(C) 2013 Marnix P. de Zeeuw. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of zooplanktivorous haplochromine cichlids from Lake Victoria, Tanzania, are described and illustrated. These species closely resemble each other. Their affinities to other zooplanktivorous haplochromines from Lake Victoria are discussed. Haplochromis argens sp. n., which featured under nicknames (mainly Haplochromis “argens”) in more than 50 papers, was caught both in the Mwanza Gulf and the Emin Pasha Gulf, whereas Haplochromis goldschmidti sp. n. was only found in the Emin Pasha Gulf. Of the latter species only males are available, but it seems unlikely that it represents a case of male colour polymorphism as several presumably unrelated characters differ in sympatry between the two species, suggesting that there is no gene flow. Statistical analysis revealed that the overall difference between the two species is greater than that between the populations from the two locations. Body depth of the two species in sympatry in the Emin Pasha Gulf was more similar than that of Haplochromis goldschmidti sp. n. and the allopatric population of Haplochromis argens sp. n. from the Mwanza Gulf, which mayindicate an overall environmental effect. However, several measurements related to the width of snout and mouth differed more between the populations of the two species in sympatry than between the allopatric populations. In contrast to a group of zooplanktivorous species that recovered successfully after environmental changes in the lake, Haplochromis argens sp. n. is among a group that became extremely rare and probably is in danger of extinction; the conservation status of Haplochromis goldschmidti sp. n. is currently unknown.
Allopatric populations, Cichlidae, colour polymorphism, East Africa, endangered species, Haplochromis, zooplanktivores
In this paper two zooplanktivorous haplochromine species from Lake Victoria are described. One of these species, nicknamed Haplochromis “argens, ” was common in the Mwanza Gulf until 1985 and also caught in other areas in the Tanzanian part of the lake. Since the late 1970s, this species has been the subject of studies on ecology (e.g.
Up to the 1980s, the zooplanktivorous haplochromines were, both in number and biomass, the second most abundant group of demersal fishes in sub-littoral areas of the Mwanza Gulf. A one hour tow of a bottom otter trawl (head rope 25 m) contained on average 1140 kg of haplochromines, of which 27% (more than 100, 000 individuals) were zooplanktivores (
From 1987 to 1992, haplochromines were extremely rare in trawl catches in the Mwanza Gulf; however, with the subsequent decline of Nile perch due to heavy fishing, a slow recovery of some zooplanktivorous species was noticed (
As Haplochromis “argens” has already made its appearance in more than 50 articles, is bred in captivity by scientists and hobbyists, and currently is caught again in the lake, a formal taxonomic description is urgently needed and presented in this paper.
In the Emin Pasha Gulf, males of Haplochromis “argens” and males that resembled this species were caught in the same hauls. The latter males differed from Haplochromis “argens” in aspects of their overall nuptial body colouration, the colour of their caudal fin, and number and position of the egg spots on the anal fin (
The type specimens of both species were collected in the Tanzanian part of Lake Victoria. All type material was collected between May 1975 and August 1986, before the collapse of the haplochromines in the Tanzanian part of the lake due to the Nile perch upsurge and eutrophication (e.g.
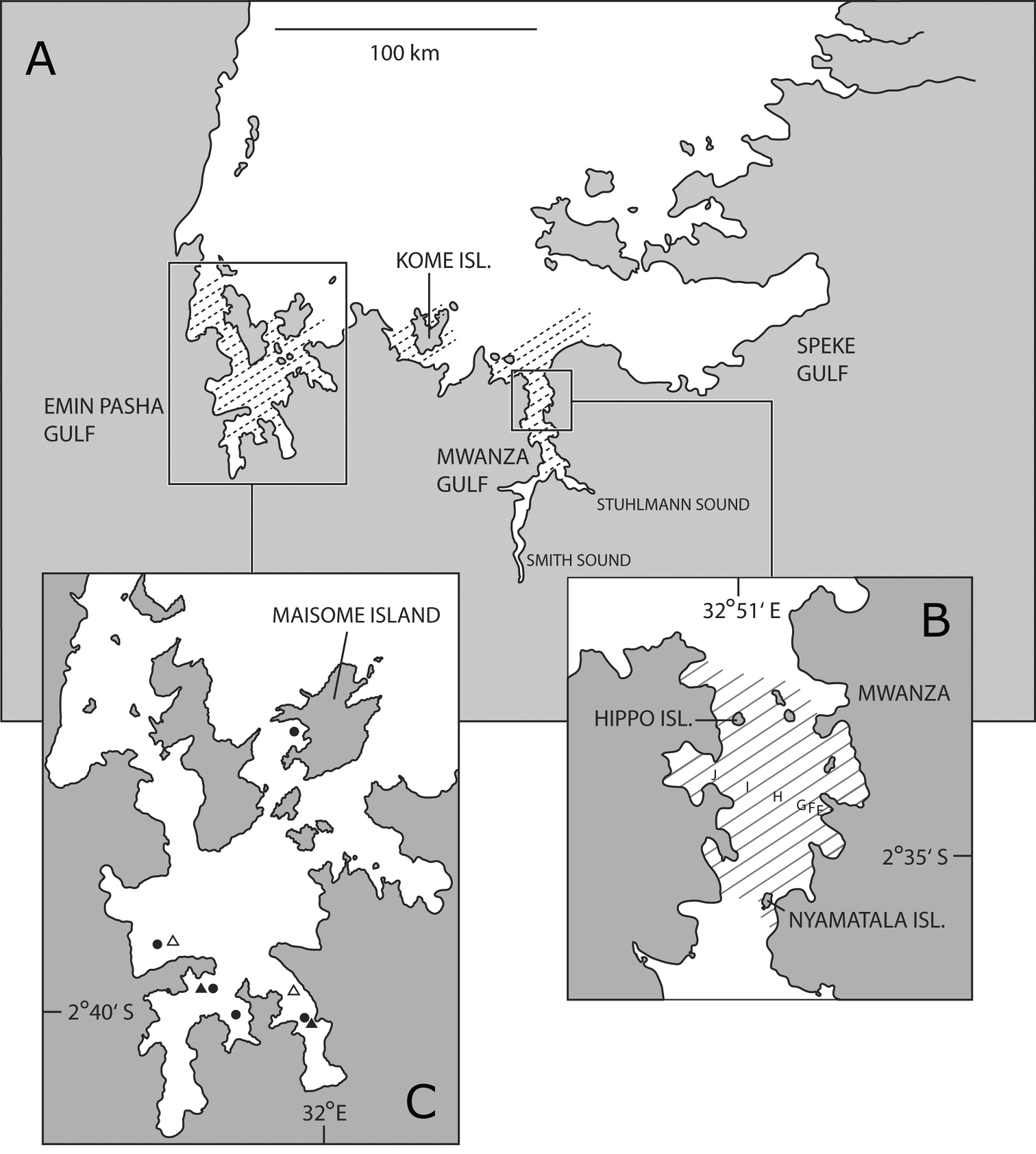
Tanzanian part of Lake Victoria. A, distribution of Haplochromis argens sp. n.(hatched area) B Mwanza Gulf with catch localities of type specimens of Haplochromis argens sp. n.(hatched area); E to J represent stations along the research transect where data on abundance of Haplochromis argens sp. n. were collected C Emin Pasha Gulf with catch localities of type specimens of Haplochromis argens sp. n. (filled circles) and type specimens of Haplochromis goldschmidti sp. n. (filledtriangles); open triangles indicate other catch localities of Haplochromis goldschmidti sp. n.
Ten male specimens of Haplochromis “argens” were dissected. The oral jaws were removed from nine specimens and the pharyngeal jaws from four specimens. From six specimens the first gill arch was dissected. Of Haplochromis “dusky argens, ” six specimens were dissected. From five specimens the oral jaws and the intestines were removed and from three specimens the pharyngeal jaws and the first gill arch. The description of the shape of the oral and pharyngeal jaws and their dentition was based on the dissected elements, as were the counts of the gill filaments.
Linear measurements and counts were collected from 57 specimens of Haplochromis “argens” and 19 specimens of Haplochromis “dusky argens.” Counts included numbers of scales, teeth, gill rakers and vertebrae, the latter obtained from radiographs. Additional specimens for which counts and measurements were not made are also designated as type specimens; these include specimens used for colour descriptions (from colour slides) and other qualitative characters, specimens used for dissection, and specimens from which tissue samples for DNA analysis had been taken. Terminology and measurements follow
Measurements of Haplochromis argens, proportional to standard length or head length. Means and standard deviations were calculated over all measured type specimens, including the holotype.
