(C) 2013 Igor M. Sokolov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Sokolov IM (2013) A new genus and eight new species of the subtribe Anillina (Carabidae, Trechinae, Bembidiini) from Mexico, with a cladistic analysis and some notes on the evolution of the genus. ZooKeys 352: 51–92. doi: 10.3897/zookeys.352.6052
One new genus and eight new species of anilline carabids are described from southern Mexico. The new genus, Zapotecanillus gen. n., is established for Z. oaxacanus (type species) sp. n., Z. nanus sp. n., Z. iviei sp. n., Z. ixtlanus sp. n., Z. montanus sp. n., and Z. kavanaughi sp. n. from the Sierra Madre de Oaxaca, Z. pecki sp. n. from the Sierra Madre del Sur, and Z. longinoi sp. n. from the Sierra Madre de Chiapas. A taxonomic key for all described species of Zapotecanillus and a cladistic analysis, based on morphological data, are provided. Morphological, behavioral and biogeographical aspects of the speciation in the genus obtained from the resulting cladogram are discussed.
Coleoptera, Adephaga, Anillina, Zapotecanillus, new genus, new species, southern Mexico, Isthmus of Tehuantepec, forest litter, identification key, cladistic analysis, syntopic speciation, allopatric speciation, biogeography
The anilline fauna of Mexico remains extremely inadequately investigated in spite of numerous publications on carabids of the region. Monographs by Jeannel, revising the world fauna of Anillina, contain no information on Mexican representatives (
Preparing a review of the Geocharidius species, I determined that anilline specimens from Oaxaca and, in part, from Chiapas, which were identified mostly as Geocharidius by different entomologists, actually belong to the undescribed genus. This paper presents the results of a taxonomic study of this genus.
Material. This study is based on examination of 150 specimens of a new genus, representing nine species, eight of which are described as new. The material was borrowed from and/or deposited in the following institutions, identified in the text by the following associated codens:
CAS California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California, U.S.A. 94118 (D. H. Kavanaugh, Curator)
CMNC Canadian Museum of Nature, Entomology, P.O. Box 3443, Station D, Ottawa, Ontario, Canada K1P 6P4 (R. S. Anderson, Curator)
CMNH Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, U.S.A. 15213 (R. L. Davidson, Collections Manager)
KUNHM University of Kansas Natural History Museum, 1345 Jayhawk Blvd., Lawrence, Kansas, U.S.A. 66045-7593 (Z. H. Falin, Collections Manager)
MTEC Montana Entomology Collection, Montana State University, Bozeman, Montana, U.S.A. 59717 (M. A. Ivie, Curator)
NMNH Department of Entomology, United States National Museum of Natural History, Smithsonian Institution, Washington, D. C., U.S.A. 20013-7012 (T. L. Erwin, Curator)
Verbatim label data are given for type specimens of all newly described taxa, with label breaks indicated by a slash (“/”). In a case of series of KUNHM specimens with the same geographical labels but differing in various barcode numbers only, these numbers were replaced in the text by periods of ellipsis.
Measurements. All specimens were measured electronically using a Leica M420 microscope equipped with a Syncroscopy AutoMontage Photomicroscopy system (SYNCROSCOPY, Synoptics Ltd.). Measurements for various body parts are encoded as follows: LH = length of head, measured along midline from anterior margin of labrum to the virtual line, connecting posterior supraorbital setae; WH = width of head, at level of anterior supraorbital setae; WPm = maximal width across pronotum; WPa = width across anterior angles of pronotum; WPp = width across posterior angles of pronotum; LP = length of pronotum from base to apex along midline; WE = width of elytra, at level of 4th umbilicate setae; LE = length of the elytra, from apex of scutellum to apex of left elytron; SBL = standardized body length, a sum of LH, LP and LE. SBL measurements are given in mm; others are presented as nine ratios: mean widths-WH/WPm and WPm/WE and body parts-WPa/WPp, WPm/WPp, WPm/LP, WE/LE, LE/SBL, WE/SBL and LP/LE. All values are given as mean ± standard deviation.
