(C) 2013 Sergei Zonstein. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Zonstein S, Marusik YM (2013) On Levymanus, a remarkable new spider genus from Israel, with notes on the Chediminae (Araneae, Palpimanidae). ZooKeys 326: 27–45. doi: 10.3897/zookeys.326.5344
Levymanus gershomi gen. n. et sp. n., is described from southern Israel. The eye arrangement and structure of the male palp indicate that this genus belongs to Chediminae Simon, 1893. Levymanus gen. n. differs from other chedimine genera by its unusually long and slender legs, an elongate body, a unique shape of the bipartite thoracic fovea, reduced leg scopulae, smaller spinnerets, and other characters, which are presumably apomorphic. We propose two taxonomic changes: 1) based on widely spaced lateral eyes the Western African genus Badia Roewer, 1961 is transferred from Chediminae to Palpimaninae, and 2) Fernandezina gyirongensis Hu & Li, 1987 from China, based on palpal morphology, is transferred to the Asian genus Steriphopus Simon, 1887 for a new combination Steriphopus gyirongensis (Hu & Li, 1987) comb. n.
Spiders, new taxa, taxonomy, Palearctic, South-Western Asia, Near East
The Palpimanidae is a relatively small family of araneophagous spiders consisting of 131 species in 15 genera (
Specimens of the following spider taxa were studied.
Boagrius sp. aff. incisus Tullgren, 1910 (Zambia), NMHL.
Diaphorocellus sp. (two species from South Africa), MNHN, NHML.
Chedima purpurae Simon, 1873 (Morocco), MNHN.
Colopea sp. (Vietnam), MNHN.
Hybosida lesserti Berland, 1920, MNHN.
Sarascelis (six species, including types: Sarascelis chaperi Simon, 1887, Sarascelis junquai Jézéquel, 1964, Sarascelis lamtoensis Jézéquel, 1964, Sarascelis luteipes Simon, 1887 and Sarascelis rebiereae Jézéquel, 1964, and an undescribed species from Nigeria), MNHN, NHML.
Scelidocteus (five species, including types: Sarascelis baccatus Simon, 1907, Sarascelis lamottei Jézéquel, 1964, Sarascelis pachypus Simon, 1907, Sarascelis ochreatus Simon, 1907, Sarascelis vuattouxi Jézéquel, 1964), Steriphopus lacertosus Simon, 1898), MNHN, and two undescribed species from Cameroon, NHML.
Scelidomachus socotranus Pocock, 1899, NHML.
Steriphopus crassipalpis Thorell, 1895 and Steriphopus macleayi (O. Pickard-Cambridge, 1873), NHML.
The holotype and paratypes of the new taxon described here, including SEM mounts and dissected specimens were deposited in the spider collection of Tel-Aviv University, Israel (TAU) and in the Zoological Museum of the Moscow State University (ZMMU).
Photographs were taken using a Zeiss Discovery V20 stereomicroscope with a Canon PowerShot G9 camera, and an Olympus SZX16 stereomicroscope with an Olympus E-520 camera, and prepared using the CombineZP software. Scanning electron micrographs were made using the SEM JEOL JSM-5200 scanning microscope at the Zoological Museum, University of Turku, Finland. Photographs of landscapes showing the surroundings of the type locality were taken by Vasiliy Kravchenko. Background maps were obtained from the public internet source http://www.maps-for-free.com. Measurements were made to an accuracy of 0.01 mm. Lengths of leg and palp segments were measured on the dorsal side, from the midpoint of the anterior margin to the midpoint of the posterior margin. All measurements are given in millimetres.
Abbreviations used are as follows. Eyes: ALE – anterior lateral, AME – anterior median, PLE – posterior lateral, PME – posterior median; Spinnerets: ALS – anterior lateral, PLS – posterior lateral, PMS – posterior median; Is – inframamillar scutum; Bulb details: Co – conical outgrowth; Em – embolus; Eo – opening of embolus; Ed – embolic division; La – lamella; Tf – tegular fovea, Tp – tegular process; Palp and leg structures: Cs – cymbial scopula, Mc – metatarsal comb, On – onychium, Sh – spatulate hairs, Ts – tarsal scopula. Arrows indicate the elevated posterior rim of the carapace, separate small scuta of the abdomen and claw teeth.
Other used institutional acronyms are: MNHN – Muséum national d’Histoire naturelle, Paris, France; NHML – The Natural History Museum, London, UK; SMF – Senckenberg-Museum (Senckenberg Forschungsinstitut und Naturmuseum), Frankfurt am Main, Germany.
Levymanus gershomi sp. n., by monotypy.
Both the generic name and the specific epithet are given in honour and memory of Gershom Levy (1937–2009), the prominent Israeli arachnologist, for his immense contribution to Israeli and Near East arachnological research. The gender is masculine.
In general appearance, especially by the elongate body and the extended dorsal abdominal scutum, Levymanus gen. n. resembles the otiothopine genus Fernandezina Birabén, 1951 (cf.