| Holotype | Paratypes (n = 56) | Mean ± SD (n = 57) | ||
|---|---|---|---|---|
| standard length SL (mm) | 67.3 | 53.1–75.5 | 63.1 ± 5.4 | |
| body depth (BD) | %SL | 28.8 | 26.0–30.7 | 28.2 ± 1.1 |
| pectoral fin length (PFL) | %SL | 27.3 | 25.4–30.9 | 27.8 ± 1.2 |
| caudal peduncle length (CPL) | %SL | 21.7 | 18.5–23.5 | 20.7 ± 1.1 |
| caudal peduncle depth (CPD) | %SL | 9.7 | 9.1–11.1 | 10.0 ± 0.5 |
| caudal fin length (CFL) | %SL | 22.7 | 20.1–25.5 | 23.9 ± 1.0 |
| head length (HL) | %SL | 31.7 | 31.0–35.6 | 32.9 ± 0.9 |
| snout length (SnL) | %HL | 26.3 | 23.6–29.7 | 26.6 ± 1.6 |
| snout width (SnW) | %HL | 27.6 | 20.6–27.8 | 24.5 ± 1.7 |
| head width (HW) | %HL | 41.3 | 32.0–43.8 | 40.0 ± 1.8 |
| interorbital width (IOW) | %HL | 22.1 | 18.8–23.8 | 20.8 ± 1.1 |
| preorbital width (POW) | %HL | 24.4 | 20.6–25.7 | 23.4 ± 1.2 |
| lachrymal width (LaW) | %HL | 21.6 | 17.3–23.2 | 20.2 ± 1.4 |
| preorbital depth (POD) | %HL | 15.5 | 13.1–18.7 | 15.3 ± 1.2 |
| eye length (EyL) | %HL | 36.6 | 30.9–39.0 | 35.7 ± 2.1 |
| eye depth (EyD) | %HL | 33.4 | 27.4–35.2 | 31.6 ± 1.7 |
| cheek depth (ChD) | %HL | 16.9 | 11.9–20.5 | 16.7 ± 1.7 |
| lower jaw length (LJL) | %HL | 40.4 | 37.6–44.3 | 40.5 ± 1.5 |
| lower jaw width (LJW) | %HL | 13.6 | 11.0–15.6 | 13.2 ± 1.0 |
| EyD/EyL | 0.9 | 0.8–1.0 | 0.9 ± 0.04 | |
| LJL/LJW | 3.0 | 2.6–3.8 | 3.1 ± 0.27 |
Measurements were taken to the nearest 0.1 mm using digital callipers with needles glued to the ends. For comparison with earlier described haplochromine species from Lake Victoria (e.g.
A comparison was made between Haplochromis “dusky argens” and the two populations of Haplochromis “argens” from the Mwanza Gulf and the Emin Pasha Gulf. As no females are available for Haplochromis “dusky argens, ” we only compared males. To test for differences in linear measurements of the three populations in general, MANCOVA was used. ANCOVAs were used to identify differences among the three populations in specific linear measurements. Data were log-e transformed to achieve linearity. The factors “Species” (two species) and “Location” (two locations) were investigated, and log standard length (SL) was used as covariate. Parameter estimates derived from the GLM (analysis of covariance) procedure were used to define and plot the power function between SL and individual taxonomic measurements and to calculate the relative differences in individual measurements between Haplochromis “dusky argens” and the two populations of Haplochromis “argens”. For testing of interactions and main effects, Sum of Squares Type II was used. Whether the residuals followed a normal distribution was investigated with the Kolmogorov-Smirnov Test. For statistical analysis of the morphometric data, SPSS version 14.0 for Windows was used.
Live (juvenile) Haplochromis (Psammochromis) cassius Greenwood & Barel, 1978, are similar to Haplochromis “argens”. To compare Haplochromis “argens” with Haplochromis cassius which was described from only five females (some of them much larger than Haplochromis “argens”), we used measurements from six specimens of Haplochromis cassius: three specimens from the collection of Naturalis Biodiversity Center (RMNH.PISC.63199, RMNH.PISC.63200 and RMNH.PISC.74187) and three specimens from the collection of the Natural History Museum, London (BMNH 1987.2.4.1, BMNH 1987.2.4.2 and BMNH 1987.2.4.5). These specimens have a size range (67.4 – 79.3 mm SL) comparable to that of Haplochromis “argens”.
To describe changes in abundance of Haplochromis “argens” during the period 1979 to 2011, we compared the frequency of occurrence (= percentage of catches containing one or more individuals of Haplochromis “argens”) and the average numbers of individuals that were caught in trawl tows of 10 minutes duration with a small boat (20 or 25 hp) on a research transect in the Mwanza Gulf (Fig. 1; Witte et al. 1992). A bottom otter trawl (head rope 4.6 m, codend mesh 5 or 15 mm) was used during the day and a surface trawl (beam 4.5 m, codend mesh 5 mm) during the night.
Specimens referred to in this study are deposited in the Naturalis Biodiversity Center, Leiden (RMNH), the American Museum of Natural History, New York (AMNH), the Natural History Museum, London (BMNH) and the National Museum of Nature and Science, Tsukuba (NSMT), Japan.
urn:lsid:zoobank.org:act:B0288AFB-9C11-4D81-9B7B-E551951A7B2D
http://species-id.net/wiki/Haplochromis_argens
Figs 2–5, Tables 1, 2Haplochromis argens:
Haplochromis “argens”;
H. “Argens”;
Haplochromis (?) “argens”;
Haplochromis sp. “argens”;
Yssichromis argens;
Yssichromis sp. “argens”;
Type-locality. Tanzania, Lake Victoria, Mwanza Gulf (ca 2°29'–2°36'S; 32°48'–32°54'E) and Emin Pasha Gulf (ca 2°18'–2°41'S; 31°47'–31°59' E).
Holotype. RMNH.PISC.835884, ♂, 67.3 mm SL, Tanzania, Lake Victoria, Mwanza Gulf, 8.iii.1979, HEST.
Paratypes. All type specimens collected by Haplochromis Ecology Survey Team (HEST) in Mwanza Gulf, Tanzania, Lake Victoria, except where noted otherwise. Size of specimens given as standard length. RMNH.PISC.728315, ♀, 71.0 mm, 30.v.1980; RMNH.PISC.728845, 6, ♂, 74.4 mm, 31.v.1980; RMNH.PISC.73097, ♀, 71.3 mm, 27.ix.1977; RMNH.PISC.812025, ♀, 65.6 mm, 11.v.1978; RMNH.PISC.83587, ♂, 77.4 mm, 31.v.1975; RMNH.PISC.835894, ♀, 75.5 mm, 21.iv.1980; RMNH.PISC.835904, ♂, 68.2 mm, 22.iv.1980; RMNH.PISC.836064, ♂, 73.7 mm, 22.iv.1980; RMNH.PISC.836074, ♂, 65.7 mm, 15.iv.1980; RMNH.PISC.836085, ♂, 65.7 mm, 31.v.1975; RMNH.PISC.836094, ♂, 67.4 mm, 22.iv.1980; RMNH.PISC.836104, ♂, 66.2 mm, 30.ix.1977; RMNH.PISC.836114, ♂, 69.5 mm, 30.ix.1977; RMNH.PISC.836124, ♀, 71.1 mm, 30.ix.1977; RMNH.PISC.836134, ♀, 68.1 mm, 30.ix.1977; RMNH.PISC.836144, ♀, 71.9 mm, 8.ix.1977; RMNH.PISC.836154, ♂, 57.7 mm, 8.ix.1977; RMNH.PISC.836164, ♂, 70.2 mm, 19.viii.1977; RMNH.PISC.836174, ♀, 71.5 mm, 19.viii.1977; RMNH.PISC.836184, ♂, 66.1 mm, 7.ix.1977; RMNH.PISC.836194, ♂, 66.5 mm, 21.xii.1977; RMNH.PISC.836204, ♂, 65.8 mm, 10.x.1977; RMNH.PISC.836211, ♂, circa 70 mm, 30.ix.1977; RMNH.PISC.836225, ♂, 59.3 mm, 22.vi.1985, Emin Pasha Gulf; RMNH.PISC.836235, ♂, 57.2 mm, 22.vi.1985, Emin Pasha Gulf; RMNH.PISC.836245, ♂, 59.6 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836254, ♂, 58.3 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836264, ♂, 58.5 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836274, ♂, 54.3 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836284, ♂, 59.6 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836294, ♂, 55.4 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836304, ♂, 53.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836314, ♂, 66.8 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836324, ♂, 67.0 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836334, ♂, 60.4 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836344, ♂, 53.9 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836354, ♂, 62.6 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836364, ♂, 66.6 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836374, ♂, 69.2 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836384, ♂, 65.8 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836394, ♂, 61.3 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836404, ♂, 73.4 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836414, ♂, 64.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836424, ♂, 63.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836434, ♂, 63.7 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836444, ♂, 62.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836454, ♂, 64.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836464, ♂, 61.6 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836474, ♂, 59.8 mm, 25.viii.1981; RMNH.PISC.836484, ♂, 61.6 mm, 10.x.1977; RMNH.PISC.836494, ♂, 61.5 mm, 21.xii.1977; RMNH.PISC.836504, ♂, 57.7 mm, 6.iii.1979; RMNH.PISC.836514, ♂, 60.6 mm, 13.vii.1979; RMNH.PISC.836524, ♂, 58.0 mm, 1.ii.1979; RMNH.PISC.836534, ♂, 53.5 mm, 18.xi.1981; RMNH.PISC.836554, ♂, 61.8 mm, 14.v.1979; RMNH.PISC.836564, ♂, 59.0 mm, 27.iii.1979; RMNH.PISC.836574, ♂, 61.6 mm, 27.iii.1979; RMNH.PISC.836604, ♂, 56.8 mm, 12.iii.1979; RMNH.PISC.836614, ♂, 57.9 mm, 12.iii.1979; RMNH.PISC.836624, ♂, 59.7 mm, 14.v.1979; RMNH.PISC.836634, ♂, 58.6 mm, 14.v.1979; RMNH.PISC.836734, ♂, 55.5 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836881–3, ♂, 61.4 mm, 6.iii.1979; RMNH.PISC.836891–3, ♂, 62.2 mm, 14.v.1979; RMNH.PISC.836941, 5, ♂, 63.1 mm, 21.vi.1985, Emin Pasha Gulf; RMNH.PISC.836971, 5, ♂, 59.1 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.836981–3, 5, ♂, 61.0 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.837033, 6, ♂, 66.4 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.837041, 3, 6, ♂, 68.4 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.837051–3, 6, ♂, 67.5 mm, 23.vi.1985, Emin Pasha Gulf; RMNH.PISC.837061, 5, ♂, 68.8 mm, 27.vi.1985, Emin Pasha Gulf; RMNH.PISC.840675, 7, ♂, 72.1 mm, 15.viii.1986; AMNH 2550354, ♂, 62.2 mm, 14.v.1979; BMNH 2012.1.5.2 4, ♂, 62.8 mm, 3.v.1979; NSMT-P 1069594, ♂, 62.6 mm, 3.v.1979.
