(C) 2013 Fernando H. Aballay. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A key to 16 histerid species associated with decaying carcasses in Argentina is presented, including diagnoses and habitus photographs for these species. This article provides a table of all species associated with carcasses, detailing the substrate from which they were collected and geographical distribution by province. All 16 Histeridae species registered are grouped into three subfamilies: Saprininae (twelve species of Euspilotus Lewis and one species of Xerosaprinus Wenzel), Histerinae (one species of Hololepta Paykull and one species of Phelister Marseul) and Dendrophilinae (one species of Carcinops Marseul). Two species are new records for Argentina: Phelister rufinotus Marseuland Carcinops troglodytes (Paykull). A discussion is presented on the potential forensic importance of some species collected on human and pig carcasses.
Key, Histeridae, Saprininae, forensic, carcasses, Argentina
Coleoptera is one of the major orders of insects represented on carcasses and its forensic importance has been frequently documented (
Histeridae comprises 4252 species and 391 genera worldwide, grouped in 11 subfamilies (
Due to the fact that the Diptera colonize the body from the beginning of the decomposition process (
Histerid adults have been frequently mentioned in forensic studies on decomposing pig carcasses (
The objective of this paper is to provide an illustrated key to the histerid species associated with decaying carcasses in Argentina to achieve their correct identification. Additionally, diagnoses for these species are presented.
A total of 7070 specimens were collected mostly during forensic studies on decomposing pig carcasses because it is the preferred animal model for forensic entomological studies (
In addition specimens from decomposing pig carcasses were recorded in the provinces of Salta (24°54'40"S, 65°28'16"W, 1379 m altitude) and Jujuy (24°09'54.13"S, 65°18'37.73"W, 1383 m altitude), with mesic conditions. For collecting and conserving specimens the methodology followed was that by
