(C) 2013 Felipe N. Soto-Adames. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Soto-Adames FN, Taylor SJ (2013) The dorsal chaetotaxy of Trogolaphysa (Collembola, Paronellidae), with descriptions of two new species from caves in Belize. ZooKeys 323: 35–74. doi: 10.3897/zookeys.323.4950
Species diagnosis in Trogolaphysa has been based, until now, almost exclusively on number of eyes and shape of claws and mucro. Chaetotaxy, a character system important to diagnose species in other genera of scaled Entomobryoidea, has been described only for a few Trogolaphysa species. Here the complete dorsal chaetotaxy of six species of Trogolaphysa is described using the AMS and Szeptycki’s systems for head and body, respectively. A morphology-based parsimony analysis was performed to evaluate whether chaetotaxic characters overcome the influence of putatively cave adaptive convergent characters to resolve species level relationships, and to evaluate the evolution of the dorsal macrochaetae of the head. Phylogenetic analysis using only putative cave-adaptive characters support clades of unrelated taxa, but the addition of chaetotaxy overcomes the influence of convergent characters. A phylogeny based on all characters supports a trend towards reduced head macrochaetae number. Head macrochaetae are lost beginning with A3 and followed, in order, by S5, S3 and M3. In addition, a checklist of New World Trogolaphysa is provided and two new species, Trogolaphysa giordanoae sp. n. and Trogolaphysa jacobyi sp. n., are described on the basis of material collected in six caves in southern Belize.
Puerto Rico, Dicranocentruga, phylogeny, cave-adaptive characters
The collembolan fauna of Belize is among the least known of any Central American country. The Catalogue of Neotropical Collembola (
What is understood about the evolution of morphological adaptations to cave habitats in entomobryoid springtails is derived from northern temperate members of the genera Pseudosinella and Sinella (
Check-list of the species of Trogolaphysa sensu
| Species | Distribution |
|---|---|
| Trogolaphysa aelleni Yoshii, 1988 | BRA |
| Trogolaphysa belizeana Palacios-Vargas and Thibaud, 1997 | BLZ |
| Trogolaphysa berlandi (Denis, 1925) | ARG, GUF |
| Trogolaphysa bessoni Thibaud & Najt, 1989 | ECU |
| Trogolaphysa caripensis (Gruia, 1987) | VEN |
| Trogolaphysa carpenteri (Denis, 1925) | CRI, GUF, MEX, VEN |
| Trogolaphysa cotopaxiana Thibaud & Najt, 1989 | ECU |
| Trogolaphysa distinguenda (Denis, 1931) | CRI |
| Trogolaphysa ecuatorica (Palacios-Vargas, Ojeda & Christiansen, 1986) | ECU |
| Trogolaphysa geminata (Mari Mutt, 1988) | PRI |
| Trogolaphysa giordanoae Soto-Adames & Taylor sp. n. | BLZ |
| Trogolaphysa guacharo Yoshii, 1988 | CRI, VEN |
| Trogolaphysa haitica (Palacios-Vargas, Ojeda & Christiansen, 1986) | HTI |
| Trogolaphysa hauseri Yoshii, 1988 | BRA |
| Trogolaphysa hirtipes (Handschin, 1924) | ARG, BRA, VEN |
| Trogolaphysa hondurasensis (Palacios-Vargas, Ojeda & Christiansen, 1986) | HND |
| Trogolaphysa jamaicana (Palacios-Vargas, Ojeda & Christiansen, 1986) | JAM |
| Trogolaphysa jataca (Wray, 1953) | JAM, PRI |
| Trogolaphysa jacobyi Soto-Adames & Taylor sp. n. | BLZ |
| Trogolaphysa luquillensis (Mari Mutt, 1988) | PRI |
| Trogolaphysa marimutti (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa maya Mills, 1938 | CUB, DOM, MEX |
| Trogolaphysa millsi Arlé, 1939 | BRA |
| Trogolaphysa nacionalica (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa oztotlica (Ojeda & Palacios-Vargas, 1984) | MEX |
| Trogolaphysa relicta (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa riopedrensis (Mari Mutt, 1988) | PRI |
| Trogolaphysa separata (Denis, 1933) | CRI |
| Trogolaphysa strinatii Yoshii, 1988 | MEX |
| Trogolaphysa subterranea (Mari Mutt, 1988) | PRI |
| Trogolaphysa tijucana (Arlé & Guimarāes, 1979) | BRA |
| Trogolaphysa toroi (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa variabilis (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa xtolokensis (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
| Trogolaphysa yoshiia (Palacios-Vargas, Ojeda & Christiansen, 1986) | MEX |
The relationships between the genera Paronella, Troglopedetes, Trogolaphysa, and Dicranocentruga has been a source of confusion.
Here we present complete descriptions of the dorsal chaetotaxy of the head and trunk for the two new species of Trogolaphysa and for Trogolaphysa belizeana, andcompare their chaetotaxy to that of Trogolaphysa jataca (Wray, 1953), Trogolaphysa geminata (Mari Mutt, 1987[1988]) and Trogolaphysa riopedrensis (Mari Mutt, 1987[1988]), three surface species from Puerto Rico. Finally, we present a morphology-based phylogenetic analysis to assess the value of chaetotaxy in elucidating species relationships in this genus, and to evaluate the evolution of some elements of the dorsal chaetotaxy of the head.
Springtails were collected with aspirators and preserved in 70% ethanol. Samples were associated with substrate characterizations and field-collected measurements of temperature, light intensity and humidity.
Selected specimens were cleared in Nesbitt’s solution, mounted in Mark André II (
Abbreviations used for structures are: antennae (Ant.), thorax (Th.) abdomen (Abd.), extra ocular structure (EOS). Abbreviations used for names are: Avelardo Canti (AC), Gabriel Chaco (GaC), Germano Coe (GeC), William R. Elliott (WRE), Geoff B. Hoese (GBH), JoAnn Jacoby (JJ), Jean K. Krejca (JKK), Bruno K. Kuppinger (BKK), C. Marcela Ospina (CMO), Rosalio Sho (RS), Christy M. Slay (CMS), Michael E. Slay (MES), Felipe N. Soto-Adames (FSA), and Steven J. Taylor (SJT).
To protect vulnerable sites, latitude and longitude are not provided for the Belize material. These locations are controlled by, and may be requested from, the Institute of Archaeology, Belmopan, Belize (see Acknowledgements). Holotypes and paratypes of the new species are deposited in the Illinois Natural History Survey Insect Collection (INHS).
Here we describe only elements of the chaetotaxy that are modified into microchaetae, macrochaetae or sensilla (i.e., idiochaetotaxy,
The idiochaetotaxy of Trogolaphysa is reduced, and in naming body setae we assume it represents the remnant of primary chaetotaxy. The setae closest to the mesothoraxic pseudopore (Figs 12, 32, 53) are identified as m1 and m2, even though they occupy positions that in entomobryoids with more abundant idiochaetotaxy might be assigned to setae m2i and m2e, respectively. The nomenclature of setae on the fourth abdominal segment follows Szeptycki’s system (
For the labial chaetotaxy, upper case letters represent macro- or mesosetae and lower case represent microsetae, an underscore in the formula identifies ciliate setae. The eye patch of a generalized springtail comprises a group of 5 anterior and 3 posterior simple eyes, we refer to the space between these two groups of eyes as the ‘eye patch well’ to distinguish it from the inter-ocular space, which is the gap between the eye patches on either side of the head.
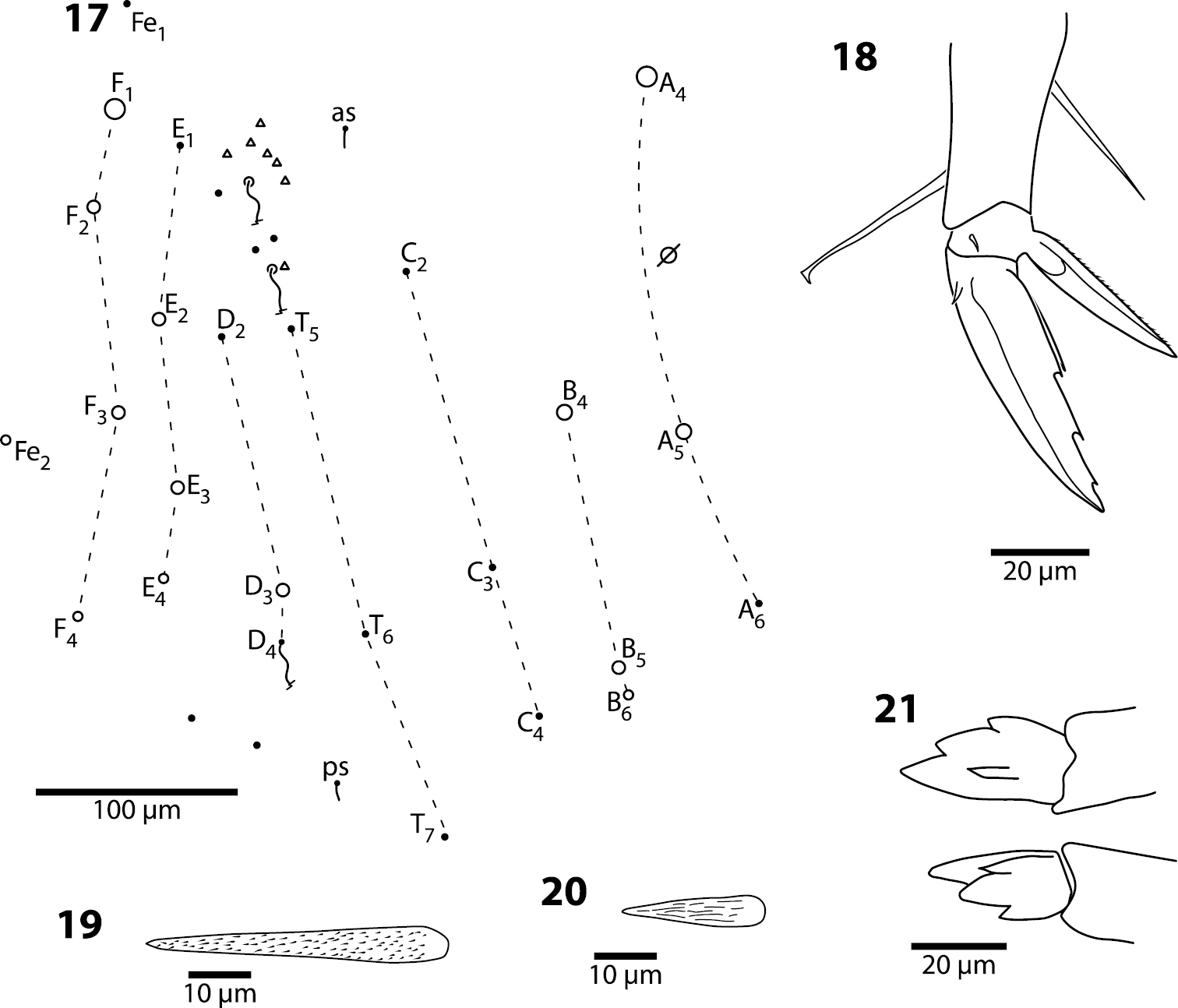
The formula of the dorsal macrochaetae of head and trunk is based on Gisin’s (1967) model, but we consider all macrochaetae associated with the bothriotricha on abdominal segments 2-4, instead of only those found between the bothriotrichal complexes. The number of macrochaetae on the head is presented as two digits; the first digit refers to macrochaetae anterior to the head sulcus (series A, M and S), the second to the posterior macrochaetae (series Ps, Pa and Pm). The macrochaetae on abdominal segment 4 are represented by three digits separated by plus (+) symbol, where the first, second and third numbers refer to the inner (series A and B), medial (assumed, in Szeptycki’s system, to be series C) and outer macrochaetae (series T, D, E, F and Fe). The last number in the macrochaeta formula may be represented by a range because the number of outer macrochaetae may be variable, as some macrochaetae external to series F appear to be added as individuals grow older. The formula is based on the relative size of the sockets and includes all macrochaetae, irrespective of whether they are large (i.e., short, thick and blunt) or small (long, slender and acuminate).
Phylogenetic trees were estimated using parsimony as implemented in PAUP 4.0* (
The habitat parameters substrate temperature, air temperature, light, and relative humidity were measured with hand held meters. Differences in abiotic parameters between habitats occupied by the two new species were tested using a Wilcoxon rank sum test in R 2.15.2 (
Paronellidae with finely denticulate scales covering dorsum of head and body, and ventral face of furcula; Ant. 4 sometimes annulated, never subdivided in two; labial seta L2 normal, not reduced; eyes 0-8; EOS present; Abd. 2-4 with 2, 3, 3 bothriothricha; manubrium without spines, dens with 1-2 rows of spines; mucro short, with 3-5 more or less evenly spaced teeth.
As currently circumscribed (
It is not known if the type species of the genus, Trogolaphysa maya Mills, 1938, has EOS, but the presence of this structure in all species discussed below, including the two troglomorphic forms, suggests it is likely also present in that species.
http://zoobank.org/3C37791A-056D-496F-87E2-58D72A355B4B
http://species-id.net/wiki/Trogolaphysa_giordanoae
Figs 1–21; Figs 22–23 (habitat)BELIZE: Toledo District: 29 km WNW of Punta Gorda, Blue Creek Cave, Hokeb Ha entrance, 11.IV.2011, SJT, MES, JJ, CMS, GBH & RS, coll.
Type material:Holotype, female on microscope slide preparation, INHS Collection Number 579, 406; Paratypes: BELIZE: Toledo District: 29 km WNW of Punta Gorda, Blue Creek Cave, Hokeb Ha entrance, 11.IV.2011, (3 in alcohol), SJT, MES, JJ, CMS, GBH & RS, coll.; 37 km WNW of Punta Gorda, cave near Pueblo Creek Cave, 16.IV.2011, (4 in alcohol-one headless), MES, JKK, CMS, GBH & GeC, coll.; 28 km NNW of Punta Gorda, Tiger Cave, 9.IV.2012, (1 on slide, 33 in alcohol), SJT, MES, JJ, CMS, GBH, BKK & GaC, coll.; 28 km NNW of Punta Gorda, Bat Cave, 10.IV.2011, (2 on slides, 29 in alcohol—some in poor condition, one headless), SJT, MES, JJ, CMS & GBH, coll.; 31 km WNW of Punta Gorda, Okebal Ha, 14.IV.2011, (3 on slides, 16 in alcohol), SJT, MES, JJ, CMS, GBH, BKK & RS, coll.
