkoi gen. et sp. n., holotype forewing PIN 3840/2603A (SEM, SE; mirror image) 10 Nanosialis ?ponomarenkoi, body with superimposed wings PIN 3840/2604A (counterpart; arrowhead, incision of 1st abdominal tergite) 11–12 Xyelidae: 11 Asioxyela paurura (Archexyelinae) forewing PIN 2785/2491 12 female Xyelinae indet., PIN 2452/582 13–14 Mesoraphidiidae: 13 female gen. indet., PIN 2997/2662, halves of ovipositor separated 14 female Nanoraphidiini indet., PIN 2784/1127. Permian of Russia (7–10), Triassic of Madygen, Kyrgyzstan (11), Jurassic of Karatau, Kazakhstan (12–14). Scale bars, 2 mm (7, 8, 11–14), 500 µm (9, 10).
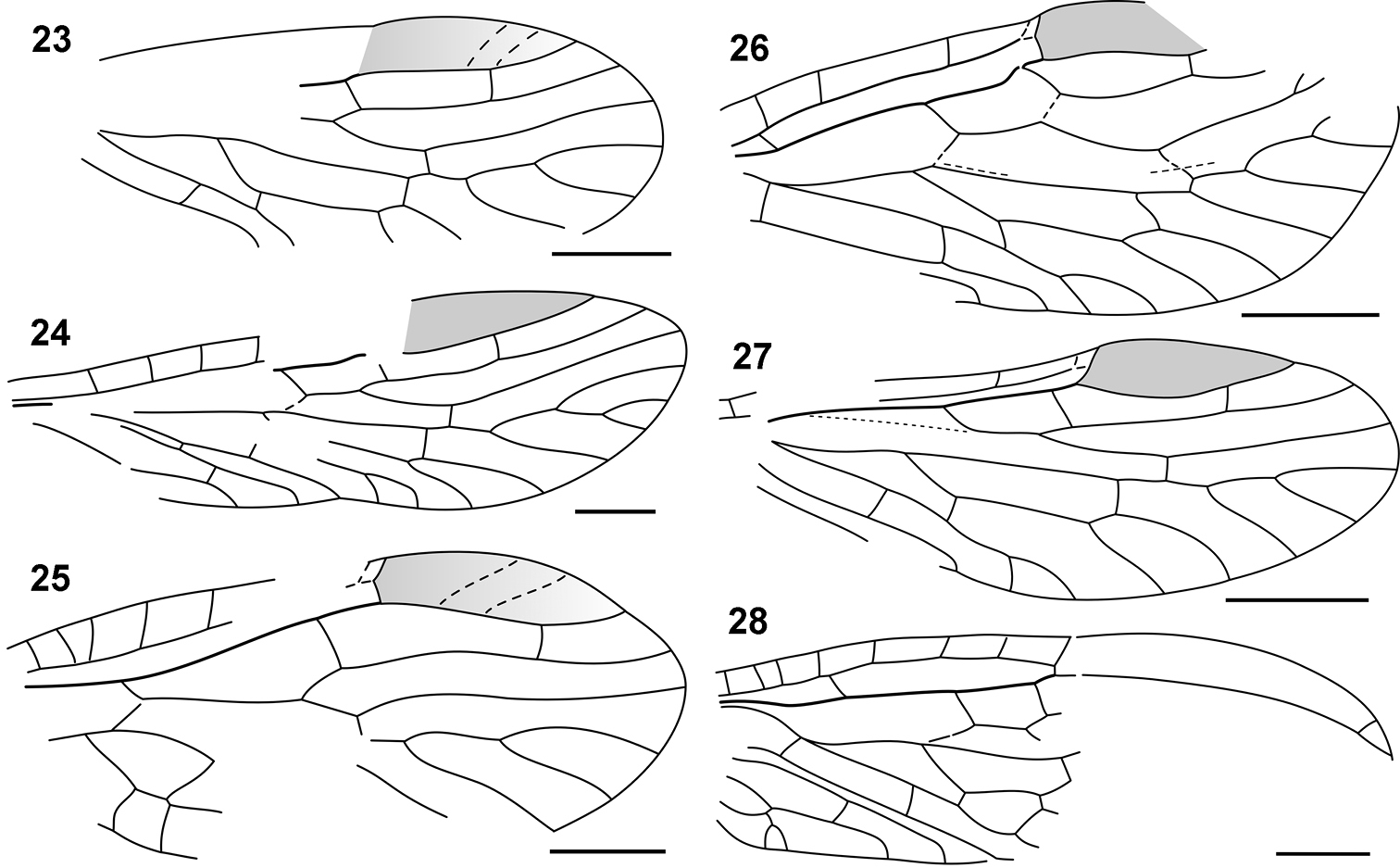
Nanosialidae: 15 Nanosialis ponomarenkoi gen. et sp. n., holotype forewing PIN 3840/2603A (mirror image) 16–17 Nanosialis ?ponomarenkoi PIN 3840/2604A: 16 body with superimposed wings (part) 17 thorax and base of abdomen (counterpart, SEM, BSE) 18 Nanosialis bashkuevi sp. n., holotype hind wing PIN 3840/2633 19 Lydasialis micheneri gen. et sp. n., holotype forewing PIN 3840/2602 20 Lydasialis ?micheneri, hind wing 3840/2601 21 Hymega rasnitsyni gen. et sp. n., holotype forewing PIN 3840/2600 22 Raphisialis martynovi gen. et sp. n., holotype forewing PIN 3840/2009 (mirror image). Scale bars, 500 µm.
