Citation: Dewulf A, De Meulemeester T, Dehon M, Engel MS, Michez D (2014) A new interpretation of the bee fossil Melitta willardi Cockerell (Hymenoptera, Melittidae) based on geometric morphometrics of the wing. ZooKeys 389: 35–48. doi: 10.3897/zookeys.389.7076
Although bees are one of the major lineages of pollinators and are today quite diverse, few well-preserved fossils are available from which to establish the tempo of their diversification/extinction since the Early Cretaceous. Here we present a reassessment of the taxonomic affinities of Melitta willardi
Bees, compression, Oligocene, wing shape, geometric morphometrics, Tertiary
Bees (Hymenoptera, Apoidea, Anthophila) are a monophyletic group of largely pollenivorous species derived from among the predatory apoid wasps (
The highly fossiliferous shales of Florissant, Colorado have revealed 34 species and 19 genera belonging to several extant bee families: Apidae, Halictidae, Melittidae, Megachilidae, and Andrenidae (
Photograph of holotype female of Melitta willardi Cockerell as preserved (UCM 18737). Specimen is preserved facing toward the viewer, with head missing (note the large opening representing the anterior thoracic fossa).
Given that Melitta willardi possesses three submarginal cells we sampled specimens from different extant subfamilies with the same arrangement of cells. All available subfamilies were included, with a maximum of 20 specimens per subfamily, and with a maximum of five specimens per species. We additionally sampled all species of Melitta available with a maximum of five specimens per species. The dataset also included eighteen extinct species [Apidae, Apinae: Anthophorula persephone Engel, 2012; Bombus randeckensis Wappler & Engel, 2012; Electrapis meliponoïdes (Buttel-Reepen, 1906); Electrapis krishnorum Engel, 2001; Electrobombus samlandensis Engel, 2001; Eufriesea melissiflora (Poinar, 1998); Melikertes stilbonotus (Engel, 2001); Melissites trigona Engel, 2001; Paleohabropoda oudardi Michez & Rasmont, 2009; Protobombus basilaris Engel, 2001; Probombus hirsutus Piton, 1940; Succinapis goeleti Engel, 2001; and Thaumastobombus andreniformis Engel, 2001; Halictidae, Halictinae: Cyrtapis anomala Cockerell, 1908; Electrolictus antiquus Engel, 2001; Halictus petrefactus Engel & Peñalver, 2006; Ocymoromelitta sorella Engel, 2002 and Ocymoromelitta florissantella (Cockerell, 1906)]. Since the holotype of Melitta willardi is a female, all individuals used in the morphometric analysis are females to avoid potential bias due to any sexual dimorphism. The assembled dataset comprised 360 specimens representing six families of Anthophila, 15 subfamilies, and 109 species (Table 1).
