(C) 2013 J. Maximilian Dehling. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Dehling JM, Sinsch U (2013) Diversity of Ptychadena in Rwanda and taxonomic status of P. chrysogaster Laurent, 1954 (Amphibia, Anura, Ptychadenidae). ZooKeys 356: 69–102. doi: 10.3897/zookeys.356.5878
We assess the diversity of Ptychadena species in Rwanda based on re-examination of voucher specimens in museum collections and our own data from recent assessment of the species composition of amphibian communities in Rwanda. We recognize five species which we allocate to the following available names: P. anchietae, P. chrysogaster, P. nilotica, P. porosissima, and P. uzungwensis. We did not find evidence for the presence of P. grandisonae and P. oxyrhynchus which have been listed for the country. The five species can be distinguished by quantitative morphometrics (discriminant analysis, success rate: 100 %) and a number of qualitative characters of external morphology. We provide an identification key to the Rwandan species and describe the morphology of each species in detail. The taxonomic status and the phylogenetic position of Ptychadena chrysogaster are further assessed based on the partial sequence of the mitochondrial 16S rRNA. The species differs genetically from available homologous sequences from congeners by an uncorrected p distance of at least 4.2 % and appears to be most closely related to specimens assigned to P. porosissima, P. mahnerti, “P. aff. uzungwensis” and “P. aff. bibroni”.
P. anchietae, P. grandisonae, P. nilotica, P. porosissima, P. uzungwensis, DNA barcoding, systematics
Ridged Frogs of the genus Ptychadena Boulenger, 1917 are widespread in sub-Saharan Africa where approximately 50 species occur. Species of the genus share a similar general appearance and many are poorly delimited, having been described based on taxonomically doubtful characters. Several species names have been erroneously considered synonyms of others, thus confusing character diagnoses in subsequent accounts; and some taxa were described based on specimens later found to represent more than one species (e.g.
In order to clarify how many and which species occur in Rwanda, we re-examined the specimens of Ptychadena in the herpetological collection of the Royal Museum for Central Africa in Tervuren, Belgium (RMCA), on which almost all previous Rwandan records are based. We herein report the results and compare the findings to our own data from recent assessment of the composition of amphibian communities at numerous locations in Rwanda. We further assess the taxonomic status and the phylogenetic position of Ptychadena chrysogaster based on examination of most of the available voucher material from Rwanda including the type series and on comparison of the partial sequence of the mitochondrial 16S rRNA gene with homologous sequences of its congeners.
We examined voucher specimens deposited at RMCA. Additional specimens including our recently collected material are deposited in the collection of the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK). See Appendix 1 for a complete list of examined specimens.
For the morphological analysis, we took the following 18 measurements to the nearest 0.1 mm using digital calipers, following
Descriptive statistics depended on the outcome of the test for normality. Normally distributed data were described by the arithmetic mean and corresponding standard error and/or range, those deviating significantly by median and range. Principal component analyses were run on the morphometric data set including 18 variables and 89 observations each (Ptychadena anchietae: 15 males, 3 females; Ptychadena chrysogaster: 13 males, 10 females; Ptychadena nilotica: 13 males, 10 females; Ptychadena porosissima: 11 males, 7 females; Ptychadena uzungwensis: 6 males, 1 female). We compared the scores obtained for the principal components 2 and 3 describing shape to distinguish taxa without a priori assignment to taxa. The morphometric distances were adjusted for SVL by calculating a linear regression of each variable against SVL and storing the residuals as representatives of size-independent shape variables. This transformed data set was used for discriminant analyses with taxa as predefined groups to optimize distinction. To account for sexual dimorphism, discriminant analyses were run separately for males (n=58) and females (n=31). Significance level was set at alpha = 0.05. All calculations were based on the procedures of the program package STATGRAPHICS centurion for Windows, version XV.
We isolated DNA from a liver tissue sample from a specimen of Ptychadena chrysogaster (ZFMK 58797), collected in southern Rwanda by H. Hinkel in 1993. DNA was used to sequence a fragment of the 16S mitochondrial rRNA gene, a universal marker to barcode amphibian species (
Examination of specimens suggested that five morphologically distinct species were present in Rwanda to which we assign the following names: Ptychadena anchietae, Ptychadena chrysogaster, Ptychadena nilotica, Ptychadena porosissima, and Ptychadena uzungwensis (Figures 1 and 2). For allocation of specimens to Ptychadena anchietae, Ptychadena nilotica, and Ptychadena porosissima and discussion thereof see
Males of Ptychadena from Rwanda in life. A Ptychadena anchietae B Ptychadena chrysogaster [Foto: E. Fischer] C Ptychadena nilotica D Ptychadena porosissima.
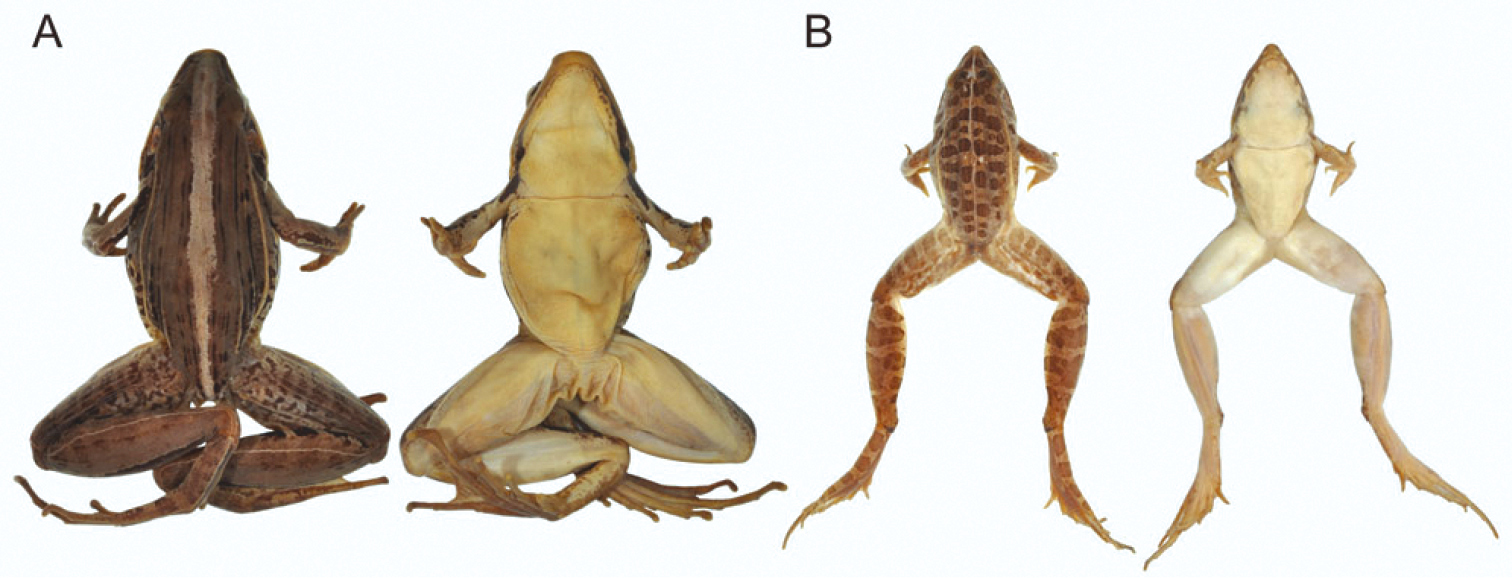
A Preserved female holotype of Ptychadena chrysogaster (RMCA 109096) from Lac Karago, Rwanda; dorsal view (left) and ventral view (right) B Preserved male specimen of Ptychadena uzungwensis (RMCA 108993-108997) from Munini, Rwanda; dorsal view (left) and ventral view (right). Not to scale.