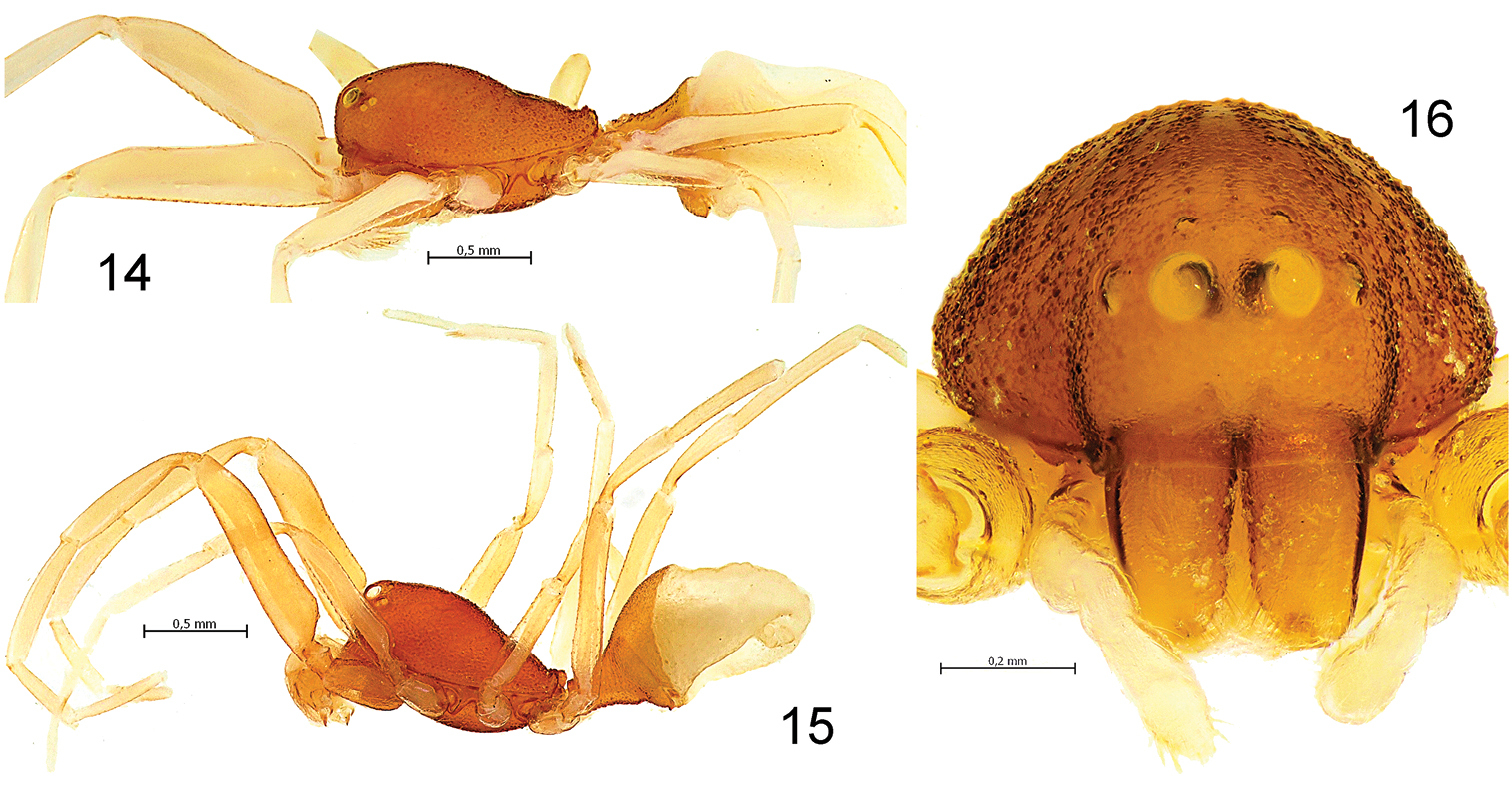
Small bicolored chedimine palpimanids with body length 2.0–2.5 in males and 2.5–3.0 in females; legs and abdomen without pattern. Carapace with corrugated cuticle, diamond-oval in dorsal view, narrowed anteriorly and posteriorly. Cephalic part somewhat raised behind eye area – slightly in males, and more noticeably in females. Thoracic fovea short and bipartite, with two separate sulci located side by side; posterior edge of carapace slightly raised (Figs 14–15). Eight eyes. ALE largest, about four to five times larger than other eyes, which are subaequal in size. ALE and PLE almost touching each other. PME widely spaced from each other, as well as from AMEs and from PLEs. Clypeus about two times higher than AME diameter. Chilum inconspicuous. Chelicerae small, equal in length with clypeus; stridulatory ridges absent; cheliceral furrow without true or peg teeth. Sternum shield-like with fine reticulation; labium about as broad at base as it is long. Prosoma posteriorly with short paired triangular extentions and narrow tubular structure (Figs 11, 13) entering pedicel tube of abdomen (= scutopetiolar apparatus sensu
Levymanus gershomi sp. n.
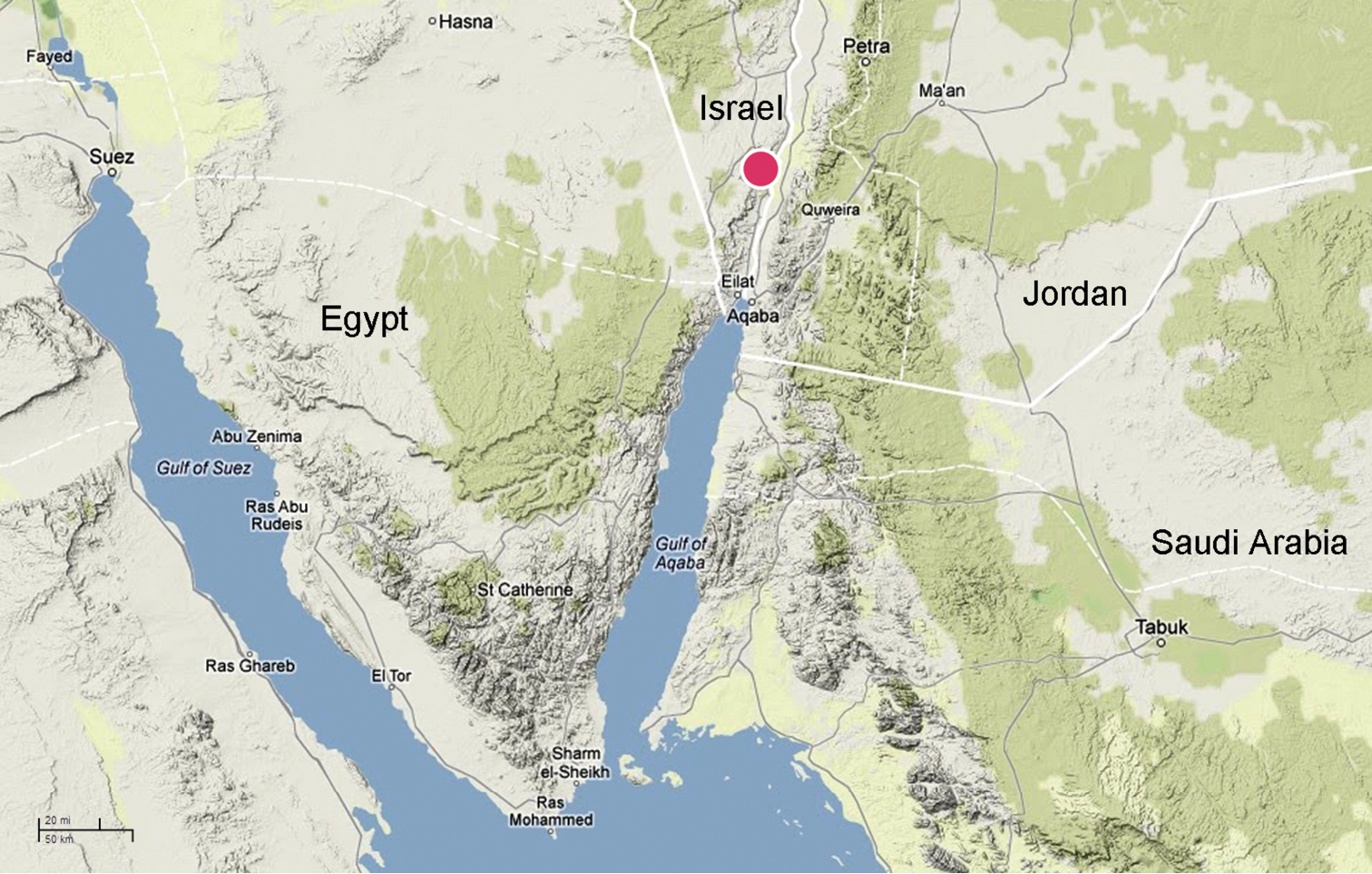
The genus is currently known only from the Arava Valley, Israel.
