(C) 2013 John D. Taylor. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Taylor JD, Glover EA (2013) New lucinid bivalves from shallow and deeper water of the Indian and West Pacific Oceans (Mollusca, Bivalvia, Lucinidae). ZooKeys 326: 69–90. doi: 10.3897/zookeys.326.5786
Four new species and a new genus of lucinid bivalves are described from shallow and deeper waters in the Indian and West Pacific Oceans. The new genus Scabrilucina (subfamily Lucininae) includes the little-known Scabrilucina victorialis (Melvill, 1899) from the Arabian Sea and Scabrilucina vitrea (Deshayes, 1844) from the Andaman Sea as well as a new species Scabrilucina melvilli from the Torres Strait off northeastern Australia. Ferrocina brunei new species (Lucininae) was recovered from 60 m near oil drilling activities off Borneo; its anatomy confirmed the presence of symbiotic bacteria. Two unusual deeper water species of Leucosphaerinae are described, both species included in on-going molecular analyses; Gonimyrtea ferruginea from 400–650 m in the southwest Pacific and Myrtina reflexa from 200–825 m off Zanzibar and Madagascar.
Bivalvia, Lucinidae, chemosymbiosis, taxonomy, new species, deep water, Indo-West Pacific
Over the last 20 years there has been much taxonomic interest in the chemosymbiotic bivalve family Lucinidae with many new genera and species described from shallow to bathyal depths of the tropical Indo-West Pacific (
In this paper a new genus is introduced to accommodate Lucina victorialis (Melvill, 1899) previously known from just a few valves collected in the Arabian Sea in the late 1800s but classified initially as Cryptodon (i.e. Thyasiridae) because of the deep sulcus and trigonal shape. Discovery in the Museum of Comparative Zoology, Harvard University of a large sample collected in the Arabian Sea as part of the 1963 International Indian Ocean Expedition has allowed re-description and assessment of the unusual features of this species. We also include within this genus the neglected species Lucina vitrea Deshayes, 1844, with new records from Southeast Asia, and another species with similar characters is newly described from the Torres Strait off northeastern Australia.
Benthic sampling at about 60 m depth off Brunei in north Borneo recovered from near oil drilling activities an unusual, small, mottled red-brown lucinid that on shell characters can be classified as a new species of Ferrocina a genus previously known from the rare type species Ferrocina multiradiata Glover & Taylor, 2007 from off Fiji and New Caledonia and another species from the Philippines (
In a molecular analysis of Lucinidae we included an individual of an undescribed genus and species dredged from over 600 m on the Chesterfield Bank west of New Caledonia (UGS-3 in
AM Australian Museum, Sydney
MCZ Museum of Comparative Zoology, Harvard University
NHMUK The Natural History Museum, London
MNHN Muséum national d’Histoire Naturelle, Paris
UMC Zoological Museum, University of Copenhagen
USNM United States National Museum of Natural History
Cryptodon victorialis Melvill, 1899. Here designated. Northern Indian Ocean.
Small to medium size, L to 40 mm, thin, semi-translucent, usually slightly higher than long, ovoid to subtrigonal, strong posterior sulcus with broad sinus at posterior margin. Sculpture of sharp, fine, commarginal lamellae. Ligament short, set in shallow resilifer. Hinge with small to vestigial cardinal teeth in both valves, lateral teeth usually absent but small anterior lateral tooth may be present in juvenile shells. Anterior adductor muscle scar long, thin, ventrally detached from pallial line for ½–¾ of length, dorsal part runs on to hinge. Interior shell with translucent spots.
Derivedfrom Latin scaber rough and Lucina, reference to the rough surface formed by closely spaced, sharp, commarginal lamellae. Feminine.
Scabrilucina can be readily distinguished from other lucinids by the subtrigonal outline with a prominent posterior sulcus, the fine, regular, sharp-edged, commarginal lamellae and the absence of radial sculpture. No preserved samples of Scabrilucina were available for molecular analysis but a small lucinid from the Philippines with some similar characters was included in a prior analysis (
The little known genus Semelilucina Cosel & Bouchet, 2008, type species Semelilucina semeliformis from offshore muddy habitats in Tanimbar, SE Indonesia, may be related to Scabrilucina. The two genera differ in shell shape but have similar sculpture of fine, regularly spaced, sharp commarginal lamellae.
http://species-id.net/wiki/Scabrilucina_victorialis
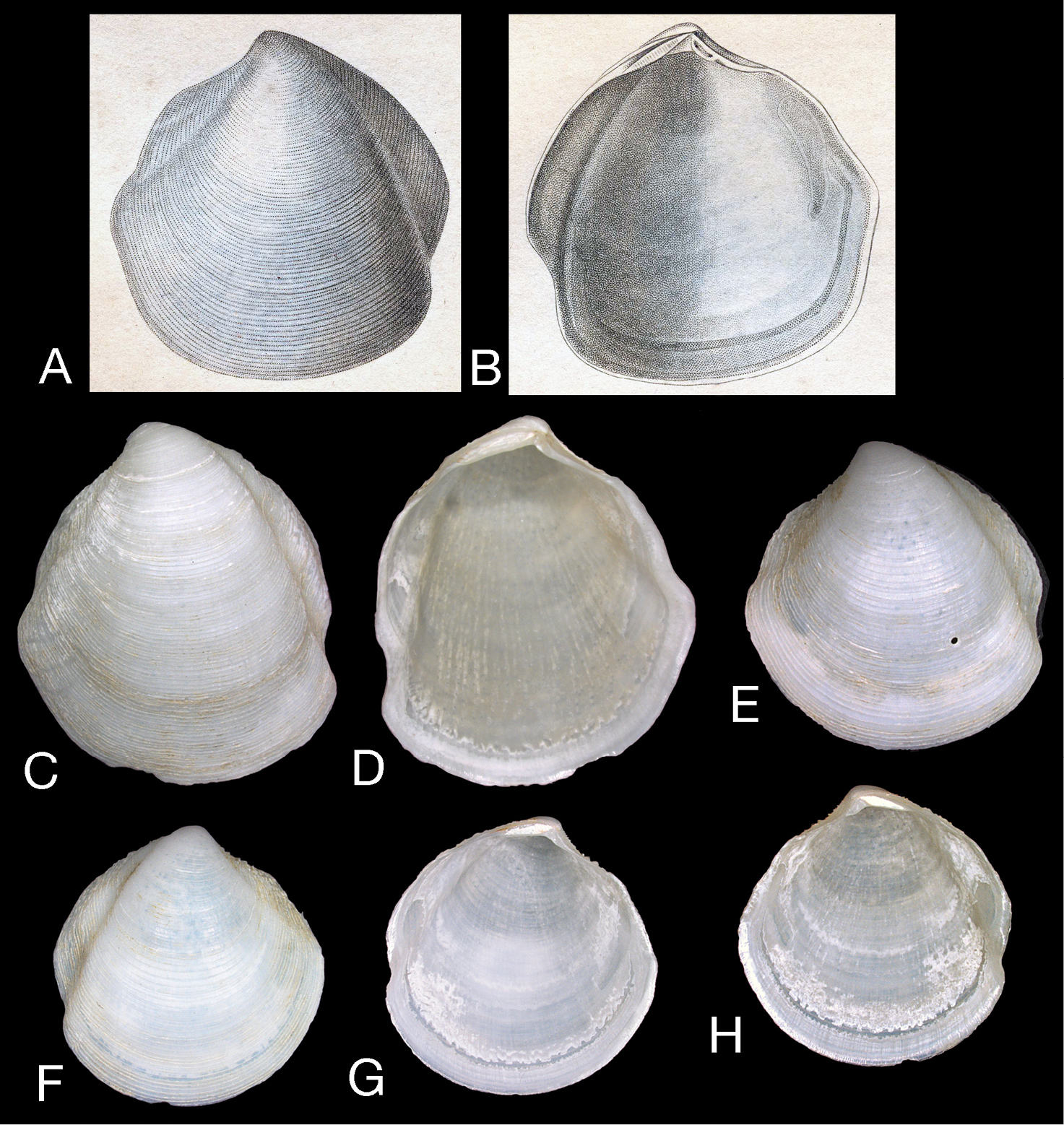
Figs 1–2, 3AHolotype, left valve (NHMUK 1899.12.18.28), H 25.3 mm, L 23.5 mm.