Dataset for the geometric morphometric analysis including 360 specimens from 109 species and 15 subfamilies. N = number of specimens.
| FAMILY | SUB-FAMILY | SPECIES | N |
|---|---|---|---|
| Andrenidae | Andreninae | Andrena bicolor Fabricius, 1775 | 5 |
| Andrena boyerella Dours, 1872 | 5 | ||
| Andrena flavipes Panzer, 1799 | 5 | ||
| Andrena fulva (Müller, 1766) | 5 | ||
| Oxaeinae | Oxaea flavescens Klug, 1807 | 1 | |
| Oxaea fuscescens Sichel, 1865 | 1 | ||
| Oxaea sp. | 1 | ||
| Protoxaea gloriosa (Fox, 1893) | 2 | ||
| Panurginae | Borgatomelissa brevipennis (Walker, 1871) | 1 | |
| Melitturga clavicornis (Latreille, 1808) | 1 | ||
| Melitturga taurica Friese, 1922 | 5 | ||
| Anthrenoïdes sp. | 2 | ||
| Parapsaenythia puncticutis (Vachal, 1909) | 2 | ||
| Apidae | Apinae | Apis florea Fabricius, 1787 | 5 |
| Bombus mendax Gerstäcker, 1869 | 5 | ||
| Melissodes confusa Cresson, 1878 | 5 | ||
| Anthophora plumipes (Pallas, 1772) | 5 | ||
| Paleohabropoda oudardi Michez & Rasmont, 2009 † | 1 | ||
| Anthophorula persephone Engel, 2012 † | 1 | ||
| Bombus randeckensis Wappler & Engel, 2012 † | 1 | ||
| Electrapis krishnorum Engel, 2001 † | 1 | ||
| Electrapis meliponoides (Buttel-Reepen, 1906) † | 1 | ||
| Electrobombus samlandensis Engel, 2001 † | 1 | ||
| Eufriesea melissiflora (Poinar, 1998) † | 1 | ||
| Melikertes stilbonotus (Engel, 2001) † | 1 | ||
| Melissites trigona Engel, 2001 † | 1 | ||
| Protobombus basilaris Engel, 2001 † | 1 | ||
| Probombus hirsutus Piton, 1940 † | 1 | ||
| Succinapis goeleti Engel, 2001 † | 1 | ||
| Thaumastobombus andreniformis Engel, 2001 † | 2 | ||
| Nomadinae | Epeolus cruciger (Panzer, 1799) | 5 | |
| Nomada fabriciana (Linnaeus, 1767) | 5 | ||
| Nomada flava Panzer, 1798 | 5 | ||
| Nomada goodeniana (Kirby, 1802) | 5 | ||
| Xylocopinae | Ceratina chloris (Illiger, 1806) | 5 | |
| Ceratina dallatorreana Friese, 1896 | 5 | ||
| Xylocopa olivieri (Lepeletier de Saint Fargeau, 1841) | 5 | ||
| Xylocopa violacea (Linnaeus, 1758) | 5 | ||
| Colletidae | Colletinae | Colletes cunicularius (Linnaeus, 1761) | 5 |
| Colletes daviesanus Smith, 1846 | 5 | ||
| Colletes succinctus (Linnaeus, 1758) | 5 | ||
| Leioproctus sp. | 5 | ||
| Diphaglossinae | Cadeguala occidentalis (Haliday, 1836) | 1 | |
| Caupolicana gayi Spinola, 1851 | 5 | ||
| Caupolicana yarrowi (Cresson, 1875) | 3 | ||
| Crawfordapis luctuosa (Smith, 1861) | 2 | ||
| Diphaglossa gayi Spinola, 1851 | 3 | ||
| Mydrosoma bohartorum Michener, 1986 | 1 | ||
| Ptiloglossa guinnae Roberts, 1971 | 1 | ||
| Ptiloglossa pretiosa (Friese, 1898) | 4 | ||
| Halictidae | Halictinae | Augochlorella striata (Packer, 1990) | 5 |
| Halictus ligatus Say, 1837 | 5 | ||
| Ruizantheda nigrocaerulea (Spinola, 1871) | 5 | ||
| Thrinchostoma kandti Blüthgen, 1930 | 5 | ||