http://zoobank.org/BBABE77C-15AE-494D-8A61-6309D6541855
http://species-id.net/wiki/Levymanus_gershomi
Figs 1–2, 10–50Male holotype, 3 ♂ and 2 ♀ paratypes from the vicinity of Qetura (Ktura), Arava Valley, Israel (29°58'N, 35°03'E), 8 May 2003, coll. E. Topel, deposited in TAU (holotype and most paratypes) and ZMMU (few paratypes).
As for the genus.
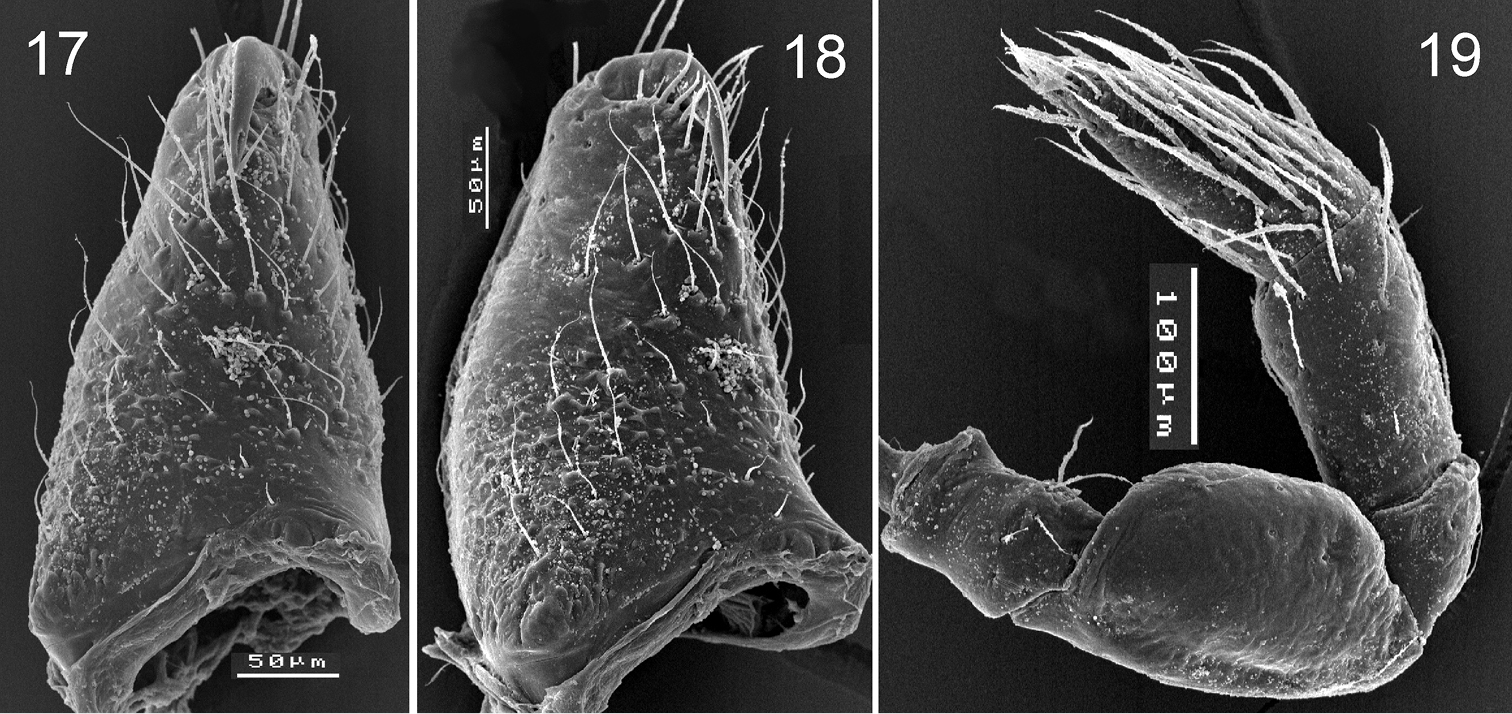
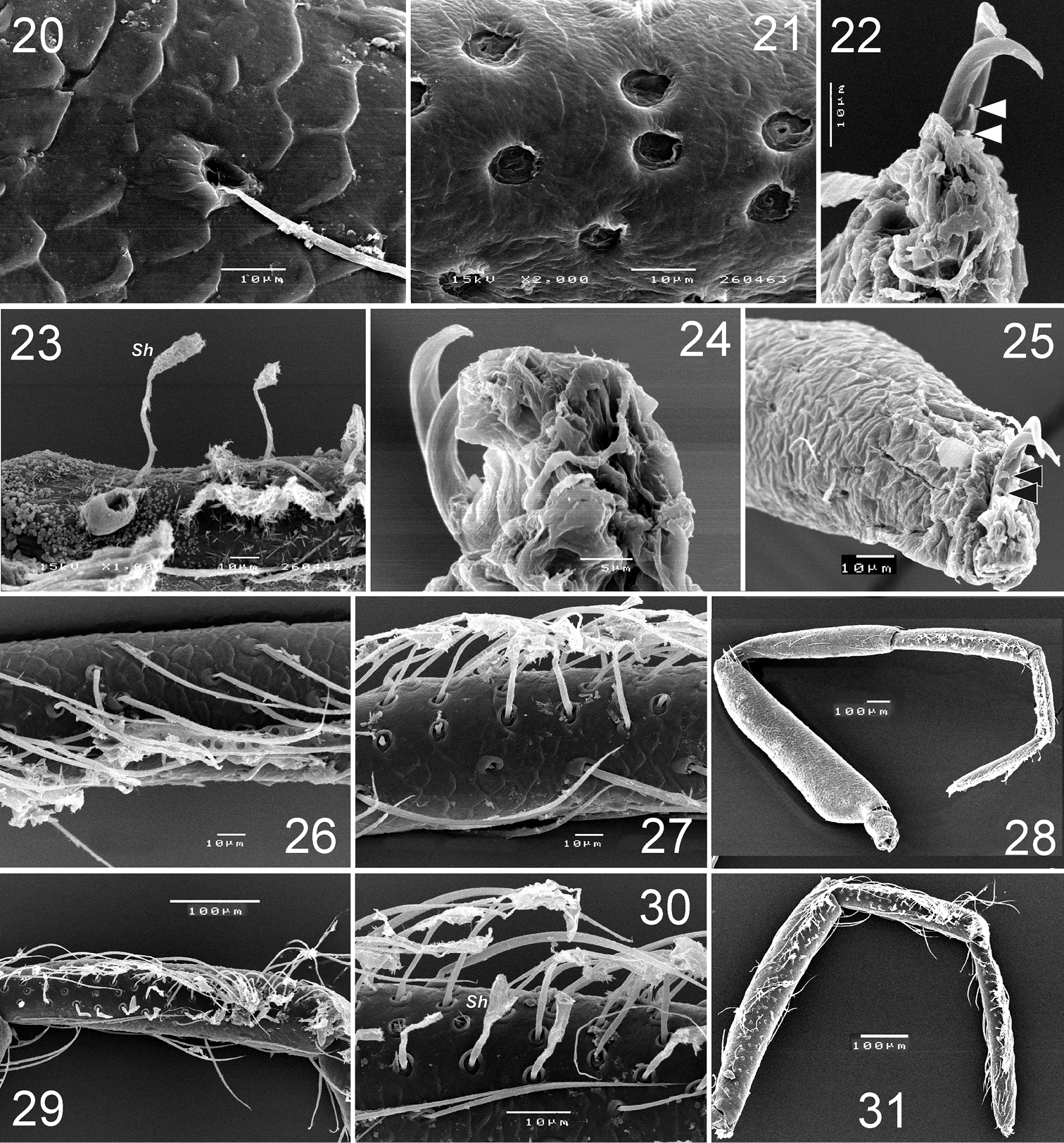
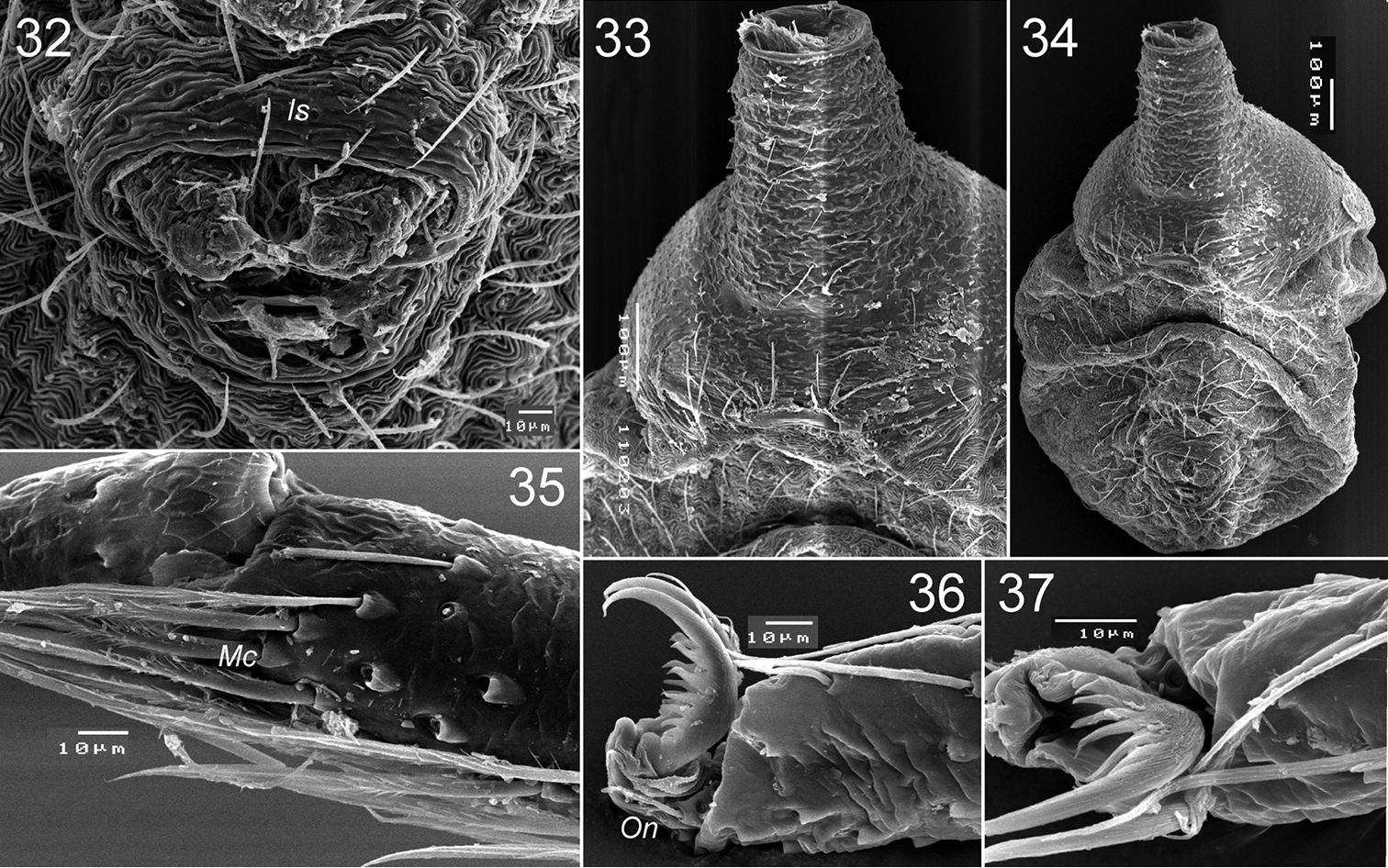
Male (holotype): Total length 2.55; carapace, sternum and labium intensive carmine-red; chelicerae, palps (entirely), coxa and femur I light reddish orange, other segments of leg I and entire legs II–IV pale yellowish red; abdomen milk-white with intensive reddish orange dorsal scutum. Carapace (Fig. 10): 1.10 long, 0.76 wide. Diameters of AME, ALE, PLE, PME: 0.10, 0.02, 0.02, 0.02. Interdistances: AME–AME 0.08, ALE–AME 0.05, ALE–PLE <0.01, PLE–PME 0.11, PME–PME 0.14. Chelicerae as shown in Figs 17–18. Sternum (Fig. 11) 0.85 long, 0.65 wide; labium 0.21 long, 0.18 wide at base. Measurements of palp and leg segments as shown in Table 1. Scopulae and tarsal claws as shown in Figs 23, 