(C) 2013 Michael W. Hastriter. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
During May 2009 and July 2011, we collected 357 mammals and examined each for ectoparasites. Among the ectoparasites collected, a new species of flea was discovered. This new species, Lentistivalius philippinensis, is described from the male sex only. Two males were recovered from two specimens of the soricid Crocidura grayi Dobson in Municipality Maria Aurora, Aurora Province, Luzon, Philippines. Additional fleas included Thaumapsylla breviceps orientalis Smit, Thaumapsylla longiforceps Traub, and Ischnopsyllus indicus Jordan. Although the latter species is common in Japan and documented in Guam (as well as mainland Southeast Asia) also on Pipistrellus javanicus (Gray), Ischnopsyllus indicus represents a new record in the Philippine Islands. The ascodipterinae (Streblidae) Maabella stomalata and Ascodipteron speiserianum Muir collected from Rhinolophus inops K. Andersen and Rhinolophus subrufus K. Andersen, respectively, also represent new host records. A key to the species of the flea genus Lentistivalius Traub is provided.
Ascodipteron, bat flies, fleas
During May 2009 and July 2011, we collected 357 mammals representing 57 species from the Philippines and examined each for ectoparasites. All but one of these mammals were collected from the island of Luzon in the northern Philippines. One bat was collected on the island of Negros in the southern Philippines. Bat flies in the families Nycteribiidae and Streblidae (Diptera) were present on many of the bat specimens, including one unusual group of endosomic flies in the subfamily Ascodipterinae (Streblidae). In addition to the ascodipterons, several species of bat fleas and a new species of flea in the Siphonapteran suborder Pygiopsyllomorpha are reported in this study. Molecular and morphological analyses of nycteribiid and other non-endosomic streblid flies from bats will be reported in a separate paper.
Mammals and their ectoparasites were surveyed at 12 field sites on the island of Luzon (Fig. 1), and one bat was collected from the island of Negros. A map for the island of Negros is not included. Mammals were captured and euthanized according to guidelines of the American Society of Mammalogists (
Sampling locations on the island of Luzon (n = number of mammals examined at each site). Stars indicate the location of ascodipterons and fleas discussed in this paper. a Mt. Pao (n=38) and Mt. Cabacan (n=38) b Mt. Cagua site 1 (n=62) and Mt. Cagua site 2 (n=32) c Casiguran (n = 16) d Maria Aurora (n=34) e Sitio Minoli (n=56) and nearby field site (n=24) f Zabali (n=39) g Tower site (n=14) h Angat (n=3); and i Burdeos (n=1). Photo courtesy of S. Villa.
Philippines, Luzon Island, Aurora Province: Sitio Minoli, Municipality San Louis (15.680°N, 121.529°E), elev. 520m, Rhinolophus subrufus K. Andersen (JAE2961), 13 VI 2009, K. Dittmar (1dealate ♀ w/o caudal disc, P-2661).
Only one Ascodipteron speiserianum was collected from the 19 Rhinolophus subrufus specimens that were examined. Ascodipteron speiserianum was documented in the Philippines in
Philippines, Luzon Island, Cagayan Province: Mt. Cagua 2, Magrafil Barangay (18.236°N, 122.104°E), elev. 680m, Rhinolophus inops K. Anderson (JAC093), 20 VII 2011, S. Villa and S. Knutie, (1 dealate ♀ w/o caudal disc, P4631); same data except Rhinolophus inops (JAC094) (1dealate ♀ with caudal disc, P4632); same data except Rhinolophus inops (JAC096) (1 dealate ♀ w/o caudal disc and 1 dealate ♀ with caudal disc, P4640); and same data except Rhinolophus inops (JAC097) (1dealate ♀ with caudal disc, 2 dealate ♀♀ w/o caudal discs, P4636).
Maabella is a widespread monotypic genus.
