(C) 2013 Zhiliang Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The preimaginal stages including egg, mature larva and pupa of Pseudaspidapion botanicum Alonso-Zarazaga & Wang, 2011 were described and figured, diagnostic characters of larva and pupa were discussed, and corresponding biological information was supplied. The nomenclature of frontal setae in the larva compared with curculionid weevils, the absence of the hypopharyngeal bracon in the larva, and the metafemoral setae in the pupa were discussed. Common and different characters among the larvae of Pseudaspidapion botanicum, Aspidapion radiolus (Marsham, 1802) and Aspidapion aeneum (Fabricius, 1775) were also provided.
Apionidae, Pseudaspidapion botanicum, larva, pupa, morphology, biology
Pseudaspidapion botanicum Alonso-Zarazaga & Wang, 2011 belongs to the tribe Aspidapiini Alonso-Zarazaga, 1990. There are 6 species of the genus Pseudaspidapion Wanat, 1990 recorded from China, 2 of which are only known from female specimens, and almost all of which were described without biological information apart from Pseudaspidapion botanicum (Alonso-Zarazaga et al., 2011). Additionally, no species of Pseudaspidapion have been previously studied for developmental stages.
The knowledge of weevil larvae is still very low compared with that of the adults because of a variety of reasons (
Therefore, in order to supplement to the available data of Pseudaspiapion for distinguishing this oriental genus from other lingeages in Aspidapiini (Aspidapion Schilsky, 1901, Flavopodapion Korotyaev, 1987 and Harpapion Voss, 1966) (