The only known body fossil of Parasialidae (Fig. 7) is small, short-bodied, somewhat dorsoventrally depressed, with a large, markedly transverse head, and small, very short pronotum, short legs, and rather short abdomen consisting of well sclerotized segments. Its male genitalia are not unlike those of some megalopterans (and symphytans): without prominent genital capsule, with gonocoxites directed caudad and clavate gonostyles directed mediad (
Differences of Hymenoptera from Megaloptera in the forewing structure are all associated with functional two-wingedness acquired by hymenopterans (
The metanotum in Symphyta is equipped with cenchri, which are two blister-like lobes, each interlocking with a field of modified microtrichia (spinarea) on the underside of the forewing anal area in repose (
In the very rich Late Permian insect fauna from Isady, northern European Russia (Sukhona River, Vologda Region; Severodvinian, correlated to Wuchiapingian, ~258 million years ago;
Four suborders: Archimegaloptera, Megaloptera s.str., Siarapha subordo n., Raphidioptera.
As for the family.
Parasialidae Ponomarenko, 1977
Medium-sized insects (wings 6.5–17 mm long). Sc joining base of pterostigma; RP origin rather distal; RP and MA deeply forked; rp-ma crossvein present; MP once forked (much beyond R fork). Free base of MA developed as crossvein originating from M stem. Anal area at least 1/2 wing length. In forewing, RP+MA angled forwards at base of pterostigma, M and CuA forming long anastomosis (ending nearly level to R fork), M arched forwards distad of anastomosis, free base of MA just beyond M+CuA fork, and CuA forked. In hind wing (Fig. 6), M and CuA connected by very oblique arculus, CuA simple, and anal area variable (narrow in smaller species and broadened in larger species). Nygmata present. Veins and wing membrane evenly covered with short hairs. Short-bodied, somewhat dorsoventrally depressed. Head large, markedly transverse. Pronotum small, very short; pterothorax homonomous; legs short. Abdomen rather short, with short, well sclerotized segments. Male genitalia without prominent genital capsule, gonocoxites directed caudad, clavate gonostyles directed mediad.
Parasialis Ponomarenko, 1977 (Lower to ?Upper Permian of Eurasia; 4 species; Figs 1, 5, 7, 8), Sojanasialis Ponomarenko, 1977 (Middle Permian of Soyana; monobasic).
In the wing structure Parasialidae are similar to Sialidae, but in the latter the R and MP forks are more proximal in the forewing, and the nygmata are absent.
The hind wings of Parasialidae differ from the forewings in the basal mcu crossvein (arculus) developed instead of M+CuA anastomosis, and CuA unbranched. The hind wing anal area is expanded, with up to six unbranched anal veins in larger parasialids, but relatively small in the smallest parasialid, Parasialis rozhkovi (likewise in Sialidae the extent of the hind wing anal area depends on the body size and abdomen mass, so that e.g. in males of smaller species of Indosialis the fore and hind wings have anal areas of equal size).
As for the family.
Nanosialidae fam. n.
Nanosialis gen. n.