Illustrations. Digital photographs of the dorsal habitus of new species were taken with the AutoMontage system using a Leica M420 microscope. Line drawings of selected body parts were made using a camera lucida on an Olympus BX 50 microscope or grids on a Labomed Lx400 compound microscope. Scanning electron micrographs were made either with coating on a LEO 1450VP SEM or without coating using low vacuum mode on an ESEM FEI Quanta 200.
Dissections. Dissections were made using standard technique. Genitalia were dissected from the abdomens of specimens previously softened in boiling water for 20–30 minutes. Contents of the abdomen were cleared using boiling 10% KOH for 2–3 minutes to remove internal tissues, and then washed in hot water before examination. After examination, genitalia were mounted on plastic transparent boards in dimethylhydantoin formaldehyde resin (DMHF) and pinned beneath the specimen. In some species, investigation of body parts was undertaken in the following way. The whole specimen was cleared, using boiling 10% KOH for ~5 minutes, then washed and dissected in the typical way. Disassembled body parts from one specimen were placed on plastic transparent board, properly oriented, mounted in DMHF and pinned together with the specimen labels.
Type material. I had no opportunity to investigate the type material of the Mexican species of Anillina described by A. Vigna Taglianti, so, Mexanillus sbordonii is known to us only by the original description. The concept of Geocharidius used here, is based on the investigation of a long series (>20 specimens) of Geocharidius integripennis (Bates) (Terry Erwin’s identification) from the Quiché Department of Guatemala, which is not the type locality of the species (the latter is located within neighboring Totonicapán Dept.); but these specimens exhibit features that closely match diagnostic features of the genus, mentioned in the literature (
Terms. Terms used in the paper are largely of general use and follow the literature (
Species ranking. Species recognition is in accordance with our previous approach (
Arrangement of taxa in the text. Taxonomic treatments of species in the text follow mostly the geographical basis. The species sequence starts with the type species, and each following species is more distant from the latter geographically, and, presumably, genetically. Within the Sierra Madre de Oaxaca the sequence generally corresponds to the virtual movement along the Tuxtepec – Oaxaca road in SW direction.
Descriptions. The scheme of descriptions follows that of Ball and Shpeley (
Maps. Maps were downloaded from the web-site: http://www.maps-for-free.com/ and adjusted with the help of Photoshop software.
Cladistic analysis. Morphological data were used to reconstruct the phylogenetic relationships among species of Zapotecanillus. The analysis was based on the assumption that the ancestral lineage of Mesoamerican anillines was represented by a true litter-dwelling, but not endogean, species. Accordingly, as outgroup taxa, two litter species from the anilline genera Nesamblyops and Geocharidius were chosen for analysis. The geographically proximate Geocharidius phineus Erwin from Guatemala represents the globose species of the genus and is confined to the litter of mid-altitudinal forests (
Zapotecanillus oaxacanus sp. n., by present designation.
The name Zapotecanillus derives from the Zapotecs, the name of the indigenous people living in the territory of Oaxaca during historic times, and the generic name Anillus Jacquelin du Val, the type genus of the subtribe.
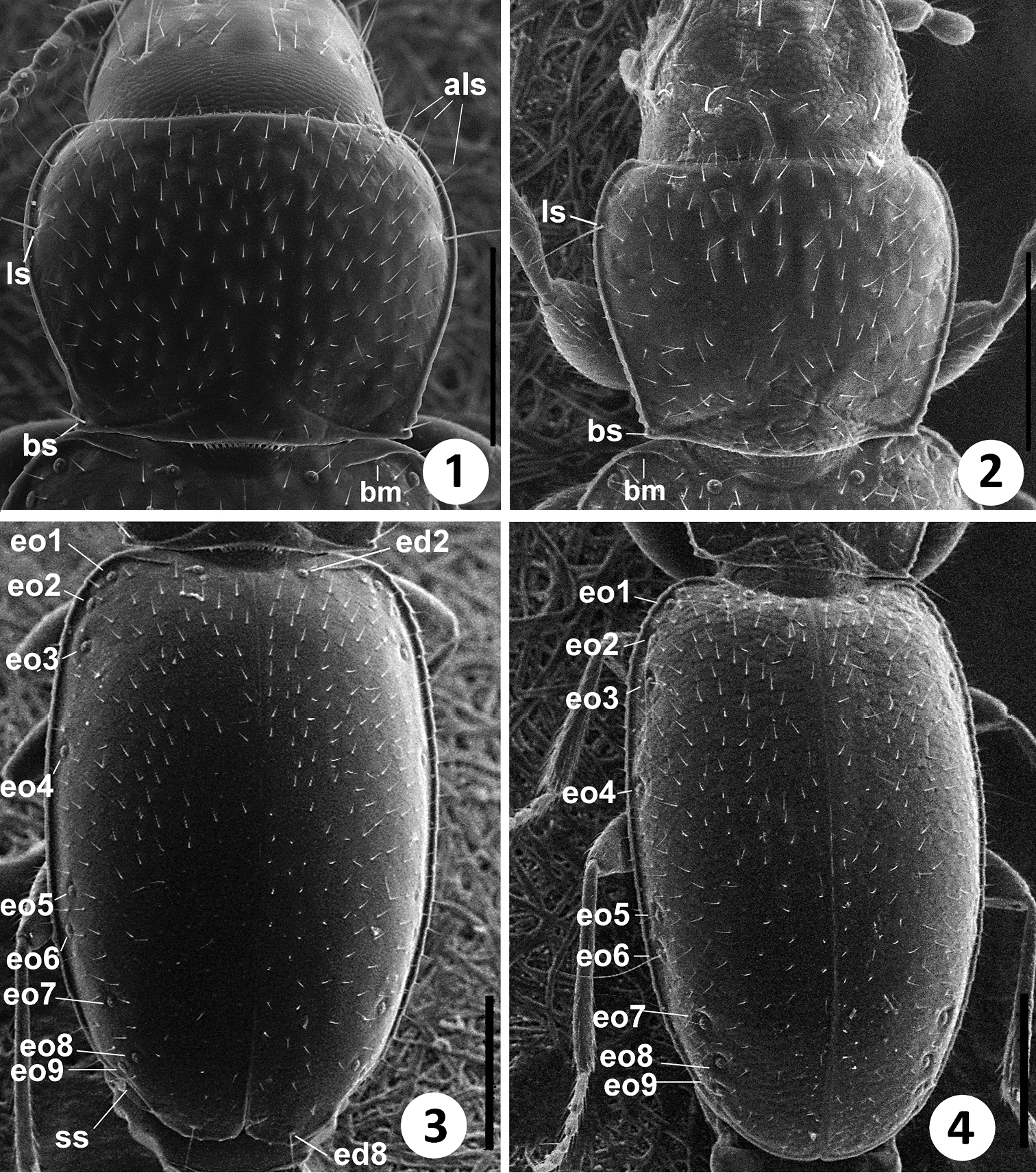
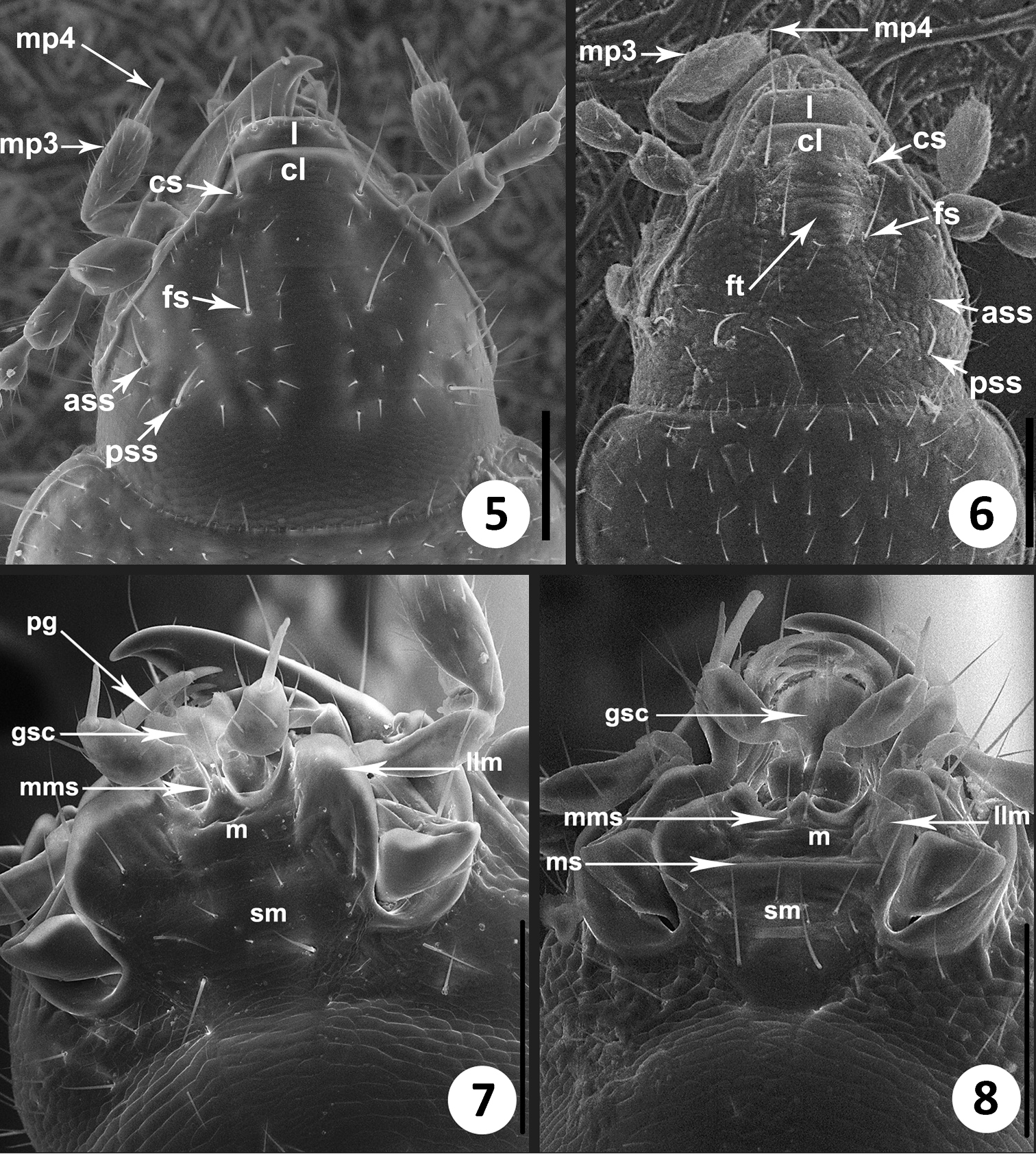
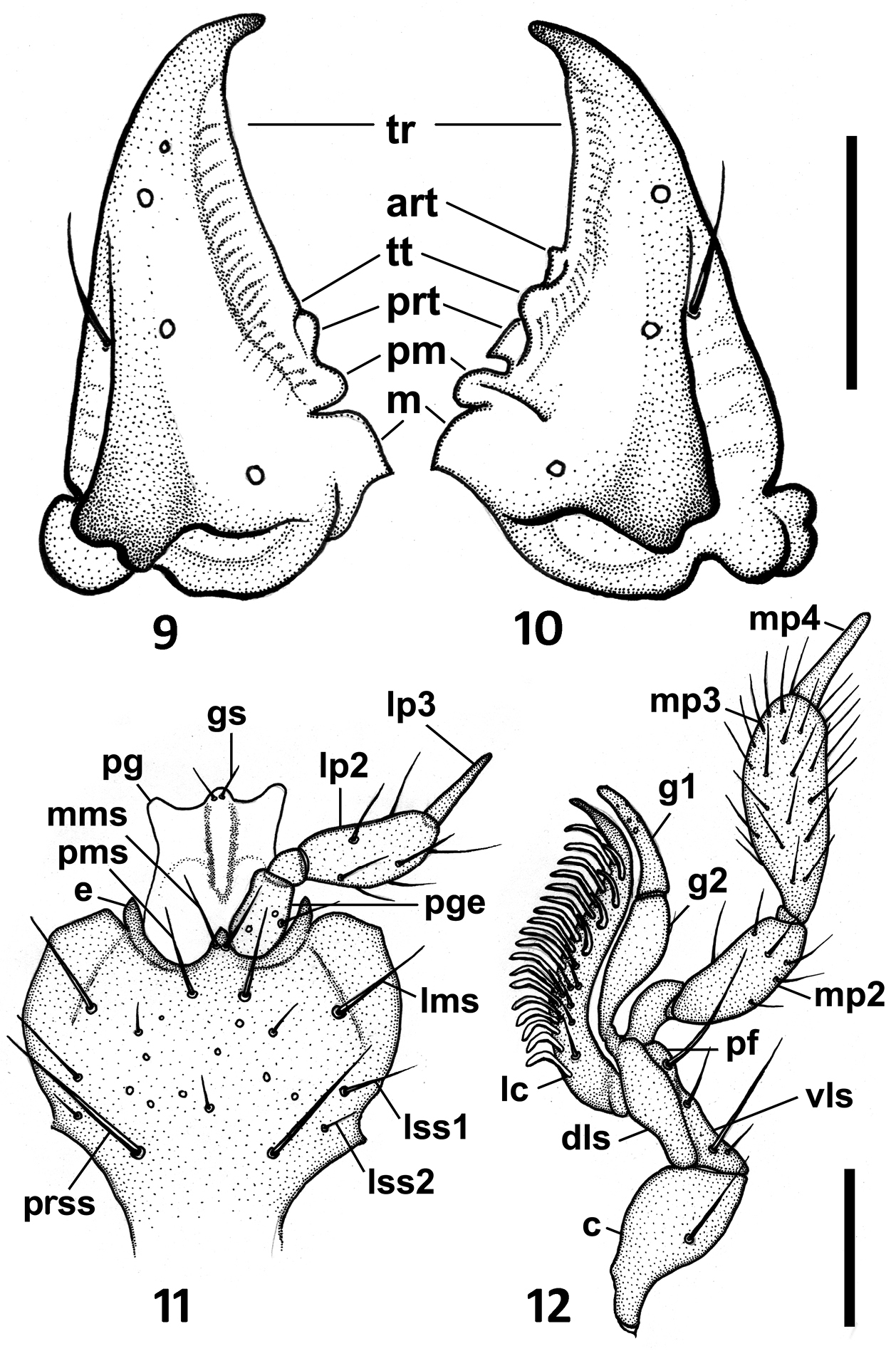
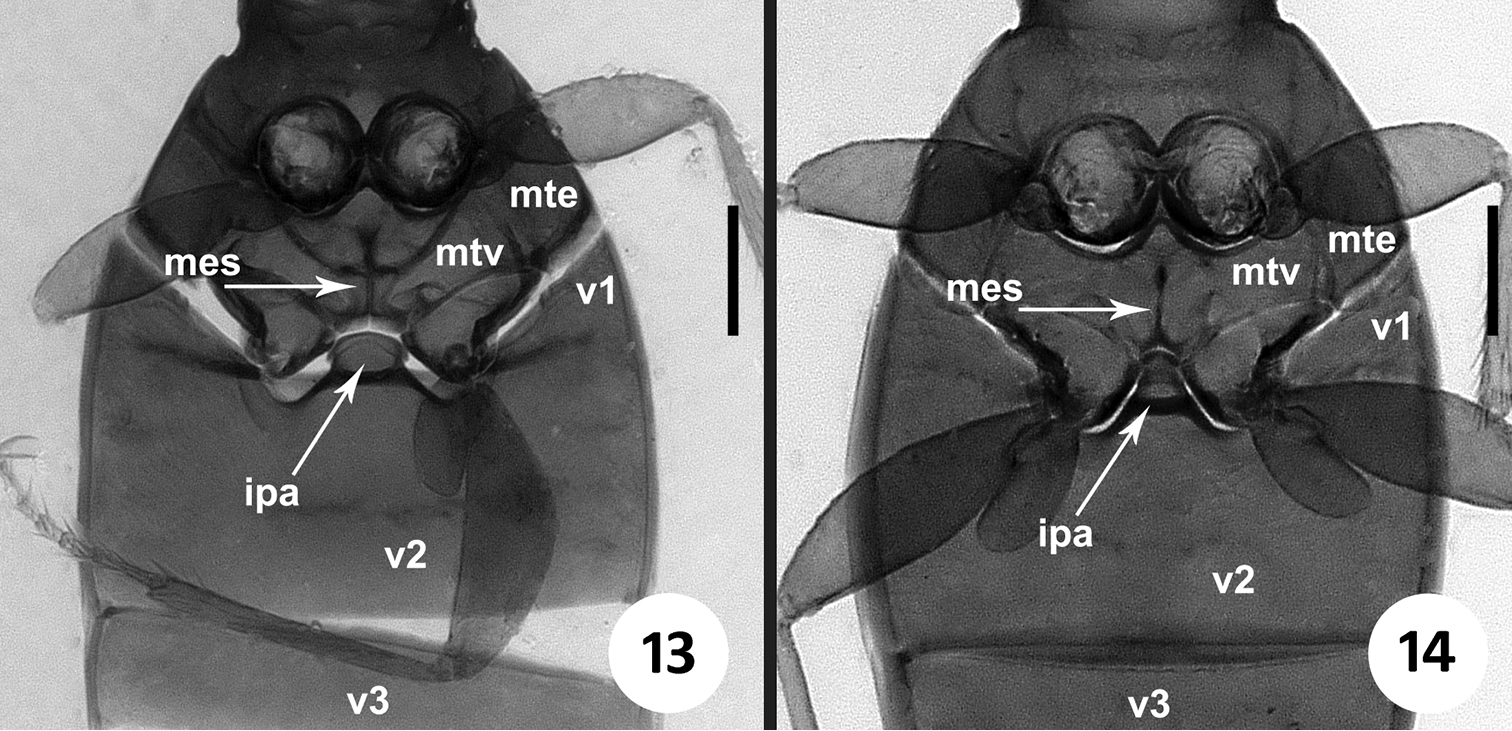
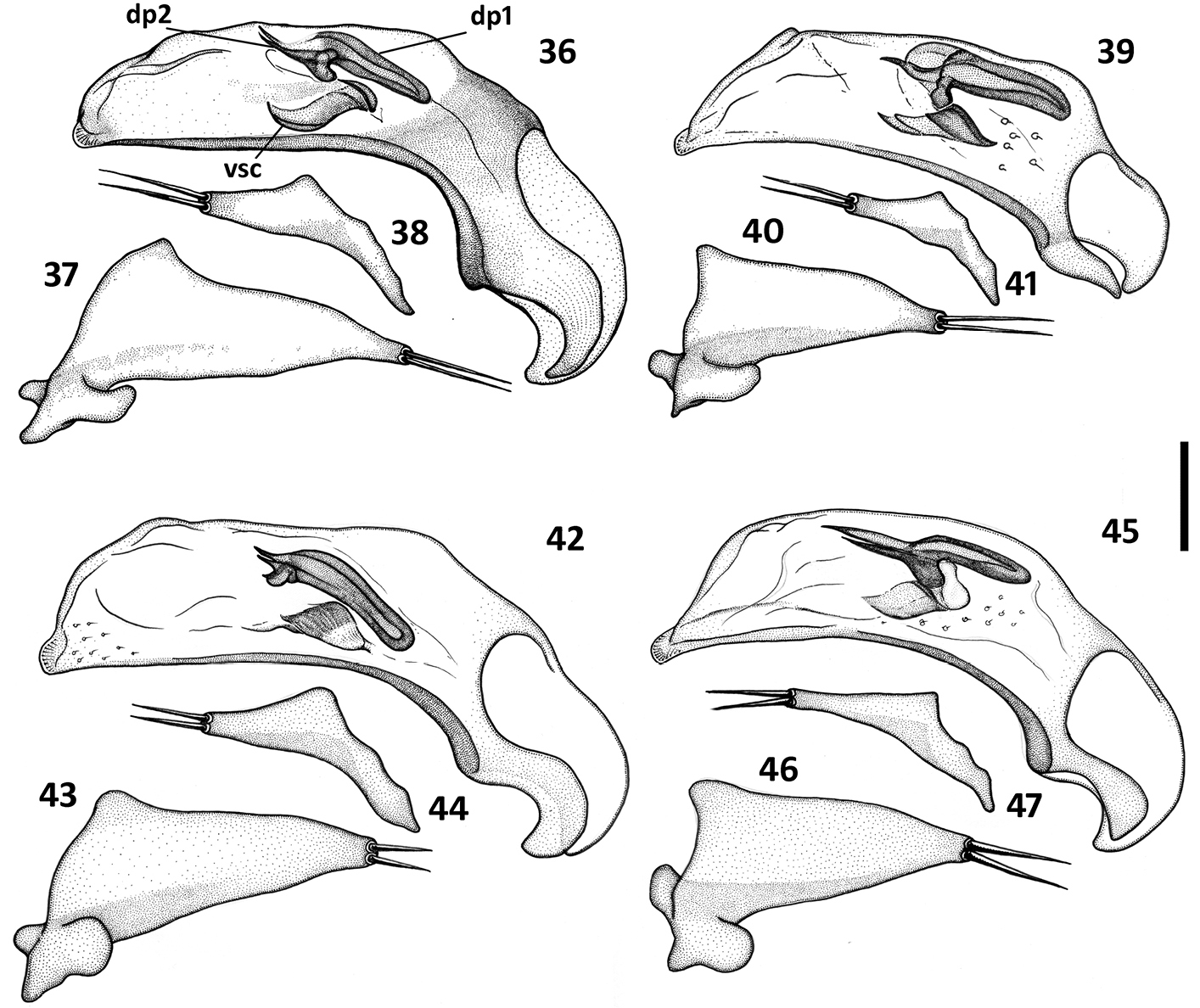
The members of this genus are distinguished from the other North and Central American representatives of Anillina by the following combination of characters: frontal area of head flat, without a median tubercle; maxillary palps with palpomere 4 longer than 1/3 of palpomere 3; labium with glossal sclerite with short but distinct paraglossae, and with mentum and submentum fused, without mental-submental suture; pronotum forward of the lateral seta and towards anterior angles with a row of elongate setae; elytra without fixed discal setae and with 8th and 9th pores of umbilicate series much closer to each other than the 7th pore is to the 8th (i.e. the “geminate” condition). The most distinctive features of the representatives of the new genus, easily distinguishing them from the species of Geocharidius, are the presence of elongate setae at the anterior angles of pronotum forward of the lateral pronotal setae (cf. Fig. 1 versus Fig. 2), a longer maxillary palpomere 4 (cf. Fig. 5 versus Fig. 6), the absence of suture between the mentum and the submentum (cf. Fig. 7 versus Fig. 8), the “geminate” state of the 8th and 9th pores of umbilicate series (cf. Fig. 3 versus Fig. 4), the cross-shaped metendoventrite and the truncate intercoxal process between the hind legs (cf. Fig. 13 versus Fig. 14).