Other Histeridae specimens were obtained using three kinds of collecting procedures, the first was conducted on human corpses at different places in Mendoza province authorized by the Medical Forensic Committee of Mendoza; the second was conducted in field trips in different Argentinean provinces on carcasses of cow (Bos taurus), horse (Equus caballus), donkey (Equus asinus), dog(Canis familiaris), snake (not identified), Geoffroy´s cat(Leopardus geoffroyi), llama(Lama glama), guanaco (Lama guanicoe), vicuña (Vicugna vicugna), sheep (Ovis orientalis), fox (Lycalopex griseus), lesser rhea (Pterocnemia pennata), rat (Eligmodontia typus) all found outdoors; the third type of collection was using traps baited with rotting flesh of chicken, squid and sardine in different provinces of Argentina.
Voucher specimens are deposited in the entomological collections of the Instituto Argentino de Investigaciones de las Zonas Áridas (Mendoza, Argentina) and Museo Nacional de Historia Natural (Santiago, Chile).
Specimens were cleaned with water and detergent using a Haier ultrasonic cleaner. Diagnoses were made using a Bausch and Lomb stereomicroscope with magnification between 45× and 60×. Measurements (given in millimeters) were taken with an ocular micrometer. Body length was measured from anterior angle of pronotum to elytral apex, without including head and abdominal terga (propygidium and pygidium) and defined as follows: small 0.5–1.9 mm, medium 2.0–3.9 mm and large 4.0–8.0 mm. Body width was measured at maximum width of elytra, in humeral part. Terminology follows
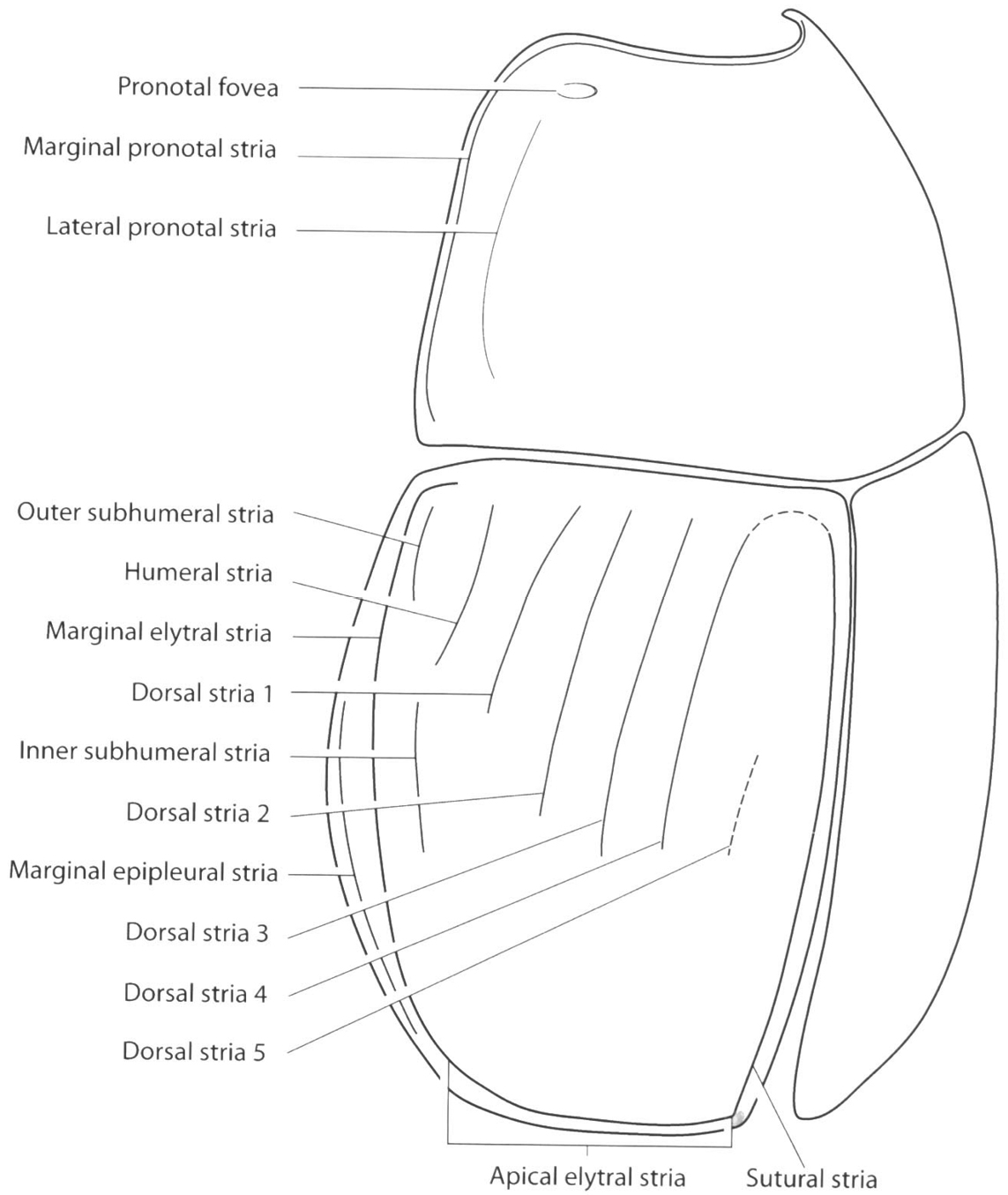
Saprininae, schematic. Pronotum and elytra, oblique lateral view (taken from
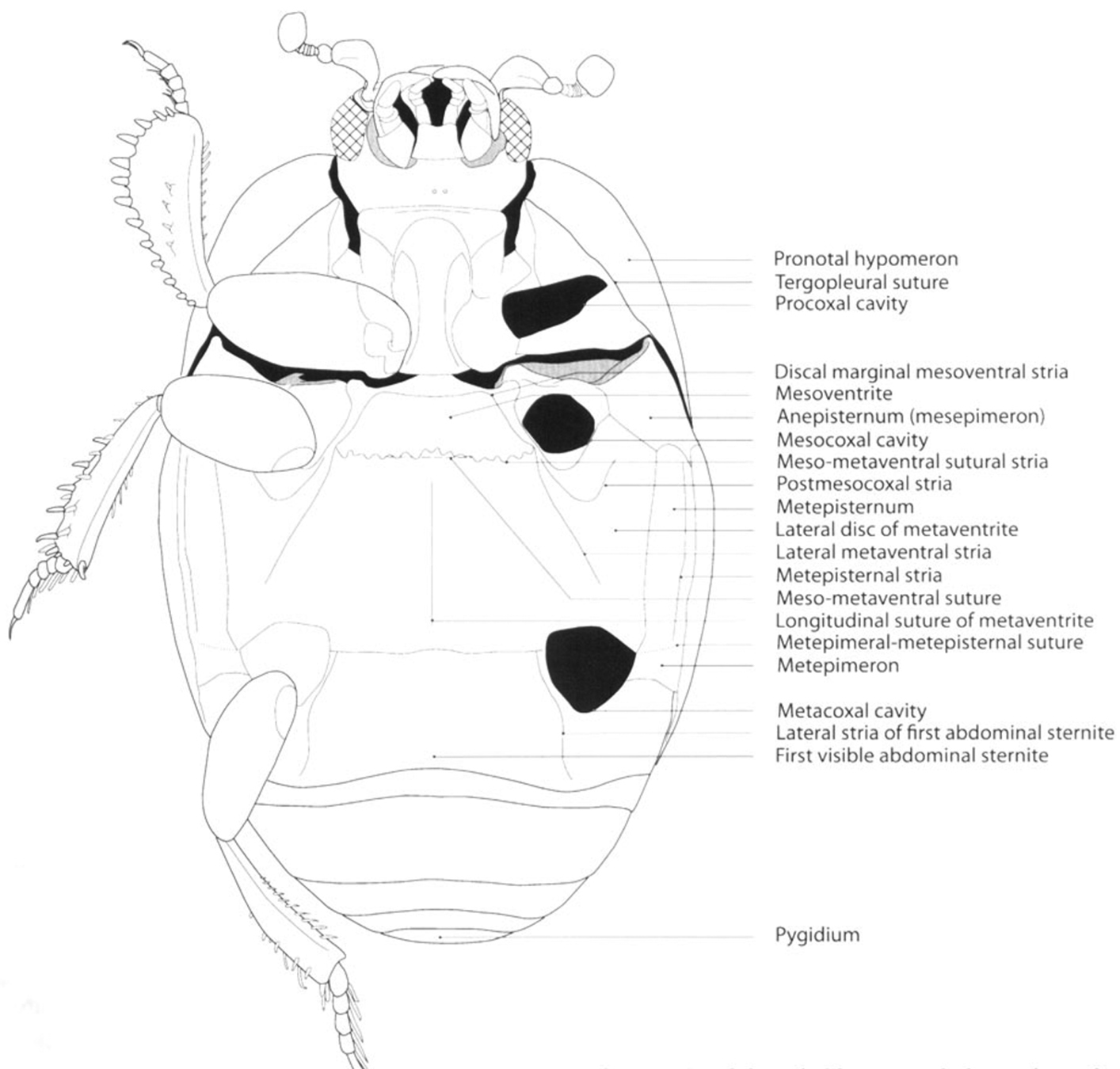
Saprininae, schematic. Habitus, ventral view (taken from
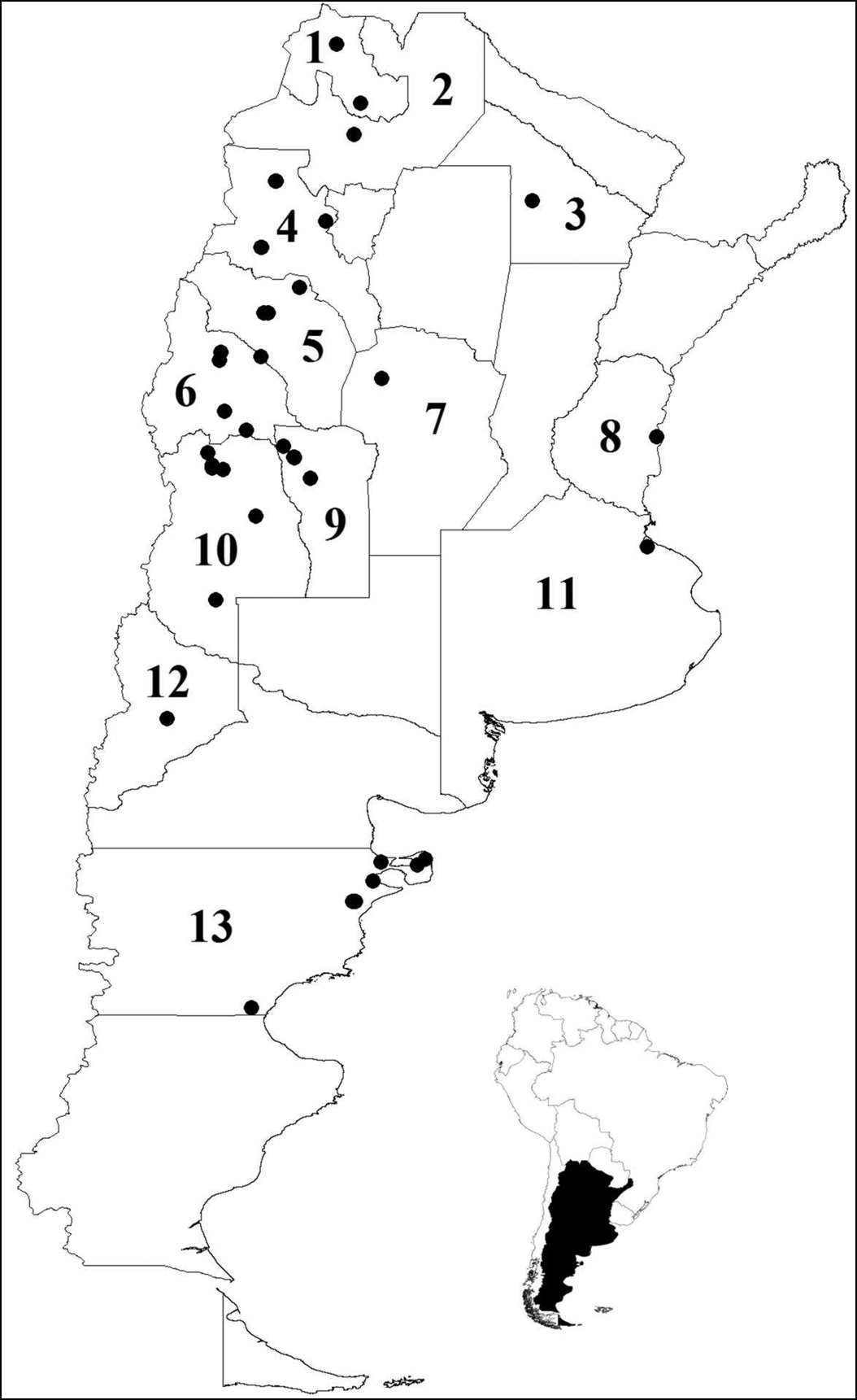
The list of histerids of forensic importance in Argentina comprises 16 species distributed in 13 provinces (Table I). In order to enable a more accurate use of the key, diagnosis of each species with habitus photographs are provided.
