(C) 2013 Sergey G. Ermilov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Ermilov SG, Weigmann G, Tolstikov AV (2013) Morphology of adult and juvenile instars of Galumna obvia (Acari, Oribatida, Galumnidae), with discussion of its taxonomic status. ZooKeys 357: 11–28. doi: 10.3897/zookeys.357.6404
The adult instar of the oribatid mite, Galumna obvia (Berlese, 1914), is redescribed in detail, on the basis of specimens from Finland. The morphology of juvenile instars of G. obvia is described and illustrated for the first time, and compared to that of other species of the family Galumnidae. The position of the insertion of the lamellar seta in adults proved variable in studied European populations, being either on or medial to the lamellar line. Since the genera Galumna and Pergalumna are currently distinguished only by the relative positions of the seta and line, specimens of G. obvia in some populations show an intermediate situation between other studied Galumna species – with lamellar seta on or lateral of lamellar line – and Pergalumna with lamellar seta at a distinct distance medially of lamellar line. A detailed reevaluation of the two genera is needed.
Oribatida, Galumna obvia, morphology, supplementary description, juvenile instars, ontogeny, insertions of lamellar setae
The oribatid mite Galumna obvia (Oribatida, Galumnidae) was described by
The original descriptionand several redescriptions of adult Galumna obvia (see below, Taxonomic history) were incomplete, lacking measurements of morphological structures and information about leg setation and solenidia, and morphology of the gnathosoma. Further, lateral and ventral views of the idiosoma, which have important traits in this family, were insufficiently studied and illustrated. The present paper provides a detailed description and illustrations of Galumna obvia on the basis of 10 specimens collected in Finland as a reference population. Our data and a literature review show variation in the insertion of lamellar setae, relative to the lamellar line; since this insertion is considered important in distinguishing Galumna from Pergalumna, we discuss the generic position of Galumna obvia.
Additionally, we described and illustrated the morphology of juvenile instars of Galumna obvia. The family Galumnidae comprises more than 450 species, however, the full series of juvenile instars has been studied in detail only for eight species: Acrogalumna longiplumna (Berlese, 1904) (
Also, the juvenile instars have been described incompletely and/or illustrated in several species briefly, namely: Acrogalumna longiplumna (
Specimens of Galumna obvia were collected at the following locality: Finland, 64°24'10.78"N, 25°26'7.86"E, Päijänne National Park, Virmailansaari Island, near Padasjoki, 80 m a.s.l., Piceetum vaccinioso-hylocomiosum plant association, moss cover on stones and soil litter, 15.07.2013, collected by Andrei V. Tolstikov. The material collected in the field contained 10 adults, five larvae, two protonymphs and one deutonymph.