SEM images of body parts of Zapotecanillus and Geocharidius. Pronotum: 1 Zapotecanillus oaxacanus 2 Geocharidius integripennis. Elytra: 3 Zapotecanillus oaxacanus; 4 Geocharidius integripennis. als – anterior lateral pronotal setae; ls – midlateral pronotal seta; bm – basal margin; bs – basilateral pronotal seta; ed2 – scutellar seta; ed8 – apical seta; eo1-9 – setae 1–9 from the umbilicate series; ss – subapical sinuation. Scale bar= 0.2mm.
SEM images of structural features of Zapotecanillus and Geocharidius. Head, dorsal aspect. 5 Zapotecanillus oaxacanus 6 Geocharidius integripennis. Labium, ventral aspect 7 Zapotecanillus oaxacanus 8 Geocharidius integripennis. ass – anterior supraorbital seta; cl – clypeus; cs – clypeal seta; fs – frontal seta; ft – frontal tubercle; gsc – glossal sclerite; l – labrum; llm – lateral lobe of mentum; m – mentum; mms – medial mental setae; mp3 – maxillary palpomere 3; mp4 – maxillary palpomere 4; ms – mental-submental suture; pg – paraglossa; pss – posterior supraorbital seta; sm – submentum. Scale bar = 0.1mm.
Size. SBL range 1.01–1.55 mm.
Habitus. Body form weakly to moderately convex, ovoid or subparallel (Figs 24–31).
Color. Body bicolored (Fig. 24) or monocolorous (Figs 25–31), brunneorufous, rufotestaceous or testaceous, appendages testaceous.
Microsculpture. Dorsal microsculpture of polygonal sculpticells on head, pronotum and elytra. Mesh pattern varies on different body parts. On head, sculpticells transverse, 2-3 times wider than long (Fig. 5). On pronotum, sculpticells longer, 1.5–2 times wider than long. On elytra, sculpticells form mostly isodiametric mesh pattern. Development of microsculpture on pronotum varied among different species.
Luster. Body surface shiny.
Macrosculpture. Body surface sparsely and finely punctate.
Vestiture. Body surface covered with sparse yellow setae of moderate length. Anterior angles of pronotum bear several long setae laterally, which are two times longer than adjacent vestiture (Fig. 1, als).
Fixed setae. Primary head setae include a pair of clypeal (cs), a pair of frontal (fs) and two pairs of supraorbital (ass and pss) setae (Fig. 5). Mentum with three pairs of long primary (medial, paramedial and lateral) setae (Fig. 11, mms, pms, lms). Medial mental setae located on mental tooth, not near its base on mentum (Fig. 7, mms). Submentum with two pairs of long primary setae in two rows (lss1, prss) and 1 additional pair of shorter setae (lss2) located laterally (Fig. 11). Maxilla with long stipetal and palpiferal setae (Fig. 12). Pronotum with two long primary lateral setae (middle, ls, and basal, bs) on each side (Fig. 1). Elytra lack discal setae (Fig. 3), but with scutellar (ed2) and apical (ed8) setae. Last two (8th and 9th) pores (eo8 and eo9) of umbilicate series much closer to each other than 7th (eo7) pore is to 8th (Fig. 3). Fifth visible sternite of male with two and of female with four setae along the posterior margin.
Drawings of the mouthparts of Zapotecanillus oaxacanus. 9 left mandible, dorsal aspect 10 right mandible, dorsal aspect 11 labium, ventral aspect 12 right maxilla, ventral aspect. art – anterior retinacular tooth; c – cardo; dls – dorsal lobe of stipes; e – epilobe of mentum; gs – glossal seta; g1 – galeomere 1; g2 – galeomere 2; lc – lacinia; lms – lateral mental seta; lp2 – labial palpomere 2; lp3 – labial palpomere 3; lss1 – lateral submental seta 1; lss2 – lateral submental seta 2; m – molar tooth; mms – medial mental seta; mp2 – maxillary palpomere 2; mp3 – maxillary palpomere 3; mp4 – maxillary palpomere 4; pf – palpifer; pg – paraglossa; pge – palpiger; pm – premolar tooth; pms – paramedial mental seta; prss – primary submental seta; prt – posterior retinacular tooth; tr – terebral ridge; tt – terebral tooth; vls – ventral lobe of stipes. Scale bars = 0.1mm (Figs 9–10); 0.05mm (Figs 11–12).