| Cyrtapis anomala Cockerell 1908 † | 1 | ||
| Electrolictus antiquus Engel 2001 † | 1 | ||
| Halictus petrefactus Engel & Peñalver 2006 † | 1 | ||
| Ocymoromelitta florissantella Cockerell 1906 † | 1 | ||
| Ocymoromelitta sorella Engel, 2002 † | 1 | ||
| Nomiinae | Dieunomia nevadensis (Cresson, 1874) | 1 | |
| Halictonomia decemmaculata (Friese, 1900) | 2 | ||
| Lipotriches australica (Smith, 1875) | 1 | ||
| Lipotriches modesta (Smith, 1862) | 5 | ||
| Nomia melanderi Cockerell, 1906 | 1 | ||
| Nomia diversipes Latreille, 1806 | 5 | ||
| Pseudapis diversipes (Latreille, 1806) | 5 | ||
| Nomioidinae | Ceylalictus variegatus (Olivier, 1789) | 5 | |
| Nomioides facilis (Rossi, 1853) | 5 | ||
| Nomioides minutissimus (Rossi, 1790) | 1 | ||
| Rophitinae | Systropha curvicornis (Scopoli, 1770) | 2 | |
| Systropha maroccana Warncke, 1977 | 3 | ||
| Systropha pici Pérez, 1895 | 2 | ||
| Systropha planidens Giraud, 1861 | 5 | ||
| Systropha sp. | 5 | ||
| Megachilidae | Fideliinae | Fidelia kobrowi Brauns, 1905 | 5 |
| Fidelia paradoxa Friese, 1899 | 5 | ||
| Fidelia villosa Brauns, 1902 | 1 | ||
| Fideliopsis major (Friese, 1911) | 2 | ||
| Melittidae | Meganomiinae | Meganomia andersoni (Meade-Waldo, 1916) | 2 |
| Meganomia binghami (Cockerell, 1909) | 5 | ||
| Melittinae | Rediviva intermixta (Cockerell, 1934) | 5 | |
| Rediviva longimanus Michener, 1981 | 3 | ||
| Melitta americana Smith, 1853 | 3 | ||
| Melitta arrogans Smith, 1879 | 5 | ||
| Melitta bicollaris Warncke, 1973 | 5 | ||
| Melitta californica Viereck, 1909 | 1 | ||
| Melitta cameroni (Cockerell, 1910) | 5 | ||
| Melitta dimidiata Morawitz, 1876 | 5 | ||
| Melitta eickworti Snelling & Stage, 1995 | 3 | ||
| Melitta ezoana Yasumatsu & Hirashima, 1956 | 5 | ||
| Melitta haemorrhoidalis (Fabricius, 1775) | 5 | ||
| Melitta hispanica Friese, 1900 | 5 | ||
| Melitta harrietae (Bingham, 1897) | 5 | ||
| Melitta japonica Yasumatsu & Hirashima, 1956 | 4 | ||
| Melitta magnifica Michez, 2012 | 3 | ||
| Melitta melittoides (Viereck, 1909) | 2 | ||
| Melitta melanura (Nylander, 1852) | 5 | ||
| Melitta murciana Warncke, 1973 | 5 | ||
| Melitta seitzi Alfken, 1927 | 1 | ||
| Melitta schultzei Friese, 1909 | 1 | ||
| Melitta sibirica (Morawitz, 1888) | 5 | ||
| Melitta aegyptiaca (Radoszkowski, 1891) | 5 | ||
| Melitta leporina (Panzer, 1799) | 5 | ||
| Melitta maura (Pérez, 1896) | 5 | ||
| Melitta nigricans Alfken, 1905 | 5 | ||
| Melitta schmiedeknechti Friese, 1898 | 5 | ||
| Melitta tricincta Kirby, 1802 | 5 | ||
| Melitta avontuurensis Michez & Kuhlmann, 2014 | 1 | ||
| Melitta richtersveldensis Michez & Kuhlmann, 2014 | 5 | ||
| Total = 360 | |||
