(C) 2013 Eli M. Sarnat. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The intent of this paper is to facilitate future research of the Solomon Islands ant fauna by providing the first comprehensively researched species inventory in over 75 years. The species list presented here includes the names of all ant species recorded from the islands that are available in the literature together with specimen records from several museum collections and new records from our 2008 Makira field expedition. All the names of described species presented are valid in accordance with the most recent Formicidae classification. In total, the checklist is composed of 237 species and subspecies (including 30 morphospecies) in 59 genera representing nine subfamilies. We report that the recent field expedition added 67 new species records to Makira and 28 new species records to the Solomon Islands. Our research recovered species occurrence records for 32 individual islands and five island groups. The five islands with the highest number of recorded species are: Makira (142 spp.), Guadalcanal (107 spp.), Malaita (70 spp.), Santa Isabel (68 spp.), and Rennell (66 spp.). Based on our results, we discuss the taxonomic composition of the archipelago’s ant fauna, which islands are most in need of additional sampling, and the importance of establishing biodiversity baselines before environmental threats such as the invasive ant Wasmannia auropunctata cause irrevocable harm to the native biodiversity.
Biogeography, checklist, Makira Island, Pacific Islands, Solomon Islands, species distributions, taxonomy, Formicidae
The intent of this paper is to facilitate future research of the Solomon Island ant fauna and that of the larger Pacific Island region by providing the first comprehensively researched species list in over 75 years (
Faunal lists are also necessary for recognizing biodiversity blind spots and identifying which regions and islands are most in need of additional sampling. Increasing environmental threats such as deforestation, mining, agriculture and the spread of invasive species give urgency to surveying these poorly sampled regions. In order to assess how these threats affect native biodiversity, it is important to establish baseline inventories before local populations and endemic species are driven extinct.
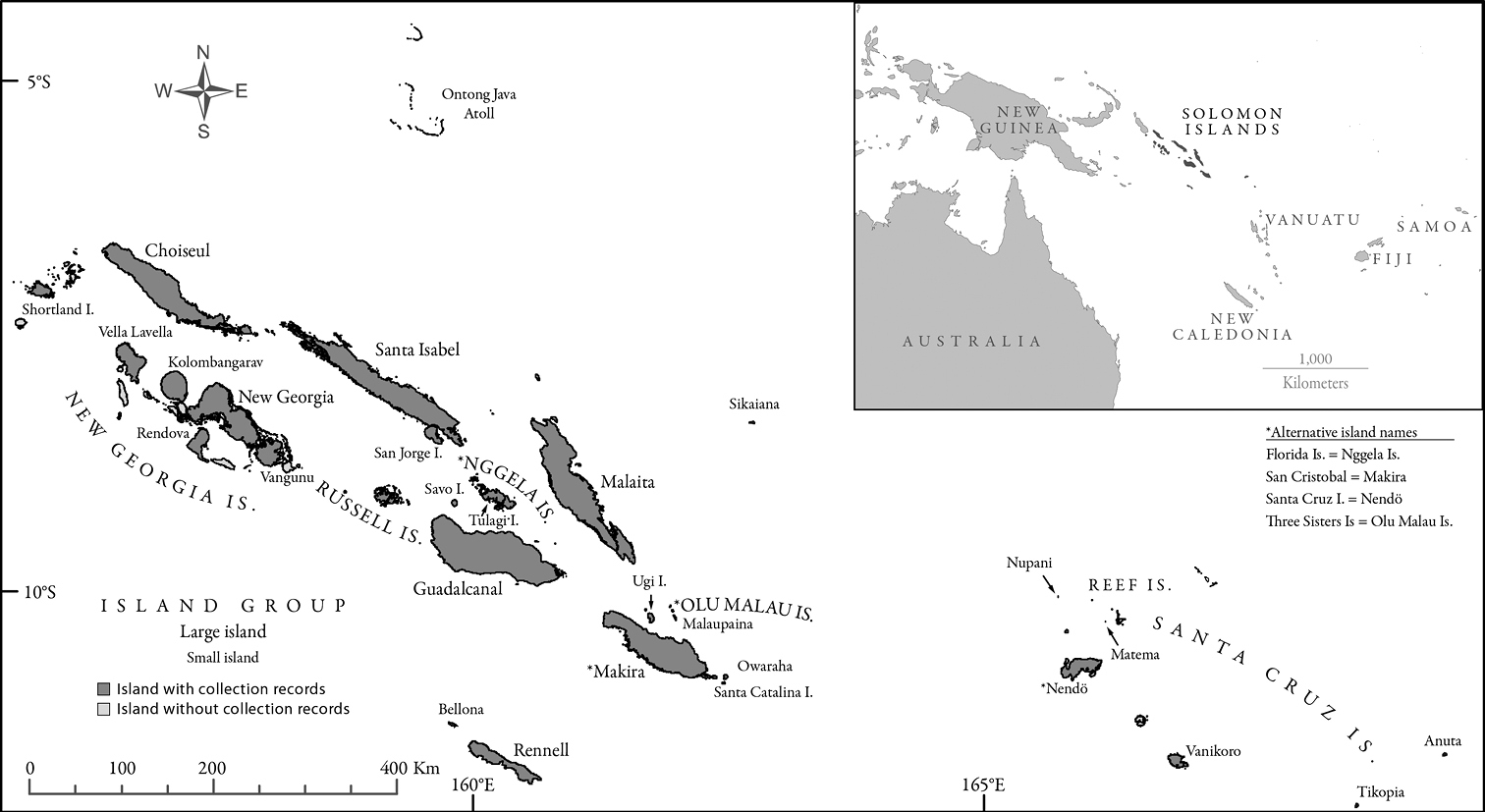
The Solomon Islands is a nation in the Southwest Pacific that is composed of seven large islands, a dozen mid-sized islands and over a thousand smaller islands (Figure 1). These islands, which comprise a total land area of 27, 556 km2, are situated between the latitudes 5° and 13°S, and longitudes 155° and 169°E. The major central islands include the Shortlands, Choiseul, the New Georgias, Santa Isabel, the Russells, Guadalcanal, the Nggelas (Floridas), Malaita, Makira (San Cristóbal), and Olu Malau (Three Sisters). Rennell and Bellona are southern outlying islands situated along the northern margin of the Coral Sea Basin. Northern outlying islands include Sikaiana and the Ontong Java Atoll, which are on the southwestern edge of the Ontong Java Plateau. The eastern outlying islands of the Santa Cruz group are politically part of the Solomon Islands, but are geologically linked to the islands of Vanuatu (
Map of the Solomon Islands. The map presents all islands and island groups for which ant species were recorded. Each island/island group from which ant species are known is labeled with the geographic name and filled darker grey. Islands for which no ant records appear in the literature are unlabeled and filled with lighter grey. Relevant historic island names from the colonial era are presented with their contemporary counterparts.
The Solomons consist of a double chain of islands separating the Pacific Plate to the north from the Australian Plate to the south (
Bougainville, which lies to the west, belongs politically to Papua New Guinea but is geographically part of the Solomon Islands. The next closest neighbor nation is Vanuatu, which lies southeast of the main archipelago and nearly due south of the Santa Cruz Is.
The climate of the Solomon Islands is characterized as humid with a mean temperature of 27 °C (80 °F) and relatively few fluctuations of temperature or weather. The cooler and drier part of the year occurs from June through August, and the warmer and wetter season occurs from September through May. The annual rainfall is approximately 3050 mm (120 in).
The first ants described from the Solomon Islands were authored by
H.
W.M. Wheeler’s first contribution to the Solomon Island ant fauna was his description of Opisthopsis manni based on specimens collected by Mann from Malaupaina (
William Brown treated many Solomon Island taxa in his revisions (
Research on economically important ants involved in coconut production was an active field in the Solomons from the 1930’s through the 1960’s (
Philip J.M. Greenslade has arguably collected more thoroughly across the Solomons than anyone since Mann. Greenslade published seven papers between 1964 and 1988 based on fieldwork he conducted in the Solomons (
E.O. Wilson included many species from the Solomon Islands during his revisionary work of the Melanesian ant fauna, including species currently in the genera Amblyopone, Leptogenys, Platythyrea and Stigmatomma (1958a); Ponera, Cryptopone, Hypoponera, Pachycondyla and Rhytidoponera (1958b); Anochetus and Odontomachus (1959c); and Cerapachys (1959d).
Wilson’s (1962) paper on the ants of Rennell and Bellona Islands examined specimens collected from three sources: a Danish Expedition (
Robert Taylor, in addition to describing Problomyrmex salomonis (
In order to compile a comprehensive and accurate inventory of ant species recorded from the Solomon Islands, we researched taxonomic names that were associated with the region in the literature. We reviewed the names of all taxa that were originally described from Solomons, reviewed specimen records from Antweb.org, reviewed the species list for the Solomon Islands presented on Antwiki <http://www.antwiki.org/Solomon_Islands> , searched the Formis database (
Names were eliminated where we found evidence of misidentification or geographic inconsistencies such as geographic names erroneously considered as belonging to the Solomon Islands. We also reconciled situations in which different authors may have referred to the same species by different valid names. For example, there were instances in which we believe one author referred to a taxon using its specific name, and another author referred to the same taxon by its infraspecific name. In cases such as these, and in the absence of additional evidence, we use the infraspecific name. We also note which other names we interpret as referring to the same taxon, and which publications those names occur in.