Head. Anterior margin of clypeus (cl) straight (Fig. 5). Frontal area flat without tubercle (ft) medially near frontoclypeal suture. Fronto-lateral carinae distinct and long.
Eyes. Eyes absent.
Antennae. Submoniliform, 11-segmented, extended to about posterior margin of pronotum. Antennomeres 1 and 2 elongate, of equal length and 1.4–1.5 times longer than antennomere 3, which is only slightly elongate and 1.1–1.2 times longer than antennomere 4. Antennomeres 4 to 10 globose, last antennomere (11) conical and 1.6-1.8 times longer than penultimate antennomere.
Labrum. Labrum (l) transverse with straight, entire anterior margin with six setae apically, increasing in size from the central pair outwards (Fig. 5).
Mandibles. General plan of Bembidion type (
Maxillae. Maxillary palps (Fig. 12) similar to Bembidion (
Labium. Labium (Fig. 7) with mental tooth; mentum and submentum fused, without mental-submental suture (ms) and with moderately enlarged lateral mental lobes, which are translucent along the lateral margins (llm). Glossal sclerite (gsc) with short but distinct paraglossae (pg) laterally and with two setae apically. Central area of mental-submental complex with a field of pores and 1-2 pairs of shorter setae additionally (Fig. 11).
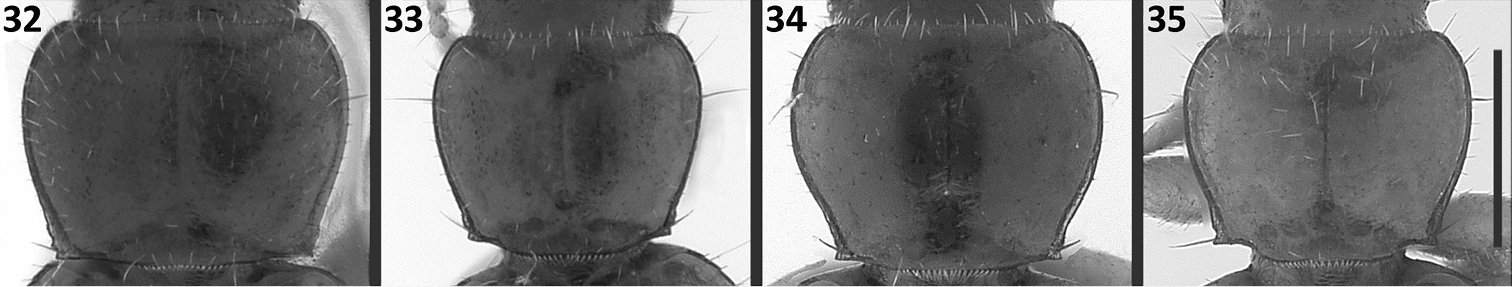
Prothorax. Pronotum cordiform, moderately convex, not sinuate (Figs 32–33) or slightly sinuate posteriorly (Figs 34–35). Basal margin of pronotum either straight (Fig. 32), or oblique laterally (Figs 33–34), in one species bisinuate laterally, at posterior angles (Fig. 35). Anterior angles indistinct, broadly rounded. Posterior angles denticulate, without or with 1-2 small denticles in front of the angles. Widths across anterior margin and between posterior angles of approximately equal length (WPa/WPp varies from 0.96 to 1.04 among species).
Scutellum. Externally visible, triangular, with narrowly rounded apex.
Elytra. Elytra of moderate length (LE/SBL from 0.57 to 0.58 among species) without visible interneurs (Fig. 3). Humeri rounded to form right angle with longitudinal axis of body. Basal margination (bm) distinct and long, reaches half the distance between humeral angle and scutellar pore (Fig. 1). Apical half of elytra with shallow but evident subapical sinuation (ss) (Fig. 3).
Hind wings. Absent.
Pterothorax (Fig. 13). Metaventrite (mtv) short, distance between meso- and metacoxae about of the diameter of mesocoxa. Metanepisternum (mte) short, subquadrate, with anterior and outer margins of equal length. Metendoventrite (mes) cross-shaped with long lateral arms.
Images of metaventrite and first abdominal ventrites of Zapotecanillus and Geocharidius. 13 Zapotecanillus oaxacanus 14 Geocharidius integripennis. ipa – intercoxal process of abdominal ventrite 2; mes – metendosternite; mte – metanepisternum; mtv – metaventrite; v1-v3 – abdominal ventrites 1–3. Scale bar = 0.1mm.
Legs. Legs of moderate length, not elongate. Prothoracic legs of males variable in structure of tarsomere 1. Typically, 1st protarsomere markedly dilated apico-laterally with two rows of oval articulo-setae (
Structural features of front legs of Zapotecanillus. Left protibia of Zapotecanillus oaxacanus: 15 ventral aspect 16 tarsal claws 17 lateral aspect 18 dorso-lateral aspect. Left protibia of Zapotecanillus nanus: 19 dorsal aspect. Male left protarsi, ventral aspect: 20 Zapotecanillus nanus 21 Zapotecanillus iviei 22 Zapotecanillus oaxacanus 23 Zapotecanillus kavanaughi. ac – antenna cleaner; as – articulo-seta, asp – anterior spur; asr – anterior setal row; cls – clip setae; psp – posterior spur; psr – posterior setal row; sb – setal band; ta1 – tarsomere 1; tbn – tibial notch. Scale bars = 0.1mm (Figs 15, 17–19); 0.05mm (Figs 20–23).
Habitus images of Zapotecanillus species. 24 Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National), paratype 25 Zapotecanillus nanus (MEXICO, Oaxaca, 18.7mi N Valle National), paratype 26 Zapotecanillus ixtlanus (MEXICO, Oaxaca, 37mi S Valle National), paratype 27 Zapotecanillus iviei (MEXICO, Oaxaca, 2mi S Cerro Pelon), paratype 28 Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan), paratype 29 Zapotecanillus montanus (MEXICO, Oaxaca, 52mi N Oaxaca), paratype 30 Zapotecanillus pecki (MEXICO, Oaxaca, 3.5mi S Suchixtepec), paratype 31 Zapotecanillus longinoi (MEXICO, Chiapas, Sierra Morena), paratype. Scale bar = 0.5mm.
