(C) 2013 Jiří Šmíd. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Šmíd J, Moravec J, Kratochvíl L, Gvoždík V, Nasher AK, Busais SM, Wilms T, Shobrak MY, Carranza S (2013) Two newly recognized species of Hemidactylus (Squamata, Gekkonidae) from the Arabian Peninsula and Sinai, Egypt. ZooKeys 355: 79–107. doi: 10.3897/zookeys.355.6190
A recent molecular phylogeny of the Arid clade of the genus Hemidactylus revealed that the recently described H. saba and two unnamed Hemidactylus species from Sinai, Saudi Arabia and Yemen form a well-supported monophyletic group within the Arabian radiation of the genus. The name ‘Hemidactylus saba species group’ is suggested for this clade. According to the results of morphological comparisons and the molecular analyses using two mitochondrial (12S and cytb) and four nuclear (cmos, mc1r, rag1, rag2) genes, the name Hemidactylus granosus Heyden, 1827 is resurrected from the synonymy of H. turcicus for the Sinai and Saudi Arabian species. The third species of this group from Yemen is described formally as a new species H. ulii sp. n. The phylogenetic relationships of the members of ‘Hemidactylus saba species group’ are evaluated and the distribution and ecology of individual species are discussed.
Reptilia, Gekkonidae, molecular phylogeny, Arabia, Red Sea, Hemidactylus saba species group , Hemidactylus granosus Heyden, 1827 , Hemidactylus ulii sp. n.
The genus Hemidactylus Oken, 1817, the second most species-rich genus of Gekkonidae (122 currently valid species;
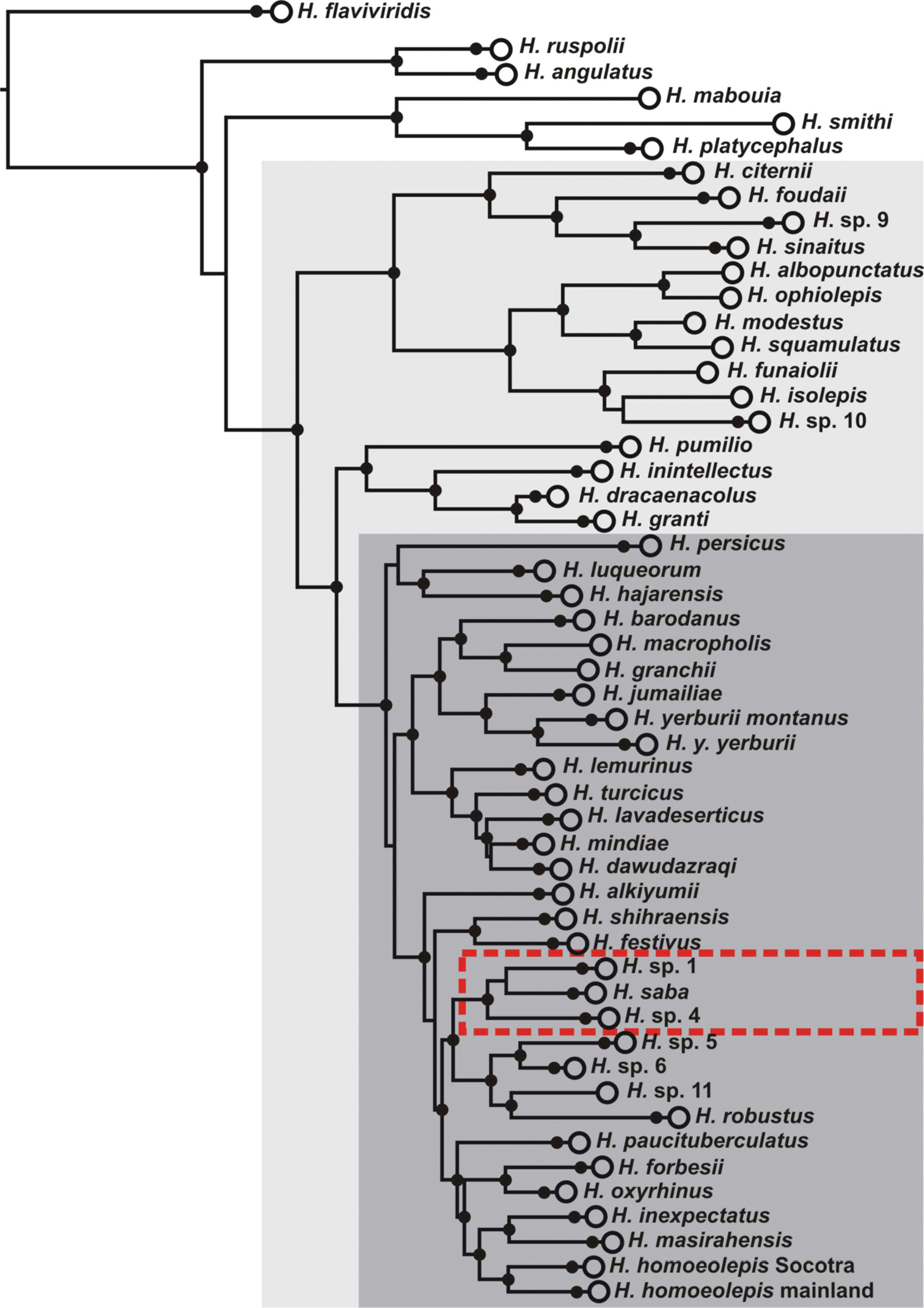
Phylogeny of the Hemidactylus Arid clade (light grey rectangle) modified after
The discovery of a monophyletic species group consisting of one recently described and two newly recognized species calls upon a more thorough study of the nomenclatural status, evolutionary relationships, taxonomy and distribution of its members based on further genetic and morphological data. The present study focuses on this task.
In order to resolve the phylogenetic relationships between the two newly recognized Hemidactylus species and Hemidactylus saba based on genetic data, a dataset containing only representatives of these three species was assembled. Apart from the data used by
List of material used for the phylogenetic analyses. Holotype of Hemidactylus ulii sp. n. and Hemidactylus saba are in bold. The column ‘Loc. No’ refers to the locality number as shown in Fig. 6.
| Species | Code | Museum number | Country | Locality | Loc. No | Lat, Long | 12S | cytb | cmos | mc1r | rag1 | rag2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemidactylus granosus | Sher10660 | SMB 10660 | Egypt | Ayoun Musa | 1 | 29.875, 32.649 | JQ957071 | JQ957216 | JQ957148 | JQ957282 | - | JQ957409 |
| Hemidactylus granosus | Hd41 | NMP6V70163/2 | Egypt | Sharm el Sheik; Sinai | 2 | 27.885, 34.317 | KC818724 | HQ833759 | JQ957148 | - | KC818981 | KF647606 |
| Hemidactylus granosus | Hd96 | NMP6V70163/1 | Egypt | Sharm el Sheik; Sinai | 2 | 27.885, 34.317 | KC818724 | HQ833759 | - | - | - | KF647607 |
| Hemidactylus granosus | Hd97 | NMP6V70163/3 | Egypt | Sharm el Sheik; Sinai | 2 | 27.885, 34.317 | KC818724 | HQ833759 | - | - | - | KF647608 |
| Hemidactylus granosus | HSA63 | ZFMK 94084 | Saudi Arabia | Al Wajh | 3 | 26.208, 36.4976 | KC818724 | HQ833759 | KF647576 | KF647589 | KF647596 | KF647610 |
| Hemidactylus granosus | HSA64 | ZFMK 94085 | Saudi Arabia | Al Wajh | 3 | 26.208, 36.4976 | KF647571 | - | - | - | - | - |
| Hemidactylus granosus | HSA65 | ZFMK 94086 | Saudi Arabia | 15 km S of Al Wajh | 4 | 26.123, 36.5689 | KF647570 | KF647581 | KF647574 | KF647590 | KF647601 | KF647610 |
| Hemidactylus granosus | HSA66 | ZFMK 94087 | Saudi Arabia | 15 km S of Al Wajh | 4 | 26.123, 36.5689 | KC818724 | - | - | - | - | - |
| Hemidactylus granosus | HSA67 | ZFMK 94088 | Saudi Arabia | 15 km S of Al Wajh | 4 | 26.123, 36.5689 | KF647569 | - | - | - | - | - |
| Hemidactylus granosus | HSA68 | TUZC-R8 | Saudi Arabia | 15 km S of Al Wajh | 4 | 26.123, 36.5689 | KF647570 | - | - | - | - | - |
| Hemidactylus granosus | HSA69 | ZFMK 94089 | Saudi Arabia | 15 km S of Al Wajh | 4 | 26.123, 36.5689 | KF647570 | - | - | - | - | - |
| Hemidactylus granosus | HSA70 | TUZC-R9 | Saudi Arabia | 72 km N of Umluj | 5 | 25.614, 36.9867 | KF647569 | KF647582 | JQ957148 | KF647591 | KF647600 | KF647609 |
| Hemidactylus granosus | HSA62 | TUZC-R10 | Saudi Arabia | 180 km W of Hail | 6 | 26.883, 40.0874 | KF647569 | KF647585 | JQ957148 | KF647588 | KF647602 | KF647609 |
| Hemidactylus granosus | HSA61 | IBES10001 | Saudi Arabia | Al Ghat | 7 | 26.054, 45.0003 | KF647569 | KF647585 | JQ957148 | KF647588 | KF647599 | KF647610 |
| Hemidactylus granosus | HSA57 | IBES10183 | Saudi Arabia | 30 km NE of Alhawiyah | 8 | 21.624, 40.7094 | KF647568 | KF647580 | - | - | KF647597 | KF647610 |
| Hemidactylus granosus | HSA58 | ZFMK 94090 | Saudi Arabia | 30 km NE of Alhawiyah | 8 | 21.624, 40.7094 | KF647569 | - | - | - | - | - |
| Hemidactylus granosus | HSA59 | TUZC-R11 | Saudi Arabia | 30 km NE of Alhawiyah | 8 | 21.624, 40.7094 | KF647569 | - | - | - | - | - |
| Hemidactylus granosus | HSA60 | IBES10344 | Saudi Arabia | 30 km NE of Alhawiyah | 8 | 21.624, 40.7094 | KF647569 | KF647583 | - | - | KF647598 | KF647610 |
| Hemidactylus granosus | HSA54 | IBES10150 | Saudi Arabia | 20 km S of Ashayrah | 9 | 21.602, 40.6911 | KF647568 | KF647584 | KF647576 | KF647588 | KF647595 | KF647609 |
| Hemidactylus granosus | HSA55 | ZFMK 94091 | Saudi Arabia | 20 km S of Ashayrah | 9 | 21.602, 40.6911 | KF647569 | KF647584 | KF647575 | KF647588 | KF647596 | KF647610 |
| Hemidactylus granosus | HSA56 | IBES10363 | Saudi Arabia | 20 km S of Ashayrah | 9 | 21.602, 40.6911 | KF647569 | - | - | - | - | - |
| Hemidactylus granosus | ZFMK 87236 | ZFMK 87236 | Saudi Arabia | Taif National Wildlife Research Center | 10 | 21.25, 40.96 | KF647569 | - | - | - | - | - |
| Hemidactylus saba | BJ27 | NHM-BS N41914 | Yemen | Marib | 17 | 14.9, 45.5 | KF647567 | - | KF647573 | - | - | KF647605 |
| Hemidactylus saba | BJ28 | NHM-BS N41913 | Yemen | Marib | 17 | 14.9, 45.5 | KF647567 | KF647579 | KF647573 | KF647586 | - | KF647605 |
| Hemidactylus saba | BJ29 | NHM-BS N41912 | Yemen | Marib | 17 | 14.9, 45.5 | KF647567 | - | KF647573 | KF647587 | KF647594 | KF647605 |