Comparative material for the taxonomic discussion originates from one Portuguese and some German locations:
Ribeira de Aljezur, Atlantic coast area of West-Algarve, Portugal, 37.347°N, 8.846°W, floodplain forest, 2011. Weigmann’s collection (Galumna tarsipennata);
River Oder Valley, Criewen; North-East Germany, 53.012°N, 14.233°E, moist deciduous forest, 1999. Weigmann’s collection (Galumna obvia);
“Berlin 1”; Berlin-Lübars, 52.62°N, 13.37°E, moist meadow, 1986. Weigmann’s collection (Galumna obvia);
“Berlin 2”; Postfenn, 52.498°N, 13.24°E, degraded moor, 1997. Weigmann’s collection (Galumna obvia);
“Berlin 3”; Berlin-Spandau, Teufelsbruch, 52.579°N, 13.205°E, moor area, 1997. Weigmann’s collection (Galumna obvia, Galumna alata);
“Berlin 4”; Berlin-Charlottenburg, 52.5°N, 13.35°E, park forests, 1995. Weigmann’s collection (Galumna lanceata);
“Oldesloe”; Brenner Moor, near Oldesloe, Schleswig-Holstein, North-West Germany, 53.78°N, 10.33°E, salty moor complex, 1973. Weigmann’s collection (Galumna obvia).
Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. All body measurements are presented in micrometers. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the ventral plate. Notogastral width refers to the maximum width in dorsal aspect. Lengths of body setae were measured in lateral aspect. Formulae for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formulae for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus. General terminology used in this paper mostly follows that summarized by Grandjean (see Travé and Vachon for references),
Oribates obvius Berlese, 1914: 119, pl. 1: 1. (type locality: Florence, Italy)
Galumna obvius:
Galumna obvia:
Galumna obvium:
Galumna “elimata” sensu van der Hammen 1952; nec
There is some confusion regarding the validity of Galumna obvia, which was declared as junior synonym of Galumna elimata (described as Oribates elimatus Koch, 1841 in CMA 31.5) firstly by
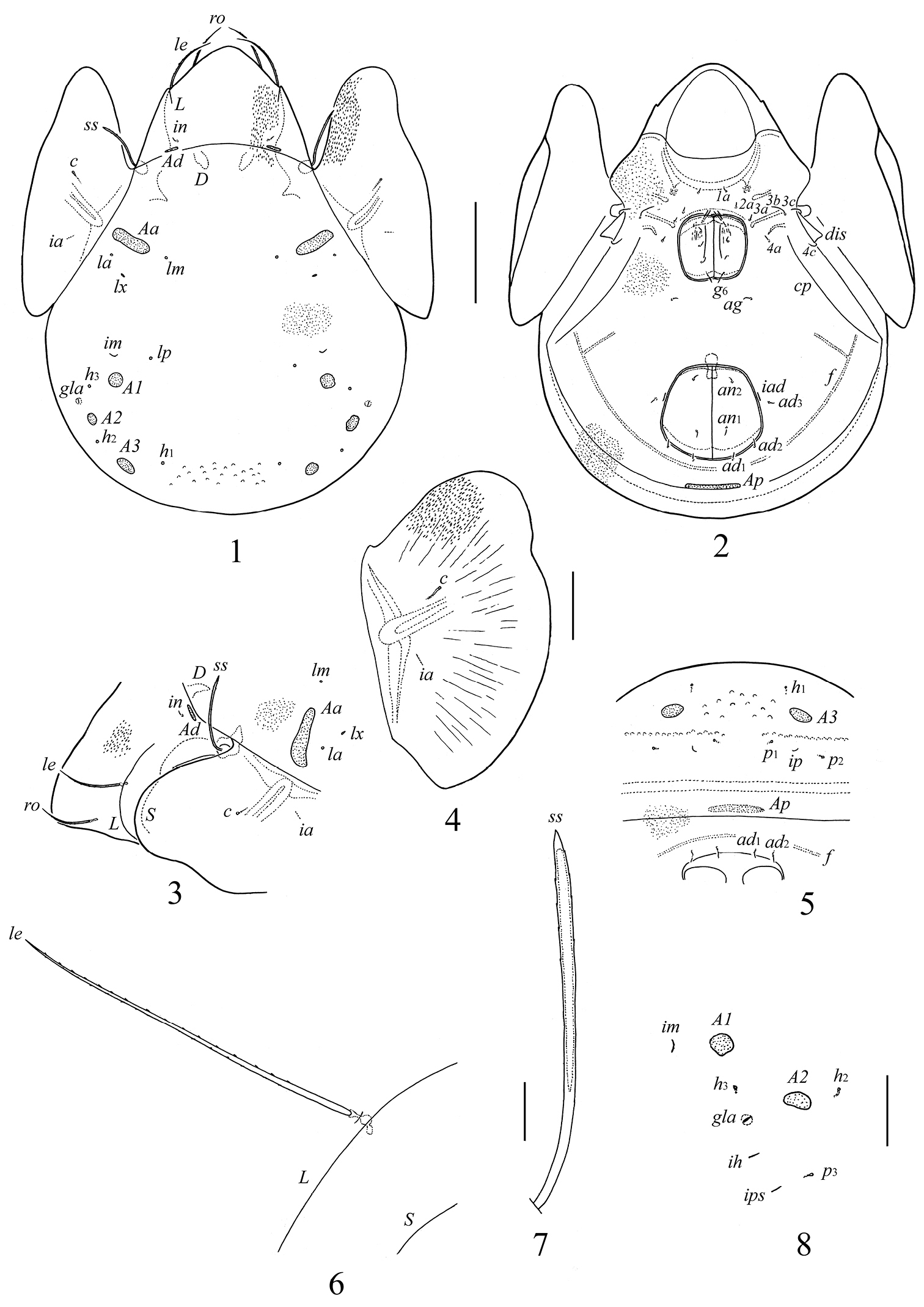
Figs 1–20
Galumna obvia, adult: 1 dorsal view 2 ventral view (gnathosoma and legs not shown) 3 anterior part of body, lateral view 4 pteromorph 5 posterior view 6 lamellar seta and parts of lamellar and sublamellar lines 7 sensillus 8 dorso-lateral part of notogaster, lateral view. Scale bars 200 μm (1–3, 5), 100 μm (4), 20 μm (6–8).
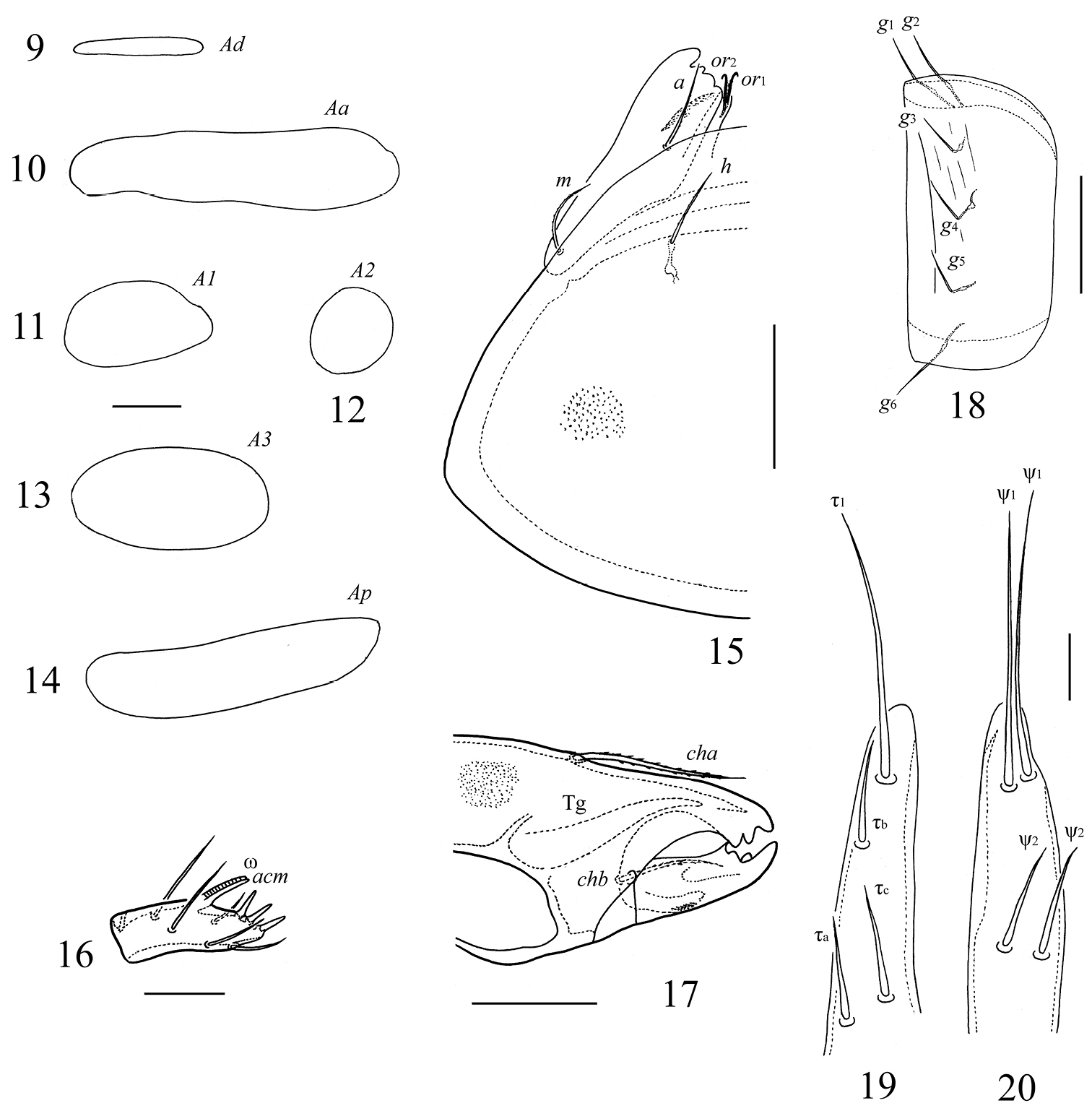
Galumna obvia, adult: 9 porose area Ad 10 porose area Aa 11 porose area A1 12 porose area A2 13 porose area A3 14 porose area Ap 15 subcapitulum, right half, ventral view 16 palptarsus 17 anterior part of chelicera 18 genital plate, left 19–20 lobes of ovipositor. Scale bars 20 μm (9–14, 16, 19, 20), 50 μm (15, 17, 18).
Measurements. Body length 846–898, width 630–647 (10 specimens, three males and seven females).
Integument. Body color brown to brownish-black. Body surface microfoveolate (well visible under high magnification); foveolae rounded (diameter up to 1) or represented by short lines (on prodorsum and pteromorphs). Posterior part of ventral plate with long, light furrow (f), located posterior and lateral to anal plates. Genital plates with one strong longitudinal fold located medial to genital setae; additional, short, weakly visible folds present in several specimens.