Specimens examined of larvae and pupae are deposited in the Institute of Zoology, Chinese Academy of Sciences, Beijing (IZCAS).
Descriptions were made and photographs were taken with a CCD Qimagine MicroPublisher 5.0 RTV mounted on a Zeiss SteREO Discovery. V12 microscope. Extended focus images were generated with Auto-Montage Pro 5.03.0061 and edited with Adobe Photoshop CS5 if required. Microscopic slides were studied under a Leica DM 2500 microscope and photos were taken with a Nikon CoolPix 5400. Drawings were made from the original photographs by using the software Adobe Illustrator CS5, or directly by using a drawing tube attached to the microscope.
Nomenclature of the larval chaetotaxy mainly follows
Egg: round, yellowish to white, diameter ca. 0.2–0.3mm.
Mature larva: Meaurements (mm): Body length: ca. 2.0–3.0, width: ca.1.0–1.5; Capsule length (in front view): ca. 0.4–0.45, width ca. 0.4–0.44.
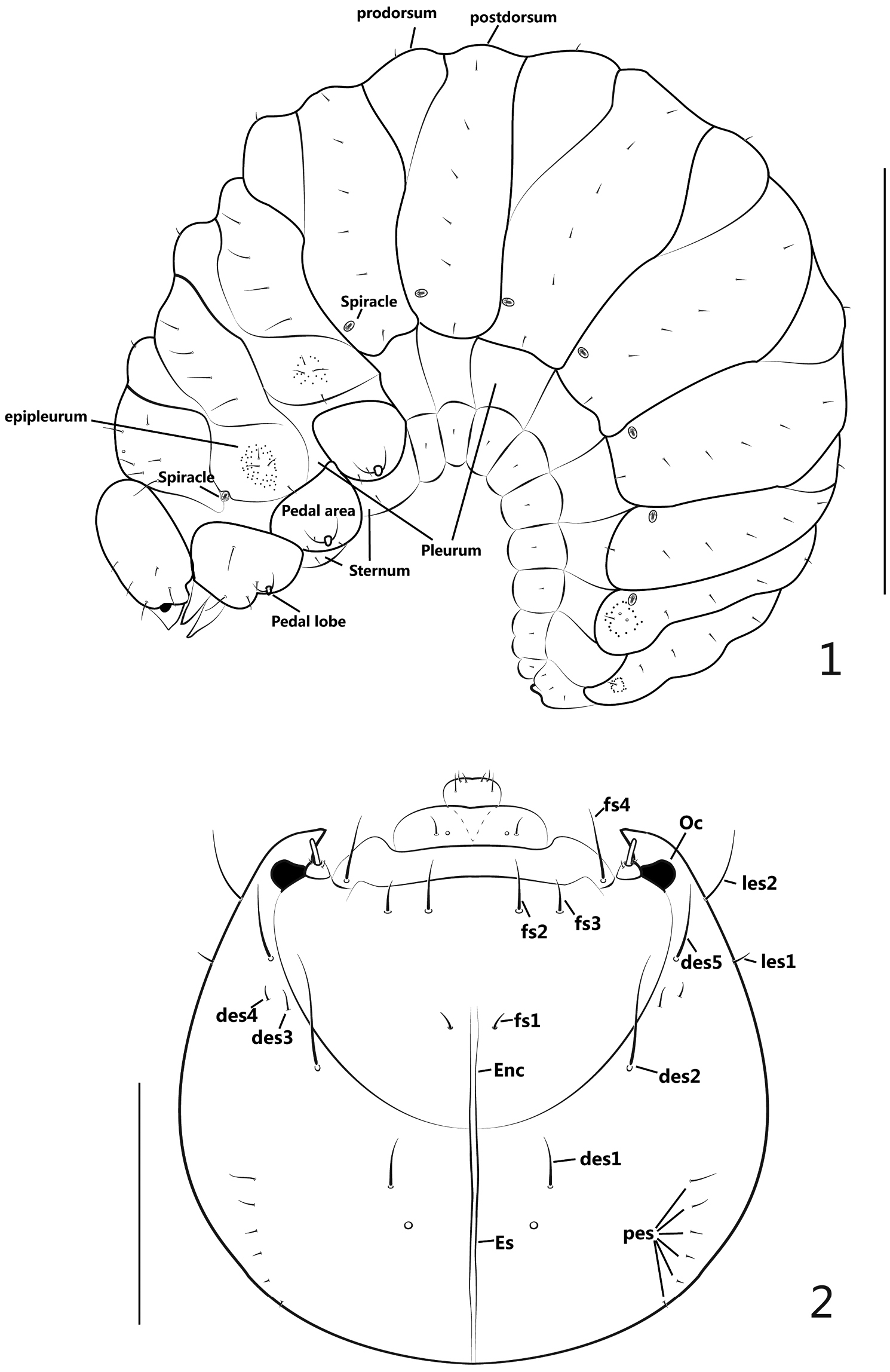
General appearance (Figure 1): Body plump and distinctly curved (C-shaped), with a large quantity of white fat inside, sub-cylindrical, with a comparatively small, yellowish to pale brown head; cuticle minutely spiculate, without visible pigmented, sclerotized areas; body segments with very short setae, pedal lobes in conspicuous knobs.
Larva. 1 mature larva, lateral view 2 head, dorsal view. Scales: 1: 1mm. 2: 0.2mm.