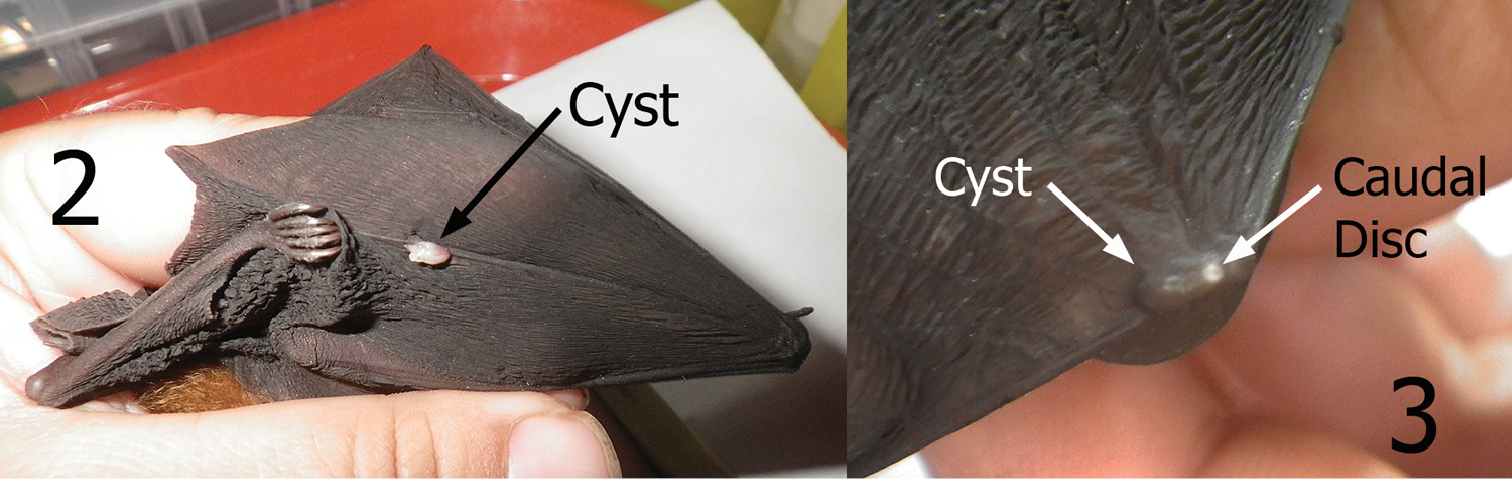
Cysts containing neosomes of Maabella stomalata. 2 Cyst located on dorsal surface directly over wing digit number five of Rhinolophus sp. (species undetermined) 3 Cyst located on dorsal surface directly over the radius-ulna/thumb joint of Rhinolophus inops. Arrow depicts caudal disc (spiracle breathing structure) protruding through the skin.
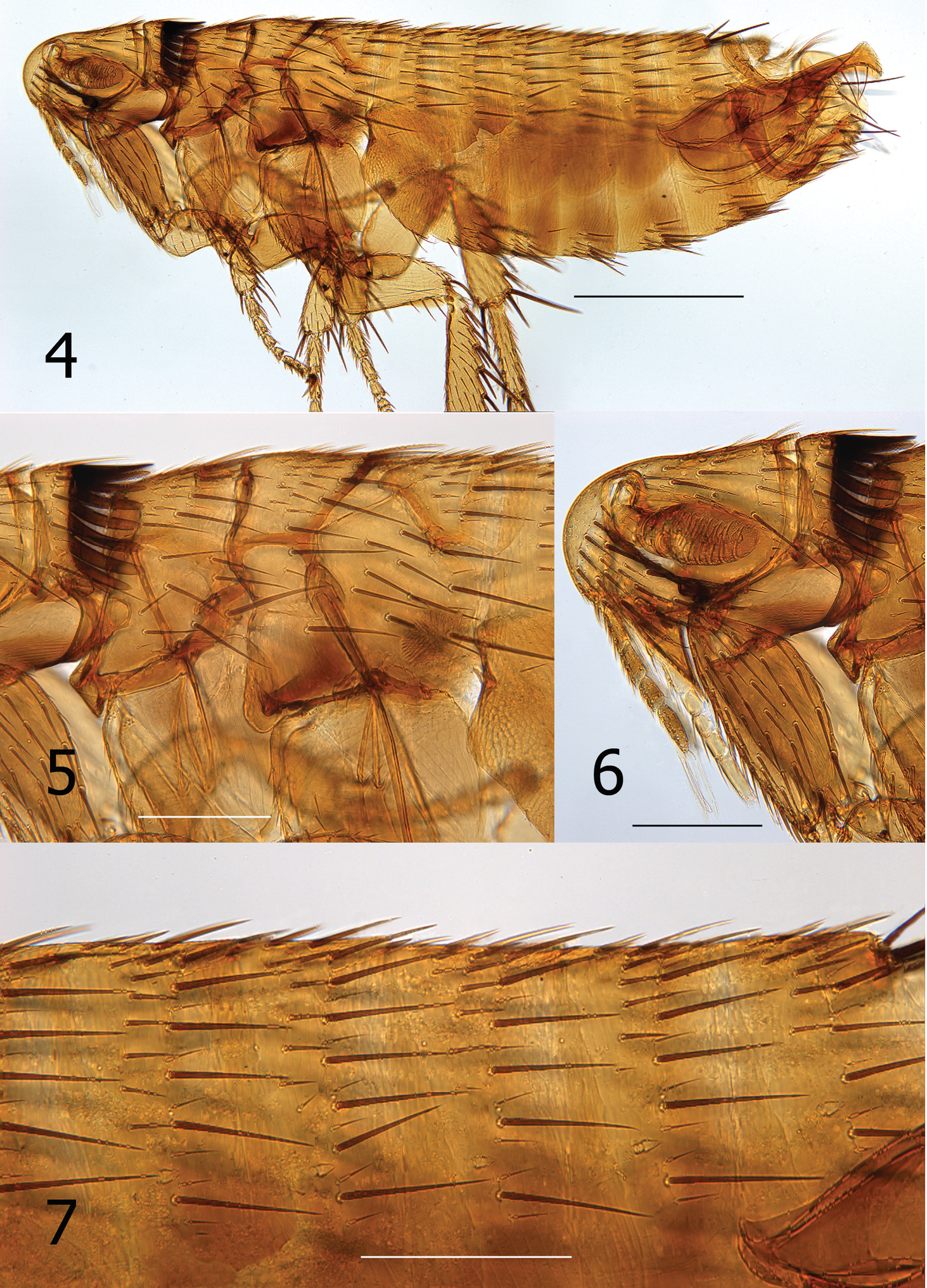
Lentistivalius philippinensis sp. n. (P2316) 4 Overview, male holotype 5 Thorax 6 Head, pronotum, forecoxa 7 Abdominal tergites. (Scale: Fig. 4 = 100 µ; Figs 5–7 = 200µ).
http://species-id.net/wiki/Thaumapsylla_breviceps_orientalis
Philippines, Luzon Island, Aurora Province: Sitio Minoli, Municipality San Louis (15.680°N, 121.529°E), elev. 520m, Eonycteris robusta Miller (JAE2944), 12 VI 2009, K. Dittmar, (1♂, P2650); same except Eonycteris spelaea Dobson (JAE3021), 16 VI 2009 (1♀, P2766); same except Eonycteris spelaea (JAE3023) (1♀, P2770); same except Rousettus amplexicaudatus (JAE3027) (1♀, P2775); same except Eonycteris spelaea (JAE3040) (1♂, P2779); Luzon Island, Ilocos Norte Province, Adams village, Mt. Pao, (18.438°N, 120.878°E), elev. 750m, Rousettus amplexicaudatus (NCA049), 22 VI 2011, S. Villa, (2♀♀, P4196); Luzon Island, Ilocos Norte Province, Adams village, Mt. Cabacan, (18.449°N, 120.894°E), elev. 475m, Rousettus amplexicaudatus (NCA125), 30 VI 2011, S. Villa, (1♀, P4318); Luzon Island, Cagayan Province, Barangay Magrafil (closest city Gonzaga), Mt. Cagua, (18.219°N, 122.111°E), elev. 780m, Rousettus amplexicaudatus (JAC036), 11 VII 2011, S. Villa and S. Knutie, (1♂, P4496).
The two populations of Thaumapsylla breviceps are recognized (Thaumapsylla breviceps breviceps Rothschild, 1907 and Thaumapsylla breviceps orientalis Smit, 1954). The nominate species is found in southern portions of Africa and the other in the Oriental and Australasian regions.