1 dissected to describe oral jaws; 2 dissected to describe pharyngeal jaws; 3 dissected to count gill filaments; 4 proportional measurements taken (Table 1); 5colour picture available; 6colour picture of anal fin available; 7 specimen of which Dr E. Verheijen, Royal Belgian Institute of Natural Sciences, has taken a tissue sample for DNA analysis.
Small sized (< 8 cm SL), slender (BD < 31% SL), micrognathic (LJL < 45% HL) zooplanktivorous Haplochromis species with slightly curved to straight dorsal head profile. Relatively long and slender, mainly bicuspid, teeth in oral jaws. Premaxillary dentigerous area extending almost to caudal end of dentigerous arm. Both males and females silvery with conspicuously ivory-white lips. Three to five, generally faint vertical stripes on flank; faint traces of a dark mid-lateral band occasionally present. Males with yellow to greenish sheen on flank.
Proportional measurements of type material given in Table 1.
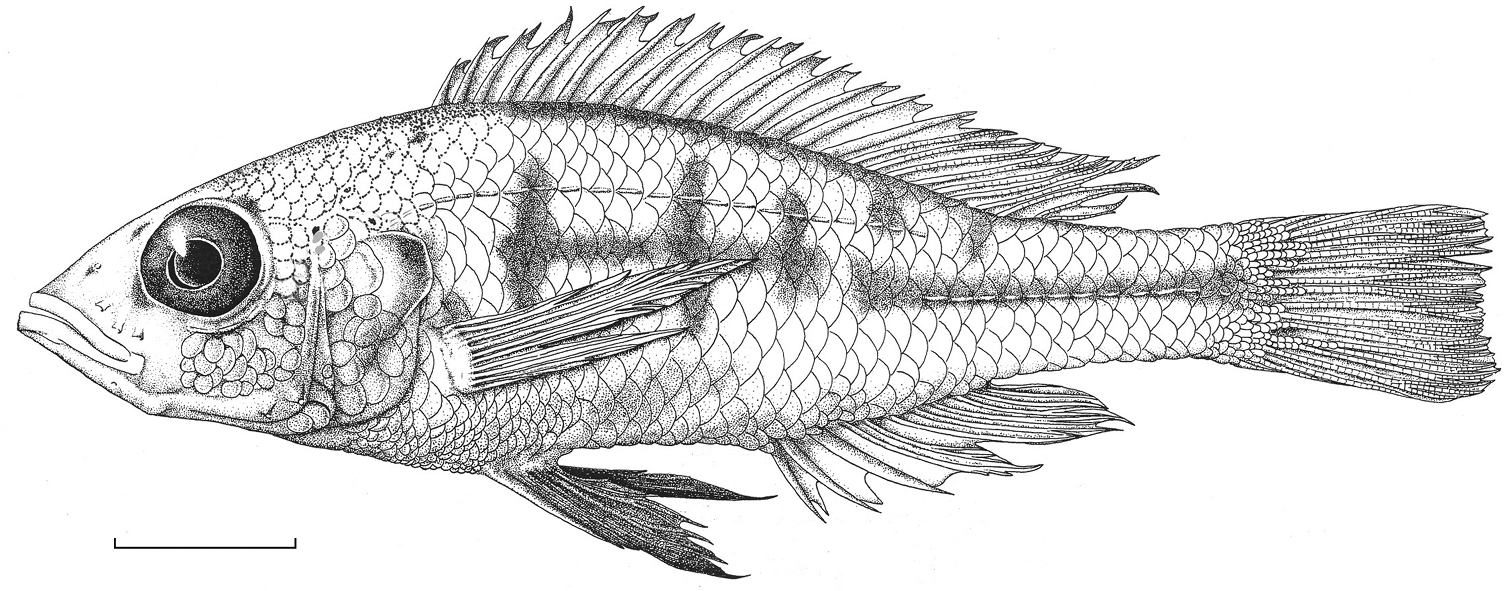
Habitus. See Fig. 2. Body slender. Dorsal head profile straight to slightly curved, occasionally moderately curved. Premaxillary pedicel slightly prominent. Mouth oblique. Lips not thickened. Medial part of premaxilla slightly expanded. Caudal part of maxilla not bullate. Vertical through caudal tip of maxilla running through iris, just rostral to pupil. Lateral snout outline isognathous and obtuse, in larger specimens slightly prognathous. Jaws equal anteriorly or lower jaw slightly protruding. Mental prominence slightly pronounced. Retro-articular processes of right and left mandible touching each other, interrupting ventral body outline. Eye approximately circular and medium to large. Generally an aphakic aperture present in pupil. Cephalic lateral line pores not enlarged.
Habitus of Haplochromis argens sp. n. ♂ (holotype, RMNH.PISC.83588). Scale bar equals 10 mm. Drawing by I. Westbroek, missing (= dotted) scales added by M. van Oijen.
Scales. Cheek, gill cover and rostral part of dorsal head surface covered with cycloid scales. Nape and rostral part of dorsum with mixture of cycloid and weakly ctenoid scales. Chest with ctenoid, weakly ctenoid and some cycloid scales. Scales on remaining part of body mainly ctenoid. Scales on chest smaller than those on ventral and ventro-lateral part of body; size transition gradual. Small elongated scales on basal quarter to half of caudal fin. Three to seven (mode 6) scales between upper lateral line and dorsal-fin origin, four to eight (mode 6) between pectoral- and pelvic-fin bases.
Fins. Pelvic fins just reaching or slightly surpassing rostral-most point of anal-fin origin. Pelvic fins with first soft rays slightly produced in both sexes, in males occasionally filamentous. Caudal tip of anal fin not reaching caudal-fin origin. Caudal-fin outline truncate to slightly emarginate.
Gill apparatus. Description based on lateral gill rakers and lateral hemibranch of first gill arch. Number of gill rakers on lower part of gill arch 11–12. Lower two to three rakers reduced (= very short), next one to two short, followed by two to six slender and longer ones. Remaining rakers hooked, bifid, trifid or quadrifid. Rakers generally closely set, viz. touching each other over major part of length. Number of gill filaments 94 to 106.
Viscera. Ratio between intestine length and SL: 1.0–1.4 (n = 25).