Northern Arabian Sea: 141 valves (MCZ 362493), 47 miles E of Duhat Sharjah, Oman, Arabian Sea, 50.5–52 fthms (92–95 m), Anton Brunn Cruise, 4b stn 255A (25°50'N, 57°07'E, 30 November 1963). 2 valves (USNM 716871), localityas above, Anton Brunn station 255A. 1 valve (NHMUK 99.2.18.10)25 fthms (45.7 m), 26°23'N, 54°53'E, Melvill collection. 1 valve (NHMUK) off Gwadhur (Gwadar), Pakistan, 70 fthms (128 m), Townsend collection. 4 valves, NHMUK, Gulf of Oman, Townsend collection. 1 valve (NHMUK 1906.10.12.90) Arabian Gulf, 47 fthms, Investigator station 346, (
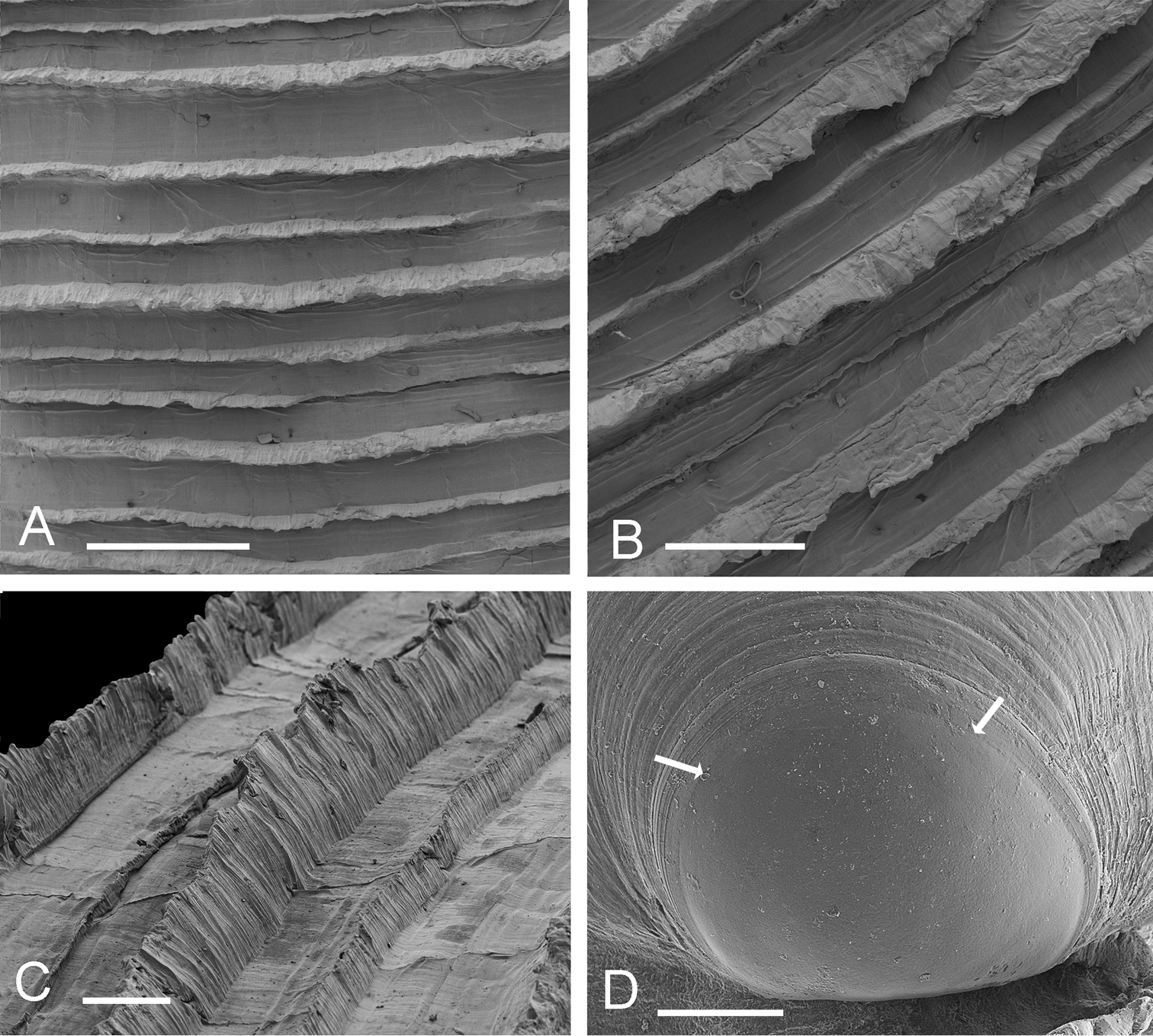
Shell white, thin, often semi-translucent, H to 43 mm, L to 39 mm, higher than long, H/L = 1.03±SD 0.03 (n=17), moderately inflated T/L 0.27±SD 0.014 (n=17), outline subtrigonal in larger shells, juvenile shells subcircular. Posterior sulcus, prominent, deeply incised, with marginal sinus; anterior sulcus narrow and shallow. Sculpture of fine, closely spaced (200–500 µm apart), thin, sharp-edged, striated, commarginal lamellae (Figs 2A–C) that narrow to around 30 µm at the distal edges and are slightly elevated along the posterior and anterior dorsal margins. Fine sediment is frequently trapped between lamellae. Protoconch (Fig. 2D): PI 150 mm, PI + PII 165 µm, PII with 2-3 growth increments. Lunule long, lanceolate and asymmetric, slightly larger in left valve. Ligament short, set in narrow groove. Hinge plate narrow, LV with 2 cardinal teeth, the anteriormost reduced and often obscured by ventral extension of lunule (Fig. 1M–P), lateral teeth absent; RV with 1-2 small cardinal teeth, sometimes obscure, small anterior lateral tooth sometimes visible in younger shells (Fig. 1P). Anterior adductor muscle scar long, thin, tapering at ventral tip, detached for ½–⅔ length and diverging ventrally at angle of 25–30° from pallial line, posterior adductor scar ovate. Pallial line broad, continuous, inner shell surface rough with many small translucent circular spots representing mantle attachment points and prominent radial grooves. Track of pallial blood vessel visible. Inner shell margin smooth.