Taxonomic affinities of the fossil were evaluated based on wing shape. Wing venation is used widely in insect taxonomy and can provide many informative features for phylogenetic analyses and for many Late Paleozoic taxa is sometimes the only form of available data (e.g.
The right forewings of 360 female specimens were initially photographed using an Olympus SZ010 binocular coupled with a Nikon D70 camera. Photographs were gathered in one TPS file using tps-UTIL 1.56 (
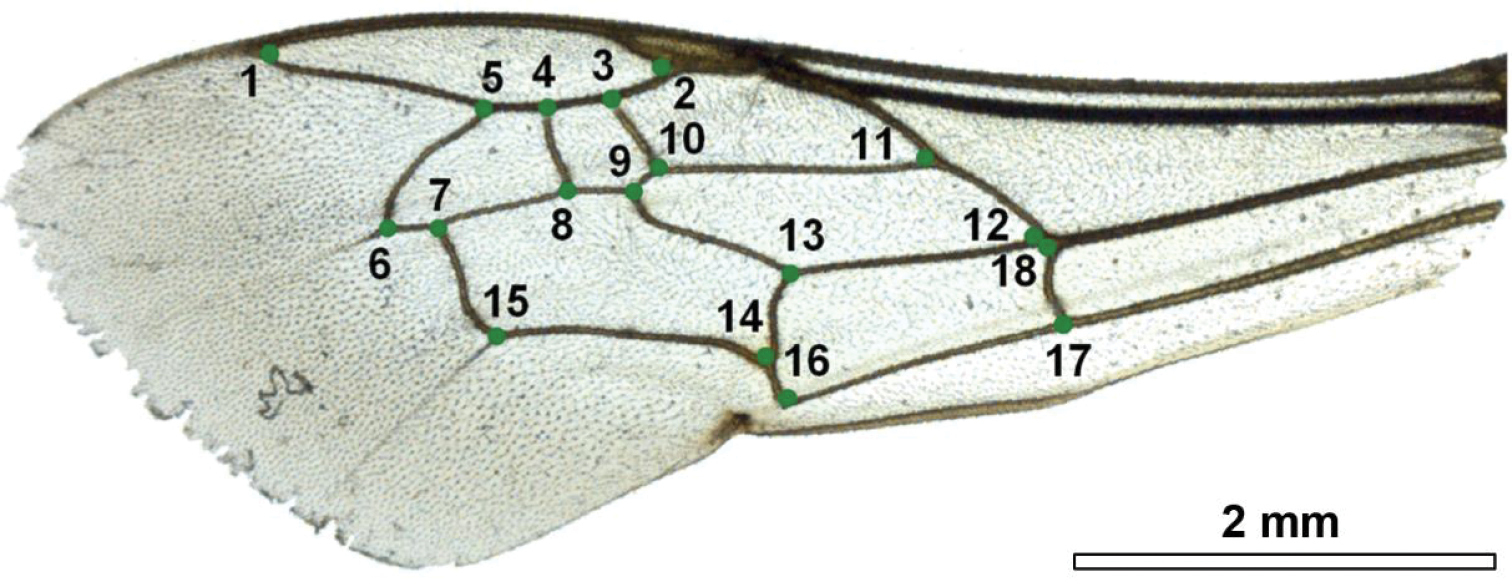
Right forewing of a female of Melitta leporina (Panzer) with the 18 landmarks indicated to describe the shape.
Prior to the assignment of the fossil, shape variation within the reference dataset and discrimination of the different taxa was assessed by Linear Discriminant Analyses (LDA) of the projected aligned configurations of landmarks, with subfamily levels as a priori grouping by using the software R version 3.0.2 (
Taxonomic affinities of the fossil were assessed based on their score in the predictive discriminant space of shapes. After superimposition of the 368 landmark configurations (i.e. corresponding to the reference dataset and the fossil), aligned coordinates of the 360 specimens from the reference dataset were used to calculate the LDA. We included a posteriori the eight aligned landmark configurations of Melitta willardi in the computed LDA space as “unknown” specimens and calculated their score. Assignments of the fossil configurations were estimated by calculating the Mahalanobis Distance (MD) between “unknowns” and group mean of each subfamily. We also calculated posterior probabilities of assignment to confirm the assignment to one taxon.
In order to assess the taxonomic affinities of Melitta willardi with the family Andrenidae, PCA was computed to visualize shape affinities between the fossil and andrenid subfamilies.
The regression coefficient between the Procrustes distances and the Euclidean distances is close to 1 (0.9999). This means that the linear tangent space closely approximates the shape space, thereby permitting us to be confident in the variation amplitude of our dataset.
In LDA space with subfamily a priori grouping, discrimination of the 15 groups are effective, with a cross-validated HR of 98.61% (e.g., 5 misclassified specimens), and 10 of the 15 subfamilies that account for a HR of 100% (Table 2). Other subfamilies have a HR between 90% and 99%. Due to sampling size within groups, the HR drastically drop down when a single specimen is misclassified. This is the case for the five groups with HR lower than 100%. Cross-validation assignment (Table 2) allows us to be confident in the group discrimination at subfamily level.
Cross-validation assignment in LDA space with subfamily a priori grouping (original groups are along the rows, predicted groups are along the columns). HR = Hit ratio.