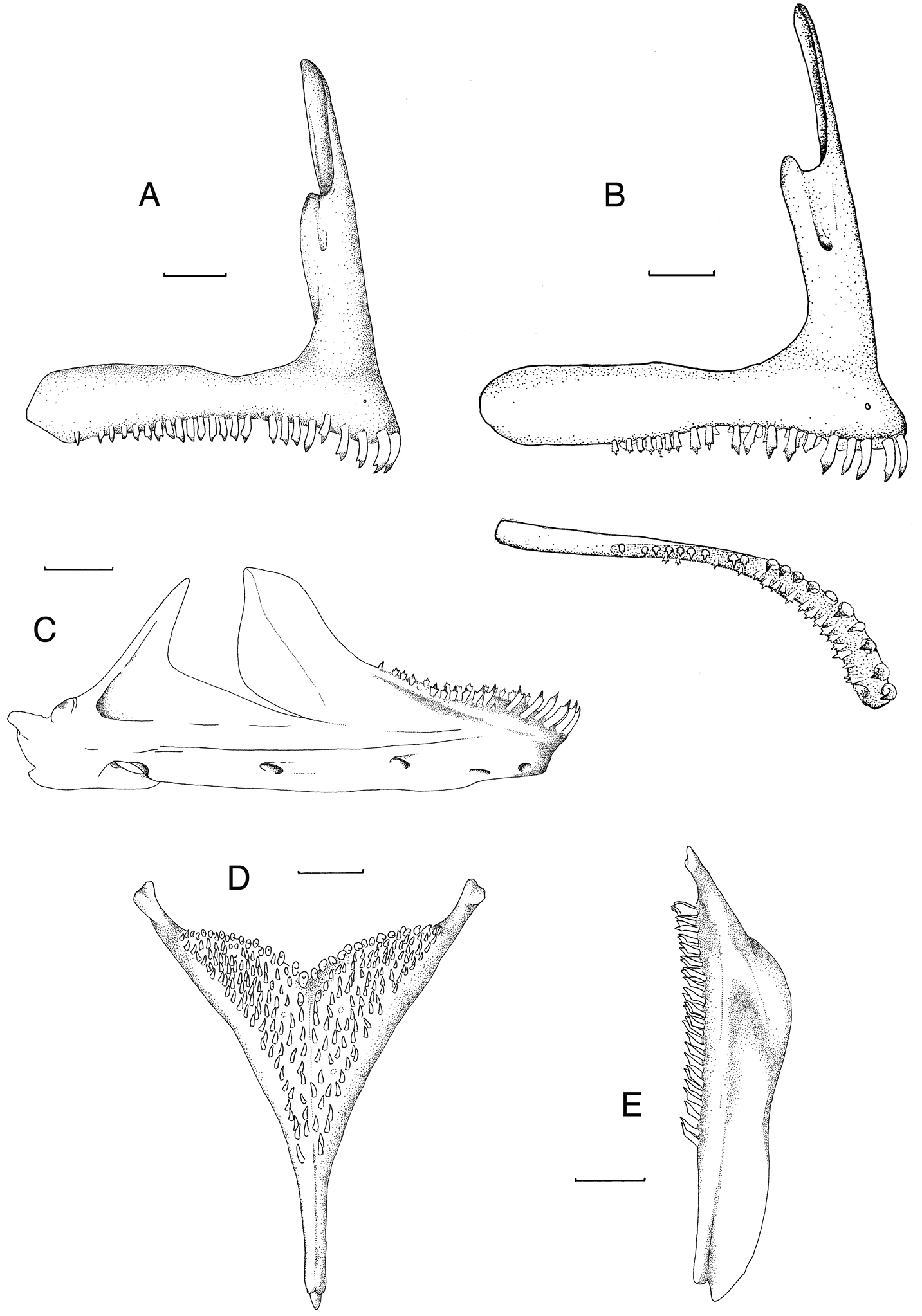
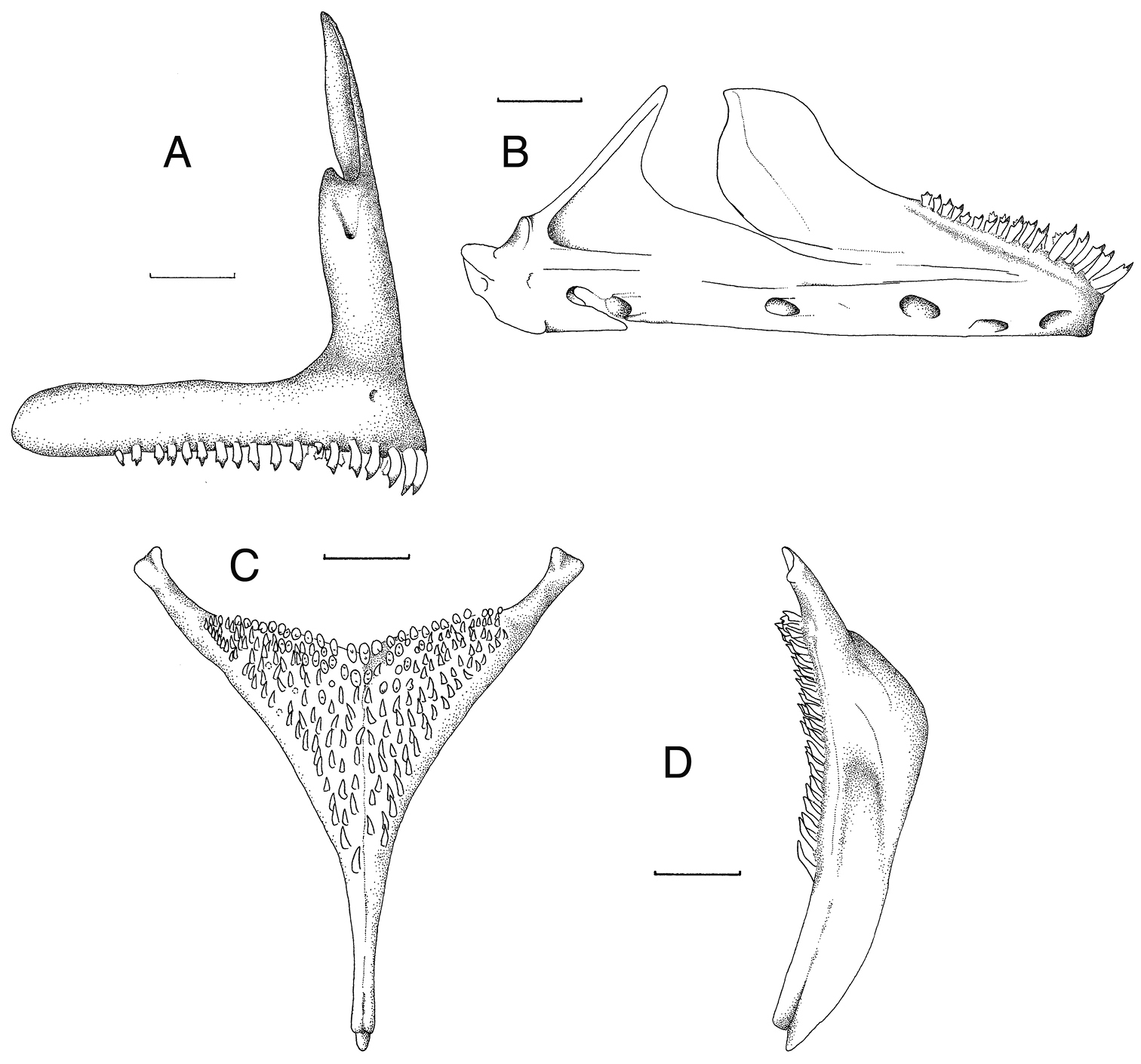
Oral jaws. (Fig. 3 A–C) Premaxillary ascending arm equal to or longer than dentigerous arm (asc./dent. arm ratio 1.0 to 1.1). Angle between the arms 77° to 81°. Symphyseal articulation facet not present. Lower jaw slightly more elongated than generalized type (length/height ratio 2.3 to 2.5). Upper half of dentary with distinct outwardly directed flare. Mental prominence slightly pronounced.
Skeletal elements of Haplochromis argens sp. n. A Right premaxilla, lateral view (RMNH.PISC.83705) B Right premaxilla, lateral (top) and occlusal (bottom) views (RMNH.PISC.83621), illustrating, for this species, a rare case of a posteriorly edentulous premaxilla C Right lower jaw, lateral view (RMNH.PISC.83697) D Lower pharyngeal element, dorsal view (RMNH.PISC. 83706) E Lower pharyngeal element, lateral view (RMNH.PISC.83706). Scale bars equal 1 mm. A, C, D drawn by I. Westbroek, B by M. van Oijen.
Oral teeth shape. (Fig. 3 A–C) Generally teeth in outer row of both premaxilla and lower jaw bicuspid or weakly bicuspid, with some unicuspid or tricuspid teeth interspersed. In specimens over 65 mm SL, weakly bicuspids and unicuspids may dominate. Major cusp of bicuspids isoscelene to subequilateral, protracted and acutely pointed. Flange generally absent, when present very small. Minor cusp weakly developed to distinct, relatively short compared to major cusp. Cusp gap wide. In labial view, neck slender to moderately slender, crown not or slightly expanded. In lateral view, crown compressed. Outer-row teeth in both premaxilla and lower jaw recurved. Inner rows in both jaws with mainly tricuspid or weakly tricuspid teeth.
Oral teeth size. Outer-row teeth relatively long and slender, gradually decreasing in size from rostral to caudal.
Dental arcade and tooth band. (Fig. 3B) Rostrally dental arcade rounded. Outer row generally occupying almost total length of dentigerous arm of premaxilla, in two specimens (RMNH.PISC.83697 and RMNH.PISC.83621; Fig. 3B) edentulous part about 25% of arm. Outer row in lower jaw not, or just, reaching coronoid wing in most dissected specimens. In one case caudal-most tooth relatively high on coronoid wing. One or two inner rows in rostral part of both jaws, decreasing to zero in caudal part.
Teeth counts and setting. Outer row of upper jaw (l+r premaxilla) with 30–52 teeth. In both jaws outer-row teeth regularly set, their placement wider rostrally than laterally.
Tooth implantation. Outer-row teeth of premaxilla rostrally erect. Inner-row teeth recumbent. Outer-row teeth of lower jaw slightly procumbent, inner-row teeth erect.
Lower pharyngeal element. (Fig. 3 D, E) Lower pharyngeal element relatively small and slender (length/width ratio 1.2–1.3). Dentigerous area slightly broader than long (length/width ratio = 0.7–0.9). Suture straight.
Pharyngeal teeth counts. Caudal-most transverse row with about 30–38 teeth, medial longitudinal rows with eight to 11 teeth.
Pharyngeal teeth shape. Teeth in caudal-most transverse row hooked, major cusp only slightly incurved, blunt to slightly acute. Other teeth bevelled or pronounced. All teeth relatively fine and slender, medial teeth not coarser than other teeth.
Vertebrae. Total number of vertebrae in 57 specimens: 30 (12), 31 (39) or 32 (6), comprising 13–14 abdominal and 16–19 caudal vertebrae.
Live colouration males. (Fig. 4 A, B). Sexually active males with ivory to grey snout and cheek. Lips remarkably ivory-white with no or few pigment spots. Eye with grey outer ring and silver to golden inner ring. Lower jaw and interoperculum whitish. Gill cover silver, sometimes with grey to dusky flush. Dorsal head surface, dorsum and flank silvery-grey, dorsum with bluish to purplish sheen, flank with yellow to greenish sheen. Chest, belly and ventral side silvery-white.
Live colours of Haplochromis argens sp. n. A sexually active ♂, 59.3 mm SL (paratype, RMNH.PISC.83622), Emin Pasha Gulf B sexually active ♂, 72.1 mm SL (paratype, RMNH.PISC.84067), Mwanza Gulf.
