(C) 2013 Ricardo Russo Siewert. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Siewert RR, Zacca T, Dias FMS, Freitas AVL, Mielke OHH, Casagrande MM (2013) The “Taygetis ypthima species group” (Lepidoptera, Nymphalidae, Satyrinae): taxonomy, variation and description of a new species. ZooKeys 356: 11–29. doi:
A new species of Taygetis Hübner, [1819] (Lepidoptera, Nymphalidae, Satyrinae) from southeastern Brazil is described: Taygetis drogoni sp. n. In addition, T. servius Weymer, 1910 and T. fulginia d’Almeida, 1922 are resurrected from synonymy and a taxonomic discussion on the species T. ypthima Hübner, [1821] and T. rectifascia Weymer, 1907 is provided. A dichotomous key for the species is also provided.
Atlantic Forest, Euptychiina, Neotropical, Pseudodebis, Taygetis rectifascia
The butterfly subtribe Euptychiina includes over 400 described species in 45 genera, being one of the most diverse groups in the subfamily Satyrinae (Lepidoptera, Nymphalidae) (
In a recent study based on molecular data,
The genus Taygetis comprises 27 described species and several undescribed species (
Although intraspecific phenotypic variation appears to be common in several Euptychiina, in some cases hidden taxonomic diversity might be underestimated. Similar to Taygetis virgilia (see above), Taygetis ypthima is a highly variable species that has five synonymized names (
The present paper studied in detail the morphology of male and female genitalia and wing pattern variation of Taygetis ypthima, and related species, such as its sister species Taygetis rectifascia Weymer, 1907 (
Dissections of the genitalia were made following standard techniques. The abdomen was removed, soaked in a heated 10% KOH solution for 5 minutes before dissection of the genitalia to analyze its structures. Illustrations were prepared with the aid of a camera lucida attached to a stereoscopic microscope. Genitalia terminology follows
All examined material belongs to the following institutions:
DZUP Coleção Entomológica Padre Jesus Santiago Moure, Curitiba, Paraná, Brazil
UFMG Universidade Federal de Minas Gerais taxonomic collection, Belo Horizonte, Minas Gerais, Brazil
ZUEC Museu de Zoologia Adão José Cardoso, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil
ZUEC-AVLF André VL Freitas Collection, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil
SMFL Lepidoptera collection, Senckenberg-Museum, Frankfurt am Main, Germany
SMT Staatliches Museum für Tierkunde, Dresden, Germany
ZSM Zoologische Staatssammlung München, Germany
http://zoobank.org/CA6CEBB1-A525-44EE-A265-CE467707BFB5
http://species-id.net/wiki/Taygetis_drogoni
Figs 1A–D; 6A–E; 7A, BHolotype male with the following labels (separated by transverse bars): /HOLOTYPUS/ Taygetis drogoni Siewert, Zacca, Dias & Freitas det. 2013/ M(#)/ 07-II-1985 Cambuquira, M[inas] G[erais] [21°51'30"S, 45°17'28"W]. Mielke & Casagrande leg./ DZ 27.604/ (DZUP). Allotype female with the following labels (separated by transverse bars): /ALLOTYPUS/ Taygetis drogoni Siewert, Zacca, Dias & Freitas det. 2013/ F(#)/ 10-XII-1968 Camb[uquira], [Minas Gerais] [21°51'30"S, 45°17'28"W]./ Coleção H. Ebert/ DZ 27.607/ (DZUP).
BRAZIL – Minas Gerais: Alfenas – 14-XII-2011, 1 female, Brito leg., JCI2.1-130 (ZUEC); 08-II-2012, 1 male, Brito leg., JCI3.2-225 (ZUEC). Cambuquira – 6-X-1968, 1 female, Ebert leg., ex-coll. Ebert, DZ 5.501 (DZUP); 10-XII-1968, 6 males, Ebert leg., ex-coll. Ebert, DZ 27.606, DZ 27.607, DZ 27.614, DZ 27.619, DZ 27.623, DZ 27.624 (DZUP); 900 m, 15-IV-1969, 3 males, Ebert leg., ex-coll. Ebert, DZ 27.605, DZ 26.419, DZ 27.620 (DZUP); 2-7-XI-1969, 1 female, Ebert leg. (SMFL); 15-V-1981, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.618 (DZUP); 7-II-1985, 5 males, Mielke & Casagrande leg. DZ 27.621, DZ 27.431, DZ 27.616, DZ 27.626, DZ 27.627 (DZUP). Caraça - Santa Barbara, 1500 m, 1-5-II-1985, 1 male and 1 female, Mielke & Casagrande leg. DZ 27.625, DZ 27.629 (DZUP). Carmo do Rio Claro – 1-VIII-1948, 1 male, Carvalho & Alceu leg. DZ 27.617 (DZUP). Nova Lima – APE Manancial Mutuca, Parque Estadual da Serra do Rola Moça, 1-V-2009, 1 male, Silva leg., DNA voucher PM 10-02 (ZUEC-AVLF). São Roque de Minas – Parque Nacional Serra da Canastra, 9-IV-1999, 1 male, without collector, UFMG ILE 1300506 (UFMG); 19-21-IV-1999, 2 males, without collector, UFMG ILE 1300504, 1300507 (UFMG). São Paulo: São Luiz do Paraitinga – 800 m, 22-IV-2004, 1 male, Ribeiro leg., ZUEC LEP 6.548 (ZUEC); 28-IX-2004, 1 male, Ribeiro leg., ZUEC LEP 6.548 (ZUEC); 29-IX-2004, 1 male, Ribeiro leg., ZUEC LEP 7.003 (ZUEC); 12-I-2005, 1 female, Ribeiro leg., ZUEC LEP 6.724 (ZUEC); 18-II-2005, 2 males, Ribeiro leg., ZUEC LEP 6.666, ZUEC LEP 6.691 (ZUEC).