Trogolaphysa giordanoae sp. n. is unique among species with 6–8 eyes in having 5 dorsal head macrochaetae, 3 metathoracic macrochaetae and 4 inner macrochaetae on Abd. 4. Among species with known dorsal chaetotaxy, the new species is most similar to Trogolaphysa riopedrensis, but the two species are easily distinguished by the combination of characters given above and by the presence of a relatively shorter mucro in the new species (Table 2). Additional diagnostic characters distinguishing the new species from all other New World Trogolaphysa with 6–8 eyes and capitate/spatulate tenent hair are presented in Table 2. Among the species described before the introduction of chaetotaxy, the new species is most similar to Trogolaphysa distinguenda (Denis, 1931), but the two species can be separated by the presence of a relatively long mucro with 5 teeth in distinguenda, and a 4-toothed short mucro in Trogolaphysa giordanoae sp. n. Trogolaphysa belizeana is the only other New World Trogolaphysa with 3 metathoracic macrochaetae. However, Trogolaphysa belizeana is a troglobiont (sensu
Diagnostic table for species of Trogolaphysa with 6–8 eyes and capitate or spatulate tenent hair.
| Species | Mucronal teeth | Mucro length/ Width dens apex | Inner ungual teeth | Dorsal head macrochaetae | Th. 2 Macrochaetae | Th. 3 Macrochaetae | Abd. 4 Inner large macrochaetae |
|---|---|---|---|---|---|---|---|
| Trogolaphysa giordanoae sp. n. | 4 | 1.8 | 4 | 5 | 7 | 3 | 4 |
| Trogolaphysa riopedrensis | 4 | 2.9 | 4 | 7 | 7 | 0 | 4 |
| Trogolaphysa geminata | 4 | 2.2 | 4 | 6 | 7 | 0 | 3 |
| Trogolaphysa jataca | 4 | 2.9 | 4 | 7 | 7 | 0 | 3 |
| Trogolaphysa carpenteri |
4 | 3.5 | 3 | 2 | 0 | 0 | 0 |
| Trogolaphysa relicta | 4 | 2.7 | 3 | 0 | 0 | 0 | 0 |
| Trogolaphysa subterranea | 4 | 2.7 | 3 | 3 | 7 | 0 | 3 |
| Trogolaphysa cotopaxiana | 5 | 3.6 | 4 | 2 | 3 | 0 | 3 |
| Trogolaphysa distinguenda | 5 | 3.3 | 4 | ? | ? | ? | ? |
† Most characters based on
Size. Body length up to 2.1 mm.
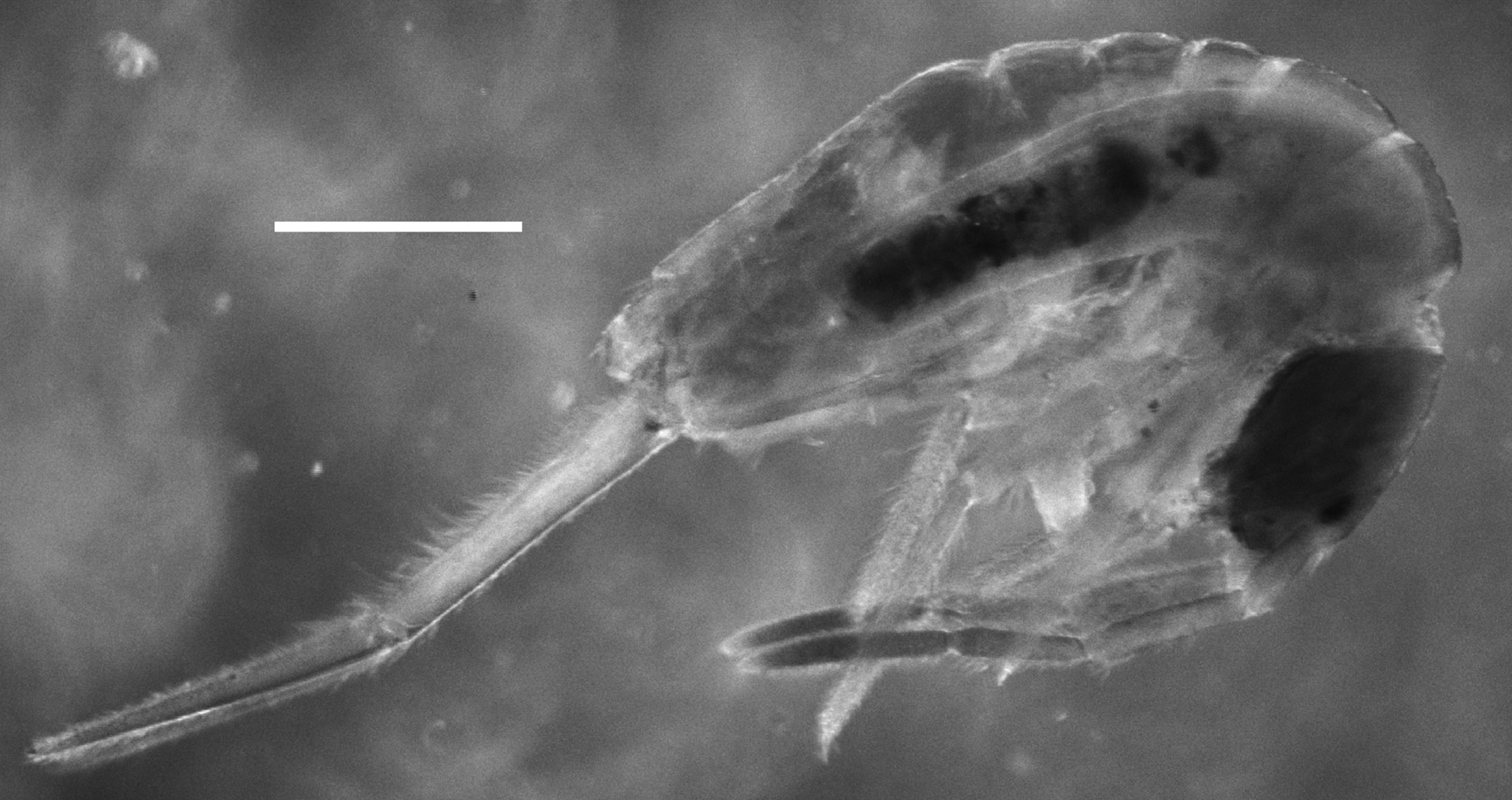
Color. Pattern, if any, obscured by green dye present in the alcohol in which specimens were preserved (Fig. 1).
Trogolaphysa giordanoae sp. n. habitus, scale=0.5 mm.
Scale distribution. Scales dark brown, present on Ant. 1-2 and base of Ant. 3, more abundant on dorsal face than on ventral face of segment. Scales absent from ventral tube, legs and dorsal face of manubrium.
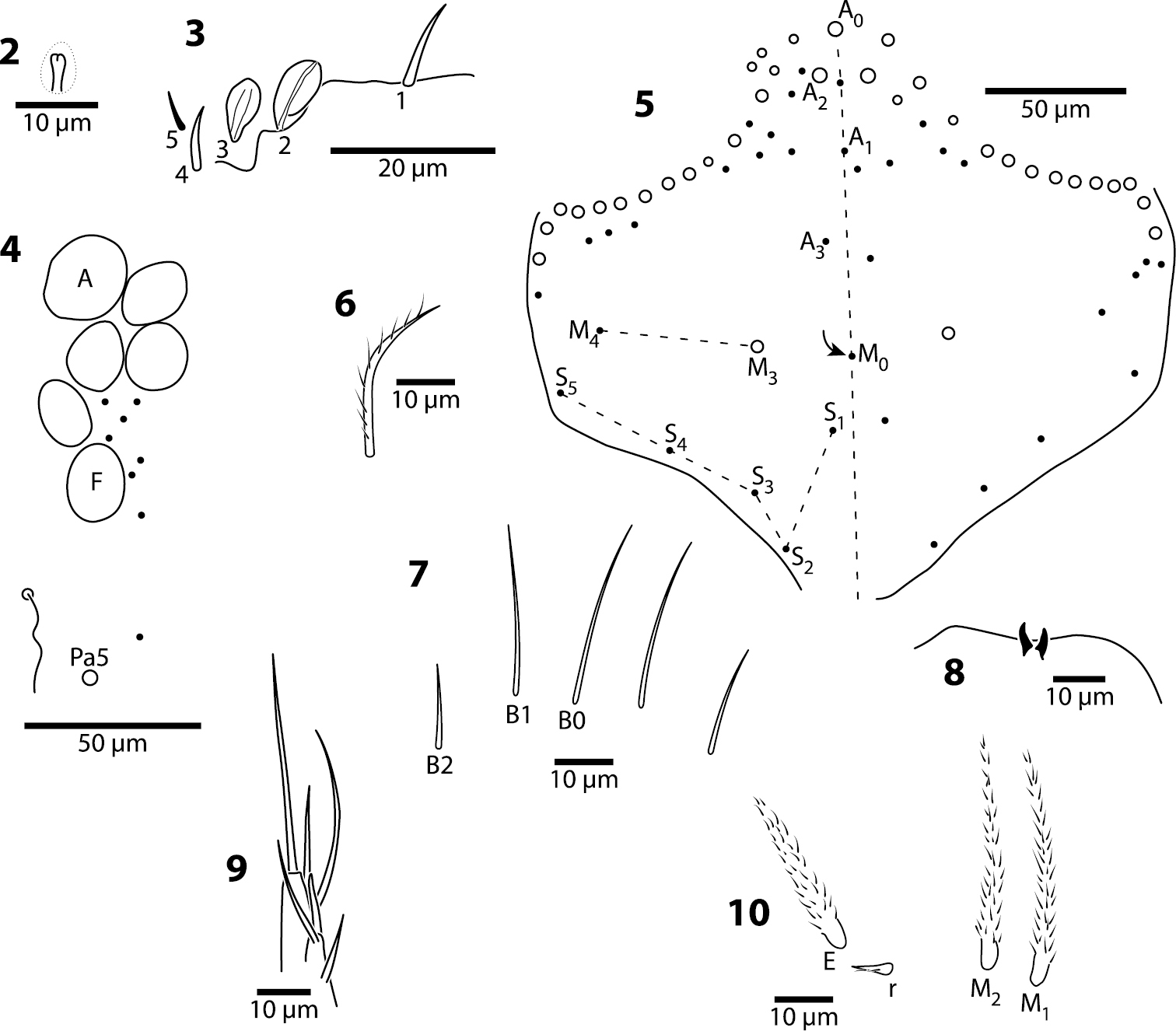
Head. Antenna/cephalic diagonal ratio 2.0–2.5 (Fig. 1). Apical bulb of Ant. 4 absent; subapical sensillum capitate (Fig. 2), fully contained in circular depression; guard sensillum absent. Sense organ of Ant. 3 (Fig. 3) with sensilla 1 and 4 acuminate, thin-walled and translucent; sensillum 5 acuminate, dark (light dense), shorter than 1 and 4; sensilla 2–3 wide, leaf-like, resting in shallow grooves. Eyes 6+6 (Fig. 4), chaetotaxy of eyepatch well with 4, sometimes 6 ciliate setae, and 1 seta posterior to eye F. Head dorsally with 5 macrochaetae (A0, A2, M3, Pa5 and Pm3 — Figs 4–5). Series M with 2 setae (M3–4); series S with 5 setae (S1–5); seta M0 seen only in one individual; S0 absent. Prelabral setae serrate (Fig. 6). Labral setae smooth: setae in rows A and C subequal; seta B2 distinctly shorter than setae B0 and B1 (Fig. 7). Distal margin of labrum with 1+1 medial hooks, papillae absent (Fig. 8). Apical and subapical setae of maxillary palp smooth; sublobular plate with 2 seta-like appendages. Lateral process of labial papilla E weakly bent dorsally, barely reaching apex of papilla (Fig. 9). Labial triangle setae as M1M2rEL1–2A1–5 (Fig. 10); r short, stout and sparsely ciliate; L1 inserted close to E and distant from L2 when compared to other entomobryoids (Fig. 11). Postlabium covered by setae and scales, all postlabial setae ciliate, modified setae absent. Columns ICELO with 42221 setae (Fig. 11): column I with posterior seta detached from main group and much longer than anterior setae. Ventral cervical setae usually 8+8.
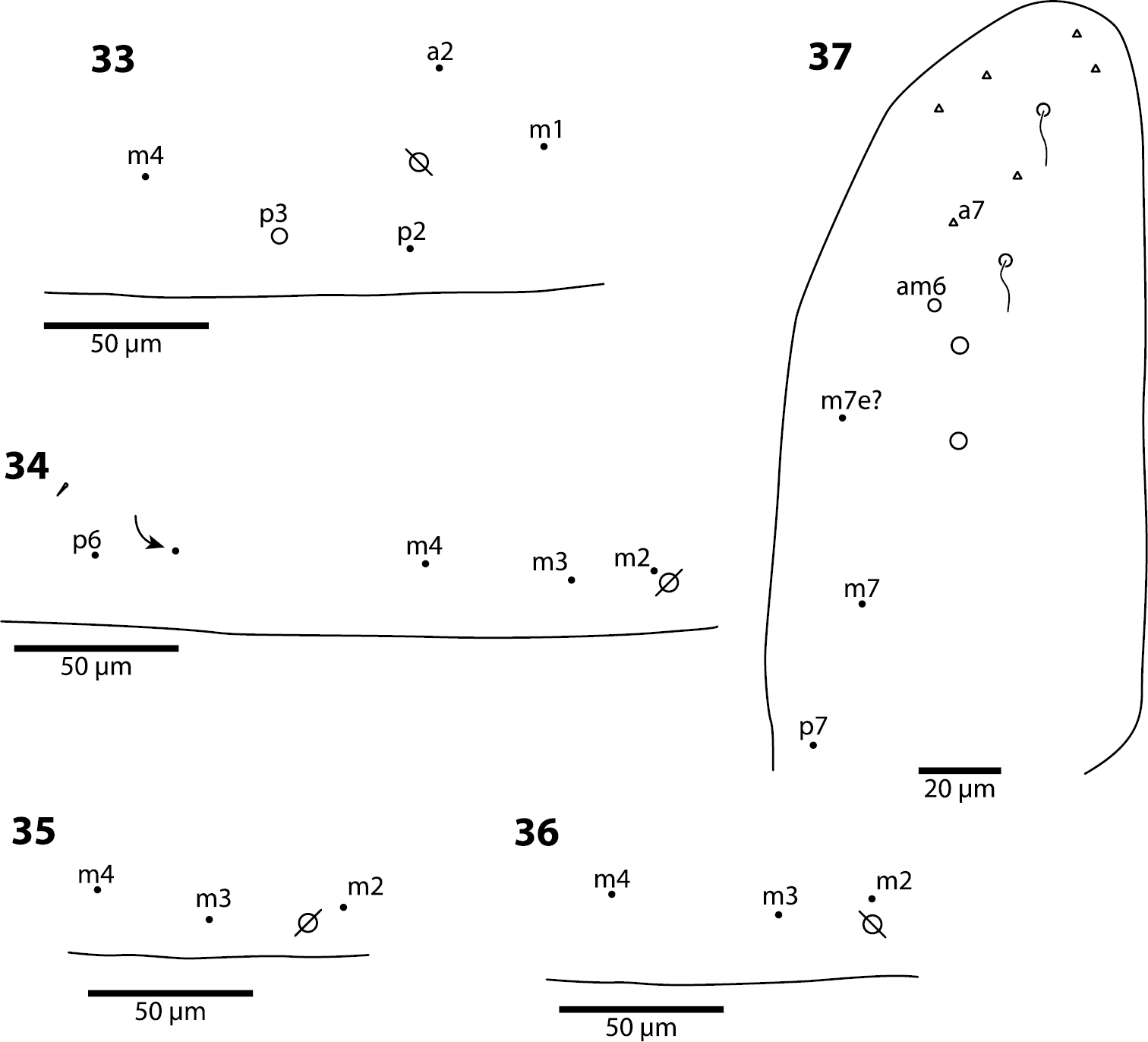
Trogolaphysa giordanoae sp. n., circles are macrochaetae, filled circles are ciliate microchaetae2 Antennal segment 4, subapical sensillum 3 Antennal segment 3, sense organ 4 Eyepatch and associated setae, 5 Head dorsal chaetotaxy, line represents dorsal sulcus 6 Prelabral seta 7 Labral row B setae 8 Distal margin of labrum 9 Labial papilla E 10 Posterior setae of labial triangle.