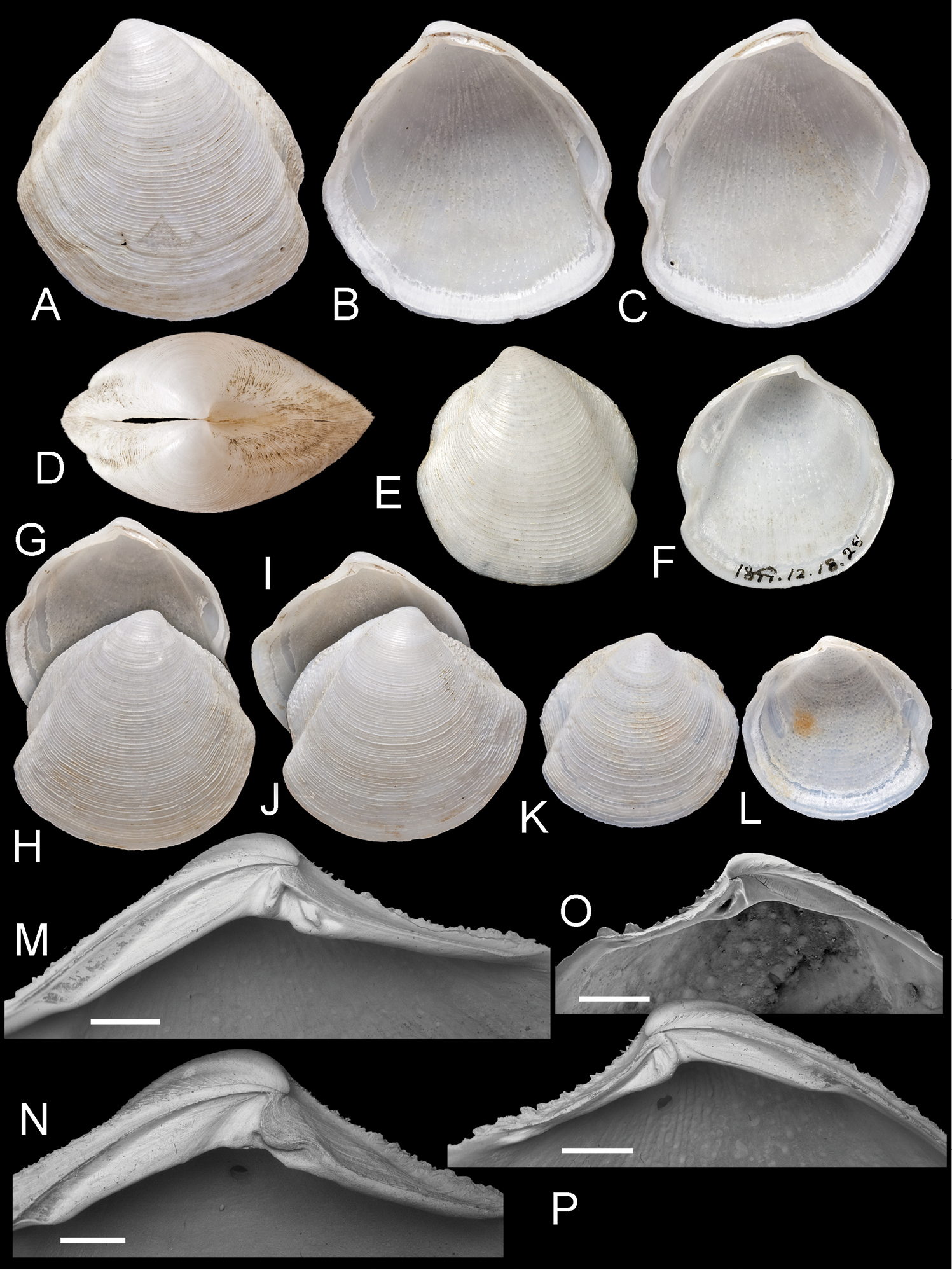
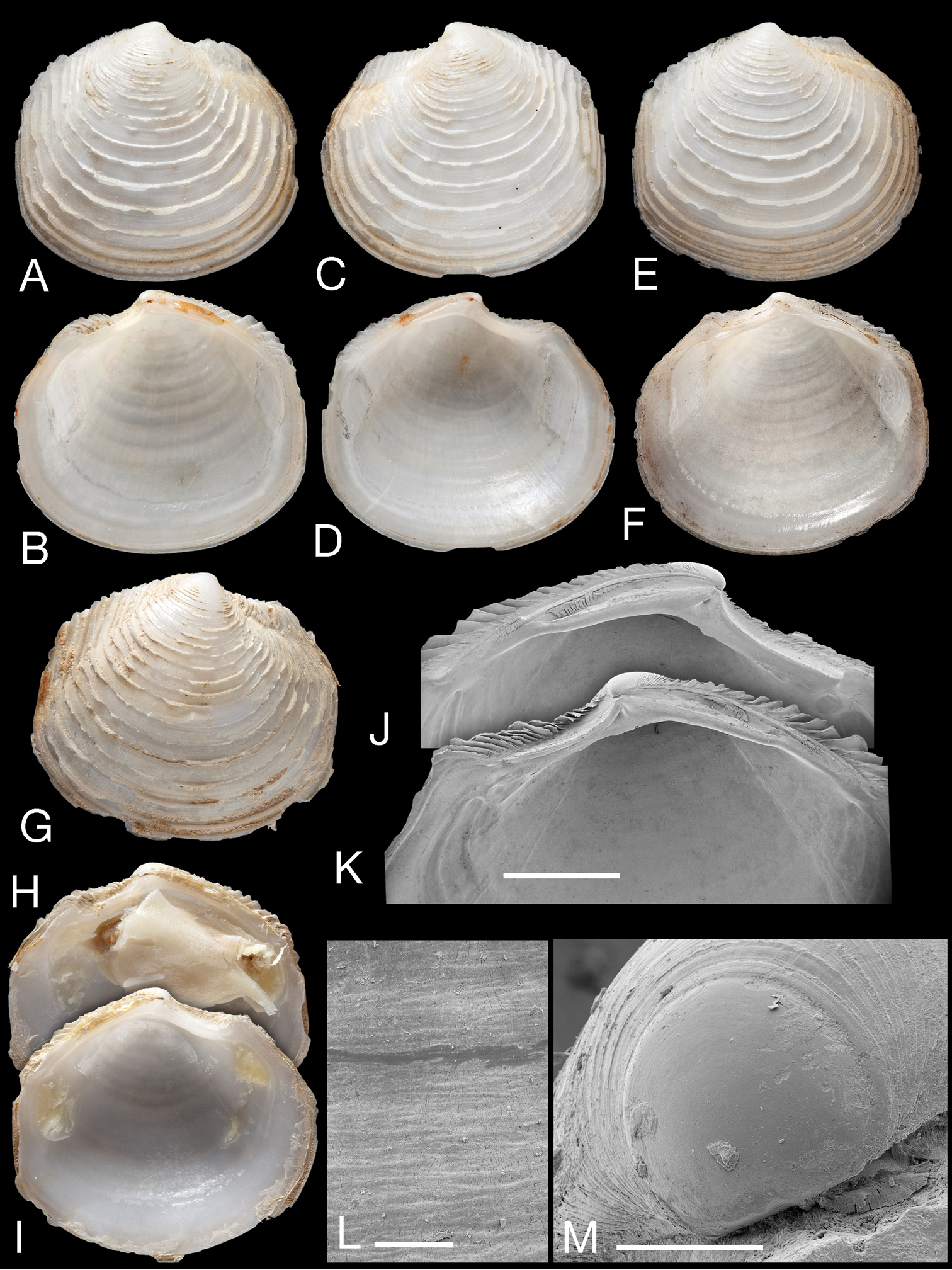
Scabrilucina victorialis (Melvill, 1899) except where otherwise stated all specimens MCZ 362493. A–C exterior of left valve and interiors of right and left valves, L = 40.4 mm D Dorsal view of A–C E–F Holotype Cryptodon victorialis Melvill, 1899, NHMUK 1899.12.18.28 exterior and interior of left valve, L = 23.5 mm G–H Interior and exterior of right valve L = 32.1 mm I–J Interior and exterior of right valve, L = 36.2 mm K–L Exterior and interior of juvenile right valve, L= 22.9 mm M Detail of hinge teeth of left valve. Scale bar = 1 mm N Hinge of left valve Scale bar = 1 mm. O Hinge of right valve juvenile shell, NHMUK Scale bar 1 mm P Hinge of right valve. Scale bar = 1 mm.
Scabrilucina victorialis shell features. A–C MCZ 362493 A Commarginal lamellae. Scale bar = 1 mm. B Detail of commarginal lamellae. Scale bar = 500 µm. C Detail of commarginal lamellae. Scale bar = 200 µm. D Protoconch NHMUK. Arrows mark boundary between PI and PII. Scale bar = 50 µm.
