(C) 2013 Cecilia Waichert. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Waichert C, Pitts JP (2013) Two new species of Abernessia Arlé (Pompilidae, Ctenocerinae). ZooKeys 353: 71–79. doi: 10.3897/zookeys.353.6223
Two new species are added to the rare pompilid genus Abernessia Arlé. Abernessia capixaba sp. n. and A. giga sp. n. are described and illustrated. This is the first record of the genus from the states of Espírito Santo and Minas Gerais, Brazil. The genus now contains four species. A brief discussion of generic characters, illustrations, and a key to the known species of Abernessia are provided.
Ctenocerinae, Neotropical, new record, spider wasp, taxonomy, key
The spider wasps (Pompilidae) form a cosmopolitan family comprised of approximately 5, 000 species and more than 120 genera (
Ctenocerinae has 18 described genera and is found in South America, Africa, and Australia (
The Neotropical genus Abernessia was described by
Abbreviations used in the descriptions are the same as those used by
The males described here were collected as part of the project “N.E.S.H. – Nucleus of Excellence in Systematics of Hymenoptera: broadening agricultural and environmental frontiers of Espírito Santo”, grant FAPES/CNPq #52263010/2011, coordinated by Dr. Celso O. Azevedo.
Material examined is deposited in Coleção Entomológica da Universidade Federal do Espírito Santo (UFES), Vitória, Brazil and Zoological Museum University of Copenhagen (ZMUC), Copenhagen, Denmark, as indicated.
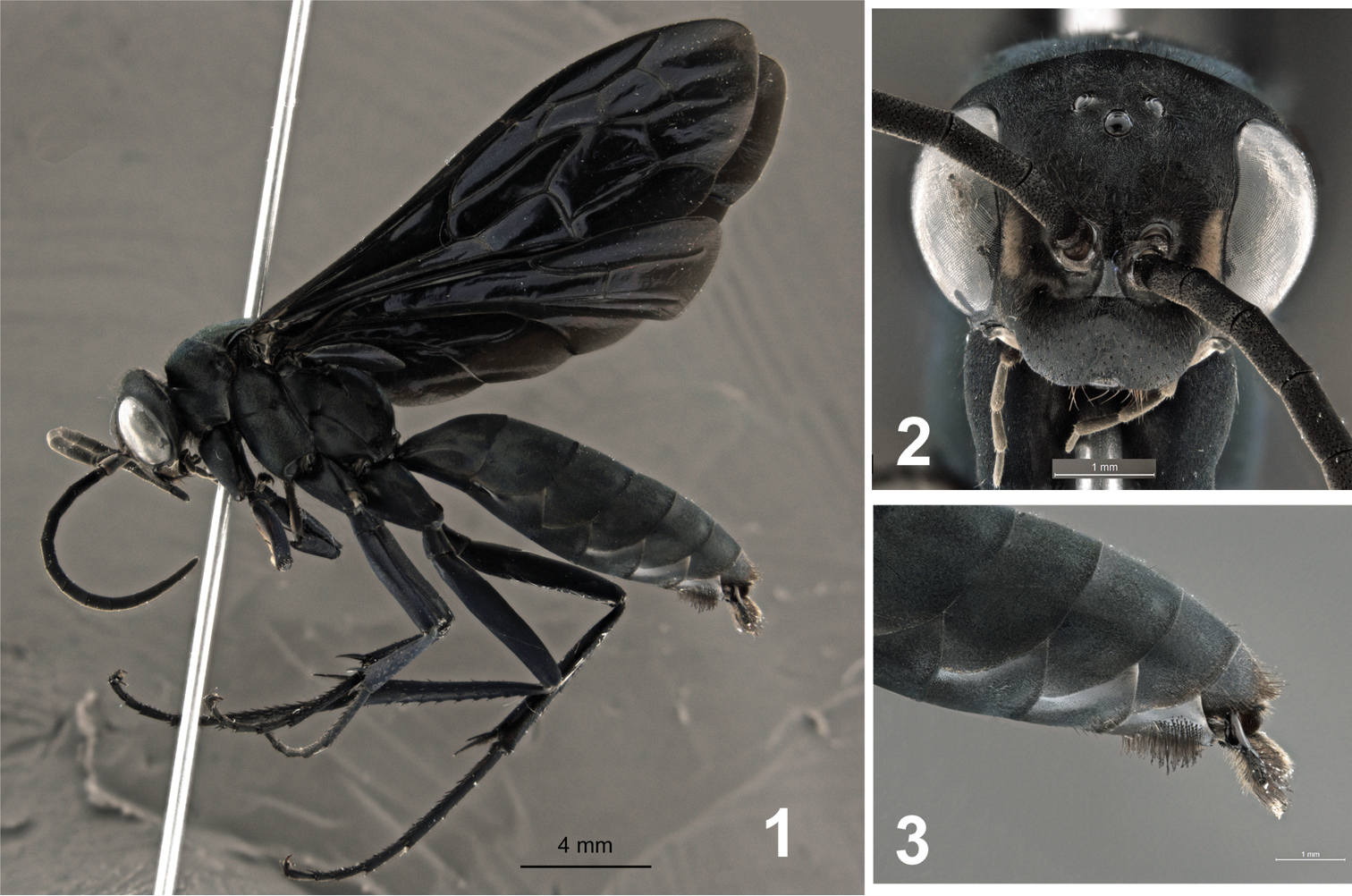
♂ (Figs 1–3), pinned, with genitalia in a separate vial, labeled “BRAZIL: E[spírito] S[anto], Laranja da Terra, Joatuba, Fazenda Betzel, 19°50'25"S, 40°49'40"W, Malaise Bosque 9, 280–430 m, 05–12.x.2012, M.T. Tavares & eq. col. (UFES #135382)”.
Male holotype of Abernessia capixaba sp. n. 1 Lateral habitus 2 Head, frontal view 3 Apex of metasoma in lateral view.
2♂: BRAZIL: E[spírito] S[anto], Laranja da Terra, Joatuba, Fazenda Betzel, 19°50'25"S, 40°49'40"W, 280–430 m, 05-12.x.2012, M.T. Tavares & eq. col., Malaise Bosque 3 (1♂) (UFES #134333), Malaise Bosque 12 (1♂) (UFES #134542).