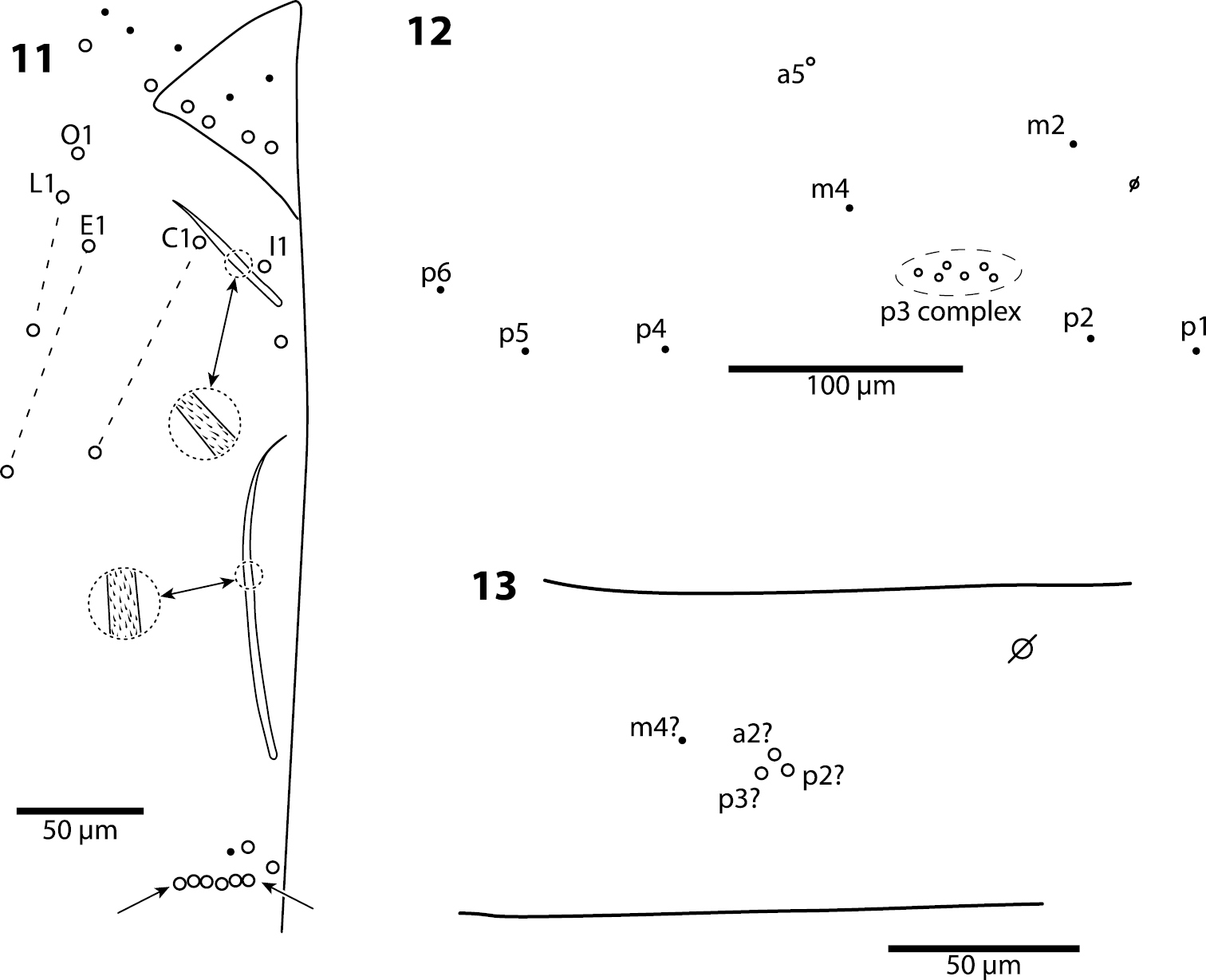
Trogolaphysa giordanoae sp. n. 11 Postlabium, circles are ciliate setae, filled circles are smooth setae, arrows point at ventral cervical setae 12 Mesothorax, dorsal chaetotaxy, circles are macrochaetae, filled circles are microchaetae 13 Metathorax, dorsal chaetotaxy, circles are macrochaetae, filled circles are microchaetae, seta a6 present but not shown.
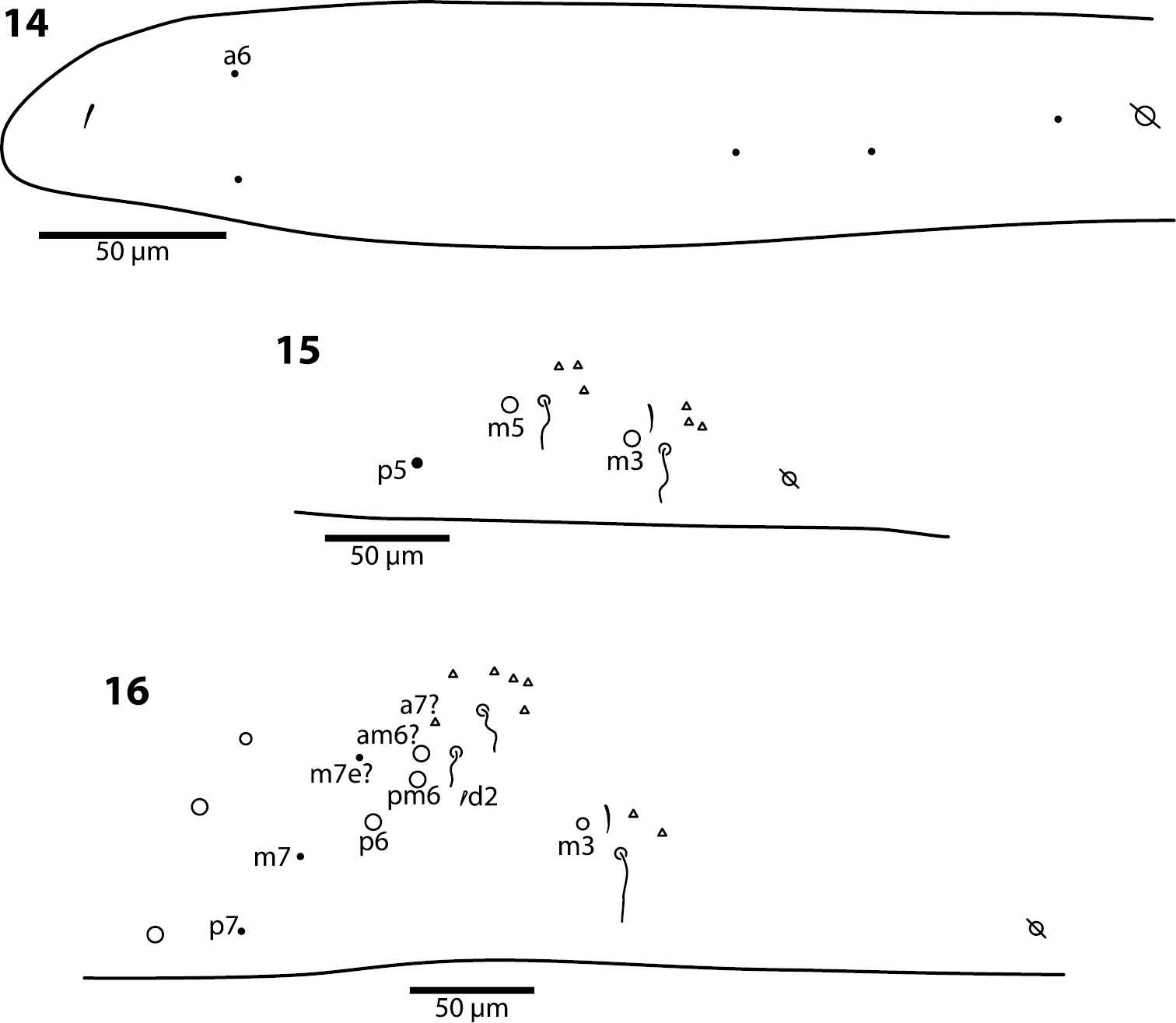
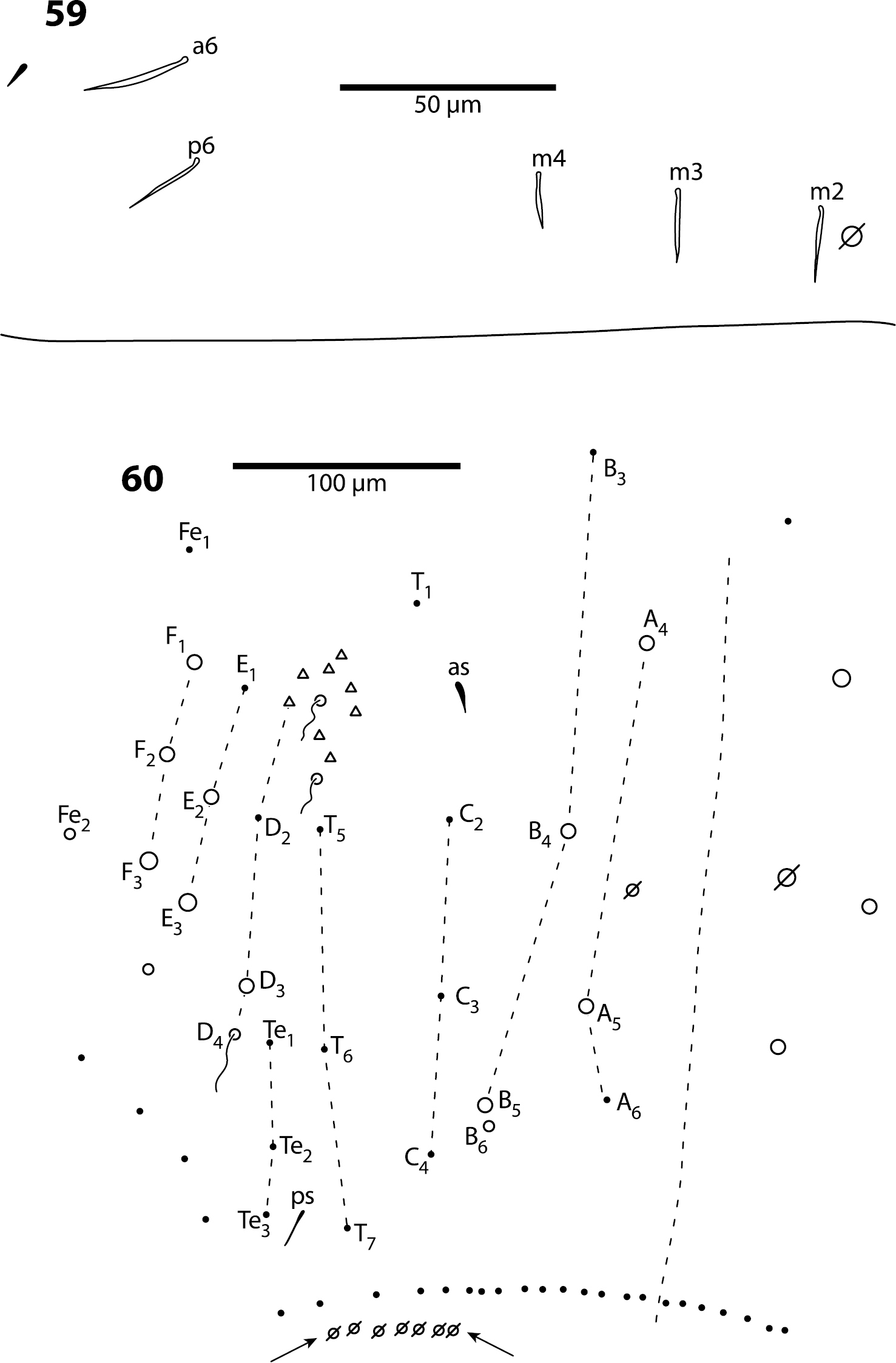
Body. Mesothoracic hood not developed. Complete dorsal macrochaetae as 32/73/0245+0+9. Mesothorax with 1 anterior (a5) and 6 posterior (p3 complex) macrochaetae arranged as is typical for genus (Fig. 12); microchaetae m2, m4, p1, p2, p4, p5 and p6 present. Inner chaetotaxy of metathorax with 3 macro- and 1 microchaetae (Fig. 13). First abdominal segment with 1 anterior (a6) and 4 posterior setae arranged in a single row (Fig. 14). Second abdominal segment (Fig. 15) inner bothriotrix with 3 fan-shaped setae, one microsensillum and macrochaeta m3; outer bothriotrix with 3 fan-shaped setae and macrochaeta m5; setae a6, m6 and p5 present. Third abdominal segment (Fig. 16) inner bothriotrix complex with 2 fan-shaped setae, 1 sensillum, and macrochaeta m3; external bothriotricha with 7 fan-shaped setae, and macrochaetae am6, pm6 and p6; sensillum d2 present, inserted near pm6. Fourth abdominal segment with 5 inner and 9 outer macrochaetae (Fig. 17): large inner macrochaetae A4, A5, B4, and B5 present; B6 a small macrochaeta; large outer macrochaetae D3, E2, E3, F1, F2, and F3 present; macrochaetae E4, F4 and one other seta probably homologous to Fe4, small. Anterior and medial bothriotricha with 7 and 3 fan-shaped supplementary setae, respectively. Posterior bothriotrix, corresponding to D4, without associated supplementary setae. Posterior setae 19–21+19–21. Intersegmental membrane between Abd. 4–5 with 4–10 lenticular organs (as in Trogolaphysa riopedrensis, Fig. 60).
Trogolaphysa giordanoae sp. n. Dorsal chaetotaxy of abdominal segments 1–3, triangles are fan-shaped setae, circles are macrochaetae, filled are circles ciliate microchaeta14 First abdominal segment 15 Second abdominal segment 16 Third abdominal segment.
Legs. Trochanteral organ with up to 36 setae. Metathoracic claw complex as in Fig. 18. Tenent hair weakly spatulate. Smooth posterior setae on metathoracic legs 0.76× as long as unguiculus. Unguis with 4 inner teeth: 1 basal tooth sometimes appearing slightly larger than other, both paired teeth ending near middle of inner edge; proximal unpaired tooth as large as basal paired teeth, ending on distal half of inner edge; distal unpaired tooth smallest of all inner teeth and ending on distal fourth of inner edge. Outer tooth ending on basal quarter of outer ungual edge. Unguiculus lanceolate, with outer margin serrate.
Trogolaphysa giordanoae sp. n. 17 Fourth abdominal segment dorsal chaetotaxy, diameter of circle is approximately proportional to size of macrochaeta 18 Metathoracic claw complex 19 Dens basal spine, outer row 20 Dens basal spine, inner row 21 Mucro.
Ventral tube. Anterior face with 3+3 or 4+4 distal macrochaetae; lateral and posterior setae not seen clearly.
Furcula. Dens with 2 rows of ciliate spines: inner row with 35–42 spines; outer row with 25–28 spines. Basal outer spines longest (Figs 19–20). Mucro with 4 short, stout teeth (Fig. 21), ratio mucro length/width of dens tip 1.2–1.8×; basal outer tooth reaches to at least half length of basal inner tooth.
This species is dedicated to Rosanna Giordano, the senior author’s wife, for her years of support and contributions to science.
The species is known only fromBelize
Trogolaphysa giordanoae sp. n. is a guanophile, recorded from entrance, twilight (Fig. 22) and dark zones of caves (6.7, 53.3 & 40.0 % of 15 collections, respectively), often in association with fruit bat or other guano (Fig. 23) (noted for 40% of 15 collections). It was commonly found on the floor of caves (76.9% of 13 collections where position was noted), but also on cave walls (23.1% of 13 collections where position was noted).
Trogolaphysa giordanoae sp. n. paratype habitat Okebal Ha entrance/twilight zone. Specimens were collected from a small pile of fruit bat guano near the researchers in the foreground, below a bat roost site. Sample site was much darker than it appears in this enhanced image. Photo courtesy of MES.