In addition to the valid names, we also use morphospecies codes to refer to presumptive species that either we or previous authors were unable to determine. The morphospecies code is ‘BP’ (The administrative code for the Solomon Islands) followed and a unique two-digit number (e.g. ‘Camponotus sp. BP01’).
Bougainville is considered to belong geographically but not politically to the Solomons. As such we do not include species recorded from Bougainville that have not also been reported from at least one of islands to its east.
In addition to basing the present study on the aforementioned published records, we also include records from our own recent survey of the Solomons. Three of the authors (E.P.E., E.M.S., J.F.) collected ants in the Solomons from 30 January to 9 February, 2008. Aside from a few collections made on Mt. Austen (Guadalcanal I.), the survey primarily focused on Makira Island (formerly San Cristóbal) where we trekked and collected from Kirakira on the coast to the interior village of Maraone, reaching a maximum elevation of 912 m. Survey methods included hand collection and litter sifting along standardized transects using Winkler extraction bags. All specimens were collected into and stored in 95% ethanol. Pinned specimens were identified using the available literature and compared to type and determined material at the United States National Museum of Natural History (USNM), Washington D.C., USA, and the Museum of Comparative Zoology (MCZC), Cambridge, Massachusetts, USA. These two collections are the primary depositories for Mann’s type material and also include type material designated by W.L. Brown, W.M. Wheeler and E.O. Wilson. We include the species records from this survey with the literature records.
Occurrence data of ant species on individual islands and island groups were compiled from the relevant literature. More detailed data with literature references for each species-island occurrence is available from the authors upon request. A map of the Solomon Islands (Figure 1) is also presented in which the name of every island and island group from which ant species have been recorded is labeled. The constituent islands comprising the listed island groups are presented in Table 1. In addition to including all taxa from Appendixes 1 and 2, we also include taxa from the 2008 survey of Makira that remain undetermined but might belong to previously described species. Inclusion of these additional taxa may weakly bias the observed species richness of Makira towards a higher value, but exclusion of these taxa would cause an even greater bias towards a lower value.
Island groups and their constituent islands.
| Island Group | Islands |
|---|---|
| Santa Cruz Is. | Anuta, Nendö (Santa Cruz), Nupani, Reef Is., Tikopia, Vanikoro |
| Olu Malau Is. (Three Sisters) | Malaupaina |
| Nggela Is. (Florida Is.) | Nggela Sule (Florida), Tulagi |
| New Georgia Is. | Kolombangarav, New Georgia, Rendova, Vangunu, Vella Lavella |
| Reef Is. | Matema |
Number of presumptive native species from Appendix 1 for each genus (arranged from greatest to least). Diverse genera with well-established subgenera are nested under the genus name and the species number of each is presented in parentheses.
| Genus (Subgenus) | Native spp. | %Total |
|---|---|---|
| Polyrhachis | 30 | 14 |
| Polyrhachis (Myrma) | (7) | – |
| Polyrhachis (Cyrtomyrma) | (5) | – |
| Polyrhachis (Chariomyrma) | (4) | – |
| Polyrhachis (Hedomyrma) | (4) | – |
| Polyrhachis (Myrmhopla) | (3) | – |
| Polyrhachis (Myrmatopa) | (2) | – |
| Polyrhachis (Myrmothrinax) | (1) | – |
| Polyrhachis (Hirtomyrma) | (1) | – |
| Pheidole | 15 | 7 |
| Camponotus | 14 | 7 |
| Camponotus (Colobopsis) | (5) | – |
| Tetramorium | 11 | 5 |
| Vollenhovia | 11 | 5 |
| Pachycondyla | 9 | 4 |
| Strumigenys | 9 | 4 |
| Crematogaster | 7 | 3 |
| Crematogaster (Crematogaster) | (5) | – |
| Crematogaster (Orthocrema) | (2) | – |
| Gnamptogenys | 6 | 3 |
| Cryptopone | 5 | 2 |
| Hypoponera | 5 | 2 |
| Myrmecina | 5 | 2 |
| Nylanderia | 5 | 2 |
| Ponera | 5 | 2 |
| Acropyga | 4 | 2 |
| Cerapachys | 4 | 2 |
| Eurhopalothrix | 4 | 2 |
| Leptogenys | 4 | 2 |
| Myopias | 4 | 2 |
| Odontomachus | 4 | 2 |
| Anochetus | 3 | 1 |
| Rogeria | 3 | 1 |
| Adelomyrmex | 2 | 1 |
| Arnoldius | 2 | 1 |
| Cardiocondyla | 2 | 1 |
| Carebara | 2 | 1 |
| Colobostruma | 2 | 1 |
| Iridomyrmex | 2 | 1 |
| Podomyrma | 2 | 1 |
| Prionopelta | 2 | 1 |
| Pristomyrmex | 2 | 1 |
| Proceratium | 2 | 1 |
| Rhytidoponera | 2 | 1 |
| Solenopsis | 2 | 1 |
| Stigmatomma | 2 | 1 |
| Turneria | 2 | 1 |
| Amblyopone | 1 | <1 |
| Anonychomyrma | 1 | <1 |
| Discothyrea | 1 | <1 |
| Lordomyrma | 1 | <1 |
| Monomorium | 1 | <1 |
| Myopopone | 1 | <1 |
| Oecophylla | 1 | <1 |
| Opisthopsis | 1 | <1 |
| Paraparatrechina | 1 | <1 |
| Philidris | 1 | <1 |
| Platythyrea | 1 | <1 |
| Probolomyrmex | 1 | <1 |
| Stereomyrmex | 1 | <1 |
| Tapinoma | 1 | <1 |
| Tetraponera | 1 | <1 |
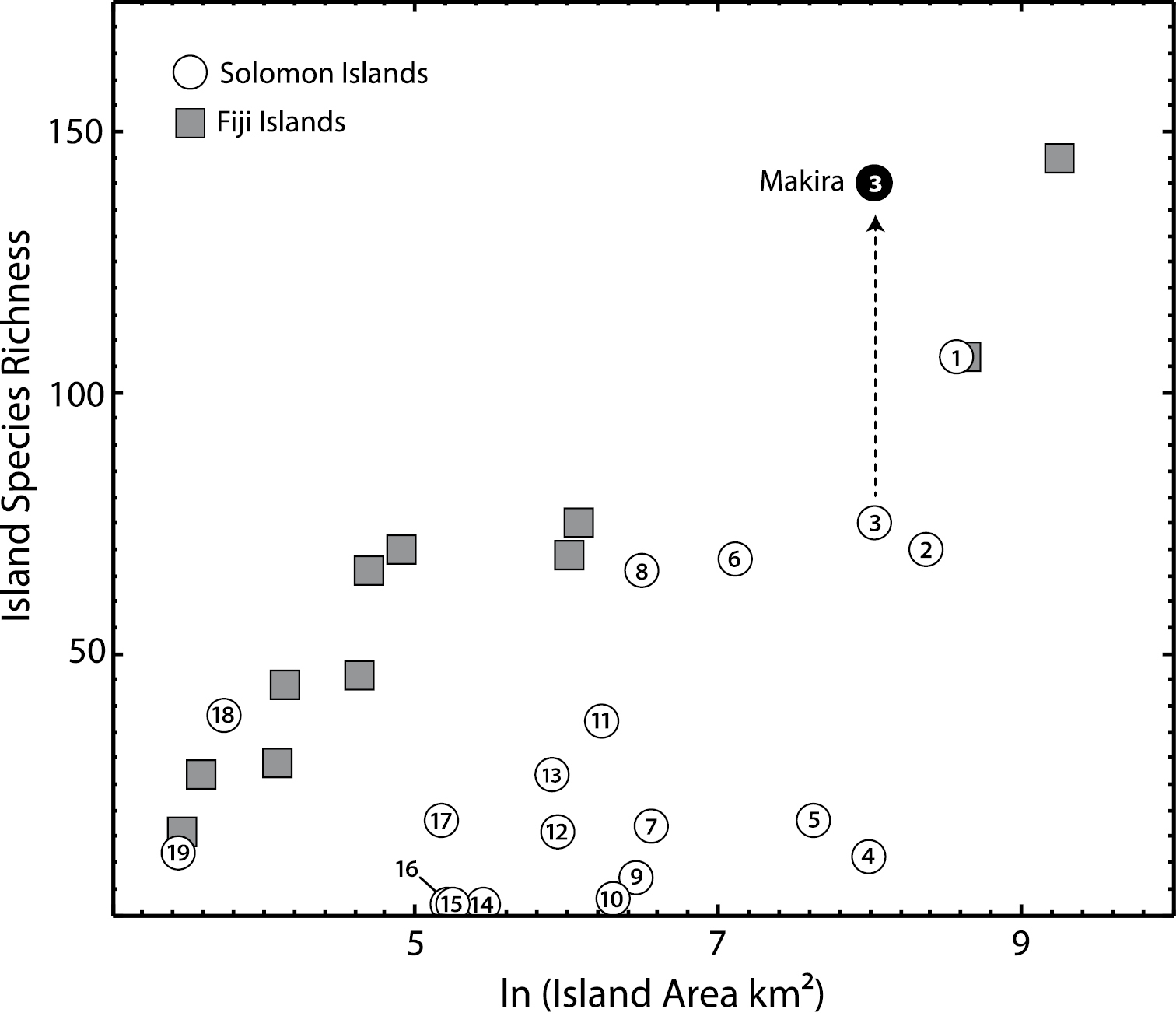
We used our data compilation to estimate in a general sense how undersampled the Solomon Islands are for ants. First, we compared the species richness of individual islands in the Solomons with counts of the Fijian islands, which were the target of recent intensive sampling and taxonomic analysis (
We present a list of nine subfamilies, 60 genera and 215 valid ant species and subspecies for the Solomon Islands based on our review of the literature and our recent collections from Makira (Appendix 1). We also present a list of 23 presumptively undescribed species that have also been recorded from the Solomons (Appendix 2). The generic composition and diversity of the Solomons is presented in Table 1. In total, our research suggests that the Solomon Islands support at least 237 unique ant taxa. The full species list with associated images and specimen data is available on Antweb.org <http://www.antweb.org/solomons.jsp> .