26–31 and 22, 24, 25, 36, 37 respectively. Scarcely distributed scopular hairs approximately as long as metatarsus and tarsus width (Figs 26–31) At least metatarsus III with comb of setae (Fig. 35). Tarsi I–IV with claw tufts, better developed on tarsi I–II (Figs 22, 24). Tarsal claws I–II with few teeth (Figs 22, 25), III–IV with more and longer teeth (Figs 36, 37). Spinnerets, pedicel tube, and ventral parts of abdominal scutum as shown in Figs 32–34.
Spiders belonging to the subfamily Chediminae; habitus in lateral (1, 2, 6), dorsal (3–5, 7, 9) and ventral (8) view. 1, 2 Levymanus gershomi sp. n., holotype male and paratype female, respectively 3 Boagrius sp. aff. incisus, female 4 Scelidocteus sp. aff. vuattouxi, male 5, 6 Sarascelis chaperi, female and male, respectively 7 Scelidomachus socotranus, conspecific male 8, 9 Steriphopus macleayi, holotype male (scale bar = 1 mm).
Levymanus gershomi sp. n., male (10, 11) and female paratypes (12, 13). 10, 12 Carapace, dorsal view 11, 13 Sternum, labium, maxillae and chelicerae; ventral view (scale bar = 0.2 mm).