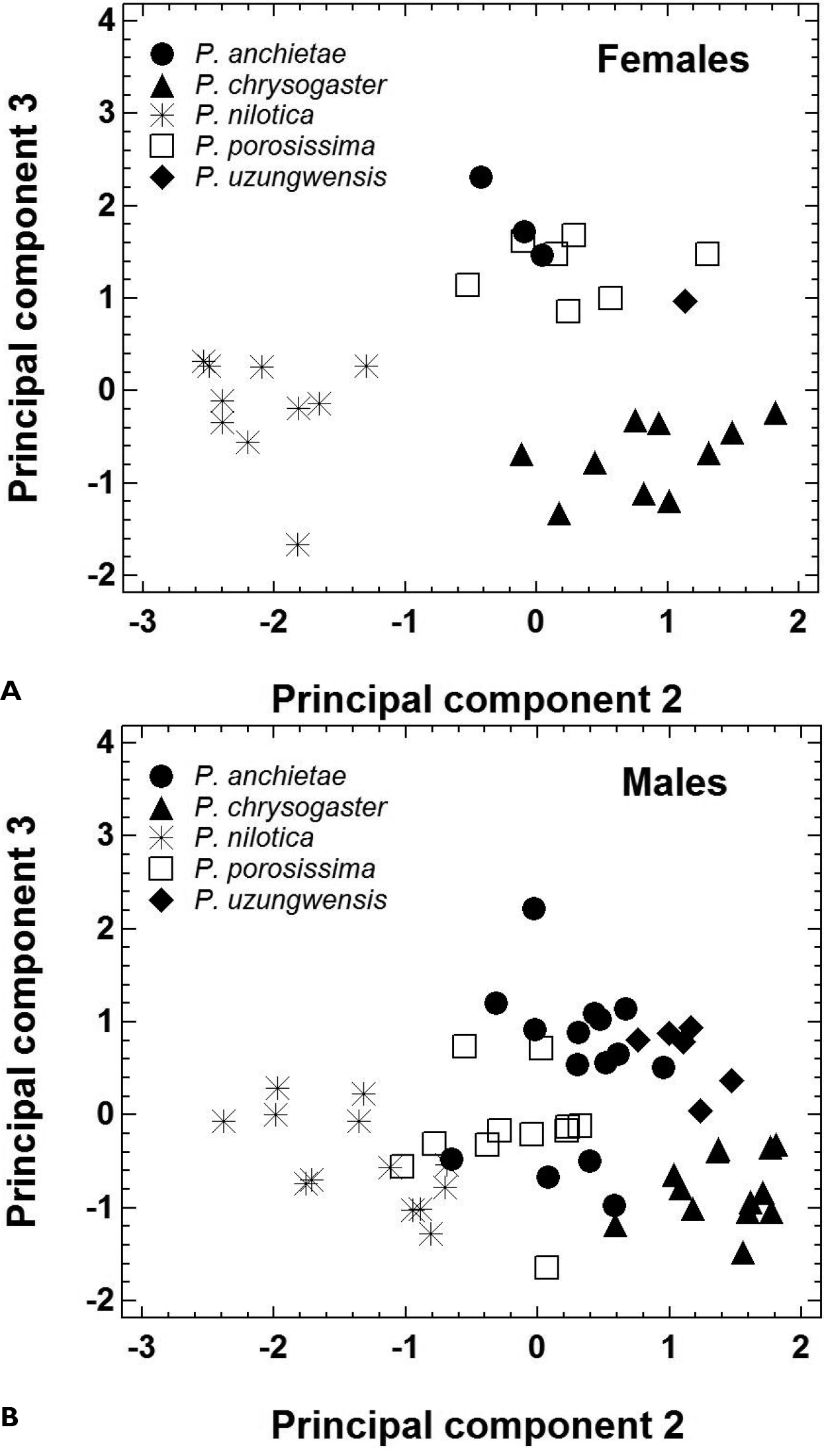
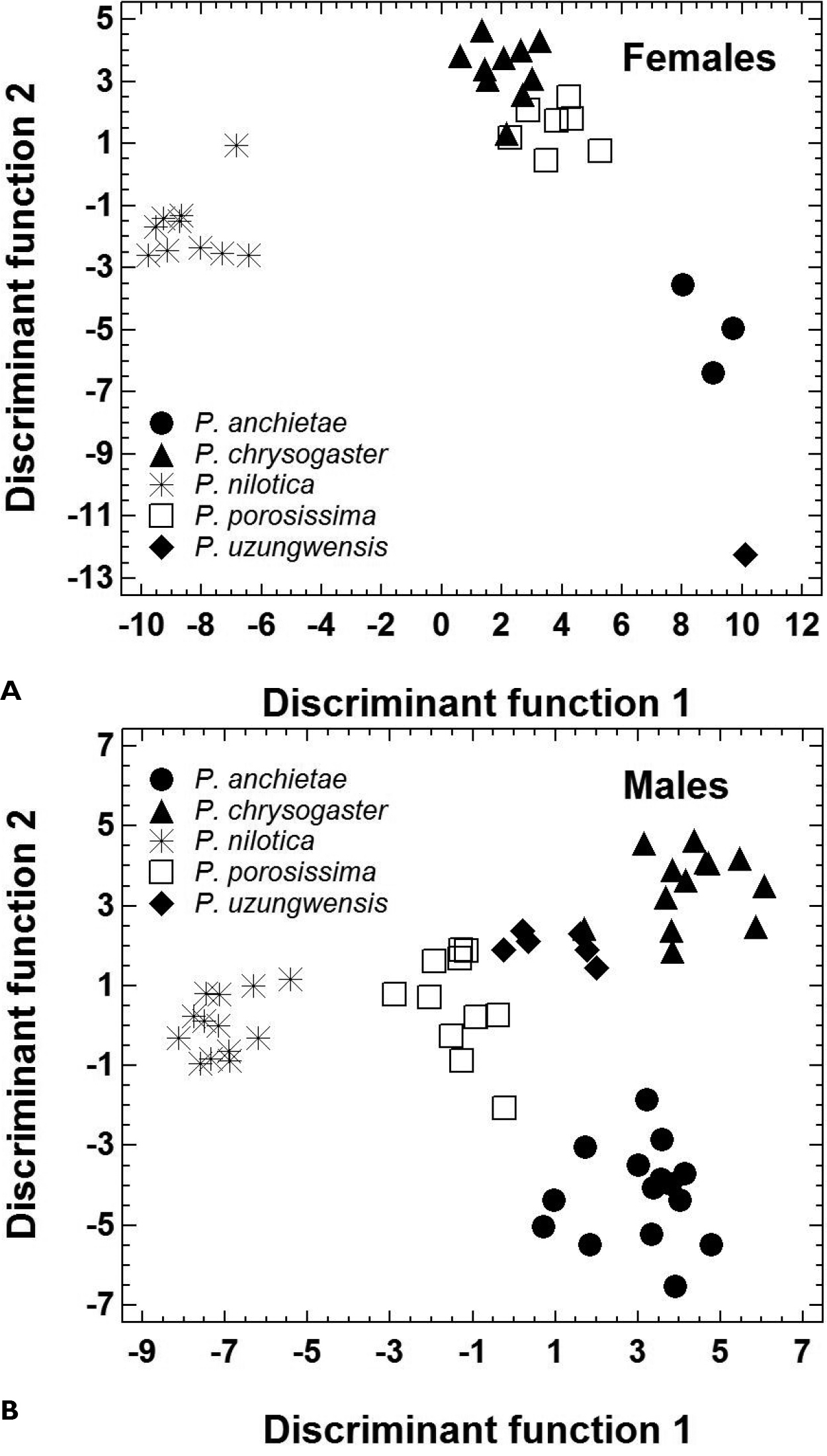
The morphometric features of the five species are summarized in Table 2. Principal component analysis yielded three PCs accounting for 89.6% of total variation (Table 3A). PC1 represented variation in size, whereas the shape-related PC2 and PC3 were mainly loaded by features describing head morphology (Table 3B). In females, PC 2 unequivocally distinguished Ptychadena nilotica from the other taxa, and PC 3 unequivocally distinguished Ptychadena chrysogaster from Ptychadena anchietae, Ptychadena porosissima, and Ptychadena uzungwensis (Figure 3A). Also, Ptychadena anchietae and Ptychadena uzungwensis could be distinguished from each other. However, both species were represented by only few individuals (three and one, respectively) in the analysis. The two species did not differ significantly in shape from Ptychadena porosissima (Figure 3A). A similar pattern was observed in the analysis of the males (Figure 3B) but males of all five species were generally more similar to each other in shape. Males of Ptychadena nilotica could be distinguished unequivocally from males of Ptychadena chrysogaster and Ptychadena uzungwensis but not from some of the males of Ptychadena anchietae and Ptychadena porosissima (Figure 3B). Males of Ptychadena chrysogaster could be distinguished from males of all other species but some of the males of Ptychadena anchietae and Ptychadena uzungwensis were very similar in shape (Figure 3B). Males of Ptychadena anchietae did not differ significantly in shape from males of Ptychadena nilotica, Ptychadena porosissima, and Ptychadena uzungwensis. Gender-specific discriminant analyses based on the residuals of 17 SVL-adjusted morphometric variables had a classification success of 100 % among the five species in both males and females (Table 4A, B, C, Figure 4A, B).
Morphological shape differentiation among 89 specimens representing five Ptychadena species, as assessed by principal component analysis (Table 3). A Individual scores obtained for 31 females B Individual scores obtained for 58 males.
Morphological shape differentiation among 89 specimens representing five Ptychadena species, as assessed by discriminant analyses (Statistical details are given in Table 4). A Individual scores obtained for 31 females B Individual scores obtained for 58 males.
The five Rwandan species can be distinguished unequivocally from each other using a combination of qualitative morphological characters (Table 1, Figure 5; see also
Volar view of hands (top) and plantar view of feet (bottom) of males of Ptychadena anchietae (a), Ptychadena chrysogaster (b), Ptychadena nilotica (c), Ptychadena porosissima (d), and Ptychadena uzungwensis from Rwanda. See also Table 1.
Distinguishing qualitative characters of Ptychadena species from Rwanda.
| Species | Ptychadena anchietae | Ptychadena chrysogaster | Ptychadena nilotica | Ptychadena porosissima | Ptychadena uzungwensis |
|---|---|---|---|---|---|
| relative length of Toes III and V | tips reaching to knee or slightly beyond, distal subarticular tubercle never reaching knee | tips reaching beyond knee, distal subarticular tubercle reaching knee | tips reaching beyond knee, distal subarticular tubercle reaching knee or beyond | tips reaching to knee or slightly beyond, distal subarticular tubercle never reaching knee | tips reaching to knee or slightly beyond, distal subarticular tubercle never reaching knee |
| position of vocal sac aperture | inferior, at ventral edge of arm insertion | inferior, at ventral edge of arm insertion | superior, above dorsal edge of arm insertion | inferior, at ventral edge of arm insertion | semi-inferior, at level of centre of arm insertion |
| spiny tubercles on venter | absent | present in males, very small | absent | present in males, comparatively large | present in males, very small |
| median dorsal ridge on snout | absent | absent | absent | absent | present |
| outer metatarsal tubercle | very faintly visible | very faintly visible, rarely distinct | distinctly present, rarely faintly visible | faintly visible, rarely distinct | faintly visible |
| inner metatarsal tubercle size (Fig. 5) | about half the length of metatarsus of Toe I | less than half the length of metatarsus of Toe I | less than half the length of metatarsus of Toe I | more than half the length of metatarsus of Toe I | about half the length of metatarsus of Toe I |
| supernumerary metacarpal tubercles (Fig. 5) | one below each finger | one below each finger | only one below Finger IV, often indistinct | one below Fingers I, II, and IV; two, rarely one below Finger III | one below Fingers I and IV, two below Finger II, two to four below Finger III |
| palmar and thenar tubercles (Fig. 5) | inner and outer palmar tubercle more or less equal in length; thenar tubercle oval, slightly longer than palmar tubercles | outer palmar tubercle longer than inner; thenar tubercle elongate, about as long as outer palmar tubercle | outer palmar tubercle longer than inner; thenar tubercle elongate, about as long as outer palmar tubercle | outer palmar tubercle longer than inner; thenar tubercle elongate, longer than outer palmar tubercle | inner and outer palmar tubercle more or less equal in length; thenar tubercle elongate, longer than palmar tubercles |
| toe webbing (Fig. 5) | I0.5-2II0.5-2III(0.5-1)-2IV2-0.5V | I2-2.5II(1.5-1.75)-3III(2-2-)(3.25-3+)IV3-(1.5-2)V | I(1.5-1.75)-(2-2.25)II1.5-(2.75-3)III(1.75-2)-3IV2.75-(1-1.5)V | I(1.75-2)-2.25II1.5-3III1.75-(3-3.25)IV3-(1-1.5)V | I2-(2.25-2.5)II1.5-3-III(1.75-2-)-3IV3-(1+-1.25)V |
| ventral colouration | head white, trunk yellow | head and trunk yellow | head white, mottled with grey; trunk yellow | head and trunk yellow | colours in life unreported |
| dark brown stripe on preaxial side of tibia | absent | present, continuous or almost continuous | absent in most specimens; few specimens with dark mottling, not forming continuous stripe | absent | absent |
| light tibial line (Figs 1 & 2) | absent | usually present, rarely absent | present or absent | present | absent |
| light dorsal band | absent | usually present, rarely absent | present or absent | present or absent | present |
| dark spots on dorsum (Figs 1 & 2) |
usually absent; if present, small and narrow | usually present, small and narrow, sometimes forming longitudinal lines; rarely absent | present, large and wide, sometimes fused with neighboring ones | present, large and wide, sometimes fused with neighboring ones | present, large and wide, often fused with neighboring ones |
| light, prominent dorsolateral fold (Figs 1 & 2) | usually absent | present | present | present | present |
| Colour pattern on postaxial side of femur | irregularly delimited, reticulated, longitudinal bands, alternately yellow and dark brown coloured | irregularly delimited, reticulated, longitudinal dark bands on light background; colours in life unreported | relatively sharply delimited longitudinal bands, alternately yellow and black coloured | yellow spots diffusely arranged in longitudinal rows on dark brown background | irregularly delimited, reticulated, longitudinal light bands on dark background; colours in life unreported |
Morphometric features of Ptychadena species from Rwanda. Data are given as arithmetic means and minimum and maximum values (in mm).