We excluded the following taxa from the list as they were reported from Bougainville but not from within the political boundaries of the Solomon Islands: Cryptopone crassicornis (Emery), Polyrhachis aurea (Mayr), Polyrhachis obliqua Stitz, and Polyrhachis salomo subsp. hiram Forel.
The following taxa were reported from the Solomon Islands, but are not believed to occur there either because the records were based on misidentified material or erroneous interpretation of locality data.
Camponotus pallens (Le Guillou, 1842): 316. Type locality: Tonga, Vavao. The website Antwiki.org, accessed 5 October 2012, listed this species under its Solomon Island webpage. The list was generated by extracting all species for which the Solomon Is. were listed as the type locality from the Bolton Catalog (
Camponotus reticulatus Roger, 1863: 139. Type locality: Sri Lanka. The first record of Camponotus reticulatus Roger appeared in
Hypoponera pallidula (Emery, 1900): 320. Type locality: New Guinea.
Leptogenys laeviceps (Smith, 1857): 69. Type locality: Borneo.
Odontomachus haematodus (Linnaeus, 1758): 582. Type locality: “America meridionali.” It is presumed that specimens referred to as Odontomachus haematodus by
Odontomachus insularis Guérin-Méneville, 1844: 423. Type locality: Cuba.
Pheidole punctulata Mayr, 1866: 899. Type locality: South Africa.
Philidris cordata (Smith, F. 1859): 137. Type locality: Indonesia, Aru I. In his introduction,
Tetramorium obesum André, 1887: 294. Type locality: India.
We collected a total of 67 described species and 30 presumptive species that are either undescribed or that we were unable to determine. Based on comparisons with type material, previously determined material and literature review, we suspect approximately 15 of the presumptive species are new to science. These taxa are included in Appendix 2. The survey added 67 new species records to Makira of taxa included in Appendixes 1 and 2, bringing the total number of species known from the island to 142. The survey also added 28 new species records to the Solomon Islands. Of these, six are previously described species (including three introduced species), and the remainder of species are included in Appendix 2.
Our research recovered species occurrence records for 32 individual islands and five island groups out of the approximately 75 named small to large individual islands and approximately 12 named island groups. These occurrence records are presented in Appendix 3. The 261 taxon names include the 215 described species and subspecies from Appendix 1, the 22 presumptive undescribed species from Appendix 2, and 24 additional morphospecies that likely represent a mixture of previously described species and undescribed species. This latter group is restricted to specimens collected during the 2008 Makira survey. The five islands with the highest number of species records, listed from greatest to least, are: Makira (142 spp.), Guadalcanal (107 spp.), Malaita (71 spp.), Santa Isabel (68 spp.), and Rennell (66 spp.). Fourteen individual islands have occurrence records for between 11–38 species. Thirteen individual islands have occurrence records for between 1–8 species.
The ten most widely distributed species, with the number of islands each is reported from, are: Odontomachus simillimus (27), Anoplolepis gracilipes (18), Camponotus bedoti (17), Nylanderia vaga (15), Anochetus graeffei (13), Eurhopalothrix procera (13), Myopopone castanea (13), Oecophylla smaragdina subnitida (13), Pachycondyla stigma (13), Philidris myrmecodiae (13). One hundred seven of the species and morphospecies included in Appendix 3 are only reported from single islands.
In total, our research suggests that the Solomon Islands support at least 237 unique ant species and subspecies. The poor sampling of many islands–some of which are quite large–and the unexamined material at the ANIC suggests that the true number is likely much greater. For example, our eight days of intensive hand collection and Winkler extractions on Makira added 67 new species records to the island (including all morphospecies) and 28 new records to the archipelago. Prior to the survey, Makira Island’s 75 species records were the second highest of the entire archipelago. Choiseul Island by comparison is approximately equal in area to Makira and closer to New Guinea, but the ant fauna of the island is virtually unknown with only eleven species recorded in the literature. There are approximately as many species known from the islands of Santa Isabel and Malaita as there are from Rennell, despite the substantially larger area of the former islands and their closer proximity to other large islands within the archipelago. The difference is that although no ant specialists have thoroughly sampled Rennell, general entomologists have collected there and the ant specimens of those surveys were the subject of several faunistic reviews (
Compared to Fijian islands of similar size, known species richness is generally much lower for individual islands within the Solomons, despite the fact that Fiji is much more isolated in the Pacific (Figure 2). This is likely due to relative sampling intensity of the two areas. Fiji has recently received intensive sampling efforts (
The relationship between islands area and known species richness. The figure presents individual islands in the Solomon (circles) and Fijian (squares) archipelagos, illustrating the undersampling of most Solomon Islands relative to the better collected Fiji Islands. For Makira, we present known species richness before (open circle) and after (filled circle) our recent collecting expedition. Numbers: 1 Guadalcanal 2 Malaita 3 Makira 4 Choiseul 5 New Georgia 6Santa Isabel 7 Kolombangara 8 Rennell 9 Vella Lavella 10 Vangunu 11 Nendö (Santa Cruz) 12 Rendova 13 Nggela Sule 14 Shortland 15 Vanikoro 16 San Jorge 17 Russell Is. 18 Ugi 19 Savo.
The species list compiled from our research suggests several interesting taxonomic patterns. For example, species richness across the 51 native ant genera of the Solomons appears uneven. The 30 Polyrhachis species represent 14% of the total native species. The nine most diverse genera (Polyrhachis, Pheidole, Camponotus, Tetramorium, Vollenhovia, Pachycondyla, Strumigenys, Crematogaster, and Gnamptogenys) collectively contain over half of the total native species, while fifteen genera are represented by a single native species.
Why is Polyrhachis so strongly represented in the Solomons? These results are likely biased to some extent by idiosyncratic collecting and taxonomic study. Besides the work of Mann, and to a lesser extent Greenslade, most of the collections from the Solomons have been made by more generalist collectors, which tend to take larger, more conspicuous ants that forage on and nest in vegetation–all of which are characteristic of Polyrhachis. Furthermore, Rudolf Kohout, who has access to the considerable collection of Solomons material at the ANIC, has devoted much of his taxonomic efforts towards revising the Polyrhachis of the Indo-Australian region (
Pachycondyla (9 native spp.), Crematogaster (7 native spp.)and Gnamptogenys (6 native spp.) are also among the most diverse ant genera in the Solomon Islands, but are either absent from or poorly represented in more easterly archipelagos. Fiji, for example, supports a single native Gnamptogenys species (Gnamptogenys aterrima Mann), and does not support any native Pachycondyla or Crematogaster species (
While additional sampling may prove otherwise, the current analysis of the Solomons ant fauna does not appear to support the type of in situ single-lineage radiations that characterize much of the Fijian ant fauna to the east. Parallels to the dramatic radiations of the Pheidole roosevelti group (
The importance of establishing baseline faunal inventories for the entire Solomon Island archipelago and its constituent islands is especially important when considering the growing environmental impacts resource extraction, plantation agriculture and invasive species are having on native biodiversity. Perhaps the greatest threat to native ant species in the Solomons is the spread of the Little Fire Ant (
We would like to express our gratitude to the national government, provincial governments and kind people of the Solomon Islands. The research was supported by the NSF graduate research fellowships (E.M.S. and E.P.E), the U. of Michigan UROP program (E.P.E. and B.D.B.), and NSF DEB 1145989 (E.P.E). We thank Rudy Kohout for identification of Polyrhachis species, Steve Shattuck for providing specimen records from ANIC and reviewing the manuscript, and an anonymous reviewer for providing comments on the manuscript.