Prodorsum. Rostrum broadly rounded. Rostral (ro) and lamellar (le) setae setiform, barbed. Interlamellar setae (in) short, thin, smooth. Sensilli (ss) long, with weakly developed, elongate, barbed head pointed distally. Exobothridial setae absent. Relative length of prodorsal setae: ss ≈ le > ro > in; measurements given in Table 1. Lamellar (L) and sublamellar (S) lines distinct, parallel. Insertions of lamellar setae located medially to the lamellar lines, very close to them. Porose areas Ad (28–45 × 4–8), transversely oriented, thin, located posterolateral to interlamellar setae.
Comparison of body setae measurements of Galumna obvia during ontogeny
| Character | Larva | Protonymph | Deutonymph | Adult |
|---|---|---|---|---|
| n=5 | n=2 | n=1 | n=10 | |
| Length of prodorsal setae: | ||||
| – rostral setae | 53–61 | 57–61 | 69 | 86–98 |
| – lamellar setae | 45–53 | 53–57 | 61 | 143–164 |
| – interlamellar setae | 32–36 | 36–45 | 41 | 8–12 |
| – sensilli | 73–82 | 77–86 | 102 | 143–164 |
| – exobothridial setae | 32–36 | 45–49 | 45 | Absent |
| Length of gastronotic setae: | ||||
| – c3 | 45 | 45 | 45 | Absent |
| – h2 | 20–24 | 4–6 | 8 | Absent |
| – p2, p3 | Absent | 14–16 | 20 | Absent |
| – other gastronotic setae | 4 | 4–6 | 8 | Absent |
| Length of epimeral setae | 8–12 | 12–16 | 16 | 3b, 3c, 4c (28–36); 1a, 2a, 4a, 4b (12–20) |
| Length of anogenital setae: | ||||
| – genital setae | Absent | 12–16 | 16 | 12–20 |
| – aggenital setae | Absent | Absent | 16 | 8–16 |
| –anal setae | Absent | Absent | Absent | 8–16 |
| – adanal setae | Absent | Absent | 16 | 8–16 |
Notogaster. Anterior notogastral margin well developed. Dorsophragmata (D) of medium size. Notogastral setae represented by 10 pairs of alveoli. Four pairs of porose areas present: Aa (77–131 × 16–32) transversly oriented, elliptical to weakly boot-shaped; A1 (24–57 × 24–32) and A2 (24–53 × 20–28) round or oval; A3 (28–69 × 20–36) oval. All porose areas well visible, but without distinct margins. Alveoli of setae la inserted posterior to Aa. Median pore absent. All lyrifissures distinct; im located anterior to A1. Opisthonotal gland openings (gla) located anterolateral to A2.
Gnathosoma. Subcapitulum longer than wide (188–200 × 172–176). Subcapitular setae (a, m, h) similar in length (28–36), setiform, slightly barbed. Adoral setae (or1, or2) (16–20) setiform, barbed. Palps (147–155) with setation 0–2–1–3–9(+ω); solenidion straight. Chelicerae (225–241) with two setiform, barbed setae; cha (65–73) longer than chb (45–53). Trägårdh’s organ (Tg) distinct, elongate conical.
Epimeral and lateral podosomal regions. Apodemes 1, 2, sejugal and 3 well visible. Seven pairs of setiform, smooth epimeral setae observed; setal formula: 1–1–3–2. Setae 3b, 3c and 4c longer than 1a, 2a, 4a and 4b (Table 1). Discidia (dis) triangular. Circumpedal carinae (cp) distinct.