| Hemidactylus ulii sp. n. | JS48 | NMP6V 74834/1 | Yemen | Wadi Zabid | 11 | 14.147, 43.517 | KC818730 | KC818881 | KC818789 | KC818943 | KC819001 | KC819062 |
| Hemidactylus ulii sp. n. | JS49 | NMP6V 74834/2 | Yemen | Wadi Zabid | 11 | 14.147, 43.517 | KC818731 | KC818882 | KC818789 | - | KF647603 | KF647614 |
| Hemidactylus ulii sp. n. | JS45 | not collected | Yemen | Al Hababi | 12 | 13.333, 43.722 | KC818728 | KC818878 | - | - | - | KF647612 |
| Hemidactylus ulii sp. n. | JS46 | NMP6V 74833/1 | Yemen | Al Hababi | 12 | 13.333, 43.722 | KC818728 | KC818879 | KC818789 | - | - | KF647613 |
| Hemidactylus ulii sp. n. | JS47 | NMP6V 74833/2 | Yemen | Al Hababi | 12 | 13.333, 43.722 | KC818729 | KC818880 | KC818789 | KC818942 | KC819001 | KC819061 |
| Hemidactylus ulii sp. n. | JS37 | NMP6V 74832/1 | Yemen | 3 km S of Najd an Nashamah | 13 | 13.358, 43.957 | KC818727 | KC818876 | KF647578 | KC818943 | - | KF647611 |
| Hemidactylus ulii sp. n. | JS38 | NMP6V 74832/2 | Yemen | 3 km S of Najd an Nashamah | 13 | 13.358, 43.957 | KC818727 | KC818877 | KC818789 | KF647593 | - | KF647614 |
| Hemidactylus ulii sp. n. | JS32 | NMP6V 74835 | Yemen | 35 km W of Lahij | 14 | 13.032, 44.558 | KC818726 | KC818875 | KC818788 | KC818941 | KC819000 | KC819060 |
| Hemidactylus ulii sp. n. | BJ09 | NHM-BS N41916 | Yemen | Radman | 15 | 14.1, 45.283 | KF647572 | - | KF647577 | KF647592 | - | KC819059 |
| Hemidactylus ulii sp. n. | JS17 | NMP6V 74831/1 | Yemen | Al Hadr | 16 | 13.877, 45.8 | KC818725 | KC818874 | KC818787 | KC818940 | KC818999 | KC819059 |
| Hemidactylus ulii sp. n. | JS18 | NMP6V 74831/2 | Yemen | Al Hadr | 16 | 13.877, 45.8 | KC818725 | - | KC818789 | - | KF647604 | KC819059 |
| Hemidactylus angulatus | JS123 | NMP6V 74845/2 | Ethiopia | Arba Minch | - | 6.034, 37.564 | KC818659 | KC818807 | KC818747 | KC818903 | KC818956 | KC819018 |
| Hemidactylus flaviviridis | JS111 | not collected | Pakistan | Okara | - | 30.811, 73.457 | KC818676 | KC818822 | JQ957126 | JQ957253 | KC818965 | KC819026 |
| Hemidactylus flaviviridis | JS113 | not collected | India | Haridwar | - | 29.964, 78.201 | KC818676 | KC818823 | JQ957126 | JQ957253 | KC818966 | KC819027 |
| Hemidactylus flaviviridis | JS119 | not collected | Oman | Jalan Bani Bu Hassan | - | 22.089, 59.278 | JQ957119 | JQ957183 | KC818754 | KC818911 | KC818967 | KC819028 |
The final dataset consisted of 36 ingroup individuals. Specimen numbers, localities, and GenBank accession numbers of all genes sequenced are presented in Table 1. The alignment of all concatenated genes was 4012 bp long. The software jModelTest 2.1.1 (
Maximum likelihood analyses of both datasets were performed in RAxML 7.0.3 (
The BI analyses were run in MrBayes 3.2.1 (
Heterozygous positions in nuclear genes were identified based on the presence of double peaks in chromatograms and using the Heterozygote Plugin in Geneious. For the purpose of haplotype network construction, haplotypes from sequences with more than one heterozygous position were resolved in PHASE 2.1.1 (
Material for morphological comparison included 225 specimens of 8 Hemidactylus species and one subspecies (Appendix) and was obtained from the following collections: National Museum Prague, Czech Republic (NMP); Natural History Museum in Braunschweig, Germany (NHM-BS); Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany (SMF); Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany (ZFMK); Museo Civico di Storia Naturale “Giacomo Doria”, Genova, Italy (MSNG); Museo Civico di Storia Naturale di Milano, Milano, Italy (MSNM); Museo Civico di Storia Naturale, Carmagnola, Italy (MCCI); Università di Firenze, Museo Zoologico “La Specola”, Firenze, Italy (MZUF); British Museum of Natural History, London, UK (BMNH); California Academy of Sciences, San Francisco, USA (CAS); Taif University Zoological Collection, Taif, Saudi Arabia (TUZC); Institute of Evolutionary Biology Collection, Barcelona, Spain (IBES); Tomas Mazuch private collection, Dříteč, Czech Republic (TMHC); L. Kratochvíl collection (JEM); J. Šmíd collection (JS); Sherif Baha El Din private collection, Cairo, Egypt (SMB). Names of localities and governorates are spelled according to Google Earth (http://www.google.com/earth/). All coordinates are in WGS84 geographic coordinate system. Table of localities in a CSV text format and high-resolution photographs of all individuals analyzed in this study (397 pictures in total) have been deposited in MorphoBank (Project 1006; http://www.morphobank.org).
The following measurements were taken with Powerfix digital calliper to the nearest 0.1 mm: snout-vent length (SVL), measured from tip of snout to vent; head length (HL), measured from tip of snout to retroarticular process of jaw; head width (HW), taken at the widest part of the head; head depth (HD), maximum depth of head; left eye diameter (E), measured horizontally; axilla-groin distance (AG), measured from posterior end of front limb insertion to anterior end of hind limb insertion; tail length (TL), measured from vent to tip of original tail. In addition to these metric characters, the following meristic characters were examined using a dissecting microscope: number of upper and lower labials (left/right); contact of nasals; number of infralabials in contact with first postmentals; mutual position of first postmentals; number of longitudinal rows of enlarged dorsal tubercles; number of lamellae under the first and fourth toe including unpaired proximal ones; and number of preanal pores in males. Terminology and diagnostic characters follow
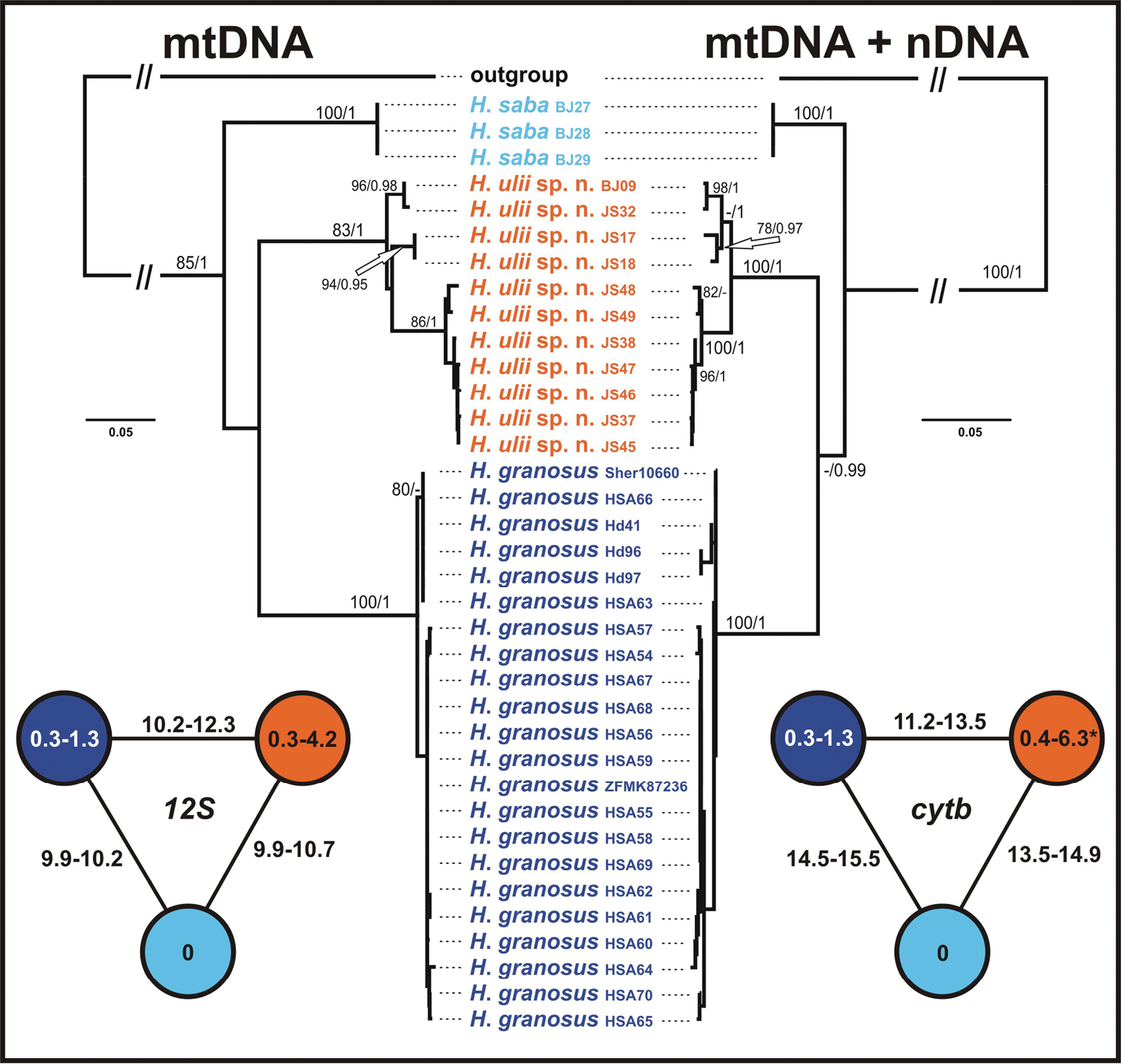
Phylogenetic analyses of both datasets resulted in trees presented in Fig. 2. Tree topology remains congruent with that showed in
Maximum likelihood trees of mtDNA and mtDNA + nDNA datasets of the ‘Hemidactylus saba species group’. ML bootstrap values/Bayesian posterior probabilities are indicated by the nodes. Hemidactylus flaviviridis and Hemidactylus angulatus were used as outgroups. At the sides, schematic networks showing intra- and interspecific uncorrected p distances (in %) in the sequences of 12S and cytb. * intraspecific distances within Hemidactylus ulii sp. n. are based on an alignment of 1127 bp, all other values for cytb are calculated for an alignment of 307 bp.