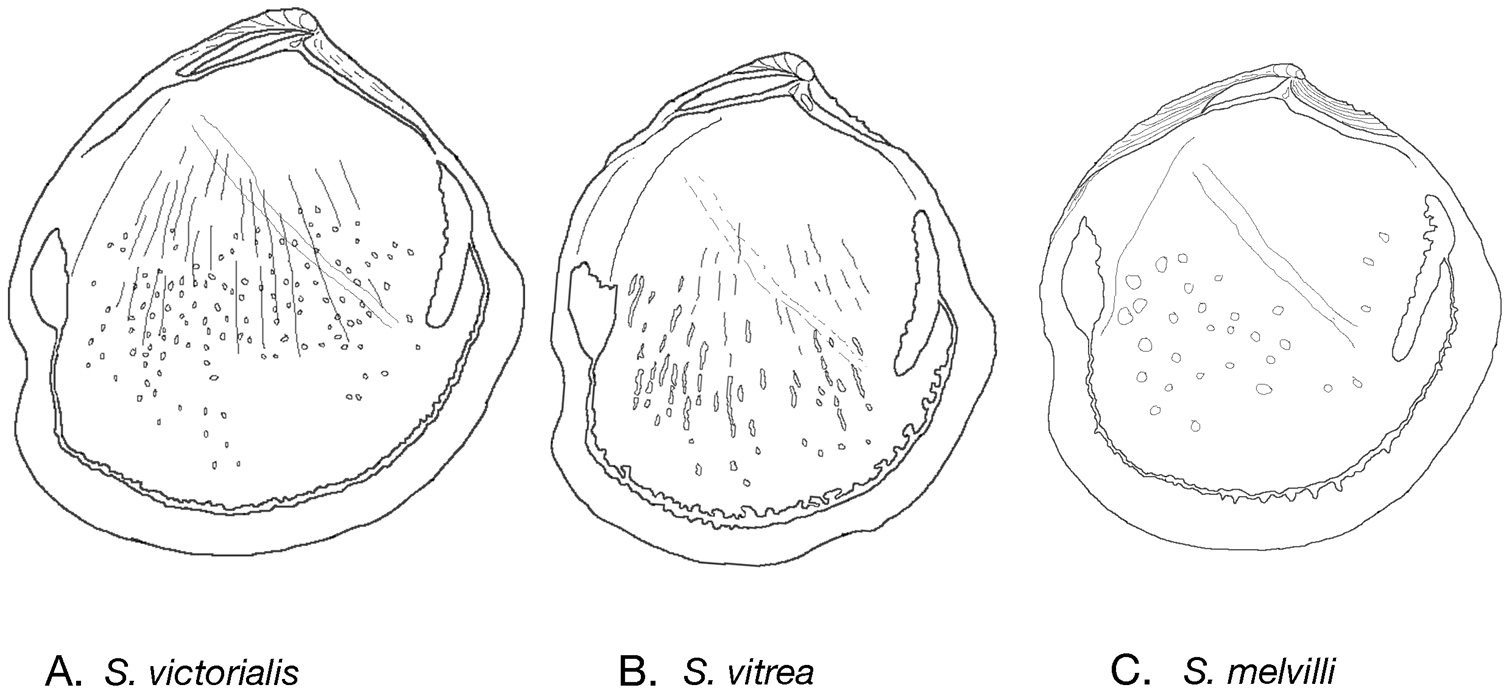
Internal drawings Scabrilucina species. A S. victorialis B Scabrilucina vitrea C Scabrilucina melvilli.
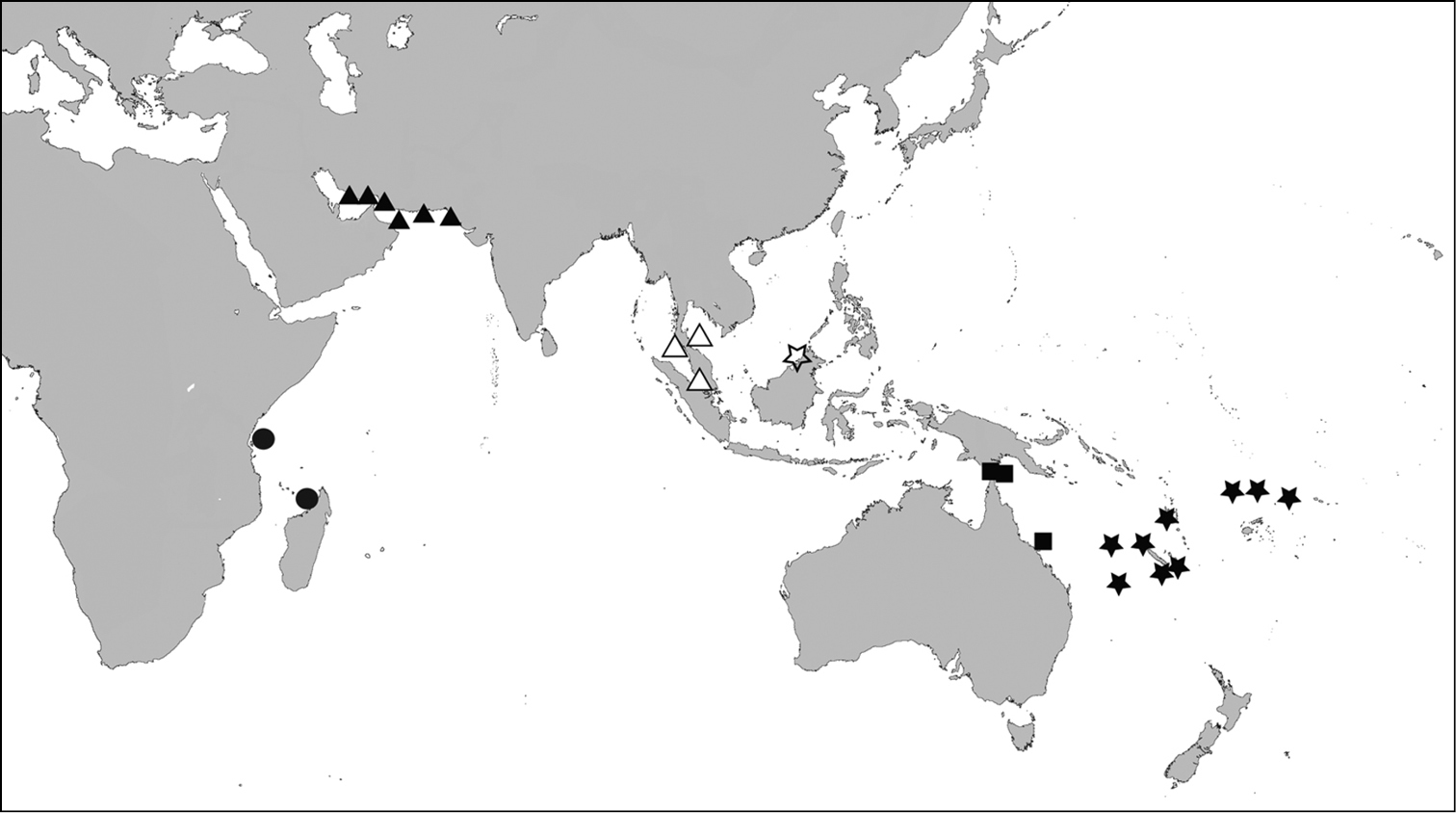
50 – ca 150 m in offshore muds, northern Arabian Sea, Gulf of Oman, northeastern Arabian Gulf (Fig. 4).
Distribution map - Scabrilucina victorialis, solid triangles; Scabrilucina vitrea, open triangles; Scabrilucina melvilli, solid squares; Gonimyrtea ferruginea, solid stars; Myrtina reflexa, solid circles, Ferrocina brunei open star.
This species is known only from shells. Scabrilucina victorialis is characterised by the deep cleft of the posterior sulcus and the fine, sharp, commarginal lamellae. Scabrilucina vitrea (see below) from off Sumatra and Gulf of Thailand is smaller, taller, and thinner shelled. The shell shape and the deep posterior sulcus superficially resemble some Thyasiridae such as Conchocele and this influenced Melvill’s initial placement in Cryptodon. Scabrilucina victorialis is also similar to Scabrilucina melvilli (new species below) from Australia that is distinguished by its smaller size, less deeply incised posterior sulcus and more widely spaced commarginal lamellae. Many of the shells of Scabrilucina victorialis from off Oman (MCZ 362493) and Scabrilucina vitrea from Thailand are penetrated by narrow, straight-sided holes ca 450 µm diameter (Figs 1A, C, 5E) comparable with those resulting from octopus predation (
Scabrilucina vitrea (Deshayes, 1844) A–B Scanned images of the original illustrations of Lucina vitrea from Deshayes, 1844 pl. 106, length 22 mm C–H specimens from Andaman Sea, Thailand (ZMC) C–D Exterior and interior left valve L = 21.9 mm E Exterior of left valve, L = 16.8 mm F–H Exterior of RV, interiors of left and right valves, L = 16.8 mm.
http://species-id.net/wiki/Scabrilucina_vitrea
Figs 3B, 5Not located.Original descriptionL 22 mm, H 20 mm.