| Andreninae | Apinae | Colletinae | Diphaglossinae | Fideliinae | Halictinae | Meganomiinae | Melittinae | Nomadinae | Nomiinae | Nomioidinae | Oxaeinae | Panurginae | Rophitinae | Xylocopinae | HR (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andreninae | 20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Apinae | - | 33 | - | 1 | - | - | - | - | - | - | - | - | - | - | - | 97 |
| Colletinae | - | - | 20 | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
| Diphaglossinae | - | 1 | - | 19 | - | - | - | - | - | - | - | - | - | - | - | 95 |
| Fideliinae | - | - | - | - | 13 | - | - | - | - | - | - | - | - | - | - | 100 |
| Halictinae | - | 1 | - | - | - | 24 | - | - | - | - | - | - | - | - | - | 96 |
| Meganomiinae | - | - | - | - | - | - | 7 | - | - | - | - | - | - | - | - | 100 |
| Melittinae | - | - | - | - | - | - | - | 117 | - | - | - | - | - | - | - | 100 |
| Nomadinae | - | - | - | - | - | - | - | - | 20 | - | - | - | - | - | - | 100 |
| Nomiinae | - | - | - | - | - | - | - | - | - | 20 | - | - | - | - | - | 100 |
| Nomioidinae | - | - | - | - | - | - | - | - | - | - | 11 | - | - | - | - | 100 |
| Oxaeinae | - | - | - | - | - | - | - | - | - | - | - | 5 | - | - | - | 100 |
| Panurginae | 1 | - | - | - | - | - | - | - | - | - | - | - | 10 | - | - | 91 |
| Rophitinae | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 16 | - | 94 |
| Xylocopinae | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 20 | 100 |
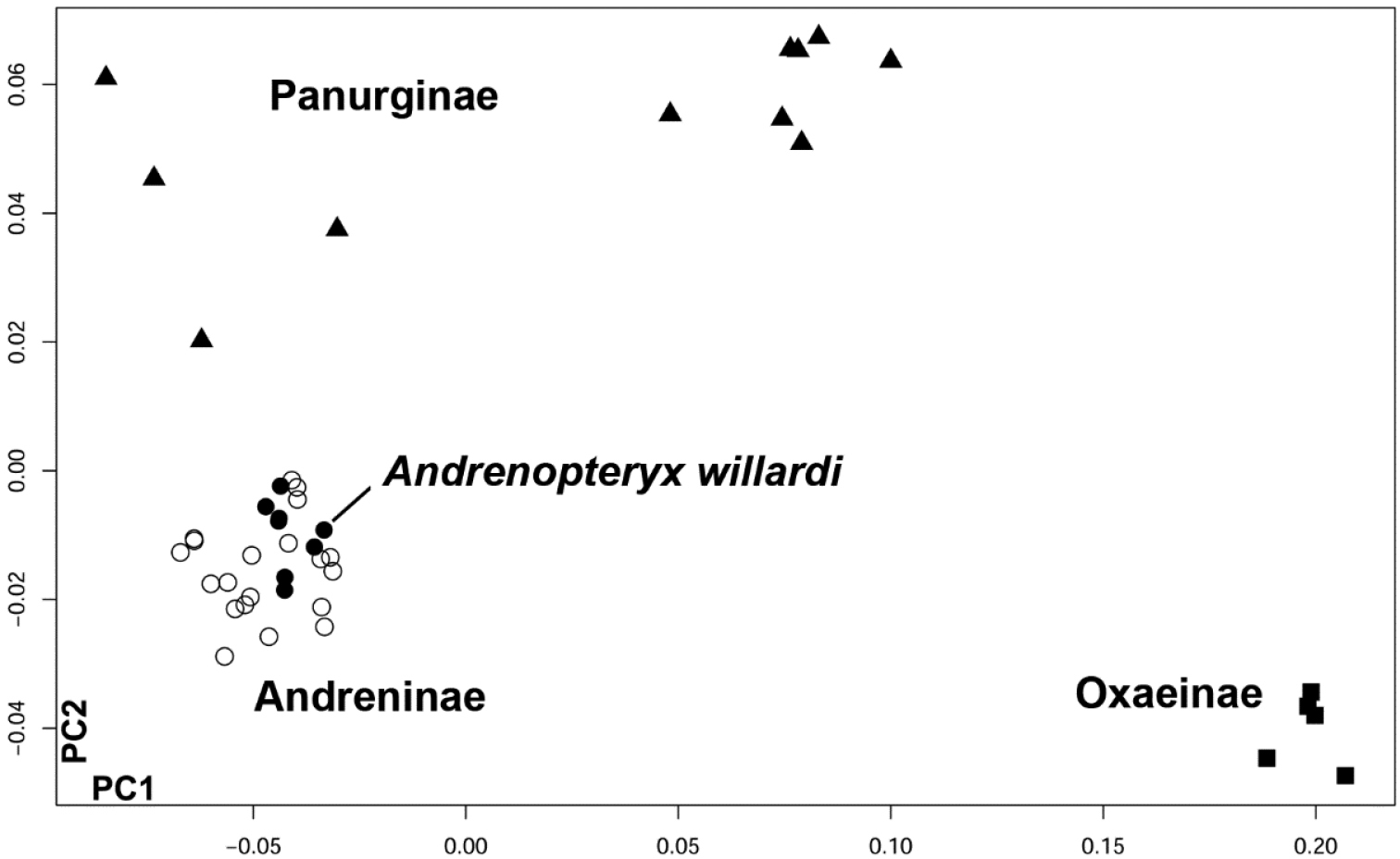
All of the 109 specimens of Melitta were correctly classified to their original taxon (Melittinae) in the leave-one-out cross-validation procedure. However, the eight landmark configurations of Melitta willardi are assigned to Andreninae (MDs = 2.72 – 4.84; PPs = 0.9999 – 1). Taxonomic affinities of the fossil were also assessed based on non-supervised analyses within Andrenidae. In the morphometric space defined by the PCA, the fossil is undoubtedly clustered with the subfamily Andreninae (Figure 3).