None of the three species on which Thaumapsylla breviceps orientalis occurred represent new host records.
Philippines, Luzon Island, Ilocos Norte Province, Adams village, Mt. Pao, (18.438°N, 120.878°E), elev. 750m, Eonycteris robusta (NCA055), 23 VI 2011, S. Villa, (1♂, P4222); Luzon Island, Ilocos Norte Province, Adams village, Mt. Cabacan, (18.449°N, 120.894°E), elev. 475m, Eonycteris robusta (NCA081), 27 VI 2011, S. Villa, (1♀, P4253); same data except Rousettus amplexicaudatus (NCA125), 30 VII 2011, S. Villa, (1♀, P4318).
Thaumapsylla longiforceps is not as widespread in the Oriental region as Thaumapsylla breviceps orientalis. These two species may occur on the same host as we found a female of each flea species on host NCA125 (Rousettus amplexicaudatus). This species commonly occurs on pteropodid bats (fruit bats) but has also been documented on vespertilionid and rhinolophid bats.
Philippines, Negros Island, Mt. Bungal, Northern Negros Natural Park (10.674°N, 123.189°E), elev. 1200m, Pipistrellus javanicus (Gray) (JAE3252), 23 VII 2009, J. Esselstyn, (1♂).
Ischnopsyllus indicus has been documented in China, Taiwan, Vietnam, India, Guam, Sri Lanka, and Japan from a number of vespertilionid bat species; however, this is the first record of Ischnopsyllus indicus in the Philippines. Finding this flea in the Philippines is no surprise, since Ischnopsyllus indicus was documented in Guam by
urn:lsid:zoobank.org:act:5E6F547E-51A0-40C2-A292-DDEB2B2A2D12
http://species-id.net/wiki/Lentistivalius_philippinensis
Figs 4–15Holotype male (P2316), Philippines, Luzon Island, Aurora Province: Camp 1, Municipality Maria Aurora (15.685°N, 121.343°E), elev. 507m, Crocidura grayi Dobson (JAE2825), 25 V 2009, K. Dittmar and V. Tkach; 1♂ paratype (P2211), same data except Crocidura grayi (JAE2785), 22 V 2009. Holotype deposited in the Carnegie Museum of Natural History, Pittsburgh, PA and male paratype in the Brigham Young University flea collection, Monte L. Bean Life Science Museum, Provo, UT.
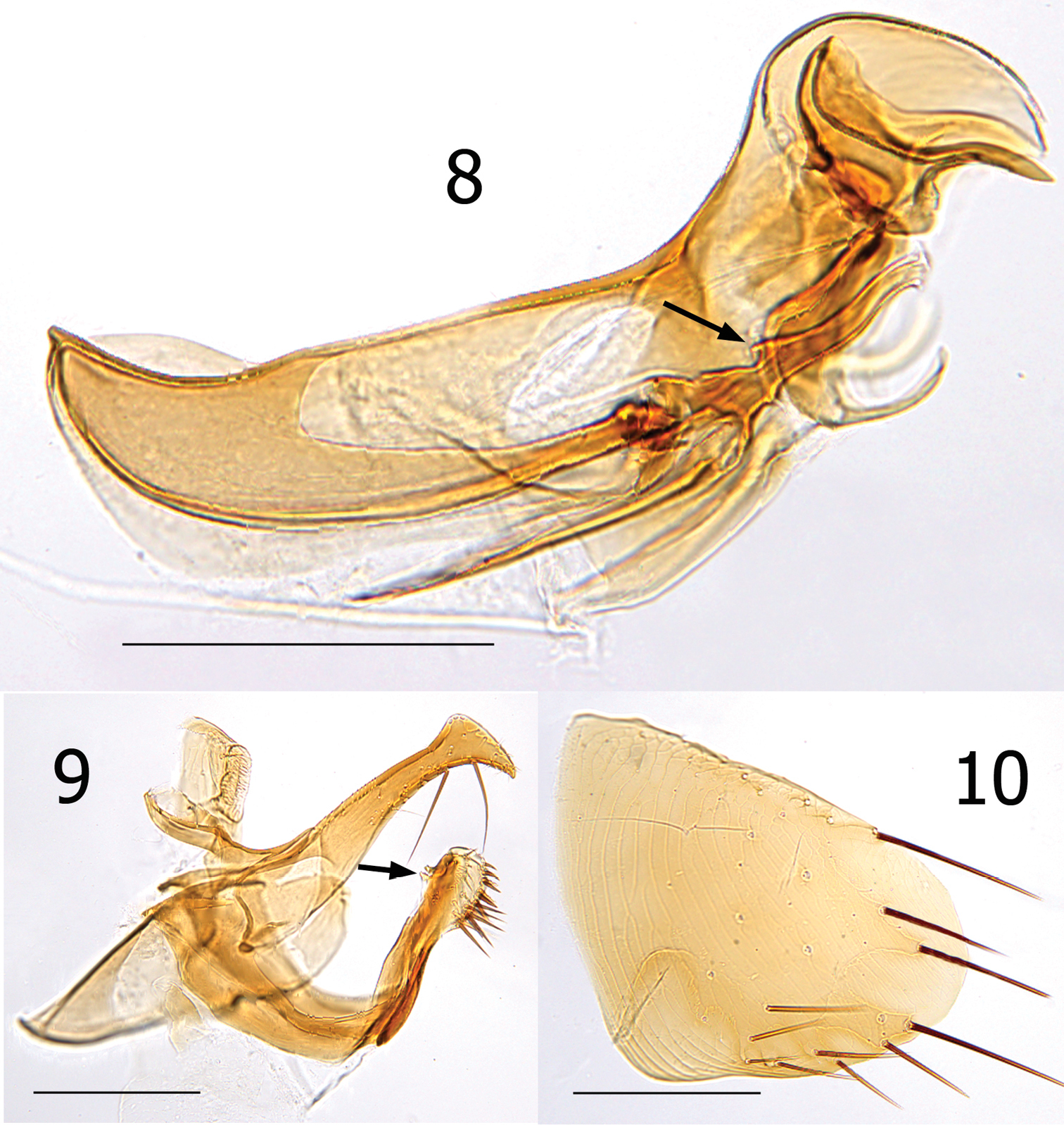
Female unknown. Male easily distinguished from all species except Lentistivalius aestivalis by the presence of a prominent spur along dorsal margin at base of sclerotized inner tube (Fig. 8). Further distinguished from Lentistivalius aestivalis by the narrow width of the distal half of the crochet (Fig. 8); width does not exceed width of sclerotized inner tube in the new species whereas it does in Lentistivalius aestivalis. Other distinguishing features include the shapes of the distal arm of S-IX, crochet, and Ford’s sclerite (Figs 8–9).