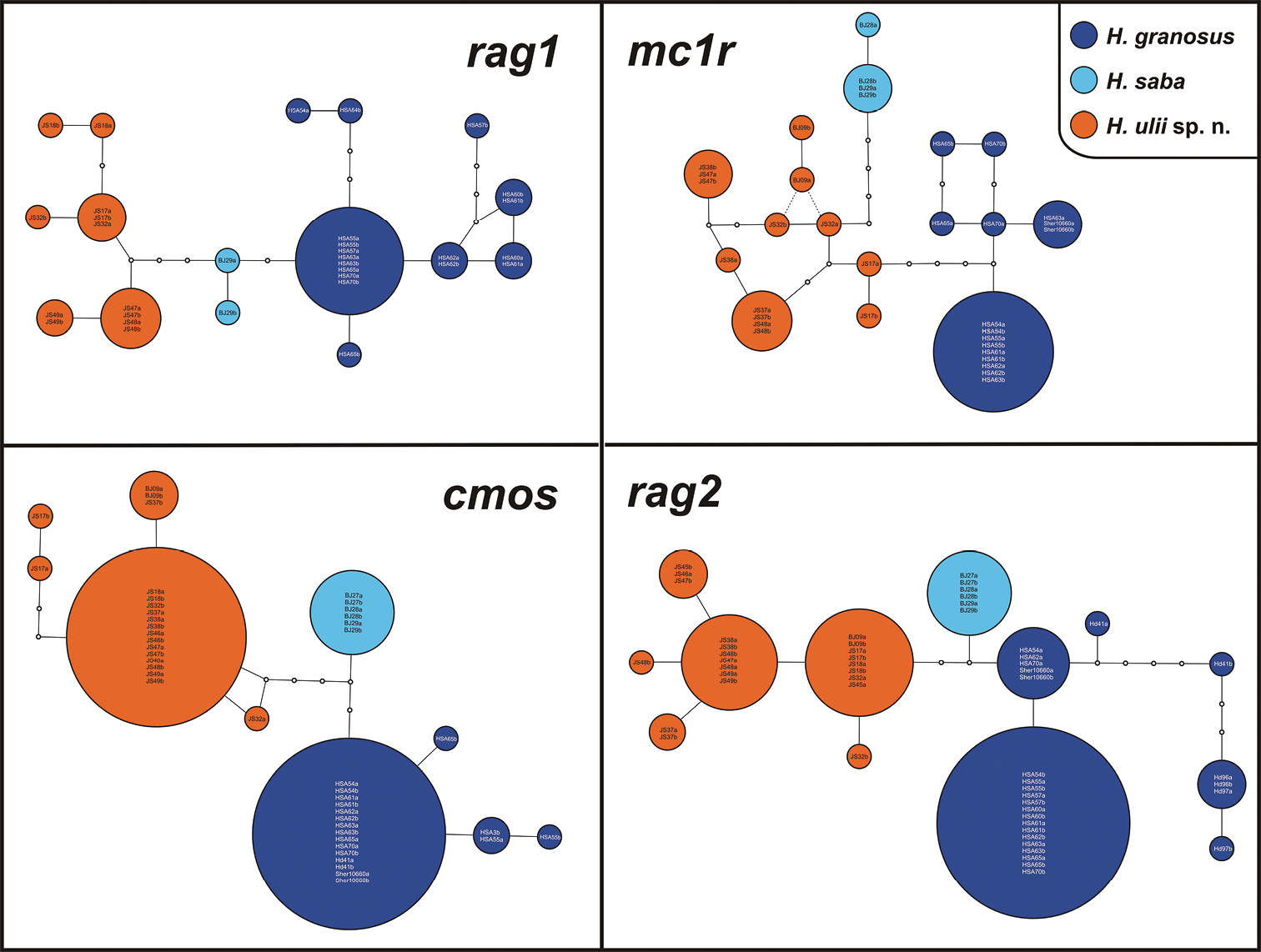
Nuclear allele networks of the four loci analyzed (cmos, mc1r, rag1, rag2). Circle sizes are proportional to the number of alleles. Small white circles represent mutational steps. Position of alleles BJ09a and BJ09b in the mc1r network is indicated by dashed lines because the sequence of the sample BJ09 (voucher NHM-BS N41916) was 108 bp shorter than the rest of the alignment and haplotype network reconstructions based on both 666 bp and 558 bp alignments linked these alleles to JS32b and JS32a, respectively.
The results of the molecular analyses, together with a unique combination of morphological features (see below) confirm the earlier conclusion that the newly recognized Hemidactylus sp. 1 and Hemidactylus sp. 4 represent two separate species, whose taxonomy and nomenclature need to be resolved.
http://species-id.net/wiki/Hemidactylus_granosus
Figs 4, 5SMF 8723 (lectotype, adult male), Petr. Arabica [Arabia petraea], collected by E. Rüppell in 1827 (MorphoBank M305565–M305594); NMP6V 70163/1 (adult female, MorphoBank M305520–M305528), NMP6V 70163/2 (adult male, MorphoBank M305529–M305542), NMP6V 70163/3–4 (adult females, MorphoBank M305543–M305554, M305555–M305564), Egypt, South Sinai governorate, Sharm el-Sheikh (27.885°N, 34.317°E), ca. 30 m a.s.l., collected by R. Kovář and R. Víta in 1996; ZFMK 94084, ZFMK 94085 (adult females, MorphoBank M305744–M305760, M305761–M305775), Saudi Arabia, Tabuk province, Al Wajh (26.2076°N, 36.4976°E), 5 m a.s.l., 31. V. 2012; ZFMK 94086 (adult female, MorphoBank M305778–M305791), ZFMK 94088, ZFMK 94089 (adult males, M305793–M305799, M305807, M305822–M305827, M305828–M305841), Saudi Arabia, Tabuk province, 15 km S of Al Wajh (26.1226°N, 36.5689°E), 25 m a.s.l., 31. V. 2012; TUZC-R10 (adult female, MorphoBank M305728–M305743), Saudi Arabia, Hail province, 180 km N of Hail (26.8831°N, 40.0874°E), 1020 m a.s.l., 30. V. 2012; IBES10183, TUZC-R11 (adult males, MorphoBank M305656–M305671, M305688–M305701), ZFMK 94090, IBES10344 (adult females, MorphoBank M305672–M305687, M305702–M305717), Saudi Arabia, Makkah province, 30 km NE of Alhawiyah (21.6244°N, 40.7094°E), 1295 m a.s.l., 28. V. 2012; IBES10150, IBES10363 (adult males, MorphoBank M305615–M305628, M305643–M305655), ZFMK 94091 (adult female, MorphoBank M305629–M305642), Saudi Arabia, Makkah province, 20 km S of Ashayrah (21.6022°N, 40.6911°E), 1316 m a.s.l., 28. V. 2012. All Saudi specimens were collected by M. Shobrak, S. Carranza and T. Wilms.
SMB 10660, Egypt, Suez governorate, Ayoun Musa (29.875°N, 32.649°E), ca. 12 m a.s.l., collected by S. Baha El Din, date unknown; TUZC-R9, Saudi Arabia, Tabuk province, 72 km N of Umluj (25.614°N, 36.9867°E), 19 m a.s.l., 31. V. 2012; IBES10001, Saudi Arabia, Riyadh province, Al Ghat (26.0545°N, 45.0003°E), 776 m a.s.l., 29. V. 2012; ZFMK 94087, TUZC-R8, Saudi Arabia, Tabuk province, 15 km S of Al Wajh (26.1226°N, 36.5689°E), 25 m a.s.l., 31. V. 2012; ZFMK 87236, Saudi Arabia, Makkah province, Taif National Wildlife Research Center (21.25°N, 40.96°E), 25. VI. 2007 by T. Wilms. These specimens were used for the molecular analyses only.