Pronotum images of Zapotecanillus species. 32 Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National) 33 Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan) 34 Zapotecanillus iviei (MEXICO, Oaxaca, 2mi S Cerro Pelon) 35 Zapotecanillus pecki (MEXICO, Oaxaca, 3.5mi S Suchixtepec). Scale bar = 0.25mm.
Abdominal ventrites. Five visible abdominal ventrites: 2nd ventrite longest (Fig. 13), more than 3 times longer than 3rd or 4th, 3rd and 4th equal in length; the last, 5th, approximately 1.5 times longer than 4th. Intercoxal process (ipa) of 2nd ventrite broad, truncate anteriorly (Fig. 13).
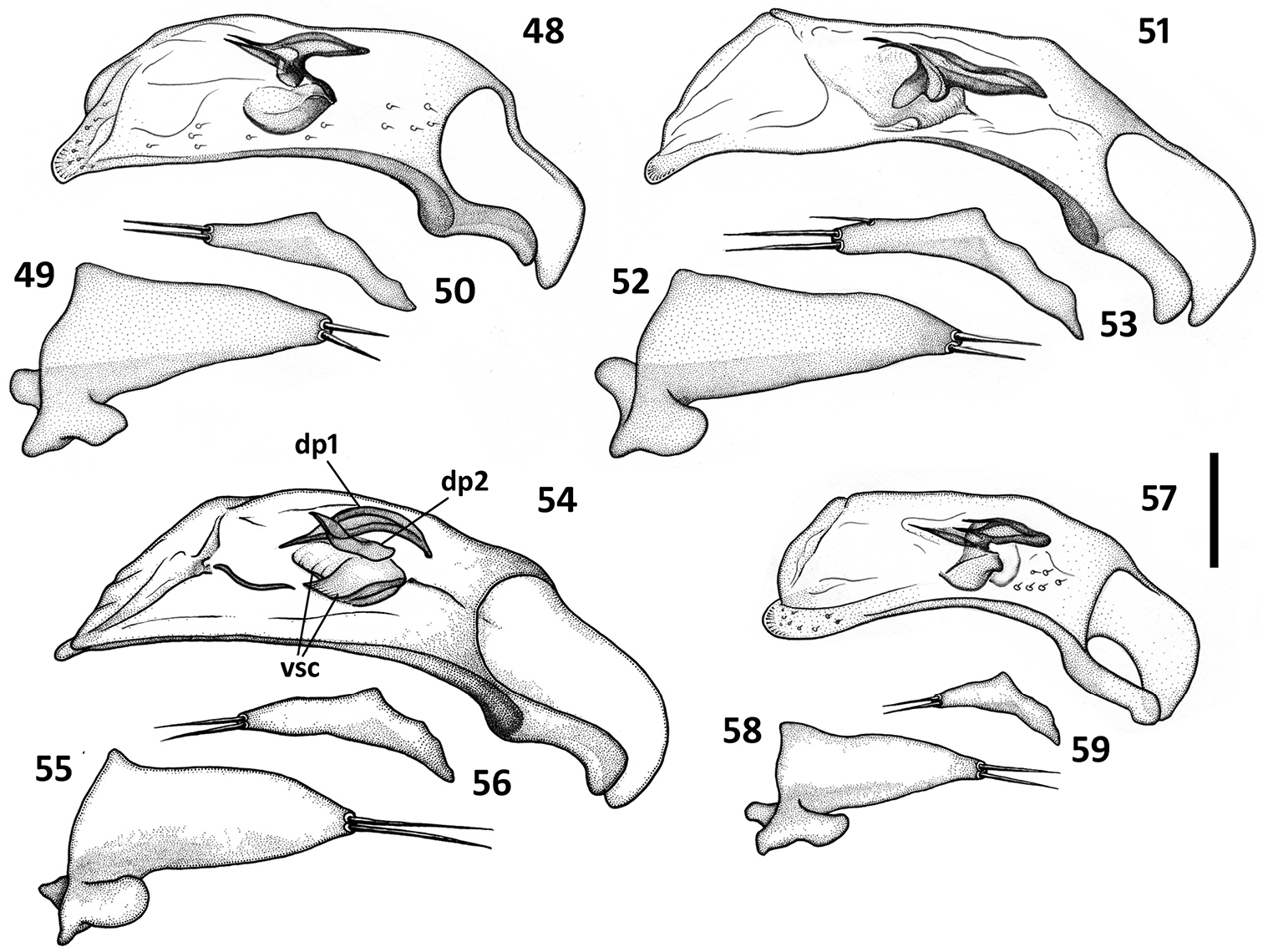
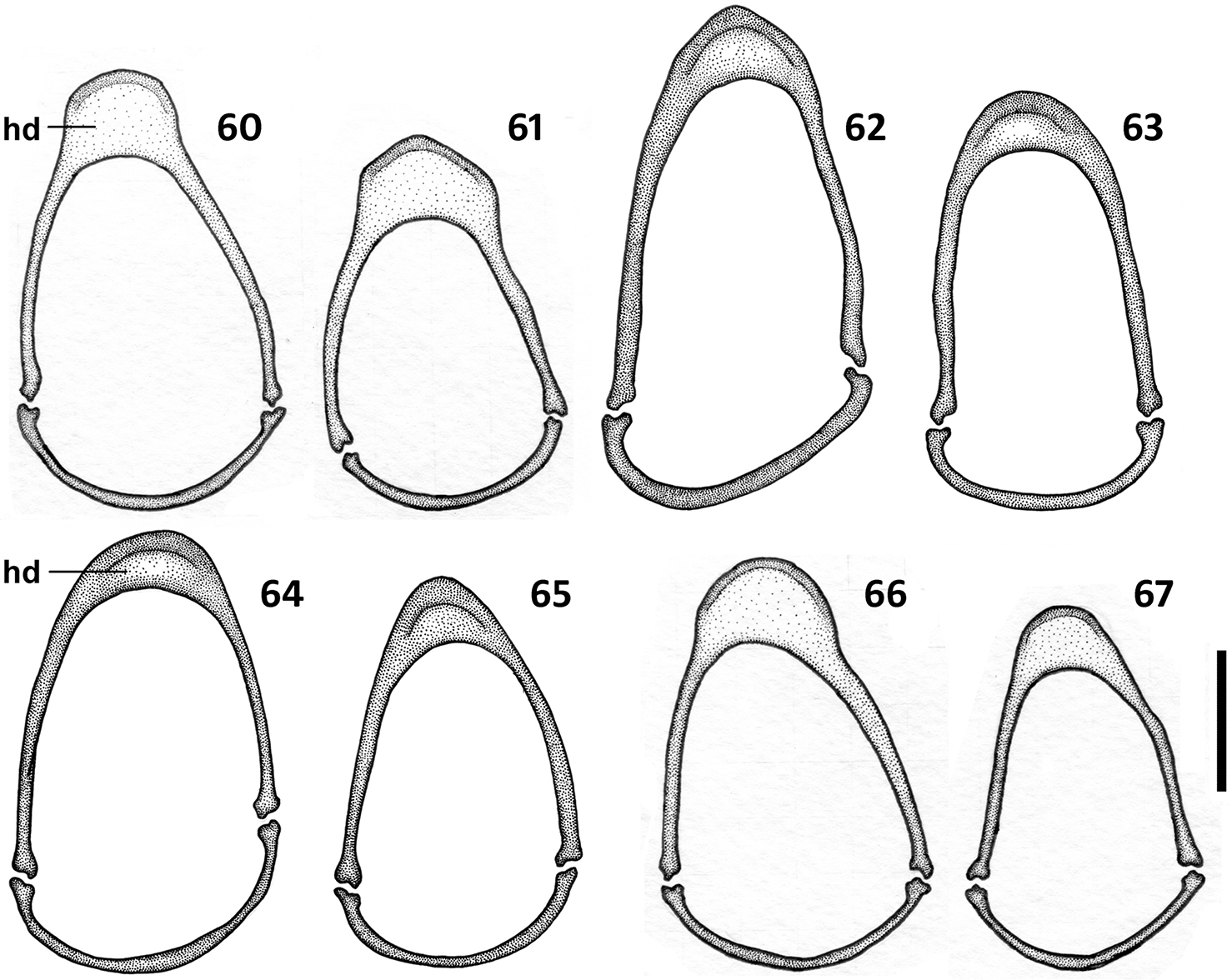
Male genitalia (Figs 36–59). Median lobe of aedeagus anopic, elongate, twisted and slightly arcuate. Internal sac with two groups of copulatory sclerites: dorsal group represented by 2 plates, and ventral group represented by weakly sclerotized fold or folds. Dorsal plate 1 (dp1) in form of an elongate plate, rounded or pointed at basal end, and tapered into a needle-like structure apically. Dorsal plate 2 (dp2) much smaller than plate 1, also needle-attenuated apically, curved and enlarged towards base; coplanarly adjoined to dorsal plate 1 apically in lateral view and divergent from plate 1 basally as a ventrally directed protuberance; can be seen as a separate structure in some species (Figs 54, 57). Ventral sclerites (vsc) of varied shape, dependent on development of sclerotization. Additional spines or scaled membranous fields of internal sac are absent. Parameres typically bisetose, except right paramere of Zapotecanillus pecki 3-setose (Fig. 53). Left paramere large and broad, either evenly tapered to apex (Figs 43, 46) or with short attenuation before setal attachment (Figs 37, 40). Ring sclerite broadly ovate with transverse handle-like extension of varied length and shape (Figs 60–67, hd).
Illustrations of male aedeagus of Zapotecanillus species. Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National) 36 median lobe, right lateral aspect 37 left paramere, left lateral aspect 38 right paramere, right lateral aspect. Zapotecanillus nanus (MEXICO, Oaxaca, 18.7mi N Valle National) 39 median lobe, right lateral aspect 40 left paramere, left lateral aspect 41 right paramere, right lateral aspect. Zapotecanillus ixtlanus (MEXICO, Oaxaca, 37mi S Valle National) 42 median lobe, right lateral aspect 43 left paramere, left lateral aspect 44 right paramere, right lateral aspect. Zapotecanillus iviei (MEXICO, Oaxaca, 2mi S Cerro Pelon) 45 median lobe, right lateral aspect 46 left paramere, left lateral aspect 47 right paramere, right lateral aspect. dp1 – dorsal plate 1; dp2 – dorsal plate 2; vsc – ventral sclerite. Scale bar = 0.05mm.
Illustrations of male aedeagus of Zapotecanillus species. Zapotecanillus montanus (MEXICO, Oaxaca, 52mi N Oaxaca) 48 median lobe, right lateral aspect 49 left paramere, left lateral aspect 50 right paramere, right lateral aspect. Zapotecanillus pecki (MEXICO, Oaxaca, 3.5mi S Suchixtepec) 51 median lobe, right lateral aspect 52 left paramere, left lateral aspect 53 right paramere, right lateral aspect. Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan) 54 median lobe, right lateral aspect 55 left paramere, left lateral aspect 56 right paramere, right lateral aspect. Zapotecanillus longinoi (MEXICO, Chiapas, Sierra Morena) 57 median lobe, right lateral aspect 58 left paramere, left lateral aspect 59 right paramere, right lateral aspect. dp1 – dorsal plate 1; dp2 – dorsal plate 2; vsc – ventral sclerites. Scale bar = 0.05mm.
Illustrations of ring sclerite of Zapotecanillus species, male genitalia, dorsal aspect. 60 Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National) 61 Zapotecanillus nanus (MEXICO, Oaxaca, 18.7mi N Valle National) 62 Zapotecanillus ixtlanus (MEXICO, Oaxaca, 37mi S Valle National) 63 Zapotecanillus iviei (MEXICO, Oaxaca, 2mi S Cerro Pelon) 64 Zapotecanillus pecki (MEXICO, Oaxaca, 3.5mi S Suchixtepec) 65 Zapotecanillus montanus (MEXICO, Oaxaca, 52mi N Oaxaca) 66 Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan) 67 Zapotecanillus longinoi (MEXICO, Chiapas, Sierra Morena). hd – handle of ring sclerite. Scale bar = 0.1mm.