Taygetis drogoni sp. n. is very similar to Taygetis ypthima, differing from the latter by the following characters: forewing with pale brown dorsal post discal band less contrasting than in Taygetis ypthima; underside pale post discal band slightly constricted at M3, tapering abruptly in CuA1-CuA2 and becoming conspicuously thinner or even absent from CuA2 to the inner margin; hind wing underside with the discal line evenly curved and regular, extending from the costal margin to 1A; and dark post discal line straight and only slightly irregular. Tegumen larger and protruding; valva stouter and shorter, with a larger dorsal rough area. Signa dorsal; laterally, sternum VIII not fused with tergum VIII; lamella antevaginalis without process.
Head. Brown. Post-genal area light brown. Eye glabrous, brown. Antennae without scales at apical third, mostly light brown; club dark brown with last flagellomere light brown. Labial palpus mixed with brown and light brown, with elongated scales at first and second segment; about 1.5 times total length of eye; third segment thin, same size as first. Thorax. Uniformly brown. Legs brown; meso- and metathoracic femurs light brown on inside. Forewing, size and shape: length: 34.5–37.0 mm (n = 23). Triangular, costal margin convex, apex pointed, outer margin convex, tornus rounded, inner margin straight. Forewing upper side (Fig. 1A, C). Mostly brown, darker along outer margin. Forewing under side (Figs 1B, D). Background brown, lighter at wing base. Dark brown scales at end of discal cell and whitish on transverse veins. Dark spot at base of M2. Costal margin to external margin with reticulated markings. Apex rufous brown. Submarginal band whitish, from costal to inner margin, with reduced creamy ocelli in spaces R5-M1, M1-M2, M2-M3 and M3-CuA1; proximal border of submarginal band irregular with dark brown scaling, distal border of marginal band inconspicuous. Marginal line brown. Fringe light brown. Hind wing shape: Costal margin convex, apex rounded, external margin convex in M1-M2, projections at CuA1, CuA2 and 2A, with a developed one at M3. Inner margin curved at base, slightly straight towards tornus. Hind wing upper side (Fig. 1A, C). Mostly brown, darker along outer margin. Hind wing under side (Fig. 1B, D). Background rufous brown, discal line dark brown and irregular. Dark brown spot at base of M2. Submarginal band with reduced creamy ocelli, in spaces Rs-M1, M1-M2, M2-M3 and M3-CuA1, ocellus on CuA1-CuA2 developed. Post discal line straight and dark brown. Proximal border of submarginal band along post discal line forming a 2 mm wide reddish fascia. Distal border of marginal band inconspicuous. Marginal line brown, with distal area reddish. Fringe light brown. Abdomen. Dorsally brown, ventrally light brown.
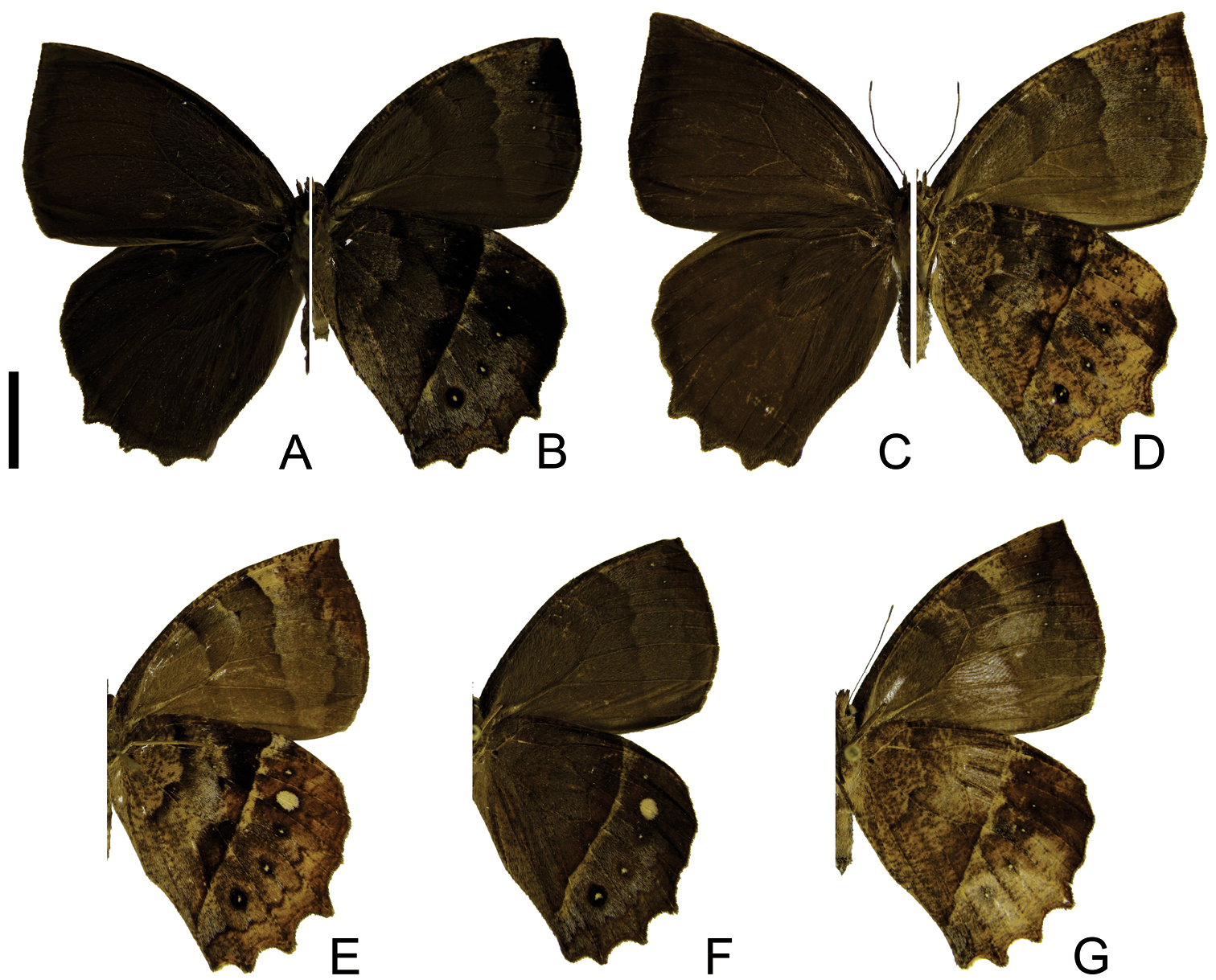
Adults of Taygetis drogoni sp. n. A–B male A dorsal view B ventral view C–D female C dorsal view D ventral view. Scale bar = 1 cm.