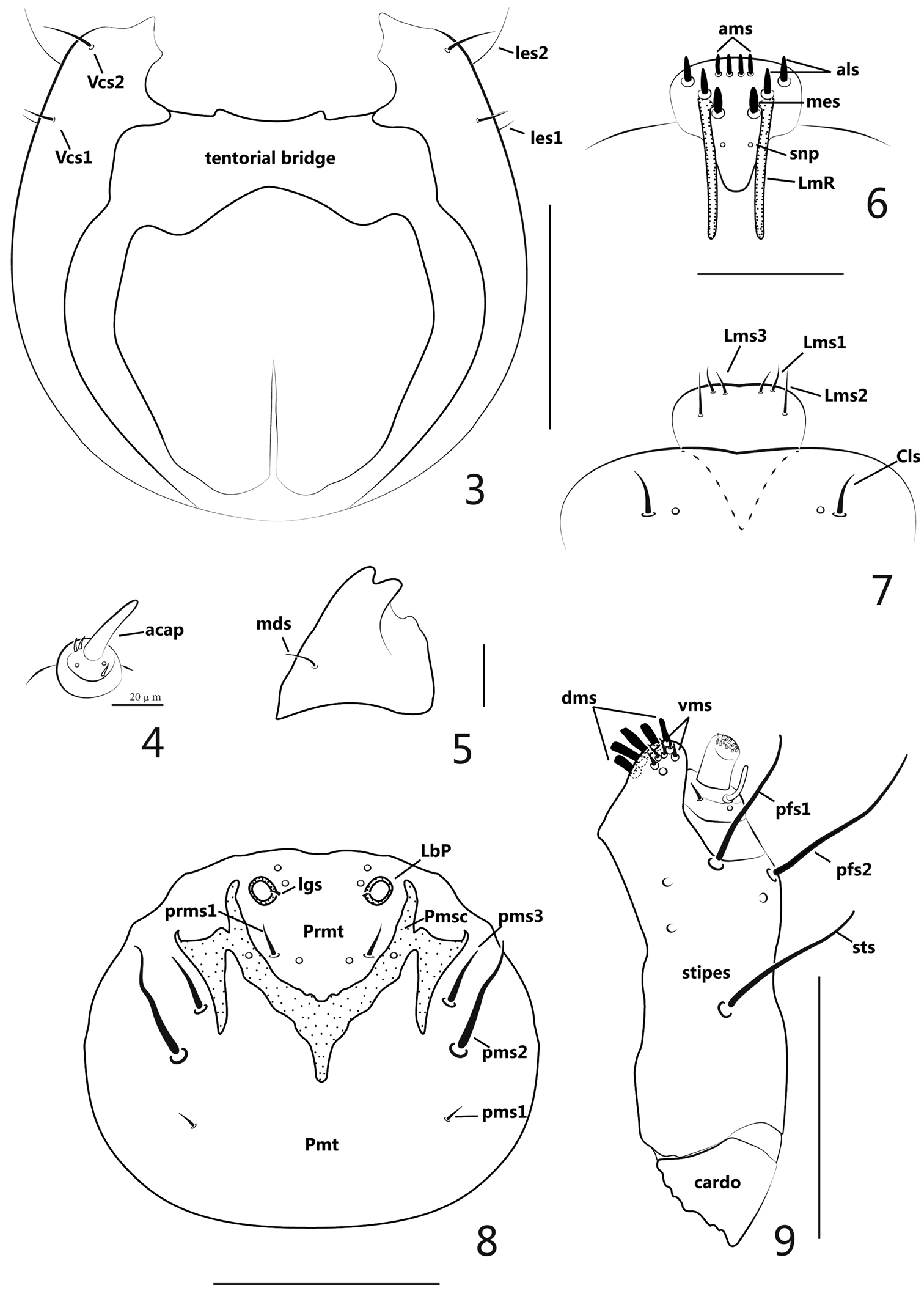
Head (Figures 2–3) moderately retracted within prothorax, epicranial line distinct, undivided, wide; frontal lines distinct, narrow, completely extended to mandibular joint; endocarina short, extended to 1/2 the length of frons; epicranium with 2 pairs of lateral setae (les), les1 short, les2 long, more than 3 times longer of les1; dorsal epicranium with 5 pairs of setae (des), des1 short, about half as long as des2, des3 and des4 much shorter, des4 a bit longer than des3, and des5 as long as des2; posterior epicranium with 6 pairs of setae (pes), pes1 shortest, pes2-6 gradually longer than the former one; frons with 4 pairs of setae (fs), fs1 very short, adjacent to end of epicranial line, fs2-3 located near epistoma, nearly transversely aligned, fs2 longer than fs3, fs4 laterally positioned at epistoma close to antennae, about 1.5 × as long as fs2; ventral epicranium with 2 pairs of lateral setae (Vcs), correspondingly situated near les; postoccipital condyles absent, tentorial bridge wide with 2 small but moderately acute anterior projections and 2 large, obtuse-angled posterior projections; hypopharyngeal bracon absent; clypeus transverse, bearing 1 pair of setae (cls), inner side of cls bearing 1 pair of sensilla; antenna reduced to 1 article (Figure 4), with rod-like accessory sensory appendage (acap) more than 3x as long as wide, with 3 spinose projections and 2 sensilla; ocellus present, evidently projected, externally close to antenna.