Pelvic fins black; in specimens of Emin Pasha Gulf, medial side sometimes red. Anal fin rostrally faintly to distinctly red, rest of fin hyaline. One to two dark yellow to orange egg dummies with hyaline ring present on caudal part of anal fin. Caudal fin orange-red to wine-red. Dorsal fin hyaline with red streaks and spots. Lappets hyaline or reddish, rostral lappets sometimes dusky.
Dark grey to blackish markings: Nostril-, interorbital-, and supraorbital stripes, sometimes rather distinct. Lachrymal stripe distinct, but relatively short (i.e. small blotch at caudal end of lachrymal generally not reaching caudal tip of maxilla), sometimes extending over iris. Irregular preopercular vertical bar generally present. Opercular blotch distinct. Three to five, generally faint vertical stripes on flank. Traces of dark-grey mid-lateral band occasionally present.
Live colouration females. Live females basically with same colours as males, lacking bluish-purplish and yellow-greenish sheens and distinct red colouration in fins, but sometimes with faint red flush in caudal fin. In females upper lip usually with more pigment than in males. Of markings on head, only lachrymal stripe and opercular blotch distinct. Mid-lateral band sometimes more distinct than in males, vertical stripes faint.
Preserved colouration of males and females. (Fig. 5) Body light brown, dorsally darker than ventrally. Snout, lips and lower jaw coloured as old ivory. Fins hyaline and light grey-brown in both sexes, except for black pelvic fins in adult males. Same markings, but slightly more distinct, as in live specimens.
Preserved colours of Haplochromis argens sp. n. ♂ 67.3 mm SL (holotype, RMNH.PISC.83588). Scale bar equals 10 mm.
Haplochromis argens is only known from the Tanzanian part of Lake Victoria. Specimens were caught in the Mwanza Gulf (from entrance of Stuhlmann Sound to entrance of gulf in north), in the south-western part of the Speke Gulf (near its entrance), in the area around Kome Island, and in the Emin Pasha Gulf (Fig. 1).
Haplochromis argens is a pelagic species from the littoral and sub-littoral zone. At night the species is virtually restricted to the two upper metres of the water column (
During 1979–1982, Haplochromis argens was present in 70% of the bottom-trawl tows by day and in 100% of the surface trawl tows at night; the mean numbers of individuals per tow ranged from 6.2 to 18.3, respectively (Table 2). In 1987–1988, it occurred in 3% of the bottom trawl tows and the mean number of individuals per tow was 0.03; the species was absent in surface trawls. Thirty-four bottom-trawl tows in 1990–1999 captured no Haplochromis argens. From 2001 to 2011 more than 150 bottom-trawl tows contained about 15 individuals of Haplochromis argens, corresponding to a decline in catch per unit effort of more than 50 times compared to the 1979–1982 captures; no individuals were caught with surface trawls.
Frequencies of occurrence (Foo) in trawl tows and mean numbers (± SD) per tow of Haplochromis argens. Tows of 10 minutes duration were made with bottom trawls (day) at stations E to J (depth range 7–14 m) and with surface trawls (night) at station G (14 m deep); in the period 1990-1999 no surface trawls were made; n indicates number of trawl tows.
| Station | Bottom trawl day | 1979–1982† n = 104 | 1987–1988† n = 29 | 1990–1999‡ n = 34 | 2001–2011§ n > 150 |
|---|---|---|---|---|---|
| E–J | Foo | 70 % | 3 % | 0 % | < 10 % |
| Mean nr | 6.2 ± 12.2 | 0.03 ± 0.19 | 0 | < 0.1 | |
| Surface trawl night | 1981/1982 n = 8 | 1987-19888| n = 26 | - | 2001-20111§ n = 15 | |
| G | Foo | 100 % | 0 % | - | 27 % |
| Mean nr | 18.3 ± 12.4 | 0 | - | 0.5 ± 0.9 |
† Data from
Food. Before the ecological changes in Lake Victoria, the diet of Haplochromis argens comprised mainly zooplankton during the day; Chaoborus larvae were important at night (
Breeding. Haplochromis argens is a female mouth brooder. Spawning sites are located at depths < 9 m (
In reference to the silver male colouration, Haplochromis argens was given the nickname “argens” under the false assumption it was Latinized Greek for silver. Since this species is well known under its cheironym, we think it is best to upgrade the nickname to the species’ epithethon.
The zooplanktivorous species Haplochromis (Yssichromis) laparogramma Greenwood & Gee, 1969, Haplochromis (Yssichromis) pyrrhocephalus Witte & Witte-Maas, 1987 and Haplochromis (Yssichromis) heusinkveldi, Witte & Witte-Maas, 1987, have shorter bicuspid teeth in the oral jaws than Haplochromis argens, and generally the premaxillary dentigerous arm is edentulous over the caudal 1/4 -1/3 versus the dentigerous portion extending almost to the caudal end of the dentigerous arm. The dental features of the zooplanktivorous/insectivorous Haplochromis tanaos van Oijen & Witte, 1996 and Haplochromis thereuterion van Oijen & Witte, 1996, are more or less similar to those of Haplochromis argens, but the former two species have more unicuspids. Haplochromis argens is further distinguished from these and other species by its colouration. Sexually active males of Haplochromis tanaos are dark blue, the females silvery with a distinct mid-lateral band and slightly less distinct dorso-lateral band. Sexually active males of Haplochromis thereuterion are black, the females coloured like females of Haplochromis tanaos (
urn:lsid:zoobank.org:act:06762084-8C0C-49CC-A48D-065244CF8457
http://species-id.net/wiki/Haplochromis_goldschmidti
Figs 6–9, Table 3Haplochromis “dusky argens”;
Tanzania, Lake Victoria, Emin Pasha Gulf (ca 2°35'–2°41'S; 31°47'–31°59'E).
RMNH.PISC.835733, ♂, 60.3 mm SL, 23.vi.1985, HEST.
Collected by the Haplochromis Ecology Survey Team (HEST). Size of specimens given as standard length. RMNH.PISC.804804, ♂, 53.6 mm, 23.vi.1985; RMNH.PISC.80481, ♂, 56.8 mm, 23.vi.1985; RMNH.PISC.804824, ♂, 63.4 mm, 23.vi.1985; RMNH.PISC.804834, ♂, 52.9 mm, 23.vi.1985; RMNH.PISC.804844, ♂, 65.3 mm, 23.vi.1985; RMNH.PISC.804854, ♂, 69.2 mm, 23.vi.1985; RMNH.PISC.804934, ♂, 50.7 mm, 23.vi.1985; RMNH.PISC.804944, ♂, 52.2 mm, 23.vi.1985; RMNH.PISC.804955, ♂, 58.7 mm, 23.vi.1985; RMNH.PISC.804965, ♂, 57.4 mm, 23.vi.1985; RMNH.PISC.804975, ♂, 55.0 mm, 23.vi.1985; RMNH.PISC.804985, ♂, 60.4 mm, 23.vi.1985; RMNH.PISC.804995, ♂, 67.4 mm, 23.vi.1985; RMNH.PISC.805015, ♂, 61.1 mm, 23.vi.1985; RMNH.PISC.805025, ♂, 62.6 mm, 23.vi.1985; RMNH.PISC.805035, ♂, 57.7 mm, 23.vi.1985; RMNH.PISC.805075, ♂, 52.8 mm, 23.vi.1985; RMNH.PISC.805085, ♂, 62.9 mm, 23.vi.1985; RMNH.PISC.805125, ♂, 59.3 mm, 23.vi.1985; RMNH.PISC.805135, ♂, 63.6 mm, 23.vi.1985; RMNH.PISC.805145, ♂, 61.7 mm, 23.vi.1985; RMNH.PISC.835743, ♂, 62.6 mm, 23.vi.1985; RMNH.PISC.835754, ♂, 57.5 mm, 23.vi.1985; RMNH.PISC.83576, ♂, 58.2 mm, 23.vi.1985; RMNH.PISC.835773, ♂, 62.5 mm, 23.vi.1985; RMNH.PISC.835783, ♂, 69.2 mm, 23.vi.1985; RMNH.PISC.835793, ♂, 64.9 mm, 23.vi.1985; RMNH.PISC.835803, ♂, 62.9 mm, 23.vi.1985; RMNH.PISC.835813, ♂, 51.7 mm, 23.vi.1985; RMNH.PISC.835823, ♂, 52.4 mm, 23.vi.1985; RMNH.PISC.835833, ♂, 57.7 mm, 23.vi.1985; RMNH.PISC.835843, ♂, 55.4 mm, 23.vi.1985; RMNH.PISC.835853, ♂, 61.9 mm, 23.vi.1985; RMNH.PISC.835863, ♂, 65.4 mm, 23.vi.1985; RMNH.PISC.836643, ♂, 56.2 mm, 23.vi.1985; RMNH.PISC.836653, ♂, 63.2 mm, 23.vi.1985; RMNH.PISC.836663, ♂, 65.5 mm, 23.vi.1985; RMNH.PISC.836673, ♂, 60.7 mm, 23.vi.1985; RMNH.PISC.836683, ♂, 64.9 mm, 23.vi.1985; RMNH.PISC.836693, ♂, 65.8 mm, 23.vi.1985; RMNH.PISC.836703, ♂, 60.0 mm, 23.vi.1985; RMNH.PISC.836951, 2, 4, ♂, 56.2 mm, 23.vi.1985; RMNH.PISC.836961, 4, ♂, 58.0 mm, 23.vi.1985; RMNH.PISC.836991, 5, ♂, 61.5 mm, 23.vi.1985; RMNH.PISC.837001, 5, ♂, 55.3 mm, 23.vi.1985; RMNH.PISC.837012, 5, ♂, 61.6 mm, 23.vi.1985; RMNH.PISC.837021, 2, 5, ♂, 61.1 mm, 23.vi.1985; AMNH 2550365, ♂, 61.4 mm, 23.vi.1985; BMNH 2012.1.5.34, ♂, 61.6 mm, 22.vi.1985; NSMT-P 1069604, ♂, 64.8 mm, 22.vi.1985.