Trogolaphysa giordanoae sp. n. on old feces in Tiger Cave. Photo courtesy of GBH.
http://zoobank.org/5F865EE9-B5E0-4844-8482-902F8E9EA2B2
http://species-id.net/wiki/Trogolaphysa_jacobyi
Figs 24–43; Fig. 44 (habitat)BELIZE: Toledo District: 32 km WNW of Punta Gorda, Yok Balum Cave, 13.IV.2012, SJT, MES, JJ, CMS, GBH & AC, coll.
Holotype, female on microscope slide preparation, INHS collection number 579, 407; BELIZE: Toledo District: 32 km WNW of Punta Gorda, Yok Balum Cave, 13.IV.2012, SJT, MES, JJ, CMS, GBH & AC, coll.; Paratypes: BELZE: Toledo District: 32 km WNW of Punta Gorda, Yok Balum Cave, 13.IV.2012, (2 adults & 1 juvenile on slides, 3 adults or subadults & 3 juveniles in alcohol), SJT, MES, JJ, CMS, GBH & AC, coll.; 37 km WNW of Punta Gorda, cave near Pueblo Creek Cave, 16.IV.2011, (1 adult on slide—without legs), MES, JKK, CMS, GBH & GeC, coll.
Trogolaphysa jacobyi sp. n. is the only member of the genus that is blind, has 3-toothed mucro and unguis, and has a single macrochaeta on the metathorax. Trogolaphysa belizeana is the only other New World Trogolaphysa lacking eyes and having a 3-toothed mucro, but it differs from Trogolaphysa jacobyi sp. n. in having 3 metathoracic macrochaetae (1 in Trogolaphysa jacobyi sp. n.), in the arrangement and identity of inner macrochaetae on Abd. 4 (cf. Figs 38, 49 see discussion below), in having few postlabial scales (absent in Trogolaphysa jacobyi sp. n.) and setae (many in Trogolaphysa jacobyi sp. n., cf. Figs 30, 46), in the presence of sensillum d2 on Abd. 3 (absent in Trogolaphysa jacobyi sp. n.), in the absence of unpaired ungual teeth (1 tooth in Trogolaphysa jacobyi sp. n.) and in having a typical lanceolate unguiculus (basally swollen in Trogolaphysa jacobyi sp. n.). Table 3 provides a list of characters that distinguish Trogolaphysa jacobyi sp. n. from all other New World Trogolaphysa lacking eyes and having paired basal ungual teeth inserted near the basal fourth of the inner edge.
Diagnostic table for blind species of Trogolaphysa with basal paired ungual teeth originating on basal fourth of inner edge of claw.
| Species | Mucronal teeth | Inner ungual teeth | Unguiculus shape | Mesothorax macrochaetae | Metathorax macrochaetae | 4th Abdominal segment large inner macrochaetae |
|---|---|---|---|---|---|---|
| Trogolaphysa jacobyi sp. n. | 3 | 3 | basally swollen | 4 | 1 | A5, B4, B5 |
| Trogolaphysa belizeana | 3 | 2 | lanceolate | 4 | 3 | A4, A5, B5 |
| Trogolaphysa haitica | 4 | 2 | lanceolate | 0 | 0 | 0 |
| Trogolaphysa ecuatoriana | 5 | 2 | basally swollen | 0 | 0 | 0 |
| Trogolaphysa bessoni | 5 | 2 | basally swollen | 3 | 0 | apparently A5, B4, B5 |
Size. Body length up to 2.0 mm.
Color. Living specimens yellowish, with pigment only on a small eyepatch and mesothorax (Fig. 24). Specimens in alcohol white, without trace of pigment.
Trogolaphysa jacobyi sp. n. habitus, photographed in Yok Balum Cave.
Scale distribution. Scales transparent, present on Ant. 1–2. Scales absent from postlabial region of head, ventral tube, legs and dorsal face of manubrium.
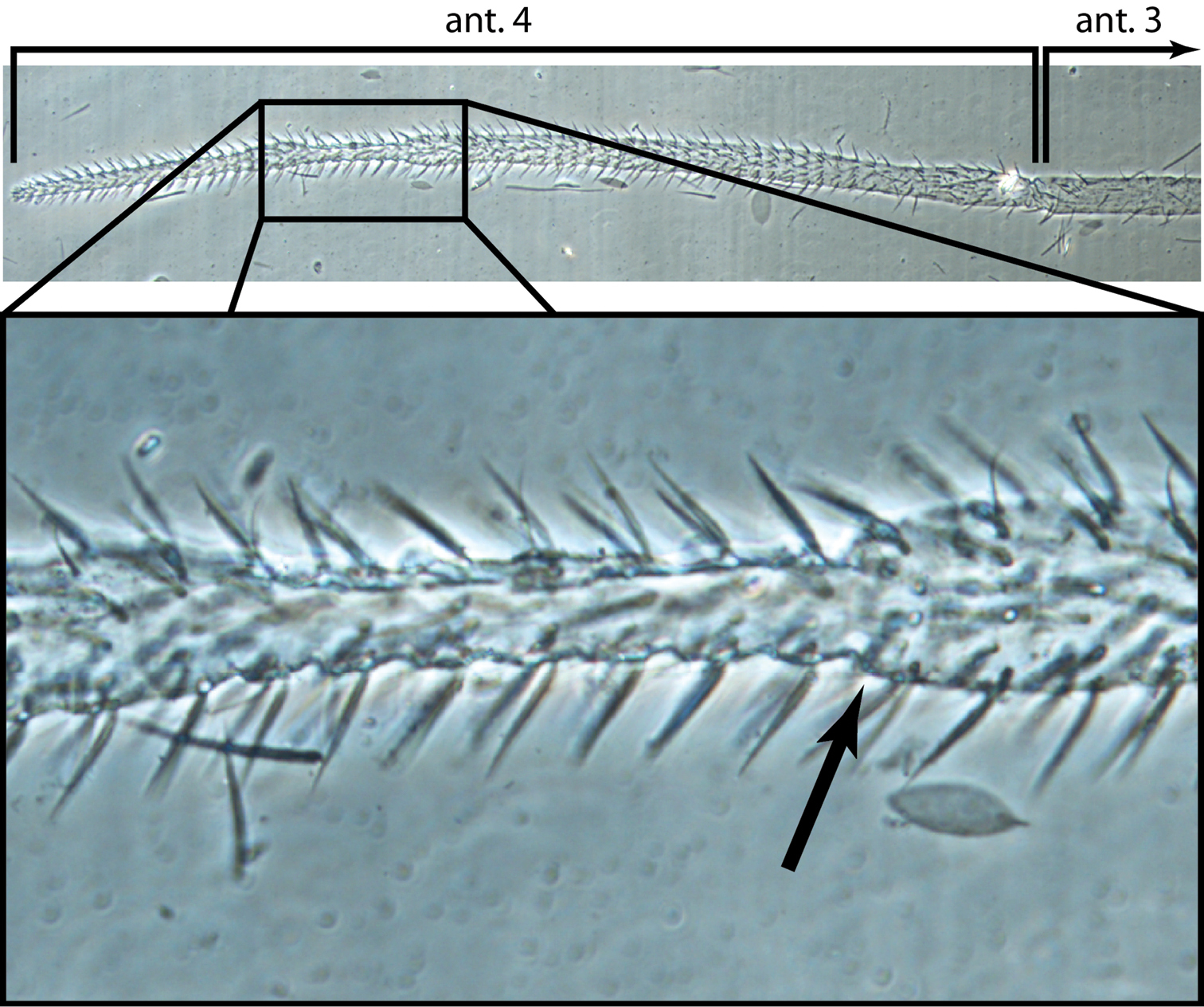
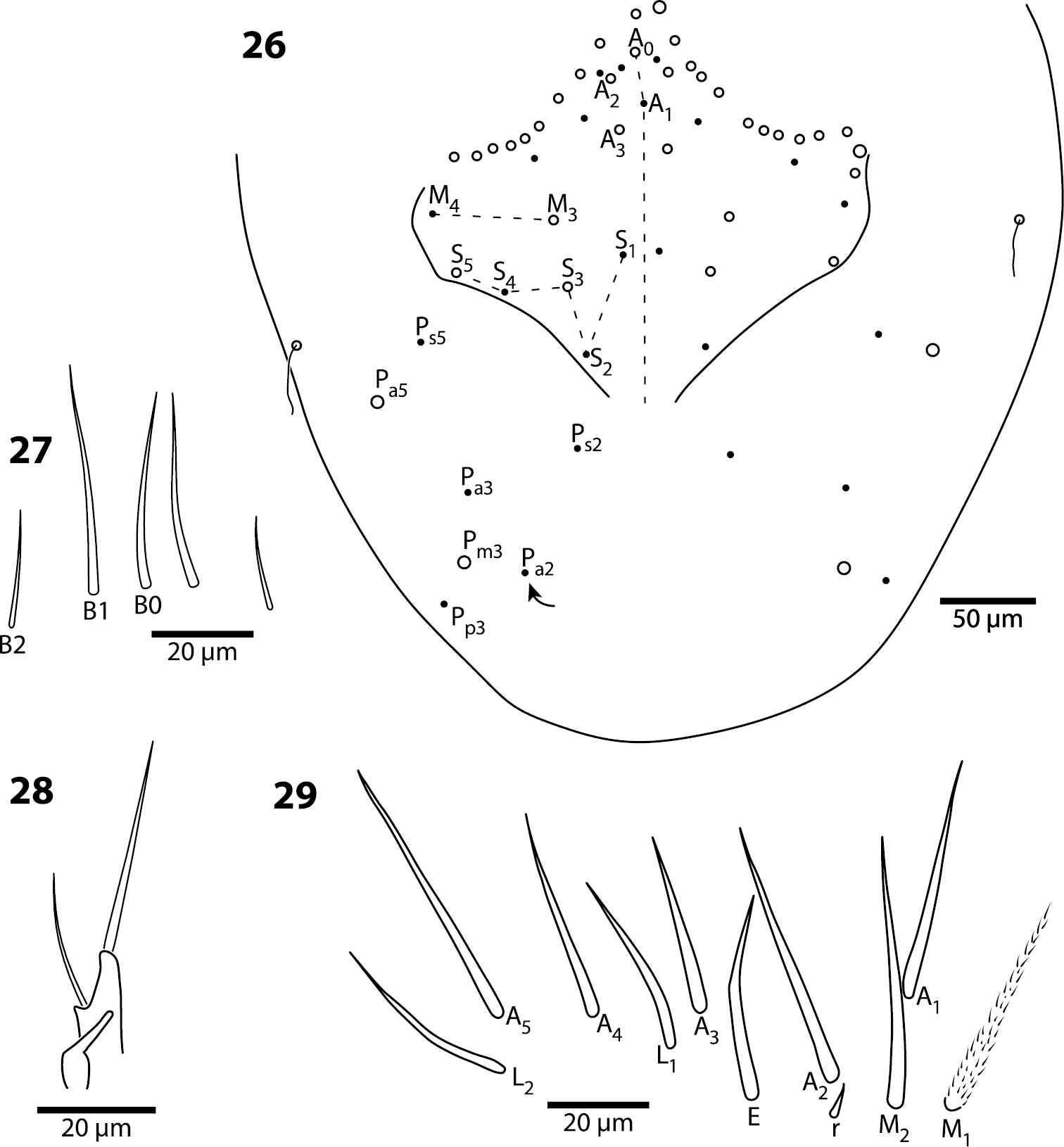
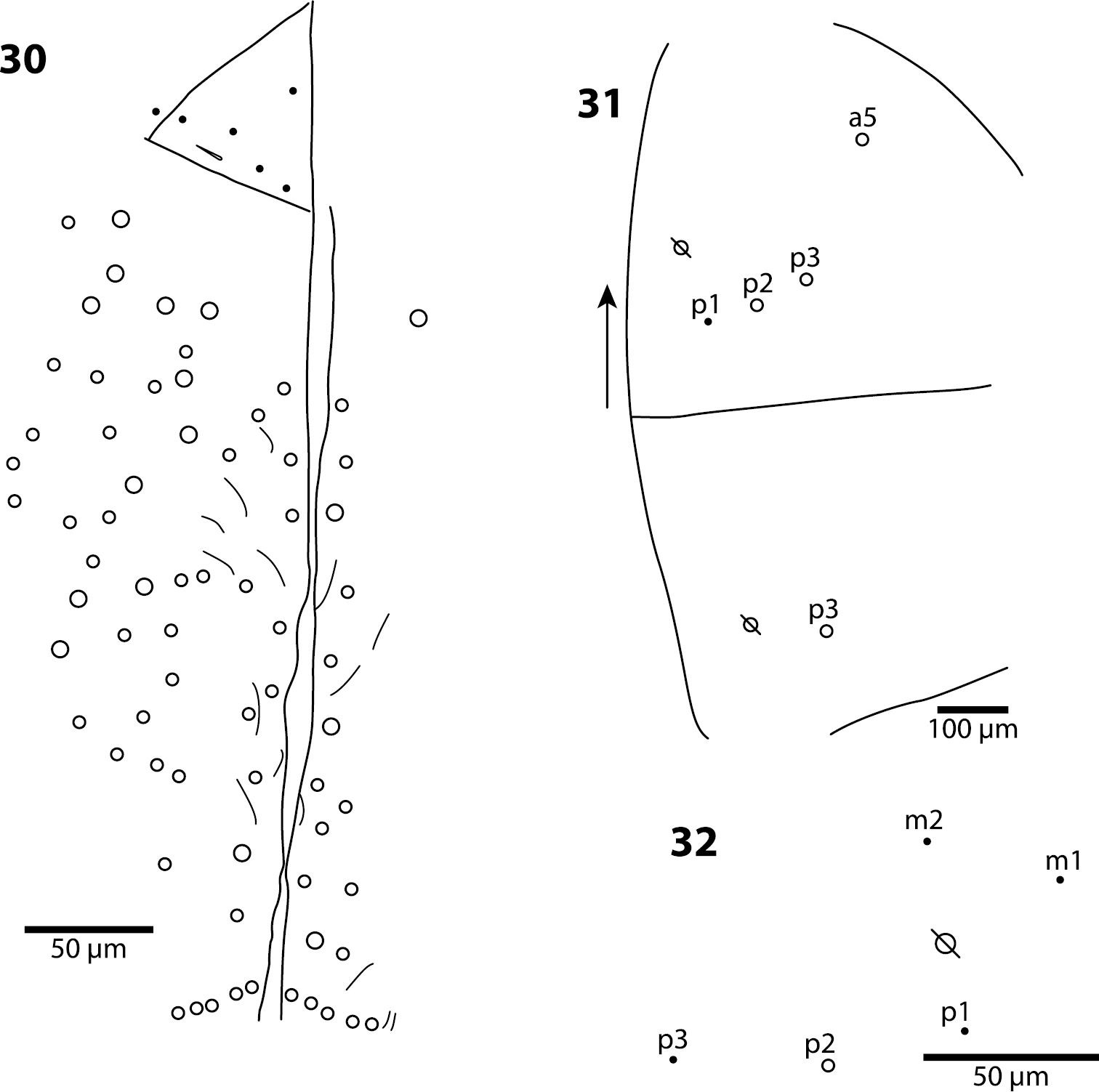
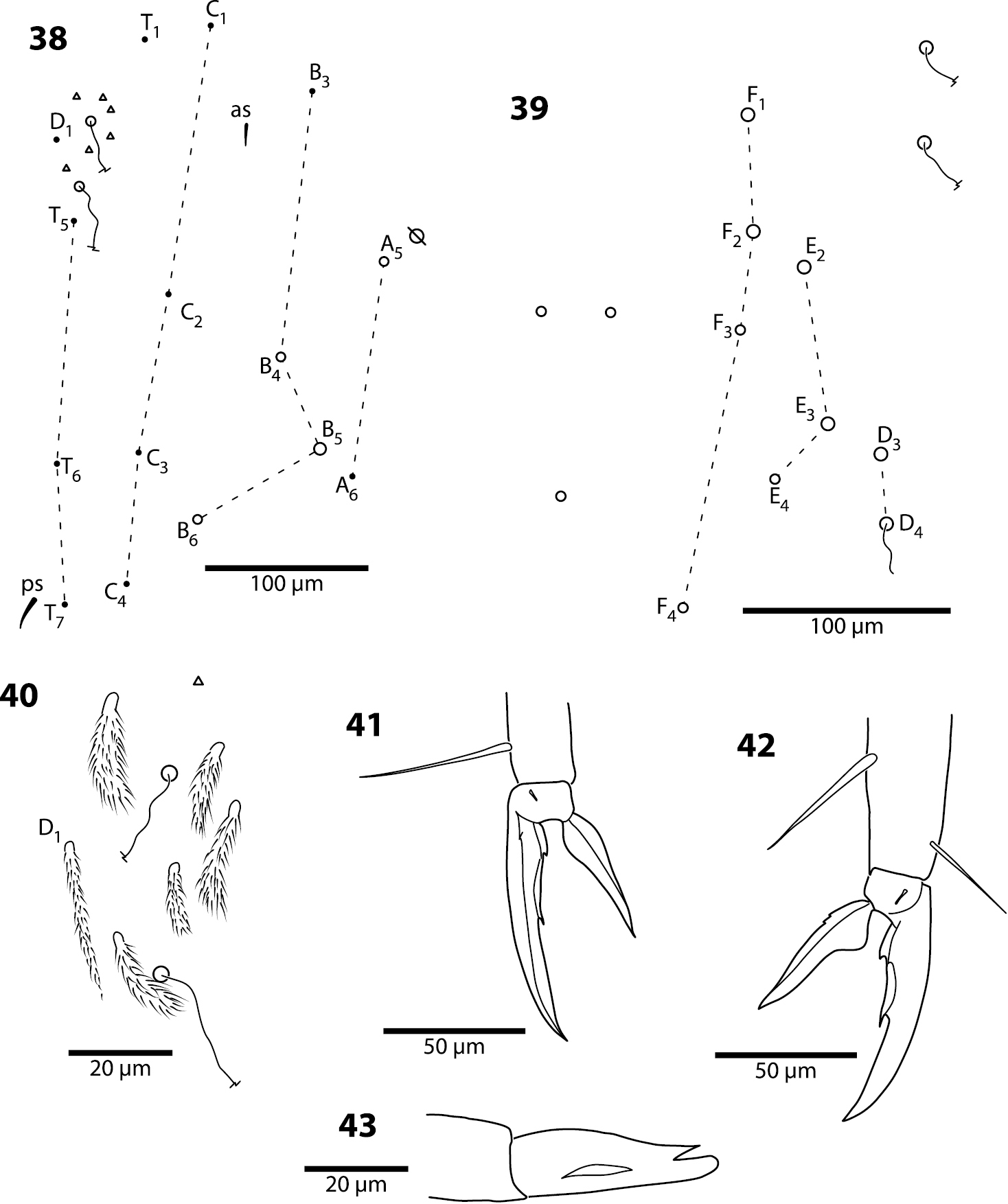
Head. Antenna/cephalic diagonal ratio up to 5.8 (Fig. 24). Fourth antennomere with incomplete but clear constriction near middle, with many shallow whorls of setae (Fig. 25); apical bulb absent; subapical sensillum not seen. Sense organ of Ant. 3 with sensilla 1 and 4 short, acuminate, thin-walled and translucent; sensillum 5 acuminate, dark and shorter than 1 and 4; sensilla 2–3 broad, leaf-like, resting in shallow grooves. Eyes not seen on slide-mounted specimens, but 1–2 pigment patches visible in life (Fig. 24). Head dorsally with 8 macrochaetae (A0, A2, A3, M3, S3, S5, Pa5 and Pm3 — Fig. 26). Seta M4 displaced laterally towards cephalic sulcus. Series S with setae S1–5; S0 absent, macrochaeta S3 displaced anteriorly, away from cephalic sulcus (cf. Figs 5, 26). Prelabral and all labral setae smooth: setae within row A and C subequal; seta B2 shorter than B0 and B1 (Fig. 27). Distal margin of labrum smooth, papillae absent. Apical and subapical setae of maxillary palp smooth; sublobular plate without seta-like appendages. Lateral process of labial papilla E weakly bent dorsally and not nearly reaching apex of papilla (Fig. 28). Labial triangle setae as M1M2rEL1–2A1–5 (Fig. 29), seta M1 ciliate, all others smooth; r short; A2 close to r, L1 close to E and distant from L2. Postlabium without scales, polychaetotic, uniformly covered with many large and small, weakly ciliate or smooth setae (Fig. 30); modified setae absent. Columns ICELO ill defined due to polychaetosis. Ventral cervical setae usually 6+6.
Trogolaphysa jacobyi sp. n. Fourth antennal segment showing constriction and incomplete suture (arrow).
Trogolaphysa jacobyi sp. n. 26 Head dorsal chaetotaxy 27 Labral setae on row B 28 Lateral process of labial papilla E 29 Labial triangle.
Trogolaphysa jacobyi sp. n. 30 Labial triangle and postlabium, open and filled circles represent ciliate and smooth setae, respectively 31 Thorax macrochaetae 32 Mesothorax, detail of inner chaetotaxy on a different individual.