List of valid species recorded from the Solomon Islands arranged by subfamily, genus and species. (*) Species known to be introduced to the Solomons from outside the Pacific region. ‘Year’ refers to the first year the species was reported from the Solomon Islands. References are arranged in chronological order. Footnotes appended to reference codes indicate that the author misidentified the species or associated it with a different valid name. Reference codes:(1)
| Taxon | Author | Year | Reference |
|---|---|---|---|
| Amblyoponinae | |||
| Amblyopone australis | Erichson, 1842: 261 | 1919 | 3, 8, 9, 13, 17, 38, 81 |
| Myopopone castanea | (Smith, F. 1860): 105 | 1919 | 3, 8, 9, 13, 17, 21, 81 |
| Prionopelta majuscula | Emery, 1897b: 595 | 2008 | 60, 81 |
| Prionopelta opaca | Emery, 1897b: 596 | 1976 | 34, 60, 79, 81 |
| Stigmatomma celata | (Mann, 1919): 279 | 1919 | 3, 8, 13, 17, 21, 34, 38, 81 |
| Stigmatomma gnoma | Taylor, 1979: 829 | 1978 | 38, 81 |
| Cerapachyinae | |||
| Cerapachys inconspicuus | Emery, 1901: 153 | 1919 | 3, 8, 9, 18, 20, 34 |
| Cerapachys pawa | Mann, 1919: 277 | 1919 | 3, 8, 20, 32 |
| Cerapachys terricola | Mann, 1919: 277 | 1919 | 3, 8, 32, 79 |
| Dolichoderinae | |||
| Anonychomyrma dimorpha | (Viehmeyer, 1912): 7 | 1919 | 3, 8, 9, 79 |
| Arnoldius pusillus | (Mayr, 1876): 83 | 1959 | 15 |
| Iridomyrmex anceps | (Roger, 1863a): 164 | 1919 | 3, 6, 8, 15, 36, 81 |
| Iridomyrmex pallidus | Forel, 1901: 22 | 1963 | 81 |
| Iridomyrmex rufoniger | (Lowne, 1865): 279 | 1919 | 3, 8 |
| Ochetellus glaber* | (Mayr, 1862): 705 | 2009 | 79 |
| Philidris myrmecodiae | (Emery, 1887): 249 | 1919 | 3, 6, 8, 15, 16, 291, 301, 681, 341, 361, 79 |
| Tapinoma (Micromyrma) indicum timidum | Santschi, 1928 | 1959 | 15 |
| Tapinoma melanocephalum* | (Fabricius, 1793): 353 | 1934 | 6, 8, 15, 18, 34, 36, 73, 81 |
| Tapinoma minutum | Mayr, 1862: 703 | 1967 | 26 |
| Technomyrmex albipes* | (Smith, F. 1861): 38 | 1910 | 1, 3, 8, 15, 18, 34, 36, 56, 79, 81 |
| Technomyrmex vitiensis | Mann, 1921: 473 | 2008 | 79 |
| Turneria dahlii | Forel, 1901: 17 | 1959 | 18, 34, 42, 81 |
| Turneria pacifica | Mann, 1919: 361 | 1919 | 3, 42, 81 |
| Ectatomminae | |||
| Gnamptogenys albiclava | (Mann, 1919): 283 | 1919 | 3, 8, 11, 17, 54 |
| Gnamptogenys crenaticeps | (Mann, 1919): 285 | 1919 | 3, 8, 11, 17, 54, 79 |
| Gnamptogenys lucida | (Mann, 1919): 285 | 1919 | 3, 8, 11, 17, 54 |
| Gnamptogenys malaensis | (Mann, 1919): 281 | 1919 | 3, 8, 11, 17, 54, 79 |
| Gnamptogenys preciosa | Lattke, 2004: 66 | 2004 | 54, 81 |
| Gnamptogenys solomonensis | Lattke, 2004: 66 | 2004 | 54, 81 |
| Rhytidoponera araneoides | (Le Guillou, 1842): 317 | 1910 | 1, 3, 14, 17, 79, 81 |
| Rhytidoponera chalybaea | Emery, 1901b: 51 | 1959 | 15 |
| Formicinae | |||
| Acropyga acutiventris | Roger, 1862: 243 | 1919 | 3, 8, 53, 79, 81 |
| Acropyga lauta | Mann, 1919: 365 | 1919 | 3, 8, 53, 79, 81 |
| Acropyga oceanica | Emery, 1900: 333 | 2008 | 79 |
| Acropyga pallida | (Donisthorpe, 1938): 598 | 1965 | 81 |
| Anoplolepis gracilipes* | Smith, F. 1857: 55 | 1919 | 3, 6, 8, 15, 15, 18, 29, 30, 68, 34, 36, 81 |
| Brachymyrmex obscurior* | Forel, 1893: 345 | 1976 | 34, 79 |
| Camponotus (Myrmamblys) bedoti | Emery, 1893: 196 | 1919 | 3, 6, 8, 15, 182, 342, 362 |
| Camponotus chloroticus | Emery, 1897b: 574 | 1959 | 15 |
| Camponotus elysii | Mann, 1919: 372 | 1919 | 3, 8 |
| Camponotus guppyi | Mann, 1919: 370 | 1919 | 3, 8 |
| Camponotus loa | Mann, 1919: 373 | 1919 | 3, 8 |
| Camponotus loa belli | Mann, 1919: 375 | 1919 | 3, 8 |
| Camponotus novaehollandiae | Mayr, 1870: 939 | 1919 | 3 |
| Nylanderia bourbonica* | (Forel, 1886): 210 | 1959 | 15, 34, 36, 81 |
| Nylanderia braueri glabrior | (Forel, 1902): 490 | 1954 | 81 |
| Nylanderia dichroa | Wheeler 1934: 181 | 1934 | 6, 8, 81 |
| Nylanderia manni | Donisthorpe, 1941: 41 | 1941 | 72, 15, 36 |
| Nylanderia obscura bismarckensis | (Forel, 1901): 26 | 1919 | 3, 6, 8 |
| Nylanderia stigmatica | Mann, 1919: 367 | 1919 | 3, 8, 62, 79, 81 |
| Nylanderia vaga* | (Forel, 1901): 26 | 1934 | 6, 8, 18, 26, 34, 36, 79, 81 |
| Nylanderia vividula* | (Nylander, 1846): 900 | 1919 | 3, 15, 79 |
| Oecophylla smaragdina subnitida | Emery 1892: 565 | 1910 | 1, 3, 6, 8, 153, 163, 293, 303, 683, 363, 793, 813 |
| Opisthopsis manni | Wheeler, W.M. 1918: 361 | 1918 | 2, 3, 8, 15 |
| Paraparatrechina minutula | (Forel, 1901): 25 | 1919 | 3, 8, 15, 34, 79, 81 |
| Paratrechina longicornis* | (Latreille, 1802): 113 | 1919 | 3, 8, 15, 34, 79, 81 |
| Plagiolepis alluaudi* | Emery, 1894: 71 | 1959 | 15 |
| Polyrhachis (Myrma) andromache | Roger, 1863b: 8 | 1959 | 34, 18, 345, 79 |
| Polyrhachis (Hedomyrma) annae | Mann, 1919: 377 | 1919 | 3, 6, 8, 15, 18, 34 |
| Polyrhachis (Chariomyrma) arcuata acutinota | Forel, 1901: 31 | 1934 | 6 |
| Polyrhachis (Hedomyrma) campbelli | Mann, 1919: 376 | 1919 | 3, 8, 79 |
| Polyrhachis (Myrmothrinax) dahlii | Forel, 1901: 30 | 1919 | 3, 8, 9, 64 |
| Polyrhachis (Cyrtomyrma) emeryana | Mann, 1919: 390 | 1919 | 3, 8, 55 |
| Polyrhachis (Cyrtomyrma) fulakora | Mann, 1919: 389 | 1919 | 3, 8, 15, 55 |
| Polyrhachis (Hedomyrma) geminata | Mann, 1919: 376 | 1919 | 3, 8, 79 |
| Polyrhachis greensladei | Kohout, 1990: 503 | 1990 | 74 |
| Polyrhachis (Myrma) ithona | Smith, F., 1860: 99 | 1934 | 6, 8 |
| Polyrhachis (Cyrtomyrma) johnsoni | Mann, 1919: 390 | 1919 | 3, 8, 55 |
| Polyrhachis (Chariomyrma) kaipi | Mann, 1919: 382 | 1919 | 3, 6, 8, 79 |
| Polyrhachis (Myrma) labella brunneipes | Wheeler, 1934 | 1934 | 6, 8 |
| Polyrhachis (Myrma) litigiosa | Emery, 1897b: 581 | 1919 | 3, 8, 79 |
| Polyrhachis (Myrma) malaensis | Mann, 1919: 386 | 1919 | 3, 8 |
| Polyrhachis nofra | Bolton, 1975: 9 | 1975 | 31 |
| Polyrhachis (Myrmatopa) osae | Mann, 1919: 384 | 1919 | 3, 6, 8, 9, 15 |
| Polyrhachis pacifica | Kohout, 2006: 140 | 2006 | 55 |
| Polyrhachis (Chariomyrma) rere | Mann, 1919: 381 | 1919 | 3, 6, 8, 15 |
| Polyrhachis (Myrmhopla) saevissima argentea | Mayr, 1862: 82 | 1919 | 3, 8, 9 |
| Polyrhachis (Myrma) salomo | Forel, 1910: 87 | 1910 | 1, 3, 8, 15 |
| Polyrhachis (Hedomyrma) santschii | Mann, 1919: 375 | 1919 | 3, 8 |
| Polyrhachis setosa | Kohout, 2006: 141 | 2006 | 55 |
| Polyrhachis (Myrma) similis | Viehmeyer, 1912: 8 | 1919 | 3, 8 |
| Polyrhachis (Cyrtomyrma) ugiensis | Mann, 1919: 389 | 1919 | 3, 8, 55, 79 |
| Polyrhachis (Myrmatopa) ulysses | Forel, 1910: 91 | 1910 | 1, 3, 8 |
| Polyrhachis (Cyrtomyrma) undulata | Kohout, 2006: 142 | 2006 | 55, 79 |
| Polyrhachis (Myrmhopla) wheeleri | Mann, 1919: 387 | 1919 | 3, 8, 9 |
| Myrmicinae | |||
| Cardiocondyla kagutsuchi* | Terayama, 1999: 100 | 2009 | 79 |
| Cardiocondyla nivalis | Mann, 1919: 317 | 1919 | 3, 8, 34, 36 |
| Cardiocondyla nuda | (Mayr, 1866): 508 | 1959 | 15, 34, 36, 51, 59 |
| Carebara atoma | (Emery, 1900): 328 | 1919 | 3, 8, 34, 36, 79 |
| Carebara viehmeyeri | (Mann, 1919): 331 | 1919 | 3, 8, 79 |
| Colobostruma foliacea | Emery, 1897a: 573 | 2000 | 48, 81 |
| Crematogaster (Crematogaster) abrupta | Mann, 1919: 320 | 1935 | 8, 15, 61 |
| Crematogaster (Crematogaster) elysii | Mann, 1919: 319 | 1935 | 8, 3, 61 |
| Crematogaster (Crematogaster) foxi | Mann, 1919: 321 | 1935 | 8, 3, 61 |
| Crematogaster (Crematogaster) nesiotis | Mann, 1919: 322 | 1935 | 8, 3, 61 |
| Crematogaster (Crematogaster) obnigra | Mann, 1919: 323 | 1919 | 3, 15, 61 |
| Crematogaster (Orthocrema) scita | Forel, 1902: 409 | 1959 | 15 |
| Crematogaster (Orthocrema) wheeleri | Mann, 1919: 318 | 1935 | 8, 3, 61 |
| Eurhopalothrix brevicornis | (Emery, 1897a): 572 | 1977 | 36, 80, 28, 82 |
| Eurhopalothrix greensladei | Taylor, 1968: 342 | 1968 | 28, 82 |
| Eurhopalothrix isabellae | (Mann, 1919): 357 | 1919 | 3, 8, 22, 80, 28, 82 |
| Eurhopalothrix procera | (Emery, 1897a): 572 | 1919 | 3, 8, 22, 28, 79, 81, 82 |
| Lordomyrma epinotalis | (Mann, 1919): 343 | 1919 | 3, 8, 34, 58, 79 |
| Monomorium australicum | Forel, 1907:20 | 1919 | 3, 8, 15, 34, 36 |
| Monomorium destructor* | (Jerdon, 1851): 105 | 1959 | 18, 34 |
| Monomorium floricola* | (Jerdon, 1851): 107 | 1959 | 15, 34, 36, 41, 79, 81 |
| Monomorium pharaonis* | (Linnaeus, 1758): 580 | 1919 | 3, 8, 15, 34, 41, 81 |
| Myrmecina modesta | Mann, 1919: 335 | 1919 | 3, 8, 346 |
| Myrmecina modesta subarmata | Mann, 1919: 337 | 1919 | 3, 8 |
| Myrmecina transversa | Emery, 1897a: 582 | 2008 | 79 |
| Pheidole belli | Mann, 1919: 306 | 1919 | 3, 8 |