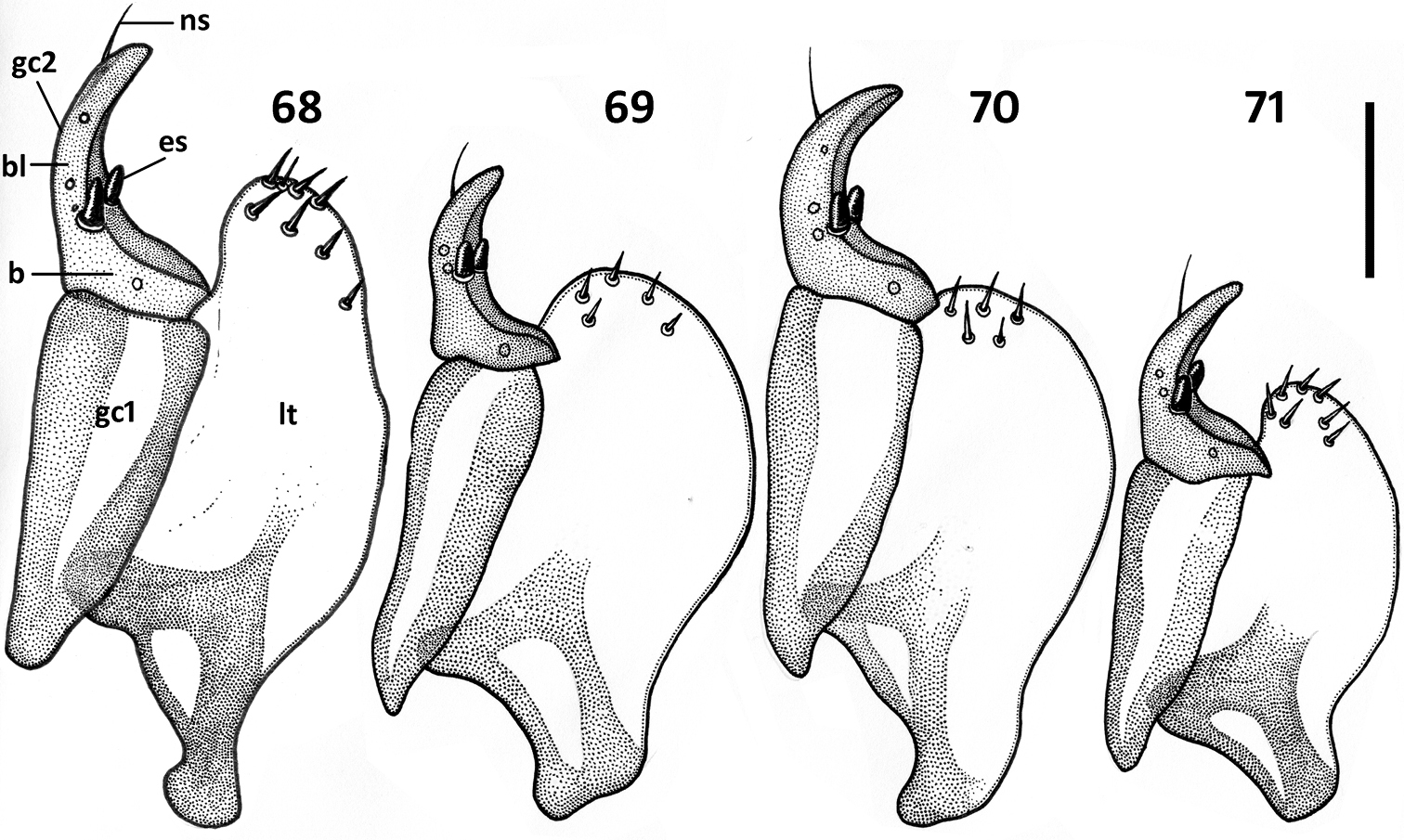
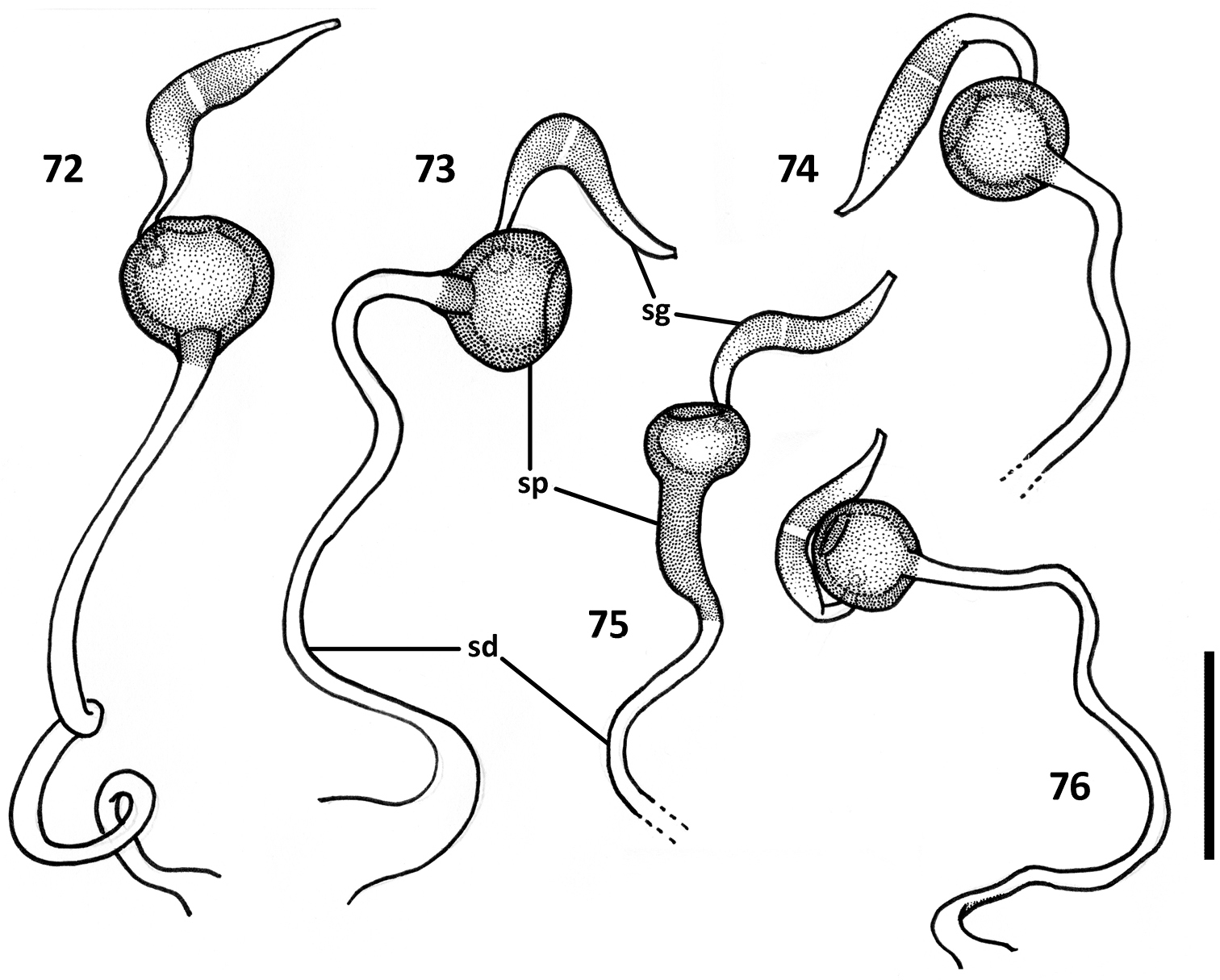
Female genitalia (Figs 68–76). Ovipositor sclerites: Gonocoxite 1 asetose (gc1). Gonocoxite 2 triangular (gc2), 1.6–1.8 times longer than its basal width, slightly to moderately curved, with 2 lateral ensiform (es) and apical nematiform (ns) setae. Laterotergite (lt) with 5–8 setae. Internal genitalia with spermatheca sclerotized, rufous, spherical and ball-shaped in most species, fusiform with a bulb-like enlargement apically in Zapotecanillus kavanaughi (Fig. 75).
Illustrations of ovipositor sclerites of Zapotecanillus species. 68 Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National) 69 Zapotecanillus nanus (MEXICO, Oaxaca, 15.1mi N Valle National) 70 Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan) 71 Zapotecanillus longinoi (MEXICO, Chiapas, Sierra Morena). b – base of gonocoxite 2, bl – blade of gonocoxite 2, es – ensiform seta; gc1 – gonocoxite 1; gc 2 – gonocoxite 2; lt – laterotergite; ns – nematiform seta. Scale bar = 0.05mm.
Illustrations of spermatheca, spermathecal duct, and spermathecal gland of Zapotecanillus species. 72 Zapotecanillus oaxacanus (MEXICO, Oaxaca, 18.7mi N Valle National) 73 Zapotecanillus nanus (MEXICO, Oaxaca, 15.1mi N Valle National) 74 Zapotecanillus iviei (MEXICO, Oaxaca, 2mi S Cerro Pelon) 75 Zapotecanillus kavanaughi (MEXICO, Oaxaca, 14km N San Juan) 76 Zapotecanillus longinoi (MEXICO, Chiapas, Sierra Morena). sd – spermathecal duct; sg – spermathecal gland; sp – spermatheca. Scale bar = 0.05mm.
The new genus includes eight species: Zapotecanillus oaxacanus sp. n., Zapotecanillus nanus sp. n., Zapotecanillus ixtlanus sp. n., Zapotecanillus iviei sp. n., Zapotecanillus montanus sp. n., Zapotecanillus pecki sp. n., Zapotecanillus kavanaughi sp. n., and Zapotecanillus longinoi sp. n.
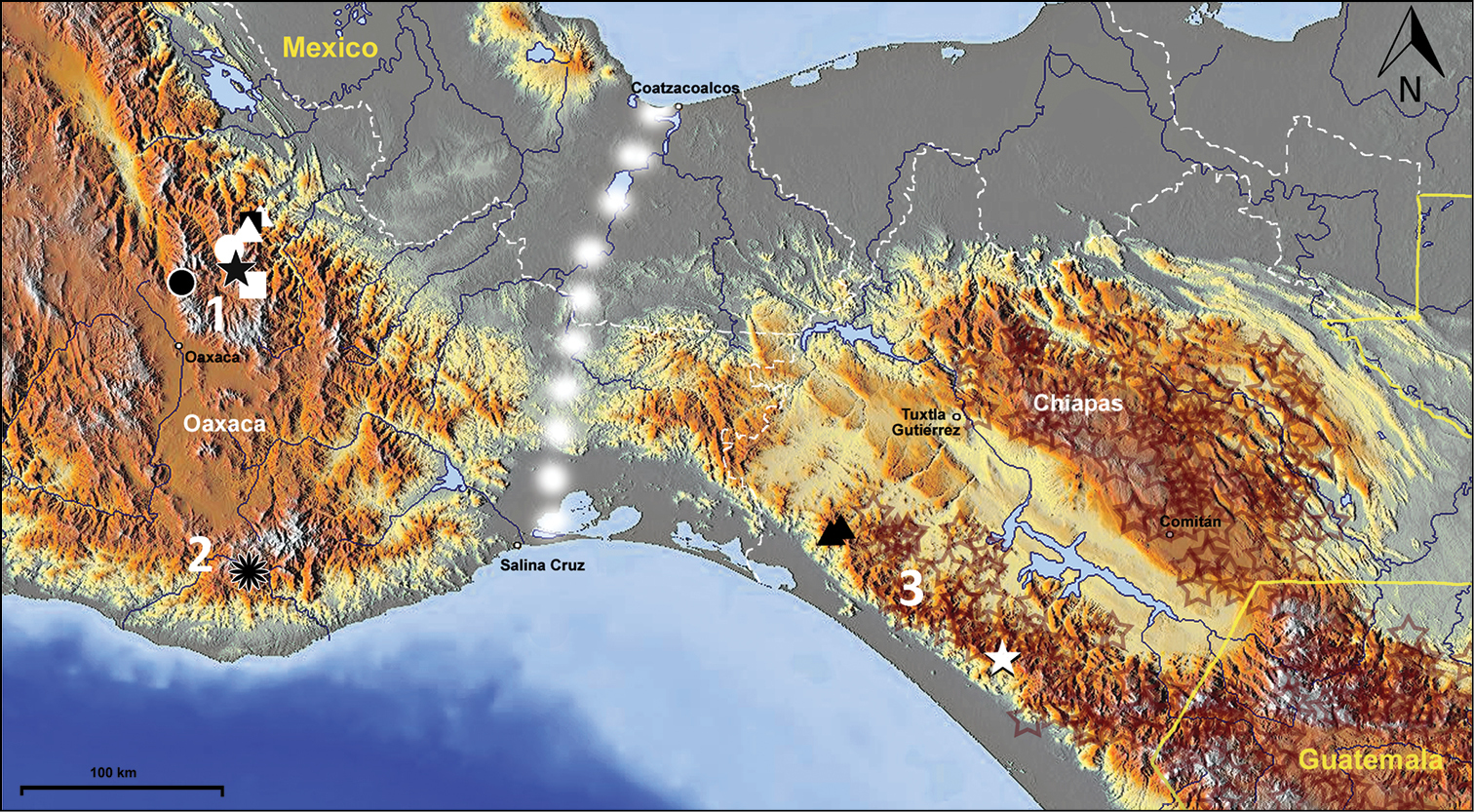
The species of this genus are known from three mountain ranges of Mexico: the Sierra Madre de Oaxaca, the Sierra Madre del Sur and the Sierra Madre de Chiapas, within the states of Oaxaca and Chiapas (Fig. 77). This type of distribution best fits
Map of southern Mexico and adjacent part of Guatemala, showing positions of locality records for the species of Zapotecanillus. Zapotecanillus oaxacanus – black quadrangle; Zapotecanillus nanus – white triangles; Zapotecanillus ixtlanus – white circle; Zapotecanillus iviei – black star; Zapotecanillus kavanaughi – black circle; Zapotecanillus montanus – white quadrangle; Zapotecanillus pecki – black flower; Zapotecanillus longinoi – black triangles; Zapotecanillus sp. – white star. Brown stars – range of Geocharidius species (original data). 1 – Sierra Madre de Oaxaca; 2 – Sierra Madre del Sur; 3 – Sierra Madre de Chiapas. White dots – the Isthmus of Tehuantepec.