| Morphometric character | Ptychadena anchietae | Ptychadena chrysogaster | Ptychadena nilotica | Ptychadena porosissima | Ptychadena uzungwensis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males N = 15 |
Females N = 3 |
Males N = 75/14* |
Females N = 23/11* |
Males N = 13 |
Females N = 10 |
Males N = 11/20* |
Females N = 9 |
Males N = 6 |
Females N = 1 |
|
| Snout-vent length | 40.4 (38.0–42.4) |
49.0 (46.7–51.3) |
43.3 (36.3–49.5) |
53.7 (48.0–57.7) |
42.0 (37.2–45.2) |
49.1 (45.6–53.1) |
41.2* (37.3–44.5) |
46.4 (39.0–52.1) |
34.7 (33.3–35.7) |
43.3 - |
| Hindlimb length | 79.6 (74.2–84.9) |
98.9 (96.0–101.2) |
88.1 (83.5–93.0) |
108.2 (102.3–114.1) |
77.2 (70.1–85.2) |
89.6 (78.0–103.9) |
78.2* (72.8–85.5) |
88.3 (74.3–94.1) |
66.7 (61.9–72.7) |
81.2 - |
| Femur length | 23.0 (21.9–24.5) |
29.0 (28.3–29.6) |
23.9 (22.5–25.0) |
29.9 (28.1–32.0) |
21.5 (19.4–24.0) |
25.5 (23.0–28.5) |
21.7* (20.2–24.1) |
24.4 (19.8–27.6) |
18.7 (17.2–19.6) |
23.1 - |
| Tibiofibula length | 26.3 (24.4–28.0) |
33.1 (31.9–33.8) |
28.4 (24.6–32.0) |
35.2 (32.4–38.5) |
23.4 (21.1–26.1) |
27.5 (23.6–32.1) |
24.7* (23.3–26.5) |
28.7 (25.2–31.0) |
21.7 (20.3–23.4) |
26.9 - |
| Tarsus length | 34.5 (31.6–36.5) |
42.7 (40.6–44.1) |
40.9* (38.9–44.1) |
49.3* (46.5–51.3) |
36.2 (32.4–40.1) |
42.8 (35.9–49.6) |
35.5 (33.3–38.8) |
40.0 (32.6–43.5) |
30.5 (27.9–32.2) |
36.0 - |
| Foot length | 24.7 (22.4–26.1) |
30.5 (29.1–31.3) |
28.4 (24.2–30.4) |
34.5 (32.9–36.1) |
25.7 (22.8–28.2) |
29.3 (25.6–33.9) |
24.4 (23.0–26.7) |
27.4 (23.1–29.3) |
21.0 (19.5–21.9) |
28.6 - |
| Forelimb length | 16.9 (15.9–18.1) |
21.0 (20.0–21.7) |
18.3* (17.4–19.1) |
22.0* (20.9–23.9) |
17.7 (16.0–19.3) |
20.5 (17.9–23.7) |
17.2 (16.0–18.6) |
18.7 (16.1–20.9) |
13.5 (12.8–14.4) |
16.3 - |
| Hand length | 10.0 (9.5–10.7) |
12.3 (12.1–12.6) |
10.4* (9.6–11.1) |
12.4* (11.8–13.3) |
10.4 (9.5–11.6) |
11.9 (10.6–13.8) |
9.7 (8.9–10.8) |
10.7 (9.1–11.7) |
7.9 (7.4–8.5) |
9.0 - |
| Head width | 13.8 (12.5–15.4) |
16.7 (16.4–17.0) |
13.9 (12.8–14.7) |
16.9 (15.4–17.5) |
13.8 (12.4–15.9) |
16.4 (14.2–18.8) |
14.1 (13.1–15.0) |
15.1 (12.5–17.1) |
11.4 (11.0–11.7) |
13.8 - |
| Head length | 15.5 (14.1–17.6) |
18.7 (18.2–19.1) |
15.6* (14.7–16.4) |
18.6* (17.9–19.8) |
16.2 (14.7–18.4) |
18.3 (16.6–20.4) |
15.5 (14.2–17.8) |
17.1 (13.6–19.1) |
13.3 (12.3–14.0) |
16.1 - |
| Interorbital distance | 2.8 (2.4–3.1) |
3.1 (3.0–3.2) |
3.5* (3.1–3.9) |
4.1* (3.8–4.5) |
2.2 (1.9–2.5) |
2.5 (2.2 - 2.7) |
2.6 (2.3–2.7) |
3.1 (2.8–3.6) |
2.7 (2.4–3.1) |
2.9 - |
| Eyelid width | 2.8 (2.5–3.1) |
3.2 (3.0–3.3) |
2.7* (2.4–3.0) |
3.3* (2.9–3.7) |
2.7 (2.3–3.2) |
3.0 (2.4–3.5) |
2.8 (2.3–3.1) |
3.1 (2.6–3.5) |
2.6 (2.3–2.8) |
2.4 - |
| Eye diameter | 4.3 (3.6–4.9) |
5.2 (5.0–5.5) |
4.3 (3.7–4.9) |
5.0 (4.6–5.6) |
4.5 (4.1–5.1) |
5.1 (4.8–5.5) |
4.2 (3.9–4.5) |
4.6 (3.9–5.1) |
3.9 (3.6–4.1) |
4.5 - |
| Tympanum diameter | 3.3 (2.9–3.6) |
4.1 (3.7–4.6) |
3.7 (3.2–4.2) |
4.5 (4.3–5.1) |
3.7 (3.3–4.1) |
4.1 (3.7–4.7) |
3.1 (2.9–3.49 |
3.6 (3.3–3.9) |
2.9 (2.5–3.3) |
3.5 - |
| Eye–nostril distance | 4.1 (3.7–4.3) |
5.2 (4.9–5.7) |
3.9* (3.5–4.4) |
4.6* (4.0–5.0) |
3.6 (3.4–4.0) |
4.2 (3.7–4.9) |
3.5 (3.1–3.9) |
4.2 (3.6–4.7) |
3.5 (3.2–3.6) |
4.2 - |
| Snout–nostril distance | 3.5 (2.9–4.0) |
4.4 (4.3–4.5) |
3.9* (3.5–4.3) |
4.3* (3.9–4.9) |
3.4 (2.9–3.8) |
3.7 (3.3–4.1) |
3.4 (2.9–3.7) |
4.1 (2.8–4.7) |
3.6 (3.2–3.9) |
4.3 - |
| Internarial distance | 3.9 (3.5–4.3) |
4.8 (4.7–4.9) |
4.3* (4.0–4.5) |
4.9* (4.5–5.2) |
3.4 (3.0–3.6) |
3.7 (2.0–4.5) |
3.6 (3.4–4.0) |
4.3 (3.7–4.7) |
3.0 (2.9–3.2) |
3.7 - |
| Snout length | 7.5 (6.6–8.1) |
9.5 (9.0–10.1) |
7.4* (7.0–7.9) |
8.7* (8.4–9.4) |
7.0 (6.3–7.7) |
7.9 (7.1–9.3) |
7.0 (6.3–7.8) |
8.1 (6.2–10.1) |
6.7 (6.4–7.0) |
8.0 - |
Principal component Analysis based on 18 standardized morphometric features of 89 specimens belonging to five Ptychadena species from Rwanda. Morphometric parameters accounting strongly for discrimination among species are highlighted in bold.
| A: Statistical significance | |||
| Principal component | Eigen-value | Relative percentage | Cumulative percentage |
| 1 | 13.84 | 76.9 | 76.9 |
| 2 | 1.46 | 8.1 | 85.0 |
| 3 | 0.82 | 4.6 | 89.6 |
| B: Standardized coefficients of the principal components | |||
| Parameter | Principal component 1 | Principal component 2 | Principal component 3 |
| Snout-vent length | 0.256 | -0.107 | -0.084 |
| tibiofibula length | 0.253 | 0.212 | -0.011 |
| foot length | 0.255 | 0.046 | -0.278 |
| tarsus + foot length | 0.257 | 0.056 | -0.246 |
| total hindlimb length | 0.261 | 0.109 | -0.137 |
| thigh length | 0.260 | 0.048 | -0.033 |
| forearm + hand length | 0.253 | -0.164 | -0.210 |
| hand length | 0.244 | -0.231 | -0.139 |
| head width | 0.243 | -0.224 | -0.016 |
| head length | 0.232 | -0.319 | 0.105 |
| interorbital distance | 0.171 | 0.578 | -0.237 |
| upper eyelid width | 0.201 | -0.142 | 0.272 |
| horizontal eye diameter | 0.211 | -0.325 | 0.162 |
| horizontal tympanum diameter | 0.231 | -0.112 | -0.284 |
| eye to nostril distance | 0.228 | 0.016 | 0.353 |
| nostril to snout distance | 0.195 | 0.326 | 0.412 |
| snout length | 0.234 | 0.070 | 0.469 |
| internarial distance | 0.227 | 0.324 | 0.072 |
Gender-specific discriminant functions based on 17 SVL-adjusted morphometric features (residuals) to distinguish among five Ptychadena species from Rwanda. Statistical significance:
| Discriminant function | Eigen-value | Relative percentage | Canonical correlation | Wilks Lambda | Chi-squared | Degrees of freedom | Statistical significance |
|---|---|---|---|---|---|---|---|
| Male 1 | 19.79 | 58.76 | 0.975 | 0.0003 | 362.2 | 68 | P < 0.0001 |
| Male 2 | 8.49 | 25.23 | 0.945 | 0.0079 | 222.6 | 48 | P < 0.0001 |
| Male 3 | 3.29 | 9.77 | 0.875 | 0.0750 | 119.0 | 30 | P < 0.0001 |
| Male 4 | 2.10 | 6.24 | 0.823 | 0.3223 | 52.0 | 14 | P < 0.0001 |
| Female 1 | 45.49 | 65.38 | 0.989 | 0.00005 | 187.7 | 68 | P < 0.0001 |
| Female 2 | 14.84 | 21.33 | 0.967 | 0.0023 | 114.7 | 48 | P < 0.0001 |
| Female 3 | 6.87 | 9.89 | 0.934 | 0.0377 | 62.2 | 30 | P < 0.0001 |
| Female 4 | 2.36 | 3.40 | 0.838 | 0.2973 | 23.0 | 14 | P = 0.0596 |
Gender-specific discriminant functions based on 17 SVL-adjusted morphometric features (residuals) to distinguish among five Ptychadena species from Rwanda. Morphometric parameters accounting strongly to discrimination among species are highlighted in bold. Standardized coefficients of the discriminant functions:
| parameter (residuals) | discriminant function 1 (males) |
discriminant function 2 (males) |
discriminant function 3 (males) |
discriminant function 4 (males) |
discriminant function 1 (females) |
discriminant function 2 (females) |
discriminant function 3 (females) |
discriminant function 4 (females) |
|---|---|---|---|---|---|---|---|---|
| tibiofibula length | 0.909 | -0.221 | 0.302 | 0.871 | 0.824 | -0.143 | 0.196 | 0.384 |
| foot length | 0.268 | -0.253 | 0.292 | -1.332 | 2.079 | -3.834 | -2.308 | -0.942 |
| tarsus + foot length | -0.798 | 2.031 | -0.026 | 1.308 | -3.311 | 4.158 | 1.119 | -0.410 |
| total hindlimb length | 0.310 | -0.029 | -0.147 | -0.929 | -0.617 | 0.188 | 0.361 | 0.999 |
| thigh length | 0.161 | -1.001 | 0.367 | -0.108 | 0.750 | 0.009 | -0.268 | 0.196 |
| forearm + hand length | -0.172 | 0.142 | -1.406 | -0.005 | 0.468 | -0.492 | 0.483 | 0.105 |
| hand length | -0.669 | -0.506 | 0.383 | -0.189 | -0.879 | -0.007 | 0.088 | 0.328 |
| head width | 0.009 | -0.137 | -0.370 | 0.326 | -0.778 | 0.997 | 0.472 | 0.534 |
| head length | -0.282 | -0.341 | 0.252 | -0.389 | -0.293 | -1.250 | -0.563 | -0.734 |
| interorbital distance | 0.366 | 0.144 | 0.285 | 0.136 | 1.030 | 0.561 | -0.269 | -0.019 |
| upper eyelid width | -0.022 | 0.315 | -0.221 | 0.328 | 1.579 | 0.473 | 0.810 | -0.234 |
| horizontal eye diameter | -0.296 | -0.048 | 0.420 | -0.193 | 1.013 | 0.232 | 0.808 | 0.089 |
| eye to nostril distance | 0.417 | -0.532 | 0.473 | -0.287 | 1.016 | -0.555 | 0.242 | -0.166 |
| nostril to snout distance | 0.072 | 0.529 | 0.225 | 0.124 | 1.162 | -0.599 | 0.727 | -0.818 |
| snout length | -0.291 | -0.063 | -0.123 | 0.422 | -0.176 | 0.035 | -0.337 | 0.767 |
| internarial distance | 0.666 | 0.135 | -0.861 | -0.567 | 0.594 | 0.087 | -0.026 | -0.213 |
| horizontal tympanum diameter | -0.052 | -0.157 | 0.707 | -0.187 | -1.365 | 0.088 | -0.621 | 0.488 |
| Constant | 0.909 | -0.222 | 0.302 | 0.871 | 0.824 | -0.143 | 0.196 | 0.384 |
Gender-specific discriminant functions based on 17 SVL-adjusted morphometric features (residuals) to distinguish among five Ptychadena species from Rwanda. Classification success.