This species can be recognized by the following unique combination of characters: the integument is black with scale-like setae reflecting greenish metallic (Fig. 1); the face has small whitish spots on inner margin of the eyes (Fig. 2); and the wing is darkened without pale maculations (Fig. 1).
Body length 2.00 cm; fore wing 1.82 cm; maximum wing width 0. 57 cm.
Coloration. Integument black with pale yellow maculation on inner margin of eyes; body covered with pubescence with bluish-green metallic reflections (Fig. 1); clypeus, antennae, labial and maxillary palpi black; wings black with weak purple reflections; veins dark castaneous; legs with greenish-purple-blue reflections.
Head. Head wide; TFD 1.2 × FD; MID 0.7 × FD; punctuation conspicuous, small, shallow. Ocelli in obtuse angle; lateral ocelli closer to each other than to compound eyes; POL 0.8 × OOL. Mandible narrow, base about 2.0 × wider than apex, with two sharp apical teeth; 1/3 of base covered by thin copper pubescence. Clypeus undifferentiated from frons, flat, bilobed, apical median margin invaginated (Fig. 2); clypeal lobes rounded (Fig. 2); LC 0.4 × WC. Labrum partially exposed. Maxillary beard not present. Flagellum elongate; length of second flagellomere 2.5 × width; ratio of the scape, pedicel, and flagellomeres 1-2 11:4:14:15; WA3 0.5 × LA3; LA3 0.4 × UID; scape with erect setae on internal margin. Torulus circular, antennal scrobe large.
Mesosoma. Pronotum not elongated (Fig. 1), width 3.3 × length; posterior margin arched, anterior margin slightly invaginated medially; propodeal disc with thin-shallow median sulcus. Notauli shallow, present on 1/5 of anterior margin. Postnotum striated. Propodeum with punctures small, almost inconspicuous under setae; propodeal disc covered with short-apressed pubescence, setae equally abundant on propodeal disc; propodeal disc slightly elevated medially, edges of disc rounded. Wing elongate; maximum width 0.3 × length; third submarginal cell about as long as second submarginal cell; second recurrent vein straight, meeting third submarginal cell half distance from base to apex of cell (Fig. 1). Fore tibia with short, sharpened spines, posterior edge angulated. Front tarsal claw bifid, mid and fore claw dentate. Tarsi spinose.