Recent examination (by JŠ) of four specimens collected by Rüppell (SMF 8723–8726) has shown that one of them [SMF 8723 designated by
Hemidactylus granosus is a member of the ‘Hemidactylus saba species group’ within the Arabian radiation of the Arid clade as evidenced by the mtDNA and nDNA analyses. The species has the following combination of molecular and morphological characters: (1) Uncorrected genetic distance from Hemidactylus saba: 9.9–10.2% in 12S, 14.5–15.5% in cytb; from Hemidactylus sp. 4: 10.2–12.3% in 12S, 11.2–13.5% in cytb; (2) small size, SVL 39.0–53.2 mm in males, 40.6–53.3 mm in females; (3) rather elongated head, head length 24–28% of SVL, head width 68–86% of head length, head depth 33–47% of head length; (4) tail length 107–130% of SVL; (5) uppermost nasals separated by a small shield in 89% of specimens; (6) large anterior postmentals in wide mutual contact, and always in contact with the 1st and 2nd lower labial; (7) 9–11 upper labials; (8) 7–9 lower labials; (9) 14–15 longitudinal rows of enlarged, subtriangular, distinctly keeled dorsal tubercles; (10) 7–8 lamellae under the 1st toe and 10–13 under the 4th toe; (11) ca. 6–8 tail segments bearing 6 pointed tubercles; (12) 4–7 preanal pores in males forming a continuous row on the left and right side; (13) subcaudals enlarged; (14) in life, dorsum pale buff with dark brown spots tending to form transverse bands or X-shaped markings, dark horizontal stripe in prefrontal and temporal region, tail with ca. 10–13 dark brown transverse bands, venter white.
SMF 8723, adult male [erroneously determined as female by
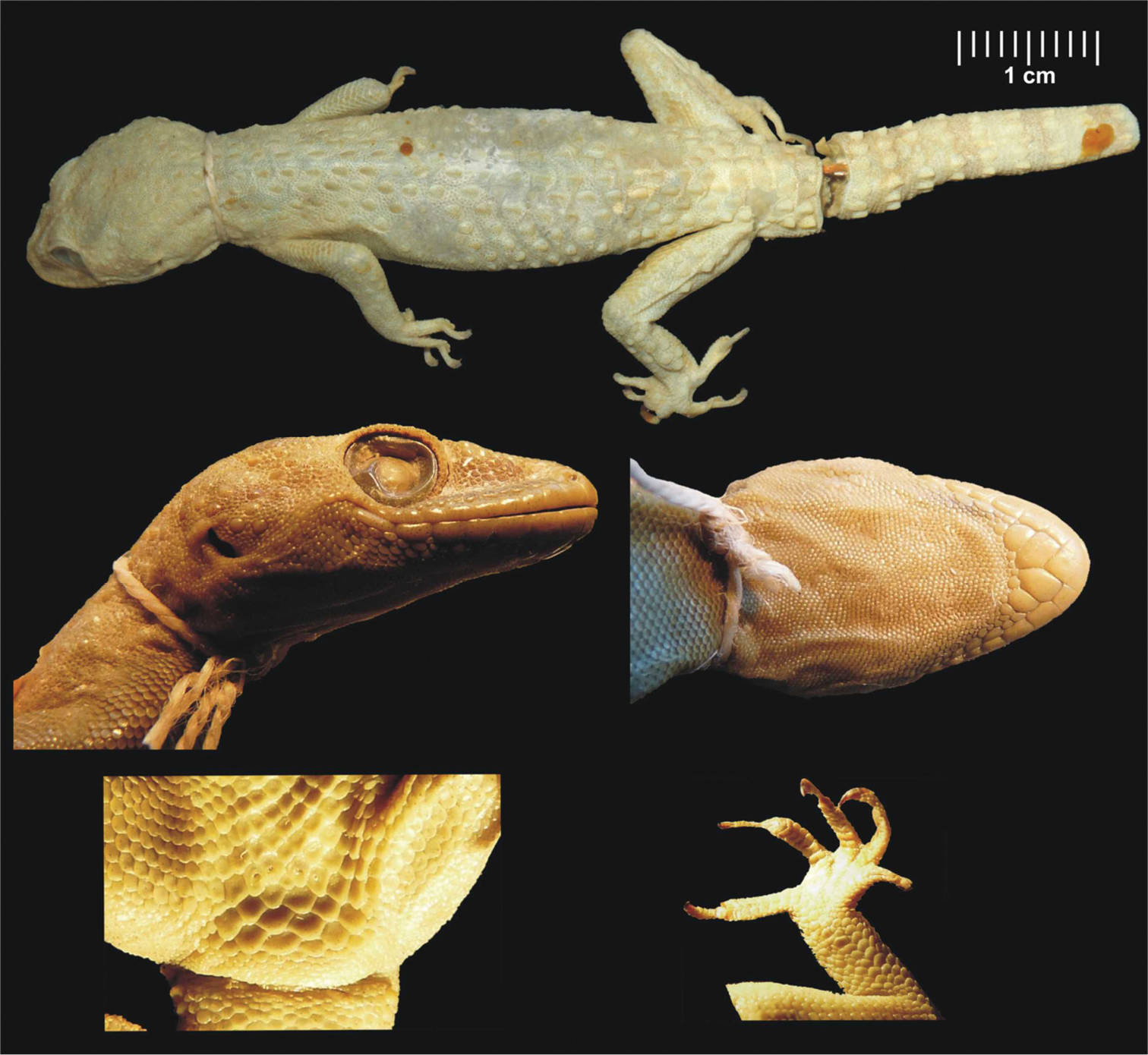
Male lectotype of Hemidactylus granosus (SMF 8723) from Sinai, Egypt. General habitus, lateral and ventral view of the head, precloacal region with preanal pores, right hind leg. Scale refers to the uppermost picture only.
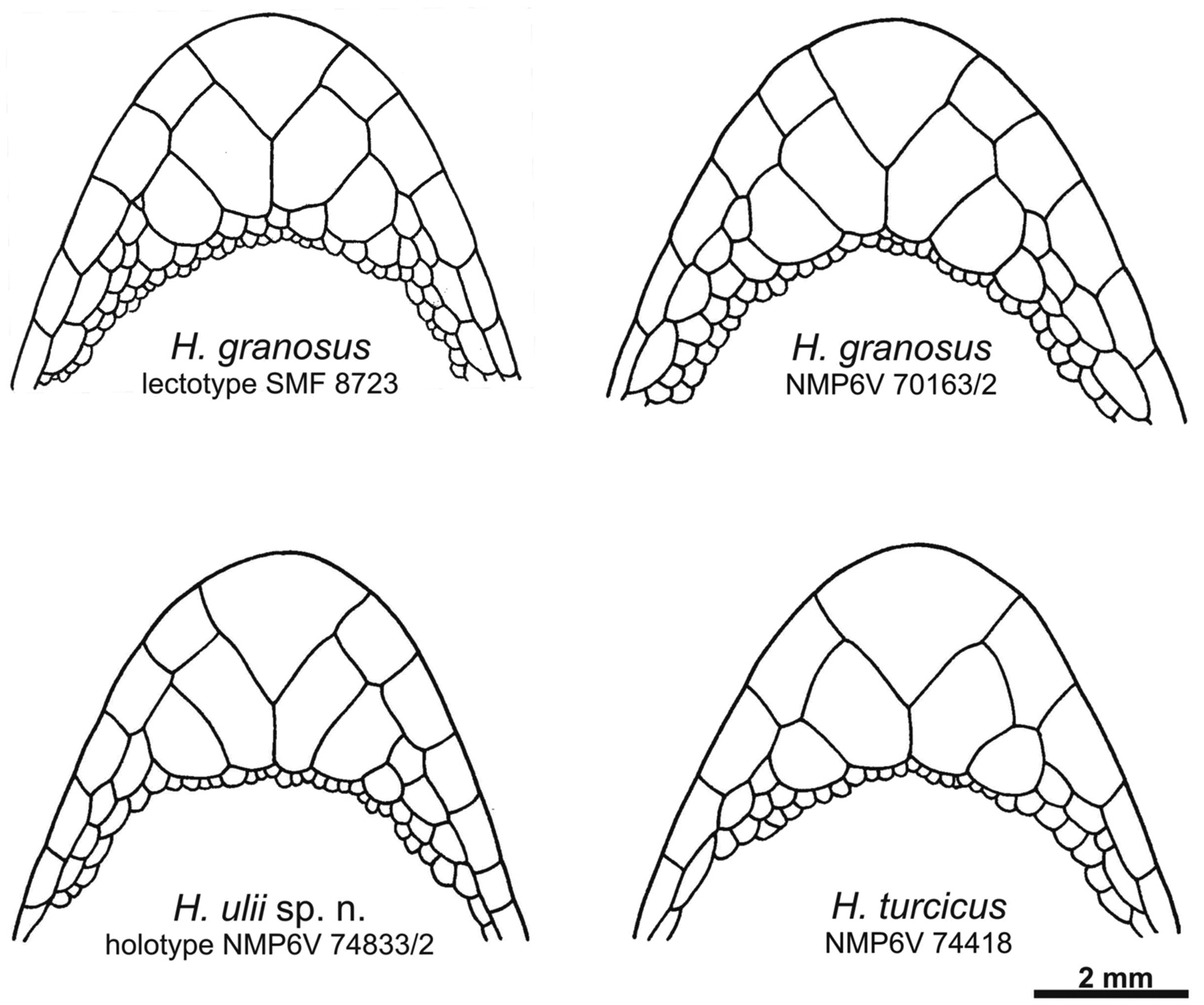
Schematic drawing of the chin region of the lectotype and a new specimen from Sinai of Hemidactylus granosus, the holotype of Hemidactylus ulii sp. n., and Hemidactylus turcicus from Sinai.