| predicted species actual species |
Ptychadena anchietae | Ptychadena chrysogaster | Ptychadena nilotica | Ptychadena porosissima | Ptychadena uzungwensis |
|---|---|---|---|---|---|
| Ptychadena anchietae. male female |
15 (100%) 3 (100%) |
0 | 0 | 0 | 0 |
| Ptychadena chrysogaster. male female |
0 | 13 (100%) 10 (100%) |
0 | 0 | 0 |
| Ptychadena nilotica. male female |
0 | 0 | 13 (100%) 10 (100%) |
0 | 0 |
| Ptychadena porosissima. male female |
0 | 0 | 0 | 11 (100%) 7 (100%) |
0 |
| Ptychadena uzungwensis. male female |
0 | 0 | 0 | 0 | 6 (100%) 1 (100%) |
| 1 | external vocal sac apertures and nuptial pads on dorsal side of metacarpals and phalanges of Fingers I–III present | adult males...2 |
| – | external vocal sac apertures and nuptial pads absent | adult females and subadults...6 |
| 2 | vocal sac aperture superior; only one supernumerary metacarpal tubercle proximal to Finger IV, often indistinct; longitudinal, alternately black and yellow coloured bands on postaxial side of femur (spiny tubercles on venter absent; inner metatarsal tubercle less than half the length of metatarsus of Toe I; distal subarticular tubercles of Toes III and V reaching to knee; toe webbing I(1.5–1.75)-(2–2.25)II1.5-(2.75–3)III(1.75–2)-3IV2.75-(1–1.5)V; ventral side of head white, mottled with grey) | Ptychadena nilotica |
| – | vocal sac aperture inferior or semi-inferior; at least one supernumerary metacarpal tubercle proximal to each finger; colouration on postaxial side of femur different | 3 |
| 3 | spiny tubercles on venter absent; toe webbing reaching distal phalanx on postaxial sides of Toes I, II, and III and on preaxial side of Toe V; external dorsal ridge usually not light and prominent (vocal sac aperture inferior; distal subarticular tubercles of Toes III and V never reaching knee; inner metatarsal tubercle about half the length of metatarsus of Toe I; ventral side of head white, trunk yellow; light tibial line and light dorsal band absent; dark spots on dorsum usually absent, if present, small and narrow; irregularly delimited, reticulated, longitudinal, alternately yellow and dark brown coloured bands on postaxial side of femur) | Ptychadena anchietae |
| – | spiny tubercles on venter present; toe webbing not reaching distal phalanges on toes; external dorsal ridge light and prominent | 4 |
| 4 | median dorsal ridge extending to level between nostrils on dorsal side of snout; vocal sac aperture semi-inferior; two supernumerary metacarpal tubercles proximal to Finger II; inner and outer palmar tubercle more or less equal in length; inner metatarsal tubercle about half the length of metatarsus of Toe I | Ptychadena uzungwensis |
| – | median dorsal ridge extending to level between eyelids only; vocal sac aperture inferior; one supernumerary metacarpal tubercle proximal to Finger II; outer palmar tubercle longer than inner; inner metatarsal tubercle either more than or less than half the length of metatarsus of Toe I | 5 |
| 5 | foot large, tips of Toes III and V reaching distinctly beyond knee, their distal subarticular tubercles reaching knee; ventral tubercles tiny, hardly visible with naked eye; inner metatarsal tubercle less than half the length of metatarsus of Toe I; dark brown stripe present on preaxial side of tibia; thenar tubercle approximately as long as outer palmar tubercle; webbing not reaching beyond distal subarticular tubercle on postaxial side of Toe III; dorsal spots small and narrow; irregularly delimited, reticulated, longitudinal dark bands on light background on postaxial side of femur | Ptychadena chrysogaster |
| – | foot smaller, tips of Toes III and V at most reaching slightly beyond knee, their distal subarticular tubercles not reaching knee; ventral tubercles large, visible with naked eye, palpable with finger; inner metatarsal tubercle more than half the length of metatarsus of Toe I; dark brown stripe absent on preaxial side of tibia; thenar tubercle longer than outer palmar tubercle; webbing reaching beyond distal subarticular tubercle on postaxial side of Toe III; dorsal spots large and wide; yellow spots, diffusely arranged in longitudinal rows on dark brown background on postaxial side of femur | Ptychadena porosissima |
| 6 | median dorsal ridge extending to level between nostrils on dorsal side of snout; two supernumerary metacarpal tubercles proximal to Finger II (inner metatarsal tubercle about half the length of metatarsus of Toe I; distal subarticular tubercles of Toes III and V not reaching to knee; toe webbing I2-(2.25–2.5)II1.5-3-III(1.75–2)-3IV3-(1+–1.25)V; light tibial line absent; light dorsal band present; dark spots on dorsum large and wide, often fused with neighboring ones; light, prominent dorsolateral fold present) | Ptychadena uzungwensis |
| – | median dorsal ridge extending to level between eyelids only; one or no supernumerary metacarpal tubercle proximal to Finger II | 7 |
| 7 | toe webbing reaching to distal phalanx on postaxial sides of Toes I, II, and III and on preaxial side of Toe V; light prominent external dorsal ridge usually absent; inner metatarsal tubercle about half the length of metatarsus of Toe I (tips of Toes III and V at most reaching slightly beyond knee, their distal subarticular tubercles not reaching knee; ventral side of head white, trunk yellow; light tibial line and light dorsal band absent; dark spots on dorsum usually absent, if present, small and narrow; irregularly delimited, reticulated, longitudinal, alternately yellow and dark brown coloured bands on postaxial side of femur) | Ptychadena anchietae |
| – | toe webbing not reaching to distal phalanx on toes; light prominent external dorsal ridge present; inner metatarsal tubercle either less than or more than half the length of metatarsus of Toe I | 8 |
| 8 | inner metatarsal tubercle more than half the length of metatarsus of Toe I; tips of Toes III and V at most reaching slightly beyond knee, their distal subarticular tubercles not reaching knee; thenar tubercle longer than outer palmar tubercle; yellow spots, diffusely arranged in longitudinal rows on dark brown background on postaxial side of femur | Ptychadena porosissima |
| – | inner metatarsal tubercle less than half the length of metatarsus of Toe I; tips of Toes III and V reaching distinctly beyond knee, their distal subarticular tubercles reaching knee; thenar tubercle about as long as outer palmar tubercle; colouration on postaxial side of femur not consisting of spots | 9 |
| 9 | dorsal spots small and narrow; one supernumerary metacarpal tubercle proximal to each finger; ventral side of head and chest yellow; dark brown stripe present on preaxial side of tibia; irregularly delimited, reticulated, longitudinal dark bands on light background on postaxial side of femur; webbing not reaching beyond subarticular tubercle on Toe I | Ptychadena chrysogaster |
| – | dorsal spots large and wide; only one supernumerary metacarpal tubercle proximal to Finger IV, often indistinct; ventral side of head and chest white; dark brown stripe on preaxial side of tibia absent, few specimens with dark mottling, not forming continuous stripe; longitudinal, alternately black and yellow coloured bands on postaxial side of femur; webbing reaching beyond subarticular tubercle on Toe I | Ptychadena nilotica |
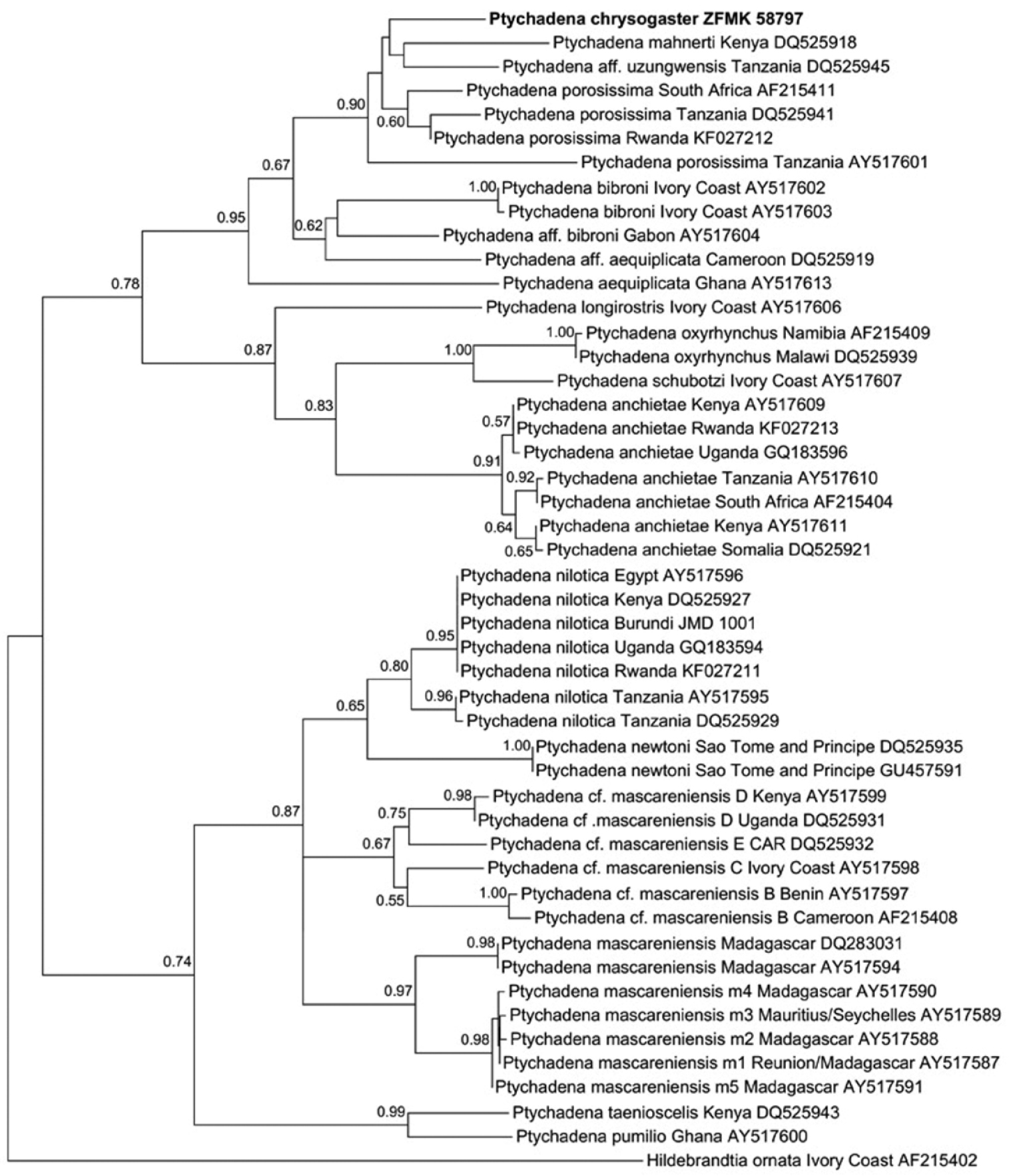
Comparison of the mitochondrial 16S rRNA gene sequences corroborated the status of Ptychadena chrysogaster as a distinct species. The partial sequence of this species differed from all available comparative sequences by an uncorrected p distance of at least 4.2 %. The p distance to sequences from Rwandan specimens of Ptychadena anchietae and Ptychadena nilotica was 13.1–13.3 % and 13.6 %, respecitively. The lowest values were observed in comparison with specimens assigned to “Ptychadena aff. uzungwensis” (4.2 %), “Ptychadena porosissima” from South Africa and Rwanda (4.7–4.9 %), “Ptychadena aff. porosissima” from Tanzania (6.0–6.9 %), “Ptychadena mahnerti” (6.2 %), and “P. aff. bibroni” from Gabon (6.9 %). The consensus tree yielded by Maximum-Likelihood analysis indicated that Ptychadena chrysogaster is most closely related to the aforementioned species (Figure 6). The clade consisting of these species is well supported by bootstraping (value 0.90;