Male genitalia (Fig. 6A–E). Tegumen dorsally convex, subtriangular in lateral view, ventral projection wide; appendix angular reduced. Uncus straight, down curved at apex and dorsally keeled. Gnathos larger than uncus; straight and projected dorsally, without a ventral projection at base. Anterior projection of saccus cylindrical, length equal to tegumen. Valva subrectangular, with dorsal projections at apical third; costa developed and subtriangular; ventrally covered by setae. Aedeagus straight, thin and larger than valva; opening of aedeagus almost the total length of posterior portion.
Female genitalia (Fig. 7A, B). Tergum VIII squared. Papillae anales with setae at distal portion, 2/3 higher than longer, sclerotized at basal half. Sterigma sclerotized, formed by a round lamella antevaginalis and a membranous pocket between ostium bursae and papilla anales. Bursa copulatrix totally membranous, with a pair of signa dorsally; ductus bursae thinned, with apical third sclerotized, about three times length of bursa copulatrix.
The specific epithet refers to Drogon, one of the three dragons of Daenerys Targaryen, a fictional character from the George R. R. Martin’s novel “A Song of Ice and Fire”.
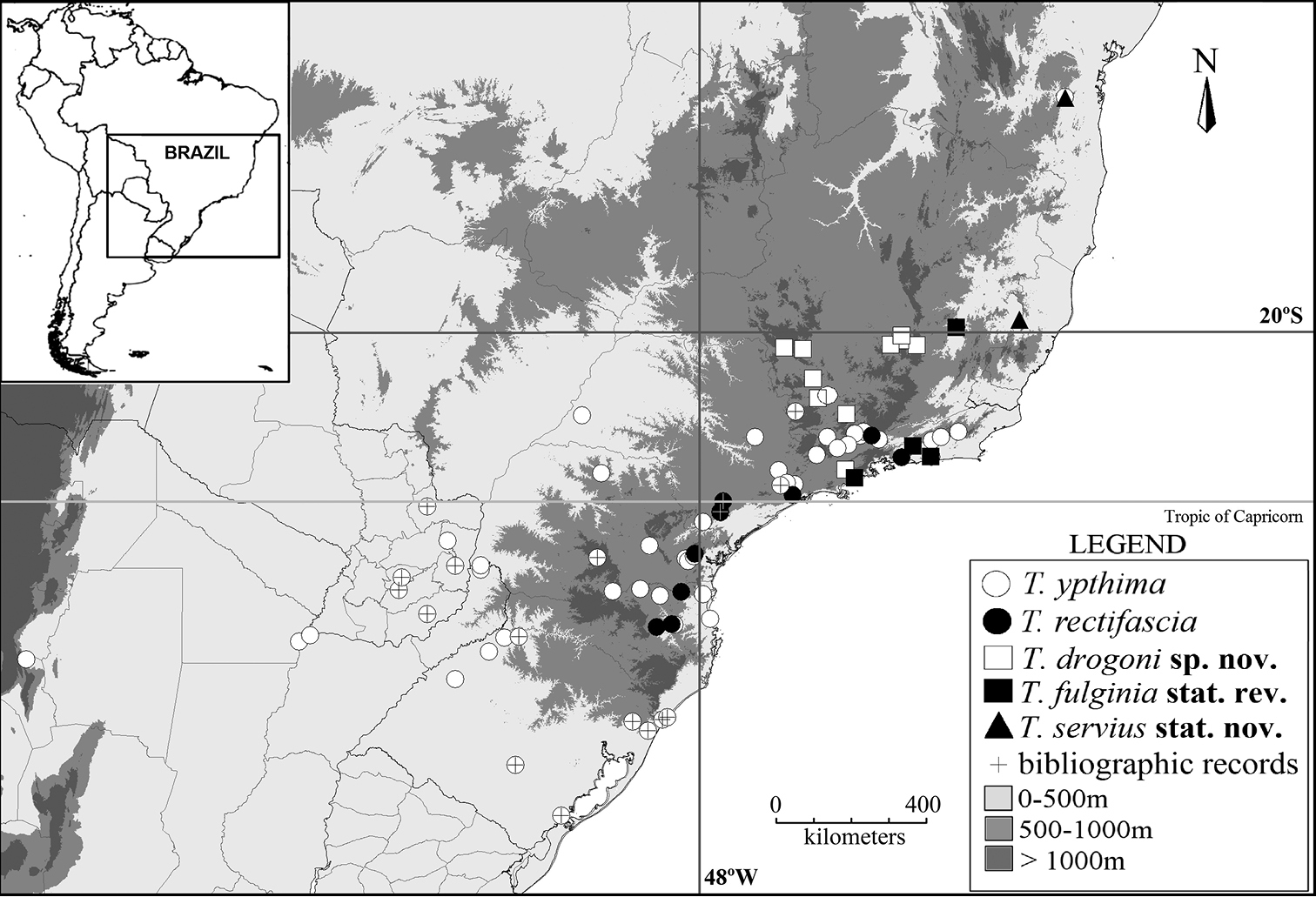
(Fig. 8). This species occurs in southeastern Brazil (Minas Gerais and São Paulo) at elevations from 800 to 1, 500 m a.s.l.