Minute insects (wings 2.5–4.5 mm long). Sc joining base of large pterostigma. RP origin distal; ir1 crossvein at base of pterostigma. RP and MA simple (sometimes MA with small fork); rp-ma crossvein absent; MP1 with 3–4, MP2 with 2 branches; CuA apparently simple or with terminal fork. MP fork level to, or just before R fork. M and CuA forming X-junction or very short anastomosis much before R fork (M stem arched towards CuA distad of junction). In forewing, RA sometimes with break at base of pterostigma. Free base of MA developed as crossvein originating from base of MP1 (in hind wing sometimes absent). Hind wing similar to forewing, with narrow anal area. Nygmata absent. Veins beset with strong setae; wing membrane bare. Body short. Pterothorax heteronomous: metanotum smaller and much shorter than mesonotum, without scutoscutellar sutures. Abdomen with short segments; 1st tergite with posteromedian notch.
Two subfamilies.
The body structure is known for the type genus only; the degree of pterothoracic heteronomy and first abdominal tergite division may vary among genera, like with modern genera of some neuropteran families.
In the structure of the proximal wing part (especially in the course of M, oblique direction and position of MA, shape of cells) Nanosialidae are similar to Mesoraphidiidae, but in the latter the pterothorax is always homonomous, anal area is much shorter, pterostigma is displaced distally, and RP+MA is usually more branched.
Among isolated wings of Nanosialidae, those having a shorter anal area, narrower costal area, and more delicate membrane are interpreted as the hind wings.
Pterostigma lanceolate to triangular, moderately elongate, dark. Anal area ~1/2 wing length, with two anal veins.
Nanosialis gen. n., Lydasialis gen. n., Hymega gen. n.
Nanosialis ponomarenkoi sp. n.
Distinct in the long 1mp cell, distal R fork, numerous Sc veinlets, and triangular pterostigma.
Type species and Nanosialis bashkuevi sp. n.
Named after Greek nanos (dwarf) and Sialis; gender feminine.
The apparent CuA (probable CuA2) is simple in Nanosialis ponomarenkoi forewing, but bears a terminal fork in Nanosialis bashkuevi hind wing. This may be an element of the fore/hind wing heteronomy, like in many mesoraphidiids (Mesoraphidia inaequalis, Mesoraphidia pterostigmalis, etc.).
http://zoobank.org/1E1AC81B-0F3B-45EE-8492-4A94F47DE977
http://species-id.net/wiki/Nanosialis_ponomarenkoi
Figs 3, 9, 10, 15–17, 23, 32, 33Forewing PIN 3840/2603A (part and counterpart).
Forewing 3.0 mm long, elongate (2.7:1), narrowly rounded posterior to MA apex; 7 Sc veinlets; pterostigma distally translucent, with 2 veinlets inside; RP base and crossveins ir1, ir2 transverse, not far separated; ir1 at base of pterostigma; mp cell single, rather long; MP1 3-branched, pectinate backwards; MP2 2-branched; CuA simple; anal area 1/2 wing length; two anal veins (2A+3A with 3 branches), delimiting one anal cell.
Adult PIN 3840/2604A (part and counterpart; head, prothorax, legs, right wings, and apex of abdomen beyond 6th segment, missing; left forewing and hind wing plaited and superimposed, as clearly seen in their pterostigmal areas). Tentatively assigned to the same species on account of similar size and reconstructed forewing venation (differing from the holotype in the larger mp cell and more separated ir1 and ir2 crossveins). Hind wing with well-developed pterostigma. Body as preserved ~2 mm long, somewhat depressed dorsoventrally. Mesoscutum 0.7 mm wide, transverse oval (1.9:1), deeply convex; narrow anterior zone laterally cut off by deep grooves; adjacent third steeply sloped, with semicircular median lobe delimited by arched lines; mesoscutellum low triangular, delimited by deep grooves, with posterior margin slightly arched; mesopostnotum rather narrow. Metascutum 0.55 mm wide, ×1.3 narrower and twice shorter than mesoscutum, subtriangular, more flat, with anterior margin concave, area of metascutellum slightly upturned (no scutoscutellar sutures), posterior margin subangulate; metapostnotum very narrow, arched. Abdomen ~0.7 mm wide, well sclerotized; 1st tergite short (especially medially), broadly sinuate anteriorly, with deep semicircular posterior notch nearly dividing it medially; 2–6th tergites longer, transverse (~2.5:1).
Named after the paleoentomologist Alexander Ponomarenko.
Nanosialidae venation: 23 Nanosialis ?ponomarenkoi, forewing, reconstructed 24 Nanosialis bashkuevi sp. n., hind wing 25 Hymega rasnitsyni gen. et sp. n., forewing PIN 3840/2604A, reconstructed 26 Lydasialis micheneri gen. et sp. n., forewing 27 Lydasialis ?micheneri, hind wing PIN 3840/2601 28 Raphisialis martynovi gen. et sp. n., forewing, reconstructed. Scale bars, 500 µm.