Levymanus gershomi sp. n., male (14) and female paratype (15, 16). 14, 15 Habitus, lateral view 16 Carapace and chelicerae, frontal view (scale bar = 0.5 mm for 14, 15; 0.2 mm for 16).
Levymanus gershomi sp. n., male (17, 18) and female paratype (18). 17, 18 right chelicera, frontal (17) and prolateral (18) view 19 right palp, femur to tarsus, retrolateral view. (scale bar = 0.05 mm for 17, 18; 0.1 mm for 19).
Levymanus gershomi sp. n., paratype male, leg I, prolateral view. 20 femur 21 patella 22 tarsus, showing claws with teeth 23 tibia, showing spatulate hair 24 tarsus, showing claws and claw ‘pillow’ 25 tarsus, showing claws and ‘pillow’ 26 tarsus, showing scopula 27 tibia, showing scopula 28 whole leg, showing scopula and enlarged patella 29, 30 metatarsus I, showing scopula 31 tibia to tarsus, showing scopula (scale bar = 0.1 mm for 20–23, 25–27, 30; 0.05 mm for 24; 0.2 mm for 29, 31).
Levymanus gershomi sp. n., paratype male. 32 spinnerets, ventral view 33 epigastral scutum, ventral view 34 abdomen, ventral view 35 distal part of metatarsus III, showing metatarsal comb, ventral view 36 distal part of tarsus III, showing onichum and claws, ventral view 37 same, prolateral view (scale bar = 0.01 mm for 32, 35–37; 0.1 mm for 33, 34).