This species has presumably not been recognized in the past because of the intrinsic phenotypic variation within Taygetis and, in particular, within Taygetis ypthima, the most similar and probably closest species to Taygetis drogoni sp. n. The species appears cited as Taygetis ypthima in
BRASIL – Bahia: Jitaúna – 25-III-1969, 1 female, Ebert leg., ex-coll. Ebert, DZ 27. 720 (DZUP). Rio de Contas, 150 m, 4-III-1969, 1 female, H. Ebert leg., ex-coll. Ebert, DZ 27.721 (DZUP). Minas Gerais: Alfenas – 27-I-2012, 1 male, Brito leg., VPI2.2-214 (ZUEC). Camanducaia – Monteverde, 1650m, 8-II-1979, Ebert leg., ex-coll. Ebert, DZ 27.646 (DZUP); Cambuquira – 10-XII-1968, 1 male and 1 female, Ebert leg., ex-coll. Ebert, DZ 27.432, DZ 26.433 (DZUP); 12-X-1968, 1 female, Ebert leg., ex-coll. Ebert, DZ 26.434 (DZUP); 15-IX-1969, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.647 (DZUP); Conceição dos Ouros – Rio Sapucaí, 24-II-1968, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.648 (DZUP); Marliéria – Parque Estadual do Rio Doce, 200 m, 08-IX-1972, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.718 (DZUP); 16-III-1972, 1 male, H. & H. D. Ebert leg., ex-coll. Ebert, DZ 27.719 (DZUP). Itamonte – Vargem Grande, 1600m, 17-II-2010, 1 male, Mielke & Casagrande leg., DZ 27.554 (DZUP); NE side of Itatiaia, 1300m, II-1959, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.671 (DZUP); Passa Quatro – Paiolinho, Fazenda Serra Fina, 1600m, 16-II-2010, 1 male, Mielke & Casagrande leg., DZ 27.645 (DZUP); Virgínia – Fazenda dos Campos, 1500m, 13-15-II-2010, Mielke & Casagrande leg., DZ 27.540 (DZUP). Rio de Janeiro: Nova Friburgo – 1000m, 23-I-1983, 1 female, O.-C. Mielke leg., DZ 27.644 (DZUP); Itatiaia – 900m, 23-I-1936, 1 male, Gagarin leg., ex-coll. Gargarin, DZ 27.472; II-1958, 2 male, Ebert leg., ex-coll. Ebert, DZ 27.634, DZ 27.635 (DZUP); 1600m, 14-II-1956, 1 male and 2 females, Ebert leg., ex-coll. Ebert, DZ 27.637, DZ 27.638, DZ 27.639 (DZUP); Parque Nacional do Itatiaia, Maromba, 1100m, 06-09-II-2011, 1 female, Freitas leg., ZUEC LEP 5.372 (ZUEC); Petrópolis – Independência, 900m, 13-III-1933, 1 male, Gargarin leg., ex-coll. Gagarin, DZ 27.655 (DZUP); Rio de Janeiro – 14-XI-1920, 1 male, D’Almeida leg., ex-coll. D’Almeida, DZ 27.654 (DZUP); Teresópolis – 1600m, 20-II-1967, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.637 (DZUP). São Paulo: Apiaí – IV-1972, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.636 (DZUP); Campos do Jordão – I.1966, 5 males and 3 females, without collector, DZ 27.369, DZ 27.579, DZ 27.588, DZ 27.589, DZ 27.591, DZ 27.597, DZ 27.602, DZ 27.603 (DZUP); 1600-2000m, 8-12-II-1982, 2 males, Mielke & Casagrande, DZ 27.587, DZ 27.595 (DZUP); Parque Estadual Campos do Jordão, 1950m, 10-II-1968, 2 males, Mielke, Brown & Laroca leg., DZ 27.593, DZ 27.600 (DZUP); 1800m, 11-12-I-2001, 1 female, Brown & Freitas leg. (ZUEC-AVLF); Capão Bonito – Fazenda Intervales, Sede, 950-1100m, 30-XII-1989, 1 female, Freitas leg. (ZUEC-AVLF); 15-II-2000, 2 females, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 1.776 (ZUEC); 13-XII-2000, 1 male and 1 female, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 4.731, ZUEC LEP 4.732 (ZUEC); 5-6-XII-2001, 1 male and 1 female, Brown & Freitas leg. (ZUEC-AVLF); 15-I-2002, 1 female, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 802 (ZUEC); 17-I-2003, 2 males and 2 females, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 1.028, ZUEC LEP 1.547, ZUEC LEP 1.548, ZUEC LEP 1.549 (ZUEC); 19-I-2003, 3 males, Brown, Freitas & Uehara-Prado leg., ZUEC LEP 1.180, ZUEC LEP 1.286, ZUEC LEP 1.305 (ZUEC); Cotia – Morro Grande, 900-1100m, 15-III-2000, 1 female, Uehara-Prado & Freitas leg. (ZUEC-AVLF); 22-XII-2000, 1 female, Brown & Uehara-Prado leg. ZUEC LEP 1.781 (ZUEC); Imbariê – 7-I-1956, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.633 (DZUP); Jundiaí – Serra do Japi, 11-V-2012, 1 female, Santos leg., BLU 246 (ZUEC); Piquete – Barreira de Piquete, 1400-1600m, 15-II-1984, 2 males, Mielke & Casagrande leg., DZ 27.585, DZ 27.596 (DZUP); Presidente Venceslau – without date, 2 males and 4 females, D’Almeida leg., DZ 27.656, DZ 27.657, DZ 27.658, DZ 27.659, DZ 27.660, DZ 27.661 (DZUP); Rio Claro – 60m, 6-I-1964, 5 