Larva. 3 head, ventral view 4 antenna 5 left mandible 6 epipharynx 7 labrum and clypeus 8 labium 9 right maxilla. Scales: 3: 1mm. 4: 0.02mm. 5: 0.05mm. 6–7: 0.05mm. 8–9: 0.1mm.
Mouthparts (Figures 5–9): labrum (Figure 7) sub-semicircular, with 3 pairs of setae (Lms), Lms1 and Lms3 short, close to distal margin of labrum, Lms2 distinctly longer and centrally localized; epipharynx (Figure 6) with 2 long, stout lateral rods (LmR) surpassing the suture between epistoma and clypeus, 2 pairs of anterolateral setae (als), 2 pairs of anteromedian setae (ams), 1 pair of median setae (mes), all epipharyngeal setae mentioned above stout, short and apically rounded, 1 pair of epipharyngeal sensory pores (snp) between pair of LmR; mandibles asymmetric, left mandible (when mouthparts viewed anteriorly) (Figure 5) apically bidentate, about as long as wide, cutting edge with 1 weakly prominent, rounded tooth, its laterodorsal surface with 1 mandibular seta (mds), without visible sensilla, right mandible with cutting edge nearly straight; labium (Figure 8) almost membranous except sclerotized area; labial palpus (LbP) vestigial, its apex a bit higher than surface of labium, like two big socket pores; premental sclerite (Pmsc) distinctly dilated, “Y” shaped, with 1 pair of sensilla, outer margins of Pmsc with exteneded sclerotized piece, irregularly triangular; ligulate area with 1 pair of tiny setae (lgs) and 2 pairs of sensilla; prementum (Prmt) with 1 pair of setae (prms) and 1 pair of sensilla, prms1 long and stout, prms2 quite short, close to inner side of labial palpi; postmentum (Pmt) with 3 pairs of setae (pms), pms1 shortest and fine, pms2 longest and stout, pms3 a bit shorter than pms2; maxillary palpus (MxP) (Figure 9) with 2 segments, basal segment with 1 long and basally curved accessory process, 1 short inner seta, 1 sensillum close to accessory process, apical segment cylindrical and apically flattened with dense crenulate setae; mala with 5 dorsal robust setae (dms) and 4 shorter, more acute ventral setae (vms), 1 sensillum; stipes (st) bearing 1 stipital setae (sts), 2 palpiferal setae (pfs) and 3 sensilla, all 3 setae extremely thick and long, sts basally medioventral, pfs1 apically medioventral, pfs2 lateroventral; cardo completely divided from stipes.
Setae of thorax and abdomen (Figure 1) described for one side only.
Thorax: pronotal shield simple without fold, unsclerotized; meso- and metanotum each with 2 folds, divided into prodorsum (fold1) and postdorsum (fold2); spiracle laterally intersegmental between pro- and mesothorax, bicameral; prothoracic epipleurum indistinct, meso- and metathoracic epipleura with outline distinct, centrally tuberculate around setae; pedal area well-defined, pedal lobe (papillae) present, 2-segmented; pronotum with 6 setae (pns): 4 longitudinally aligned and close to anterior margin, another 2 nearly at middle area; meso- and metanotum both with 9 setae: prodorsum with 1 short setae (prs), epipleurum with 4 setae, 3 setae surrounded by numerous small, tuberculate projections, another situated close to pleurum; postdorsum with 4 relatively longer setae (pds), longitudinally aligned; pedal area with 3-4 setae; sternum with 1 tiny seta.