Anogenital region. Six pairs of genital (g1–g6), one pair of aggenital (ag), two pairs of anal (an1, an2) and three pairs of adanal (ad1–ad3) setae setiform, thin, smooth (Table 1). Anterior edge of genital plates with two setae. Adanal setae ad3 inserted laterally or slightly postero-laterally to lyrifissures iad. Postanal porose area (Ap, 73–110 × 12–24) transversly oriented, oblong. Ovipositor of typical form for Galumnidae (
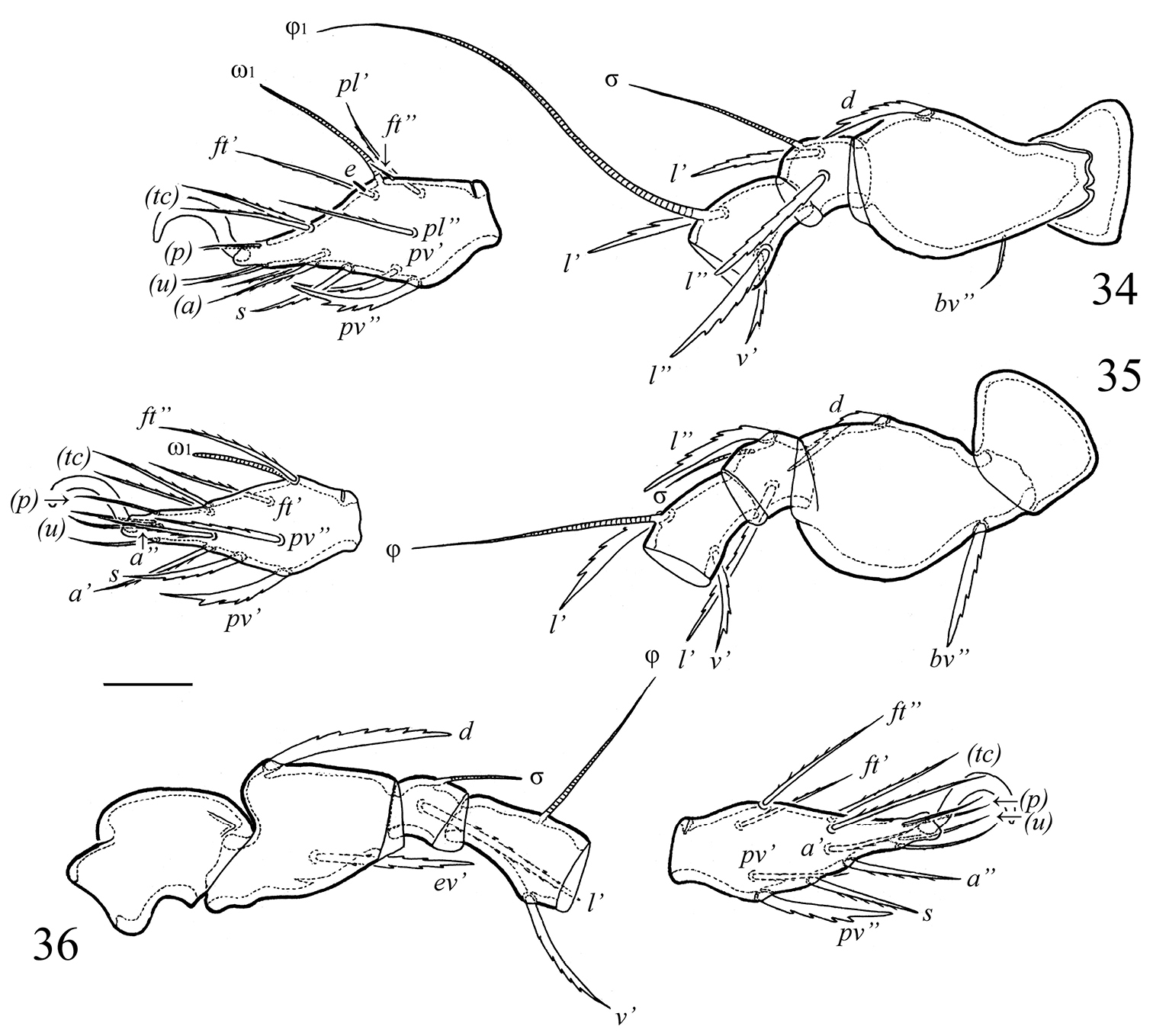
Legs. Morphology of leg segments, setae and solenidia typical for Galumnidae (
Leg setation and solenidia of adult Galumna obvia
| Leg | Trochanter | Femur | Genu | Tibia | Tarsus |
|---|---|---|---|---|---|
| I | v’ | d, (l), bv’’ | (l), v’, σ | (l), (v), φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), v’, (pl), l’’, e, ω1, ω2 |
| II | v’ | d, (l), bv’’ | (l), v’, σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2 |
| III | v’ | d, ev’ | l’, σ | l’, (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v’ | d, ev’ | d, l’ | l’, (v), φ | ft’’, (tc), (p), (u), (a), s, (pv) |
Roman letters refer to normal setae (e to famulus), Greek letters to solenidia. Single prime (’) marks setae on anterior and double prime (’’) setae on posterior side of the given leg segment. Parentheses refer to a pseudosymmetrical setae
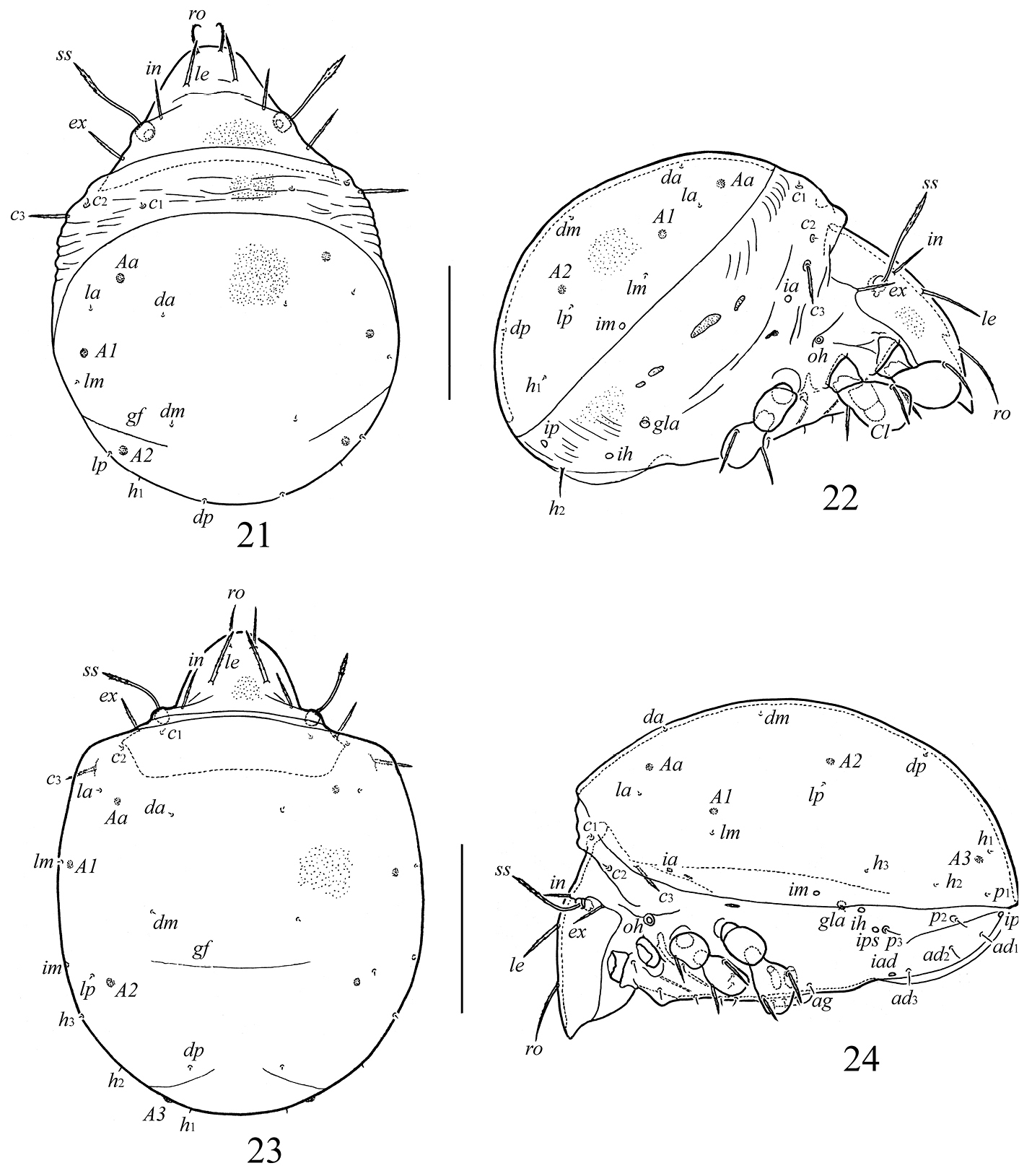
(Figs 21–30)
Galumna obvia, juvenile instars: 21 larva, dorsal view 22 larva, lateral view (gnathosoma and legs except basal parts not shown) 23 deutonymph, dorsal view 24 deutonymph, lateral view (gnathosoma and and legs except basal parts not shown). Scale bars 100 μm (21, 22), 200 μm (23, 24).