Body. Mesothoracic hood not developed. Complete dorsal macrochaetae as 62/41/0244+0+9-11. Mesothorax with 1 anterior (a5) and usually 3 posterior (p1–3) macrochaetae forming an arch (Fig. 31); some individuals with only mesothoraxic macrochaeta p2 (Fig. 32); microchaetae m1, m2, m4, p4 and p5 present. Metathorax with 1 macro- and 5 microchaetae (Fig. 33). First abdominal segment seta a6 absent; 4 posterior setae arranged in a single row (Fig. 34–36). Inner bothriotrix complex of Abd. 2 with 3 fan-shaped setae, one microsensillum and macrochaeta m3; outer bothriotrix with 3 fan-shaped setae and macrochaeta m5; setae a6, m6 and p5 present. Inner bothriotrix complex of Abd. 3 with 3 fan-shaped setae, one sensillum and macrochaetae m3; external bothriotrichal complex (Fig. 37) with 6–7 fan-shaped setae, macrochaetae am6, pm6 and p6; sensillum d2 absent. Fourth abdominal segment with 4 inner (Fig. 38) and 9–11 outer (Fig. 39) macrochaetae: inner macrochaetae A5, B4, and B5 large, B6 small; B5 displaced towards A6 instead of B6; (Fig. 38). Outer macrochaetae D3, E2, E3, F1, and F2 large; small outer macrochaetae E4, F3, F4 and 3 others probably belonging to series Fe present. Abd. 4 anterior and medial bothriotricha with 4 and 2 fan-shaped supplementary setae, respectively (Fig. 39). Posterior bothriotrix corresponds to D4, without associated supplementary setae. Posterior setae 6–7+6–7. Intersegmental membrane between Abd. 4–5 with 4–7 lenticular organs.
Trogolaphysa jacobyi sp. n., open and filled circles represent macro- and microchaetae, respectively, triangles represent fan-shaped microchaetae. 33 Metathorax, detail of inner chaetotaxy, seta a6 present but not shown 34 First abdominal segment, chaetotaxy, arrow points at seta seen in a single individual 35–36 First abdominal segment, alternative insertions of seta m2 37 Third abdominal segment, setae associate with lateral bothriotricha.
Trogolaphysa jacobyi sp. n., symbols as in previous plate. 38 Fourth abdominal segment, inner chaetotaxy 39 Fourth abdominal segment, outer macrochaetae 40 Fourth abdominal segment anterior bothriotrichal complex 41 Prothoracic claw complex 42 Metathoracic claw complex 43 Mucro.
Legs. Trochanteral organ with up to 25 setae. Claw complex as in Figs 41–42. Tenent hair acuminate, longer on L1 than L3. Smooth posterior setae on metathoraxic legs as long as unguiculus. Unguis with 3 inner teeth: basal teeth small, subequal and ending on basal fourth of inner edge; unpaired tooth distinctly larger than basal teeth, ending near middle of inner ungual edge. Outer tooth absent on all claws; lateral teeth present only on pro- and mesothoracic legs, and ending on basal quarter of outer edge of unguis (Fig. 41). Unguiculus basally swollen, with basal fifth of outer margin weakly serrate.
Anterior face with 2+2 distal macrochaetae; lateral and posterior setae not seen.
Furcula. Dens with 2 rows of finely ciliate spines, number of spines per row unclear on all specimens examined, but inner row with at least 36 spines. Mucro elongate and slender, with 3 teeth, basal inner tooth absent (Fig. 43): ratio mucro length/width of dens tip 2.3–2.8 (mode=2.4).
The species is known only from caves in southern Belize
Trogolaphysa jacobyi sp. n. is a troglobiont (sensu
It could be argued that the constriction of the fourth antennomere places this species in Troglopedetes. However, the presence of a well-developed ciliate labial seta L2, the incomplete nature of the constriction on Ant. 4, and the similarity with Trogolaphysa belizeana (presumably with complete, unconstricted Ant. 4, and therefore an uncontested Trogolaphysa) suggest that Trogolaphysa jacobyi sp. n. should be retained in Trogolaphysa. Additionally, the fact that all other Troglopedetes species are restricted to the Old World have prompted us to retain the new species in Trogolaphysa.
This species is dedicated to JoAnn Jacoby, the junior author’s wife, in gratitude for her enthusiasm and assistance in the planning and execution of field-work in the caves of Belize and in many earlier excursions.
This species is a troglobiont, and all 5 collections (11 individuals) were taken in the dark zone (0 lux) on the floor (Fig. 44), often (80% of collections) in wet conditions associated with flowstone or calcite and drip pools, sometimes with scattered cricket droppings.
Type locality for Trogolaphysa jacobyi sp. n. in the dark zone of Yok Balum. Photo courtesy of MES.
http://species-id.net/wiki/Trogolaphysa_belizeana
Figs 45–52Two paratypes; Belize: Cayo District, Actun Chapal cave, 7 km SE of Benque Viejo del Carmen, 10.XII.1992, W.R. Elliott.
Additions to the original description.
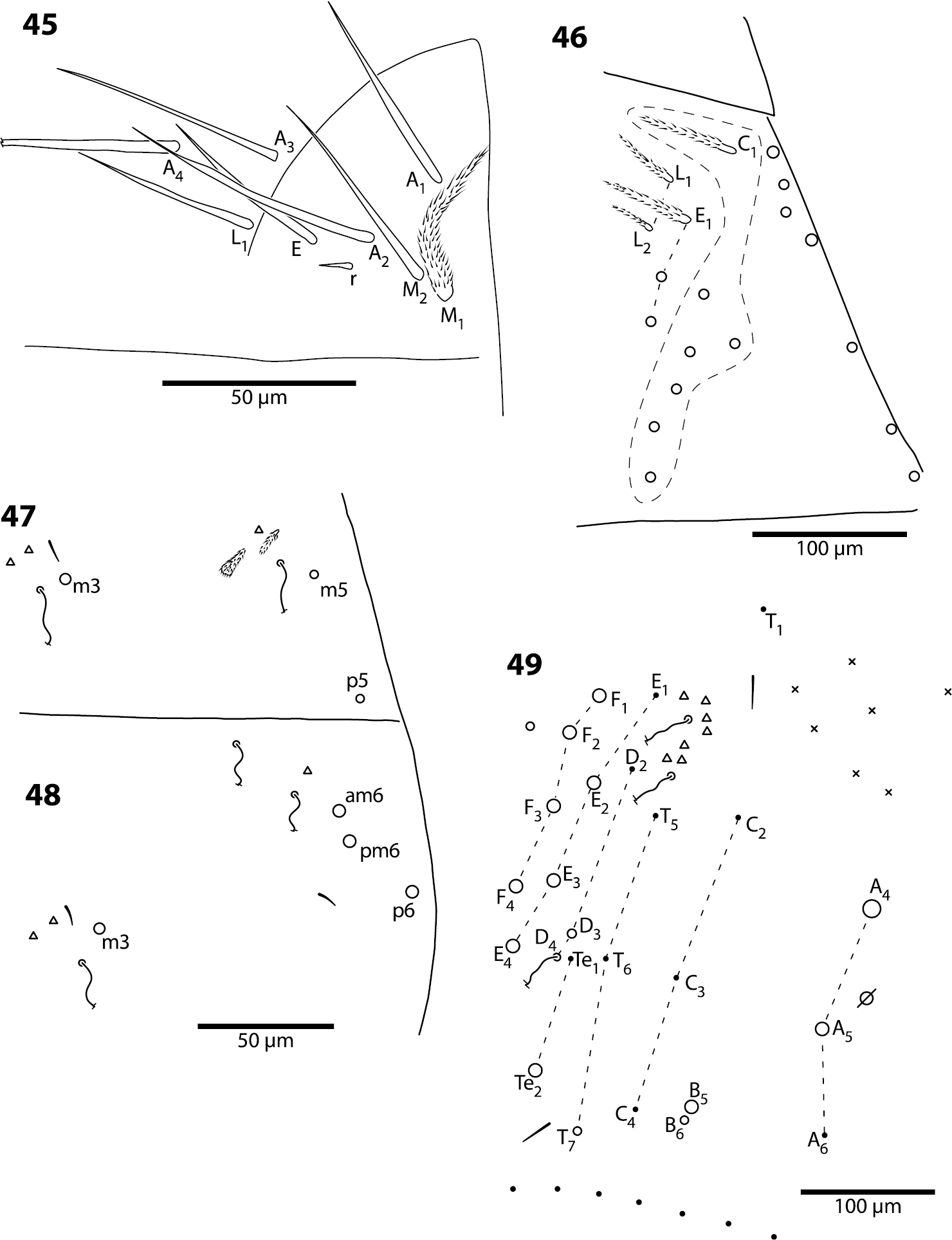
Head. Dorsal chaetotaxy of the head identical to that of Trogolaphysa jacobyi sp. n., with macrochaetae A0, A2, A3, M3, S3, S5, Pa5 and Pm3. Labral margin smooth. Sublobular plate of outer maxillary lobe without setae-like appendages. Labial papilla E with lateral appendage reaching tip of papilla; 5 proximal smooth labial setae present, seta z (
Trogolaphysa belizeana 45 Labial triangle 46 Postlabial chaetotaxy 47 Chaetotaxy of second abdominal segment 48 Chaetotaxy of third abdominal segment 49 Complete chaetotaxy of fourth abdominal segment, x represent sensilla-like setae.