Abdomen: tergites I-VI with 2 folds, prodorsum(fold1) with 1 seta on disc, postdorsum(fold2) with 6 setae, shorter than thoracic setae and longitudinally aligned; tergites VII-VIII undivided with 5 setae, longitudinally aligned, basal 1 seta surrounded with a circle of sparse tubercles; tergite IX undivided and greatly reduced with 3 setae; 7 spiracles present, size similar, bicameral, anterolaterally located on tergites I-VII, respectively; pleura I-VIII without setae, each sternum with 1 seta.
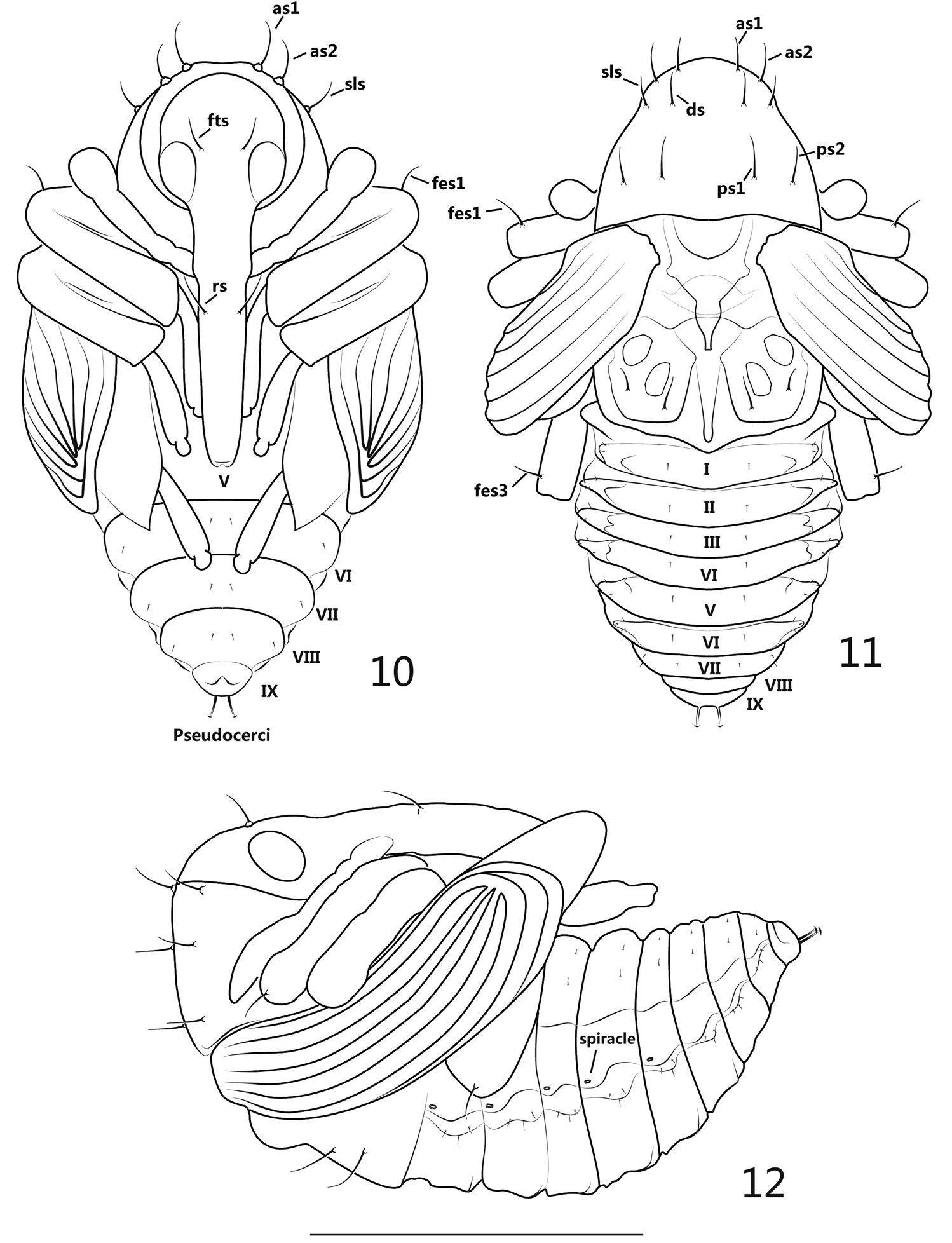
Pupa (Figures 10–12): Measurements (mm): length: ca. 1.9–2.0, width: ca. 1.3.