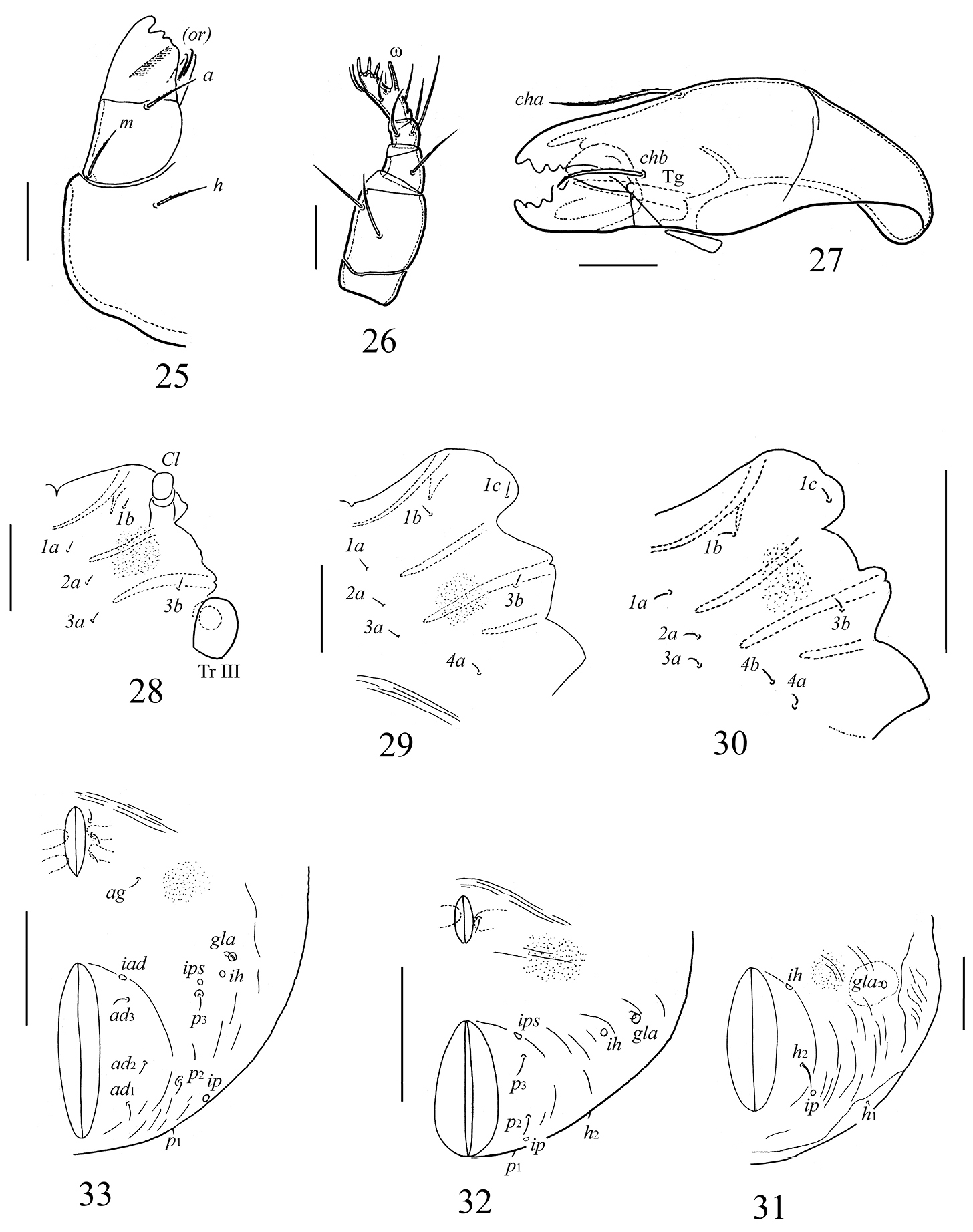
Galumna obvia, juvenile instars: 25 subcapitulum, right half, ventral view 26 palp 27 chelicera 28 epimeral region of larva 29 epimeral region of protonymph 30 epimeral region of deutonymph 31 anogenital region of larva 32 anogenital region of protonymph 33 anogenital region of deutonymph. Scale bars 20 μm (25–27), 50 μm (28, 29, 31), 100 μm (30, 32, 33).
Dimensions. Length: larva 344–352 (five specimens), protonymph 431, 435 (two specimens), deutonymph 564 (one specimen). Width: larva 246–254, protonymph 332, 336, deutonymph 431.
Integument. Prodorsum, gastronotic shield, gnathosoma and legs light brownish; dorsosejugal and epimeral regions and lateral sides colorless to yellowish. Sclerotized body cuticle microfoveolate (diameter foveolae up to 1); soft dorsosejugal, lateral and anogenital regions region with some folds.
Prodorsum. Relatively short, about 1/2 length of gastronotic region. Rostrum broadly rounded. Rostral, lamellar, interlamellar and exobothridial setae setiform, barbed, inserted on small tubercles. Sensilli with long stalk and weakly developed, lanceolate, barbed head. Relative length of prodorsal setae: ss > ro > le > in ≈ ex; measurements compared in Table 1.