According to the label information, specimens of the new genus were taken from the leaf litter within the 1200–3000m range of altitudes at the Sierra Madre de Oaxaca, and in mesophyll and cloud forests within the 1330–2140m range of altitudes at the Sierra Madre de Chiapas. Collecting dates are May, June, July and August. One specimen from the Sierra Madre de Oaxaca was taken “under bark hardwood”.
The position of Zapotecanillus within the North and Central American Anillina is unclear at present, and awaits molecular data analysis or further discoveries and subsequent morphological analyses of the Middle American anilline taxa. Members of this new genus differ principally from those of the southern stock of Middle American anilline genera (Geocharidius Jeannel, Honduranillus Zaballos, Mexanillus Vigna Taglianti) in having a different arrangement of the last three pores of the umbilicate series and the fused labium, and from geographically proximate Geocharidius and Mexanillus in having distinct paraglossae. They differ from members of the northern stock of North American anilline genera (Anillaspis Casey, Anillinus Casey, Anillodes Jeannel, Micranillodes Jeannel, Serranillus Barr) in lacking fixed discal pores on the elytra.
The key provided below allows distinguishing members of the new genus from those of the other continental North and Middle American anilline genera:
| 1 | Elytra without fixed discal setae (Figs 3–4). Mexico and Central America | 2 |
| – | Elytra with 3 pairs of fixed discal setae. North of Mexico | other genera (Anillaspis Casey, Anillinus Casey, Anillodes Jeannel, Micranillodes Jeannel, Serranillus Barr) |
| 2 | Labium fused, without mental-submental suture (Fig. 7). Pores 8 and 9 of umbilicate series geminate, much closer to each other, than 8th to 7th (Fig. 3) | Zapotecanillus, gen. n. |
| – | Labium free, with distinct mental-submental suture (Fig. 8). Pores 7, 8 and 9 of umbilicate series equidistant (Fig. 4) | 3 |
| 3 | Pronotum convex. Glossal sclerite without distinct paraglossae (Fig. 8). Size smaller, less than 2.7mm | 4 |
| – | Pronotum subdepressed. Glossal sclerite with distinct paraglossae (as in Fig. 7). Size larger, 3mm | Honduranillus Zaballos |
| 4 | Length greater than 2.2 mm. Head with impressed, subparallel frontal furrows and shortened latero-frontal carinae. Appendages, especially tarsi, elongate; 1st tarsomeres of middle and hind legs very long, longer than the length of tarsomeres 2-4 combined. Troglobitic beetles | Mexanillus Vigna Taglianti |
| – | Length less than 1.9 mm. Head with faint and divergent frontal furrows and long latero-frontal carinae. Appendages of standard length, 1st tarsomere of middle and hind legs shorter than tarsomeres 2-4 combined. Litter-dwelling beetles | Geocharidius Jeannel |
http://zoobank.org/86234081-8CB0-45D0-861D-721A0C05174A
http://species-id.net/wiki/Zapotecanillus_oaxacanus
Figs 1, 3, 5, 7, 9–13, 15–18, 22, 24, 32, 36–38, 60, 68, 72, 77, 90–92, 94HOLOTYPE, male, in NMNH, point-mounted, labeled: \MEXICO. Oaxaca 18.7mi S Valle Nacional 5200’ 17 Aug.1973\ A.Newton Collector \ Loan from NMNH 2051867\. PARATYPES (8 ex., 4♂2♀ were dissected), labeled same as a holotype, except two specimens, which have an additional label: \Geocharidius n. sp. det. T.L.Erwin 76\ each, where italicized font means handwritten (deposited in CAS, NMNH).
The specific epithet is a Latinized adjective in the masculine form based on Oaxaca, the state of Mexico from which the new species is described.
Mexico, Oaxaca, 18.7mi S from Valle Nacional.
Adults of this new species can be distinguished easily from those of other species of the genus by the following combination of external characters: bicolored and robust appearance, comparatively small head and distinctly transverse pronotum.
Size. Medium-sized for the genus (SBL range 1.30-1.36 mm, mean 1.33±0.049 mm, n=8).
Habitus. Body form (Fig. 24) moderately convex, slightly elongate (WE/SBL 0.41±0.09), head narrow for genus compared to pronotum (WH/WPm 0.69±0.015), pronotum wide compared to elytra (WPm/WE 0.81±0.017).
Color. Body bicolored: head and pronotum brunneorufous, elytra rufotestaceous, appendages testaceous.
Microsculpture. Disc of pronotum with well-developed microsculpture.
Prothorax. Pronotum (Fig. 32) relatively long (LP/LE 0.44±0.006) and markedly transverse (WPm/LP 1.33±0.022), with lateral margins straight and moderately constricted posteriorly (WPm/WPp 1.32±0.034). Basal margin straight. Contour of posterior angles nearly rectangular (100–110°) with 1–2 small denticles in front of the angles.
Elytra (Fig. 3). Convex, not depressed along suture, comparatively wide (WE/LE 0.73±0.013). Margins rounded, slightly divergent in basal half, evenly rounded to apex in apical half, maximal width of elytra at midpoint.
Legs. 1st male protarsomere only slightly dilated (Fig. 22).
Male genitalia. Median lobe of aedeagus (Fig. 36), with short and transverse apex, broadly rounded at tip. Dorsal plate 1 long, with apical pointed attenuation of moderate length. Dorsal plate 2 joined to plate 1 at its middle ventrally, where it forms a distinct protuberance. Ventral sclerites elongate, with subparallel sides and obliquely stretched from dorsal plates towards ventral margin of median lobe. Right paramere short and moderately wide (Fig. 38). Left paramere with distinct apical constriction (Fig. 37). Ring sclerite with long handle-like extension, widely rounded apically (Fig. 60).
Female genitalia. Gonocoxite 2 rather long, with slightly curved blade and rounded apex (Fig. 68). Laterotergite with 7-8 setae. Spermatheca standard for genus (Fig. 72).
Geographical distribution. This species is known only from the type locality in the Sierra de Juárez Range, a part of the Sierra Madre de Oaxaca, within the high course of the Rio de Valle Nacional (Figs 77 and 94, black quadrangles).
Way of life. Specimens of this species were collected at an altitude of 5200 feet (1600 m).
Relationships. The armature of internal sac of Zapotecanillus oaxacanus males is nearly indistinguishable from that of Zapotecanillus nanus and Zapotecanillus ixtlanus males, described below, clearly suggesting both of them as closest relatives. The former species is sympatric with Zapotecanillus oaxacanus and, based on the same label data, may also be syntopic (i.e., their members may occur together in the same habitat). See also Fig. 90 for cladistic affinities.
http://zoobank.org/E3677571-83C4-4836-9252-BC1B5C11129E
http://species-id.net/wiki/Zapotecanillus_nanus
Figs 19, 20, 25, 39–41, 61, 69, 73, 77, 90–92, 94HOLOTYPE, male, in NMNH, point-mounted, labeled: \ MEX. Oax., 15mi. S. Valle Nacional, 4000’ 21.V.1971 S.Peck Ber.204, leaf litter \ Borrowed Ex. J.M. Campbell \ USNMNH 2051867\. PARATYPES (27 ex., 4♂4♀ were dissected), 4 ex. labeled same as holotype, except one specimen, which has an additional label: \Geocharidius n. sp. det. T.L.Erwin 76\, where italicized font means handwriting.; 12 ex. labeled: MEXICO: Oaxaca, 15mi. S. Valle Nacional, 4000’ 21 May 1971, leaf litter, S. Peck \ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\; 1 ex. labeled: \ MEXICO: Oaxaca 15.1mi S Valle Nacional 4300’ VIII-11-18-1973 \ under bark hardwood A.Newton \ Borrowed ex. MCZ \ Loan from USNMNH 2051867\; 10 ex. labeled: \ MEXICO. Oaxaca 18.7mi S Valle Nacional 5200’ 17 Aug.1973\ A.Newton Collector \ Loan from USNMNH 2051867\ (deposited in CAS, CMNH, NMNH).
The specific epithet is a Latin adjective, nanus, in the masculine form, meaning dwarf, miniature, and refers to the small size of the beetles.
Mexico, Oaxaca, 15mi S from Valle Nacional.
Adults of this new species are distinguished from those of other species of the genus by the combination of small size and brunneorufous color; and males can be further distinguished by the shape of the median lobe (Fig. 39).
Size. Small for genus (SBL range 1.02–1.16 mm, mean 1.10±0.049 mm, n=12).
Habitus. Body form (Fig. 25) moderately convex, slightly elongate (WE/SBL 0.41±0.09), head of normal proportions for genus (WH/WPm 0.75±0.016), pronotum narrow compared to elytra (WPm/WE 0.75±0.028).
Color. Body monocolorous, brunneorufous, appendages testaceous.
Microsculpture. Microlines partially effaced on disc of pronotum.
Prothorax. Pronotum relatively long (LP/LE 0.43±0.020) and moderately transverse (WPm/LP 1.26±0.030), with margins straight and markedly constricted posteriorly (WPm/WPp 1.38±0.044). Basal margin slightly oblique laterally. Posterior angles small, contour of posterior angles obtuse (112–126°) without or with 1 small denticle in front of the angles.