| Pheidole erato | Mann, 1919: 307 | 1919 | 3, 8 |
| Pheidole fuscula | Emery, 1900: 325 | 1919 | 3, 8 |
| Pheidole isis | Mann, 1919: 311 | 1919 | 3, 8 |
| Pheidole isis taki | Mann, 1919: 314 | 1919 | 3, 8, 79 |
| Pheidole megacephala* | (Fabricius, 1793): 361 | 1910 | 17, 6, 8, 15, 26, 30, 34, 81 |
| Pheidole mendanai | Mann, 1919: 311 | 1919 | 3, 8 |
| Pheidole nindi | Mann, 1919: 314 | 1919 | 3, 8, 34, 36, 79 |
| Pheidole oceanica | Mayr, 1866: 510 | 1919 | 3, 8, 15, 18, 34, 36, 79 |
| Pheidole philemon | Forel, 1910: 44 | 1910 | 1, 3, 8, 15, 79 |
| Pheidole sexspinosa | Mayr, 1870: 977 | 1919 | 3, 8, 34, 36, 79 |
| Pheidole sexspinosa fuscescens | Emery, 1900: 323 | 1919 | 3, 8, 18 |
| Pheidole umbonata | Mayr, 1870: 978 | 1919 | 3, 8, 15, 18, 34, 36 |
| Podomyrma basalis salomo | Mann, 1919: 333 | 1919 | 3, 8 |
| Podomyrma basalis woodfordi | Mann, 1919: 334 | 1919 | 3, 8 |
| Pristomyrmex levigatus | Emery, 1897a: 583 | 1919 | 3, 52, 79 |
| Pristomyrmex obesus | Mann, 1919: 339 | 1919 | 3, 8, 80, 52 |
| Rogeria megastigmatica | Kugler, C. 1994: 35 | 1994 | 45, 79 |
| Rogeria stigmatica | Emery, 1897: 589 | 1919 | 3, 8, 34, 45 |
| Romblonella elysii | (Mann, 1919): 346 | 1919 | 3, 8, 44 |
| Solenopsis geminata* | (Fabricius, 1804): 423 | 1977 | 36 |
| Solenopsis papuana | Emery, 1900: 330 | 1919 | 3, 79 |
| Solenopsis pawaensis | Mann, 1919: 329 | 1919 | 3, 79 |
| Stereomyrmex dispar | (Wheeler, W.M. 1934): 175 | 1934 | 6, 18, 34, 44 |
| Strumigenys chyzeri | Emery, 1897a: 576 | 1919 | 3, 48, 79 |
| Strumigenys decollata | Mann, 1919: 353 | 1919 | 3, 8, 12, 48 |
| Strumigenys emmae* | Emery, 1890: 70 | 1976 | 34, 36, 48, 81 |
| Strumigenys eurycera | Emery, 1897a: 581 | 2000 | 48, 81 |
| Strumigenys frivaldszkyi | Emery, 1897: 580 | 1976 | 34, 48, 79 |
| Strumigenys godeffroyi* | Mayr, 1866: 516 | 1919 | 3, 15, 34, 36, 47, 48, 79 |
| Strumigenys karawajewi | (Brown, 1948): 44 | 1976 | 34, 46, 48, 79, 81 |
| Strumigenys membranifera* | (Emery, 1869): 24 | 2000 | 48, 36, 81 |
| Strumigenys mocsaryi | (Emery, 1897a): 580 | 2000 | 48 |
| Strumigenys rogeri* | Emery, 1890: 68 | 2000 | 48 |
| Strumigenys szalayi | Emery, 1897: 578 | 2000 | 48, 79 |
| Strumigenys undras | Bolton, 2000: 752 | 2000 | 48 |
| Strumigenys yaleopleura | Brown, 1988: 41 | 2000 | 48 |
| Tetramorium antennatum | (Mann, 1919): 350 | 1919 | 3 |
| Tetramorium aspersum | (Smith, F. 1865): 72 | 1919 | 3, 6, 8, 35, 79 |
| Tetramorium bicarinatum* | (Nylander, 1846): 1061 | 1919 | 38, 68, 88, 158, 348, 35, 36 |
| Tetramorium carinatum | (Smith, F. 1859): 148 | 1919 | 3, 8 |
| Tetramorium insolens | (Smith, F., 1861) | 1934 | 6, 8, 18, 34, 35 |
| Tetramorium lanuginosum* | Mayr, 1870: 976 | 1935 | 8, 69 |
| Tetramorium mayri | (Mann, 1919: 351) | 1919 | 3, 8, 79 |
| Tetramorium melanogyna | Mann, 1919: 345 | 1919 | 3, 8, 79 |
| Tetramorium mutatum | Bolton, 1985: 247 | 1919 | 3, 8, 69 |
| Tetramorium pacificum | Mayr, 1870: 976 | 1934 | 6, 8, 18, 34, 35 |
| Tetramorium salomo | Mann, 1919: 344 | 1935 | 8, 35, 79 |
| Tetramorium simillimum* | (Smith, F. 1851): 118 | 1959 | 15, 34, 35, 36, 79 |
| Tetramorium tonganum | Mayr, 1870: 976 | 1919 | 3, 8, 15, 18, 34, 35 |
| Tetramorium vombis | Bolton, 1976: 358 | 1985 | 39, 349, 69 |
| Vollenhovia dentata | Mann, 1919: 325 | 1919 | 3, 8, 24, 79 |
| Vollenhovia dentata marginata | Mann, 1919: 327 | 1919 | 3, 8, 24 |
| Vollenhovia elysii | Mann, 1919: 327 | 1919 | 3, 8, 24 |
| Vollenhovia foveaceps | Mann, 1919: 328 | 1919 | 3, 8, 24 |
| Vollenhovia loboii | Mann, 1919: 324 | 1919 | 3, 8, 24 |
| Vollenhovia oblonga | (Smith, F. 1861): 46 | 1959 | 18, 34, 43 |
| Vollenhovia oblonga pedestris | (Smith, F. 1860): 107 | 1919 | 3, 8, 15, 79 |
| Vollenhovia subtilis | Emery, 1887: 454 | 1919 | 3, 8 |
| Wasmannia auropunctata* | (Roger, 1863a): 183 | 1984 | 40, 75, 76, 77, 79 |
| Ponerinae | |||
| Anochetus cato | Forel, 1901: 6 | 1919 | 3, 8, 17, 19, 79, 81 |
| Anochetus graeffei | Mayr, 1870: 961 | 1919 | 3, 8, 15, 17, 19, 34, 36, 65, 79, 81 |
| Anochetus isolatus | Mann, 1919: 302 | 1919 | 3, 8, 17, 19, 34, 37, 65, 79, 81 |
| Cryptopone butteli | Forel, 1913: 9 | 1965 | 81 |
| Cryptopone crassicornis | (Emery, 1897): 533 | 1965 | 81 |
| Cryptopone fusciceps | (Emery, 1900): 321 | 1919 | 3, 4, 8, 14, 17, 81 |
| Cryptopone testacea | (Emery, 1893): cclxxv | 1919 | 3, 4, 8, 14, 17, 32, 81 |
| Hypoponera biroi | (Emery, 1900): 7 | 1959 | 17, 34 |
| Hypoponera confinis | (Roger, 1860): 284 | 1959 | 17 |
| Hypoponera pallidula | (Emery, 1900): 320 | 1919 | 3, 8, 9 |
| Hypoponera papuana | (Emery, 1900): 319 | 1919 | 3, 8, 79 |
| Hypoponera pruinosa | (Emery, 1900): 319 | 1919 | 3, 8, 9, 14, 17, 34, 79 |
| Hypoponera punctatissima* | (Roger, 1859): 246 | 1976 | 34, 79 |
| Hypoponera ragusai* | (Forel, 1899): 28 | 1919 | 3, 8, 14, 17, 36 |
| Hypoponera sororcula | (Wilson, 1958a): 338 | 1958 | 14, 17 |
| Leptogenys diminuta | (Smith, F. 1857): 69 | 1919 | 3, 8, 17, 79 |
| Leptogenys foreli | Mann, 1919: 297 | 1919 | 3, 8, 13, 17, 1810, 3410 |
| Leptogenys oresbia | Wilson, 1958b: 131 | 1958 | 311, 13, 17 |
| Leptogenys truncata | Mann, 1919: 26 | 1919 | 3, 17 |
| Odontomachus malignus | Smith, F. 1859: 144 | 1919 | 3, 17, 1812, 19, 33, 3412, 63, 81 |
| Odontomachus rufithorax | Emery, 1911: 534 | 1919 | 3, 17, 19, 33, 81 |
| Odontomachus saevissimus | (Smith, F. 1858) | 1959 | 15, 33, 81 |
| Odontomachus simillimus | (Smith, F. 1858): 80 | 1910 | 113, 314, 614, 814, 1514, 17, 18, 19, 26, 34, 36, 79, 81 |
| Pachycondyla acuta | Emery, 1900 | 1958 | 14, 17 |
| Pachycondyla aequalis | (Mann, 1919): 289 | 1919 | 3, 8, 14, 17, 79 |
| Pachycondyla croceicornis | (Emery, 1900): 315 | 1919 | 3, 14, 17, 36, 79 |
| Pachycondyla darwinii | (Forel, 1893): 460 | 1959 | 17 |
| Pachycondyla exarata | Emery, 1901b: 156 | 1919 | 3, 8 |
| Pachycondyla manni | (Viehmeyer, 1924): 228 | 1924 | 71, 14, 17 |
| Pachycondyla melancholica | Smith, F. 1865: 71 | 1919 | 3 |
| Pachycondyla papuana | (Viehmeyer, 1914): 608 | 1919 | 3, 9 |
| Pachycondyla sheldoni | (Mann, 1919): 292 | 1919 | 3, 8, 14, 17 |
| Pachycondyla stigma* | (Fabricius, 1804): 400 | 1919 | 3, 8, 9, 15, 17, 18, 34, 79 |
| Platythyrea parallela | (Smith, F., 1859): 143 | 1919 | 3, 9, 17 |
| Ponera clavicornis | Emery, 1900: 317 | 1919 | 3, 8, 10, 25, 3415, 81 |
| Ponera incerta | (Wheeler, W.M. 1933): 18 | 1959 | 17, 25, 81 |
| Ponera swezeyi | (Wheeler, W.M. 1933): 16 | 2009 | 79 |
| Ponera szaboi | Wilson, 1957: 371 | 1976 | 34 |
| Ponera tenuis | (Emery, 1900): 321 | 1965 | 81 |
| Proceratiinae | |||
| Discothyrea clavicornis | Emery, 1897b: 593 | 1919 | 3, 8, 9, 17, 81 |
| Probolomyrmex salomonis | Taylor, 1965: 358 | 1965 | 23, 66, 81 |
| Proceratium austronesicum | De Andrade, in |
2003 | 50, 81 |
| Proceratium papuanum | Emery, 1897b: 592 | 2003 | 50, 81 |
| Pseudomyrmecinae | |||
| Tetraponera laeviceps | (Smith, F. 1859): 145 | 1919 | 3, 8, 49 |
Presumed undescribed species recorded from the Solomon Islands arranged by species name. The ‘Year’ column refers to the year the species was first recorded from the Solomon Islands. Reference codes are the same as those used in Appendix 11.
| Taxon | Notes | Year | Reference |
| Adelomyrmex sp. BP02 | nr. hirsutus | 2008 | 79 |
| Adelomyrmex sp. BP03 | as “Adelomyrmex (Arctomyrmex) sp.” | 1976 | 34 |
| Arnoldius sp. BP01 | as “nr. flavus” | 1959 | 15 |
| Camponotus sp. BP02 | nr. guppyi | 2008 | 79 |
| Camponotus sp. BP05 | nr. elysii | 2008 | 79 |
| Camponotus sp. BP06 | as “Camponotus (Colobopsis) sp. A” | 1976 | 34 |
| Camponotus sp. BP07 | as “Camponotus (Colobopsis) sp. B” | 1976 | 34 |
| Camponotus sp. BP08 | as “Camponotus (Colobopsis) sp. C” | 1976 | 34 |
| Camponotus sp. BP09 | as “Camponotus (Colobopsis) spp. (2)” | 1959 | 18, 34 |
| Camponotus sp. BP10 | as “Camponotus (Colobopsis) spp. (2)” | 1959 | 18, 34 |
| Cerapachys sp. BP01 | as “Cerapachys? (Syscia) sp. 1” | 1959 | 18, 34 |
| Colobostruma sp. BP01 | nr. foliacea | 2008 | 79 |
| Cryptopone sp. BP01 | nr. testacea | 2008 | 79 |
| Myopias sp. BP01 | 2008 | 79 | |
| Myopias sp. BP02 | 2008 | 79 | |
| Myopias sp. BP03 | 2008 | 79 | |
| Myopias sp. BP04 | as “Myopias cf. tenuis” | 1983 | 39 |
| Myrmecina sp. BP01 | 2008 | 79 | |
| Myrmecina sp. BP03 | 2008 | 79 | |
| Pheidole sp. BP02 | 2008 | 79 | |
| Pheidole sp. BP12 | nr. mendanai | 2008 | 79 |
| Pheidole sp. BP13 | as “Pheidole (Pheidolacanthinus) sp.” | 1976 | 34 |