Numbers of setae described indicate only one side unless otherwise stated. Head (Figs 4, 6). Frons smoothly rounded; punctate area extensive anterior to frontal row of six moderately heavy setae. Ocular and genal rows: three setae each. Seven supernumerary setae between frontal row and ocular row. Two labral setae. Four minute setae line ventral rim of antennal fossa anterior to eye. Maxillary palpus extends to mid coxa; labial palpus of five segments (excluding basal segment); apical segment longest. Labial palpus extending ¾ length of forecoxa. Darkly pigmented eye contiguous with genal margin; with ventral sinus. Post-antennal area with four rows setae (3, 4, 1, 5 + intercalaries). Numerous setulae along antennal fossa. Four lateral setae on scape; four short apical setae on pedicel. Clavus not extending beyond caudal margin of head. Thorax (Figs 4, 5). Pronotum with 18 ctenidia (both sides); each outside tooth much smaller than others. Longest ctenidia twice length pronotum, about equal vertical length pronotum; each tooth divergent, curved dorsally. Two rows setae; anterior row with two setae. Meso- and metanota each with three rows setae. Metanotum with single sharp hyaline spine at ventrocaudal margin. Prosternosome without notch for 1st link-plate; not extended ventrally on ventral margin. Mesosternum reduced; extending ventrally between coxae as triangular projection. Mesepisternum with three setae; mesepimeron with six setae, single posterior seta largest. Pleural rod, bifurcate dorsally. Metasternum rounded; metepisternum with single large seta and single minute seta. Squamulum long, narrow. Pleural arch well developed; pleural ridge more robust dorsally. Well defined suture between lateral metanotal area and metepisternum. Metepimeron with three vertical rows setae (3, 4, 3), all below level pointed spiracular atrium; posterior setae longest. Legs (Figs 12–15). Forecoxa heavily adorned with setae. Anterior margins meso- and metacoxae with numerous setae along lower two thirds. Oblique lateral sulcus of mesocoxa complete. Two setae each guarding femorotibial joints of all three tibiae; outer short, spiniform, inner seta many times longer. Lateral surface of hind femur with coarse horizontal parallel sculpturing; mesal surface with broader vertical parallel sculpturing (perpendicular to longitudinal axis of femur). First tarsal segment of foreleg with unique set of three long setae along posterior margin. Tarsal segments 1-4 each leg progressively shorter (proximal to distal) than preceding segment. First tarsal segment hind leg nearly as long as segments 2–4. Dorsal margins all tibia with seven notches; setae per notch metatibia (2, 2, 1, 2, 2, 1, 3). Lateral surface metatibia covered with usual setae; none enlarged or shifted towards dorsal notches. Six