‘les mers de Sumatra’
Thailand: 2 paired valves and 5 valves (ZMC), Andaman Sea, 27-35 m, 7°00'15"N, 99°21'42"E, 5th Thai Danish Expedition stn 1052 & 1054, sandy mud with dead shells, 10 February 1966. 1 valve (ZMC) Gulf of Thailand, 30 fthms (54 m) 10°04.10'N, 100°10.1E, Naga Expedition 1960, stn 60–853. Malaysia: 1 valve (NHMUK 163529), Malacca, Cuming Collection.
Shell white, L to 22.0 mm, H to 25.1 mm, slightly higher than long, H/L 1.08 ± SD 0.05 (n=5), very thin shelled, translucent, prominent posterior and anterior sulcus, umbones prominent. Sculpture of fine, thin commarginal lamellae, lamellae slightly irregular and elevated at posterior and anterior dorsal area, radial sculpture absent. Lunule broad, lanceolate, slightly impressed. Hinge extremely narrow, right valve with single cardinal tooth, lateral teeth absent, left valve with 2 small cardinal teeth, lateral teeth absent. Anterior adductor scar long, narrow, pointed, detached for 2/3rds of length, posterior scar ovoid, pallial line entire with dorsal elevations, close to shell margin, inner shell surface with many fine points of mantle attachment, shell surface glossy outside the pallial line.
Andaman Sea, Straits of Malacca and Gulf of Thailand (Fig. 4).
Similar to Scabrilucina victorialis but smaller and less trigonal in outline of the adult shell, with finer commarginal sculpture and thinner shell.
The type material of Scabrilucina vitrea has not been located but the original figures of Deshayes are clear (Figs 5A, B). The type locality of Sumatra is close to that of the other samples mentioned here. As far as we are aware, except for a listing in
http://zoobank.org/EC1E8F2B-0F9E-4DE1-8DE7-4C26695A61FB
http://species-id.net/wiki/Scabrilucina_melvilli
Figs 3C, 6Holotype: 1 whole shell (AM C. 360708), H 21.2 mm, L 21.0, T (single valve) 5.2 mm. Paratypes: single left valve, H 20.5mm, L 18.3 mm, juvenile shells 4 LV and 4 RV, locality as holotype (AM C. 479181).
Northeastern Australia, Queensland, E of Bowen 19°45.7'S, 148°19'E, 46 m, thin, grey mud, coll. PH Colman & F Rowe.
Australia, Queensland: 6 valves (AM C. 036166), Albany Passage, Cape York Peninsula, 10°45'S, 142°37'E, 4–14 fthms (7–26 m), mud & sand, coll. C. Hedley & A. Mc Culloch. (3 LV, 3 RV+ juveniles. Left valves – H 26.3 mm, L 24.3 mm; H 24.3 mm, L 23.2 mm; H 23.3 mm, L 20.9 mm; right valves - 19.0 mm L 18.6 mm; H 18.7 mm, L 17.2 mm). 1 valve (AM C. 360707), Horn Island, Torres Strait, 10°35.6S, 142°14.6'E, mangroves and sand flats, near jetty, coll. W.F. Ponder & I. Loch. (1 LV H 18.6 mm, L 17.3 mm).
Shell fragile, thin, white, semi-translucent, subtrigonal, L to 26.3 mm, H to 26.3 mm, higher than long H/L = 1.05 ± SD 0.04 (n = 9), moderately inflated T/L = 0.27 ± 0.02 (n = 9). Posterior sulcus broad, shallow with marginal sinus. Anterior dorsal area weakly defined. Sculpture (Fig. 6K, L) of regularly spaced, low, thin, sharp, commarginal lamellae with faint radial folds in interspaces. Commarginals more closely spaced in larger specimens. Commarginal lamellae elevated to scales along dorsal margin. Protoconch (Fig. 6M): 150 µm, PII a narrow rim. Lunule short, lanceolate, with more prominent growth increments in LV. Ligament short, set in groove. Hinge plate narrow (Fig. 6I, J), LV with two small cardinal teeth, anterior tooth larger, RV with single cardinal tooth, in larger specimens teeth sometimes obscure. Small anterior lateral present in RV of juveniles. Anterior adductor scar long, thin, detached ventrally from pallial line for ¾ of length at angle of 25°. Posterior adductor scar ovoid. Pallial line narrow, entire. Shell inside pallial line with small round points of mantle attachment, radial grooves, track of pallial blood vessel visible as shallow groove. Shell margin smooth.
Scabrilucina melvilli sp. n. A–C Holotype AMS C. 360708 exterior of left valve and interior of left and right valves, L = 21 mm D–E paratype AMS C. 360708 exterior and interior of left valve, L= 18.3 mm F–G paratype AMS 036166 exterior and interior of left valve, L= 23.1 mm H Dorsal view of holotype I–J Detail of hinge teeth of right and left valves of juvenile shells. Scale bar = 1 mm K External sculpture. Scale bar = 1 mm L Detail of commarginal lamellae. Scale bar = 200 µm M Protoconch. Arrow marks boundary between PI and PII. Scale bar = 50 µm.
Torres Strait, northeastern Australia (Fig. 4) shallow water mud to 50 m.
See above for comparison with Scabrilucina victorialis.
Named for James Cosmo Melvill (1845–1929), British malacologist who described many IWP species. Noun in genitive case.
Ferrocina multiradiata Glover & Taylor, 2007 by original designation.
Shell to 20 mm, thin, subovate, posteriorly truncate, sculpture of numerous fine, often indistinct radial ribs crossed by fine commarginal threads. Hinge plate thin, small single cardinal tooth in RV, two cardinals in LV, lateral teeth very small or obsolete. Anterior adductor scar short, detached for 1/3 of length. Interior shell margin finely to coarsely dentate. Colour pale orange to rusty red-brown, sometimes blotchy.