Measurements (in mm): SVL 51.5, HL 12.9, HW 9.8, HD 6.0, E 3.3, AG 23.7.
Paralectotype SMF 8724 differs from other individuals of Hemidactylus granosus in having relatively high head (HD 50% of HL), lower number of lower labials (6), uppermost nasals in wide contact, first postmentals in contact with 1st lower labials, and 2 preanal pores.
Hemidactylus granosus can be distinguished from other member of the ‘Hemidactylus saba species group’ and from other congeners distributed in Sinai and the Red Sea coast by the following set of characters (see also Table 2).
Morphological comparison among members of the ‘Hemidactylus saba species group’ and with other Hemidactylus species from Sinai and SW Yemen. The values are given as follows: sample size, mean ± standard deviation above, min. – max. value below.
| Species / Character | Hemidactylus saba species group | Hemidactylus robustus | Hemidactylus turcicus | Hemidactylus mindiae | Hemidactylus jumailiae | Hemidactylus yerburii yerburii | Hemidactylus yerburii montanus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemidactylus granosus | Hemidactylus saba | Hemidactylus ulii sp. n. | ||||||||||||||||
| Upper labials | 18 | 9.4 ± 0.5 | 3 | 9.3 ± 0.8 | 10 | 9.3 ± 0.8 | 27 | 9.4 ± 0.7 | 33 | 8.2 ± 0.5 | 5 | 10.8 ± 0.8 | 18 | 9.8 ± 0.7 | 51 | 10.3 ± 0.7 | 57 | 10.2 ± 0.7 |
| 9–11 | 8–10 | 8–10 | 8–11 | 7 - 10 | 10 - 12 | 8–12 | 9–12 | 8–12 | ||||||||||
| Lower labials | 18 | 7.4 ± 0.4 | 3 | 7.7 ± 0.6 | 10 | 8.0 ± 0.6 | 27 | 7.7 ± 0.6 | 33 | 6.7 ± 0.5 | 5 | 8.1 ± 0.4 | 18 | 8.2 ± 0.6 | 51 | 7.9 ± 0.5 | 57 | 7.8 ± 0.6 |
| 7–9 | 7–8 | 7–9 | 6–9 | 6–8 | 7–9 | 7–10 | 6–9 | 6–10 | ||||||||||
| Nasals in contact (%) | 18 | 11 | 3 | 33.3 | 10 | 40 | 27 | 22.2 | 33 | 21.2 | 5 | 0 | 18 | 5.5 | 51 | 7.8 | 57 | 5.3 |
| 1st postmental in contact with 2nd lower labial (%) | 18 | 100 | 3 | 33.3 | 10 | 100 | 27 | 70.3 | 33 | 12.1 | 5 | 80 | 18 | 83.3 | 51 | 98 | 57 | 89.5 |
| Rows of dorsal tubercles | 18 | 14.1 ± 0.2 | 3 | 14 ± 0.0 | 10 | 14.1 ± 1.0 | 27 | 14.8 ± 1.2 | 33 | 13.8 ± 0.7 | 5 | 12.4 ± 0.9 | 15 | 14 ± 1.4 | 46 | 15.3 ± 1.1 | 53 | 15.2 ± 1.2 |
| 14–15 | 14–14 | 12–16 | 13–18 | 12–16 | 12–14 | 12–16 | 13–18 | 12–18 | ||||||||||
| Pores | 8 | 5.6 ± 1.1 | 1 | 6 | 2 | 8 ± 0.0 | 9 | 6.1 ± 0.8 | 13 | 7.2 ± 1.4 | 1 | 4 | 9 | 7.2 ± 1.1 | 23 | 13.7 ± 2.2 | 27 | 11.2 ± 1.1 |
| 4–7 | 8 - 8 | 5–8 | 6–10 | 6–9 | 10–18 | 9–13 | ||||||||||||
| Lamellae under 1st toe | 18 | 7.4 ± 0.5 | 3 | 8.2 ± 0.3 | 10 | 5.4 ± 0.5 | 27 | 6.1 ± 0.5 | 32 | 6.5 ± 0.5 | 5 | 6.2 ± 0.3 | 18 | 6.9 ± 0.7 | 51 | 6.7 ± 0.4 | 57 | 6.3 ± 0.4 |
| 7–8 | 8–9 | 5–6 | 5–8 | 6–7 | 6–7 | 6–8 | 6–8 | 5–7 | ||||||||||
| Lamellae under 4th toe | 18 | 11.5 ± 0.7 | 3 | 11.2 ± 0.3 | 10 | 8.6 ± 0.5 | 27 | 10.1 ± 0.7 | 32 | 9.7 ± 0.6 | 5 | 10 ± 0.0 | 18 | 10.9 ± 0.8 | 51 | 10.4 ± 0.6 | 57 | 10.2 ± 0.5 |
| 10 - 13 | 11–12 | 8–9 | 8–12 | 8–11 | 10–10 | 9–12 | 9–12 | 9–11 | ||||||||||
| SVL (males) | 8 | 46.8 ± 5.9 | 1 | 58.3 | 2 | 38.6 ± 2.6 | 8 | 41.8 ± 2.3 | 13 | 46.0 ± 5.8 | 1 | 49.3 | 8 | 48.4 ± 4.1 | 23 | 58.5 ± 7.1 | 25 | 56.5 ± 5.7 |
| 39.0–53.2 | 36.8–40.4 | 37.0–43.7 | 37.3–54.1 | 40.0–54.2 | 43.6–74.9 | 45.2–65.3 | ||||||||||||
| SVL (females) | 10 | 49.0 ± 3.5 | 2 | 53.5 ± 7.9 | 2 | 40.1 ± 0.9 | 16 | 43.6 ± 4.7 | 18 | 49.2 ± 5.1 | 4 | 46.2 ± 11.4 | 8 | 48.6 ± 3.3 | 23 | 55.7 ± 5.3 | 30 | 52.6 ± 5.1 |
| 40.6–53.3 | 47.9–59.1 | 39.4–40.7 | 32.7–50.1 | 39.4–56.2 | 35.6–56.6 | 43.1–54.0 | 43.6–62.1 | 42.4–64.1 | ||||||||||
From Hemidactylus saba by having distinctly keeled dorsal tubercles (smooth in Hemidactylus saba), and lower number of lamellae under the 1st toe (7–8 vs. 8–9).
From Hemidactylus sp. 4 (described below) by its larger size (max. SVL 53.2 mm vs. 40.4 mm in males, 53.3 mm vs. 40.7 mm in females), in having more frequently separated uppermost nasals (100% vs. 60% of specimens), lower number of preanal pores in males (4–7 vs. 8), and higher number of lamellae under the 1st (7–8 vs. 5–6) and 4th (10–13 vs. 8–9) toe.
From Hemidactylus flaviviridis by its smaller size (max. SVL 53.2 mm in males and 53.3 mm in females vs. up to 90 mm [
From Hemidactylus mindiae by the lower number of supralabials (9–11 vs. 10–12), by having anterior postmentals in wide contact (punctual in Hemidactylus mindiae) and keeled dorsal tubercles (smooth in Hemidactylus mindiae).
From Hemidactylus robustus by the larger size of males (max. SVL 53.2 mm vs. 43.7 mm), longer tail (tail length 53.0–64.8 mm vs. 40.9–48.7 mm), and lower number of preanal pores in males (4–7 vs. 5–8).
From Hemidactylus turcicus by its higher number of upper labials (9–11 vs. 7–10), in having anterior postmentals more frequently in contact with 2nd lower labial (100% vs. 12.1%), in having anterior postmentals in wide mutual contact behind the mental scale (contact punctual in 67% specimens of Hemidactylus turcicus), and by the lower number of preanal pores in males (4–7 vs. 6–10).
Specimens with intact tail vary in number of tail segments bearing 6 pointed tubercles (7–8). The original portion of the tail of the female NMP6V 70163/4 is very wide at the base, separated from cloacal region by a basal constriction. One specimen (IBES10212) is the only animal with 15 longitudinal rows of enlarged tubercles. Another one (IBES10284) has uppermost nasals in wide contact. Most striking is the variation in the number of preanal pores in males. Whereas the lectotype and the only male from Sinai (NMP6V 70163/2) have both 4 pores, all males from Saudi Arabia have 6–7 pores. There seems to be clinal variability in this character, males from NW of the known range (Fig. 6) possess only 4 preanal pores, all animals from the eastern Red Sea coast in Saudi Arabia have 6 pores and a single individual from the southern limit of the range has 7 pores.