lateral plantar bristles each distitarsus. Fifth segment of fore and mid distitarsi with four spiniform preapical plantar bristles; hind distitarsus with two small preapical plantar bristles. Three proximal lateral plantar bristles more robust than distal three pairs; third and fourth at same level, third inside and fourth outside. Unmodified Abdominal Segments (Figs 4, 7). Tergites I-VII with three rows setae; anterior row one to two setae. Terga II-V with single apical pigmented spinelet. Two antesensilial bristles; lateral twice length of mesal. Sternum II, three ventral setae; single small seta near rod-like fourth link-plate. Dorsocephalic margin S-II heavily sclerotized; incrassation at fourth link-plate. Sterna III-VII with four setae main row; 5–12 scattered setae preceding main rows. Modified Abdominal Segments (Figs 4, 8–10). Tergum VIII reduced, with two small dorsal setae; spiracle VIII large, equal to convex sensilium. Subsensilial sclerite present; bearing two setae. Sternum VIII largely covering T-IX, S-IX, and aedeagus; numerous setae on apical two thirds. Proximal arm of S-IX apically broad and blunt, fused with manubrium. Distal arm of S-IX strongly sclerotized along ventral margin; apex expanded, club-like. Club with small lateral patch of setulae; oblique line of eight setae (distal six fine, proximal two long, pigmented), ventroapical margin with six setae (distal four short, spiniform, proximal two long, all darkly pigmented). Lacking apical lobe; subapical lobe present on anterior margin. Terminal portion of basimere of T-IX bilobed; L1 modified long extension of apodeme of T-IX paralleling telomere and L2 large rounded lobe bearing two acetabular bristles (ventral short and dorsal long). Telomere narrows from proximal to stiva; stiva expanded dorsoapical angle forming a near right angle. Four long setae on ventral margin of stiva. Fulcral sclerite truncate; very developed. Aedeagus (Fig. 8). Aedeagal apodeme broad, upturned apically. Dorsal margin with thick sclerotization preceding arched median dorsal lobe. Crescent sclerite small, capsule small, satellite sclerite thin, short. Y sclerite reduced. Penis rods thick, short; not reaching end of aedeagal apodeme. Virga ventralis short, thick; half length of aedeagal apodeme. Sclerotized inner tube undulate; prominent dorsal spur at base. Ventral armature absent. Crochet broad at base, abruptly narrowing, scythe-like. Phylax thick, sclerotized. Alpha portion of Ford’s sclerite massive; securifer sharp, hook-like. Tendon of phylax and Ford’s sclerite visible.
Lentistivalius philippinensis sp. n., male paratype (P2211). 8 Aedeagus 9 Tergum IX and Sternum IX 10 Sternum VIII. (Scale: 200µ).