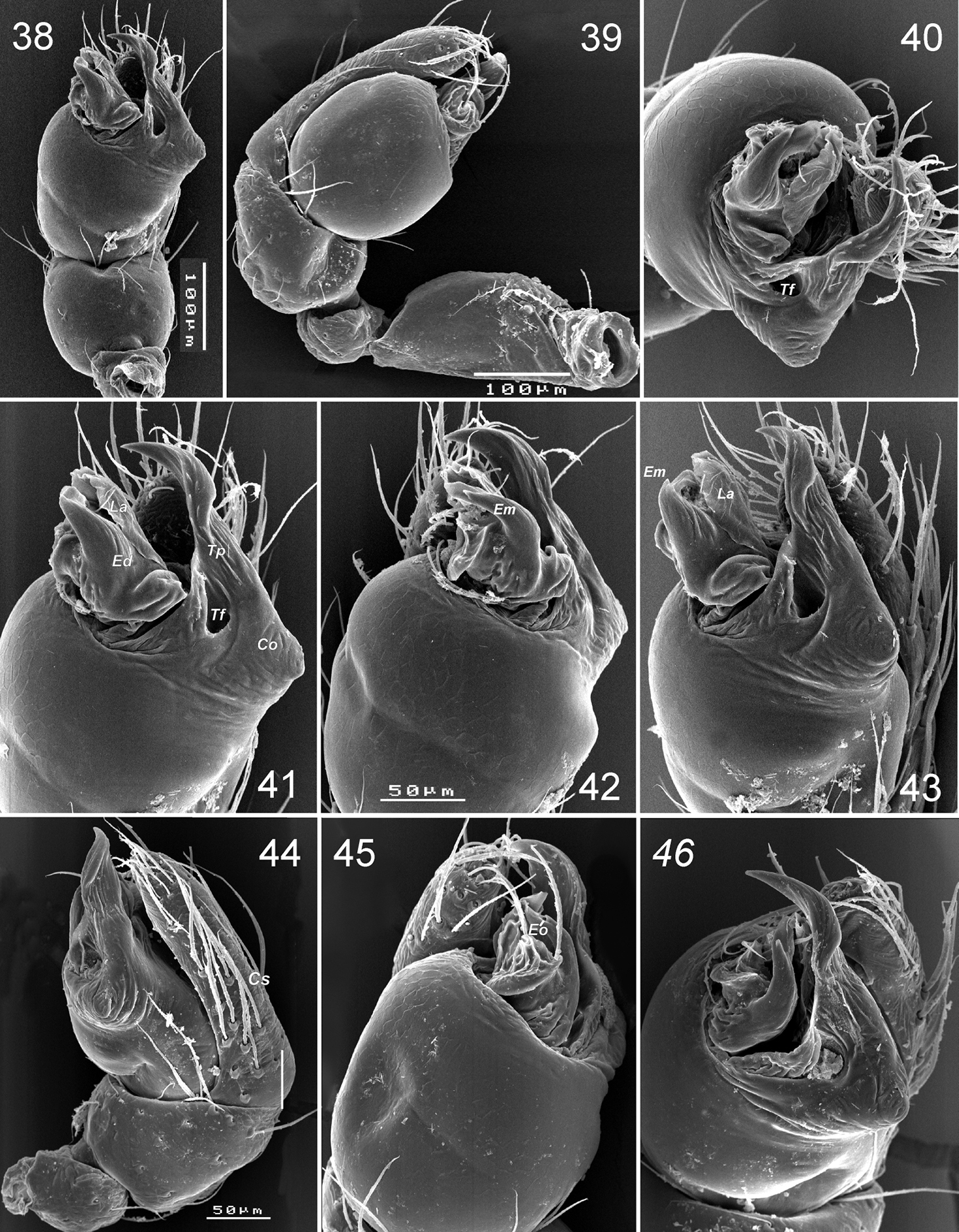
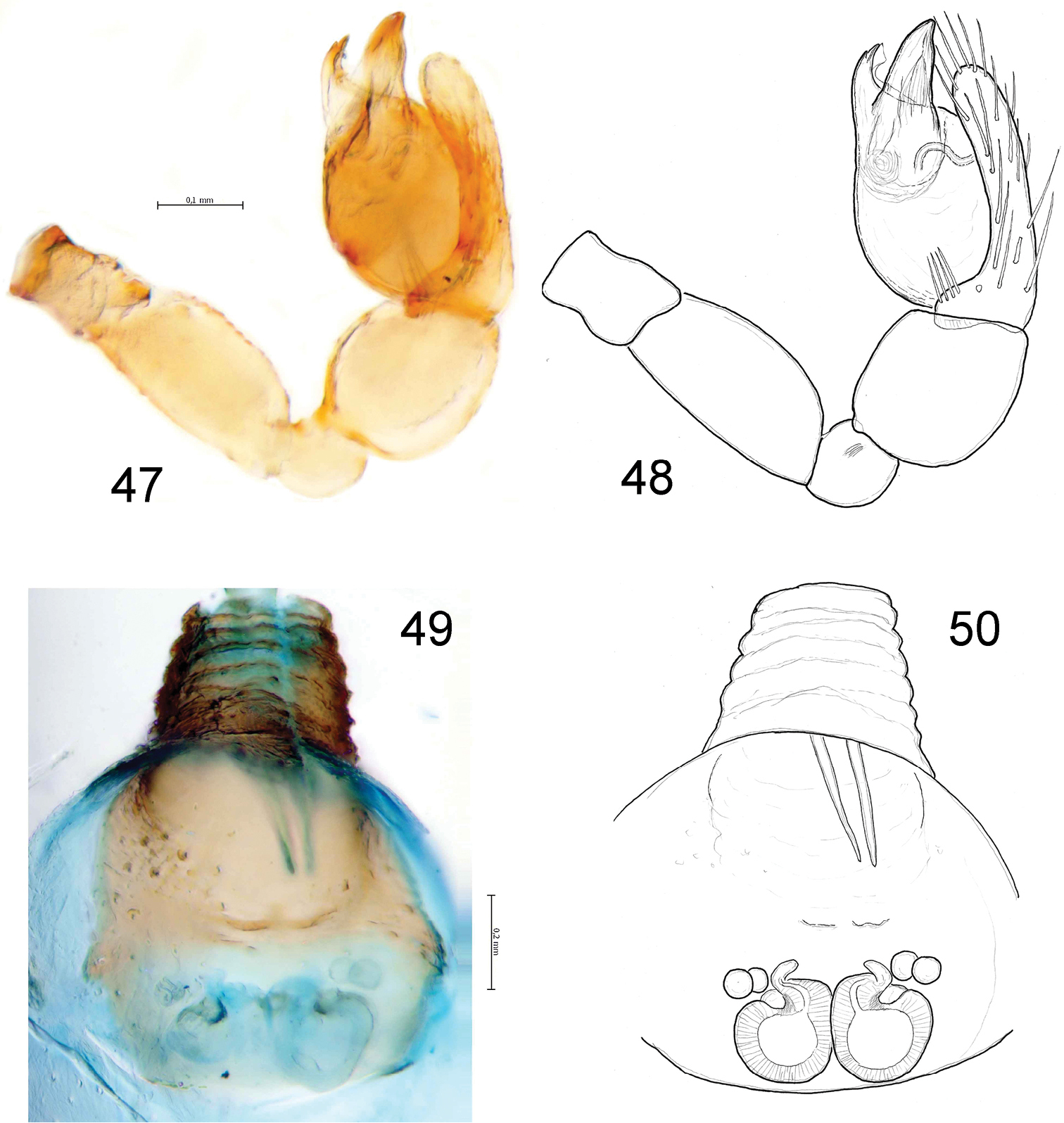
Palp (Figs 38–48): femur short and swollen, 2 times longer than wide, subequal in length to tibia and slightly shorter than cymbium. Patella globular. Tibia without apophyses, strongly swollen, 1.5 times longer than wide, 1.3 times wider than femur. Cymbium narrow, shorter than bulb, without outgrowths, with two clusters of hairs on prolateral side (Figs 39, 46). Bulb as wide (in widest part) as long (not counting embolic division and tegular process), tegulum with strong and long retrolateral tegular process (Tp); base of process with conical outgrowth (Co) and deep furrow (Tf); embolus fused with other sclerites of the embolic division (Ed). Embolic division, attached to tegulum by a flexible membrane, and bears embolus (Em) with retrolateral outgrowth (Eo), and lamella (La) located between embolus and cymbium.