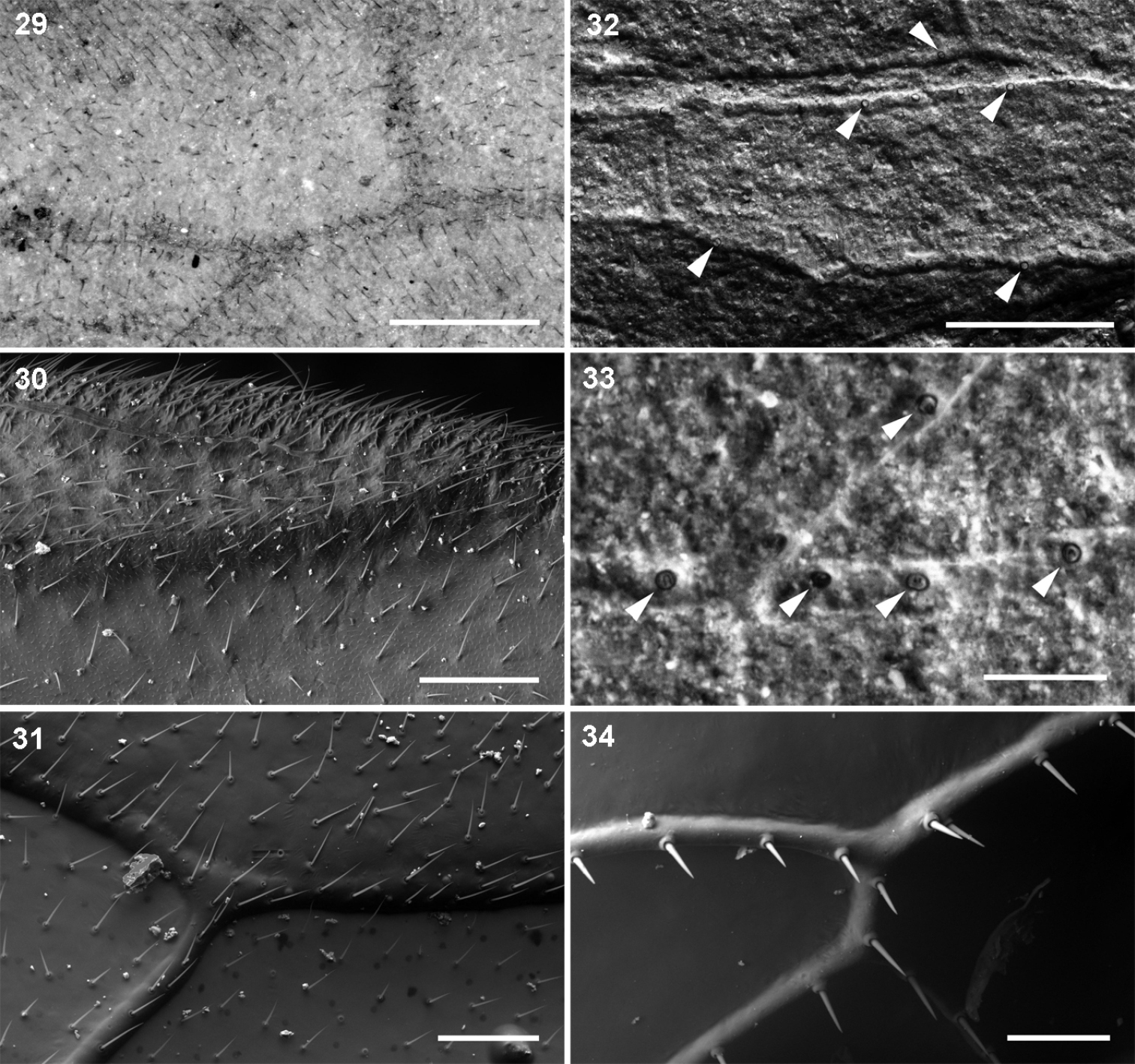
Hairs and setae on forewings: 29–31 uniform hair cover on veins and membrane: 29 Parasialidae, Parasialis rozhkovi holotype 30 Sialidae, Sialis sp. 31 Xyelidae, Pleroneura coniferarum 32–34 strong setae on veins only: 32–33 Nanosialidae, Nanosialisponomarenkoi gen. et sp. n., holotype (arrowheads, bases of strong setae on veins) 34 Raphidiidae, Raphidia sp., strong erect setae on veins. Recent (30, 31, 34); SEM, BSE (30–34). Scale bars, 200 µm (29, 30, 32), 100 µm (31, 34), 50 µm (33).
http://zoobank.org/98E8549D-8C7F-4AF4-A1C9-FEF4892CC044
http://species-id.net/wiki/Nanosialis_bashkuevi
Figs 18, 24Hind wing PIN 3840/2633 (part and counterpart), interpreted as a hind wing due to the delicate, crimpled wing membrane.