The unusual and rare genus Ferrocina was first recognised from Vanuatu, Fiji and New Caledonia at depths from 80–400 m (
http://zoobank.org/1954D59B-0F70-481B-9FE9-159FDF23DD24
http://species-id.net/wiki/Ferrocina_brunei
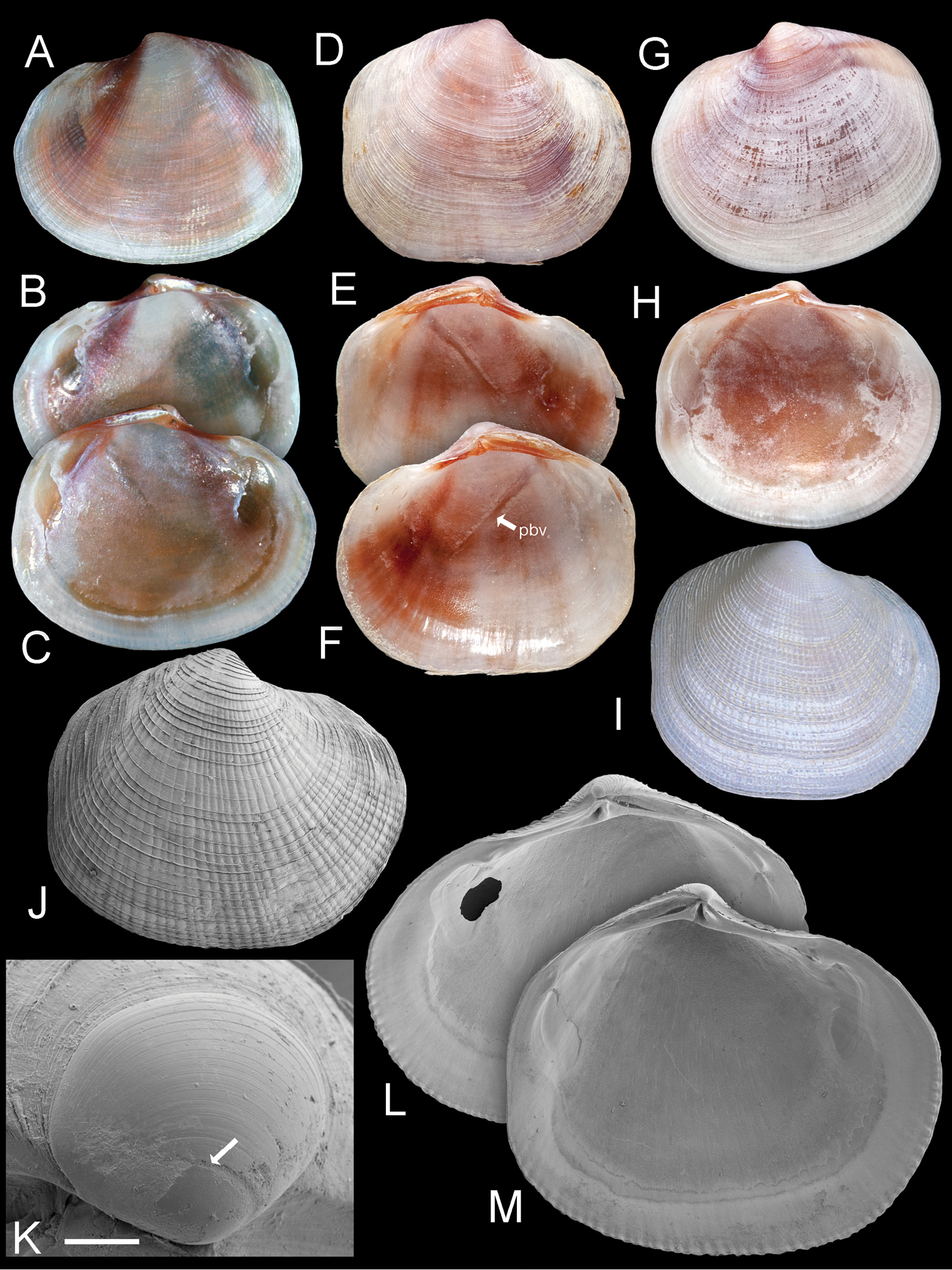
Figs 7, 8, 9CHolotype: whole shell NHMUK 20130122 L 8.2 mm, H 6.7 mm, T 1.6 mm; Paratypes: NHMUK 20130123, figured L 8.4 mm, H. 7.3 mm, T 2.1 mm; L 8.9 mm, H 7.8 mm, T 2.1 mm, non figured 19 v.
Brunei, 05°21'12"N, 111°26'21"E, 63 m, muddy sand near oil drilling rig.
Shell small, H to 7.8 mm, L to 8.9 mm, T to 2.1 mm, longer than high H/L = 0.89 ± 0.034 (n=13). Colour grey-white with patches, streaks or stripes of rusty red, more pronounced dorsally, including lunule, occasionally whole shell red-brown; internally red brown particularly at anterior. Sculpture of numerous (ca 40) low, radial ribs that divide and intercalate, crossed by very fine, widely spaced commarginal lamellae. Shallow posterior sulcus and posterior dorsal area with slightly elevated commarginal lamellae, radial ribs absent. Anterior dorsal area also without ribs. Protoconch (Fig. 7K) PI = 82 µm, PI+ PII = 159 µm, PII with many growth increments. Lunule short lanceolate, slightly impressed, asymmetrical, greater part in right valve, brown coloured. Ligament set in shallow groove. Hinge plate thin (Figs 7L, M), RV with single cardinal tooth and small anterior and posterior lateral teeth; LV with two cardinal teeth, anteriormost is larger and faint sockets for anterior and posterior lateral teeth. Anterior adductor muscle scar broad, short, detached for ½ of length at an angle of 25°. Pallial blood vessel trace prominent, terminates ventral to anterior adductor scar. Pallial line entire. Inner shell margin denticulate.
Ferrocina brunei sp. n. A–C Holotype NHMUK 20130122 Exterior of left valve and interior of right and left valves, L = 8.2 mm D–F Paratype NHMUK 20130123 exterior of right valve and interior of left and right valves, L = 8.4 mm. pbv trace of pallial blood vessel G–H Paratype NHMUK 20130123 exterior and interior of left valve, L = 8.9 mm I Exterior of right valve of white form NHMUK 20130123, L = 7.9 mm J SEM of right valve L = 6.0 mm K Protoconch, arrow at PI /PII junction. Scale bar = 50 µm L–M Interior of right and left valves L = 5.2 mm.
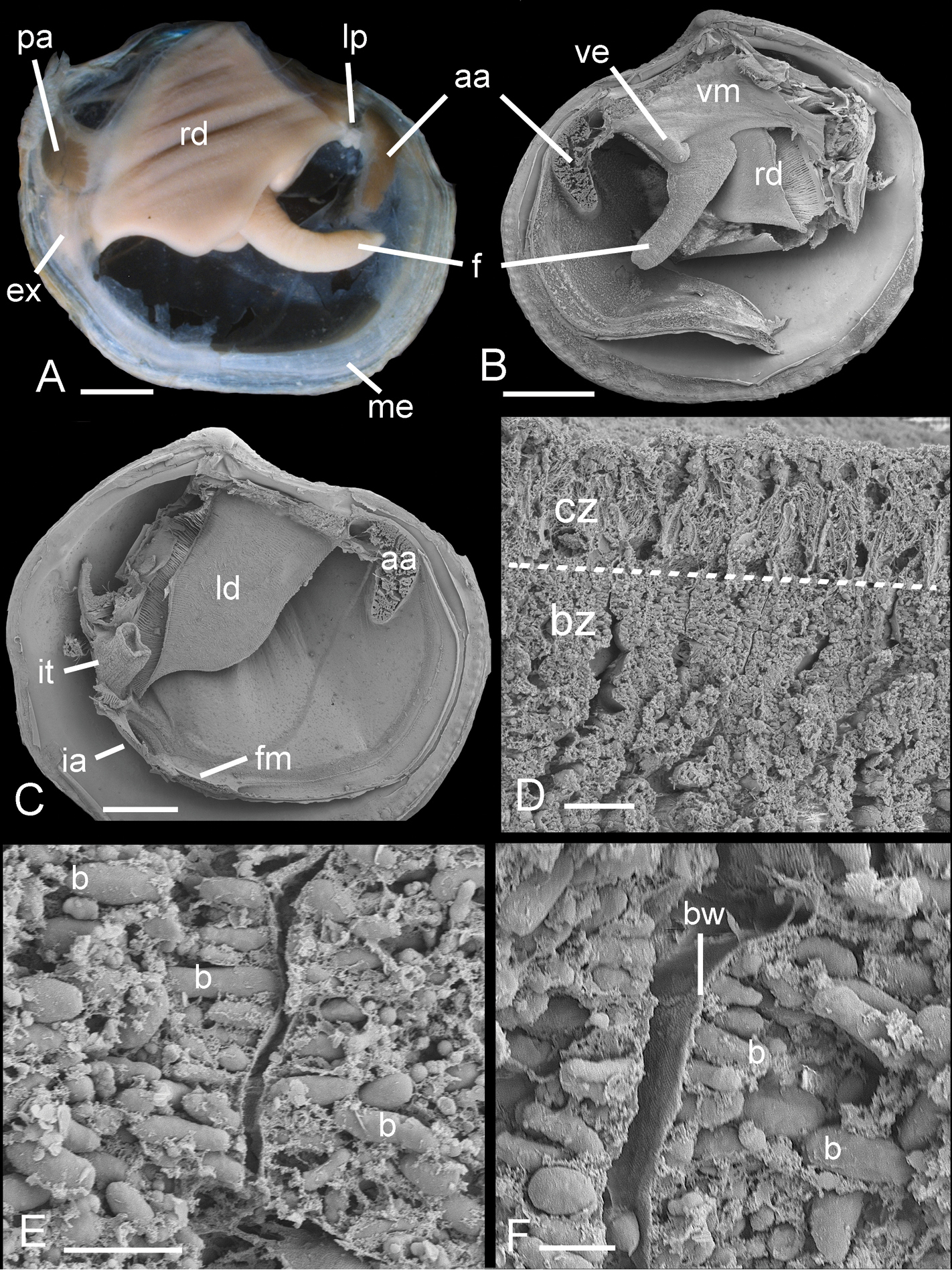
Ctenidia comprising inner demibranchs (Fig. 8A), pink, thick, occupying about ½ of mantle cavity. Foot cylindrical with small heel. Labial palps small ridges. Visceral mass anterior to foot laterally extended into pair of ramshorn-like coiled structures (Fig. 8B). Posterior exhalant aperture with inverted tube (Fig. 8C), inhalant aperture with small papillae, short section of fused mantle ventral to aperture. Ctenidial filaments with thick bacteriocyte zone with bacteriocytes packed with short rod-shaped bacteria 2-5 µm long and 1-2 µm wide (Figs 8D–F). Bacteria aligned with long axes normal to apical surfaces of bacteriocytes.