| 1. | Prosternal lobe present (Fig. 3) | 2 |
| 1´ | Prosternal lobe absent (Fig. 4) | 4 |
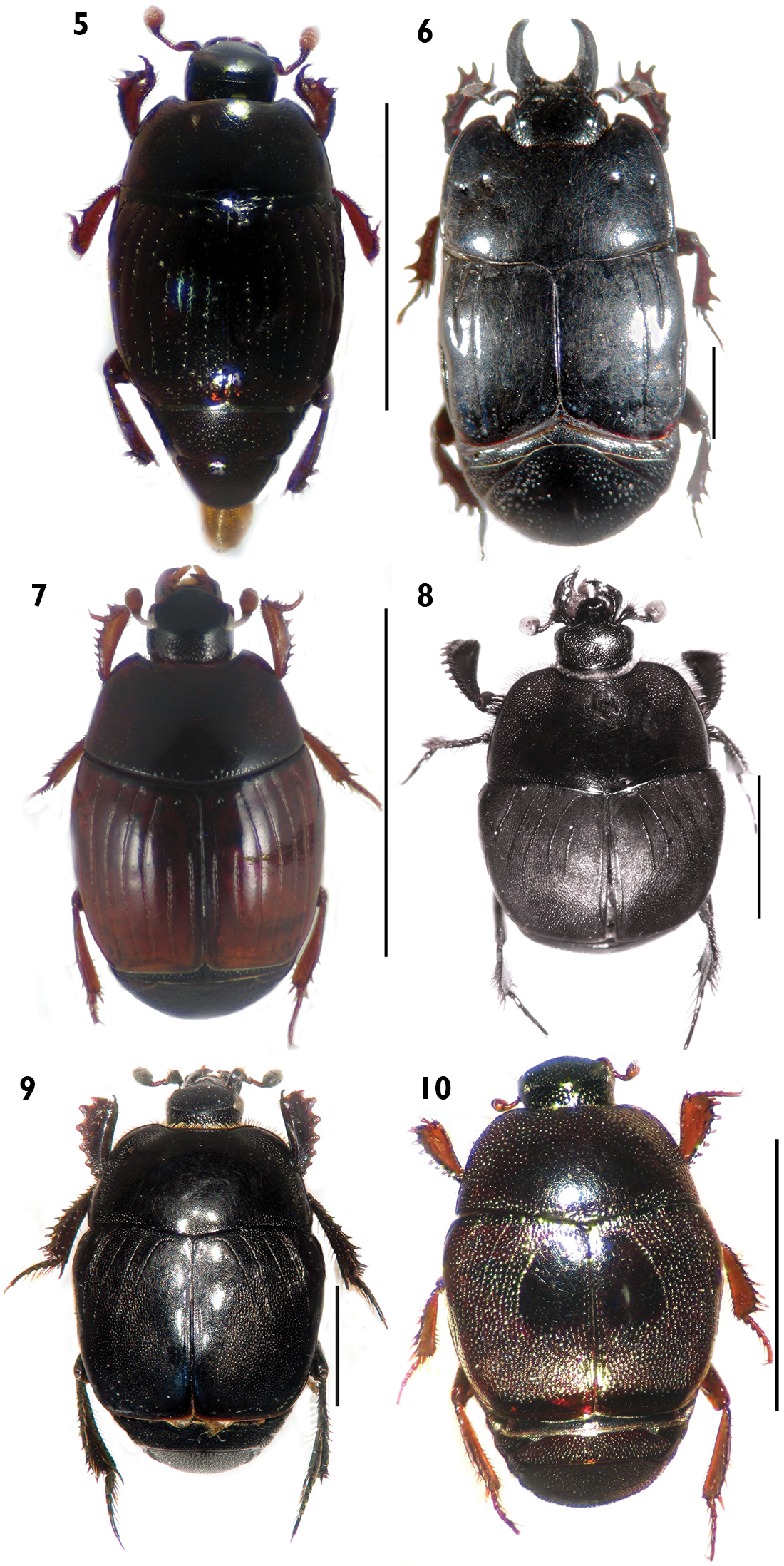
| 2. | Labrum with setae (Fig. 5) | Carcinops (Carcinops) troglodytes (Paykull) |
| 2´ | Labrum without setae | 3 |
| 3 | Head prognathous, not retractile; mandibles long, prominent, as long as head; pronotum and elytra lacking punctures; length greater than 6.9 mm (Fig. 6) | Hololepta (Leionota) reichii Marseul |
| 3´ | Head hypognathous, retractile; mandibles short, as long as half of head; pronotum and elytra with finer and sparse punctation; length less than 1.5 mm (Fig. 7) | Phelister rufinotus Marseul |
| 4 | Pronotal hypomeron setose in dorsal view | 5 |
| 4´ | Pronotal hypomeron glabrous in dorsal view | 10 |
| 5 | Elytronwith five dorsal striae, the fifth between the fourth dorsal and sutural striae (Fig. 8) | Euspilotus (sensu stricto) lacordairei (Marseul) |
| 5´ | Elytronwith four dorsal striae, fifth stria absent (Figs 9, 10, 13–15) | 6 |
| 6 | Elytron black, lacking spots (Figs 9, 10) | 7 |
| 6´ | Elytronblack with orange, yellow or white spots (Figs 13–15) | 8 |
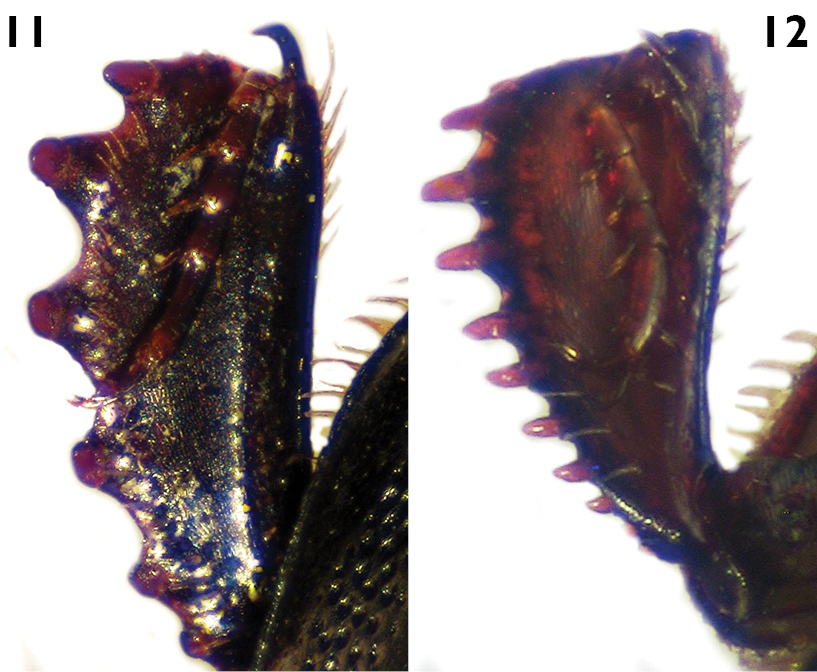
| 7 | Outer margin of protibiae with teeth much expanded and 6 denticles (Fig. 11); elytron with coarse and dense punctation, with a shining area with finer and sparse punctation between the fourth dorsal and sutural striae, narrowed apically; length greater than 4.4 mm (Fig. 9) | Euspilotus (sensu stricto) patagonicus (Blanchard) |
| 7´ | Outer margin of protibiae with teeth moderately expanded and 7-8 denticles (Fig. 12); elytronwith very coarse and dense punctation, with a shining impunctate area between the fourth dorsal and sutural striae, wider apically; length less than 2.9 mm (Fig. 10) | Xerosaprinus (Xerosaprinus) diptychus (Marseul) |
| 8 | Elytral spot with a digitiform projection towards apex (Fig. 13) | Euspilotus (sensu stricto) richteri Lewis |
| 8´ | Elytral spot straight on distal edge (Figs 14–15) | 9 |
| 9 | Elytral spot with two digitiform projections anterad, the outer one close to but not reaching the basal edge (Fig. 14) | Euspilotus (sensu stricto) lepidus (Erichson) |
| 9´ | Elytral spot with three digitiform projections anterad, away from basal edge (Fig. 15) | Euspilotus (sensu stricto) ornatus (Blanchard) |
| 10 | Anterior half of elytronwith very coarse and dense punctation, with a shining impunctate area between the fourth dorsal and sutural striae (Fig. 16) | Euspilotus (Hesperosaprinus) caesopygus (Marseul) |
| 10´ | Anterior half of elytronwith finer and sparse punctation, lacking shining impunctate areas (Figs 17–22) | 11 |
| 11 | Dorsal elytral striae 3–4 present, well demarcated on anterior half (Figs 17–20) | 12 |
| 11´ | Dorsal elytral striae 3 absent or marked as a row of impressed punctures on basal area, stria 4 present or reduced to a rounded arch basally connected to the sutural stria (Figs 21–22) | 15 |
| 12 | Pronotum with a single fovea on each side close to anterior angles or with a longitudinal lateral depression on each side close to lateral margins with coarse and dense punctation (Figs 17–18) | 13 |
| 12´ | Pronotum lacking fovea or longitudinal lateral depression (Figs 19–20) | 14 |