Body. Dorsal macrochaeta formula as 62/3–43/0343+0+11. Mesothorax with macrochaetae p2, p3 and a5, and microchaetae m4 and p5 clearly visible; setae p1, m2 and p6 obscured. Metathorax with 3 macro- and 1 microchaetae arranged as in Trogolaphysa giordanoae sp. n. (Fig. 13). Abd. 1 with at least three inner microchaetae, apparently without a6, but lateral field of segment not clearly visible. Abd. 2 chaetotaxy normal (Fig. 47): with bothriotricha m2 and a5, sensillum as, macrochaetae m3 and m5, setae a6, m6 and p5, and fan-shaped supplementary setae around bothriotrichal complexes. Abd. 3 (Fig. 48) with insertion of bothriotricha m2, a5 and m5, macrochaetae m3, a7, pm6 and p6, and sensillum d2 normally placed. Chaetotaxy of Abd. 4 as in Fig. 49: inner macrochaetae A4, A5, B5 and B6 present, B6 smallest; outer macrochaetae T7, D3, E2, E3, E4, F1, F2, F3, F4, one member of series Fe and one posterior setae of unclear homology present; relative position of bothriotricha normal; microchaeta B4 absent, microchaeta Te1 present. Posterior setae 7+7. Intersegmental membrane between Abd. 4–5 with at least 4 lenticular organs, actual number of organs unclear due to folding of membrane.
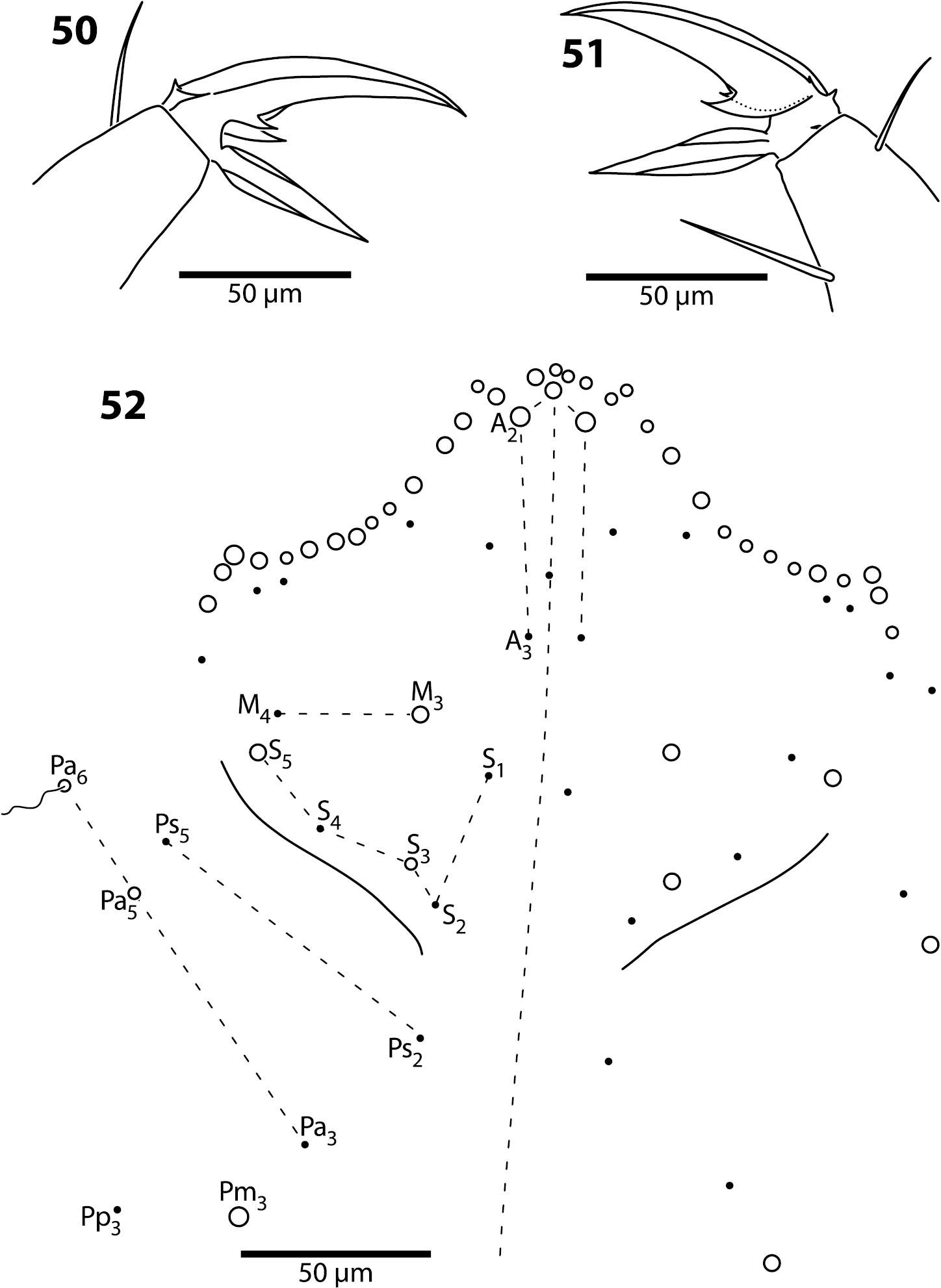
Legs. Claw complex of pro- and metathoracic legs as in Figs 50–51. Tenent hair acuminate. Outer and lateral unguis teeth small, inconspicuous; inner paired teeth with one tooth slightly, but clearly larger, unpaired teeth absent. Unguiculus lanceolate.
Trogolaphysa belizeana (50, 51) and Trogolaphysa jataca (52) 50 Prothoracic claw 51 Metathoracic claw 52 Dorsal chaetotaxy of head.
Ventral tube. With 2+2 distal macrochaetae on anterior face.
The paratypes examined differ from the original description of the species in having labial seta L2 smooth instead of ciliate, in having only 2 posterior mesothoraxic macrochaetae, in the claws having lateral teeth and in the number of bothriotricha on Abd. 2 and Abd. 4.
Variation in the number of mesothoraxic macrochaetae is also seen in Trogolaphysa jacobyi sp. n and may be related to post-embryonic development. The chaetotaxy of Abd. 2 in fig. 12 of
Puerto Rico: Isabela, Guajataca Commonwealth Forest, Rd. 446, 18.41263°N, 66.96887°W, top of mogote at crossroad between trails 6, 25 & 26, leaf litter, 15.V.2009, F. Soto (2 specimens); Cayey, Rd. 4471, Km 4.1, leaf litter, 18.VI.1998, F. Soto (1 specimen); Mayagüez, University of Puerto Rico, secondary forest east of Biology Building, 18.21350°N, 67.13774°W, royal palm (Roystonea borinquena O.F. Cook) leaf litter, III.2009, M. Ospina (3 specimens).
Additions to the original description.
Head. Dorsal chaetotaxy as in Fig. 52: macrochaetae A0, A2, M3, S3, S5, Pa5 and Pm3 present; 1+1 microchaetae inserted near A1. Postlabium with all setae ciliate; columns ICELO with 41232; posterior setae on column I detached from anterior group.
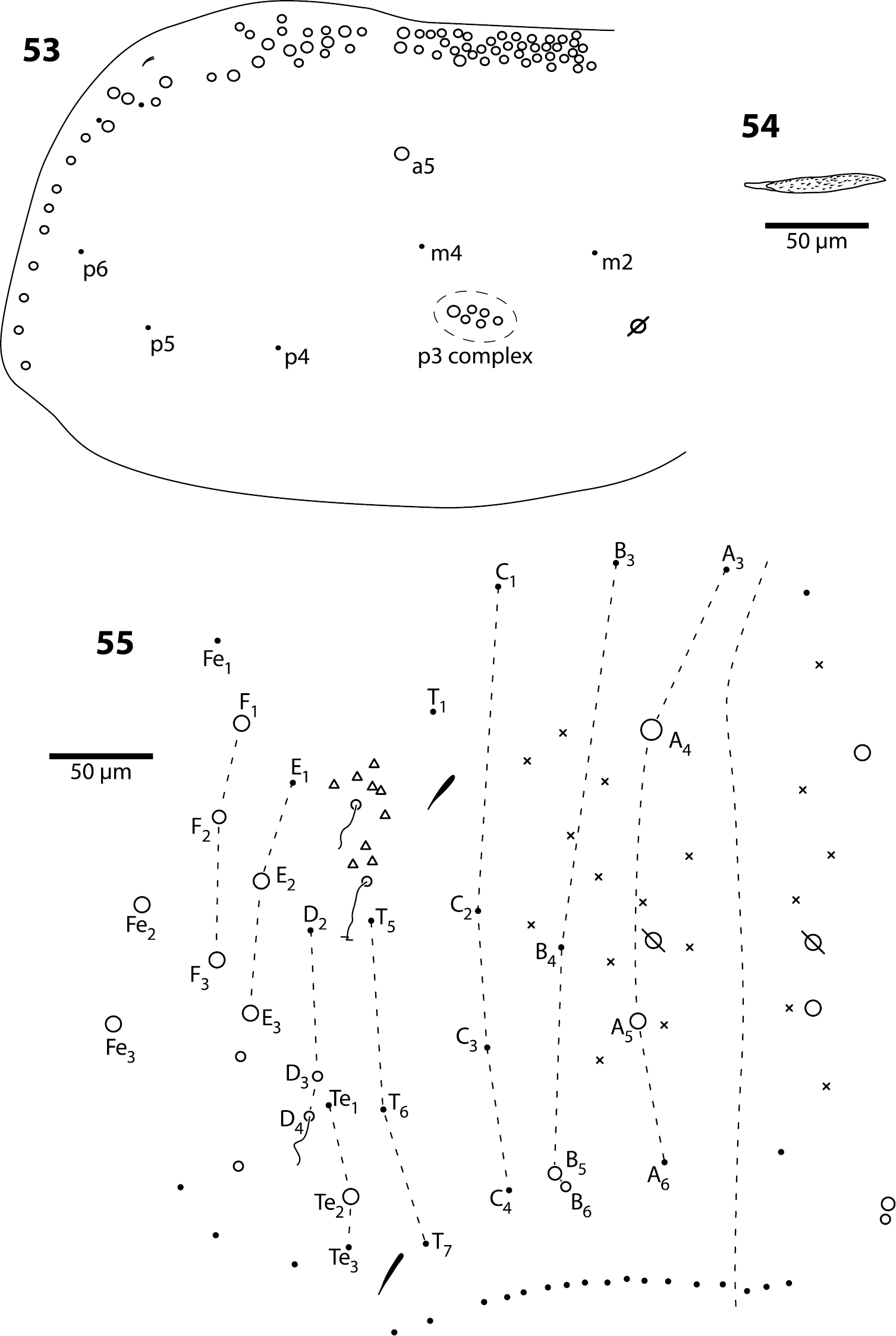
Body. Mesothorax (Fig. 53) with one anterior (a5) and six posterior macrochaeta; microchaetae m2, m4, p5 and p6 present; microchaetae p1 and p2 absent. Metathorax with 4 inner microchaetae, as in Trogolaphysa riopedrensis (Fig. 58). Abd. 1 with 4 posterior setae; seta a6 absent. Abd. 2 and 3 as in Trogolaphysa giordanoae sp. n. (Figs 15, 16); Abd. 2 seta p5 fusiform, with enlarged socket (Fig. 54). Abd. 4 as in Fig. 55: inner macrochaetae A4, A5, B5 and B6 present; macrochaetae Te2, D3, E2, E3, F1–3 present; 4 other lateral and posterior small macrochaetae present. Posterior setae 13–14+13–14. Intersegmental membrane between Abd. 4–5 with 4 lenticular organs.
Trogolaphysa jataca 53 Mesothorax chaetotaxy 54 Second abdominal segment seta p5 55 Complete chaetotaxy of fourth abdominal segment.
Ventral tube. Anterior face with 3+3 distal macrochaetae; smaller individuals with 2+2 macrochaetae.
Puerto Rico: Maricao, Maricao Commonwealth Forest, near observation tower on Rd. 120, 18.14444°N, 66.97962°W, leaf litter, 8.VI.1998, F. Soto (1 specimen); Mayagüez, University of Puerto Rico, secondary forest east of Biology Building, 18.21350°N, 67.13774°W, royal palm (Roystonea borinquena) leaf litter, III.2009, M. Ospina (3 specimens).
Additions to the original description.
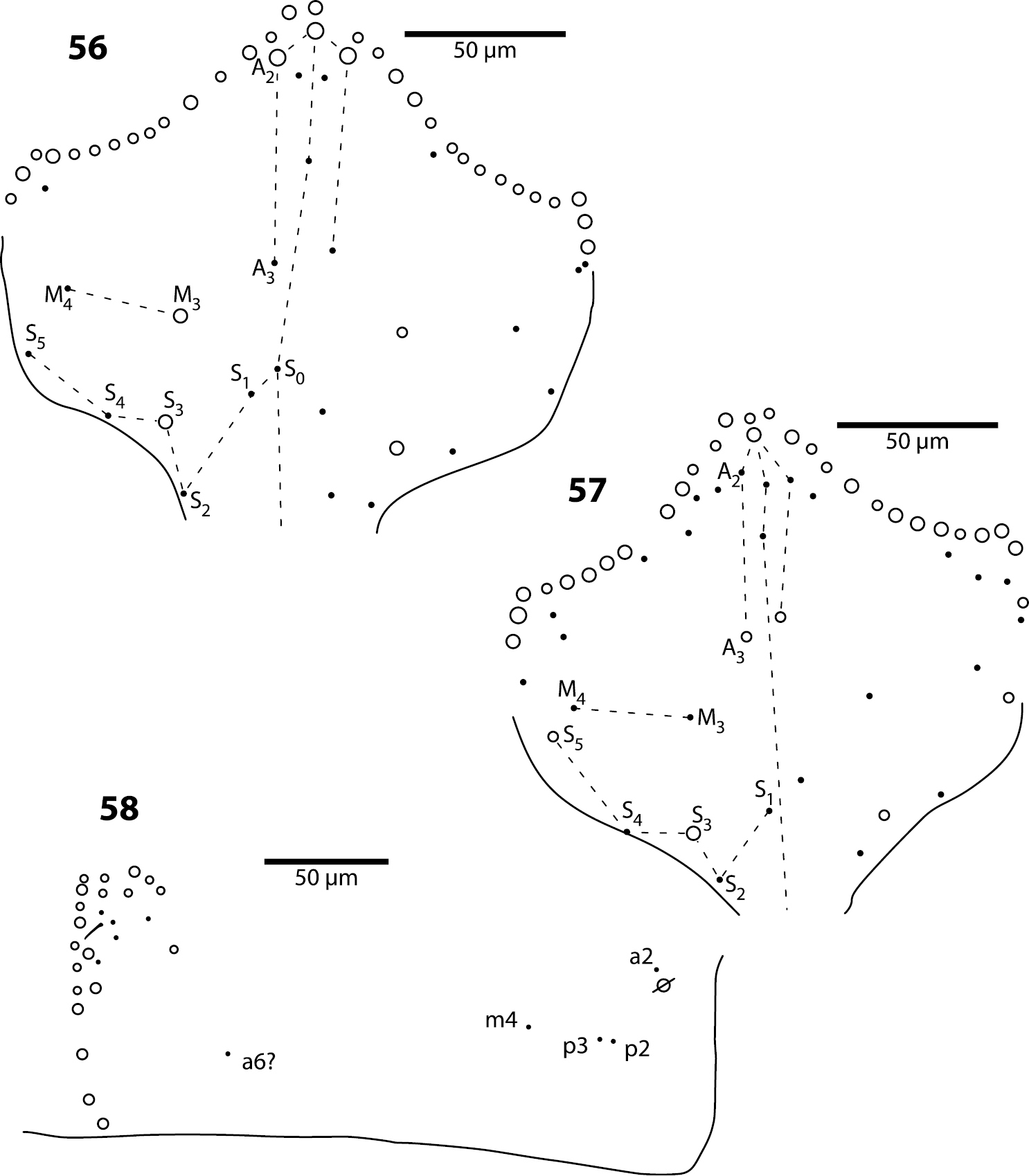
Head. Dorsal chaetotaxy as in Fig. 56: macrochaetae A0, A2, M3, S3, Pa5 and Pm3 present. Postlabium with all setae ciliate; columns ICELO with 41232; posterior setae on column I detached from anterior group.