1 dissected to describe the oral jaws and contents of stomachs and intestines; 2 dissected to describe pharyngeal jaws and to count gill filaments; 3 proportional measurements taken; 4 colour picture available; 5 colour picture of anal fin available.
Small sized (< 7 cm SL), slender (BD < 28% SL), micrognathic, zooplanktivorous Hapolochromis species (LJL < 45% HL in all but one specimens) with a slightly curved to straight dorsal head profile. Mainly bicuspid teeth in oral jaws. Generally premaxillary dentigerous arm edentulous over caudal 1/5 - 1/4. Males silvery with dusky flush on chest, flank, ventral side and ventral half of caudal peduncle.
Proportional measurements of type material provided in Table 3.
Measurements of Haplochromis goldschmidti sp. n., proportional to standard length or head length. Means and standard deviations were calculated over all measured type specimens, including the holotype.
| Holotype | Paratypes (n = 18) (n = 18) | Mean ± SD (n = 19) (n = 19) | ||
|---|---|---|---|---|
| SL (mm) | 60.3 | 51.7–69.2 | 61.2 ± 4.6 | |
| BD | %SL | 25.2 | 25.0–27.3 | 26.2 ± 0.7 |
| PFL | %SL | 27.4 | 24.2–29.3 | 27.4 ± 1.3 |
| CPL | %SL | 19.4 | 17.8–22.6 | 20.2 ± 1.1 |
| CPD | %SL | 9.0 | 8.5–10.4 | 9.6 ± 0.5 |
| CFL | %SL | 23.9 | 22.2–25.2 | 24.4 ± 0.8 |
| HL | %SL | 30.5 | 31.0–34.6 | 32.9 ± 0.9 |
| SnL | %HL | 26.6 | 23.7–29.7 | 26.4 ± 1.3 |
| SnW | %HL | 26.1 | 24.1–27.8 | 25.8 ± 1.2 |
| HW | %HL | 42.9 | 37.2–44.2 | 40.6 ± 1.7 |
| IOW | %HL | 21.7 | 19.3–21.7 | 20.7 ± 0.8 |
| POW | %HL | 26.1 | 22.1–27.3 | 24.1 ± 1.4 |
| LaW | %HL | 22.3 | 19.8–24.4 | 22.0 ± 1.3 |
| POD | %HL | 13.6 | 11.7–18.0 | 14.4 ± 1.8 |
| EyL | %HL | 35.9 | 32.7–40.2 | 36.5 ± 2.2 |
| EyD | %HL | 34.2 | 28.5–35.0 | 32.1 ± 1.8 |
| ChD | %HL | 14.1 | 13.1–16.3 | 15.0 ± 1.1 |
| LJL | %HL | 42.1 | 39.0–45.6 | 41.0 ± 1.6 |
| LJW | %HL | 12.5 | 11.7–18.0 | 14.0 ± 1.5 |
| EyD/EyL | 1.0 | 0.8–0.9 | 0.9 ± 0.0 | |
| LJL/LJW | 3.4 | 2.2–3.6 | 3.0 ± 0.3 |
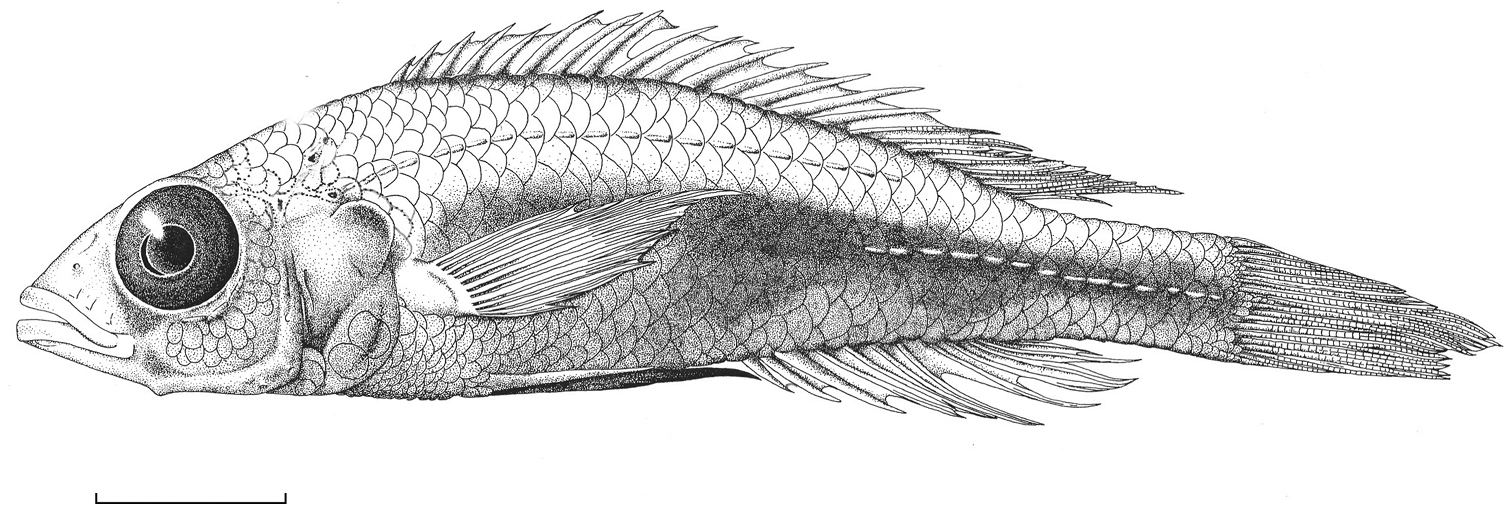
Habitus. See Fig. 6. Body slender. Dorsal head profile straight to slightly curved. Premaxillary pedicel slightly prominent. Mouth oblique. Lips not thickened. Medial part of premaxilla slightly expanded to expanded. Caudal part of maxilla not bullate. Vertical through caudal tip of maxilla running through iris, just rostral to pupil. Lateral snout outline isognathous and obtuse, in large specimens sometimes slightly prognathous. Jaws equal anteriorly or lower jaw slightly protruding. Mental prominence slightly pronounced. Retro-articular processes of right and left mandible touching each other, interrupting ventral body outline. Eye approximately circular (occasionally slightly elongated) and medium to large in size. Distinct aphakic aperture present in pupil. Cephalic lateral line pores not enlarged.
Habitus of Haplochromis goldschmidti sp. n. ♂ (holotype, RMNH.PISC.83573). Scale bar equals 10 mm. Drawing by I. Westbroek, missing (= dotted) scales added by M. van Oijen.
Scales. Cheek, gill cover, and rostral part of the dorsal head surface with cycloid scales. Nape and rostral part of dorsum with a mixture of cycloid and weakly ctenoid scales. Chest mainly with ctenoid scales, occasionally weakly ctenoid or cycloid. Scales on remaining part of body mainly ctenoid. Scales on chest smaller than those on ventral and ventro-lateral part of body; size transition gradual. Small elongated scales on basal quarter to one third of caudal fin. Four to 5.5 (mode 5) scales between upper lateral line and dorsal-fin origin, four to seven (mode 6) between between pectoral- and pelvic-fin bases.
Fins. Pelvic fins just reaching or slightly surpassing rostral-most point of anal-fin origin. Pelvic fins with first soft rays slightly produced, occasionally filamentous. Caudal tip of anal fin not reaching caudal-fin origin. Caudal-fin outline truncate to slightly emarginate.
Gill apparatus. Description of gill apparatus based on lateral gill rakers and lateral hemibranch of first gill arch. Number of gill rakers on lower part of first gill arch 10–12 (one specimen with 13). Lower two to three rakers reduced (= very short), next one to two short, followed by two to seven slender and longer ones. Remaining rakers hooked, bifid, or trifid. Generally rakers closely set, viz. touching each other over major part of length. Number of gill filaments 87 to 93.
Viscera. Ratio between intestine length and SL: 1.0–1.2 (n = 5).
Oral jaws. (Fig. 7 A, B) Premaxillary ascending arm equal to or longer than dentigerous arm (asc./dent. arm ratio 1.0 to 1.1). Angle between arms 77° to 81°. Symphyseal articulation facet not present, lower jaw slightly more elongated than generalized type (length/height ratio 2.3 to 2.8). Upper half of dentary with distinct outwardly directed flare. Mental prominence slightly pronounced.
Oral teeth shape. (Fig. 7 A, B) Teeth of outer row in both jaws mainly bicuspid, with some unicuspid or tricuspid teeth interspersed. Major cusp of bicuspids isoscelene to subequilateral, protracted and acutely pointed. Flange occasionally present on major cusp. Minor cusp short compared to major cusp. Cusp gap rather narrow. In labial view neck moderately slender to normal, crown moderately expanded. In lateral view, crown compressed. Outer-row teeth in both premaxilla and lower jaw recurved. Inner rows in both jaws with mainly tricuspid or weakly tricuspid teeth.