males and 1 female, Ebert leg., ex-coll. Ebert, DZ 27.392, DZ 5.500, DZ 27.663, DZ 27.630, DZ 27.631, DZ 27.632 (DZUP); 16-V-1965, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.662 (DZUP); Salesópolis – Estação Biológica da Boraceia, 900m, 21-II-2006, 1 male, Uehara-Prado & Freitas leg. (ZUEC-AVLF); 27-III-2006, 1 male, Uehara-Prado & Freitas leg. (ZUEC-AVLF); Umuarama – 1800m, 8-15-III-1937, 8 males and 2 females, Gargarin leg., ex-coll. Gagarin, DZ 27.584, DZ 27.586, DZ 27.590, DZ 27.592, DZ 27.593, DZ 27.594, DZ 27.598, DZ 27.599, DZ 27.601, DZ 27.632 (DZUP). Paraná: Foz do Iguaçu – 250m, 17-II-1969, 3 males, Moure & Mielke leg., DZ 26.741, DZ 1.626, DZ 27.368 (DZUP); 10-X-1969, 1 male, Krause leg., DZ 26.740 (DZUP); Parque Nacional do Iguaçu, 21-24-IV-1996, 2 females, Mielke & Casagrande leg., DZ 26.743, DZ 26.769 (DZUP); Curitiba – 900m, 20-III-1980, 1 male, O. Mielke leg., DZ 27.405 (DZUP); Uberaba, Tirol das Torres, 900m, 5-II-2010, 1 female, O. Mielke leg., DZ 26.742 (DZUP); Rolândia – Rio Tibagi, 750m, XII-1941, 1 male, Waltz leg., DZ 27.397 (DZUP). Santa Catarina: Canoinhas – I, 1 male, Pohl leg., DZ 27.667 (DZUP); 16-IX-1941, 1 male, Schimith leg., DZ 27.668 (DZUP); Ibirama – I, 1 male, Pohl leg., DZ 27.666 (DZUP); VIII, 1 female, Pohl leg., DZ 27.670 (DZUP); XII, Pohl leg., DZ 27.669 (DZUP); Itaiópolis – 26-III-1937, 1 male, D’Almeida leg., ex-coll. D’Almeida, DZ 27.549 (DZUP); Itajaí– Agrolândia, 400m, II-1973, 1 female, Wulff leg., DZ 27.665 (DZUP); Joinville – 5-III-1974, 1 male, O. Mielke leg., DZ 26.778 (DZUP); São Bento do Sul – Rio Vermelho, 850m, 10-IV-1980, 1 female, Rank leg., DZ 27.640 (DZUP); 950m, 23-I-1982, 1 male, Rank leg., DZ 26.815 (DZUP); 650m, 30-I-1982, 1 male, Rank leg., DZ 26.816 (DZUP); 850m, 7-XII-1969, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.649 (DZUP); 8-I-1971, Ebert leg., ex-coll. Ebert, DZ 27.643 (DZUP); 10-I-1971, 1 female, Ebert leg., ex-coll. Ebert, DZ 27.641 (DZUP); 7-VIII-1971, Ebert leg., ex-coll. Ebert, DZ 27.650 (DZUP); 3-X-1971, 1 female, Ebert leg., ex-coll. Ebert, DZ 5.499 (DZUP); 5-XII-1969, 1 male and 1 female, Ebert leg., ex-coll. Ebert, DZ 27.642, DZ 27.651 (DZUP); 6-XII-1969, 1 male and 1 female, Ebert leg., ex-coll. Ebert, DZ 26.429, DZ 27.653 (DZUP); 4-III-1980, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.652 (DZUP); 8-V-1980, 1 male, Rank leg., DZ 27.664 (DZUP); Taió – February, 1 male, Pohl leg., DZ 27.666 (DZUP). Rio Grande do Sul: São José do Inhacorá – Alto Uruguai, 2-V-1980, 1 male, Steiniger leg., DZ 26.770 (DZUP). PARAGUAY – General Dias: Itaquiri – 400m, 15-20-I-1980, 5 males and 5 females, O.-C. Mielke & Myers leg., DZ 27.708, DZ 27.709, DZ 27.710, DZ 27.711, DZ 27.712, DZ 27.713, DZ 27.714, DZ 27.715, DZ 27.716, DZ 27.717 (DZUP). ARGENTINA – Corrientes: Santo Tomé – I-1924, 1 male, D’Almeida leg., ex-coll. D’Almeida, DZ 27.522 (DZUP). Misiones: General Manuel Belgrano – Almirante Brown, Reserva Yacutinga, 2-5-III-2007, 1 male, Mielke & Casagrande leg., DZ 27.531 (DZUP). Tucumán: Ibatim – Pueblo Viejo, 850m, 25-I-1970, 1 female, O. Mielke leg., DZ 27.707 (DZUP).
Taygetis ypthima can be distinguished from Taygetis drogoni by the forewing underside submarginal band, not conspicuously constricted at M3; the proximal line is oblique in M3-CuA1 to the direction of the base of the wing, disjointed of the remainder of the line from CuA1 to the inner margin; submarginal band irregular, but about the same width from M1 to the inner margin, sometimes slightly wider at M3-CuA1; hind wing underside with the discal line curved and irregular, extending from the costal margin to the inner margin; and proximal line of the submarginal band distinctly curved and irregular. Tegumen smaller; valva thinner and longer, with a smaller dorsal rough area. Signa ventral; laterally, sternum VIII fused with tergum VIII; lamella antevaginalis with two lateral process.
Adults of Taygetis ypthima. A–B male A dorsal view B ventral view C–D female C dorsal view D ventral view E–H variations in ventral view. Scale bar = 1 cm.
(Fig. 8). Occurs in northeastern, southeastern and southern Brazil, and also in Paraguay and Argentina, from sea level to 2000 m a.s.l. Based on label data, adults are present all year round.