Gastronotic region. Dorsal gastronotic region with large, well-bordered shield (macrosclerite) in all juvenile instars. Transversal gastronotic furrow present (gf), poorly visible. Lateral sides with several small, elongate sclerites (smaller and weakly visible in nymphs than in larva). Larva with 11 pairs of gastronotic setae, proto- and deutonymphs with 15 pairs. Setae c1–c3 and p2–p3 (in deutonymph) on small sclerites. Gastronotic shield with seven pairs of setae (da, dm, dp, la, lm, lp, h1) in larva, 10 pairs (da, dm, dp, la, lm, lp, h1–h3, p1) in proto- and deutonymphs. Gastronotic setae c3 longest, straight, barbed; h2 in larva shorter, setiform, slightly barbed; p2, p3 in proto- and deutonymph setiform, smooth; other setae very short, thin, smooth (Table 1). Porose areas rounded, poorly visible: larva with three pairs (6–8, Aa, A1, A2), proto- (8) and deutonymph (12) with four pairs (A3 present additionally). Cupules ia, im and ip clearly visible. Humeral organ (oh) well developed.
Gnathosoma. Similar to that of adult instar.
Epimeral region. Setal formulae for epimeres: larva 3–1–2 (larval seta 1c scale-like, covering tip of retracted Claparède’s organ); protonymph 3–1–2–1; deutonymph 3–1–2–2. Epimeral setae setiform, smooth (Table 1).
Anogenital region. Ontogenetic genital, aggenital, adanal, anal formulae (larva to deutonymph): 0–1–3, 0–0–1, 0–0–3, 0–0–0, respectively. All setae setiform, thin, smooth (Table 1). Paraproctal setae absent. Cupules ih, ips, iad and opisthonotal gland openings clearly visible, appearing in normal ontogenetic pattern.
Legs. Ontogeny of leg setae and solenidia given in Table 3.