Distinct from the type species in the larger size and more abundant vein branching.
Hind wing ~4.4 mm long, elongate (~3.2:1), acutely rounded at MA apex; 5? Sc veinlets (four preserved); pterostigma rather evenly suffused, without distinct veinlets; MP1 4-branched; CuA with terminal fork.
Named after the paleoentomologist Alexei Bashkuev.
Lydasialis micheneri sp. n.
Very long 1mp cell; few Sc veinlets; in forewing, RA with a break at nodus, and RP section distal to RP+MA desclerotized.
Monobasic.
Named after Lyda and Sialis; gender feminine.
http://zoobank.org/91351955-95EF-402F-8EA2-8A42A49BCFE9
http://species-id.net/wiki/Lydasialis_micheneri
Figs 19, 20, 26, 27Forewing PIN 3840/2602 (the base and most of the anal area missing).
Forewing ~2.8 mm long, broad (~2.4:1), obliquely rounded apically; costal space moderately narrow, with 3 Sc veinlets; pterostigma wide; RA with break at nodus; R fork rather distal; RP base and crossveins ir1, im1, im2, mcu oblique; ir1 at base of pterostigma; RP section between RP+MA and ir1 desclerotized; MA with terminal fork; 1mp cell single, very long; MP1 4-branched, pectinate backwards; MP2 2-branched; CuA with terminal fork beyond mcu.
Hind wing PIN 3840/2601 (base and most of anal area missing, 1A tucked under), interpreted as a hind wing due to the delicate, finely longitudinally wrinkled wing membrane. Tentatively assigned to the same species on account of similar size, few Sc veinlets, a wide pterostigma, and very long 1mp cell. Hind wing ~2.6 mm long, elongate (~2.8:1), narrowly rounded apically; costal space narrower, probably with 3 Sc veinlets; pterostigma wider, rather evenly suffused, without distinct veinlets; no break on RA; only ir1 somewhat oblique; MA simple; MP1 3-branched; MP2 2-branched; CuA with terminal fork.
Named after the hymenopterist Charles D. Michener.
Hymega rasnitsyni sp. n.
Forewing with R fork and ir1 crossvein proximal, costal space wide, 1mp cell very short (apparently more than one mp cell), and two mcu crossveins.
Monobasic.
Named after Hymenoptera and Megaloptera; gender feminine.
http://zoobank.org/0C81D97C-5273-437E-943A-BCC84D2870B6
http://species-id.net/wiki/Hymega_rasnitsyni
Figs 21, 25Forewing PIN 3840/2600 (part and counterpart; costal and mp areas torn off and overturned, base and cubitoanal area missing).
Forewing ~3.4 mm long, broad (~2.5:1); costal space wide, with more than 5 Sc veinlets; pterostigma large, distally translucent, with 2 veinlets inside; R fork proximal; RP base short; ir1 oblique, distant from pterostigma; MP1 4-branched; 1mp cell very short; two crossveins mcu.
Named after the paleoentomologist Alexander Rasnitsyn.
Raphisialis gen. n.
Pterostigma sickle-shaped, very elongate. Anal area shorter than 1/2 wing length, with three anal veins.
Monobasic.
Raphisialis martynovi sp. n.
Forewing with cell 1mp short (apparently several mp cells) and two mcu crossveins.
Monobasic.
Named after Raphidia and Sialis; gender feminine.
http://zoobank.org/F829E139-1436-4496-8F46-D465BA24BFF3
http://species-id.net/wiki/Raphisialis_martynovi
Figs 22, 28Forewing PIN 3840/2009 (part and counterpart; anal area and incomplete distal part tucked under).
Forewing ~3.9 mm long, elongate (~3.2:1); costal space moderately narrow, with 7 Sc veinlets; pterostigma long, narrow, sickle-shaped, unpigmented; RA with small fork beyond it; R fork distal; ir1 at base of pterostigma; 1mp cell short (apparently at least three mp cells); two crossveins mcu; anal area shorter than 1/2 wing length; three anal veins (3A with terminal fork), delimiting two anal cells.