| 13 | Pronotum with a single depression on each side close to anterior angles, with coarse and dense punctation; distal half of elytra, propygidium and pygidium with ocellate punctation, a small puncture within a large puncture (Fig. 17) | Euspilotus (Hesperosaprinus) strobeli (Steinheil) |
| 13´ | Pronotum with a longitudinal lateral depression on each side, with coarse and dense punctation; distal half of elytra, propygidium and pygidium with regular punctation (Fig. 18) | Euspilotus (Hesperosaprinus) pavidus (Erichson) |
| 14 | Elytron with inner subhumeral stria; length greater than 2.5 mm (Fig. 19) | Euspilotus (Hesperosaprinus) modestus (Erichson) |
| 14´ | Elytron lacking inner subhumeral stria; length less than 2.2 mm (Fig. 20) | Euspilotus (Hesperosaprinus) parenthesis (Schmidt) |
| 15 | Pronotum with marginal stria away from lateral margin; pygidium with a transverse subapical groove not reaching lateral margins (Fig. 21) | Euspilotus (Hesperosaprinus) connectens (Paykull) |
| 15´ | Pronotum with marginal stria very close to lateral margin; pygidium with a transverse subapical groove reaching lateral margins (Fig. 22) | Euspilotus (Hesperosaprinus) azureus (Salberg) |
http://species-id.net/wiki/Carcinops_troglodytes
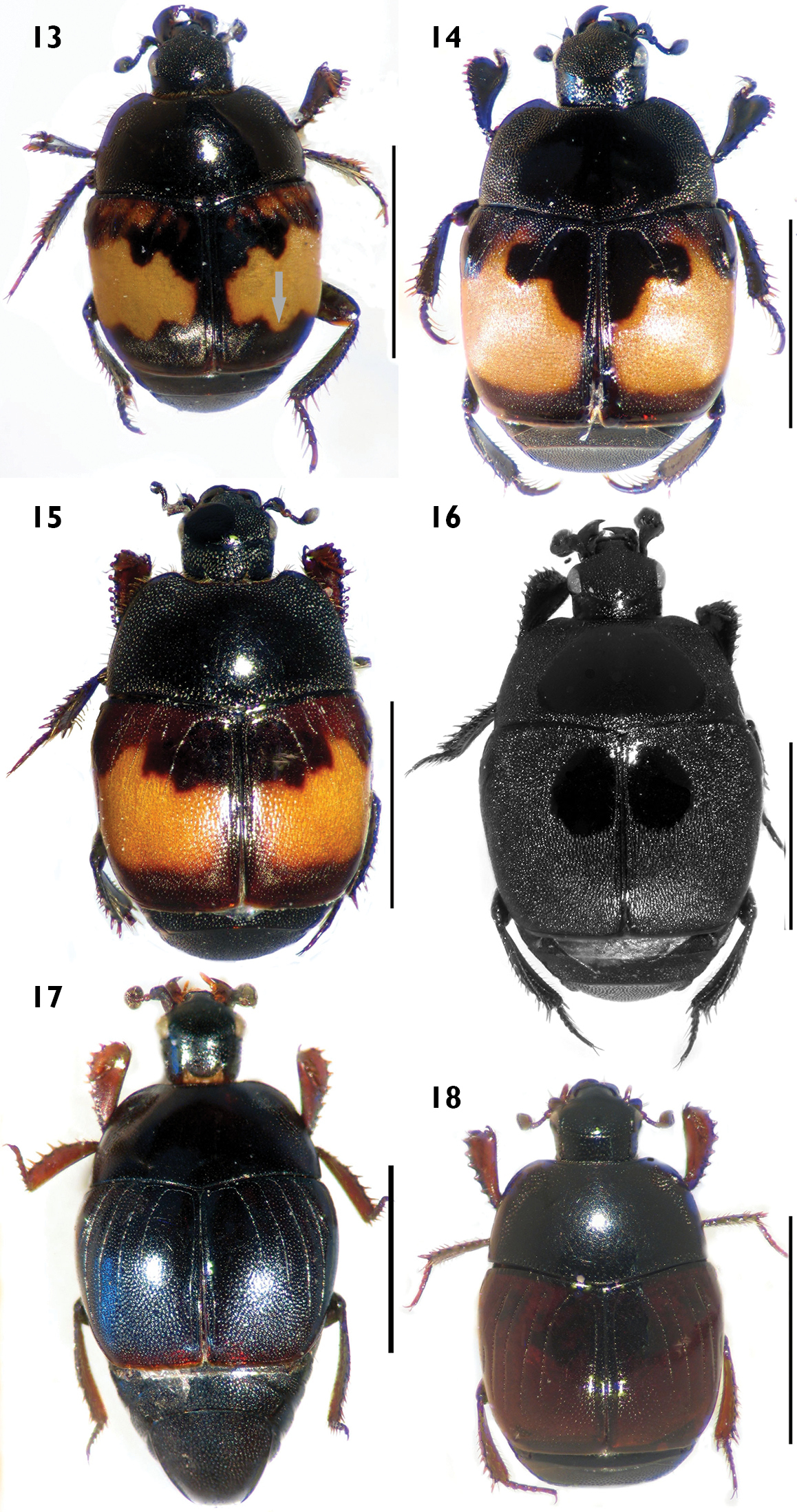
Figures 3, 5Small size (length: 2.1–2.3 mm, width: 1.4–1.6 mm). Body oval, elongated, parallel, black, shiny, with reddish legs. Pronotum with finer and sparse punctation, longer on lateral area, with a large puncture on medial part close to posterior margin. Pronotal hypomeron glabrous in dorsal view. Elytron with finer and sparse punctation in intervals; dorsal elytral striae 1–5 complete, well demarcated with punctures, sutural stria present, reduced on basal part. Pygidium without grooves. Protibiae with teeth expanded and 2 short, separated denticles and a long apical spur; proximal half of outer margin serrate, with small spurs.
Large size (length: 6.9 mm, width: 5.3 mm). Body black, shiny, depressed, elongated, parallel, head prognathous, not retractile, mandibles long, prominent, as long as head. Pronotum lacking punctures, with marginal stria well demarcated, in males ending in a fovea on anterior angles. Pronotal hypomeron glabrous in dorsal view. Elytron lacking spot and punctures, with only two dorsal striae, first stria reduced to anterior half, second complete, almost reaching apex. Propygidium larger than pygidium, pygidium without grooves. Protibiae with four teeth, the two distal ones longer.
Argentina, Brazil, French Guiana, Mexico and Central America (
Habitus in dorsal view. 5Carcinops (Carcinops) troglodytes 6 Hololepta (Leionota) reichii. 7 Phelister rufinotus 8 Euspilotus (s. str.) lacordairei 9 Euspilotus (s. str.) patagonicus 10 Xerosaprinus (Xerosaprinus) diptychus. Scale bars: 2 mm. Scale bars: 2 mm.
Small size (length: 1.5 mm, width: 1.3 mm). Body oval, black, shiny, with elytron reddish or black rufescent. Pronotum with finer and sparse punctation, larger on medial part close to posterior margin. Pronotal hypomeron glabrous in dorsal view. Elytron with finer and sparse punctation in intervals; dorsal elytral striae 1–4 complete, fifth present on distal half and with a large basal puncture; sutural stria present on distal half. Pygidium with finer and dense punctation and without grooves. Protibiae with outer margin not expanded and with 7 separated denticles.