Maximum likelihood phylogram of species in the genus Ptychadena and Hildebrandtia ornata as outgroup, based on comparison of 548 base pairs of the mitochondrial 16S rRNA gene. Included are specimens from Rwanda and samples taken from GenBank (see
Among the examined material we identified five distinct species of Ptychadena: Ptychadena anchietae, Ptychadena chrysogaster, Ptychadena nilotica, Ptychadena porosissima, and Ptychadena uzungwensis. The five species are distinguishable from each other unambiguously using quantitative morphometric as well as qualitative morphological characters. The comparison of the partial 16S rRNA sequence of a specimen of Ptychadena chrysogaster with sequences from congeners corroborated its distinct specific status. Sequences from specimens of Ptychadena anchietae, Ptychadena nilotica, and Ptychadena porosissima from Rwanda differ from each other considerably by an uncorrected p distance of more than 10 % (
We did not find any Ptychadena individual collected in Rwanda which was assignable to Ptychadena oxyrhynchus in the collections of the RMCA and the ZFMK. Three specimens from Kisenyi (= Gisenyi, nowadays Rubavu; RMCA 51565–67), Rwanda, had been deposited under the name Ptychadena oxyrhynchus but were re-identified as males of Ptychadena anchietae.
There is no specimen of Ptychadena grandisonae among the material Laurent collected in Rwanda.
The available evidence indicates that only five species of Ptychadena occur in Rwanda. At present, three of these species (Ptychadena anchietae, Ptychadena nilotica, and Ptychadena porosissima) are widespread and can be found abundantly in both wetlands of the eastern lowland between 1300 and 2000 m elevation which drains into the Nile River and the western lowland on the shore of Lake Kivu which drains into the Congo River. The species inhabit higher elevations of up to 2300 m in deforested, cultivated areas, but are absent from dense forest habitats at similar elevations which at present only remain in the Volcano and Nyungwe National Parks and in the Gishwati Forest. Ptychadena uzungwensis is known from Rwanda from only few specimens, five males from “Kumunini” [= Munini, South Province, 2°42'S, 29°32'E] and a female from “Astrida” [= Butare/Huye, South Province, 2°36'S, 29°44'E], collected in 1952 and 1951, respectively, and has not been found since. Assuming the species is still extant in Rwanda, its distribution is apparently restricted to the south of the country.
There are large series of Ptychadena chrysogaster from various localities in Rwanda in the collection of the RMCA (Appendix 1), collected by Laurent in 1951–1952, indicating that the species was abundant at that time. We repeatedly conducted surveys at several of these localities including the type locality at Lac Karago (1°37'S, 29°30'E) but did not encounter individuals of Ptychadena chrysogaster. Our survey periods (February to April, September to October) were at similar times of the year to those of Laurent (Janurary, February, and October). Species of Ptychadena are among the most conspicuous frogs in areas they inhabit, usually occurring in high numbers and easy to detect. Although the absence of a species from a certain area cannot be proven ultimately, our observations indicate that Ptychadena chrysogaster has disappeared from these areas or at least is much less common than it used to be. The human population in Rwanda has grown from little more than 2 million people in 1950 to approximately 11 million in 2011 (
Our recent efforts to untangle the diversity of Ptychadena in Rwanda are a first step to clarify the complicated taxonomy of the genus in sub-Saharan Africa. The results of our studies show that species of Ptychadena can be easily distinguished, if standardized diagnostic schemes are applied, which has also been demonstrated by previous studies (e.g.
Permission for field studies, handling and exportation of specimens was kindly issued by the Rwanda Development Board - Tourism & Conservation (RDB; officer in charge: A. Mudakikwa). We would like to thank S. Seidel and K. Rembold (Butare Field Station, University of Koblenz) and E. Fischer (Department of Biology, University of Koblenz) for logistic support. E. Fischer also provided a photograph of Ptychadena chrysogaster. B. Dumbo (Bukavu, DRC) helped during field surveys. A. Hochkirch, M. Veith and S. Lötters (Department of Biogeography, University of Trier) permitted the use of their laboratory facilities for the molecular analysis of tissue samples and gave an introduction to the requisite techniques. We would like to thank S. Naber, K. Fischer, and P. Willems (Department of Biogeography, University of Trier) for various help during laboratory work. D. Meirte and G. Cael (MRAC), and D. Rödder and W. Böhme (ZFMK) provided working space and facilitated examination of material under their care. JMD’s visit to the RMCA was funded by the Synthesys Project (http://www.synthesys.info) which is financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” programme (BE-TAF-2107). For helpful comments on an earlier draft of the manuscript we would like to thank D. C. Blackburn (California Academy of Sciences), S. Lötters, and an anonymous reviewer.
Ptychadena anchietae: Butare [= Huye], Rwanda (ZFMK 94575–89; twelve males, three females); “Kisenyi (Kivu)” [= Gisenyi/Rubavu, Rwanda] (RMCA 51565–67; three males).
Ptychadena chrysogaster: „Lac Karago, alt. 2250 m, terr. De Kisenyi“, Rwanda (RMCA 109096, one female, holotype; RMCA 109097–109113, sixteen males, paratypes); “Lulenga, Kivu”, DRC (RMCA 3452–69, eleven males, one female, paratypes; 2518–2521, four males, paratypes; 2759–68, one female, nine males, paratypes; 1748–55, seven males, one female, paratypes); “Gatsibu”, Rwanda (RMCA 36844, one male, paratype; 36866, one male, paratype); “Ruhengeri, riv. Moklungwa, alt. 1800–1825 m.), Ruanda” (RMCA 42011–13, three females, paratypes); “Lac Gando, alt. 2400 m, Ruanda” (RMCA 42025–28, four males, paratypes); “Region de Mulera, alt. 1800–2000 m”, Rwanda (RMCA 41987–88, one male, one female, paratypes); “Kasenze (versant S. Karisimbi)”, Rwanda (RMCA 42022–23, two males, paratypes); “entre Managna et Tshengelero, alt. 1750–2000 m”, DRC (RMCA 41965–41970, four males, one female, paratypes); “Kundhuru-Tshuve (col. Gahinga-Sabinyo), alt. 2600 m”, Rwanda (RMCA 41982–83, one male, one female, paratypes; RMCA 41984–86, three males, paratypes); “Ruhengeri, sources Kirii, alt. 1800–1825 m”, Rwanda (RMCA 41991, female, 41992–93, two males, paratypes); “Riv. Rodahira, afflt. de la riv. Fuku, s/afflt. de la riv. Rutshuru, près de Rutshuru, alt. 1250 m”, DRC (RMCA 116959, one male); “Nyabitsindi, entre le Visoke et le Musule, alt. 2400 m”, Rwanda (RMCA 42016–17, one male, one female, paratypes); “Kibga, riv. Suza, versant Sud Visoke, alt. 2400 m”, Rwanda (RMCA 42018, one male, paratype); “Dubi versant S. Visoke, Ruanda” (RMCA 42019–21, one female, two males, paratypes); “Shamuheru, Nyamuragira, alt. 1843 m”, DRC (RMCA 42031–32, one male, one female, paratypes); “Munagana, marais de Maziba, alt 2000 m”, Uganda (RMCA 41979–80, one male, one juvenile, paratypes); “Ilega (versant S. Karisimbi), Rubinda, alt. 2400 m”, Rwanda (RMCA 42024, subadult, paratype); “Kagogo, Lac Bulera, terr. de Ruhengeri, alt. 1870 m (Ruanda)” (RMCA 109121, one female; 109122, one male, paratypes); “Remera, Lac Luhondo, alt. 1770 m, terr. de Ruhengeri (Ruanda)” (RMCA 109114–120, three males, four females, paratypes); “Mutabonika, près de Ngabitsindi entre le Visoke et le Musule, alt. 2400 m” (RMCA 42014, one female; 42015, one male; paratypes); “Bitare, alt. 1650 m, Terr. de Kitega, Urundi” [= Burundi] (RMCA 109161–62, two males, paratypes); “Vyuya, Terr. de Bururi, alt. 1900 m (Urundi)” [=Burundi] (RMCA 109163–66, two males, paratypes); “Astrida, alt. 1750 m. (Ruanda)” [=Butare/Huye, Rwanda] (RMCA 109139–141, two females, one male, paratypes); “Tare, Busanza, région d’Astrida, alt. 1700 m (Ruanda)” (RMCA 109142, one female; 109143–147, four males, one female, paratypes); Mugatemba, Pref. Gikongoro, Rwanda (ZFMK 58747, one female); Nyungwe-Wald, Mukina (Kitabi), Rwanda (ZFMK 58797, one male); Cyamudongo, Rwanda (ZFMK 58847–850); Nyakalengijo, Mt Ruwenzori, Uganda (ZFMK 63239–241).