Skeletal elements of Haplochromis goldschmidti sp. n. A Right premaxilla, lateral view (RMNH.PISC.83695) B Right lower jaw, lateral view (RMNH.PISC.83695) C Lower pharyngeal element, dorsal view (RMNH.PISC.83701) D Lower pharyngeal element, lateral view (RMNH.PISC.83701). Scale bars equal 1 mm. Drawn by I. Westbroek.
Oral teeth size. Outer-row teeth relatively slender, gradually decreasing in size from rostral to caudal.
Dental arcade and tooth band. Rostrally dental arcade rounded. Outer row occupying 3/4 to 4/5 of premaxillary dentigerous arm. One to two inner rows in both jaws.
Teeth counts and setting. Outer row of upper jaw (l+r premaxilla) with 33–47 teeth. In both jaws outer-row teeth regularly set. Teeth set wider rostrally than laterally.
Tooth implantation. Outer-row teeth of premaxilla rostrally erect. Inner-row teeth recumbent. Outer-row teeth of lower jaw slightly procumbent, inner-row teeth erect.
Lower pharyngeal element. (Fig. 7 C, D) Lower pharyngeal element relatively small and slender (length/width ratio 1.2–1.3). Dentigerous area slightly broader than long (length/width ratio 0.7–0.9). Suture straight.
Pharyngeal teeth counts. Caudal-most transverse row with 25–34 teeth, medial longitudinal rows with nine to 10 teeth.
Pharyngeal teeth shape. Teeth in caudal-most transverse row hooked, major cusp only slightly incurved, blunt to slightly acute. Other teeth bevelled or pronounced. All teeth relatively fine and slender, medial teeth not coarser than other teeth.
Vertebrae. Total number of vertebrae in 19 specimens: 30 (2), 31 (16) or 32 (1), comprising 13–14 abdominal and 16–18 caudal vertebrae.
Live colouration males. (Fig. 8 A, B) Sexually active males with grey-white snout, cheek and gill cover. Lips grey-white, generally with distinct black pigment spots. Eye with grey outer ring and silver inner ring. Dorsal head surface and dorsum silvery-grey. Chest, ventral side, flank and ventral part of caudal peduncle silvery-grey with dusky flush. Flush most distinct on flank and caudal peduncle and occasionally absent on ventral side, sometimes extending over suboperculum, interoperculum, branchiostegal membrane and lower jaw.
Live colours of Haplochromis goldschmidti sp. n. A sexually active ♂, 53.6 mm SL (paratype, RMNH.PISC.80480) B sexually active ♂, 57.5 mm SL (paratype, RMNH.PISC.83575).
Pelvic fins black. Anal fin rostrally hyaline-grey with bluish sheen, remaining part hyaline. One to two pale-yellow to yellow egg dummies surrounded by hyaline ring on caudal part of anal fin. Caudal fin hyaline with bluish sheen; dusky flush on caudal peduncle may extend over rostral part of caudal fin. Dorsal fin hyaline with bluish sheen and faint dusky lappets.
Dark grey to blackish markings: Faint nostril-, interorbital- and supraorbital stripes sometimes present. Lachrymal stripe occasionally slightly longer than in Haplochromis argens, but often less distinct. Preopercular vertical stripe generally not clear. Opercular blotch present.
Preserved colouration of
(Fig. 9) Body light brown. Chest, ventral side, flank and ventral part of caudal peduncle dusky to dark brown. Dark brown colour on caudal peduncle sometimes giving impression of broad mid-lateral band. Fins transparent to light grey-brown except for pelvics, which are black in adult males. Same markings present as in live specimens.
Preserved colours of Haplochromis goldschmidti sp. n. ♂ 60.3 mm SL (holotype, RMNH.PISC.83573). Scale bar equals 10 mm.
Haplochromis goldschmidti is only known from the southern part of the Emin Pasha Gulf of Lake Victoria (Fig. 1).
Haplochromis goldschmidti was caught over mud bottoms at depths of 4–10 m.
Stomach and intestines of five examined specimens caught by day contained mainly zooplankton (mainly copepods, but also some cladocerans) and some insects (Chaoborus larvae and pupae).
Based on the egg dummies on the anal fin of males, Haplochromis goldschmidti is probably a female mouth brooder.
This species is named in honour of Dr Tijs Goldschmidt in appreciation for his work on haplochromine cichlids of Lake Victoria. As a member of the Haplochromis Ecology Survey Team, Tijs Goldschmidt worked in Tanzania (1981–1986) on the ecology and evolution of zooplanktivorous and detritivorous cichlids. Haplochromis goldschmidti is one of the species on which he based his theory on the possible role of egg-dummy divergence in speciation of haplochromines (
Sexually active males of Haplochromis goldschmidti and Haplochromis argens are very similar, but distinguishable by live colouration. Haplochromis goldschmidti has a dusky flush on its flank and Haplochromis argens a yellow to greenish sheen. In contrast to Haplochromis argens, red is generally absent on the fins of Haplochromis goldschmidti, only occasionally very faint traces of red are present on the caudal and dorsal fins. Haplochromis goldschmidti has plain yellow egg spots, while the egg spots of Haplochromis argens are orange yellow (
Adjusted means (rounded to the nearest 0.1 mm), their differences (in %) and significance levels of the ANCOVAs of linear measurements. Both populations of Haplochromis argens are compared to Haplochromis goldschmidti. The mean values represent antilogged adjusted means calculated from the ANCOVA analyses (sample mean adjusted for a common mean standard length and a common regression line for the three groups). Adjusted means and differences are not applicable (NA) when the slopes of the relationships differ (i.e., in case of a significant interaction between location and SL). Estimated differences were calculated from adjusted means. Parameter estimates were derived from the GLM (analysis of covariance) procedure. Significance levels (P) of the effect of species (Spec.), location (Loc.) and the interaction between SL and location (Loc. * SL) are given when below 0.05.
| Haplochromis argens | Haplochromis argens | Haplochromis goldsch | Haplochromis argens | Haplochromis argens | Spec. | Loc. | Loc. * SL | |
|---|---|---|---|---|---|---|---|---|
| Mwanza | Emin P | Emin P | Mwanza | Emin P | ||||
| Mean | Mean | Mean | % diff. | % diff. | P | P | P | |
| BD | NA | NA | NA | NA | NA | < 0.001 | 0.041 | 0.031 |
| PFL | 17.6 | 16.9 | 16.9 | 4.1 | 0.0 | ns | < 0.001 | ns |
| CPL | 12.9 | 12.9 | 12.4 | 3.3 | 3.3 | 0.012 | ns | ns |
| CPD | 6.3 | 6.0 | 6.0 | 5.5 | 0.0 | ns | < 0.001 | ns |
| CFL | 15.0 | 14.6 | 15.1 | -0.6 | -3.2 | 0.007 | 0.016 | ns |
| HL | 20.4 | 20.4 | 20.4 | 0.0 | 0.0 | ns | ns | ns |
| SnL | 5.4 | 5.4 | 5.4 | 0.0 | 0.0 | ns | ns | ns |
| SnW | 5.1 | 4.8 | 5.3 | -2.7 | -9.0 | < 0.001 | < 0.001 | ns |
| HW | NA | NA | NA | NA | NA | ns | < 0.001 | < 0.001 |
| IOW | 4.3 | 4.2 | 4.2 | 2.7 | 0.0 | ns | 0.011 | ns |
| POW | 4.7 | 4.7 | 4.9 | -3.5 | -3.5 | 0.004 | ns | ns |
| LaW | 4.2 | 4.0 | 4.5 | -7.3 | -10.4 | < 0.001 | 0.046 | ns |
| POD | 3.1 | 3.1 | 2.9 | 5.4 | 5.4 | 0.030 | ns | ns |
| EyL | NA | NA | NA | NA | NA | ns | < 0.001 | < 0.001 |
| EyD | 6.5 | 6.5 | 6.5 | 0.0 | 0.0 | ns | ns | ns |
| ChD | 3.5 | 3.1 | 3.1 | 11.7 | 0.0 | ns | < 0.001 | ns |
| LJL | NA | NA | NA | NA | NA | ns | 0.039 | 0.040 |
| LJW | 2.8 | 2.6 | 2.8 | -2.1 | -9.1 | 0.001 | 0.003 | ns |
Sexually active males of Haplochromis goldschmidti are distinguished from Haplochromis tanaos and Haplochromis thereuterion, two other slender zooplanktivores, by colouration. A distinct mid- and dorsal-lateral band is absent in males of Haplochromis goldschmidti, whereas they are present in Haplochromis tanaos and Haplochromis thereuterion (sometimes difficult to distinguish in the latter). A nape band is lacking in Haplochromis goldschmidti, but present in Haplochromis tanaos.
The MANCOVA of the linear measurements of males of Haplochromis goldschmidti and both populations of Haplochromis argens shows that SL is a significant covariate (P < 0.001), explaining the largest part of the variance (F = 81.406). The two species differ significantly (species effect, P < 0.001), and the same holds for the populations from the Mwanza Gulf and the Emin Pasha Gulf (location effect, P = 0.007), with the effect of species explaining more of the variance (F = 9.262) than the effect of location (F = 2.494). There is also a significant interaction between SL and location, however it does not explain much of the variance (F = 2.545).