Named after the paleoentomologist Andrey Martynov.
I consider Nanosialidae derivatives of Parasialidae, not vice versa, because of simplified venation, heteronomous pterothorax, the lack of nygmata (so far as known, never restored after being lost), and the M stem arched towards CuA after short M+CuA junction (interpreted as remnants of a longer anastomosis like in parasialids). Lydasialis shows a pronounced transverse flexion at the base of the forewing pterostigma, RA having a break (articulation) there (Figs 19, 26), the condition broadly similar to that shared by Parasialidae and Symphyta, which have RP+MA angled there instead. The only known nanosialid body fossil (tentatively assigned to Nanosialis ponomarenkoi; Figs 10, 16, 17) resembles parasialids in having a short body and the hind wings retaining the pterostigma, butits metanotum is narrower and much shorter than mesonotum and lacks scutoscutellar sutures, and the first tergite of abdomen is divided medially.
Nanosialids share several characters with hymenopterans: RP+MA two-branched (occasionally MA with short fork, e.g. in aberrant specimens); rp-ma crossvein absent (restored in some Xyelidae: Triassic Madygenius and recent Macroxyela –
Nanosialidae are distinct from Parasialidae + Hymenoptera and similar to Mesoraphidiidae (Jurassic–Cretaceous; Fig. 4) and other primitive snakeflies in the structure of the proximal wing part (especially in the course of M, position and oblique direction of MA), MP forked proximally (more branched than RP+MA, whereas in hymenopterans MP is simple and RP+MA forked), shape and number of cells, absence of nygmata, and also in the short, stiff, erect setae along veins, and bare wing membrane. Secondary shortening of the M+CuA anastomosis in Nanosialidae and Raphidioptera is associated with shortening of the M stem itself, bringing the MP fork close to MA base; the evidence of a formerly longer anastomosis is the M stem arched close to CuA beyond the M+CuA in Nanosialidae.
The genus Raphisialis (Raphisialinae; Figs 22, 28) is additionally similar to mesoraphidiids in the rather short anal area and long, sickle-shaped pterostigma (unpigmented, as in several Mesoraphidia spp.). This incompletely known genus is not separated at the family level because the gap between it and Nanosialis is partly filled with Hymega (Figs 21, 25) having ashort 1mp cell (probably several mp cells) and two mcu crossveins like in Raphisialis.
Despite these similarities, Nanosialidae are distinct from Raphidioptera in the longer anal area, more proximal position of pterostigma, less branched RP+MA, and, most importantly, the heteronomous pterothorax, so they cannot be assigned to this order as currently understood. This Permian family is likely to be ancestral to snakeflies, which are still unknown from the Triassic. The striking resemblance between Nanosialidae and Mesoraphidiidae casts doubt on the primitiveness of Jurassic Priscaenigmatidae, considered to be the most basal Raphidioptera (
The aforementioned venation features shared by hymenopterans and nanosialids but not parasialids seem to be associated with miniaturization and likely are homoplasies appearing in closely related lineages, i.e. “underlying synapomorphies” (
Minute nanosialids, with their veins beset with stiff, erect setae and the wing membrane bare, both like in snakeflies (Figs 32–34), were surely terrestrial. In Parasialidae, the wing membrane and veins are densely covered with short decumbent hairs (Fig. 29). Such a uniform hair cover occurs on the wings of both amphibiotic Megaloptera and terrestrial Hymenoptera (Figs 30, 31) and gives no clue to the life mode of parasialids.
Female genitalia of nanosialids and parasialids are unknown. If Parasialidae were amphibiotic, like the present-day Megaloptera, their ovipositor is likely to have been more or less reduced, suggesting a subsequent restoration of ovipositor in Hymenoptera, Raphidioptera, and possibly in Nanosialidae.