Ferrocina brunei sp. n. A Body from right side. Scale bar = 1 mm B Body from left side, mantle and left demibranch removed showing foot and visceral extension. Scale bar = 1 mm C Body from right side with visceral mass, right demibranch and mantle removed. Scale bar = 1 mm D Section through part of demibranch showing ciliated and bacteriocyte zones. Scale bar = 20 µm E Part of ctenidial filament in bacteriocyte zone showing bacteria. Scale bar = 5 µm F Detail of bacteriocytes and bacteria aligned normal to the apical cell wall. Scale bar = 2 µm. aa, anterior adductor muscle. b, bacteria. bw, bacteriocyte apical wall. bz, bacteriocyte zone. cz, ciliated zone. ex, exhalant aperture. f, foot. fm, fused mantle. ia, inhalant aperture. it, inverted tube of posterior exhalant aperture. ld, left demibranch. lp, labial palps. me, mantle edge. pa, posterior adductor muscle. rd, right demibranch. ve, visceral extension. vm, visceral mass.
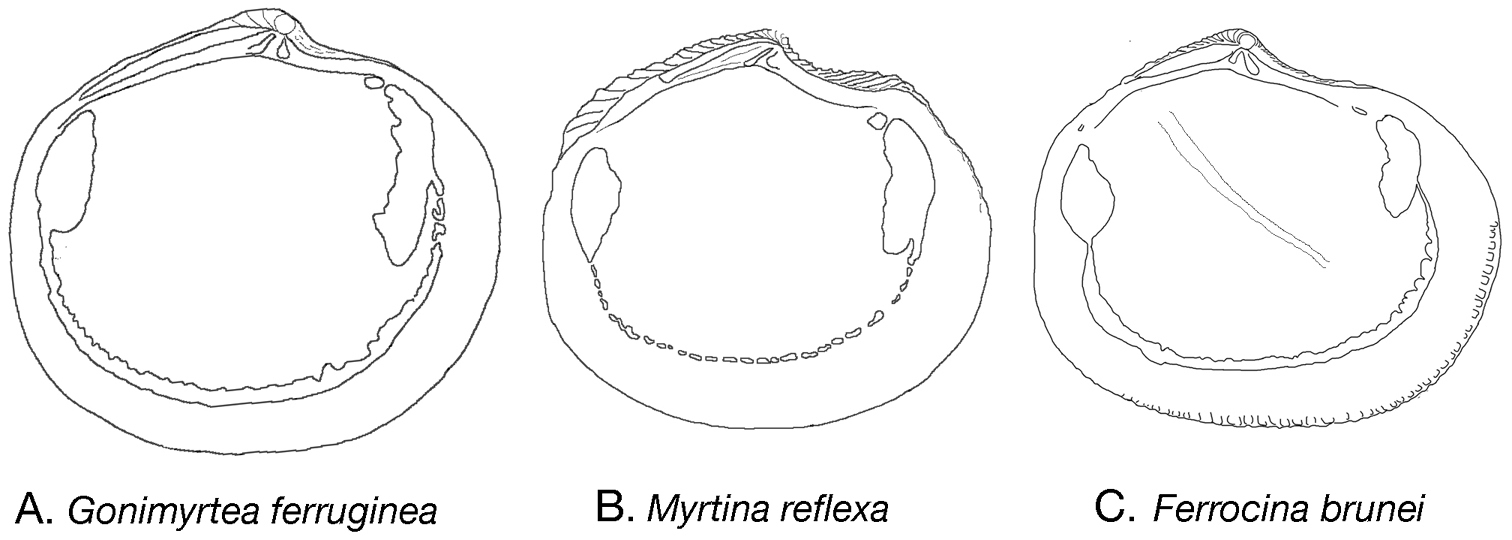
Internal outline drawings of A Gonimyrtea ferruginea B Myrtina reflexa C Ferrocina brunei. Not to scale.
Known only from type locality (Fig. 4).
Named for Sultanate of Brunei. Noun in apposition.
Ferrocina brunei is similar to the type species Ferrocina mutiradiata from Fiji but has much less prominent radial ribs, a less strongly denticulate shell margin and is smaller (shell length to 9 mm compared to 18 mm).
The anatomy is similar to most Lucinidae and bacterial symbiosis is confirmed by the presence of abundant bacteria in the ctenidial filaments. A distinctive feature of the anatomy is the bilateral ramshorn-like extension of the visceral mass anterior to the foot. We have observed similar structures only in Bathyaustriella thionipta Glover, Taylor & Rowden, 2004 from a hydrothermal vent on the Kermadec Ridge and in a Ferrocina species recently discovered off the southern USA in the Western Atlantic (unpublished observations). The function of this structure is unknown but thin sections of the structure in Bathyaustriella thionipta showed that it consisted of diverticula of the digestive gland.
Loripes concinna Hutton, 1885. Original designation.
Shells small, subcircular to ovate, higher than long, inflated. Sculpture of closely spaced thin, low commarginal lamellae. Right valve with single cardinal tooth and small anterior and posterior laterals sometime present; left valve with two cardinal teeth and small anterior and posterior laterals sometimes present. Lunule narrow lanceolate. Ligament short, curved. Anterior adductor scar detached for about 1/5–1/2 of length.
There has been much confusion concerning the concept of this genus following from
http://zoobank.org/4BD0BF6A-71CE-47B0-8C70-5D0D2D148BFB
http://species-id.net/wiki/Gonimyrtea_ferruginea
Figs 9A, 10Holotype: paired valves, live collected (MNHN IM-2009-10376) L 24.5 mm H 23.2 mm T whole shell 15.0 mm. Paratypes: 1 paired valve (MNHN IM-2009-10376) L 24.0 mm H 22.0 mm T 6.5 mm (single valve); 1 pv (MNHN IM-2009-10376) L 15.9mm H 14.2 mm T 7.7 mm.
New Caledonia, Chesterfield Bank, 19°35'S, 158°48'E, 680–722 m. N/O Alis campagne EBISCO 2005 stn CP2614.