General appearance: theca transparent with semitransparent setae, setae greatly reduced in number, inner body pure white.
pupa. 10 ventral view 11 dorsal view 12 lateral view. Scales: 10–12: 1mm.
Rostrum: in ventral view, apex reaching ventrite V, mesorostrum visibly dilated, mandibular theca weakly projected; 1 pair of rostral setae (rs) positioned in front of antennal insertion. Head: frons with 1 pair of setae (fts), about as long as rostral setae, situated at the level of hind margin of eyes; antennae basally situated near prosternum and apically extended to propleurum, sub-parallel to protibia. Thorax: in dorsal view, pronotum with 2 pairs of apical setae (as), 1 pair of sublateral setae (sls), 1 pair of discal setae (ds) and 2 pairs of posterolateral setae (pls), these long, stout and similarly elongated setae forming 2 longitudinal rows on pronotum, 3 positioned in each row and transversely localized; in ventral view, as1, as2 and sls completely visible, distinctly longer than vs and fas; mesonotum without setae; metanotum bearing 2 pairs of setae near lateral and hind margins of metanotum, respectively, subequal in length to pronotal setae. Legs: in ventral view, metatibiae and femora covered by pterothecae, front and middle legs and meta-tarsomere visible, in lateral view, pro- and mesofemora both covered by pterothecae; pro- and metafemora apically bearing 1 slightly outcurved seta; each seta mentioned above arising from a tuberculate base. Abdomen: in ventral view, ventrites V-IX visible, in dorsal view ventrites I-VI about equal in length and width, ventrites VII-IX clearly and gradually reduced; 5 spiracles present, positioned on pleura I-V, bicameral; peudocerci narrow, elongate and slightly outcurved in ventral and dorsal views, subterminally positioned at abdominal segment IX, a bit shorter than femoral setae, apex lenticular; in ventral view, the completely visible ventrites VI-VIII with 2 pairs of setae on each segment, 1 pair situated medially, another pair situated laterally; in dorsal view, tergites I and VII each bearing 3 pairs of setae, 1 pair situated medially, 2 pairs situated laterodorsally; tegites II-VI each bearing 4 pairs of setae, 1 pair situated medially, 3 situated laterodorsally; tergites VIII-IX without clearly visible setae; all abdominal setae short and thin.
Biological information: Pseudaspidapion botanicum was collected from Grewia biloba G. Don var. parviflora (Bunge) Hand.-Mazz (Malvaceae: Grewioideae). The adults feed on leaves and buds of their host while they mate and oviposit in the bud (Alonso-Zarazaga and Wang 2011). After dissection of some 300 buds, we have found that 1-4 oviposition holes can be found on one bud surface, but only 1–2 eggs or larvae per bud are normally found, and 3 or more eggs or larvae were quite rare (Figures 13–15). The mature larva almost completely consumes the internal organs of the bud and then constructs a chamber for pupation (Figure 16), so those buds parasitized by Pseudaspidapion botanicum will never blossom. In normal conditions, Pseudaspidapion botanicum can seriously reduce the fructification rate of Grewia biloba, but the damage done seems not to seriously harm the plant’s reproductive rate, and the vegetative growth is not decreased by feeding on other areas. Furthermore, in late July, most of the adults disappear from their host and migrate to some other plants in sunny areas. The same case has been reported by other authors, for example, Ehret (1983) reported that apionid weevils sought shrubs and tree crowns to avoid unfavorable weather when growth of their host plants ceased temporarily or permanently, whether during the growth season or at the end of it. We have not yet had an opportunity to study the overwintering behavior of this species.