Lentistivalius philippinensis sp. n., male holotype (P2316). Sensilium, dorsal and ventral anal lobes, and subsensilial sclerite (arrow). (Scale: 100µ).
Lentistivalius philippinensis sp. n., male paratype (P2211). 12 Lateral of hind femur, longitudinal parallel sculpturing 13 Mesal view of hind femur, vertical parallel sculpturing 14–15 Lentistivalius philippinensis, sp. n., male holotype (P2316) 14 Hind tibia 15 Hind tarsi. (Scale: Figs 12–13 = 100µ; Figs 14–15 = 200µ)
The new species bears the name of the country from which it was collected.
Seven species of Lentistivalius are currently recognized (including this new species). Lentistivalius is primarily a parasite of Southeast Asian murids and soricids, although one species (Lentistivalius insolli) is definitively a bird parasite documented from 18 different species of birds (
| l | Pronotum with one row of setae | 2 |
| 1’ | Pronotum with two rows of setae (anterior row may only be comprised of one or two small setae dorsally) | 7 |
| 2(1) | Males | 3 |
| 2’ | Females | 5 |
| 3(2) | Spiniform setae on ventroapical margin of the distal arm of S-IX appear grouped in a dense patch (India, Nepal, Sri Lanka) | Lentistivalius ferinus |
| 3’ | Spiniform setae dispersed evenly in a row | 4 |
| 4(3’) | Ventroapical margin of distal arm of S-IX flat in lateral aspect (Tanzania, Zaire) | Lentistivalius alienus |
| 4’ | Ventroapical margin convexly rounded (China) | Lentistivalius occidentayunnanus |
| 5(2’) | Middle lobe on caudal margin of S-VII large and acutely triangular (China) | Lentistivalius occidentayunnanus |
| 5’ | Middle lobe smaller and not acutely triangular | 6 |
| 6(5’) | Caudal margin of S-VII with strongly lobed ventral lobe; several setae on lobe (India, Nepal, Sri Lanka) | Lentistivalius ferinus |
| 6’ | Lobe weakly indicated; setae clearly not present on weak lobe (Tanzania, Zaire) | Lentistivalius alienus |
| 7(1’) | Males | 8 |
| 7’ | Females (females of Lentistivalius philippinensis unknown) | 11 |
| 8(7) | Combination of a substantial spur at the base and dorsal surface of the sclerotized inner tube (s.i.t.) and the width of the distal half of the crochet no wider than the width of the s.i.t. (Luzon, Philippines) | Lentistivalius philippinensis sp. n. |
| 8’ | Sclerotized inner tube with, or without spur; if spur is present, distal half of crochet is distinctly wider than s.i.t. | 9 |
| 9(8’) | Stiva of telomere with an angular bulge (near right angle) at dorsoapical angle; not rounded as usual (Japan) | Lentistivalius aestivalis |
| 9’ | Stiva without angular bulge; evenly rounded | 10 |
| 10(9’) | Pronotal ctenidia arranged close together and not noticeably reflexed upward towards their apices (Malaysia, Vietnam) | Lentistivalius insolli |
| 10’ | Pronotal ctenidia separated slightly; diverging towards apices (Borneo) | Lentistivalius vomerus |
| 11(7’) | Caudal margin of S-VII without distinct sinus (Malaysia, Vietnam) | Lentistivalius insolli |
| 11’ | Caudal margin of S-VII with deep sinus (as deep as wide) | 12 |
| 12(11’) | Undulate dorsal lobe on caudal margin of S-VII with single subtending sinus (Borneo) | Lentistivalius vomerus |
| 12’ | Dorsal lobe subtended by two sinuses, each separated by a lobe (Japan) | Lentistivalius aestivalis |
We thank N. Antoque, J. A. Cantil, K. Dittmar, S. Knutie, V. Tkach, S. Villa, and J. Esselstyn for assistance in collecting specimens used in this study. We also express our appreciation to J. Esselstyn for providing crucially important mammal identifications. To Scott Villa we are indebted for his permission to use the parasitized bat wing images that he photographed on-site. This study was partially funded by NSF 0743491, Biotic Survey & Inventories to R. Brown, S. E. Bush, D. H. Clayton, and R. G. Moyle.