Levymanus gershomi sp. n., palp of paratype male 38 palp, ventral view 39 whole palp, prolateral view 40 same, apical view 41 terminal part of bulbus, ventral view 42, 43 same, prolateral-ventral view 44 same, ventral-retrolateral view 45 palp, retrolateral view 46 same, apical-retrolateral view. 39, 44–46 palp with embolic division sunken into tegulum (scale bar = 0.1 mm for 38, 39; 0.05 mm for 40–46).
Levymanus gershomi sp. n., male (47, 48) and female paratypes (49, 50). 47, 48 palp, ventral view 49, 50 epigyne; ventral view (scale bar = 0.2 mm).
Female (paratype): coloration as in male. Total length 2.93. Carapace (Fig. 12): 1.34 long, 0.92 wide. Diameters of AME, ALE, PLE, PME: 0.10, 0.03, 0.02, 0.02. Interdistances: AME–AME 0.08, ALE–AME 0.05, ALE–PLE <0.01, PLE–PME 0.10, PME–PME 0.12. Sternum (Fig. 13) 0.94 long, 0.80 wide; labium 0.22 long, 0.24 wide at base. Measurements of palp and leg segments as shown in Table 1. Spermathecae weakly sclerotised, round, touching each other, each spermatheca with a pair of accessory glands (Figs 49, 50). Due to the weak sclerotisation the outline of the spermathecae and their ducts are not very clear.
Levymanus gershomi, gen. et sp. n., palp and leg measurements (in mm). Male holotype and female paratype (in parentheses).
| Palp | Leg I | Leg II | Leg III | Leg IV | |
|---|---|---|---|---|---|
| Femur | 0.19 (0.29) | 1.10 (1.36) | 0.78 (0.97) | 0.78 (0.82) | 1.08 (1.19) |
| Patella | 0.11 (0.12) | 0.88 (1.09) | 0.53 (0.69) | 0.36 (0.49) | 0.47 (0.65) |
| Tibia | 0.18 (0.20) | 0.71 (0.84) | 0.53 (0.73) | 0.51 (0.68) | 0.69 (0.96) |
| Metatarsus | 0.42 (0.53) | 0.47 (0.70) | 0.60 (0.64) | 0.85 (1.11) | |
| Tarsus | 0.27 (0.25) | 0.43 (0.55) | 0.36 (0.49) | 0.32 (0.39) | 0.39 (0.46) |
| Total | 0.75 (0.86) | 3.54 (4.37) | 2.67 (3.58) | 2.57 (3.02) | 3.48 (4.37) |
Carapace length in males 1.01–1.12, in females 1.22–1.34. Coloration varies very slightly, recently moulted specimens are lighter.
The species is known only from the type locality (Qetura) represented by an extra-arid stony desert at 200–500 m above sea level. All specimens were collected with pitfall traps.
Of the three recognized palpimanid subfamilies, the Palpimaninae was reviewed by
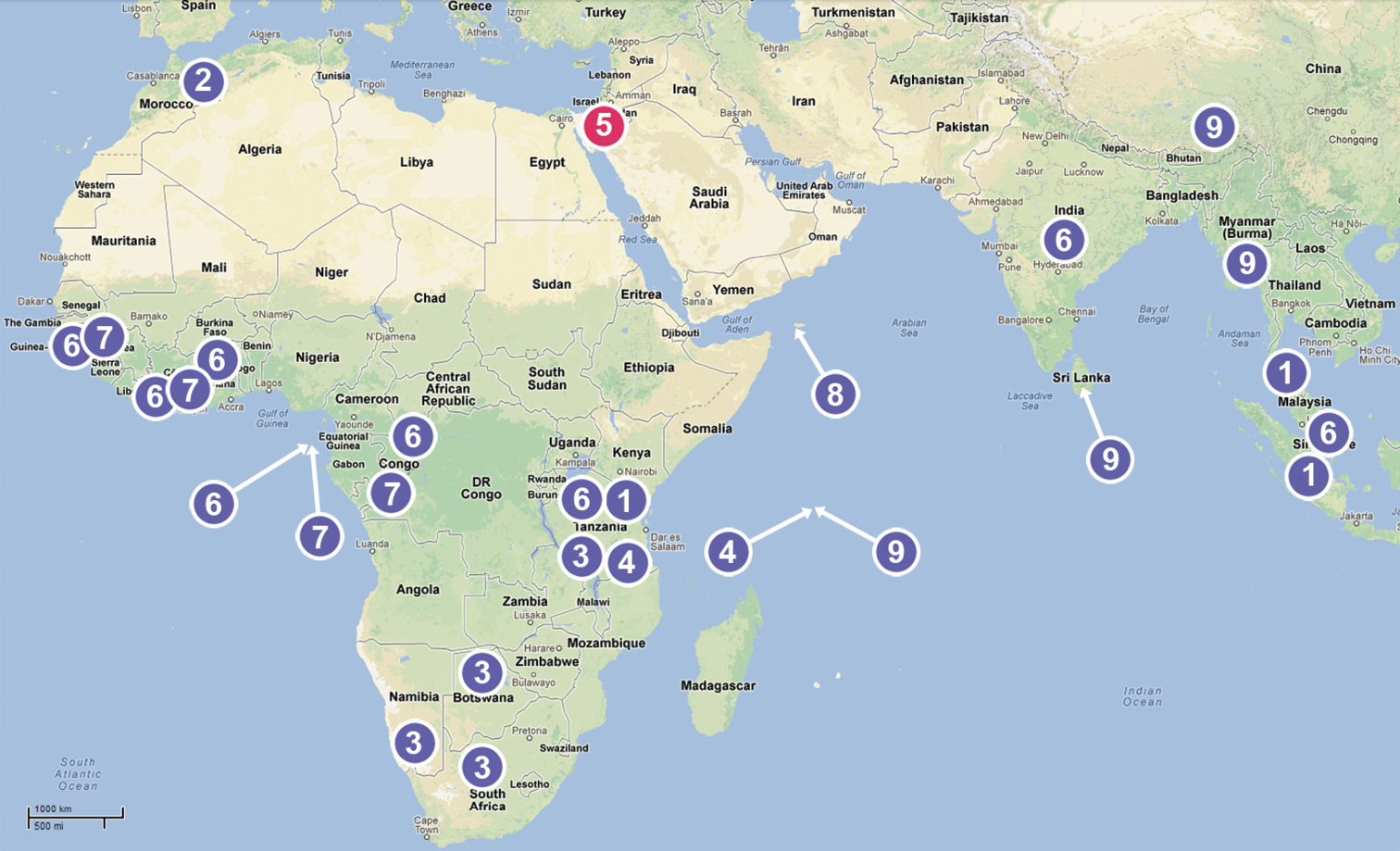
The currently known Chediminae are distributed mainly within the Paleotropical area (Fig. 51). This subfamily includes nine genera: Badia Roewer, 1961 (West Africa), Boagrius Simon, 1893 (East Africa and South-East Asia), Chedima Simon, 1873 (Morocco), Diaphorocellus Simon, 1893 (South and South-East Africa), Hybosida Simon, 1898 (East Africa, Seychelles), Sarascelis Simon, 1887 (tropical parts of Africa and Asia), Scelidocteus Simon, 1907 (West Africa), Scelidomachus Pocock, 1899 (Socotra) and Steriphopus Simon, 1893 (Seychelles and South Asia). Almost all of them are undoubtedly correctly placed in this subfamily, apart from Badia Roewer.