Metasoma. Metasoma covered by short, scale-like setae. Sternum 7 covered by thick, long setae, marginal setae longer than remaining setae, apex of setae sinuous and dilated (Fig. 3).
Genitalia. (Figs 4–6) Parapenial lobe bifid; lobe wide, short, almost shield-like; outer apex lanceolate, higher than inner apex; inner apex rounded, broad. Dorsal lobe of digitus slightly longer than edeagus, apex wide, rounded, with small extension ventrally; ventral lobe of digitus, short, length 0.3 × paramere length, spatulate, base with long, thin setae (Figs 4, 5). Aedeagus short, total length 0.6 × length of paramere + gonobase, split, lateral margins rolling inwards, apex rounded (Figs 4, 5). Paramere as long as aedeagus, constricted on base, wide apically; apex lanceolate, covered with long setae, inner face flat, outer rounded; setae long, longer marginally, apex dilated. Subgenital plate elongate, wide; apex narrower, rounded; apical margin of apex polished, glabrous; abundant setae, long, thin along entire length (Fig. 6).
Male genitalia, paratype of Abernessia capixaba sp. n. 4 Dorsal view 5 Ventral view 6 Genital plate.
No significant morphological variation was observed.
The specific epithet refers to the type locality. Capixaba refers to a person born in Espírito Santo State, Brazil.
Males of Abernessia capixaba are distinguished from those of Abernessia prima Waichert & Pitts (2011) by the lack of pale maculation on the metasoma and the fully fuscous fore wing (Fig. 1). In Abernessia prima the fore wing is partially yellow and maculations are present on the face, metasoma, and fore wing. Finally, the setae on the subapical metasomal sternite are longer on the outer margin in Abernessia capixaba.
♀ (Figs 7–9), labeled “[BRAZIL]: Minas Gerais, Reinhardt. Mus: Drenis (ZMUC)”.
Female holotype of Abernessia giga sp. n. 7 Lateral habitus 8 Face, frontal view 9 Fore and hind wings.
This species can be recognized by the following unique combination of characters: the integument is black, with scale-like setae reflecting metallic bluish-green (Fig. 7); the antennal scape is red apically (Fig. 8); the clypeus is slightly folded ventrally on the apical-lateral margin, and the wing is dark with a large pale brown band (Fig. 9).
Body length 2.81 cm; fore wing 2.00 cm; maximum wing width 0.63 cm.
Coloration. Head black; mesosoma black, legs brown with purple reflections (Fig. 7); metasoma brown, tergites distally reddish, hypopygium reddish; body pubescence with bluish-green metallic reflections; wings dark brown with pale brown spots, hind wing with pale brown band (Fig. 9); wing venation brown, pale brown on pale spots; stigma brown (Fig. 9).
Head. Head as long as wide; TFD 1.0 × FD; MID 0.7 × FD; punctuation conspicuous, small, shallow. Pubescence abundant, short, thin, apressed, with metallic reflections from above ocelli to vertex. Eye short, HE 0.6 × FD; vertex long, distance from posterior ocellus to vertex 0.2 × FD. Ocelli in an obtuse angle; lateral ocelli closer to each other than to compound eyes; POL 0.3 × OOL. Mandible wide, elbowed, with two sharpened apical teeth; distal margin with a row of setae. Clypeus undifferentiated from frons, flat, bilobed, apical median margin invaginated; clypeal lobes rounded, sides slightly turned downwards (Fig. 8); LC 0.4 × WC. Labrum partially exposed, setose (Fig. 8). Maxillary beard not present. Flagellum elongate; length of second flagellomere 3.1 × width; ratio of the scape, pedicel, and flagellomeres 1-2 15:3:16:14; WA3 0.3 × LA3; LA3 0.4 × UID; scape curved, internal margin flat. Torulus circular, antennal scrobe large.
Mesosoma. Pronotum not elongate (Fig. 7), width 1.6 × length; posterior margin arched, anterior margin slightly invaginated medially; propodeal disc with thin-shallow median sulcus, lateral margins rounded. Notauli shallow, complete. Postnotum striated. Propodeum with punctures small, almost inconspicuous under setae; propodeal disc covered with short-apressed pubescence, setae equally abundant; propodeal disc slightly elevated medially, edges rounded. Wing long; maximum width 0.3 × length; third submarginal cell about as long as second submarginal cell; second recurrent vein straight, meeting third submarginal cell about half the distance from base to apex of cell; 2r-m straight (Fig. 9). Fore tibia with short, sharpened spines, posterior edge angulated. Front tarsal claw bifid, mid dentate (hind tarsi broken in holotype). Tarsi spinose.