From the ANCOVAs of the individual measurements, the adjusted means and their differences were calculated (Table 4).
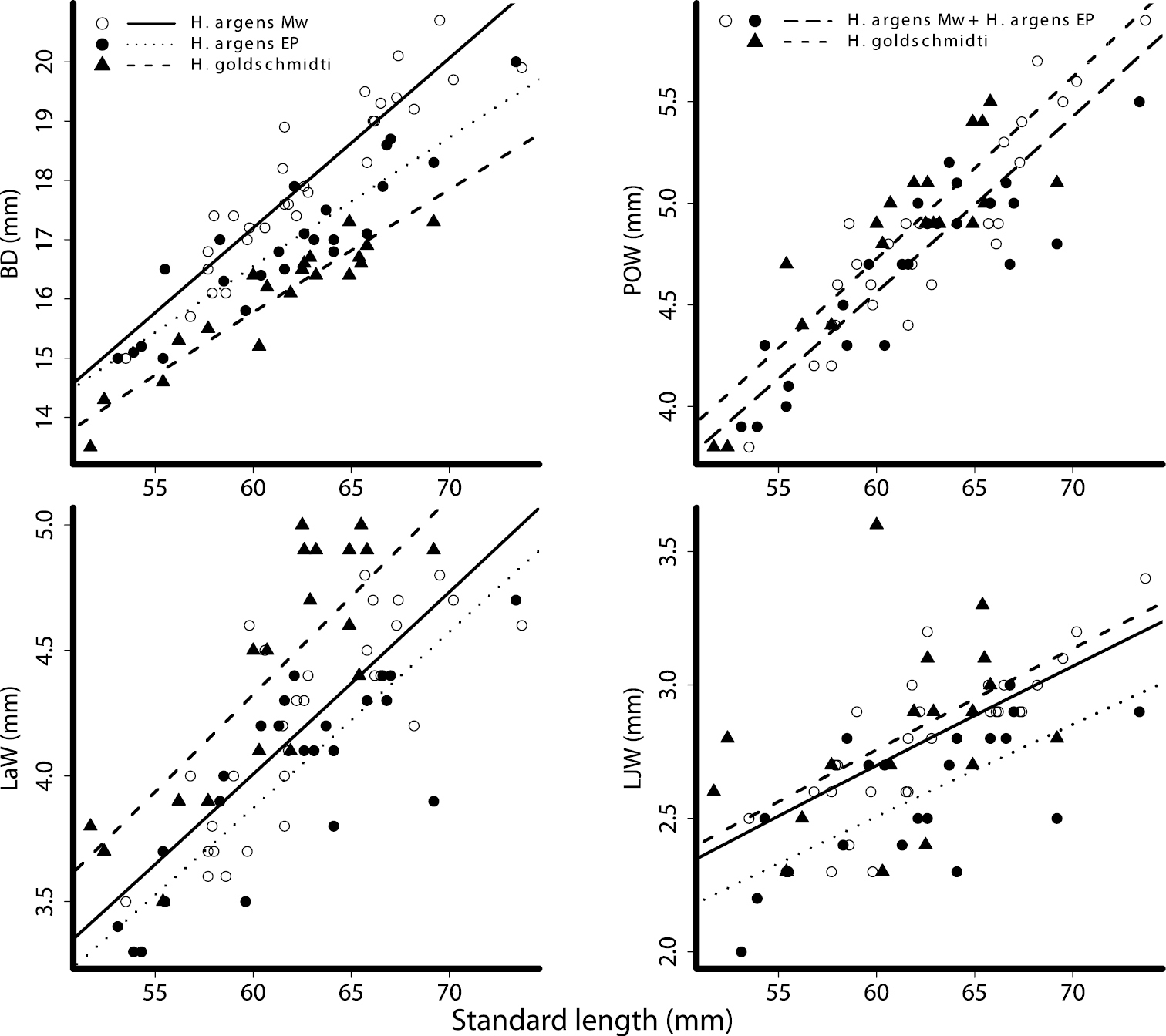
There is a significant species effect in eight (BD, CPL, CFL, SnW, POW, LaW, POD and LJW) of the 18 measurements, and in three of these measurements (CPL, POW, POD) this is the only significant effect. The other measurements (BD, CFL, SnW, LaW and LJW) also differ significantly between the two populations of Haplochromis argens (= effect of location). In addition, for BD, a significant interaction (P = 0.031) between the effects of location and SL indicate an increasing relative difference in BD (with increasing standard length) between the estimations for the populations from the different locations (Table 4, Fig. 10).
Plots of taxonomic measurements as a function of standard length. Body depth (BD), preorbital width (POW), lower jaw width (LJW) and lachrymal width (LaW). Open circles - Haplochromis argens from the Mwanza Gulf (Mw); closed circles - Haplochromis argens from the Emin Pasha Gulf (EP); triangles - Haplochromis goldschmidti. Power curves determined from GLM parameter estimates, solid line - Haplochromis argens from the Mwanza Gulf; dotted line - Haplochromis argens from the Emin Pasha Gulf; dashed line - Haplochromis goldschmidti, long dashed line (POW) - Haplochromis argens from both locations (MW + EP).
In the measurements, except for BD, in which there is a significant effect of species and location, the population of Haplochromis argens from the Emin Pasha Gulf differs more from Haplochromis goldschmidti than does the population of Haplochromis argens from the Mwanza Gulf. Especially in width measurements from the oral and suspensorial compartment, SnW, LaW and LJW, this is apparent (Fig. 10). In BD, Haplochromis argens from the Mwanza Gulf differs more from Haplochromis goldschmidti than does Haplochromis argens from the Emin Pasha Gulf (Fig. 10).
The effect of location is significant in 12 of the 18 measurements, and for PFL, CPD, IOW and ChD, this is the only significant effect; these measurements are significantly larger in Haplochromis argens from the Mwanza Gulf than in Haplochromis argens from the Emin Pasha Gulf and Haplochromis goldschmidti. In four measurements in which there is a significant effect of location, the two-way interaction is significant as well. For BD, HW and LJL, the relative difference between Haplochromis argens from the Mwanza Gulf and both Haplochromis argens and Haplochromis goldschmidti from the Emin Pasha Gulf increases with SL. For EyL, however, the relative difference between Haplochromis argens from the Mwanza Gulf and both Haplochromis argens and Haplochromis goldschmidti from the Emin Pasha Gulf decreases with SL.
In this paper, females of Haplochromis goldschmidti have not been described since it was difficult to distinguish the females from females of Haplochromis argens in the field. Samples of females, which presumably contained both Haplochromis argens and Haplochromis goldschmidti, were collected during ecological surveys in the Emin Pasha Gulf, but unfortunately these samples were mislaid or may have been lost during the many moves of the material since its collection.
Since the field research of Greenwood (many papers bundled in 1981), male colouration has become a major character in species distinction of Lake Victoria cichlids. Indeed, experimental research has shown that male colouration plays a major role in female mate choice of Lake Victoria haplochromines (
In some studies Haplochromis argens has been assigned to Yssichromis (e.g.
Haplochromis goldschmidti has the two autapomorphic characters of Yssichromis and can thus be referred to as Haplochromis (Yssichromis) goldschmidti. However, in some characters it seems to bridge the gap between Haplochromis argens and the species of the Yssichromis group, as its teeth are longer than those of the latter, but shorter than those of Haplochromis argens, and it has the medial part of the premaxilla slightly expanded to expanded. Both Haplochromis argens and Haplochromis goldschmidti lack the typical arrangement of the inner rows in the lower jaw described for Haplochromis (Yssichromis) laparogramma, Haplochromis (Yssichromis) pyrrhocephalus and Haplochromis (Yssichromis) heusinkveldi by
Considering the resemblance between Haplochromis argens and Haplochromis cassius, the latter assigned to the genus Psammochromis by
In some morphological characters (e.g. body depth), Haplochromis goldschmidti is more similar to the sympatric population of Haplochromis argens than to the allopatric population of the latter in the Mwanza Gulf, whereas for other characters (snout width, lower jaw width) the opposite is true. It has been found in earlier studies that local adaptations related to environmental conditions may cause similar morphological shifts in several species of Lake Victoria haplochromines (
Eight zooplanktivorous haplochromine species that were common on our research transect in the Mwanza Gulf (
In the successfully recovering zooplanktivorous haplochromine species, morphological changes were observed, which may have been caused by adaptive responses to the environmental changes, both through natural selection and phenotypic plasticity (
We thank our colleagues from the Haplochromis Ecology Survey Team (HEST), the Tanzania Fisheries Research Institute (TAFIRI) and the Freshwater Fisheries Training Institute at Nyegezi, for support and co-operation during the fieldwork. We wish to thank Mhoja Kayeba, the late Aloys Peter, and the other crew members of the trawlers for their skilful labour, Abby Israëls for his help with the GLM, Mark van der Loo and Edwin de Jonge for their help with producing Fig. 10, and Erik-Jan Bosch, Bas Blankevoort and Niko Korenhof for making and/or digital processing of the other figures. We are indebted to Carole Baldwin, Jay Stauffer and Lodewijk van Duuren for carefully reviewing the manuscript. The fieldwork, during which the species and the ecological data described in this paper were collected, was financially supported by The Netherlands Foundation for the Advancement of Tropical Research (WOTRO; grants W87–129, W87–161, W87–189, W84–282, W84–488, WB84–587) and by the Section for Research and Technology of the Netherlands’ Ministry of Development Co-operation.