This is the commonest and more widespread species of the group. The high intraspecific variation observed in Taygetis yphtima yield a number of descriptions of local forms or synonyms: Taygetis xantippe Butler, [1870], Taygetis ophelia Butler, 1870; Taygetis ophelia f. semibrunnea Weymer, 1910 and Taygetis ypthima [sic] ab. lineata Kivirikko, 1936, all synonyms of Taygetis ypthima (
http://species-id.net/wiki/Taygetis_rectifascia
Figs 3A–G; 6K–O; 7E, FBRASIL – Rio de Janeiro: Rio de Janeiro – Mangaratiba - 12-VIII-1926, 1 female, D’Almeida leg., ex-coll. D’Almeida, DZ 26.426 (DZUP); Itatiaia - Parque Nacional do Itatiaia, 1200m, 25-II-1959, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.490 (DZUP); 1000-1200m, 25-VII-1963, 1 male, Ebert leg., ex-coll. Ebert, DZ 26.417 (DZUP); 1100m, 4-II-1966, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.469 (DZUP); 110m, 29-III-1967, Ebert leg., ex-coll. Ebert, DZ 5.502 (DZUP); 1000-1200m, 25-VII-1968, 1 male, Ebert leg., ex-coll. Ebert, DZ 27.346 (DZUP). São Paulo: Capão Bonito – Fazenda Intervales, Sede, 950m, 28-XII-1989, 1 male, Freitas leg. (ZUEC-AVLF); 30-XII-1989, 1 male, Freitas leg. (ZUEC-AVLF); 15-II-2000, 3 males and 3 females, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 1.777, ZUEC LEP, 1.778, ZUEC LEP 1.779, ZUEC LEP 1.780, ZUEC LEP 1.782, ZUEC LEP 1.785 (ZUEC); 13-XII-2000, 3 males and 3 females, Brown, Freitas, Francini & Uehara-Prado leg., ZUEC LEP 4.730, ZUEC LEP 4.733, ZUEC LEP 4.734, ZUEC LEP 4.735, ZUEC LEP 4.736 (ZUEC); 5-6-XII-2001, 1 male and 4 females, Brown & Freitas leg. (ZUEC-AVLF); 17-20-I-2003, 3 males, Brown, Freitas & Uehara-Prado leg., ZUEC LEP 1.188, ZUEC LEP 1.189, ZUEC LEP 1.550 (ZUEC); Salesópolis – Estação Biológica da Boraceia, 900m, 28-IV-2006, 1 male, Uehara-Prado & Freitas leg. (ZUEC-AVLF); Paraná: Campina Grande do Sul – 13.III.1982, 2 males and 2 female, Mielke & Casagrande leg., DZ 26.430, DZ 27.519, DZ 27.532, DZ 27.534 (DZUP); Santa Catarina: Taió – I, 1 male, Pohl leg., DZ 27.382; Presidente Getúlio – Dalbérgia, 1 male, December, Pohl leg., DZ 26.425 (DZUP); São Bento do Sul – Rio Natal, IV-2012, 1 male, Rank leg., DZ 27.449 (DZUP).
Taygetis rectifascia can be distinguished from Taygetis fulginia and other species of the genus by the following combination of characters: forewing pointed at the apex; hind wing, with small projections at M3, CuA1 and CuA2; dorsal wings brown with thin suffused dark brown bands about 2 mm away from and along the outer margin; ventral hind wing with the proximal border of the submarginal band and post discal line straight and slightly irregular, sometimes forming a creamy white fascia of varying width. The base of the gnathos presents a ventral pointed projection, similar to Taygetis fulginia and Taygetis servius stat. n. The male genitalia differ from all other species discussed in the present paper by the shape of the valvar end, which is bifid and claw-shaped (presenting large intraspecific variation).
Adults of Taygetis rectifascia. A–B male A dorsal view B ventral view C–D female C dorsal view D ventral view E–G variations in ventral view. Scale bar = 1 cm.
(Fig. 8). Occurs in southeastern and southern Brazil (Rio de Janeiro, São Paulo, Paraná and Santa Catarina), at elevations from 300 to 1, 200 m a.s.l. Based on label data, adults are present all year round.
Despite its superficial resemblance to Taygetis fulginia, this species presents a very distinctive genitalia. Taygetis rectifascia presents strong intraspecific variation in the wing pattern, which in the past has motivated the description of several aberrations and forms: Taygetis rectifascia ab. stigma Weymer, 1907; Taygetis rectifascia ab. latifascia Weymer, 1907 (all synonyms of Taygetis rectifascia) (
Figs 4A–D, 6P-T; 7G–H
BRAZIL – Bahia: Jitaúna – 26-III-1969, 2 males and 2 females, Ebert leg., ex-coll. Ebert, DZ 26.424, DZ 27.440, DZ 27.396, DZ 27. 386 (DZUP). Espírito Santo: Baixo Guandú – 10-IV-1970, 1 male, Elias leg., DZ 26.806 (DZUP).
Taygetis servius stat. n. can be distinguished from Taygetis fulginia stat. r. and Taygetis rectifascia by the following characters: forewing rounded at the apex; dorsal wings light brown without any suffused dark brown bands along the outer margin; ventral hind wing with the proximal border of the submarginal band and post discal line straight and regular, forming a 2 mm wide creamy white fascia. The base of the gnathos presents a pointed ventral projection, as in Taygetis rectifascia and Taygetis fulginia, but differs from Taygetis rectifascia by the absence of the claw-shaped bifid valva apex, and from Taygetis fulginia by its stouter valva, with a shorter but wider distal projection of the valva. In additional, Taygetis servius is considerably smaller than the other species treated in the present paper.
Adults of Taygetis servius stat. n. A–B male A dorsal view B ventral view C–D female C dorsal view D ventral view. Scale bar = 1 cm.
(Fig. 8). Taygetis servius is known from Baixo Guandú (Espírito Santo), and from Jitaúna (Bahia). It should also be present in Minas Gerais (see below).
Taygetis servius stat. n. was described from an unknown number of specimens from Minas Gerais, Brazil, as a form of Taygetis rectifascia (Weymer, 1910: 187). Although clearly recognized as such, the illustration of Taygetis servius (Weymer, 1910: pl. 46, fig. c [3]) is placed in another plate, separated from the rest of the illustrations of Taygetis rectifascia and its forms (Weymer 1910: pl. 45, fig. a [1-2]). The description and the illustration matches exactly a series of five specimens from the states of Espírito Santo and Bahia, Brazil, deposited at the DZUP. Wing shape and pattern, also acknowledged by Weymer, and examination of the genitalia confirms it as a distinct species. The type specimen (or specimens) of Taygetis servius is missing, however, type specimens of other species of Taygetis described by Weymer in the same fascicle of Die Gross-Schmetterlinge der Erde are housed at the SMT and ZSM collections (i.e. Taygetis mermeria f. crameri (Weymer, 1910), at SMT, and Taygetina banghaasi (Weymer, 1910), at ZSM). However, previous and recent searches for type specimens carried out by G. Lamas, O.H.H. Mielke and the curators of the above cited collections did not produce any specimens (
Figs 5A–D; 6U–Y; 7I, J
Holotype male with the following labels: /HOLOTYPUS/ Taygetis fulginia d’Almeida, 1922 /M(#)/ 30-X-1921 Parada Caramujos, E. F. C. B. [Estação de Ferro Central do Brasil] [Japeri] [22°38'34"S, 43°39'10"W] Estado do Rio [de Janeiro] Ferreira d’Almeida leg. /N°5163/ DZ 27.378/ (DZUP).