Trogolaphysa geminata (56) and Trogolaphysa riopedrensis (57, 58) 56 Head dorsal chaetotaxy 57 Head dorsal chaetotaxy 58 Metathorax chaetotaxy.
Body. Mesothorax as in Trogolaphysa jataca (Fig. 53). Metathorax as in Trogolaphysa riopedrensis (Fig. 58). Abd. 1 as in Trogolaphysa riopedrensis (Fig. 59) with one anterior (a6) and 4 posterior setae. Abd. 2 and 3 as in Trogolaphysa giordanoae sp. n. (Figs 15, 16); Abd. 2 seta p5 fusiform as in Trogolaphysa jataca. Abd. 4 as in Trogolaphysa jataca (Fig. 55): inner macrochaetae A4, A5, B5 and B6 present; macrochaetae Te2, D3, E2, E3, F1, F2, F3 present; 4 other lateral and posterior small macrochaetae present. Posterior setae 13–14+13–14. Intersegmental membrane between Abd. 4–5 with 4–6 lenticular organs.
Trogolaphysa riopedrensis 59 Chaetotaxy of first abdominal segment 60 Complete chaetotaxy of fourth abdominal segment, arrows identify the lenticular organs.
Ventral tube. Anterior face with 3+3 distal macrochaetae.
http://species-id.net/wiki/Trogolaphysa_riopedrensis
Fig. 57–60Puerto Rico, Aguadilla, Caimital Alto, Villa Grajales, 18.44058°N, 67.11840°W, moist mown lawn, 9.VII.1999, F. Soto (1 specimen); USA Virgin Islands, St. Thomas, 18.35348°N, 64.93520°W, wet leaf litter, patch of forest along Rd. 33 near intersection with Rd. 40, 28.VI.2000, F. Soto (1 specimen).
Additions to the original description.
Head. Dorsal chaetotaxy as in Fig. 57: macrochaetae A0, (A2), A3, S3, S5, Pa5 and Pm3 present. Postlabium with all setae ciliate; columns ICELO with 41232; posterior setae on column C detached from anterior group.
Body. Mesothorax as in Trogolaphysa jataca (Fig. 53). Metathorax as in (Fig. 58). Abd. 1 with 1 anterior (a6) and 4 posterior setae (Fig. 59). Abd. 2 and 3 as in Trogolaphysa giordanoae sp. n. (Figs 15, 16). Abd. 4 as in Fig. 60: inner macrochaetae A4, A5, B4, B5 and B6 present; outer macrochaetae D3, E2, E3, F1, F2, F3, Fe3 present; at least one other outer macrochaeta present. Posterior setae 17+17. Intersegmental membrane between Abd. 4–5 with 4–6 lenticular organs (Fig. 60).
Ventral tube. Anterior face with 4+4 distal macrochaetae.
Remarks: The individual from St. Thomas lacks head macrochaetae A2. In the individual from Aguadilla the dorsal and outer teeth of the unguis end on the basal fourth of the claw instead of the distal half.
The dorsal chaetotaxy of Trogolaphysa has not been fully described in the context of the AMS (
Head. The dorsal chaetotaxy of the head is reduced when compared to other genera of scaled Entomobryidae (e.g., Seira, Pseudosinella; cf. Fig. 26 here to fig. 1 in
Series M includes 2 setae, probably homologous to M3–M4. In most species the lateral seta in series M is internal to S5, but in troglomorphs Trogolaphysa jacobyi sp. n. and Trogolaphysa belizeana the seta is inserted external to S5 and just internal to the dorsal cephalic suture. M0 is absent (seen only in one individual of Trogolaphysa giordanoae sp. n.), whereas M3 is often developed into a macrochaetae. Series S includes setae S1–5, S0 is absent (seen only in one individual of Trogolaphysa geminata). Among the species examined only setae S3 and S5 are modified into macrochaetae. Most setae in series S are inserted along the dorsal cephalic sulcus; the exceptions are S1, which is anterior to all others, and seta S3 when it is modified into a macrochaeta (cf., Trogolaphysa giordanoae sp. n. [Fig. 5] versus Trogolaphysa jacobyi sp. n. [Fig. 26]).
There is a pattern in the addition of macrochaetae on the interocular region of the head for species with 3–4 macrochaetae, but the pattern in not retained for species with five macrochaetae: whenever three macrochaetae are present they are always A0, A2 and M3; the species with four macrochaetae carries A0, A2 and M3 plus S3; the species with five macrochaetae have A0, A2, S3, S5, and either A3 or M3.
Series Ps includes only two setae (Ps2 and Ps5) whereas series Pa has four setae (Pa2, 3, 5 and bothriotrix Pa6), and series Pm and Pp has one seta each (Pm3 and Pp3). Posterior setae Pa5 and Pm3 are often modified into macrochaetae.
Mesothorax. The chaetotaxy of the mesothorax is reduced, as in scaled Entomobryidae (e.g., Seira, Pseudosinella [
The homologies of the posterior macrochaetae across the species examined are unclear. The presence of setae p1 and p2 in Trogolaphysa giordanoae sp. n. suggests that the cluster of six posterior macrochaetae represent a multiplication of seta p3; whereas the transformation of p1 and p2 into macrochaetae in Trogolaphysa jacobyi sp. n. and Trogolaphysa belizeana, and their absence in the surface species Trogolaphysa jataca, Trogolaphysa geminata and Trogolaphysa riopedrensis suggest that the three setae have been integrated (and duplicated) into the macrochaetal complex. We propose three hypotheses to explain the evolution of posterior macrochaeta: the macrochaetae evolved independently more than once in the genus, either as 1) a duplication of p1–3 or as 2) multiplication of p3 alone; 3) the cluster evolved only once, a duplication of p1–3, and the setae we have identified as p1 and p2 in Trogolaphysa giordanoae sp. n. are secondary and not homologous to those present in Trogolaphysa jacobyi sp. n. and Trogolaphysa belizeana. A study of the postembryonic development of these setae or molecular phylogenetic analysis may provide evidence in support one of the hypotheses proposed above.
Metathorax. The chaetotaxy of this segment is reduced to five setae (e.g., Trogolaphysa geminata, Fig. 58). The homologies of these setae are uncertain, and names provided in Fig. 58 are based on comparison with the general organization of the chaetotaxy in first instar Seira dowlingi (Wray, 1953), Heteromurus nitidus (Templeton, 1835) and Willowsia buskii (Lubbock, 1870) (
Abdomen 1. This segment also has a reduced chaetotaxy, carrying not more than six setae (Figs 14, 59). The homologies proposed are based on comparisons with first instar Seira dowlingi, Heteromurus nitidus and Willowsia buskii (
Abdomen 2–3. The chaetotaxy of these segments was previously described by
The macrochaetae on Abd. 3 appear to be homologous to m3, am6, pm6 and p6 (Fig. 16). Sensillum d2 is absent in Trogolaphysa jacobyi sp. n. (Fig. 37), in Trogolaphysa belizeana it is inserted posterior to macrochaeta pm6 (Fig. 48), whereas in Trogolaphysa giordanoae sp. n., Trogolaphysa jataca, Trogolaphysa geminata and Trogolaphysa riopedrensis it is inserted anterior to or forming a row with pm6 (Fig. 16).
Abdomen 4. The chaetotaxy of Abd. 4 is similar to that in scaled Entomobryidae and setae modified in, for example, Seira or Lepidocyrtus, can also be modified in Trogolaphysa. The chaetotaxy displays some unique peculiarities. For example, what appears to be seta B6 is, in most species, a meso- or small macrochaeta inserted just posterior to B5 (Fig. 17). In addition, the posterior bothriotrix corresponds to D4 (D3 in Seira,
The external macrochaetae in the first three rows of columns D, E and F are stable in the species of examined. All species have macrochaetae D3, E2, E3, F1 and F2. Macrochaeta F3 is present in all species except Trogolaphysa jacobyi sp. n. The number of macrochaetae external to column F and posterior to row 3 varies intra- and interspecifically. However, the lateral and posterior fields are often difficult to see in regular preparations and it is possible that some of the apparent differences are simply incomplete observations.
The number of posterior setae (per side) on Abd. 4 also varies between species: 6–7 in Trogolaphysa belizeana and Trogolaphysa jacobyi sp. n., 13–14 in Trogolaphysa jataca and Trogolaphysa geminata, 17 in Trogolaphysa riopedrensis and 19–21 in Trogolaphysa giordanoae sp. n.
The morphological information for surface species Trogolaphysa luquillensis (
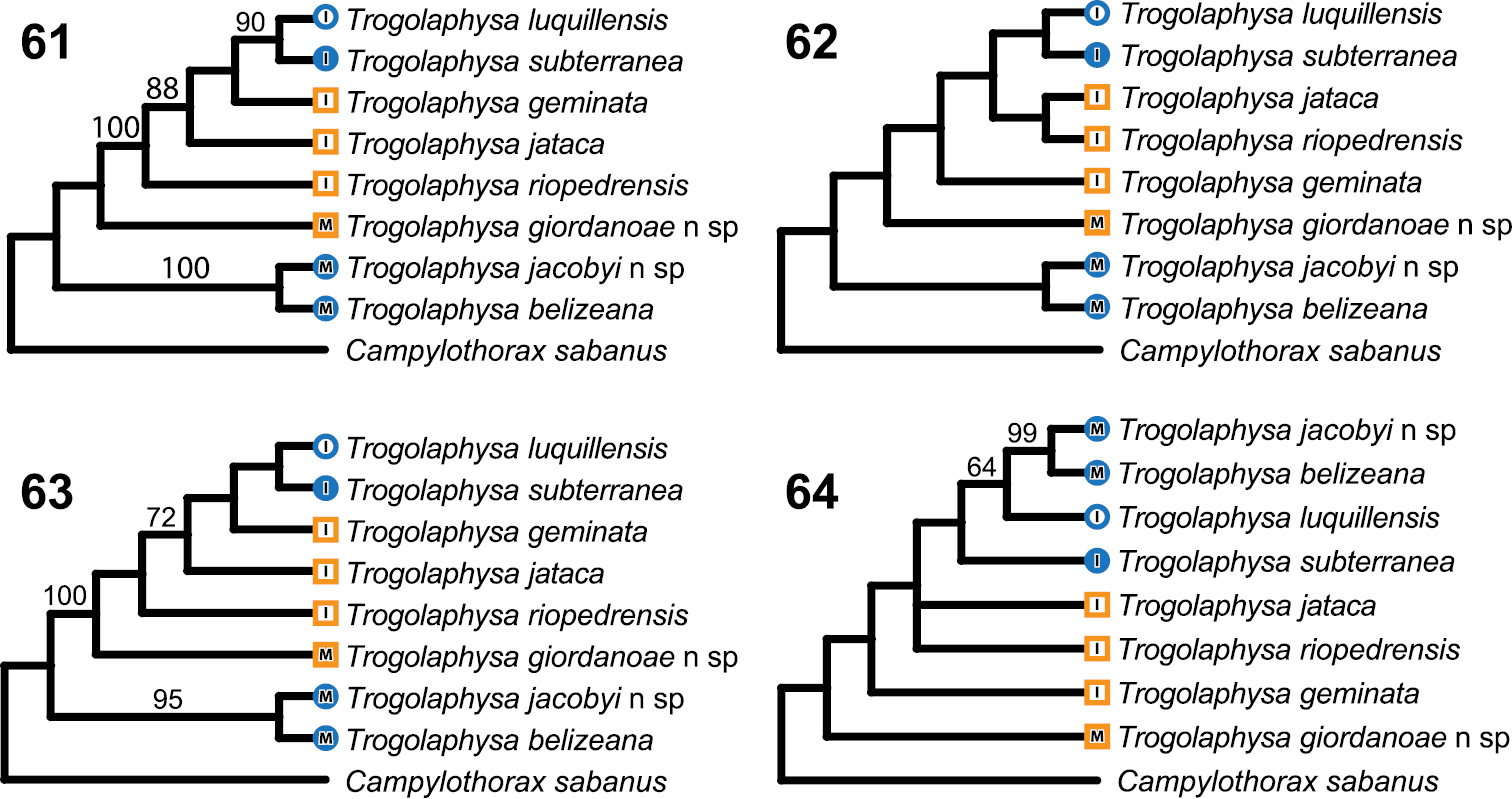
Phylogenetic analysis based on all characters supports two equally parsimonious trees (Figs 61–64) in which the two troglobiontic species from Belize form a monophyletic group and Trogolaphysa giordanoae sp. n. is placed at the base of the species from Puerto Rico. The parsimony trees support the sister species relationship between Trogolaphysa subterranea and Trogolaphysa luquillensis, but relationships between the other three species from Puerto Rico are unresolved, as Trogolaphysa riopedrensis is placed as sister to either Trogolaphysa jataca or to a clade that includes all other island species.
Cladograms. Branch lengths are arbitrary. All searches performed using branch and bound, including the bootstrap analyses. Numbers above branches are bootstrap values based on 5000 pseudoreplicates. Circles: taxa with troglomorphies, squares-not troglomorphic. Solid symbols recorded only from caves, open symbols recorded from surface. M, mainland species: I, island species 61–62 The two shortest trees found when all characters are included in the analysis 63 Shortest tree found when only chaetotactic characters are analyzed 64 Shortest tree found when only eye number, characters related to claw complex morphology and mucro are analyzed.
The apparently rare occurrence of metathoracic macrochaetae in the three Belizean species suggests a close relationship between them, but the parsimony trees show the troglobiontic species diverging before the separation of Trogolaphysa giordanoae sp. n. from the ancestor of the island species. The lack of support for the monophyly of Belizean species may be an artifact of a disproportionate contribution of characters under strong cave habitat selection to the final topology of the tree. However, parsimony analysis based only on chatotactic characters results in a single shortest tree (Fig. 63), which also supports the monophyly of troglobiontic species while retaining Trogolaphysa giordanoae sp. n. at the base of the island species clade.
To assess whether putative adaptive characters provide support for alternative relationships, we conducted a phylogenetic analysis using only eye number, ornamentation of labral papilla, and claw and mucro morphology. These characters support a single tree (Fig. 64) that places most surface forms at the base of the tree while supporting a clade comprising the cave species (Trogolaphysa jacobyi sp. n., Trogolaphysa belizeana, Trogolaphysa subterranea) and Trogolaphysa luquillensis. Trogolaphysa luquillensis is endemic to the tropical rainforest and is unique among surface species examined here in having an acuminate tenent hair and three inner ungual teeth close to each other and inserted in the basal half of the claw. These characters of the claw have been identified as adaptations to walking on water surface or other, permanently wet, surfaces such as those found in rainforest leaf litter and caves (
Evaluation of the direction of evolution of head chaetotaxy using trees in Fig. 61 and Fig. 62 supports a trend towards a reduction in number of macrochaetae. However, the pattern is equivocal because some macrochaetae may be lost independently through out the tree, depending on tree topology. For example, S5 might have been lost once and regained or it might have been lost twice independently. What is clear from this analysis is that A3 is the first macrochaetae to be lost, followed by S5, S3 and M3 (Table 4). Trogolaphysa riopedrensis is the only species in which this pattern seems to be disrupted: under either tree this species is hypothesized to have lost M3 and gain A3 independently.