Brazil (
http://species-id.net/wiki/Xerosaprinus_diptychus
Figures 10, 12Smallto medium size (length: 1.8–2.9 mm, width: 1.7–2.4 mm). Body oval, black to dark brown, shiny. Pronotum with coarse and dense punctation on anterior, lateral and basal areas, disc small, with finer and sparse punctation. Pronotal hypomeron setose in dorsal view. Elytron with coarse and very dense punctation seemingly rugose on distal half and on proximal half in intervals 1–3, with a smooth, shining area between the fourth dorsal stria, the sutural stria and the rounded arch; elytral dorsal striae 1–4 complete on anterior half, sometimes the first and third vestigial, fourth and sutural striae connected by a rounded arch. Pygidium without grooves. Protibiae with teeth moderately expanded and 7–8 denticles (Fig. 12).
http://species-id.net/wiki/Euspilotus_lacordairei
Figure 8Medium to large size (length: 3.4–4.5 mm, width: 3.3–3.8 mm). Body black reddish. Pronotum with coarse and dense punctation on anterior, lateral and basal areas, disc small, with finer and sparse punctation. Pronotal hypomeron setose in dorsal view. Elytron with coarse and dense punctation on distal half, projecting anterad in intervals 1-3, shortest in interval 4; with five dorsal striae well demarcated, 1–4 complete on anterior half, fifth reduced between the fourth dorsal and sutural striae, fourth and sutural striae connected by a rounded arch. Pygidium without grooves. Protibiae with expanded outer margin and 10–11 short, reddish denticles.
Distribution: Argentina, Bolivia and Chile (
http://species-id.net/wiki/Euspilotus_patagonicus
Figures 9, 11Large size (length: 4.4–5.8 mm, width: 3.9–4.7 mm). Body black. Pronotum with large, shiny disc, with finer and sparse punctation, lateral and basal areas with coarse and dense punctation, with a punctate depressed area on anterior angles, without punctures behind anterior margin. Pronotal hypomeron setose in dorsal view. Elytronwith coarse and dense punctation on distal half, projecting towards anterior half in intervals 1-4, not reaching inner subhumeral stria, the basal area of fourth and sutural striae, with a shining area with finer and sparse punctation between the fourth dorsal and sutural striae, narrowed apically; elytral dorsal striae 1–4 complete on anterior half, sutural stria sometimes absent in(on) basal part. Pygidium without grooves. Protibiae with teeth much expanded and 5–6 short denticles wider on base (Fig. 11).
Argentina, Bolivia and Chile (
Medium size (length: 2.3–3.8 mm, width: 2.1–3.4 mm). Body black, elytron with yellow or white spot. Pronotum with finer and sparse punctation, with a longitudinal lateral area on each side with coarse and dense punctation reaching the marginal stria, with two rows of large punctures on base. Pronotal hypomeron setose in dorsal view. Elytron with punctation coarse and dense on posterior half, finer and sparser on anterior half between intervals 2, 3 and 4; elytral dorsal striae 1–4 complete on anterior third, third stria sometimes reduced in basal area, fourth and sutural striae connected by a rounded arch; elytral spot with a digitiform projection towards apex, with two digitiform projections anterad, the outer one between the first and third dorsal elytral striae, the inner one between the fourth dorsal and sutural striae, sometimes between anterior margin, first and fourth dorsal striae and humerus with small yellow spots, making the anterior margin of the large elytral spot fuzzy. Pygidium: female with subapical groove V-shaped, male without grooves. Protibiae with outer margin expanded and 11–12 short, reddish denticles.
Argentina, Chile and Paraguay (
Habitus in dorsal view. 13 Euspilotus (s. str.) richteri 14 Euspilotus (s. str.) lepidus 15 Euspilotus (s. str.) ornatus 16 Euspilotus (Hesperosaprinus) caesopygus 17 Euspilotus (Hesperosaprinus) strobeli 18 Euspilotus (Hesperosaprinus) pavidus. Scale bars: 2 mm.
http://species-id.net/wiki/Euspilotus_lepidus
Figure 14Medium size (length: 2.3–3.3 mm, width: 1.86–2.3 mm). Body black, elytron with yellow or white spot. Pronotum with finer and sparse punctation, with a shining area on disc, with a longitudinal lateral area on each side with coarse and dense punctation, with two rows of large punctures on base. Pronotal hypomeron setose in dorsal view. Elytron with punctation coarse and dense on posterior half, finer and sparser on anterior half defining a shining area between intervals 2, 3 and 4; elytral dorsal striae 1, 2 and 4 complete on anterior half, third stria reduced to a short row of punctures on basal area, fourth and sutural striae connected by a rounded arch; elytral spot with distal margin straight and two digitiform projections anterad, the outer one between the first and second (or third) dorsal striae, the inner one towards the fourth dorsal elytral stria. Pygidium without grooves. Protibiae with outer margin expanded and 10–13 denticles.
Argentina, Bolivia, Chile, and Peru (
http://species-id.net/wiki/Euspilotus_ornatus
Figure 15Medium size (length: 2.5–3.5 mm, width: 2.3–3.2 mm). Body black, elytron with yellow or orange spot. Pronotum: disc with finer and sparse punctation, lateral areas and base with coarse and dense punctation. Pronotal hypomeron setose in dorsal view. Elytron with punctation coarse and dense on posterior half, finer and sparser on anterior half defining a shining area between intervals 3 and 4; elytral dorsal striae 1–2 and 4 complete on anterior half, third interrupted, fourth and sutural striae connected by a rounded arch; elytral spot occupying the distal half of elytron with distal margin straight and three digitiform projections anterad, the outer one between the first and second dorsal striae, the medial one between the third and fourth dorsal elytral striae, and the inner one close to the sutural elytral stria. Pygidium without grooves. Protibiae with outer margin expanded and 8–10 short, reddish denticles.
Distribution. Argentina and Chile (
http://species-id.net/wiki/Euspilotus_caesopygus
Figure 16Medium to large size (length: 3.2–4.3 mm, width: 2.7–3.7 mm). Body black. Pronotum with coarse and dense punctation, disc small, with finer and sparse punctation. Pronotal hypomeron glabrous in dorsal view. Elytron with coarse and very dense punctation seemingly rugose, dorsal elytral striae 1–4 absent or vestigial, sutural stria present, lacking rounded arch, with a shining area on anterior half between the fourth dorsal and sutural striae which presents a finer and sparse punctation visible only at 60× magnification. Pygidium: female with subapical groove, male without grooves. Protibiae with outer margin expanded and 10 short, reddish denticles.
Argentina and Bolivia (
http://species-id.net/wiki/Euspilotus_strobeli
Figure 17Medium to large size (length: 3.5–4.0 mm, width: 2.9–3.9 mm). Body black to metallic blue. Pronotum with a large, shiny disc with finer and sparse punctation, with coarse and dense punctation on lateral area and in a single depression on each side close to anterior angles. Pronotal hypomeron glabrous in dorsal view. Elytron with finer and sparse punctation in the intervals on proximal half; distal half with coarse and dense ocellate punctation, a small puncture within a large puncture, with a smooth, shining area between the fourth dorsal and sutural striae; elytral striae 1-2 almost complete, 3-4 reduced but surpassing the middle of elytron on posterior half, fourth dorsal and sutural striae connected by a rounded arch, lacking inner subhumeral stria. Pygidium with ocellate punctation and with a complete subapical groove in the middle with internal ramifications. Protibiae with outer margin expanded and 7–8 short, reddish denticles.
Argentina and South Brazil (
http://species-id.net/wiki/Euspilotus_pavidus
Figure 18Medium size (length: 2.4–3.8 mm, width: 2.1–3.2 mm). Body black with elytron dark reddish. Pronotum with large, shiny, and smooth disc with finer and sparse punctation; anterior, lateral and basal areas with coarse and dense punctation, with two longitudinal, lateral, depressed punctate areas. Pronotal hypomeron glabrous in dorsal view. Elytron with coarse and dense punctuation on distal third from interval 2 to sutural stria, on proximal half with finer and sparse punctation in intervals 1-4; elytral striae 1-2 almost complete, second longer than first, 3-4 surpassing the middle of elytron on posterior half, with inner subhumeral stria well demarcated, sometimes reduced. Pygidium with punctures, without grooves. Protibiae with outer margin expanded and 7–8 short, reddish denticles.