BRAZIL – Minas Gerais: Marliéria – Parque Estadual do Rio Doce, 250 m, 14-V-1974, 2 males, Ebert leg., ex-coll. Ebert, DZ 26.418, DZ 27.524 (DZUP); 17-V-1974, 1 male, Ebert leg., ex-coll. Ebert, DZ 26.821 (DZUP). Rio de Janeiro: Rio de Janeiro – Horto Florestal – 01-VIII-1932, 1 female, Gagarin leg., ex-coll. Gagarin, DZ 27.416 (DZUP). São Paulo: Ubatuba – Parque Estadual da Serra do Mar, Núcleo Picinguaba, 0-100m, 30-IX-2001, 1 male, Brown & Freitas leg. DNA voucher BLU 443 (ZUEC-AVLF).
Taygetis fulginia can be distinguished from Taygetis ypthima and other species of the genus by the following characters: in size it is slightly smaller, the forewing is only slightly pointed at the apex, the hind wing has smaller projections at M3, CuA1 and CuA2, similar to Taygetis servius stat. n., the dorsal wings lack suffused dark brown bands along the outer margin. The base of the gnathos presents a ventral pointed projection, as in Taygetis rectifascia and Taygetis servius stat. n., but Taygetis fulginia differs from the former by the absence of a developed dorsal projection on the valva, and from the latter by the longer and thinner distal projection of the valva, which is also longer and with a dorsally protruding area in Taygetis fulginia.
Adults of Taygetis fulginia stat. r. A–B male A dorsal view B ventral view C–D female C dorsal view D ventral view. Scale bar = 1 cm.
(Fig. 8). This species occurs in southeastern Brazil (Minas Gerais, Rio de Janeiro and São Paulo), from sea level to 250m.
The description of Taygetis fulginia was based on a single specimen in the D’Almeida collection, now deposited at DZUP (see above). This species was previously considered a synonym of Taygetis ypthima, but the morphological study confirms its specific status and indicates closer relationship with Taygetis rectifascia and Taygetis servius stat. n.
A combination of wing shape and color pattern permits identification of all five species without dissection, and genitalia of both sexes (not included here but discussed in the text) provide diagnostic characters for all species.
| 1 | Forewing upper side with suffused dark brown marginal band developed (Figs 1A, C; 2A, C); hind wing with long projections at CuA1, CuA2 and 2A (Figs 1, 2) | 2 |
| – | Forewing upper side with suffused dark brown marginal band reduced (Fig. 3A, C) or absent (Figs 4A, C; 5A, C); hind wing with short projections at CuA1, CuA2 and 2A (Figs 3, 4, 5) | 3 |
| 2 | Forewing underside submarginal band constricted at M3 and reduced or absent in CuA1-CuA2 (Fig. 1B, D); hind wing underside with the discal line evenly curved and regular; dark post discal line straight and more or less regular (Fig. 1B, D) | Taygetis drogoni sp. n. |
| – | Forewing underside submarginal band not constricted at M3 (Fig. 2B, D); hind wing underside with the discal line irregular; post discal line distinctly irregular and not straight (Fig. 2B, D) | Taygetis ypthima |
| 3 | Forewing apex conspicuously pointed (Fig. 3A–G); forewing upper side with suffused dark brown marginal band reduced (Fig. 3A, C) | Taygetis rectifascia |
| – | Forewing apex rounded (Fig. 4A–D) or slightly pointed (Fig. 5A–D); forewing upper side with suffused dark brown marginal band absent (Figs 4A, C; 5A, C) | 4 |
| 4. | Ventral hind wing with the proximal border of the submarginal band and post discal line straight and regular, forming an even 2 mm wide creamy white fascia; hind wing with projections at CuA1, CuA2 and 2A strongly reduced (Fig. 4B, D) | Taygetis servius stat. n. |
| – | Ventral hind wing with the proximal border of the submarginal band and post discal line irregular, forming an irregular creamy white fascia (Fig. 5B, D) | Taygetis fulginia stat. r. |