Ptychadena grandisonae: “Muita-Luembe, E (Angola)” (RMCA 60530, one female, holotype); ““Bitare, alt. 1650 m, Terr. de Kitega, Urundi” [= Burundi] (RMCA 109036–37, one male, one female, paratypes).
Ptychadena guibei: “Muita-Luembe, E (Angola)“ (RMCA 60535, female, holotype).
Ptychadena nilotica: Butare, Rwanda (ZFMK 94590–612, thirteen males, ten females; JMD 807, one male; EL 15-17, 25-26, 37, one male, five females); Mashyuza, Nyakabuye, Rwanda (ZFMK 58839–846); Route Kigali – Byumba, Rwanda (ZFMK 58851–852); Cyangugu, Rwanda (JMD 961, one male); Lac Karago, Rwanda (JMD 1028-1029); Bugarama, Rwanda (JMD 1074, one male); Bururi, Burundi (JMD 1001-1002, two males); Ruzizi National Park, Burundi (JMD 1015-1016, one male, one female). [JMD& EL specs currently being assigned ZFMK nos.]
Ptychadena porosissima: Butare [= Huye], Rwanda (ZFMK 94613–24, eleven males, one female); “Tare, reg. Astrida”, Rwanda (RMCA 109038, one female, holotype of Ptychadena loveridgei Laurent, 1954); “Astrida, alt. 1750 m” [= Huye], Rwanda (RMCA 109085–095, seven males, four females, paratypes of Ptychadena loveridgei); “Tare, Busanza”, Rwanda (RMCA 109039, one female, paratype of Ptychadena loveridgei); “Karambi, Terr. De Nyanza”, Rwanda (RMCA 109051–056, two females, paratypes of Ptychadena loveridgei); “Ruhengeri, riv. Moklungwa, alt. 1800–1825 m., Ruanda” (RMCA 42003–10, eight males, paratypes of Ptychadena loveridgei); “Ruhengeri, sources Kirii, alt. 1800–1825 m”, Rwanda (RMCA 41994, paratype of Ptychadena chrysogaster); “Reg. du Rwankeri, alt. 2200 m, Ruanda” (RMCA 41989, one male, paratype of Ptychadena chrysogaster).
Ptychadena taenioscelis: “Lukulu près Kiambi”, DRC (RMCA 13122, one subadult female, holotype).
Ptychadena uzungwensis: „Dabaga, Utschungwe Mts.“, Tanzania (RMCA 58843, one male, paratype); “Kumunini, Buyenza, alt. 2000 m, terr. d’ Astrida (Ruanda)” [= Munini, Rwanda] (RMCA 108993–97, five males); “Astrida, marais de la Mukura, alt. 1700 m (Ruanda)” [= Huye, Rwanda] (RMCA 108992, one female); “Bitare, alt. 1650 m, Terr. de Kitega, Urundi” (RMCA 108999–109016).
The external morphology of each species is described in the following account. Descriptions are primarily based on Rwandan material. In case of Ptychadena chrysogaster we also included data from type specimens from Burundi, Uganda, and the Democratic Republic of the Congo. The description of Ptychadena uzungwensis is based on all available material from Rwanda and a male paratype from the type locality (see Appendix 1).
Ptychadena anchietae
Body moderately sturdy, widest at temporal region, slightly tapering to groin; head large (HL/SUL 0.36–0.43 in males, 0.37–0.41 in females; HW/SUL 0.31–0.39 in males, 0.33–0.36 in females), longer than wide (HL/HW 1.01–1.26 in males, 1.11–1.13 in females); snout long (SL/HL 0.43–0.53 in males, 0.49–0.53 in females), pointed in dorsal view, rounded in profile, considerably projecting beyond lower jaw, longer than wide (SL/EE 1.08–1.24 in males, 1.30–1.33 in females); canthus rostralis distinct between eye and nostril, straight-lined; loreal region oblique, strongly concave; nostrils rounded, directed dorsolaterally; situated half-way between tip of snout and eye or closer to tip of snout than to eye (EN/NS 0.98–1.33 in males, 1.14–1.27 in females), separated from each other by distance subequal to distance between eye and nostril (NN/EN 0.87–1.05 in males, 0.85–0.96 in females); eyes directed anterolaterally, moderately protruding, relatively small (ED/HL 0.24–0.29 in males, 0.26–0.29 in females), its diameter much shorter than snout (ED/SL 0.50–0.63 in males, 0.53–0.58 in females); interorbital distance more or less equalling upper eyelid width (IO/EW 0.83–1.10 in males, 0.89–1.06 in females) and smaller than internarial distance (IO/NN 0.59–0.83 in males, 0.63–0.67 in females); tympanum and its annulus distinctly visible, separated from eye by about one-fifth to one-third of its diameter (ET/TD 0.21–0.36 in males, 0.19–0.25 in females); tympanum diameter 0.62–0.92 (in males) and 0.74–0.83 (in females) of eye diameter; upper jaw with dentition; choanae small, rounded, located far anterolaterally at margins of roof of mouth; vomer teeth in two short rows, separated from each other by distance about three times length of individual row; tongue long and narrow, bilobed for about one-sixth of its length, free distally for one-fourths its length; median lingual process absent; vocal sac in males paired, lateral; external vocal sac aperture as a longitudinal, posterolaterally orientated slit, inferior, terminating at level of ventral edge of insertion of arms; internal vocal sac apertures rounded, situated close to corner of mouth.
Dorsal surfaces of head, trunk and limbs finely shagreened with many scattered small tubercles; dorsum with five or six longitudinal dermal ridges on each side; median ridge extending from interorbital region almost to vent, postpalpebral and external ridges from level just behind posterior edge of upper eyelid to insertion of leg; laterodorsal ridge extending from level about one snout length posterior to tympanum to groin; dorsal ridges interrupted in few specimens; sacral ridge extending from about one head length anterior to vent either medially to median ridges or between median and postpalpebral ridges to vent, in few specimens absent; external ridge forming anterior part of supratympanic fold; posterior part of supratympanic fold less distinct, branching off from external dorsal ridge posterior to tympanum in wide angle and extending posterolaterally to insertion of arm; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold; ventral side of limbs and body smooth except slightly areolate postaxial side of thigh; distinct transverse fold between arms on ventral side; supratympanic fold moderately distinct, angled, extending from posterior corner of eye to point dorsal from arm insertion; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold, continued after a small gap in form of large oval tubercle dorsally of posterior end of arm insertion.
Forelimbs moderately sturdy; hand relatively small (HND/SUL 0.23–0.27 in males, 0.25–0.26 in females); tips of fingers rounded, not enlarged into disks but slightly swollen volarly; transverse dorsal skin ridge separating ultimate from other phalanges on each finger; relative length of fingers: I ≤ II < IV < III; subarticular tubercles rounded, well developed, numbering one on Fingers I and II, two on Fingers III and IV, proximal tubercles on Fingers III and IV larger and more prominent than distal ones; finger webbing absent; thenar tubercle distinct, large, flat, oval, slightly more than half as long as metacarpal of Finger I; inner palmar tubercle on proximal half of metacarpal region of Fingers II and III, rounded, flat; outer palmar tubercle on proximal half of metacarpal region of Finger IV, oval, slightly more prominent than inner palmar tubercle; one supernumerary metacarpal tubercle between palmar or thenar tubercles and proximal subarticular tubercles on each finger; callous longitudinal ridges between subarticular tubercles on Fingers III and IV and between subarticular tubercles and finger tips on all fingers; nuptial pads in males covering almost entire dorsal surfaces of Fingers I and II, and proximal portion of dorsal side of Finger III.
Hindlimbs sturdy, very long (LEG/SUL 1.87–2.11 in males, 1.96–2.13 in females); knee reaching slightly beyond insertion of forelimbs and tibio-tarsal articulation reaching almost a head length beyond tip of snout when legs are adpressed forwardly to body; tibiofibula very long (TFL/SUL 0.61–0.68 in males, 0.65–0.72 in females), longer than thigh (TFL/THL 1.09–1.19 in males, 1.13–1.16 in females); heels overlapping each other considerably when knees flexed and thighs held perpendicularly to median plane; two low longitudinal ridges on plantar side of tarsus between heel and metatarsal tubercles; foot shorter than or equal in length to tibiofibula (FOT/TFL 0.85–1.00 in males, 0.91–0.93 in females); relative length of toes: I < II < III < V < IV; toe tips rounded, not enlarged into disks but slightly swollen plantarly; transverse dorsal skin ridge separating ultimate from other phalanges on each toe; subarticular tubercles numbering one on Toes I and II, two on Toes III and V, and three on Toe IV; low callous ridges between subarticular tubercles, and between subarticular tubercles and toe tips; pedal webbing formula I 0.5-2II 0.5-2III(0.5–1)-2IV 2-0.5V; inner metatarsal tubercle moderately large, half as long as metatarsus of Toe I, oval, prominent; outer metatarsal tubercle rounded, flat, faintly visible, callous tissue weakly developed.