Distribution of extant examined andrenid (36 specimens) and the eight landmark configurations of Andrenopteryx willardi (*), along the first two PC axis (PC1 = 72%, PC2 = 11%).
Melitta willardi Cockerell, 1909.
The genus presently includes only the type species, Andrenopteryx willardi (Cockerel, 1909), comb. n.
♀: Forewing with three submarginal cells, first submarginal cell largest, second smallest; r-rs long, about as long as anterior border of second submarginal cell; anterior border of second submarginal cell not dramatically shorter than that of third submarginal cell; 1rs-m relatively straight; 2rs-m greatly arched apical in posterior half; 1m-cu entering second submarginal cell near midpoint; 2m-cu entering third marginal cell at apical third of cell length, 2m-cu relatively straight; pterostigma linear, much longer than wide, border inside marginal cell relatively straight; marginal cell with acutely rounded apex, not truncate or appendiculate, apex on costal margin, apical most abscissa Rs relatively straight such that marginal cell apex tapers gradually in width from 2rs-m to apex. Pilosity well developed; flocculus absent; scopa present on metafemur and metabasitarsus; metabasitarsus more than half as long as metatibia; pretarsal claws with minute inner tooth. ♂: Unknown.
The new genus-group name is a combination of Andrena, type genus of the subfamily Andreninae, and -pteryx, meaning “wing”. The name is feminine and refers to the “Andrena-like” venation of the wings.
The wings of Andrenopteryx gen. n. have three submarginal cells, suggesting that the genus does not probably belong to subfamilies such as Xeromelissinae, Hylaeinae, Euryglossinae (all Colletidae), Dasypodainae (Melittidae), Megachilinae (Megachilidae), or various tribes among the Apidae (i.e., Allodapini, Ammobatini, Ammobatoidini, Biastini, Boreallodapini, Caenoprosopidini, Ctenoplectrini, Neolarrini, and Townsendiellini). Furthermore, Andrenopteryx gen. n. clearly possesses pollen-collecting structures, suggesting that the fossil was probably not cleptoparasitic and accordingly those genera may also be excluded (cleptoparasitic genera occur in various families, see
Assuming that its clustering among Andrenidae is an accurate reflection of its relationships, among andrenids the three submarginals cells excludes placement among most of the Panurginae. The species has a long marginal cell with an acutely curved apex that lies along the costal margin as in Andreninae, while the other subfamilies have a marginal cell with a truncate apex (
Wing shape analyses were successfully employed in previous studies to discriminate extant bee taxa at various classificatory levels, from subspecies to tribes (e.g.,
Based on the discovery that Cockerell’s fossil Melitta is more likely an andrenine, some previous hypotheses regarding the biogeography of North American bees require reconsideration.
The bees of the Florissant shale have been ignored for a long time (
We sincerely thank the University of Colorado Museum of Natural History (Boulder, USA) and Dena Smith and Talia Karim for making it possible to study Cockerell’s holotype of Andrenopteryx willardi. We are grateful to the following curators for collection access within their respective institutions: David Notton, National History Museum (London, UK), Frederique Bakker, Naturalis Biodiversity Center (Leiden, NL), Jeannine Bortels, University of Liège (Gembloux, BE), Eliane De Coninck, Royal Museum of Central Africa (Tervuren, BE), and Wouter Dekoninck, Royal Belgian Institute of Natural Sciences (Bruxelles, BE).