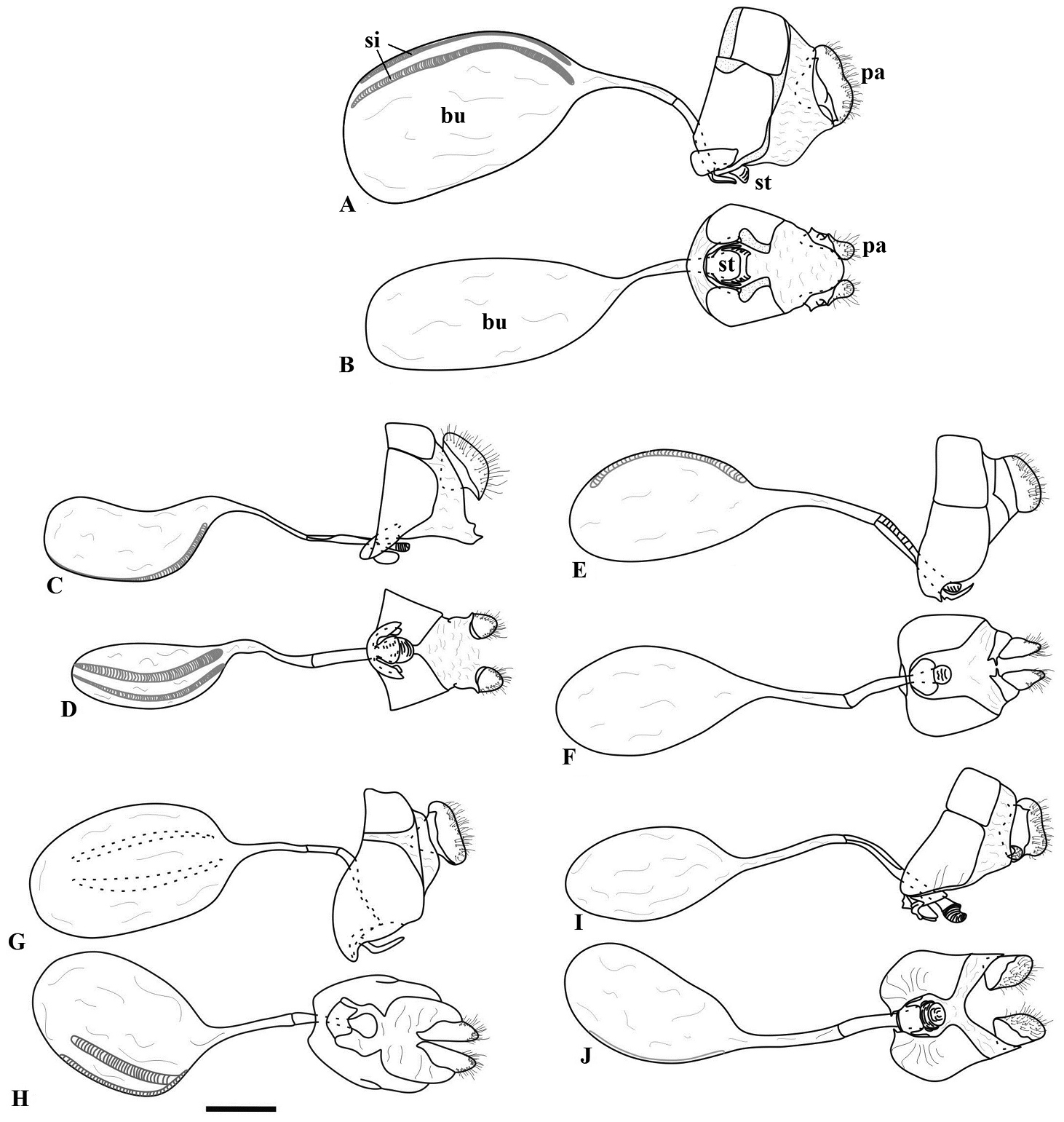
Male genitalia of Taygetis. A–E Taygetis drogoni sp. n. A lateral view B–C aedeagus: B ventral C lateral D dorsal view E ventral view F–J Taygetis ypthima F lateral view G–H aedeagus: G ventral H lateral I dorsal view J ventral view K–O Taygetis rectifascia K lateral view L–M aedeagus: L ventral M lateral N dorsal view O ventral view P–T Taygetis servius stat. n. P lateral view Q–R aedeagus: Q ventral R lateral S dorsal view T ventral view U–Y Taygetis fulginia U lateral view V–W aedeagus: V ventral W lateral X dorsal view Y ventral view. Abbreviations: aa appendix angular; gn gnathos; sa saccus; te tegumen; un uncus; va valva. Scale bar = 1 mm.
Female genitalia of Taygetis. A–B Taygetis drogoni sp. n. A lateral view B ventral view C–D Taygetis ypthima C lateral view D ventral view E–F Taygetis rectifascia E lateral view F ventral view G–H Taygetis servius stat. n. G lateral view H ventral view I–J Taygetis fulginia I lateral view J ventral view. Abbreviations: bu corpus bursae; pa papilla analis; si signa; st sterigma. Scale bar = 1 mm.
Geographical distribution of the species in the “Taygetis ypthima species group”.
The “Taygetis ypthima species group” is not part of the genus Taygetis, but in fact a clade related to the genus Pseudodebis Forster, 1964 (including Taygetomorpha L. D. Miller, 2004) in the “Pseudodebis subclade” of
The five species treated here can be easily distinguished from one another by wing pattern and genitalia, and two main subgroups can be identified based on morphology - group 1, composed of Taygetis ypthima and Taygetis drogoni sp. n., and group 2, composed by Taygetis rectifascia, Taygetis fulginia stat. r. and Taygetis servius stat. n. Species in group 2 can be distinguished from those of the group 1 by bearing shorter tails at M3, CuA1 and CuA2, by the lighter dorsal ground color of wings, and by a clear reduction (Taygetis rectifascia) or total absence (Taygetis fulginia stat. r. and Taygetis servius stat. n.) of the suffused dark brown marginal bands. The base of the gnathos in the species of the group 2 presents a conspicuous ventral projection, and there is a longer saccus and aedeagus, and a relatively shorter posterior opening of the aedeagus. Wing color and pattern present conspicuous intraspecific variability in all five species treated here, especially in Taygetis ypthima and Taygetis rectifascia (
Although species in the “Taygetis ypthima species group” are not part of the genus Taygetis (a fact also reinforced by karyological data, see
The present study revealed that morphological characters, such as wing shape and pattern, and male and female genitalia, were efficient to provide clear-cut species delimitation. However, further detailed morphological studies on Euptychiina are highly required to clarify the species and genera delimitations within the subtribe. As clear morphological keys are generated, several monophyletic groups are easily identified using informative synapomorphies (
We are grateful to Gerardo Lamas and Keith Willmott for critical reading of the manuscript. Axel Hausmann (ZSM), Marcelo Duarte (MZUSP), Fernando Silveira (UFMG), Wolfgang Nässig (SMFL), Jessie Pereira dos Santos, Mariana Monteiro de Brito and André Roberto Melo Silva provided additional distributional data and/or information about the type species. R. R. S., T. Z. and F. M. S. D. acknowledge the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for research fellowships. A. V. L. F. acknowledges support from FAPESP (Biota-Fapesp – grant 2011/50225-3), from the Brazilian Research Council – CNPq (fellowship 302585/2011-7, and “SISBIOTA-Brasil/CNPq grant 563332/2010-7), and from the National Science Foundation, USA (DEB-1256742).