Distribution of genera of the Chediminae: 1 Boagrius 2 Chedima 3 Diaphorocellus 4 Hybosida 5 Levymanus gen. n. 6 Sarascelis 7 Scelidocteus 8 Scelidomachus 9 Steriphopus.
Levymanus gershomi sp. n., distribution.
Surroundings of Qetura (Ktura), the type locality of Levymanus gershomi sp. n.
The holotype of Badia rugosa was not found. Only a single microslide containing a separate leg of this specimen is deposited in SMF (Julia Altmann, personal communication). We thus consider the holotype of Badia rugosa to be lost as is the case in several other types from the same study (see
According to the original description, Badia possesses lateral eyes, ALE and PLE, widely spaced from each other (cf.
The taxonomic position of Fernandezina gyirongensis Hu & Li, 1987 from Xizang (Tibet) also appears doubtful. Fernandezina Birabén, 1951, the genus to which this species was originally referred is known exclusively from the Neotropical Region, from Guyana to Northern Argentina (
The structure of the male bulb in this species, bearing a tegular process (see also
Within three Oriental genera of the Chediminae, Boagrius, Sarascelis and Steriphopus, the two former genera possess much larger anterior median eyes (see
The third genus, Steriphopus (described originally under Pachypus Pickard-Cambridge, 1873 nom. praeocc.), possesses smaller AMEs (like in Fernandezina gyirongensis). Strictly speaking, at the first view other characters noted and figured by
Therefore, the given species is provisionally placed in Steriphopus and the new combination is proposed: Steriphopus gyirongensis (Hu & Li, 1987), comb. n., with reservation and assumption, that this species may represent a separate chedimine genus, as yet undescribed (since we could not study the holotype, which is lost – Shuqiang Li personal communication).
The distinctive characters of the new taxon are listed and discussed below. It should be noted that within the Chediminae some characters, such as the structure of the spermathecae and fine structure of the male palp are known only for a few species described or surveyed after the 1960s (
First and foremost, Levymanus gershomi sp. n. differs from all other chedimine palpimanids by having long slender legs (A1) and an elongate body (A2) – as shown in Figs 1–2. All examined members of Chediminae may be referred to the “standard” palpimanid type with a more or less compact or robust body and considerably shorter and thicker legs, as in Figs 3–9. Among other palpimanids, some species of Fernandezina (Otiothopinae) also have somewhat longer and thinner legs and a more elongate body (
As has already been noted, Levymanus gershomi sp. n. possesses a thoracic fovea divided into two parts (A3); all other palpimanids have an entire, short fovea that may be longitudinal, transverse, pit-like, or anchor-shaped (
Other characteristic features of the new taxon are edentate chelicerae, lacking the stridulatory ridges (A4), and the considerably reduced scopula (A5) and spinnerets (A6). The presence of stridulatory organs in palpimanids is not well documented.
A dense prolateral brush of scopular hairs on the tibia, metatarsus and tarsus of leg I is very characteristic for the whole family Palpimanidae (
Although all palpimanids possess strongly reduced spinnerets (
The structure of the palp in the new taxon does not differ strongly from that in other members of the subfamily. The most significant distinction is the presence of a tegular furrow (A7) (Figs 38, 40, 41, 43), a detail that has not been found in any other examined genera of the chedimine palpimanids.
We thus conclude that Levymanus gen. n. is distinct from all other genera within the subfamily. Moreover, the above-noted characters contrast with all other genera of Chediminae taken as a whole. Currently it is not certain whether this new taxon represents a separate subfamily or whether itshould be considered only as a specialized chedimine palpimanid. This question might be answered in the course of a taxonomic revision and phylogenetic study of the Chediminae.
Jan Beccaloni (NHML), Christine Rollard and Elise-Ann LeGuin (MNHN) kindly helped us to examine the specimens used in this study. Ning Sun (Capital Normal University, Beijing, China) generously translated a description of Fernandezina gyirongensis from Chinese to English. Peter Jäger and Julia Altman (SMF) helped us with information concerning Badia rugosa. Shuqiang Li (Institute of Zoology, Chinese Academy of Sciences, Beijing, China) helpfully confirmed that the holotype of Fernandezina gyirongensis is lost. Vasiliy Kravchenko (TAU) provided us with his landscape photographs of the surroundings of Qetura. We thank David Penney (Manchester University) for his linguistic help. We are also thankful to Christian Grismado and an anonymous reviewer for their helpful comments. This study received financial support from the Ministry of Absorption, Israel and from the Russian Foundation for Fundamental Research (grants # 11–04–01716 and 12–04–01548).