Development of leg setation of Galumna obvia during ontogeny.
| Trochanter | Femur | Genu | Tibia | Tarsus | |
|---|---|---|---|---|---|
| Leg I | |||||
| Larva | – | d, bv’’ | (l), σ | (l), v’, φ1 | (ft), (tc), (p), (u), (a), s, (pv), (pl), e, ω1 |
| Protonymph | – | – | – | – | ω2 |
| Deutonymph | – | (l) | – | φ2 | – |
| Leg II | |||||
| Larva | – | d, bv’’ | (l), σ | l’, v’, φ | (ft), (tc), (p), (u), (a), s, (pv), ω1 |
| Protonymph | – | – | – | – | – |
| Deutonymph | – | l’’ | – | l’’ | ω2 |
| Leg III | |||||
| Larva | – | d, ev’ | l’, σ | v’, φ | (ft), (tc), (p), (u), (a), s, (pv) |
| Protonymph | – | – | – | – | – |
| Deutonymph | v’ | – | – | v’’ | – |
| Leg IV | |||||
| Protonymph | – | – | – | – | ft’’, (p), (u), (pv) |
| Deutonymph | – | d, ev’ | d, l’ | v’, φ | (tc), (a), s |
See Table 1 for explanations. Setae are listed only for the instar in which they first appear.
The adult specimens of Galumna obvia collected in Finland correspond to earlier redescriptions (
Juvenile instars (larva, proto- and deutonymph) of Galumna obvia correspond to those of other Galumnidae in many characters (cf.
From Pilogalumna (Pilogalumna crassiclava, Pilogalumna ornatula, Pilogalumna tenuiclava) by: the length of prodorsal setae (ro longest in Galumna obvia versus in longest in Pilogalumna species); length of gastronotic setae of c-series (c3 of medium size, c1 and c2 short, c3 > c1 ≈ c2 in Galumna obvia versus c3 and c2 of medium size, c1 short, c3 > c2 > c1 in Pilogalumna species); and number of gastronotic setae and length of setae h1 in larval instar (11 pairs – h3 absent, h1 short in Galumna obvia versus 12 pairs – h3 present, h1 of medium size in Pilogalumna species).
From Acrogalumna (Acrogalumna longipluma) by: the length of prodorsal setae (ro longest in Galumna obvia versus le longest in Acrogalumna longipluma); the length of gastronotic setae of c-serie (c3 of medium size, c1 and c2 short, c3 > c1 ≈ c2 in Galumna obvia versus c3 and c2 of medium size, c1 short, c3 > c2 > c1 in Acrogalumna longipluma).
From Allogalumna (Allogalumna alamellae) by: the length of prodorsal setae (ro > le > in in Galumna obvia versus in > ro ≈(>) le in Allogalumna alamellae); the length of gastronotic setae of c-serie (c3 of medium size, c1 and c2 short, c3 > c1 ≈ c2 in Galumna obvia versus c3 and c2 of medium size, c1 short, c3 ≈ c2 > c1 in Allogalumna alamellae).
From Galumna species: from Galumna alata by the length of prodorsal setae (ro > le > in in Galumna obvia versus in > ro > le in larva, in ≈(>) le > ro in nymphal instars in Galumna alata), the length of gastronotic setae of c-series (c3 of medium size, c1 and c2 short, c3 > c1 ≈ c2 in Galumna obvia versus c3 and c2 of medium size, c1 short, c2 > c3 > c1 in Galumna alata); from Galumna zachvatkini by the length of gastronotic setae (c3 of medium size, c1, c2 and other dorsal setae short, c3 > c1 ≈ c2 in Galumna obvia versus c1, c2, c3 and other dorsal setae well developed, of medium size, c1 ≈ c2 ≈c3 in Galumna zachvatkini), and number of gastronotic setae in larval instar (11 pairs – h3 absent in Galumna obvia versus 12 pairs – h3 present in Galumna zachvatkini).
From Pergalumna (Pergalumna nervosa) by: the length of prodorsal setae (ro > le > in in Galumna obvia versus le ≈(>) ro > in Pergalumna nervosa); the length of gastronotic setae of c-serie and number of gastronotic setae and length of setae h1 in larval instar (c3 of medium size, c1 and c2 short, c3 > c1 ≈ c2, 11 pairs setae present – h3 absent in Galumna obvia versus c3 and c2 of medium size, c1 short, c3 ≈ c2 > c1, 12 pairs setae present – h3 present in Pergalumna nervosa).
Thus, the diagnostic morphological characters of Galumnidae juvenile instars are not numerous and can be summarized as: the length of rostral, lamellar and interlamellar setae; the number of gastronotic setae in larval instar; the length of gastronotic setae of c-series, dp, h1; the presence or absence of a transverse furrow on gastronotic shield and genital and adanal macrosclerites on the ventral side in nymphal instars); and body size.