| Platythyrea sp. BP01 | as “Platythyrea sp.” | 1976 | 34 |
| Polyrhachis sp. BP01 | nr. bismarckensis | 2008 | 79 |
| Polyrhachis sp. BP03 | as “Polyrhachis (Chariomyrma) sp.” | 1976 | 34 |
| Rogeria sp. BP01 | nr. stigmatica | 2008 | 79 |
| Strumigenys sp. BP05 | nr. mocsaryi | 2008 | 79 |
| Vollenhovia sp. BP01 | nr. elysii | 2008 | 79 |
| Vollenhovia sp. BP02 | nr. loboii | 2008 | 79 |
| Vollenhovia sp. BP03 | as “Vollenhovia sp.” | 1976 | 34 |
Occurrence records of individual islands and island groups from which ant species have been recorded arranged by species name and island/island group name. The valid names refer to those presented in Appendix 1. Infraspecific names are abbreviated from trinomials to binomials composed of the genus and infraspecific name (e.g. Nylanderia obscura bismarckensis (Forel) is presented as “Nylanderia bismarckensis”). Asterisks (*) are appended to morphospecies presumed to be undescribed species (Appendix 2). Morphospecies that we were unable to determine but might represent previously described species are also presented. Individual island names appear in regular type and island group names appear in uppercase bold type. Island groups and their constituent islands from which ants have been recorded are presented in Table 1. The penultimate column ‘Solomon Is.’ includes species records for which no individual island or island group was associated (
| Taxon | Anuta | Bellona | Choiseul | Guadalcanal | Kolombangara | Makira | Malaita | Malaupaina | Matema | Mbanika | New Georgia | NEW GEORGIA IS. | NGGELA IS. | Nggela Sule | Nupani | OLU MALAU IS. | Ontong Java Is. | Owaraha | REEF IS. | Rendova | Rennell | Russell Is. | San Jorge | Santa Catalina | Santa Cruz | SANTA CRUZ IS. | Santa Isabel | Savo | Shortland | Sikaiana | Tikopia | Tulagi | Ugi | Vangunu | Vanikoro | Vella Lavella | SOLOMON IS. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acropyga | ||||||||||||||||||||||||||||||||||||||
| Acropyga acutiventris | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Acropyga lauta | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Acropyga oceanica | x | 1 | ||||||||||||||||||||||||||||||||||||
| Acropyga pallida | x | 1 | ||||||||||||||||||||||||||||||||||||
| Adelomyrmex | ||||||||||||||||||||||||||||||||||||||
| Adelomyrmex sp. BP02* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Adelomyrmex sp. BP03* | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Amblyopone | ||||||||||||||||||||||||||||||||||||||
| Amblyopone australis | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Anochetus | ||||||||||||||||||||||||||||||||||||||
| Anochetus cato | x | x | x | x | x | x | x | x | x | x | x | x | 11 | |||||||||||||||||||||||||
| Anochetus graeffei | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 13 | |||||||||||||||||||||||
| Anochetus isolatus | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Anonychomyrma | ||||||||||||||||||||||||||||||||||||||
| Anonychomyrma dimorpha | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Anoplolepis | ||||||||||||||||||||||||||||||||||||||
| Anoplolepis gracilipes | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 18 | |||||||||||||||||||
| Arnoldius | ||||||||||||||||||||||||||||||||||||||
| Arnoldius sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Arnoldius pusillus | x | 1 | ||||||||||||||||||||||||||||||||||||
| Brachymyrmex | 0 | |||||||||||||||||||||||||||||||||||||
| Brachymyrmex obscurior | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Camponotus | ||||||||||||||||||||||||||||||||||||||
| Camponotus bedoti | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 17 | ||||||||||||||||||||
| Camponotus chloroticus | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Camponotus elysii | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Camponotus guppyi | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus loa | x | x | x | x | x | x | x | x | x | x | 10 | |||||||||||||||||||||||||||
| Camponotus loa belli | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Camponotus novaehollandiae | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Camponotus sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP02* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP04 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP05* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP06* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP07* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP08* | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Camponotus sp. BP09* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Camponotus sp. BP10* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cardiocondyla | ||||||||||||||||||||||||||||||||||||||
| Cardiocondyla kagutsuchi | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cardiocondyla nivalis | x | x | x | x | x | x | x | x | 8 | |||||||||||||||||||||||||||||
| Cardiocondyla nuda | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Carebara | ||||||||||||||||||||||||||||||||||||||
| Carebara atoma | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Carebara viehmeyeri | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cerapachys | ||||||||||||||||||||||||||||||||||||||
| Cerapachys inconspicuus | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Cerapachys pawa | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Cerapachys terricola | x | x | x | x | x | x | x | 6 | ||||||||||||||||||||||||||||||
| Cerapachys sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Colobostruma | ||||||||||||||||||||||||||||||||||||||
| Colobostruma foliacea | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Colobostruma sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Crematogaster | ||||||||||||||||||||||||||||||||||||||
| Crematogaster abrupta | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Crematogaster elysii | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Crematogaster foxi | x | 1 | ||||||||||||||||||||||||||||||||||||
| Crematogaster nesiotis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Crematogaster obnigra | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Crematogaster scita | x | 1 | ||||||||||||||||||||||||||||||||||||
| Crematogaster wheeleri | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cryptopone | ||||||||||||||||||||||||||||||||||||||
| Cryptopone butteli | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cryptopone crassicornis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Cryptopone fusciceps | x | x | x | x | x | x | 5 | |||||||||||||||||||||||||||||||
| Cryptopone testacea | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Cryptopone sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Dilobocondyla | ||||||||||||||||||||||||||||||||||||||
| Dilobocondyla sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Discothyrea | ||||||||||||||||||||||||||||||||||||||
| Discothyrea clavicornis | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Eurhopalothrix | ||||||||||||||||||||||||||||||||||||||
| Eurhopalothrix brevicornis | x | x | x | x | x | x | 5 | |||||||||||||||||||||||||||||||
| Eurhopalothrix greensladei | x | 1 | ||||||||||||||||||||||||||||||||||||
| Eurhopalothrix isabellae | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Eurhopalothrix procera | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 14 | |||||||||||||||||||||||
| Gnamptogenys | ||||||||||||||||||||||||||||||||||||||
| Gnamptogenys albiclava | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Gnamptogenys crenaticeps | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Gnamptogenys lucida | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Gnamptogenys malaensis | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Gnamptogenys preciosa | x | 1 | ||||||||||||||||||||||||||||||||||||
| Gnamptogenys solomonensis | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Gnamptogenys sp. BP03 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Hypoponera | ||||||||||||||||||||||||||||||||||||||
| Hypoponera biroi | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Hypoponera confinis | x | 0 | ||||||||||||||||||||||||||||||||||||
| Hypoponera pallidula | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Hypoponera papuana | x | x | x | x | x | x | 5 | |||||||||||||||||||||||||||||||
| Hypoponera pruinosa | x | x | x | x | x | x | x | x | x | x | x | x | 11 | |||||||||||||||||||||||||