Distribution of head macrochaetae in eight species of New World Trogolaphsya.
| Species | Macrochaetae number | Macrochaeta identity | |||||
|---|---|---|---|---|---|---|---|
| Trogolaphysa jacobyi sp. n. | 6 | A0 | A2 | A3 | M3 | S3 | S5 |
| Trogolaphysa belizeana | 6 | A0 | A2 | A3 | M3 | S3 | S5 |
| Trogolaphysa riopedrensis | 5 | A0 | A2 | A3 | — | S3 | S5 |
| Trogolaphysa jataca | 5 | A0 | A2 | — | M3 | S3 | S5 |
| Trogolaphysa geminata | 4 | A0 | A2 | — | M3 | S3 | — |
| Trogolaphysa luquillensis, | 3 | A0 | A2 | — | M3 | — | — |
| Trogolaphysa giordanoae sp. n. | 3 | A0 | A2 | — | M3 | — | — |
| Trogolaphysa subterranea | 2 | A0 | A2 | — | — | — | — |
The character used by
It is possible, as proposed by
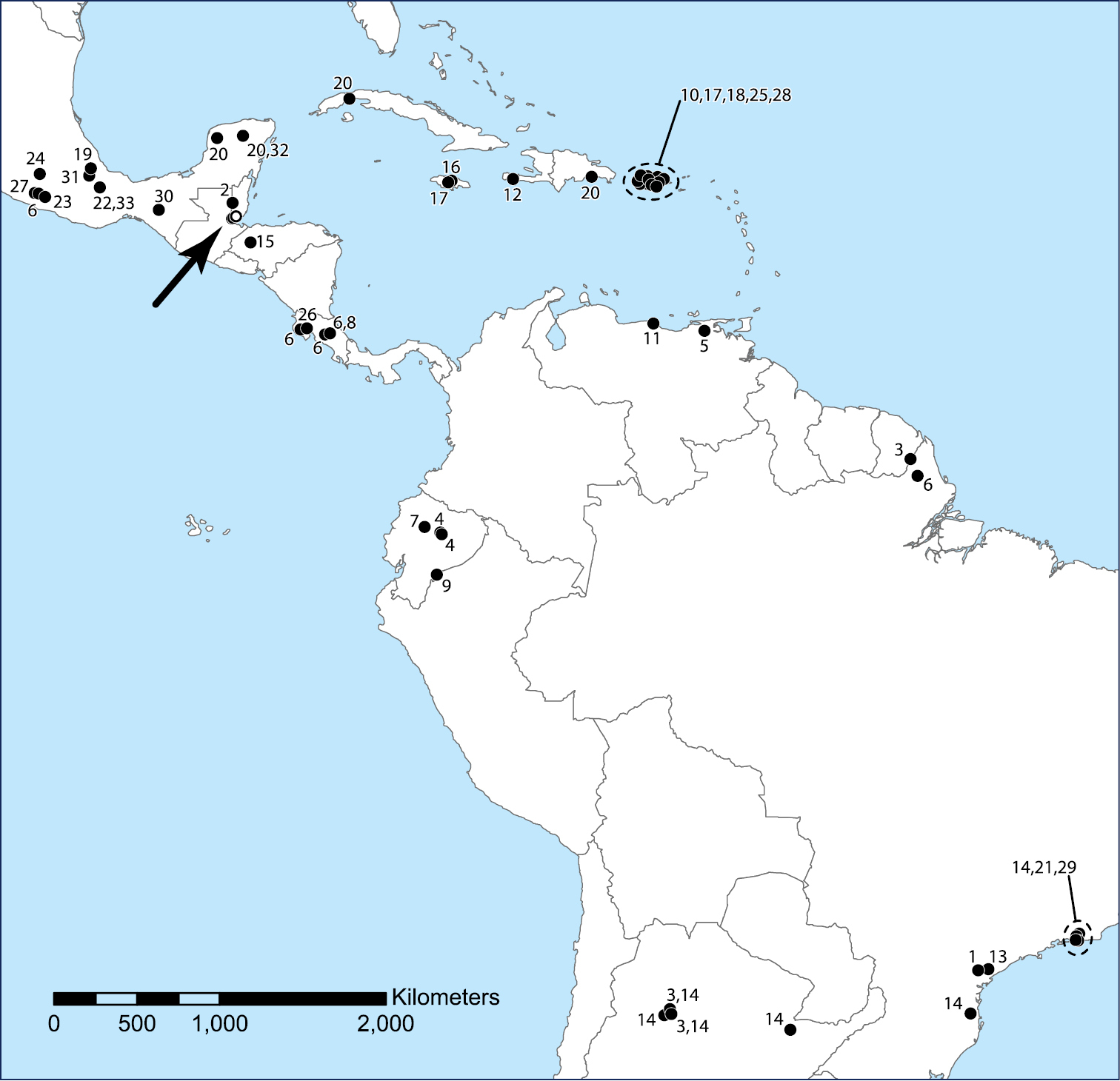
The genus Trogolaphysa has diversified in the New World from where now 35 species have been named (Table 1, Fig. 65), many of which are troglobionts or at least eutroglophiles (sensu
Central and South America and the Caribbean Islands, showing the published distributions of described New World species of the genus Trogolaphysa. Open circles (arrow): Trogolaphysa jacobyi sp. n., Trogolaphysa giordanoae sp. n. Closed circles: 1 Trogolaphysa aelleni Yoshii, 1988 2 Trogolaphysa belizeana 3 Trogolaphysa berlandi (Denis, 1925) 4 Trogolaphysa bessoni Thibaud & Najt, 1989 5 Trogolaphysa caripensis (Gruia, 1987) 6 Trogolaphysa carpenteri (Denis, 1925) 7 Trogolaphysa cotopaxiana Thibaud & Najt, 1989 8 Trogolaphysa distinguenda (Denis, 1931) 9 Trogolaphysa ecuatorica (Palacios-Vargas, Ojeda & Christiansen, 1986) 10 Trogolaphysa geminata 11 Trogolaphysa guacharo Yoshii, 1988 12 Trogolaphysa haitica (Palacios-Vargas, Ojeda & Christiansen, 1986) 13 Trogolaphysa hauseri Yoshii, 1988 14 Trogolaphysa hirtipes (Handschin, 1924) 15 Trogolaphysa hondurasensis (Palacios-Vargas, Ojeda & Christiansen, 1986) 16 Trogolaphysa jamaicana (Palacios-Vargas, Ojeda & Christiansen, 1986) 17 Trogolaphysa jataca 18 Trogolaphysa luquillensis 19 Trogolaphysa marimutti (Palacios-Vargas, Ojeda & Christiansen, 1986) 20 Trogolaphysa maya 21 Trogolaphysa millsi Arlé, 1939 22 Trogolaphysa nacionalica (Palacios-Vargas, Ojeda & Christiansen, 1986) 23 Trogolaphysa oztotlica (Ojeda & Palacios-Vargas, 1984) 24 Trogolaphysa relicta (Palacios-Vargas, Ojeda & Christiansen, 1986) 25 Trogolaphysa riopedrensis 26 Trogolaphysa separata (Denis, 1933) 27 Trogolaphysa strinatii Yoshii, 1988 28 Trogolaphysa subterranea 29 Trogolaphysa tijucana (Arlé & Guimarāes, 1979) 30 Trogolaphysa toroi (Palacios-Vargas, Ojeda & Christiansen, 1986) 31 Trogolaphysa variabilis (Palacios-Vargas, Ojeda & Christiansen, 1986) 32 Trogolaphysa xtolokensis (Palacios-Vargas, Ojeda & Christiansen, 1986) 33 Trogolaphysa yoshiia (Palacios-Vargas, Ojeda & Christiansen, 1986).
Ever since the publication of Gisin’s (1967) “systématique ideal, ” collembolan systematists have assumed that ideochaetotaxic characters are non-adaptive characters that evolve neutrally, are less prone to convergence and, therefore, more valuable for phylogenetic analysis. However, this assumption has never been tested in a phylogenetic context. The simple test performed here supports the traditional view of chaetotaxy as less vulnerable to directional convergence than characters related to claw structure. Analysis based exclusively on putative cave-adaptive characters support a clade comprising cave species from Puerto Rico and Belize, whereas analysis of chaetotaxy alone supports the placement of cave species from Puerto Rico and Belize in independent clades. Despite the clear difference in signal in the character partitions it should be noted that analysis of the complete character set results in higher bootstrap values for what is basically the chaetotaxy-only tree, than when only chaetotactic characters are analyzed. It is clear that some putative adaptive characters retain phylogenetic information concordant with chaetotaxy characters, an observation which argues in favor of the retention of all characters in the analysis. The simple test preformed here has to be expanded to include many more species, to determine if the result obtained are consistent or just an artifact of the sparse taxon sampling. It is unclear if chaetotaxy will provide sufficient characters to resolve relationships in an analysis that includes all species. In any case, there are problems related to the evolution and homology of some chaetotactic characters (e.g., posterior macrochaetae on the meso- and metathorax, and the inner macrochaetae on the fourth abdominal segment) that may be intractable on morphology-based datasets, and will require the use of putatively independent molecular characters.
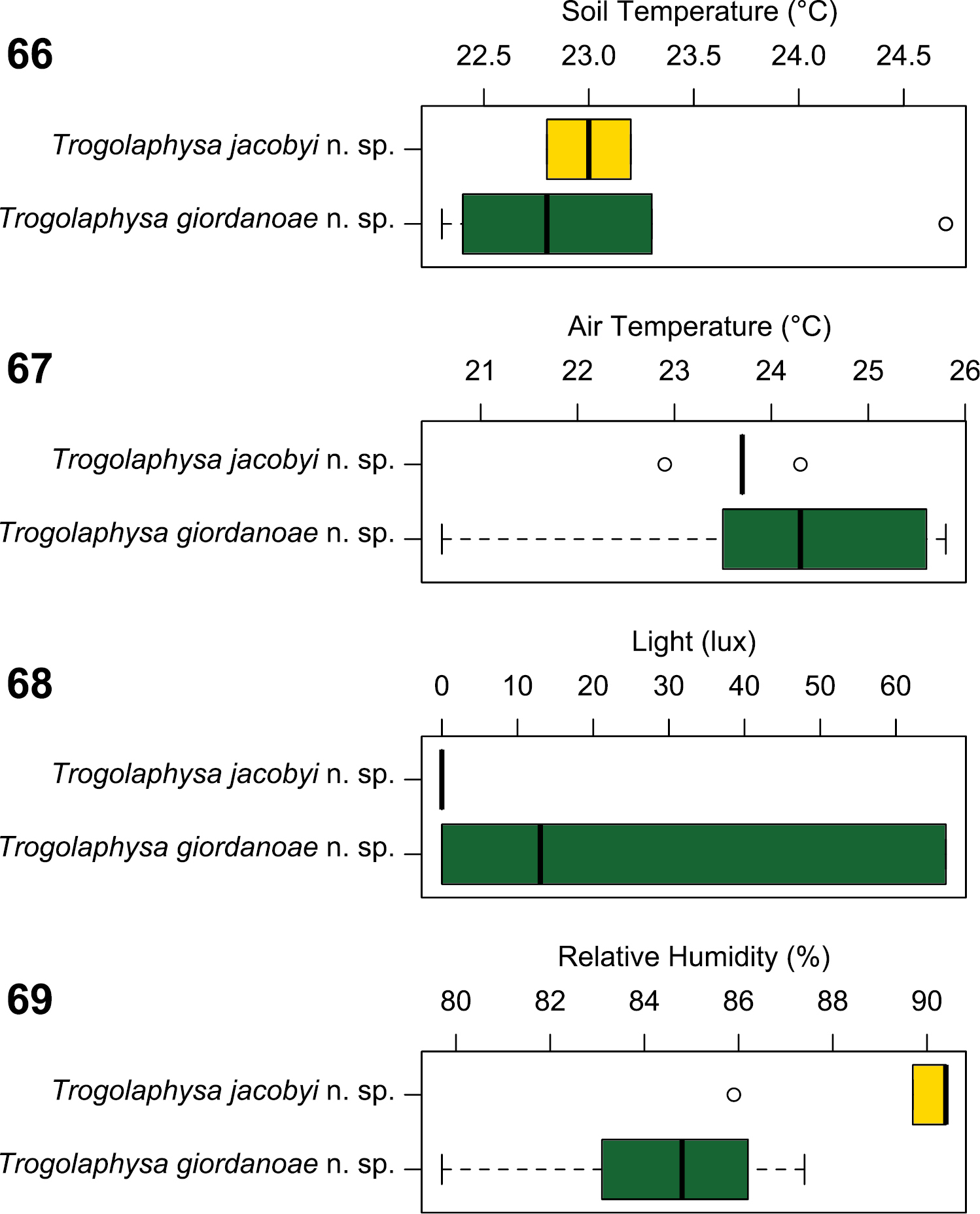
The two new species were found in conditions of similar substrate (Trogolaphysa jacobyi sp. n. mean=23.0 °C; Trogolaphysa giordanoae sp. n. mean=23.1 °C; W=11, p=0.7200) (Fig. 66) and air temperatures (Trogolaphysa jacobyi sp. n. mean=23.7 °C; Trogolaphysa giordanoae sp. n. mean=24.3 °C; W=23.5, p=0.3947) (Fig. 67), but Trogolaphysa jacobyi sp. n. was found only in complete darkness (Fig. 68), whereas Trogolaphysa giordanoae sp. n. was found at significantly brighter and varying light conditions, typically in twilight (Trogolaphysa jacobyi sp. n. mean=0.0 lux; Trogolaphysa giordanoae sp. n. mean=29.5 lux; W=12.5, p=0.0260). Trogolaphysa jacobyi sp. n. also was found primarily under conditions of significantly elevated humidity, whereas Trogolaphysa giordanoae sp. n. was more varied in the humidity levels at which it was found (Trogolaphysa jacobyi sp. n. mean=89.36 %; Trogolaphysa giordanoae sp. n. mean=84.56 %; W=65, p=0.0056) (Fig. 69). In addition, Trogolaphysa giordanoae sp. n. was frequently associated with fruit bat guano or other scat (Fig. 23). These observations support our classification of Trogolaphysa jacobyi sp. n. as a troglobiont and Trogolaphysa giordanoae sp. n. as a guanophile.
Boxplot comparisons of environmental parameters for collections of Trogolaphysa jacobyi sp. n. and Trogolaphysa giordanoae sp. n. 66 Soil temperature 67 Air temperature 68 Light 69 Relative humidity.
We thank members of the 2011 Belize biospelology expedition, Michael E. Slay, JoAnn Jacoby, Geoffrey B. Hoese, Jean K. Krejca and Christy M. Slay, who, along with the junior author, collected the species described in this paper. We thank local guides Avelardo Canti, Gabriel Chaco, Germano Coe and Rosalio Sho for taking the field crew to the caves in southern Belize. Bruno Kuppinger facilitated various logistical aspects of the Belize fieldwork. We thank the Belize Institute of Archeology, especially Dr. John Morris, Dr. Jaime J. Awe and George Thompson, and the Belize Forest Department, especially Rasheda M. Garcia and Hector Mai, for their support, advice, permissions and cooperation in undertaking research in the caves of Belize. Phil Walker, Alan Braybrooke and Keith Prufer provided valuable information about study sites. The Subterranean Ecology Institute, Inc. and the National Speleological Foundation provided financial support for fieldwork, and in-kind support was provided by the Illinois Natural History Survey, the University of Illinois, The Nature Conservancy and Zara Environmental, LLC. We thank Jim Nardi for graciously providing access to a polarized light-equipped microscope used to visualize the EOS.
Character and character states as circumscribed for phylogenetic analysis. (doi: 10.3897/zookeys.323.4950.app1) File format: Microsoft Word document (doc).
Data matrix of morphological characters used in the phylogenetic analysis. (doi: 10.3897/zookeys.323.4950.app2) File format: Microsoft Word document (doc).