Argentina, Bolivia, Brazil, French Guiana, Paraguay, Uruguay, Suriname, and Central America (
http://species-id.net/wiki/Euspilotus_modestus
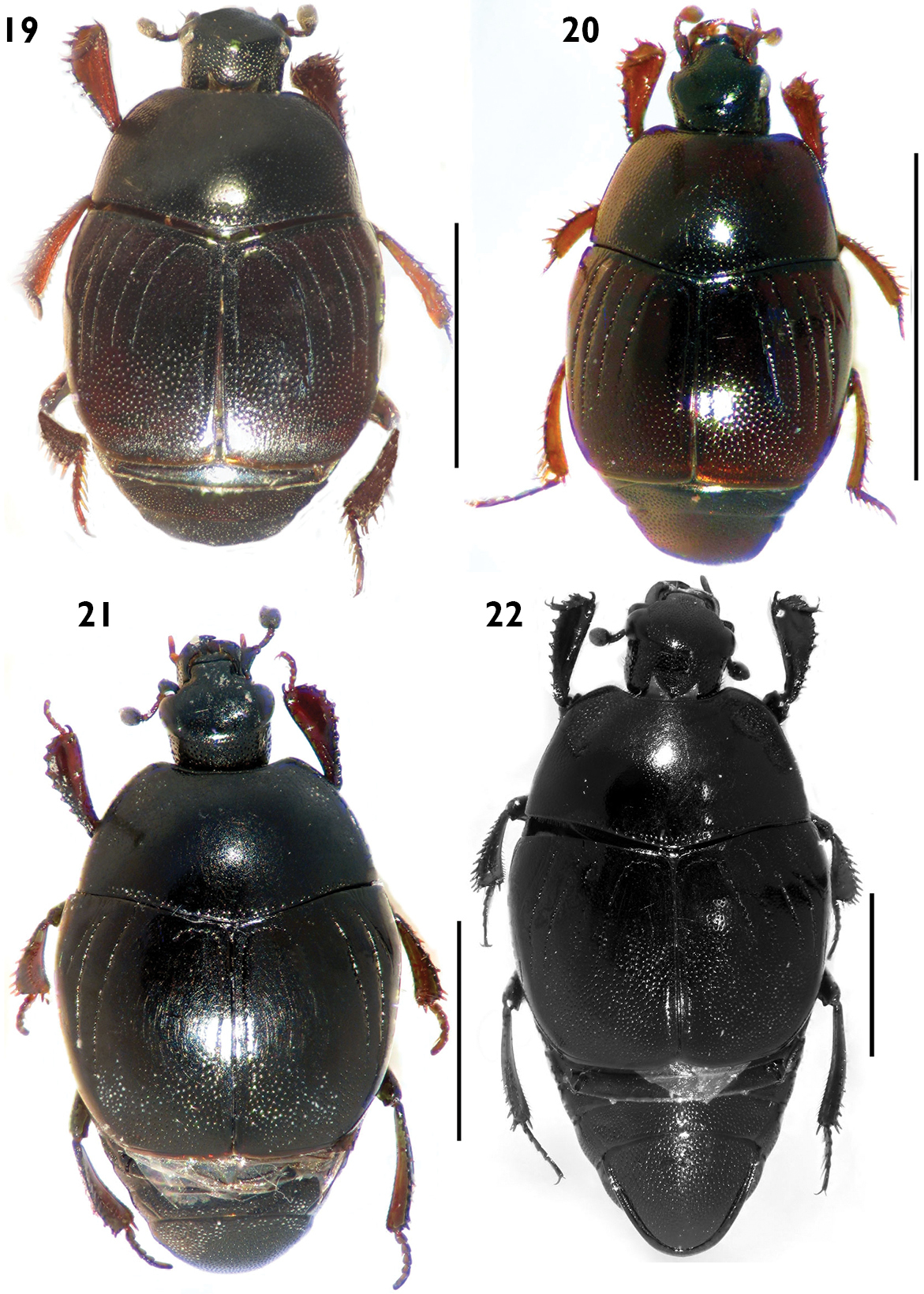
Figures 4, 19Medium to large size (length: 2.5–4.0 mm, width: 2.4–2.7 mm). Body black to reddish. Pronotum with fine and sparse punctation on disc, larger and deeper on lateral area. Pronotal hypomeron glabrous in dorsal view. Elytron with finer and sparse punctation in the intervals on proximal half; distal half with coarse and dense punctation, apically the punctures form elongate wrinkles; elytral striae 1-4 well demarcated, 1-2 surpassing the middle of elytron on posterior half, 3-4 reduced to anterior half; with inner subhumeral stria well demarcated. Pygidium with coarse and dense punctation, with two short transverse grooves or two longitudinal depressions. Protibiae with outer margin expanded and 8–9 short, reddish denticles, the basal fourth very small.
Argentina, Brazil, French Guiana, Paraguay, Uruguay and Venezuela (
Habitus in dorsal view. 19 Euspilotus (Hesperosaprinus) modestus 20 Euspilotus (Hesperosaprinus) parenthesis 21 Euspilotus (Hesperosaprinus) connectens 22 Euspilotus (Hesperosaprinus) azureus. Scale bars: 2 mm.
http://species-id.net/wiki/Euspilotus_parenthesis
Figure 20Smallto medium size (length: 1.7–2.2 mm, width: 1.3–1.8 mm). Body black reddish. Pronotum with fine and sparse punctation over the whole surface area, larger on lateral area. Pronotal hypomeron glabrous in dorsal view. Elytron with coarse and dense punctation on distal half, with finer and sparse punctation in the intervals on proximal half; elytral striae 1-2 almost complete, 3-4 reduced but surpassing the middle of elytron on posterior half; lacking inner subhumeral stria. Pygidium with coarse and dense punctation with or without a short subapical groove, if present it is parenthesis-shaped and concave anterad, not reaching lateral margin of pygidium. Protibiae with outer margin expanded and 7–8 short, reddish denticles.
Distribution. Brazil (
http://species-id.net/wiki/Euspilotus_connectens
Figure 21Medium to large size (length: 2.6–3.8 mm, width: 2.2–3.2 mm). Body black. Pronotum with a large, shiny disc with finer and sparse punctation, with coarse and dense punctation on lateral and basal areas and in a single rounded, shallow depression on each side close to anterior angles; with marginal stria away from lateral margin. Pronotal hypomeron glabrous in dorsal view. Elytron with proximal 2/3 lacking punctures, distal third with coarse and dense punctation between the second elytral dorsal and sutural striae; elytral dorsal striae 1–2 almost complete, second larger, third absent or reduced to a short row of punctures on basal area, fourth absent or reduced to a short row of punctures on basal area connected by a rounded arch with sutural stria. Pygidium with punctures and with a transverse subapical groove not reaching lateral margins. Protibiae with outer margin expanded and 7–8 short, reddish denticles.
Argentina, Brazil and Uruguay (
http://species-id.net/wiki/Euspilotus_azureus
Figure 22Medium to large size (length: 2.9–5.5 mm, width: 2.5–4.7 mm). Body black or metallic blue. Pronotum with a large, shiny disc with finer and dense punctation visible only at 60× magnification, larger on lateral areas and in a single depression on each side close to anterior angles; with marginal stria very close to lateral margin. Pronotal hypomeron glabrous in dorsal view. Elytron with finer and sparse punctation in the intervals on proximal half; distal half with coarse and dense punctation between the second interval and sutural stria; elytral dorsal striae 1–2 almost complete, third absent or reduced to a short row of punctures on basal area, fourth complete on anterior half, fourth and sutural striae connected by a rounded arch. Pygidium with punctures and with a transverse subapical groove reaching lateral margins. Protibiae with outer margin expanded and 7–13 short, reddish denticles, the most basal ones very small.