The ovipositor, transformed into a very long, unpaired organ (1st valvulae fused, 3rd valvulae fused dorsally) in living snakeflies, was much more generalized in Mesozoic Mesoraphidiidae, which are sometimes preserved with the left and right halves of the ovipositor separated (in Siboptera fornicata (Ren, 1994) and various mesoraphidiids from Karatau, Fig. 13). Therefore, the fusion of the third ovipositor valvulae, previously considered to be a synapomorphy of Neuropteroidea (
Small Jurassic mesoraphidiids have the ovipositor much shorter than the abdomen, in lateral aspect relatively wide and downcurved (like in some Xyela spp. – Xyela is from Greek xyēlē, curved knife). These small Jurassic snakeflies are short-bodied, with the subquadrate head and short pronotum and abdomen, and look remarkably similar to xyelid sawflies (Figs 12, 14). There are some other notable similarities between snakefies and hymenopterans, including the wasp-like colour pattern in some snakeflies, and the late pupa (in fact, pharate adult) capable of locomotion and with functional mandibles in Xyelidae (
Are Parasialidae, the oldest known megalopterans, also the most primitive ones? Megaloptera are still unknown from the Triassic: the only Triassic find ascribed to Megaloptera (
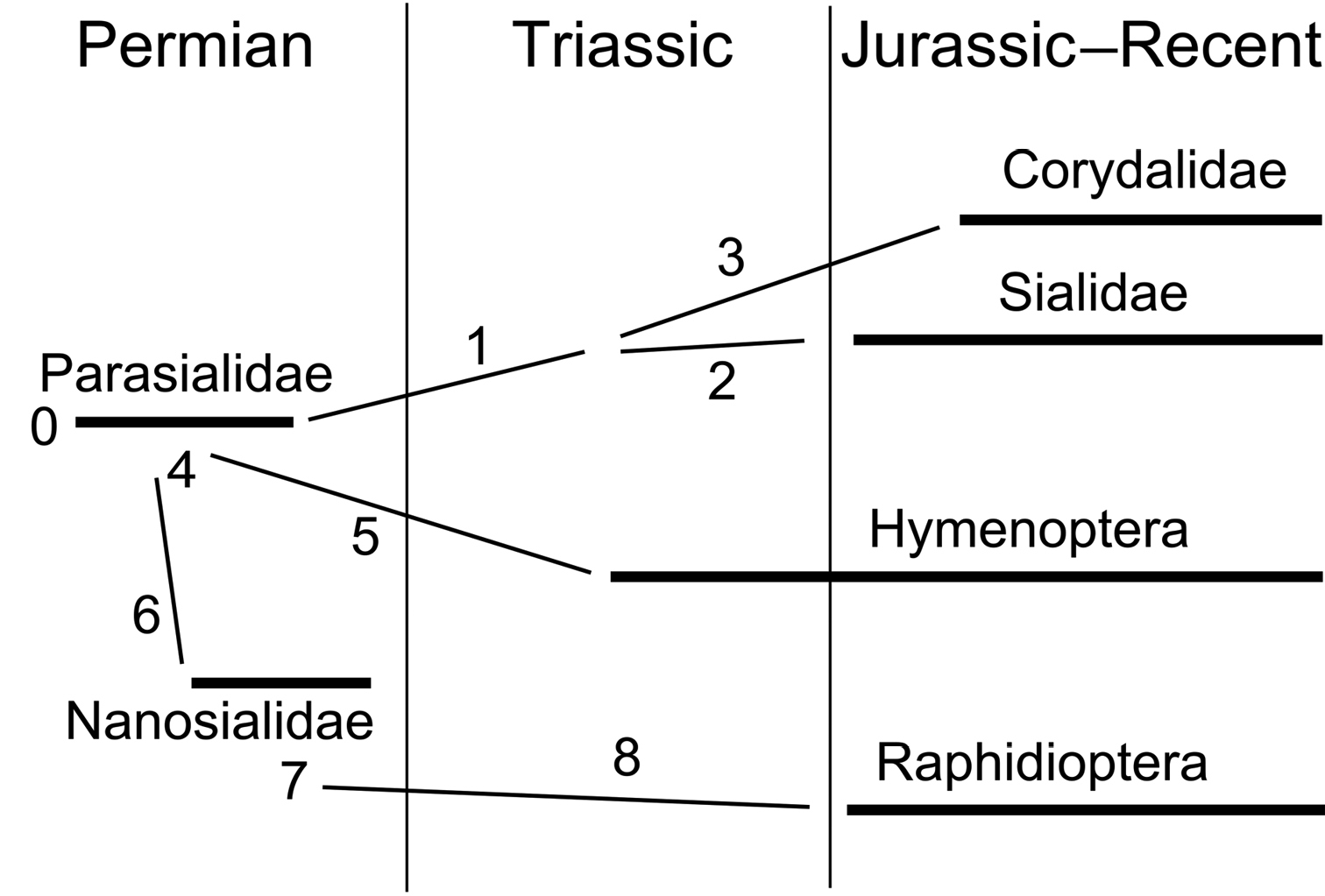
Phylogenetic diagram of Panmegaloptera and Hymenoptera (for characters see Appendix).