Ptychadena chrysogaster Laurent, 1954
Body moderately sturdy, widest at temporal region, slightly tapering to groin; head large (HL/SUL 0.34–0.38 in males, 0.33–0.37 in females; HW/SUL 0.29–0.34 in males, 0.28–0.34 in females), longer than wide (HL/HW 1.04–1.22 in males, 1.05–1.23 in females); snout long (SL/HL 0.45–0.50 in males, 0.45–0.51 in females), pointed in dorsal view, rounded in profile, considerably projecting beyond lower jaw, longer than wide (SL/EE 1.11–1.28 in males, 1.11–1.37 in females); canthus rostralis distinct between eye and nostril, straight-lined; loreal region oblique, strongly concave; nostrils rounded, directed dorsolaterally; situated more or less half-way between tip of snout and eye (EN/NS 0.87–1.14 in males, 0.93–1.24 in females), separated from each other by distance subequal to or larger than distance between eye and nostril (NN/EN 1.02–1.22 in males, 0.94–1.24 in females); eyes directed anterolaterally, moderately protruding, relatively small (ED/HL 0.27–0.32 in males, 0.26–0.30 in females), its diameter much shorter than snout (ED/SL 0.57–0.66 in males, 0.51–0.66 in females); interorbital distance larger than upper eyelid width (IO/EW 1.12–1.48 in males, 1.09–1.44 in females) and smaller than internarial distance (IO/NN 0.75–0.89 in males, 0.78–0.91 in females); tympanum and its annulus distinctly visible, separated from eye by about one-fourth to two-fifths of its diameter (ET/TD 0.24–0.38 in males, 0.26–0.40 in females); tympanum diameter slightly smaller to subequal to eye diameter (TD/ED 0.76–0.98 in males, 0.85–1.01 in females); upper jaw with dentition; choanae small, rounded, located far anterolaterally at margins of roof of mouth; vomer teeth in two short rows, separated from each other by distance about three times length of individual row; tongue long and narrow, bilobed for about one-fifth of its length, free distally for one-fourth its length; median lingual process absent; vocal sac in males paired, lateral; external vocal sac aperture as a longitudinal, posterolaterally orientated slit, inferior, terminating at level of ventral edge of ventral insertion of arms; internal vocal sac apertures rounded, situated close to corner of mouth.
Dorsal surfaces of head, trunk and limbs finely shagreened; dorsum with five or six longitudinal dermal ridges on each side, median one extending from interorbital region almost to vent, postpalpebral and external ones from level just behind posterior edge of upper eyelid to insertion of leg; laterodorsal ridge extending from level about one snout length posterior to tympanum to groin; sacral ridge extending from about one head length anterior to vent either medially to median ridges or between median and postpalpebral ridges to vent; in few specimens additional sacromedial ridge extending between sacral ridge and median ridge to vent for about half length of sacral ridge; external ridge forming anterior part of supratympanic fold; posterior part of supratympanic fold less distinct, branching off from external dorsal ridge posterior to tympanum in wide angle and extending posterolaterally to insertion of arm; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold; ventral side of limbs and body smooth except areolate proximal postaxial-ventral part of thigh; distinct transverse fold between arms on ventral side; ventral side of trunk and head densely covered with more or less evenly scattered tiny, pointed tubercles in males.
Forelimbs moderately sturdy; hand relatively small (HND/SUL 0.22–0.26 in males, 0.21–0.25 in females); tips of fingers rounded, not enlarged into disks but slightly swollen volarly; transverse dorsal skin ridge separating ultimate from other phalanges on each finger; relative length of fingers: I = II < IV < III; subarticular tubercles rounded, well developed, numbering one on Fingers I and II, two on Fingers III and IV, proximal tubercles on Fingers III and IV larger and more prominent than distal ones; finger webbing absent; thenar tubercle prominent, elongated, large, two-thirds length of metacarpal of Finger I; inner palmar tubercle on proximal third of metacarpal region of Fingers II and III, oval, flat; outer palmar tubercle on proximal half of metacarpal region of Finger IV, elongated, slightly more prominent than inner palmar tubercle; one supernumerary metacarpal tubercle between palmar or thenar tubercles and proximal subarticular tubercles on all fingers; low callous longitudinal ridges between subarticular tubercles on Fingers III and IV and between subarticular tubercles and finger tips on all fingers; nuptial pads in males covering almost entire dorsal surfaces of Fingers I and II except distal phalanx, and preaxial half of dorsal side of metacarpal of Finger III.
Hindlimbs sturdy, very long (LEG/SUL 1.94–2.27 in males, 1.93–2.14 in females); knee reaching to insertion of forelimbs and tibio-tarsal articulation reaching slightly more than a snout length beyond tip of snout when legs adpressed forwardly to body; tibiofibula very long (TFL/SUL 0.61–0.73 in males, 0.62–0.70 in females), longer than thigh (TFL/THL 1.13–1.22 in males, 1.13–1.25 in females); heels overlapping each other considerably when knees flexed and thighs held perpendicularly to median body plane; two low longitudinalridges on plantar side of tarsus between heel and metatarsal tubercles; foot subequal in length to tibiofibula (FOT/TFL 0.97–1.07 in males, 0.94–1.03 in females); relative length of toes: I < II < III < V < IV; toe tips rounded, not enlarged into disks but slightly swollen plantarly; transverse dorsal skin ridge separating ultimate from other phalanges on each toe; subarticular tubercles numbering one on Toes I and II, two on Toes III and V, and three on Toe IV; low callous ridges between subarticular tubercles, and between subarticular tubercles and toe tips; pedal webbing formula I 2-2.5II(1.5–1.75)-3III(2–2-)(3.25–3+)IV 3-(1.5–2)V; inner metatarsal tubercle elongated, prominent, small, less than half as long as metatarsus of Toe I; outer metatarsal tubercle very faintly visible, rarely prominent, small and pointed.
Ptychadena nilotica
Body moderately sturdy, widest at temporal region, slightly tapering to groin; head large (HL/SUL 0.35–0.43 in males, 0.35–0.39 in females; HW/SUL 0.30–0.37 in males, 0.32–0.35 in females), longer than wide (HL/HW 1.10–1.27 in males, 1.06–1.15 in females); snout long (SL/HL 0.38–0.46 in males, 0.40–0.45 in females), pointed in dorsal view, rounded in profile, considerably projecting beyond lower jaw, longer than wide (SL/EE 1.20–1.40 in males, 1.15–1.36 in females); canthus rostralis distinct between eye and nostril, straight-lined; loreal region oblique, moderately concave; nostrils rounded, directed dorsolaterally; situated half-way between tip of snout and eye or closer to tip of snout than to eye (EN/NS 0.99–1.18 in males, 1.05–1.34 in females), separated from each other by distance slightly less than or subequal to distance between eye and nostril (NN/EN 0.82–0.99 in males, 0.87–0.97 in females); eyes directed anterolaterally, moderately protruding, relatively small (ED/HL 0.25–0.32 in males, 0.26–0.29 in females), its diameter shorter than snout (ED/SL 0.60–0.69 in males, 0.59–0.69 in females); interorbital distance smaller to equalling upper eyelid width (IO/EW 0.64–1.03 in males, 0.72–0.90 in females) and smaller than internarial distance (IO/NN 0.55–0.74 in males, 0.57–0.72 in females); tympanum and its annulus distinctly visible, separated from eye by about one-fourth to one-third of its diameter (ET/TD 0.28–0.36 in males, 0.26–0.37 in females); tympanum diameter 0.75–0.88 (in males) and 0.77–0.89 (in females) of eye diameter; upper jaw with dentition; choanae small, rounded, located far anterolaterally at margins of roof of mouth; vomer teeth in two short rows, separated from each other by distance about three times length of individual row; tongue long and narrow, bilobed for about one-sixth of its length, free distally for one-third its length; median lingual process absent; vocal sac in males paired, lateral; external vocal sac aperture as a longitudinal, posteriorly orientated slit, situated dorsally of level of insertion of arms, parallel to mandibel and infratympanic fold, covered by narrow dermal flap on ventral edge of slit; internal vocal sac apertures rounded, situated close to corner of mouth.
Dorsal surfaces of head, trunk and limbs shagreened; dorsum with four or five longitudinal dermal ridges on each side, median one extending from interorbital region almost to vent, postpalpebral and external ones from level just behind posterior edge of upper eyelid to insertion of leg; laterodorsal ridge extending from level about one snout length posterior to tympanum to groin; in few specimens additional very short ridge present between median and postpalpebral ridge from about one snout length anterior to vent extending almost to vent; external ridge forming anterior part of supratympanic fold; posterior part of supratympanic fold less distinct, branching off from external dorsal ridge posterior to tympanum in wide angle and extending posterolaterally to insertion of arm; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold; ventral side of limbs and body smooth except areolate postaxial side of thigh; distinct transverse fold between arms on ventral side.
Forelimbs moderately sturdy; hand relatively small (HND/SUL 0.21–0.27 in males, 0.22–0.26 in females); tips of fingers rounded, not enlarged into disks but slightly swollen volarly; transverse dorsal skin ridge separating ultimate from other phalanges on each finger; relative length of fingers: I ≤ II < IV < III; subarticular tubercles rounded, well developed, numbering one on Fingers I and II, two on Fingers III and IV; proximal tubercles on Fingers III and IV larger and more prominent than distal ones; finger webbing absent; thenar tubercle prominent, elongated and narrow, comparatively small, less than half as long as metacarpal of Finger I; inner palmar tubercle small, flat, less conspicuous, on proximal one-third to one-fourth of metacarpal region of Fingers II and III, rounded, flat; outer palmar tubercle on proximal half of metacarpal region of Finger IV, prominent, elongate and narrow; single supernumerary metacarpal tubercle between outer palmar tubercle and proximal subarticular tubercle of Finger IV, often indistinct; callous longitudinal ridges between subarticular tubercles on Fingers III and IV and between subarticular tubercles and finger tips on all fingers; nuptial pads in males covering almost entire dorsal surfaces of Fingers I and II and preaxial half of dorsal surface of Finger III.
Hindlimbs sturdy, long (LEG/SUL 1.71–1.97 in males, 1.76–1.96 in females); knee reaching just behind insertion of forelimbs and tibio-tarsal articulation reaching tip of snout when legs are adpressed forwardly to body; tibiofibula moderately long (TFL/SUL 0.50–0.60 in males, 0.54–0.60 in females), longer than thigh (TFL/THL 1.04–1.12 in males, 1.09–1.12 in females); heels overlapping each other when knees flexed and thighs held perpendicularly to median plane; two low longitudinalridges on plantar side of tarsus between heel and metatarsal tubercles; foot long, longer than tibiofibula (FOT/TFL 1.07–1.14 in males, 1.03–1.10 in females); relative length of toes: I < II < III < V < IV; toe tips rounded, not enlarged into disks but slightly swollen plantarly; transverse dorsal skin ridge separating ultimate from other phalanges on each toe; subarticular tubercles numbering one on Toes I and II, two on Toes III and V, and three on Toe IV; low callous ridges between subarticular tubercles, and between subarticular tubercles and toe tips; pedal webbing formula I(1.5–1.75)-(2–2.25)II 1.5-(2.75–3)III(1.75–2)-3IV 2.75-(1–1.5)V; inner metatarsal tubercle elongated, prominent, small, less than half as long as metatarsus of Toe I; outer metatarsal tubercle rounded, flat, distinctly present, rarely faintly visible.