| Hypoponera punctatissima | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Hypoponera ragusai | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Hypoponera sororcula | x | 0 | ||||||||||||||||||||||||||||||||||||
| Hypoponera sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Hypoponera sp. BP06 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Hypoponera sp. BP07 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Hypoponera sp. BP08 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Iridomyrmex | ||||||||||||||||||||||||||||||||||||||
| Iridomyrmex anceps | x | x | x | x | x | x | x | x | x | x | x | 11 | ||||||||||||||||||||||||||
| Iridomyrmex pallidus | x | 1 | ||||||||||||||||||||||||||||||||||||
| Iridomyrmex rufoniger | x | 1 | ||||||||||||||||||||||||||||||||||||
| Leptogenys | ||||||||||||||||||||||||||||||||||||||
| Leptogenys diminuta | x | x | x | x | x | 4 | ||||||||||||||||||||||||||||||||
| Leptogenys foreli | x | x | x | x | x | 4 | ||||||||||||||||||||||||||||||||
| Leptogenys oresbia | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Leptogenys truncata | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Lordomyrma | ||||||||||||||||||||||||||||||||||||||
| Lordomyrma epinotalis | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Monomorium | ||||||||||||||||||||||||||||||||||||||
| Monomorium australicum | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Monomorium destructor | x | 1 | ||||||||||||||||||||||||||||||||||||
| Monomorium floricola | x | x | x | x | x | x | x | x | 8 | |||||||||||||||||||||||||||||
| Monomorium pharaonis | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Myopias | ||||||||||||||||||||||||||||||||||||||
| Myopias sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myopias sp. BP02* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myopias sp. BP03* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myopias sp. BP04* | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Myopopone | ||||||||||||||||||||||||||||||||||||||
| Myopopone castanea | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 13 | |||||||||||||||||||||||
| Myrmecina | ||||||||||||||||||||||||||||||||||||||
| Myrmecina modesta | x | x | x | x | x | x | x | x | 8 | |||||||||||||||||||||||||||||
| Myrmecina subarmata | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myrmecina | ||||||||||||||||||||||||||||||||||||||
| Myrmecina transversa | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myrmecina sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Myrmecina sp. BP03* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Nylanderia | ||||||||||||||||||||||||||||||||||||||
| Nylanderia bourbonica | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Nylanderia braueri glabrior | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Nylanderia dichroa | x | x | x | x | x | x | x | x | x | 9 | ||||||||||||||||||||||||||||
| Nylanderia manni | x | 1 | ||||||||||||||||||||||||||||||||||||
| Nylanderia bismarckensis | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Nylanderia stigmatica | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Nylanderia vaga | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 15 | ||||||||||||||||||||||
| Nylanderia vividula | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Ochetellus | ||||||||||||||||||||||||||||||||||||||
| Ochetellus glaber | x | 1 | ||||||||||||||||||||||||||||||||||||
| Odontomachus | ||||||||||||||||||||||||||||||||||||||
| Odontomachus malignus | x | x | x | x | x | x | x | 6 | ||||||||||||||||||||||||||||||
| Odontomachus rufithorax | x | x | x | x | x | 4 | ||||||||||||||||||||||||||||||||
| Odontomachus saevissimus | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Odontomachus simillimus | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 27 | |||||||||
| Oecophylla | ||||||||||||||||||||||||||||||||||||||
| Oecophylla subnitida | x | x | x | x | x | x | x | x | x | x | x | x | x | 13 | ||||||||||||||||||||||||
| Opisthopsis | 0 | |||||||||||||||||||||||||||||||||||||
| Opisthopsis manni | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Pachycondyla | ||||||||||||||||||||||||||||||||||||||
| Pachycondyla acuta | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Pachycondyla aequalis | x | x | x | x | x | 4 | ||||||||||||||||||||||||||||||||
| Pachycondyla croceicornis | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Pachycondyla darwinii | x | 0 | ||||||||||||||||||||||||||||||||||||
| Pachycondyla exarata | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Pachycondyla manni | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Pachycondyla melancholica | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pachycondyla papuana | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Pachycondyla sheldoni | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Pachycondyla stigma | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 13 | |||||||||||||||||||||||
| Pachycondyla sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Paraparatrechina | ||||||||||||||||||||||||||||||||||||||
| Paraparatrechina minutula | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Paratrechina | ||||||||||||||||||||||||||||||||||||||
| Paratrechina longicornis | x | x | x | x | x | x | x | x | x | x | 10 | |||||||||||||||||||||||||||
| Pheidole | ||||||||||||||||||||||||||||||||||||||
| Pheidole belli | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole erato | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole fuscula | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole isis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole taki | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole megacephala | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Pheidole mendanai | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole nindi | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Pheidole oceanica | x | x | x | x | x | x | x | x | x | x | x | 11 | ||||||||||||||||||||||||||
| Pheidole philemon | x | x | x | x | x | x | x | x | 7 | |||||||||||||||||||||||||||||
| Pheidole sexspinosa | x | x | x | x | x | x | x | x | x | x | 10 | |||||||||||||||||||||||||||
| Pheidole fuscescens | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Pheidole umbonata | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Pheidole sp. BP02* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole sp. BP09 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole sp. BP10 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole sp. BP11 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole sp. BP12* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Pheidole sp. BP13* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Philidris | ||||||||||||||||||||||||||||||||||||||
| Philidris myrmecodiae | x | x | x | x | x | x | x | x | x | x | x | x | x | 13 | ||||||||||||||||||||||||
| Plagiolepis | ||||||||||||||||||||||||||||||||||||||
| Plagiolepis alluaudi | x | 1 | ||||||||||||||||||||||||||||||||||||
| Platythyrea | ||||||||||||||||||||||||||||||||||||||
| Platythyrea parallela | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Platythyrea sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Podomyrma | ||||||||||||||||||||||||||||||||||||||
| Podomyrma salomo | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Podomyrma woodfordi | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis | ||||||||||||||||||||||||||||||||||||||
| Polyrhachis andromache | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis annae | x | x | x | x | x | x | x | x | x | x | 10 | |||||||||||||||||||||||||||
| Polyrhachis acutinota | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis argentea | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Polyrhachis brunneipes | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Polyrhachis campbelli | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis dahlii | x | x | x | x | x | x | x | x | x | 8 | ||||||||||||||||||||||||||||