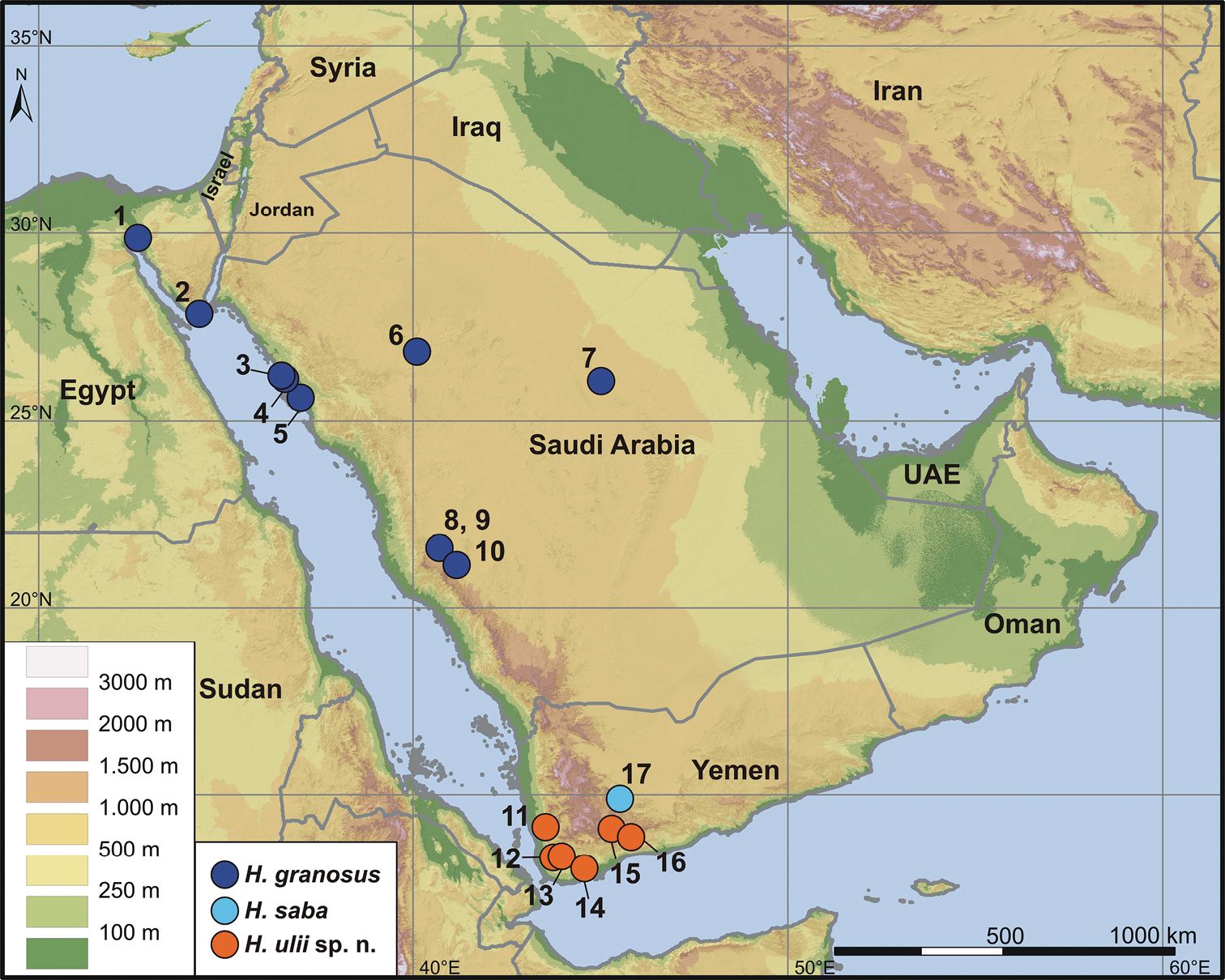
Distribution map of Hemidactylus granosus, Hemidactylus saba and Hemidactylus ulii sp. n. For the list of locality names and their corresponding numbers in the map see Table 1.
Coloration (in life) pale buff dorsally (Fig. 7). Conspicuous dark brown horizontal stripe in loreal and temporal area, terminated at the level of ear from where it continues in a series of dark patches on the neck. Four barely visible X-shaped markings on dorsum formed mainly by dark brown enlarged tubercles (first on nape, second across scapulae, third in lumbal region, and fourth just in front of the anterior insertion of hind limbs). Isolated dark brown stripe runs across body in the place of posterior insertion of hind limbs. Regenerated tails are uniformly buff from above. Dorsum, sides of chin, underside of front and hind limbs and underside of tail with faint stipple visible under magnification. Belly white. Tips of fingers and toes black behind insertion of terminal phalanges. Coloration is consistent among all specimens and varies only in distinctness of the markings.
Live specimens of Hemidactylus granosus from Saudi Arabia. A IBES10344, 30 km NE of Alhawiyah (loc. number 8) B TUZC-R10, 180 km W of Hail (6) C ZFMK 94091, 20 km S of Ashayrah (9) D ZFMK 94086, 15 km S of Al Wajh (4).
There is a very low variation in mtDNA between specimens from Sinai and Saudi Arabia (max. 1.3% in both 12S and cytb). All animals from Sinai share the same haplotypes in 12S and also cytb gene. All four nuclear loci studied show some degree of intraspecific variation (Fig. 3).
Eduard Rüppell collected the original series in 1827 when he began his marine biological studies of the Red Sea and travelled from Egypt to Eritrea. There is no specific information that he went to Arabia as well (
In 1996, when the NMP specimens were collected, the locality in Sharm el-Sheikh was formed by a crop field supplied with drain water from nearby habitations. Geckos were found during the day under unused empty barrels and also inside buildings. Other species syntopic with Hemidactylus granosus in Sharm el-Sheikh were: Hemidactylus turcicus, Chalcides ocellatus (Forskål, 1775), Stenodactylus sthenodactylus (Lichtenstein, 1823), and Ptyodactylus hasselquistii (Donndorff, 1798) (R. Víta in litt, 2013). However, when visited again in 2010, the locality had changed dramatically (R. Víta in litt, 2013). The whole area was under heavy development and the irrigation channels had disappeared. The current conditions at the place are unknown to us. In 2011 JM surveyed a neighbouring urban area east of this locality. It was covered by a mosaic of tourist resorts and abandoned ruderal plots. In dry anthropogenic habitats (e.g. rubbish dumps, road ditches, old walls and buildings, abandoned construction sites, natural but heavily disturbed open areas, etc.) dominated two very abundant gecko species. Ptyodactylus hasselquistii occupied primarily various vertical surfaces whereas Cyrtopodion scabrum (Heyden, 1827) prevailed on the ground. Tropiocolotes nattereri Steindachner, 1901 was found in dry and relatively well-preserved natural places. Hemidactylus turcicus was occasionally encountered in more humid artificial habitats in parks and hotel gardens. Specimens from Saudi Arabia were mostly collected during the day inside concrete tunnels under roads. In some of the tunnels they were syntopic with Ptyodactylus hasselquistii. One specimen was also collected on the walls of the Taif National Wildlife Research Centre, where it was also syntopic with Ptyodactylus hasselquistii.
http://zoobank.org/8E15D1BC-5D4D-4A55-AFEB-2E20FAD40112
http://species-id.net/wiki/Hemidactylus_ulii
Figs 5, 7, 8NMP6V 74833/2, adult male (MorphoBank M305892–M305902), Yemen, Ta’izz governorate, Al Hababi (13.333°N, 43.722°E), 463 m a.s.l.; collected by L. Kratochvíl, 28. X. 2007.
NMP6V 74833/1 (adult male, MorphoBank M305884–M305891), same collecting data as holotype; NMP6V 74831/1–2 (one adult and one subadult female, MorphoBank M305854–M305863, M305864–M305870), Yemen, Abyan governorate, Al Hadr (13.877°N, 45.8°E), 1151 m a.s.l., collected by L. Kratochvíl on 22. X. 2005; NMP6V 74832/1–2 (two subadult females, MorphoBank M305871–M305875, M305876–M305883), Yemen, Ta’izz governorate, ca. 3 km S of Najd an Nashamah by road (13.358°N, 43.957°E), 1182 m a.s.l., collected by L. Kratochvíl on 26. X. 2007; NMP6V 74834/1–2 (one adult and one subadult female, MorphoBank M305903–M305911), Yemen, Dhamar governorate, Wadi Zabid (14.147°N, 43.517°E), 292 m a.s.l., collected by L. Kratochvíl on 29. X. 2007; NHM-BS N41916 (juvenile, MorphoBank M305842–M305852), Yemen, Al Bayda’ governorate, Radman (14.1°N, 45.283°E), collected by W. Mustafa on 13. XI. 2007.
NMP6V 74835 (juvenile), Yemen, Lahij governorate, wadi 35 km W of Lahij (13.032°N, 44.558°E), 297 m a.s.l., collected by L. Kratochvíl on 25. X. 2007; JEM476 (juvenile), same collecting data as holotype; All juvenile specimens were used for comparison of meristic characters and included in the molecular analyses.
A small species of the ‘Hemidactylus saba species group’ withinthe Arabian radiation of the Arid clade of Hemidactylus, as evidenced by the mtDNA and nDNA analyses. The new species is characterized by the following combination of molecular and morphological characters: (1) Uncorrected genetic distances from Hemidactylus saba: 9.9–10.7% in 12S, 13.5–14.9% in cytb; from Hemidactylus granosus: 10.2–12.3% in 12S, 11.2–13.5% in cytb; (2) small size with a maximum recorded SVL 40.7 mm (36.8–40.4 mm in males, 39.4–40.7 mm in females); (3) moderately robust head, head length 28–30% of SVL, head width 70–75% of head length, head depth 37–46% of head length; (4) tail length 116% of SVL (only 1 specimen with intact tail); (5) uppermost nasals separated by a small shield (60% specimens) or in wide contact (40%); (6) large anterior postmentals in wide mutual contact in 90% of individuals, and in contact with the 1st and 2nd lower labial (scarcely and unilaterally with the 1st lower labial only); (7) 8–10 upper labials; (8) 7–9 lower labials; (9) dorsum with 12-16 longitudinal rows of enlarged, slightly keeled, conical tubercles; (10) 5–6 lamellae under the 1st toe and 8–9 lamellae under the 4th toe; (11) ca. 6–8 tail segments bearing 6 tubercles; (12) 8 preanal pores in one continuous row in males; (13) subcaudals enlarged; (14) in alcohol dorsum brownish grey with a pattern of more or less conspicuous dark transverse bands starting on the nape, tail with 9 dark brown transverse bands.
Hemidactylus ulii sp. n. can be distinguished from the other members of the ‘Hemidactylus saba species group’ and from all other congeners distributed in the region by the following combination of characters (see also Table 2):
From Hemidactylus granosus by its smaller size (max. SVL 40.4 mm vs. 53.2 mm in males, 40.7 mm vs. 53.3 mm in females), by having less frequently separated uppermost nasals (60% vs. 89% of specimens), higher number of preanal pores in males (8 vs. 4–7), and lower number of lamellae under the 1st (5–6 vs. 7–8) and 4th (8–9 vs. 10–13) toe.