All MNHN material from several deep water cruises in South west Pacific (for details see - www.mnhn.fr/musorstom/). New Caledonia, MUSORSTOM 4: 1 valve, stn CP158, 18°49'S, 163°15'E, 625 m. 2 valves, stn CP179 18°57'S, 163°14'E, 475 m.1 paired valve, stn CP 236, 22°11'S, 167°15'E, 495–550 m. BATHUS 2: 1 paired valve, stn DW740, 22°36'S, 166°27'E, 570-605 m. BATHUS 4: 1 valve, stn CP 900, 20°17'S, 163°50'E, 580 m. 2 valves, stn DW 908, 18°58'S, 163°11'E, 502–527m. 1 valve, stn CP905, 19°02'S, 163°16'E, 294–296 m. 1 valve, stn CP954, 21°44'S, 166°36'E 250–255 m. BIOCAL: 1 valve, stn DW40, 22°55'S, 167°24"E, 650 m. Chesterfield Bank, MUSORSTOM 5: 5 valves, stn 380, 19°38'S, 158°44'E, 555–570 m. 24 valves, stn 381, 19°38'S, 158°47'E, 620 m. Coral Sea, Kelso Bank, MUSORSTOM 5: 2 valves, stn 284, 24°10'S, 159°33'E, 225-230 m. Vanuatu, MUSORSTOM 8: 1 valve, stn DW1072, 15°40'S, 167°20'E, 622–625 m. SW Pacific, Wallis Island, MUSORSTOM 7: 3 valves, stn DW526, 13°13'S, 176°15'E, 355–360 m. 5 valves, stn DW527, 13°24'S, 176°15'W 540–560 m. 10 valves, stn DW 528, 13°24'S, 176°13'W, 435–515 m. 8 valves, stn DW601, 13°19'S, 176°17'W, 350 m. Bank Bayonnaise, MUSORSTOM 7: 1 valve, stn DW626, 11°54'S, 179°32W 597–600 m. 2 valves, stn CP629, 11°54'S, 179°32'W, 400–420 m. Tuscarora Bank, MUSORSTOM 7: 35 valves, stn DW556, 11°49'S, 178°18'W, 440 m.
Shell white, L to 25 mm H to 23.5 mm;subcircular, H/L 0.91 ± 0.017 (n=10), inflated T/L 0.28 ± 0.02 (n=10), sculpture of fine, low, rounded, regular, closely spaced, commarginal lamellae, radial sculpture absent, dorsal areas characteristically with ferruginous encrustations. Lunule short, lanceolate, symmetric. Protoconch (Fig. 10L): PI+PII 275 µm, PII, a narrow, 19 µm rim with fine increments. Ligament inset in narrow groove. Hinge (Figs 10J, K) LV with 2 cardinal teeth the posterior larger, can be obscure, lateral teeth not visible, RV with 2 cardinals, very small anterior lateral tooth, posterior lateral absent. Anterior adductor muscle scar long detached for ½ length, at an angle of 10–15°. Posterior adductor scar ovoid. Pallial line continuous, shell margin smooth.
Gonimyrtea ferruginea new species. A Holotype, MNHN IM-2009-10376 exterior of left valve. L = 24.5 mm B Holotype, dorsal view C Paratype MNHN IM-2009-10376 exterior right valve L = 15.9 mm D–E Paratype MNHN IM-2009-10376 exterior and interior of right valve, sequenced specimen L = 24.0 mm F–H Paratype MNHN XXXXXXX exterior of right valve and interior of left and right valves. New Caledonia MUSORSTOM 4 stn CP236, 495–550 m, L = 12.5 mm I–L from BATHUS 2: stn DW740, 22°36'S, 166°27'E, 570–605 mI Detail of sculpture, scale bar = 500 µm J, K Details of hinge teeth right and left valves. Scale bar = 1 mm. L Protoconch. Arrow marks boundary between PI and PII. Scale bar = 100 µm.
New Caledonia, Vanuatu, and banks and islands in SW Pacific, in deeper water between 350 and 650 m (Fig. 4).
Compared to the genotype, Gonimyrtea concinna from New Zealand, at depths to 200 m, Gonimyrtea ferruginea occurs in much deeper water, is larger, the lateral teeth are less distinct, and commarginal lamellae are more closely spaced. Another deeper water species of Gonimyrtea is known from the Philippines (
Both Gonimyrtea ferruginea and the new species from the Philippines typically have dense iron-rich encrustations on the dorsal areas of the shell (Fig. 10A–D). This also occurs in Dulcina guidoi from central Philippines at similar depths (
Molecular analyses show that Gonimyrtea ferruginea (as UGS-3 in
Derived from Latin ferrugineus meaning rust-coloured. Adjective in nominative singular.
Myrtina porcata Glover & Taylor, 2007 original designation.
Shell small, L to 16 mm, subcircular, posterior margin quadrate, sculpture of commarginal lamellae, elevated commarginal lamellae on dorsal margin, radial sculpture absent, lunule often strongly asymmetric, hinge with cardinal teeth in both valves, lateral teeth strong to obscure with RV anterior lateral tooth generally larger, anterior adductor muscle scar short, detached for about 1/5 length, pallial line either entire or in close, small blocks.
Eight species of Myrtina are known from the central IWP from shallow offshore to deeper water (
http://zoobank.org/C5254102-0F66-41A0-9A3E-8DB483BB7D44
http://species-id.net/wiki/Myrtina_reflexa
Figs 9B, 11Holotype: 1 whole shell, L 10.5 mm, H 9.4 mm, T 2.5 mm, NHMUK 1968738, John Murray Expedition stn 125, 825m, 5°36'12"S, 39°28'24"E off Zanzibar.
Paratypes: 2 RV, L 10.7 mm H 9.5 mm T 2.5 mm, L 11.8 mm H 10.7 mm T 2.8 mm, NHMUK 1968738 locality as holotype; 17 valves (13 RV, 4 LV), figured paratype (Fig. 11E-F) RV, L 15 mm, H 13.3 mm, NHMUK 20130121, John Murray Expedition stn 106, 183–194 m, 5°38'54"S, 39°18'42"E, off Zanzibar.
Myrtina reflexa new species. A–D Holotype, NHMUK 196838 exterior and interior of right (A–B) and left valves (C–D), L = 10.5 mm E–F Paratype NHMUK 20130121 John Murray Expedition stn 106, exterior and interior of right valve. L = 15 mm G–I Exterior of right valve and interior of right and left valves, MNHN IM 2009-8733, MIRIKY stn DW3239, 14°30'S, 47°26'E, 230–288 m, off NW Madagascar, L = 14.2 mmJ–K Details of hinge of holotype left and right valves. Scale bar = 2.0 mm L Detail of microsculpture, holotype. Scale bar = 50 µm M Protoconch of holotype. Scale bar = 100 µm.
1 whole shell (live collected) L 14.2 mm, H 12.7 mm, T 3.3 mm, MNHN IM 2009-8733, MIRIKY stn DW3239 14°30'S, 47°26'E, 230–288 m, off NW Madagascar.
Shells greyish white, H to 13.5 mm, L to 15.2 mm, T 3.8 mm, longer than high H/L = 0.91 ± 0.038 (n=8), T/L = 0.24 ± 0.018 (n=8), subovate, posterior truncate, anterior dorsal area marked by shallow double sulcus, anterior dorsal margin with elevated lamellae, umbones low, sculpture of widely spaced commarginal lamellae that are reflected ventrally, no radial sculpture. Microsculpture of fine growth increments (Fig. 11L). Lunule lanceolate, strongly asymmetric with larger part in LV. Protoconch (Fig. 11M): PI+ PII 183 µm, PII a narrow rim. Hinge (Figs 11J, K): LV with 1 thin cardinal tooth, laterals absent, RV with small, single, cardinal tooth, small anterior lateral tooth, posterior lateral teeth absent. Anterior adductor scar short, detached from pallial line for ¼ of length, posterior scar ovoid. Pallial line discontinuous in short narrow blocks. Shell interior glossy, shell margin smooth.
Western Indian Ocean, off Zanzibar and Madagascar from 200–825 m (Fig. 4).
Latin reflexa means bent or turned back, a reference to the form of the commarginal lamellae. Adjective nominative singular
This species was referred to Lucina inanis (Prashad) by
Myrtina reflexa differs from the genotype, Myrtina porcata, by the more widely spaced and reflexed commarginal lamellae. It is most similar to a new Myrtina species (
A number of colleagues provided specimens examined in this paper and we are grateful to Roger Bamber for the samples of Ferrocina brunei; Philippe Bouchet & Virginie Héros (MNHN) for the loan of Gonimyrtea specimens; Adam Baldinger and Gonzalo Giribet (MCZ) for the loan of the large sample of Scabrilucina victorialis; Antonia Vedelsby and Kathe Jensen (ZMC) for specimens of Scabrilucina vitrea andAlison Miller and Ian Loch (AMS) for loan of Scabrilucina melvilli samples.
Thanks are due to Harry Taylor (NHMUK) for macrophotography and Phil Rainbow (NHMUK) is especially acknowledged for his continuing support of our work on lucinids.