| Polyrhachis emeryana | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis fulakora | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis geminata | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Polyrhachis greensladei | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis ithona | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis johnsoni | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Polyrhachis kaipi | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Polyrhachis litigiosa | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis malaensis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis nofra | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis osae | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Polyrhachis pacifica | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis rere | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Polyrhachis salomo | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Polyrhachis santschii | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis setosa | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis similis | x | x | x | x | x | x | x | x | x | 9 | ||||||||||||||||||||||||||||
| Polyrhachis ugiensis | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Polyrhachis ulysses | x | x | x | x | 3 | |||||||||||||||||||||||||||||||||
| Polyrhachis undulata | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis wheeleri | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Polyrhachis sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis sp. BP03 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Polyrhachis sp. BP04 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Ponera | ||||||||||||||||||||||||||||||||||||||
| Ponera clavicornis | x | x | x | x | x | x | x | x | x | x | x | x | 12 | |||||||||||||||||||||||||
| Ponera incerta | x | x | x | x | x | x | x | x | 7 | |||||||||||||||||||||||||||||
| Ponera swezeyi | x | 1 | ||||||||||||||||||||||||||||||||||||
| Ponera szaboi | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Ponera tenuis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Ponera sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Ponera sp. BP02 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Prionopelta | ||||||||||||||||||||||||||||||||||||||
| Prionopelta majuscula | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Prionopelta opaca | x | x | x | x | x | x | x | x | x | x | x | 11 | ||||||||||||||||||||||||||
| Pristomyrmex | ||||||||||||||||||||||||||||||||||||||
| Pristomyrmex levigatus | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Pristomyrmex obesus | x | x | x | x | x | x | x | x | x | x | 10 | |||||||||||||||||||||||||||
| Probolomyrmex | ||||||||||||||||||||||||||||||||||||||
| Probolomyrmex salomonis | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Proceratium | ||||||||||||||||||||||||||||||||||||||
| Proceratium austronesicum | x | 1 | ||||||||||||||||||||||||||||||||||||
| Proceratium papuanum | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Rhytidoponera | ||||||||||||||||||||||||||||||||||||||
| Rhytidoponera araneoides | x | x | x | x | x | x | x | x | x | x | x | 10 | ||||||||||||||||||||||||||
| Rhytidoponera chalybaea | x | 1 | ||||||||||||||||||||||||||||||||||||
| Rogeria | ||||||||||||||||||||||||||||||||||||||
| Rogeria megastigmatica | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Rogeria stigmatica | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Rogeria sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Romblonella | ||||||||||||||||||||||||||||||||||||||
| Romblonella elysii | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Solenopsis | ||||||||||||||||||||||||||||||||||||||
| Solenopsis geminata | x | 1 | ||||||||||||||||||||||||||||||||||||
| Solenopsis papuana | x | 1 | ||||||||||||||||||||||||||||||||||||
| Solenopsis pawaensis | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Stereomyrmex | ||||||||||||||||||||||||||||||||||||||
| Stereomyrmex dispar | x | 1 | ||||||||||||||||||||||||||||||||||||
| Stigmatomma | ||||||||||||||||||||||||||||||||||||||
| Stigmatomma celata | x | x | x | x | x | x | x | x | x | x | x | 10 | ||||||||||||||||||||||||||
| Stigmatomma gnoma | x | 1 | ||||||||||||||||||||||||||||||||||||
| Strumigenys | ||||||||||||||||||||||||||||||||||||||
| Strumigenys chyzeri | x | x | x | x | x | x | x | x | x | 9 | ||||||||||||||||||||||||||||
| Strumigenys decollata | x | 1 | ||||||||||||||||||||||||||||||||||||
| Strumigenys emmae | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Strumigenys eurycera | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Strumigenys frivaldszkyi | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Strumigenys godeffroyi | x | x | x | x | x | x | x | x | x | x | x | x | 11 | |||||||||||||||||||||||||
| Strumigenys karawajewi | x | x | x | x | x | x | 5 | |||||||||||||||||||||||||||||||
| Strumigenys membranifera | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Strumigenys mocsaryi | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Strumigenys rogeri | x | 1 | ||||||||||||||||||||||||||||||||||||
| Strumigenys szalayi | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Strumigenys undras | x | 1 | ||||||||||||||||||||||||||||||||||||
| Strumigenys yaleopleura | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Strumigenys sp. BP05 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Strumigenys sp. BP06 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Tapinoma | ||||||||||||||||||||||||||||||||||||||
| Tapinoma melanocephalum | x | x | x | x | x | x | x | x | x | 9 | ||||||||||||||||||||||||||||
| Tapinoma minutum | x | 0 | ||||||||||||||||||||||||||||||||||||
| Tapinoma timidum | x | 1 | ||||||||||||||||||||||||||||||||||||
| Technomyrmex | ||||||||||||||||||||||||||||||||||||||
| Technomyrmex albipes | x | x | x | x | x | x | x | x | x | x | x | x | x | 12 | ||||||||||||||||||||||||
| Technomyrmex vitiensis | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Technomyrmex sp. BP01 | x | 1 | ||||||||||||||||||||||||||||||||||||
| Tetramorium | ||||||||||||||||||||||||||||||||||||||
| Tetramorium antennatum | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Tetramorium aspersum | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Tetramorium bicarinatum | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Tetramorium carinatum | x | x | x | x | x | x | x | x | x | 9 | ||||||||||||||||||||||||||||
| Tetramorium insolens | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Tetramorium lanuginosum | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Tetramorium mayri | x | 1 | ||||||||||||||||||||||||||||||||||||
| Tetramorium melanogyna | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Tetramorium mutatum | x | 1 | ||||||||||||||||||||||||||||||||||||
| Tetramorium pacificum | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Tetramorium salomo | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Tetramorium simillimum | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Tetramorium tonganum | x | x | x | x | x | 5 | ||||||||||||||||||||||||||||||||
| Tetramorium vombis | x | 1 | ||||||||||||||||||||||||||||||||||||
| Tetraponera | ||||||||||||||||||||||||||||||||||||||
| Tetraponera laeviceps | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Turneria | ||||||||||||||||||||||||||||||||||||||
| Turneria dahlii | x | x | x | x | 4 | |||||||||||||||||||||||||||||||||
| Turneria pacifica | x | x | x | 3 | ||||||||||||||||||||||||||||||||||
| Vollenhovia | ||||||||||||||||||||||||||||||||||||||
| Vollenhovia dentata | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Vollenhovia marginata | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Vollenhovia elysii | x | x | x | 2 | ||||||||||||||||||||||||||||||||||
| Vollenhovia foveaceps | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Vollenhovia loboii | x | x | 1 | |||||||||||||||||||||||||||||||||||
| Vollenhovia oblonga | x | x | x | x | x | x | 6 | |||||||||||||||||||||||||||||||
| Vollenhovia pedestris | x | x | x | x | x | x | x | 7 | ||||||||||||||||||||||||||||||
| Vollenhovia subtilis | x | x | 2 | |||||||||||||||||||||||||||||||||||
| Vollenhovia sp. BP01* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Vollenhovia sp. BP02* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Vollenhovia sp. BP03* | x | 1 | ||||||||||||||||||||||||||||||||||||
| Wasmannia | ||||||||||||||||||||||||||||||||||||||
| Wasmannia auropunctata | x | 1 | ||||||||||||||||||||||||||||||||||||
| Total | 8 | 32 | 11 | 107 | 17 | 142 | 71 | 29 | 7 | 2 | 18 | 40 | 43 | 28 | 4 | 35 | 4 | 8 | 10 | 16 | 66 | 18 | 2 | 4 | 32 | 37 | 68 | 12 | 2 | 1 | 1 | 21 | 38 | 3 | 2 | 7 | 60 | 217 |