From Hemidactylus saba by its smaller size (max. SVL 40.4 mm vs. 58.3 mm in males, 40.7 mm vs. 59.1 mm in females), higher number of preanal pores in males (8 vs. 6), and lower number of lamellae under the 1st (5–6 vs. 8–9) and 4th (8–9 vs. 11–12) toe.
From Hemidactylus flaviviridis by its smaller size (maximum SVL 40.4 mm in males, 40.7 mm in females vs. up to 90 mm [
From Hemidactylus jumailiae by its smaller size (max. SVL 40.4 mm vs. 54.2 mm in males, 40.7 mm vs. 54.0 mm in females), lower frequency of separated uppermost nasals (60% vs. 95%), in having conical and at least slightly keeled dorsal tubercles (vs. non-protruding and smooth tubercles), and lower number of lamellae under the 1st (5–6 vs. 6–8) and 4th (8–9 vs. 9–12) toe.
From Hemidactylus robustus by its smaller size (max. SVL 40.4 mm vs. 43.7 mm in males, 40.7 mm vs. 50.1 mm in females), and lower number of lamellae under the 4th toe (8–9 vs. 8–12).
From Hemidactylus sinaitus by the presence of enlarged tile-like subcaudals and in having separated uppermost nasals (60% vs. 9% of specimens).
From Hemidactylus yerburii montanus by its smaller size (maximum SVL 40.4 mm vs. 65.3 mm in males, 40.7 mm vs. 64.1 mm in females), lower number of preanal pores in males (8 vs. 9–13), and lower number of lamellae under the 4th toe (8–9 vs. 9–11).
From Hemidactylus yerburii yerburii by its smaller size (maximum SVL 40.4 mm vs. 74.9 mm in males, 40.7 mm vs. 62.1 mm in females), lower number of supralabials (8–10 vs. 9–12), lower frequency of having separated uppermost nasals (60% vs. 92%), lower number of preanal pores in males (8 vs. 10–18), and lower number of lamellae under the 1st (5–6 vs. 6–8) and 4th (8–9 vs. 9–12) toe.
NMP6V 74833/2, adult male. Body slightly depressed to cylindrical (Fig. 8). Upper labials 8/8, lower labials 7/7. Nostril between rostral, three nasals and in punctual contact with the first upper labial. Uppermost nasals separated by a small inserted shield. Mental almost triangular. Anterior postmentals large and very long, in wide mutual contact behind mental, in contact with the 1st lower labial (left) and the 1st and 2nd lower labials (right) (Fig. 5). Posterior postmentals smaller, in contact with the 1st and 2nd (left) and the 2nd (right) lower labial. Eye moderate (E/HL=0.24). Supraciliar granules with prominent projections, which form a comb-like structure above the eyes. Parietal and temporal region covered with round pointed regularly distributed tubercles. Ear opening oval. Dorsum with 14 longitudinal rows of enlarged, prominent, caudally pointed tubercles bearing distinct longitudinal keels. Thighs and lower legs with scattered enlarged tubercles. Tail partially regenerated from about half of its original length (estimate), original part relatively thick without basal constriction. Conical and keeled tail tubercles on tail segments forming regular whorls. Each whorl separated from the next one by four small scales. Subcaudals enlarged, tile-like. Regenerated part of the tail with small uniform scales without tubercles. Lamellae under the 1st toe 6/6, lamellae under the 4th toe 8/8. Eight preanal pores, no femoral pores or enlarged femoral scales.
Holotype of Hemidactylus ulii sp. n. (NMP6V 74833/2, male) from Al Hababi, Yemen. General habitus, lateral and ventral view of the head, precloacal region with preanal pores, right hind leg. Scale refers to the uppermost picture only.
Measurements (in mm): SVL 40.4, HL 11.5, HW 8.6, HD 5.2, E 2.8, AG 16.2.
Overall dorsal coloration brownish grey. An indistinct dark horizontal stripe in loreal and temporal area. Seven dark brown transverse bands across the nape and body, the one in scapular region being the most conspicuous. Dark brown bands also on the original part of the tail. Belly whitish.
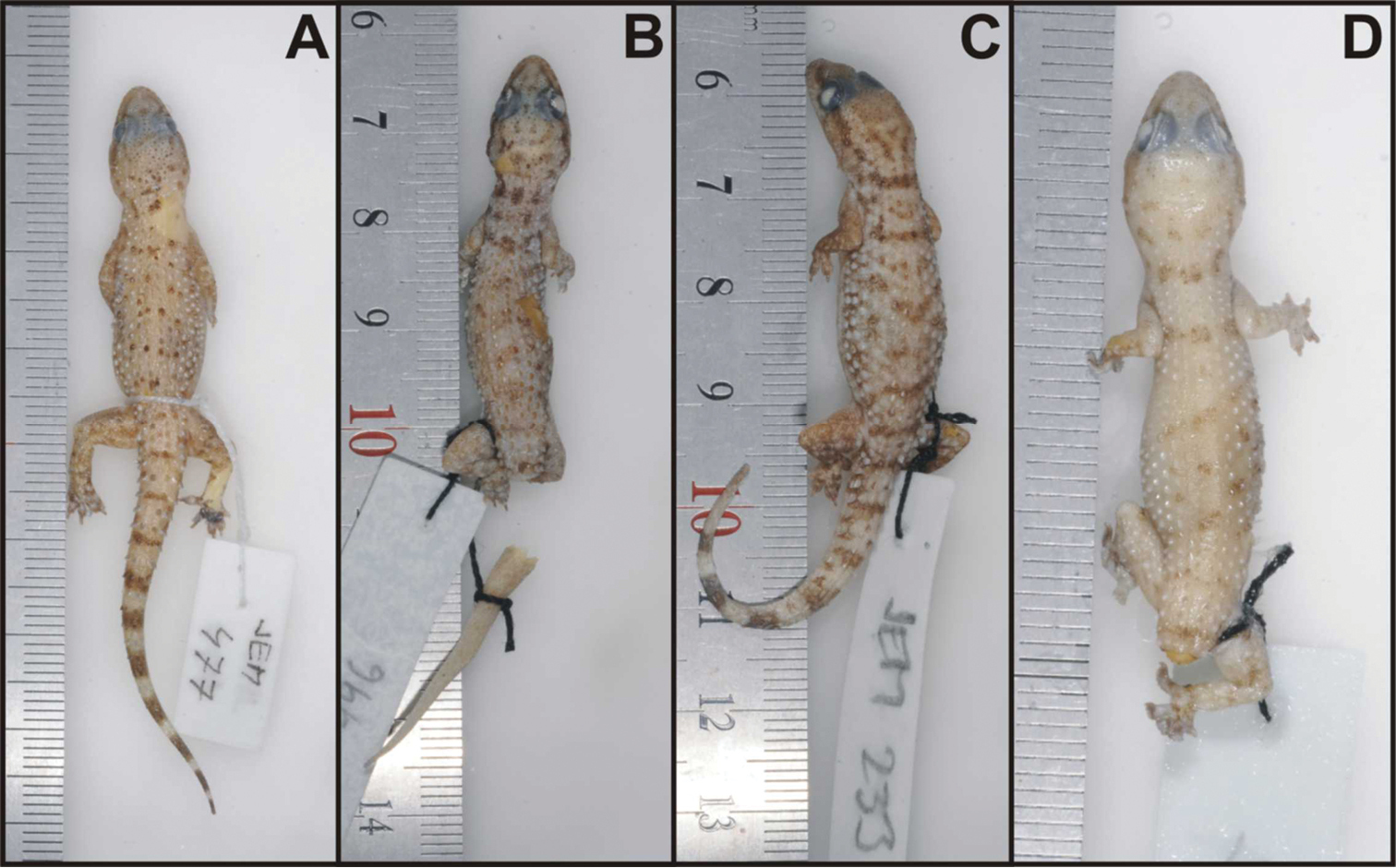
The paratypes (Fig. 9) differ from the holotype in the following features: number of upper labials 8–10; number of lower labials 7–9; four paratypes (NMP6V 74831/1, NMP6V 74832/1–2, NMP6V 748333/1) have uppermost nasals in wide contact; anterior postmentals in contact with 2nd lower labials on both sides (except of NMP6V 74832/1 where the arrangement is the same as in the holotype); longitudinal rows of enlarged tubercles 12–16; lamellae under the 1st toe 5–6, lamellae under the 4th toe 8–9. The intact tail of the paratype NMP6V 74833/1 has 7 segments bearing at least six enlarged spine-like tubercles and 9 dark brown transverse bands widening towards the tail tip.
Four (out of eight) paratypes of Hemidactylus ulii sp. n. A NMP6V 74833/1, male B NMP6V 74834/1, female C NMP6V 74831/1, female D NMP6V 74832/1, subadult female.
Measurements of paratypes (in mm): NMP6V 74831/1: SVL 40.7, HL 11.5, HW 8.2, HD 4.9, E 3.0, AG 19.0; NMP6V 74831/2: SVL 32.0, HL 9.3, HW 6.6, HD 3.7, E 2.1, AG 12.7; NMP6V 74832/1: SVL 32.7, HL 9.7, HW 7.0, HD 3.4, E 2.3, AG 14.3; NMP6V 74832/2: SVL 32.9, HL 9.3, HW 6.7, HD 3.6, E 2.4, AG 13.5; NMP6V 74833/1: SVL 36.8, HL 10.7, HW 8.0, HD 4.5, E 2.4, AG 14.1, TL 42.5; NMP6V 74834/1: SVL 39.4, HL 11.1, HW 8.1, HD 4.4, E 2.7, AG 16.7; NMP6V 74834/2: SVL 32.0, HL 9.5, HW 6.7, HD 3.9, E 2.5, AG 13.8; NHM-BS N41916: juvenile, not measured.
As already mentioned (Results), the level of genetic variability within Hemidactylus ulii sp. n. is very high. The species is divided into three well supported sublineages which reflect the geographic origin of the samples. Although there is a certain geographic separation corresponding with these sublineages, the exact limits are not distinct and also morphological variation among paratypes is not congruent with geography.
The species epithet “ulii” is a patronym for Prof. Ulrich Joger, a German herpetologist known as Uli among friends, in recognition of his important contribution to the knowledge of the herpetofauna of the Western Palearctic.