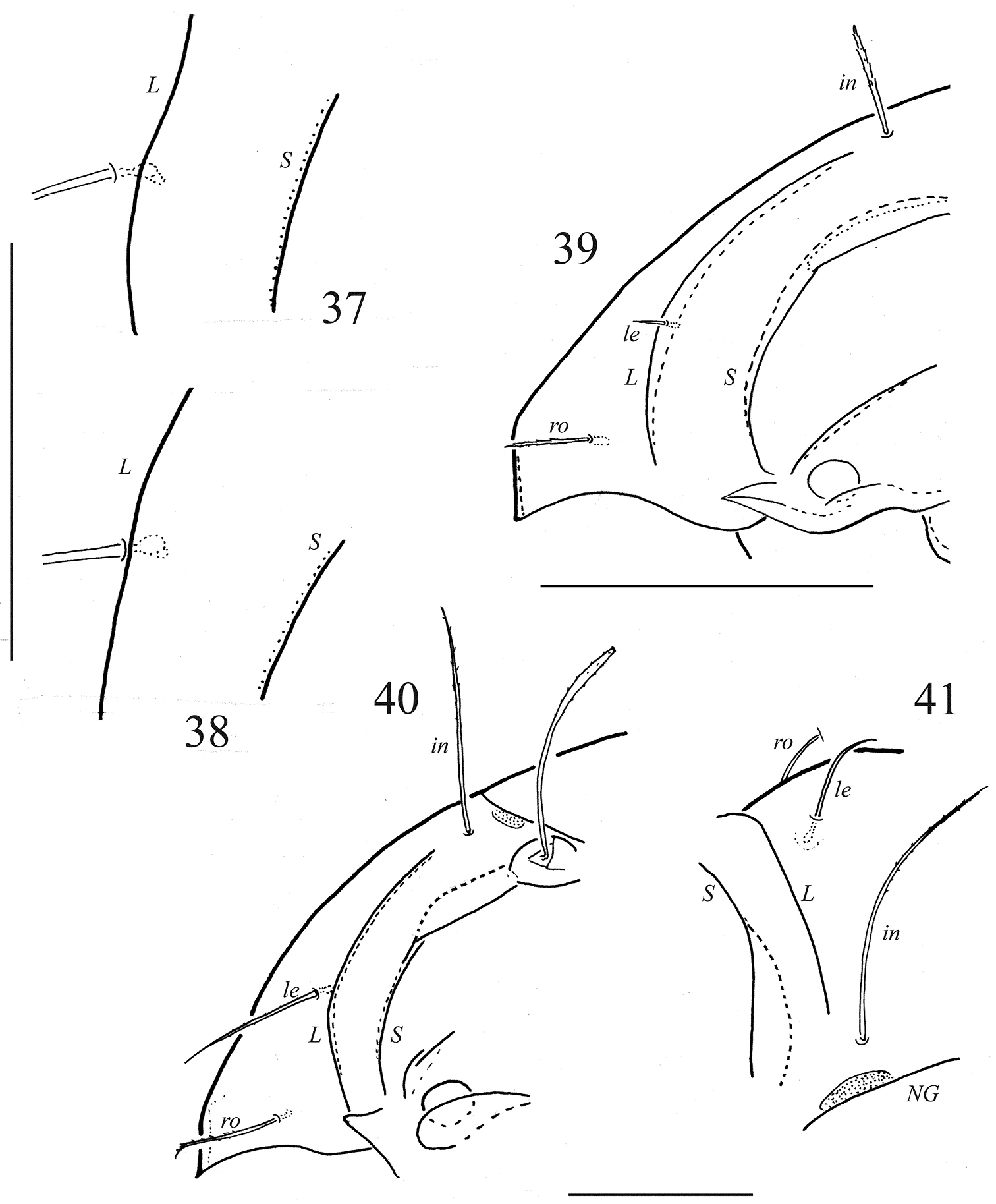
In the Finnish population of Galumna obvia, the lamellar seta (le) inserts medial to the lamellar line, at a distance of about 5 µm; no distinct variability is observed. The conventional definition of the genus Galumna includes the differential character “lamellar seta on (at) the lamellar line” in contrast to the definition of the genus Pergalumna Grandjean, 1936, originally as subgenus with the differential character “lamellar seta in some distance medially to the lamellar line” (
We compared the characters of the Finnish population of Galumna obvia with those in some other European populations, especially from northwest to northeast of Germany; and we found no convincing character combinations to exclude the Finnish population from Galumna obvia, regarding body size (indicated for German populations with 705–845 µm total body length:
Galumna obvia, juvenile instars: 34 leg I, left, antiaxial view 35 leg II, left, antiaxial view 36 leg III, right, paraxial view. Scale bar 20 μm.
Adults of Galumnidae: 37 Galumna obvia, lamellar region of prodorsum, lateral view (specimen from Berlin) 38 Galumna obvia, lamellar region of prodorsum, lateral view (specimen from Oder Valley, North-East Germany) 39 Galumna paragibbula, lateral view of prodorsum 40 Pergalumna nervosa, lateral view of prodorsum 41 Pergalumna nervosa, dorso-frontal view of left part of prodorsum (depressed mounted specimen). Abbreviation: NG– notogastral shield. Scale bar 100 μm.
These slight differences of the position of seta le raise a similar question with regard to other Galumna species. Since species descriptions, redescriptions and illustrations are often not sufficiently precise, we give only selective examples. In the type species of Galumna, Galumna alata (Hermann, 1804),
Comparing the position of seta le in strict lateral aspect in Pergalumna nervosa (Fig. 40) and in Galumna obvia (Figs 6, 37), there seems to be less difference: in both species the seta seems to be inserted a short distance medially from the lamellar line. Yet in dorso-frontal aspect without parallactic error, the distance between le and the lamellar line is about 27 µm in Pergalumna nervosa (Fig. 41), in Galumna obvia at most 6 µm.
As a preliminary conclusion, most studied Galumna species have the seta le inserted a short distance lateral to the lamellar line; in Galumna alata the seta is positioned on the line or lateral to it. Galumna obvia is the only species observed with a le insertion medial to the lamellar line or in some specimens on it. The latter two species both show some variability of the le insertion.
The differentiation of the genera Galumna and Pergalumna, defined by
An analogous case in the family Malaconothridae relates to the single argument to differentiate Malaconothrus Berlese, 1904 from Trimalaconothrus Berlese, 1916, by the typological characters “monodactylous or tridactylous legs”. This character state is easy to distinguish but obviously without phylogenetical value.
We gratefully acknowledge Prof. Dr. Roy A. Norton (State University of New York, College of Environmental Science and Forestry, Syracuse, USA) and Prof. Dr. Badamdorj Bayartogtokh (National University of Mongolia, Ulaanbaatar, Mongolia) for valuable comments. The present research was in part supported by the grant of the Academy of Finland “Linking environmental change to biodiversity change: long-term and large-scale data on European boreal forest biodiversity (EBFB)”, a Finnish-Swedish-Russian collaborative project for 2011–2015. The authors are thankful to Prof. Dr. Otto Ovaskainen and Dr. Juri Kurhinen (University of Helsinki, and Ilpo Hanski, Finnish Museum of Natural History) for the opportunity to collaborate on boreal forest soil fauna.