13 the buds of Grewia biloba 14 egg positioned among stamens in the bud 15 first instar larva 16 pupa.
The diagnostic characters of the preimaginal stages of Pseudaspidapion botanicum can be summarized as follows. Larva: (1) antennae with acap more than 3× as long as wide, 3 spinose projections and 2 sensilla (2) head with 10 des, 8 fs, 4 les, 12 pes and 4 vcs; (3) labrum with 6 lms; (4) epipharynx with 4 ams, 4 als, 2 mes and 2 snp; (5) maxilla with 1 stps, 2 pfs and 3 sensilla; (6) mala with 5 dms and 4 vms; (7) each side of Pmsc extended outward with 1 irregularly triangular sclerotization; (8) labial palpus (lbp) vestigial, pore-shaped; (9) hypopharyngeal bracon absent; (10) thoracic and abdominal spiracles 1+7 in number, both bicameral. Pupa: (1) the number of setae significantly reduced on rostrum (2rs) and head (2fts); (2) pronotum with 4as, 2sls, 2ds, 4ps; (3) only metanotum with 4 setae; (4) pro- and metafemoral apical setae present, mesofemoral setae absent; (5) in lateral view, ventrites I-V each with 1 spiracle.
In terms of
Furthermore, most authors did not mention whether mandibles are symmetric or not, and few indicated the direction of the mouthpart, which is particularly important when the mandibles are asymmetric. Inconsistent terms of morphological position resulted in those instances when “left” was identified by looking toward the body center (= proximal view) rather than away (= distal or cranial view). To avoid such ambiguities, we suggest that the left mandible should be specified and described as seen from the body center to the anterior in dorsal view.
The hypopharyngeal bracon is a bar connecting the left and right sides of the hypopharyngeal margins and supporting the hypopharynx. The presence of the bracon is one of the characters separating curculionoid larvae from those of Bruchidae and Chrysomelidae (
It is also worthy to stress that the absence of mesofemoral setae in the pupae of Apionidae has not been recorded before, while some or all femoral setae of some groups of Anthribidae, Brentinae and Curculionidae could be absent (
Additionally,
Character comparison among Aspidapion aeneum, Aspidapion radiolus and Pseudaspidapion botanicum.
| Species | Aspidapion aeneum1) | Aspidapion radiolus1) | Pseudaspidapion botanicum |
|---|---|---|---|
| head | as long as wide | wider than long | as long as wide |
| antennal accessory sensory appendage (acap) | slender, more than 2 × as long as wide | slender, more than 2 × as long as wide | rod-like, more than 3 × as long as wide |
| endocarina | long | long | short |
| left mandibular cutting edge | with a rather distinct tooth | with a strong, rounded dilatation | with a weakly prominent, rounded tooth |
| setae of labrum (lms) | 4 pairs | 4 pairs | 3 pairs |
| anterolateral setae of epipharynx (als) | 3 pairs | 3 pairs | 2 pairs |
| prms (=ventral bristles of prementum) | as close together as inner basal end of palpi | as far apart as inner basal end of palpi | as far apart as inner basal end of palpi |
1) Emeden’s key (1938)
We are grateful to Dr. Liu Ning for providing partial support for this research, Dr. Jens Prena for his valuable suggestions and the anonymous reviewer for considerably improving the manuscript. This research was supported by the National Natural Science Foundation of China (31201735/31172130/ 31210103909/J1210002), project grant CGL2010–15786 (Ministerio de Ciencia e Innovación, Spain) and the Invited Professor Award (2009) of the Chinese Academy of Sciences to the second author.