Argentina, Brazil and Venezuela (
Geographical distribution of sixteen species of Histeridae in Argentina. Provinces: 1 Jujuy: Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (s. str.) lacordairei, Euspilotus (s. str.) lepidus 2 Salta: Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (Hesperosaprinus) strobeli 3 Chaco: Euspilotus (s. str.) lacordairei 4 Catamarca: Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (Hesperosaprinus) pavidus, Euspilotus (s. str.) lacordairei, Euspilotus (s. str.) richteri 5 La Rioja: Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (s. str.) lacordairei, Euspilotus (s. str.) lepidus, Euspilotus (s. str.) richteri 6 San Juan: Euspilotus (Hesperosaprinus) modestus, Euspilotus (Hesperosaprinus) parenthesis, Euspilotus (Hesperosaprinus) pavidus, Euspilotus (s. str.) lacordairei, Euspilotus. (s. str.) ornatus, Xerosaprinus (Xerosaprinus) diptychus 7 Córdoba: Euspilotus (Hesperosaprinus) pavidus 8 Entre Ríos: Euspilotus (Hesperosaprinus) pavidus 9 San Luis: Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (Hesperosaprinus) pavidus, Euspilotus (s. str.) lacordairei, Euspilotus (s. str.) ornatus 10 Mendoza: Carcinops (s. str.) troglodytes, Euspilotus (Hesperosaprinus) azureus, Euspilotus (Hesperosaprinus) caesopygus, Euspilotus (Hesperosaprinus) connectens, Euspilotus (Hesperosaprinus) modestus, Euspilotus (Hesperosaprinus) parenthesis, Euspilotus (Hesperosaprinus) pavidus, Euspilotus (Hesperosaprinus) strobeli, Euspilotus (s. str.) lacordairei, Euspilotus(s. str.) lepidus, Euspilotus (s. str.) ornatus, Euspilotus (s. str.) patagonicus, Euspilotus (s. str.) richteri, Hololepta (Leionota) reichii, Phelister rufinotus, Xerosaprinus diptychus 11 Buenos Aires: Euspilotus (s. str.) patagonicus 12 Neuquén: Euspilotus (s. str.) patagonicus 13 Chubut: Carcinops (s. str.) troglodytes, Euspilotus (Hesperosaprinus) modestus, Euspilotus (s. str.) lacordairei, Euspilotus (s. str.) ornatus, Euspilotus (s. str.) patagonicus, Euspilotus(s. str.) richteri.
The 16 Histeridae species collected in this study on carcasses in Argentina are grouped into three of the 11 subfamilies: Saprininae (twelve species of Euspilotus Lewis and one species of Xerosaprinus Wenzel), Histerinae (one species of Hololepta Paykull and one species of Phelister Marseul) and Dendrophilinae (one species of Carcinops Marseul).
Species of Euspilotus, Xerosaprinus and Phelister have been recorded as attracted by carcasses (
Nine species of Histeridae constitute new records from the cadaveric fauna in Argentina: Euspilotus caesopygus, Euspilotus connectens, Euspilotus lepidus, Euspilotus richteri, Euspilotus strobelis, Euspilotus azureus, Hololepta reichii, Phelister rufinotus and Carcinops troglodytes. All of them were collected mostly on human and pig carcasses. The remaining seven species associated with carcasses listed in this key were recorded previously for the country in Buenos Aires (
Histeridae of forensic importance were already cited in the literature, for instance in Central Europe adults of Saprinus planiusculus Motschulsky and Saprinus semistriatus (Scriba) are predictable at a specific time period in the cadaver succession because they have a short period of residency in the carcasses depending on their specialized feeding habits, therefore they are good tools for estimating PMI indicators (
Operclipygus hospes (Lewis) was recorded from Brazil in buried bodies of rabbits in summer and autumn, and it was suggested that this species plays an important role in forensic entomology as a seasonal indicator (
Further research is necessary to establish the specific time period in the cadaver succession for which the species cited in the present article can be predictable and could be used to estimate PMI indicators based on succession patterns. In addition, immature stages can be useful in forensic entomology because they are reared within the body and collected in advanced stages of decomposition (Aballay pers. obs.) but the duration of larval development is variable and depends on the species (
Due to the limited information concerning development of larvae of Histeridae species (
List of Histeridae species collected on vertebrate carcasses and from baited traps in Argentina and their geographic distribution by provinces. * = baited traps.
| Species | N° | Substratum /carcasses | Province | Geographic Coordinates | Altitude (m) | Collector/ reference |
|---|---|---|---|---|---|---|
| Carcinops (s. str.) troglodytes | 4 | Pig | Mendoza | 32°53'49.3"S, 68°52'23.9"W | 839 |
|
| Carcinops (s. str.) troglodytes | 4 | Sheep | Chubut | 43°16'18.2"S, 65°26'23.3"W | 39 | Arriagada G. |
| Euspilotus (Hesperosaprinus) azureus | 192 | Pig | Mendoza | 32°53'58.4"S, 68°52'22.1"W | 841 |
|
| Euspilotus (Hesperosaprinus) caesopygus | 5 | Pig | Mendoza | 32°53'53.3"S, 68°52'26.2"W | 850 |
|
| Euspilotus (Hesperosaprinus) caesopygus | 2 | Human, | Mendoza | 32°32'07.5"S, 68°58'42.8"W | 1424 | Aballay F. (forensic cases) |
| Euspilotus (Hesperosaprinus) caesopygus | 3 | Pig | Jujuy | 24°09'54.1"S, 65°18'37.7"W | 1383 | Quiroga N. |
| Euspilotus (Hesperosaprinus) caesopygus | 1 | Pig | Salta | 24°54'40"S, 65°28'16"W | 1379 | Ayón R. |
| Euspilotus (Hesperosaprinus) caesopygus | 121 | Squid | La Rioja | 29°10'45.3"S, 67°37'33.9"W | 1806 | Arriagada G. |
| Euspilotus (Hesperosaprinus) caesopygus | 4 | Squid * | Catamarca | 27°36'35.4"S, 67°41'48.5"W | 1752 | Arriagada G. |
| Euspilotus (Hesperosaprinus) caesopygus | 1 | Dog | San Luis | 32°37'37.8"S, 66°54'35.5"W | 744 | Arriagada G. |
| Euspilotus (Hesperosaprinus) connectens | 12 | Pig | Mendoza | 32°53'57.6"S, 68°52'32.4"W | 847 |
|
| Euspilotus (Hesperosaprinus) modestus | 21 | Human | Mendoza | 32°49'18.4"S, 68°52'38.9"W | 788 | Aballay F. (forensic cases) |
| Euspilotus (Hesperosaprinus) modestus | 4 | Cow | San Juan | 31°59'51.1"S, 68°03'20.3"W | 541 | Arriagada G |
| Euspilotus (Hesperosaprinus) modestus | 2 | Pig | San Juan | 31°32'34.1"S, 68°34'38.2"W | 673 |
|
| Euspilotus (Hesperosaprinus) modestus | 91 | Pig | Mendoza | 32°53'53.3"S, 68°52'26.2"W | 850 |
|
| Euspilotus (Hesperosaprinus) modestus | 91 | Sardine* | Chubut | 43°16'37.1"S, 65°29'49.8"W | 68 | Arriagada G. |