Ptychadena porosissima
Body moderately sturdy, widest at temporal region, slightly tapering to groin; head large (HL/SUL 0.35–0.41 in males, 0.35–0.40 in females; HW/SUL 0.31–0.36 in males, 0.30–0.34 in females), longer than wide (HL/HW 1.02–1.19 in males, 1.03–1.21 in females); snout moderately long (SL/HL 0.38–0.49 in males, 0.43–0.51 in females), pointed in dorsal view, rounded in profile, considerably projecting beyond lower jaw, longer than wide (SL/EE 1.21–1.27 in males, 1.11–1.61 in females); canthus rostralis distinct between eye and nostril, straight-lined; loreal region oblique, strongly concave; nostrils rounded, directed dorsolaterally; situated more or less half-way between tip of snout and eye (EN/NS 0.89–1.15 in males, 0.87–1.29 in females), separated from each other by distance subequal to or larger than distance between eye and nostril (NN/EN 0.96–1.15 in males, 0.96–1.11 in females); eye directed anterolaterally, moderately protruding, relatively small (ED/HL 0.24–0.30 in males, 0.25–0.29 in females), its diameter much shorter than snout (ED/SL 0.54–0.67 in males, 0.51–0.63 in females); interorbital distance subequal to upper eyelid width (IO/EW 0.80–1.09 in males, 0.81–1.10 in females) and smaller than internarial distance (IO/NN 0.65–0.74 in males, 0.60–0.82 in females); tympanum and its annulus distinctly visible, separated from eye by about one-fourth to two-fifths of its diameter (ET/TD 0.27–0.39 in males, 0.29–0.39 in females); tympanum diameter smaller than eye diameter (TD/ED 0.68–0.81 in males, 0.70–0.84 in females); upper jaw with dentition; choanae small, rounded, located far anterolaterally at margins of roof of mouth; vomer teeth in two short rows, separated from each other by distance about three times length of individual row; tongue long and narrow, bilobed for about one-fifth of its length, free distally for one-third its length; median lingual process absent; vocal sac in males paired, lateral; external vocal sac aperture as a longitudinal, posterolaterally orientated slit, inferior, terminating at level of ventral edge of insertion of arms; internal vocal sac apertures rounded, situated close to corner of mouth.
Dorsal surfaces of head, trunk and limbs finely shagreened; dorsum with four or five longitudinal dermal ridges on each side; median ridge extending from interorbital region almost to vent, postpalpebral and external ridges from posterior edge of upper eyelid to insertion of leg; laterodorsal ridge extending from level of insertion of arm to groin; sacral ridge extending from about one head length anterior to vent to vent, in some specimens absent; external ridge forming anterior part of supratympanic fold; posterior part of supratympanic fold less distinct, branching off from external dorsal ridge posterior to tympanum in wide angle and extending posterolaterally to insertion of arm; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold; ventral side of limbs and body smooth except areolate postaxial side of thigh; distinct transverse fold between arms on ventral side; ventral side of trunk and head densely covered with more or less evenly scattered small, pointed tubercles in males.
Forelimbs moderately sturdy; hand relatively small (HND/SUL 0.22–0.26 in males, 0.22–0.24 in females); tips of fingers rounded, not enlarged into disks but slightly swollen volarly; transverse dorsal skin ridge separating ultimate from other phalanges on each finger; relative length of fingers: I ≤ II < IV < III; subarticular tubercles rounded, well developed, numbering one on Fingers I and II, two on Fingers III and IV, proximal tubercles on Fingers III and IV larger and more prominent than distal ones; finger webbing absent; thenar tubercle distinct, elongated, large, two-thirds length of metacarpal of Finger I; inner palmar tubercle on proximal third of metacarpal region of Fingers II and III, oval, flat; outer palmar tubercle on proximal half of metacarpal region of Finger IV, elongated, slightly more prominent than inner palmar tubercle; one supernumerary metacarpal tubercle between palmar or thenar tubercles and proximal subarticular tubercles on Fingers I, II, and IV, two on Finger III; callous longitudinal ridges between subarticular tubercles on Fingers III and IV and between subarticular tubercles and finger tips on all fingers; nuptial pads in males covering almost entire dorsal surfaces of Fingers I and II and preaxial half of dorsal side of Finger III.
Hindlimbs sturdy, very long (LEG/SUL 1.77–2.05 in males, 1.80–2.06 in females); knee reaching to insertion of forelimbs and tibio-tarsal articulation reaching slightly more than one snout length beyond tip of snout when legs adpressed forwardly to body; tibiofibula long (TFL/SUL 0.56–0.65 in males, 0.56–0.66 in females), longer than thigh (TFL/THL 1.08–1.18 in males, 1.05–1.26 in female); heels overlapping each other considerably when knees flexed and thighs held perpendicularly to median plane; two low longitudinal ridges on plantar side of tarsus between heel and metatarsal tubercles; foot slightly shorter than or equal in length to tibiofibula (FOT/TFL 0.94–1.01 in males, 0.92–1.01 in females); relative length of toes: I < II < III < V < IV; toe tips rounded, not enlarged into disks but slightly swollen plantarly; transverse dorsal skin ridge separating ultimate from other phalanges on each toe; subarticular tubercles numbering one on Toes I and II, two on Toes III and V, and three on Toe IV; low callous ridges between subarticular tubercles, and between subarticular tubercles and toe tips; pedal webbing formula I(1.75–2)-2.25II 1.5-3III 1.75-(3–3.25)IV 3-(1–1.5)V; inner metatarsal tubercle very prominent, shovel-like, large, more than half as long as metatarsus of Toe I; outer metatarsal tubercle faintly visible, rarely prominent and pointed.
Ptychadena uzungwensis
Body moderately sturdy, widest at temporal region, slightly tapering to groin; head large (HL/SUL 0.36–0.39 in males, 0.37 in females; HW/SUL 0.32–0.35 in males, 0.32 in female), longer than wide (HL/HW 1.06–1.23 in males, 1.16 in female); snout long (SL/HL 0.50–0.52 in males, 0.50 in female), pointed in dorsal view, rounded in profile, considerably projecting beyond lower jaw, much longer than wide (SL/EE 1.30–1.53 in males, 1.56 in female); canthus rostralis distinct between eye and nostril, straight-lined; loreal region oblique, strongly concave; nostrils rounded, directed dorsolaterally; situated more or less half-way between tip of snout and eye (EN/NS 0.91–1.03 in males, 0.97 in female), separated from each other by distance subequal to or shorter than distance between eye and nostril (NN/EN 0.82–0.94 in males, 0.89 in female); eyes directed anterolaterally, moderately protruding, relatively small (ED/HL 0.27–0.32 in males, 0.28 in female), its diameter much shorter than snout (ED/SL 0.55–0.63 in males, 0.56 in female); interorbital distance subequal to or larger than upper eyelid width (IO/EW 0.97–1.24 in males, 1.19 in female) and smaller or subequal to internarial distance (IO/NN 0.80–1.06 in males, 0.78 in female); tympanum and its annulus distinctly visible, separated from eye by about two-fifths to half of its diameter (ET/TD 0.36–0.47 in males, 0.41 in female); tympanum diameter smaller than eye diameter (TD/ED 0.70–0.83 in males, 0.77 in female); upper jaw with dentition; choanae small, rounded, located far anterolaterally at margins of roof of mouth; vomer teeth in two short rows, separated from each other by distance about two and half times length of individual row; tongue long and narrow, bilobed for about one-third of its length, free distally for one-third its length; median lingual process absent; vocal sac in males paired, lateral; external vocal sac aperture as a longitudinal, posterolaterally orientated slit, semi-inferior, terminating at level of centre of insertion of arms; internal vocal sac apertures rounded, situated close to corner of mouth.
Dorsal surfaces of head, trunk and limbs finely shagreened; dorsum with four longitudinal dermal ridges on each side; median ridge extending from dorsal side of snout between nostrils almost to vent; postpalpebral ridge extending from posterior portion of upper eyelid, external ridge from level of tympanum to insertion of leg; laterodorsal ridge extending from level of tympanum to groin; external ridge forming anterior part of supratympanic fold; posterior part of supratympanic fold less distinct, branching off from external dorsal ridge posterior to tympanum in wide angle and extending posterolaterally to insertion of arm; infratympanic fold thick and conspicuous, almost straight-lined, extending from ventral edge of eye to level of arm insertion, meeting with supratympanic fold; ventral side of limbs and body smooth except areolate postaxial side of thigh; distinct transverse fold between arms on ventral side; ventral side of trunk and head densely covered with more or less evenly scattered small, pointed, very small tubercles in males.
Forelimbs moderately sturdy; hand relatively small (HND/SUL 0.22–0.24 in males, 0.21 in female); tips of fingers rounded, not enlarged into disks but slightly swollen volarly; transverse dorsal skin ridge separating ultimate from other phalanges on each finger; relative length of fingers: II = IV ≤ I < III; subarticular tubercles rounded, well developed, numbering one on Fingers I and II, two on Fingers III and IV, proximal tubercles on Fingers III and IV larger and more prominent than distal ones; finger webbing absent; thenar tubercle distinct, elongated, large, half as long as metacarpal of Finger I; inner palmar tubercle on proximal third of metacarpal of Finger III, small, roundish, flat; outer palmar tubercle on proximal third of metacarpal of Finger IV, elongated, more prominent than inner palmar tubercle; one supernumerary metacarpal on Fingers I and IV, two on Finger II, and two to four on Finger III between palmar or thenar tubercles and proximal subarticular tubercles; callous longitudinal ridges between subarticular tubercles on Fingers III and IV and between subarticular tubercles and finger tips on all fingers; nuptial pads in males covering almost entire dorsal surfaces of metacarpal and proximal phalanx of Fingers I and II and preaxial halves of dorsal sides of metacarpal and [in Rwandan specimens but not in paratype and specimens from Kivu and Burundi] proximal portion of proximal phalanx of Finger III.
Hindlimbs sturdy, very long (LEG/SUL 1.84–2.04 in males, 1.87 in female); knee reaching to insertion of forelimbs and tibio-tarsal articulation reaching slightly more than one snout length beyond tip of snout when legs adpressed forwardly to body; tibiofibula long (TFL/SUL 0.59–0.66 in males, 0.62 in female), longer than thigh (TFL/THL 1.12–1.20 in males, 1.16 in female); heels overlapping each other considerably when knees flexed and thighs held perpendicularly to median body plane; two low longitudinal ridges on plantar side of tarsus between heel and metatarsal tubercles, postaxial one less distinct than preaxial one; foot shorter than or subequal in length to tibiofibula (FOT/TFL 0.94–1.03 in males, 1.07 in female); relative length of toes: I < II < III < V < IV; toe tips rounded, not enlarged into disks but slightly swollen plantarly; transverse dorsal skin ridge separating ultimate from other phalanges on each toe; subarticular tubercles numbering one on Toes I and II, two on Toes III and V, and three on Toe IV; low callous ridges between subarticular tubercles, and between subarticular tubercles and toe tips; pedal webbing formula I 2-(2.25–2.5)II 1.5-3-III(1.75–2-)-3IV 3-(1+–1.25)V; inner metatarsal tubercle prominent, elongated, large, half as long as metatarsus of Toe I; outer metatarsal tubercle faintly visible.