Hemidactylus ulii sp. n. is known from inland mid-altitude areas (292–1182 m) of southwestern Yemen (Fig. 6). Most specimens were collected in open dry wadis with scattered rocks and boulders, in stony deserts and also in the vicinity of villages in gardens and irrigated cropland fields.
The following reptile specieswere found to occur in sympatry with Hemidactylus ulii: Bunopus spatalurus Anderson, 1901; Hemidactylus yerburii yerburii Anderson, 1895; Pristurus crucifer (Valenciennes, 1861); Pristurus flavipunctatus Rüppell, 1835; Pristurus rupestris Blanford, 1874; Ptyodactylus sp.; Tropiocolotes scorteccii Cherchi and Spano, 1963; Acanthodactylus sp.; Chamaeleo arabicus Matschie, 1893; Pseudotrapelus sinaitus (Heyden, 1827); Trapelus flavimaculatus Rüppell, 1835; and Pelomedusa subrufa (Bonnaterre, 1789).
Previous phylogenetic studies of the Arid clade of Hemidactylus disclosed an extraordinarily rich diversity within this genus in the Arabian Peninsula (
The highlands of southwestern Saudi Arabia and Yemen are known to host a high number of endemic taxa (
We thank the following curators for granting access to collections under their care: U. Joger (NHM-BS), G. Köhler and his assistant L. Acker (SMF), R. Sindaco and G. Boano (MCCI), G. Doria (MSNG), S. Scali (MSNM), A. Nistri (MZUF), J. Vindum (CAS), B. Clarke and E. N. Arnold (BMNH), and T. Mazuch. We are very indebted to R. Kovář and R. Víta for collecting the Sinai material of Hemidactylus granosus, to S. Baha El Din for providing tissue sample of specimen SMB 10660 of the same species and to J. Červenka for field assistance in Yemen. We are grateful to two anonymous reviewers for their helpful comments. The study was supported by the NAKI project of the Ministry of Culture of the Czech Republic (# DF12P01OVV021 MKČR to JŠ and JM), by grant CGL2012-36970 to SC from the Ministerio de Economía y Competitividad, Spain (co-funded by FEDER). We are thankful to the Deanship of academic research at Taif University for funding the sample collection in Saudi Arabia (Grant no. 1-433-2108) and to Omer Baeshen, Environment Protection Agency, Sana'a, Republic of Yemen for issuing the collecting permit (Ref 10/2007).
Hemidactylus flaviviridis (8 individuals) - NMP6V 74858 (Oman, Jalan Bani Bu Hasan); NMP6V 74859/1–5 (Pakistan, Multan); NMP6V 74856 (Pakistan, Rakhni); NMP6V 74857 (Pakistan, Sukkur)
Hemidactylus jumailiae (18 individuals) - NMP6V 74818/1 (Yemen, near Al Bayda [At Dageeg]); NMP6V 74819 (Yemen, Sana’a); NHM-BS N41788, NHM-BS N41890 (paratype), NHM-BS N41891, NHM-BS N41893 (holotype), NHM-BS N41894 (paratype), NHM-BS N41897 (paratype) (Yemen, Ibb); NHM-BS N41898 (paratype, the same number as one of Hemidactylus yerburii montanus paratypes, Busais and Joger 2011b), NHM-BS N41899 (paratype) (Yemen, Thamar); BMNH1982.1143–44 (Yemen, Al Nabi Shuaib, 30 Km W. of Sana’a); BMNH1982.1145 (Yemen, Sana’a); BMNH1982.1146 (Yemen, Wadi Ahger, 45 Km. W. of Sana’a); BMNH1952.1.3.52 (Yemen, Sana’a); MSNG-YEM02, MSNG-YEM03 (Yemen, El Menghil); MCCI-R814 (Yemen, Hababah)
Hemidactylus mindiae (5 individuals) - NMP6V 71323/1–2 (Jordan, Jabal Ghazali); NMP6V 72739/1–3 (Jordan, Wadi Ramm Nughra Radet Salem)
Hemidactylus robustus (27 individuals) - SMF 8720 (lectotype), SMF 8721 (“Abyssinia” [Ethiopia and Eritrea]); SMF 8725–8726 – redetermined from Hemidactylus granosus (Egypt, Sinai); JS210, TMHC2012.07.092, TMHC2012.07.100 (Ethiopia, Jijiga), CAS130512 – redetermined from Hemidactylus macropholis as it is in the CAS catalogue (Kenya, vicinity of Mandera); NMP6V 74820 (Iran, Bandar Lengeh); NMP6V 74821/1–2 (Yemen, Wadi Zabid); NMP6V 74829 (Yemen, Bir Ali); JS144 (Kenya, Garissa); NMP6V 74867/1–3 (Oman, Muscat); NMP6V 74868 (Oman, Salalah); NMP6V 74869/1–7 (Oman, Mughsayl); NMP6V 74870/1–2 (Oman, Shisr); MCCI–R815 (Yemen, Zabid)
Hemidactylus saba (3 individuals) - NHM-BS N41912 (holotype, MorphoBank M305478–M305492), NHM-BS N41913 (paratype, MorphoBank M305493–M305504), NHM-BS N41914 (paratype, MorphoBank M305505–M305519) (Yemen, Marib)
Hemidactylus sinaitus (23 individuals) - BMNH82.8.16.27 (holotype, probably from Suakin, Sudan); BMNH97.10.28.83–85 (Sudan, Durrur, N of Suakin); BMNH97.10.28.87 (Sudan, Wadi Haifa); BMNH1974.3931 (Ethiopia, Mule River?, Danakil); BMNH1937.12.5.293–294 (Somalia, Borama district); BMNH95.5.23.7 (Yemen, Sheikh Osman, near Aden); BMNH1945.12.12.14 (Yemen, Bir Fadhl, Aden); NMP6V 74809/1–4 (Sudan, Wad Ben Naga); NMP6V 74810 (Sudan, 15 km SE Atbara); MZUF28645–646 (Yemen, Moka); MZUF10914, MSNM521 (Eritrea, Isola [island] Sheik-Said); MSNM523–524 (Eritrea, Ailet); CAS174021–022 (Sudan, Assalaya)
Hemidactylus turcicus (33 individuals) - NMP6V 34747 (Syria, Baniyas); NMP6V 34748/1–3 (Syria, Palmyra); NMP6V 34749 (Syria, Salkhad); NMP6V 70648/1–4 (Turkey, Kaş); NMP6V 70668 (Greece, Kastellorizo, St. Georgies); NMP6V 71056 (Egypt, Bahariya); NMP6V 71587/1–3 (Cyprus, Famagusta); NMP6V 71592/1–2 (Cyprus, Yali); NMP6V 72497 (Syria); NMP6V 74046/1–2 (Syria, Cyrrhus); NMP6V 74047/1–2 (Turkey, Antakya); NMP6V 74050 (Greece, Crete, Kavros); NMP6V 74131/1–3 (Syria, Palmyra); NMP6V 73626/1–3 (Turkey, Finike); NMP6V 70269 (Italy, Sardinia, Cagliari); NMP6V 72073 (Greece, Korfu, Nicos); NMP6V 74167 (Greece, Crete, Kavros); NMP6V 70667 (Greece, Kastellorizo); NMP6V 70163/5 (Egypt, Sharm el-Sheikh)
Hemidactylus yerburii yerburii (51 individuals) - NMP6V 74827/1–4 (Yemen, Jabel Habeshi); NMP6V 74825/1–2 (Yemen, Al Turbah); NMP6V 74826 (Yemen, N of Lahij, Wadi Tuban); NMP6V 74823/1–3 (Yemen, 14 km NW of Al Turbah); NMP6V 74824/1–2 (Yemen, 3 km S of Najd an Nashamah); NMP6V 74828/1–3 (Yemen, Al Hababi); NMP6V 74822/1–5 (Yemen, near Zinjubar); MSNG-YEM01 (Yemen, Ta’izz); MSNG-YEM05, MSNG-YEM06 (Yemen, Vahren); NHM-BS N41856–59, NHM-BS N41861–64, NHM-BS N41866, NHM-BS N41868–69, NHM-BS N41888 (Yemen, Tour Albaha); NHM-BS N41860 (Yemen, Lahij); NHM-BS N41871–72 (Yemen, Radfan); NHM-BS N41873 (Yemen, Shihr); NHM-BS N41875 (Yemen, Ariab); NHM-BS N41876–77, NHM-BS N41879–86 (Yemen, Lowder); NHM-BS N41887 (Yemen, Aden)
Hemidactylus yerburii montanus (57 individuals) - NMP6V 74802 (Yemen, Jabal Bura); NHM-BS N41751–52 (paratypes), NHM-BS N41758 (paratype), NHM-BS N41762–63, NHM-BS N41765–66, NHM-BS N41768–69, NHM-BS N41770 (paratype), NHM-BS N41772–74, NHM-BS N41779, NHM-BS N41783 (paratype), NHM-BS N41785 (paratype), NHM-BS N41791 (paratype), NHM-BS N41793 (paratype), NHM-BS N41797–800 (paratypes), NHM-BS N41802–06 (paratypes), NHM-BS N41807 (paratype), NHM-BS N41809 (paratype), NHM-BS N41811–15 (paratypes), NHM-BS N41818 (paratype), NHM-BS N41821 (paratype), NHM-BS N41823 (paratype), NHM-BS N41836 (holotype), NHM-BS N41839, NHM-BS N41840 (paratype), NHM-BS N41842 (paratype), NHM-BS N41843, NHM-BS N41844 (paratype), NHM-BS N41846, NHM-BS N41848, NHM-BS N41851–52, NHM-BS N41867 (paratype) (Yemen, Ibb); NHM-BS N41771 (paratype) (Yemen, Yareem); NHM-BS N41789–90 (Yemen, Thamar); NHM-BS N41833–34 (paratypes) (Yemen, Wadah); NHM-BS N41853–55 (paratypes) (Yemen, Sana’a).