| Euspilotus (Hesperosaprinus) parenthesis | 2 | Pig | San Juan | 31°32'34.9"S, 68°34'35.9"W | 674 |
|
| Euspilotus (Hesperosaprinus) parenthesis | 30 | Pig | Mendoza | 32°53'49.3"S, 68°52'23.2"W | 850 |
|
| Euspilotus (Hesperosaprinus) pavidus | 5 | Human | Mendoza | 32°56'14.2"S, 68°36'32.9"W | 653 | Aballay F. (forensic cases) |
| Euspilotus (Hesperosaprinus) pavidus | 63 | Pig | San Juan | 31°32'32.1"S, 68°34'44.8"W | 675 |
|
| Euspilotus (Hesperosaprinus) pavidus | 163 | Pig | Mendoza | 32°53'58.4"S, 68°52'22.1"W | 841 |
|
| Euspilotus (Hesperosaprinus) pavidus | 70 | Donkey | Catamarca | 26°59'22.1"S, 66°08'42.1"W | 2121 | Arriagada G. |
| Euspilotus (Hesperosaprinus) pavidus | 15 | Horse | San Luis | 32°38'43.4"S, 66°53'52.7"W | 717 | Arriagada G. |
| Euspilotus (Hesperosaprinus) pavidus | 45 | Chicken * | Córdoba | 30°44'39.8"S, 64°48'35.5"W | 480 | Arriagada G. |
| Euspilotus (Hesperosaprinus) pavidus | 100 | Cow | Entre Rios | 32°08'39.1"S, 58°13'04.3"W | 31 | Arriagada G. |
| Euspilotus (Hesperosaprinus) strobeli | 1 | Pig | Salta | 24°54'40"S, 65°28'16"W | 1379 | Ayón R. |
| Euspilotus (Hesperosaprinus) strobeli | 1 | Cow | Mendoza | 34°03'18.1"S, 67°49'13.8"W | 537 | Flores G. |
| Euspilotus (Hesperosaprinus) strobeli | 1 | Chicken * | Mendoza | 34°03'25.1"S, 67°49'11.8"W | 534 | Arriagada G |
| Euspilotus (s. str.) lacordairei | 25 | Pig | San Juan | 31°32'34.1"S, 68°34'38.2"W, | 673 |
|
| Euspilotus (s. str.) lacordairei | 2 | Pig | San Juan | 30°07'01.1"S, 68°39'43.9"W | 1144 | Aballay F. |
| Euspilotus (s. str.) lacordairei | 867 | Pig | Mendoza | 32°53'57.6"S, 68°52'32.2"W | 850 |
|
| Euspilotus (s. str.) lacordairei | 2 | Horse | San Luis | 32°38'34.4"S, 66°53'35.7"W | 720 | Arriagada G. |
| Euspilotus (s. str.) lacordairei | 2 | Cow | San Luis | 32°22'08.2"S, 67°09'37.3"W | 556 | Aballay F. |
| Euspilotus (s. str.) lacordairei | 4080 | Sardine*, Squid* | Chubut | 43°16'37.1"S, 65°29'49.8"W | 68 | Arriagada G. |
| Euspilotus (s. str.) lacordairei | 20 | Rat | Chubut | 42°24'11.1"S, 63°57'25.4"W | 6 | Cheli G |
| Euspilotus (s. str.) lacordairei | 2 | Lesser rhea | Chubut | 42°20'21.8"S, 64°49'11.2"W | 50 | Flores G;. |
| Euspilotus (s. str.) lacordairei | 2 | Sheep | Chubut | 42°20'28.8"S, 64°49'09.2"W | 48 | Flores G. |
| Euspilotus (s. str.) lacordairei | 5 | Vicuña | Jujuy | 22°44'52.4"S, 65°53'12.9"W | 3667 | Arriagada G. |
| Euspilotus (s. str.) lacordairei | 166 | Squid * | La Rioja | 29°10'43.5"S, 67°31'49.9"W | 1196 | Arriagada G. |
| Euspilotus (s. str.) lacordairei | 2 | Donkey | Catamarca | 26°59'22.1"S, 66°08'42.1"W | 2121 | Arriagada G. |
| Euspilotus (s. str.) lacordairei | 1 | Snake | Chaco | 26°30'16.3"S, 61°11'15.2"W | 124 | Arriagada G. |
| Euspilotus (s. str.) lepidus | 7 | Pig | Jujuy | 24°09'54.1"S, 65°18'37.7"W | 1383 | Quiroga N. |
| Euspilotus (s. str.) lepidus | 55 | Pig | Mendoza | 32°53'49.3"S, 68°52'23.9"W | 839 |
|
| Euspilotus (s. str.) lepidus | 10 | Squid * | La Rioja | 28°34'17.9"S, 66°47'07.4"W | 812 | Arriagada G. |
| Euspilotus (s. str.) ornatus | 2 | Pig | San Juan | 31°32'34.9"S, 68°34'35.9"W | 674 |
|
| Euspilotus (s. str.) ornatus | 48 | Pig | Mendoza | 32°53'58.4"S, 68°52'22.1"W | 841 |
|
| Euspilotus (s. str.) ornatus | 30 | Rat | Chubut | 45°49'04.7"S, 67°55'59.6"W | 680 | Cheli G. |
| Euspilotus (s. str.) ornatus | 30 | Sardine* | Chubut | 43°16'30.1"S, 65°29'26.8"W | 66 | Arriagada G. |
| Euspilotus (s. str.) ornatus | 3 | Geoffroy´s cat | San Luis | 33°08'07.5"S, 66°30'27.9"W | 551 | Arriagada G. |
| Euspilotus (s. str.) patagonicus | 4 | Rat | Chubut | 42°47'07.5"S, 65°00'43.8"W | 9 | Cheli G. |
| Euspilotus (s. str.) patagonicus | 4 | Guanaco | Mendoza | 36°03'27.5"S, 68°47'11.1"W | 1684 | Flores G., Ruiz Manzanos E. |
| Euspilotus (s. str.) patagonicus | 1 | Pig | Buenos Aires | 34°47'13.2"S, 58°26'33.1"W | 17 |
|
| Euspilotus (s. str.) patagonicus | 1 | Human | Neuquén | 38°53'54.8"S, 69°56'54.2"W | 962 |
|
| Euspilotus (s. str.) richteri | 178 | Pig | Catamarca | 26°01'38.2"S, 67°20'31.6"W | 3595 |
|
| Euspilotus (s. str.) richteri | 95 | Llama | Catamarca | 26°01'33.4"S, 67°20'42.5"W, | 3585 |
|
| Euspilotus (s. str.) richteri | 85 | Squid * | Catamarca | 27°36'30.1"S, 67°41'04.7"W | 1750 | Arriagada G. |
| Euspilotus (s. str.) richteri | 8 | Pig | Mendoza | 32°53'57.6"S, 68°52'32.4"W | 847 |
|
| Euspilotus (s. str.) richteri | 1 | Rat | Chubut | 42°16'10.4"S, 63°45'32.2"W | 40 | Cheli G. |
| Euspilotus (s. str.) richteri | 76 | Squid * | La Rioja | 28°34'17.9"S, 66°47'07.4"W | 812 | Arriagada G. |
| Hololepta (Leionota) reichii | 1 | Human | Mendoza | 32°56'14.2"S, 68°36'32.9"W | 653 | Aballay F.(forensic cases) |
| Phelister rufinotus | 11 | Pig | Mendoza | 32°53'53.3"S, 68°52'26.2"W | 850 |
|
| Xerosaprinus diptychus | 72 | Pig | San Juan | 31°32'32.1"S, 68°34'44.8"W | 675 |
|
| Xerosaprinus diptychus | 2 | Horse | San Juan | 30°13'52.3"S, 67°42'33.8"W | 1261 | Aballay F. |
| Xerosaprinus diptychus | 2 | Fox | San Juan | 30°19'01.3"S, 68°41'42.3"W | 673 | Aballay F. |
| Xerosaprinus diptychus | 114 | Pig | Mendoza | 32°53'49.3"S, 68°52'23.9"W | 839 |
|
We acknowledge Hugo San Martino and Eduardo Berté (Medical Forensic Committee of Mendoza) for the collecting permission on human carcasses; Rosana Ayón, Nancy Quiroga and Germán H. Cheli for his collecting efforts in Salta, Jujuy and Chubut provinces; Tomás Lackner for authorizing the utilization of Figs 1 and 2; Nelly Horak for correction of the English language; Mariana Chani Posse for suggestions improving the manuscript; Ana María Scollo for mounting specimens; Germán San Blas and Federico Agrain for help in taking photographs; Rodolfo Carrara for GIS assistance; Michael S. Catherino (Editor Histeridae) and two anonymous reviewers for suggestions for improving this paper. Financial support was provided by grants of the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), Argentina (“Estudio de aspectos biológicos de la entomofauna cadavérica aplicados a la entomología forense”, PICT 26000), Universidad Nacional de Quilmes to FHA and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).