Parasialidae and Nanosialidae bridge the gap between Megaloptera and Raphidioptera and demonstrate that these two orders can be treated as one. Such was the original concept of Megaloptera (
The larvae of the most basal hymenopterans supposedly developed in staminate cones of gymnosperms, and their females used their long ovipositors to lay eggs into the cones, like many xyelid sawflies still do (
Parasialids and nanosialids are found only in the richest Permian fossil insect sites of Eurasia, being rare in all of them: Chekarda (Lower Permian, Urals)—one Parasialis rozhkovi specimen per ~7, 000 total insects; Tyulkino (Lower Permian, Urals)—one Parasialis specimen per ~550 insects; Soyana (Middle Permian, northern European Russia)—14 Parasialidae specimens (3 species in 2 genera) per ~4, 000 insects; Bor Tolgoy (Upper? Permian, southern Mongolia)—two Parasialis ovata specimens (
Miniaturization can be an important source of morphological novelty, in some cases resulting in the origin of higher taxa (
After my paper was submitted, an article was published by
I am grateful to Alexei Bashkuev for preparing these interesting fossils and bringing them to my attention, to Roman Rakitov for taking SEM micrographs and helping edit the manuscript, and to two anonymous reviewers for constructive comments. The study was partially supported by RFBR, research project No. 13-04-01839. Open access to this paper was supported by the Encyclopedia of Life (EOL) Open Access Support Project (EOASP).
List of characters:
0. Characters of Parasialidae:
wings homonomous; fore and hind wing not coupled in flight;
pterostigma well developed, oval to lanceolate;
R fork rather distal; RP base crossvein-like;
MP forked distally, with 2 branches;
free MA base crossvein-like, connecting RP and M near bases;
RP+MA twice forked, dichotomous (4-branched);
one or two rp-ma crossveins;
nygmata present;
membrane and veins uniformly covered with short hairs;
forewing:
RP+MA angled at base of pterostigma (pronounced nodal flexion);
M+CuA anastomosis moderately long (its apex about level of R fork);
CuA once forked;
Cu base continued with CuP;
anal area reaching ~1/2 wing length;
at least three free anal veins;
hind wing anal area enlarged (in larger species) or not;
pterothorax homonomous;
presumably also: forewing-metanotum microtrichial coupling.
1. Synapomorphies of Sialidae + Corydalidae:
forewing R and MP forks shifted proximad;
ovipositor reduced.
2. Apomorphy of Sialidae:
nygmata lost.
3. Apomorphies of Corydalidae:
RP+MA pectinate;
forewing:
M+CuA anastomosis replaced with crossvein;
Cu base continued with CuA.
4. Homoplasies (underlying synapomorphies) of Hymenoptera and Nanosialidae + Raphidioptera:
forewing:
R fork shifted distad; crossvein ir1 near base of pterostigma;
RP+MA once forked;
rp-ma crossvein absent;
two free anal veins (2A and 3A fused at least proximally);
pterothorax heteronomous;
1st abdominal tergite with deep posteromedian notch.
5. Apomorphies of Hymenoptera:
wings heteronomous; hind wing without pterostigma, coupled to forewing in flight;
M+CuA anastomosis very long;
forewing:
free MA base replaced with RP+M anastomosis;
RP+MA fork shifted distad (to near ir2);
MP simple;
CuA1 shifted posteriad, CuA2 transverse, CuA fork narrow;
two cubitoanal braces between zigzagged CuA and anal veins: crossveins cua-cup and cup-ashifted distad and aligned across reduced distal CuP; CuA2 joining apically fused 1A and 2A+3A;
pterothorax markedly heteronomous;
microtrichial areas of metanotum modified into cenchri.
6. Synapomorphies of Nanosialidae + Raphidioptera:
pterostigma enlarged;
nygmata absent;
membrane bare; short, stiff, erect setae along veins;
M+CuA anastomosis much shortened or replaced with x-junction;
MP fork shifted proximad, MP with 5–6 branches (more branched than RP+MA);
free MA base connecting RP near base and MP near fork;
CuA1 anastomosing to MP2;
short free 3A sometimes restored (Raphisialinae, Inocelliidae);
hind wing anal area always small.
7. Synapomorphies of Raphisialinae + Raphidioptera:
pterostigma sickle-shaped, elongate;
anal area somewhat shortened.
8. Apomorphies of Raphidioptera:
RP+MA usually twice forked, dichotomous (no less than 4, rarely 3 branches);
at least one rp-ma crossvein;
pterostigma shifted distad;
anal area much shortened;
pterothorax always homonomous.