Metasoma. Metasoma long (Fig. 7), total length 1.4 × mesosoma + head lengths, wide; covered by short, scale-like setae. Apical tergite setose.
The specific epithet was taken from Greek, giga, meaning “giant” in English. It refers to the large size of the specimen.
This species can be distinguished from Abernessia irmgardae Arlé by having the wing brown, with venation both pale and dark brown. In Abernessia irmgardae the wing is yellow and the venation is only dark brown. Additionally, the eyes are convergent on vertex in Abernessia giga and the vertex expanded unlike in Abernessia irmgardae.
| 1 | Wings yellow, slightly darkened at the base; fore wing with pale brown vein; head with vertex weakly prolonged posteriorly; eyes not convergent on vertex | Abernessia irmgardae Arlé |
| – | Wings darkened, with spots in pale brown (Fig. 9) near the base; fore wing with venation both pale and dark brown; hind wing with a pale brown band; head with vertex strongly prolonged posteriorly; eyes convergent on vertex (Fig. 8) | Abernessia giga sp. n. |
| 1 | Wings pale brown near base, darkened apically; large whitish spots on inner margin of eyes, clypeus and metasoma | Abernessia prima Waichert & Pitts |
| – | Wings black with purplish reflections (Fig. 1); small whitish spots only on inner margin of eyes (Fig. 2); whitish spots absent on metasoma | Abernessia capixaba sp. n. |
Abernessia is a species-poor genus totaling four described species. All four species of Abernessia appear to be restricted to Brazil. Two species have precise collecting data (Abernessia prima and Abernessia capixaba) indicating that they were collected in ecotones of the Brazilian cerrado and Atlantic forest. Additionally, this is the first record of Abernessia in the states of Espírito Santo and Minas Gerais and the first time that more than one specimen was collected in the same locality providing the opportunity to account for morphological variation for the group. Although males and females differ in several features, all four species of Abernessia are diagnosed by the following characteristics: the clypeus is flat and undifferentiated from the face; the antennal scrobe is large (Figs 2, 8); the labrum is partially exposed with setae present apically; the metasoma is longer than the mesosoma; and the pronotal disc is flat dorsally with a median sulcus on the anterior margin. Additionally, males have yellowish pale spots on the inner side of the eyes (Fig. 2); the subapical sternite and paremere have long setae that are swollen on the apex (Fig. 3); and the genitalia has a short base, which gives a false impression of long parameres (Figs 4, 5).
We were unable to associate the sexes of the newly described species. Unfortunately, the type of Abernessia irmgardae could not be located for morphological studies. Based on the wing and body coloration, Abernessia capixaba could be the male of Abernessia irmgardae, and Abernessia prima of Abernessia giga. However, Abernessia capixaba differs from Abernessia irmgardae by lacking reddish color on the median flagellomeres and on the apex of tarsi, and by having the wings black, whereas in Abernessia irmgardae the wings are yellow but dark brown basally. Additionally, the second submarginal cell is slightly different from the type by having the vein inclined downwards, while in the female type it is bent upwards. Abernessia giga resembles Abernessia prima by having pale spots on the wing; Abernessia prima, however, has a different pattern of wing coloration and presents whitish spots on metasoma. In other genera of Pompilidae (e.g. Priocnemella Banks, Phanochilus Banks), wing venation and coloration usually matches between sexes of a single species, even when sexual dimorphism is present. Although, wing venation in Abernessia seems only slightly variable, differences between specimens are obvious. Because the dimorphism sexual is not understood in the genus yet, we believe it unwise to associate these sexes prematurely.
We are grateful to Dr. Celso Azevedo and N.E.S.H program for supporting the trip to study the UFES collection through the grant FAPES/CNPq #52263010/2011; to the ZMUC collection for loaning specimens; to Dr. Felipe Vivallo for sharing information about the type of Abernessia irmgardae; to Felipe B. Braga for kindly taking pictures of Abernessia capixaba; and to Dr. Lynn Kimsey and an anonymous reviewer for improving this manuscript. This work was supported by the National Science Foundation award DEB-0743763 to JPP and CDvD, and by the Utah Agricultural Experiment Station, Utah State UAES #8599.