Elytra. Slightly convex, not depressed along suture, comparatively wide (WE/LE 0.72±0.025). Margins rounded, moderately divergent in basal half, evenly rounded to apex in apical third, maximal width of elytra slightly behind the midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally (Fig. 20).
Male genitalia. Median lobe of aedeagus (Fig. 39), with small, slightly elongated apex, angulately rounded at tip. Dorsal plate 1 long, with apical pointed attenuation of moderate length. Dorsal plate 2 joined to plate 1 at its middle ventrally, where it forms a distinct protuberance. Ventral sclerites with sides divergent ventrally, trianguloid in shape. Right paramere short and moderately narrow (Fig. 41). Left paramere with distinct apical constriction (Fig. 40). Ring sclerite with long handle-like extension, pointed apically (Fig. 61).
Female genitalia. Gonocoxite 2 comparatively short, with slightly curved blade and narrowly rounded apex (Fig. 69). Laterotergite with 5-6 setae. Spermatheca standard for genus (Fig. 73).
The species is known from a few localities in the Sierra de Juárez Range, a part of the Sierra Madre de Oaxaca, along the ~5km stretch of the Rio de Valle Nacional (Figs 77 and 94, white triangles).
According to the label data, the elevations of localities range from 4000’ to 5200’ (1200–1600 m).
Aedeagal characters (shape of dorsal plates and left paramere) suggest that Zapotecanillus nanus is most closely related to the sympatric Zapotecanillus oaxacanus. See also Fig. 90 for cladistic affinities.
http://zoobank.org/3349A2CB-9EA6-4F1E-8472-F9C7C4854CDC
http://species-id.net/wiki/Zapotecanillus_ixtlanus
Figs 26, 42–44, 62, 77, 90, 92, 94HOLOTYPE, male, in CMNH, point-mounted, labeled: \MEXICO: Oaxaca, 32 miles S Valle Nacional, 7000 ft. 23 May 1971, ex. leaf litter, Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\. PARATYPES (17 ex., 3♂1♀ were dissected), 10 ex. labeled same as a holotype; one male labeled: 8500’, 37mi. S. Valle Nacional, Oax. Mex. V.24.1971 H.Howden \ Borrowed Ex. H.F. Howden \ Loan from USNMNH 2051867\; 3 ex. labeled: \MEXICO: Oaxaca, 37 mi. S Valle Nacional, 8500 ft. 23 May 1971, ex. leaf litter, Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\; 3 ex. labeled: \MEXICO: Oaxaca, 37 mi. S Valle Nacional, oak litter, 25 May 1971, S. Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\ (deposited in CAS, CMNH, NMNH).
The specific epithet is a Latinized adjective in the masculine form based on Ixtlan, the district of the state of Oaxaca, Mexico from which the new species is described.
Mexico, Oaxaca, 32mi. S. Valle Nacional.
Males of this new species can be distinguished from those of other species of the genus by the combination of the large size and shape of the median lobe (Fig. 42).
Size. Large for genus (SBL range 1.32–1.53 mm, mean 1.39±0.069 mm, n=12).
Habitus. Body form (Fig. 26) moderately convex, slightly elongate (WE/SBL 0.41±0.016), head of normal proportions for genus (WH/WPm 0.72±0.013), pronotum narrow compared to elytra (WPm/WE 0.72±0.038).
Color. Body monocolorous, rufobrunneous, appendages testaceous.
Microsculpture. Disc of pronotum with well-developed microsculpture.
Prothorax. Pronotum relatively small (LP/LE 0.39±0.020) and moderately transverse (WPm/LP 1.31±0.039), with margins straight and distinctly constricted posteriorly (WPm/WPp 1.41±0.050). Basal margin oblique laterally. Contour of posterior angles obtuse (111–123°) with a small denticle.
Elytra. Convex, not depressed along suture, of moderate width (WE/LE 0.70±0.023). Margins subparallel at middle, slightly divergent in basal third, evenly rounded to apex in apical third, maximal width of elytra slightly behind midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally.
Male genitalia. Median lobe of aedeagus (Fig. 42) with short semicircular apex. Dorsal plate 1 long, with short apical attenuation. Dorsal plate 2 joined to plate 1 at its middle ventrally, where it forms a distinct protuberance. Ventral sclerites slightly sclerotized, trianguloid in shape. Right paramere short and moderately wide (Fig. 44). Left paramere without apical constriction (Fig. 43). Ring sclerite with short handle-like extension, pointed apically (Fig. 62).
Female genitalia. Spermatheca standard for genus.
The species is known only from the type locality in the Sierra Juárez Range, a part of the Sierra Madre de Oaxaca (Figs 77 and 94, white circle).
Specimens were collected at altitudes 7000-8500’ (2100–2600 m) in oak litter.
Aedeagal characters (shape of the median lobe and dorsal plates) suggest that Zapotecanillus ixtlanus is closely related to Zapotecanillus oaxacanus and Zapotecanillus nanus. See also Fig. 90 for cladistic affinities.
http://zoobank.org/7B74C66E-1EEB-4706-8AFE-A7B6D9A056CC
http://species-id.net/wiki/Zapotecanillus_iviei
Figs 21, 27, 34, 45–47, 63, 74, 77, 90, 94HOLOTYPE, male, in CAS, point-mounted, labeled: \ MEX: OAXACA, 2 mi S. Cerro Pelon, 8–9000 ft. 03 JUL 1982 M.A. Ivie colr.\ ex rotten pine \ Geocharidius n. sp. det. M. A. Ivie 1983 (handwriting)\. PARATYPES (34 ex., 4♂2♀ were dissected), labeled same as a holotype (deposited in CAS, MTEC).
The specific epithet is a Latinized eponym in the genitive case, and is based on the surname of Michael A. Ivie, Associate Professor and Curator of Entomology at the Montana State University, the collector of the type series of the species.
Mexico, Oaxaca, 2 miles S Cerro Pelon.
Adults of this new species can be distinguished from those of other species of the genus by the combination of elongate habitus and comparatively narrow pronotum; and males can be further distinguished by the shape of the copulatory sclerites of the median lobe (Fig. 45).
Size. Large for genus (SBL range 1.34–1.55 mm, mean 1.43 ± 0.066 mm, n=21).
Habitus. Body form (Fig. 27) slightly convex, moderately elongate (WE/SBL 0.39±0.09), head of normal proportions for genus (WH/WPm 0.74±0.013), pronotum narrow compared to elytra (WPm/WE 0.72±0.022).
Color. Body monocolorous, rufotestaceous, appendages testaceous.
Microsculpture. Partially effaced on disc of pronotum.
Prothorax. Pronotum (Fig. 34) relatively short (LP/LE 0.39±0.011) and slightly transverse (WPm/LP 1.22±0.025), with margins slightly sinuate and markedly constricted posteriorly (WPm/WPp 1.42±0.039). Basal margin oblique laterally. Contour of posterior angles obtuse (114–124°) without or with 1 small denticle in front of the angles.
Elytra. Slightly convex, not depressed along suture, rather narrow (WE/LE 0.66±0.019). Margins subparallel at middle, slightly divergent in basal forth, evenly rounded to apex in apical forth, maximal width of elytra at midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally (Fig. 21).
Male genitalia. Median lobe of aedeagus (Fig. 45), with small, slightly elongated apex, angulately rounded at tip. Dorsal plate 1 long, with long apical attenuation. Dorsal plate 2 joined to plate 1 at its middle ventrally, where it forms a distinct protuberance. Ventral sclerites weakly sclerotized. Right paramere rather long and narrow (Fig. 47). Left paramere without apical constriction (Fig. 46). Ring sclerite with short handle, which is widely rounded apically (Fig. 63).
Female genitalia. Spermatheca (Fig. 74) standard for genus.
The species is known only from the type locality in the Sierra Juárez Range, a part of the Sierra Madre de Oaxaca (Figs 77 and 94 black star).
According to the label data (elevation ranges 2600–2700m), these beetles inhabit the pine-oak forest zone of the Sierra Madre de Oaxaca.
Externally, adults of Zapotecanillus iviei are similar to those of Zapotecanillus kavanaughi, Zapotecanillus pecki and Zapotecanillus montanus, described below, but the armature of the internal sac of the median lobe suggests closer relatedness to Zapotecanillus oaxacanus, Zapotecanillus nanus and Zapotecanillus ixtlanus. See also Fig. 90 for cladistic affinities.
http://zoobank.org/129C5EFE-EB44-4528-A228-DF4315902D76
http://species-id.net/wiki/Zapotecanillus_kavanaughi
Figs 23, 28, 33, 54–56, 66, 70, 75, 77, 90, 94HOLOTYPE, male, in CMNC, point-mounted, labeled: \MEX. Oax. 14km N SanJuan del Estado 2600m. 4-VIII.1986 H. & A. Howden\ berlese\ CMNC\. PARATYPES (8 ex., 2♂2♀ were dissected), labeled same as a holotype (deposited in CAS, CMNC).
The specific epithet is a Latinized eponym in the genitive case, and is based on the surname of David H. Kavanaugh, Senior Curator of the Entomology Department of the California Academy of Sciences, whose enthusiastic efforts in locating and borrowing the material for the current investigation were so magnanimous and productive.
Mexico, Oaxaca, 14 km N San Juan del Estado.
Adults of this new species are distinguished from those of other species of the genus by the combination of elongate habitus and comparatively narrow pronotum; and males can be further distinguished by the shape of median lobe (Fig. 54).
Size. Medium-sized for genus (SBL range 1.25–1.42 mm, mean 1.34±0.055 mm, n=9).
Habitus. Body form (Fig. 28) slightly convex, moderately elongate (WE/SBL 0.38±0.13), head of normal proportions for genus (WH/WPm 0.77±0.014), pronotum narrow compared to elytra (WPm/WE 0.74±0.020).
Color. Body monocolorous, rufotestaceous, appendages testaceous.
Microsculpture. Partially effaced on disc of pronotum.
Prothorax. Pronotum (Fig. 33) relatively short (LP/LE 0.40±0.009) and slightly transverse (WPm/LP 1.23±0.031), with margins rectilinear and distinctly constricted posteriorly (WPm/WPp 1.36±0.039). Basal margin oblique laterally. Contour of posterior angles obtuse (116–122°) with 1–2 small denticles in front of the angles.
Elytra. Slightly convex, not depressed along suture, rather narrow (WE/LE 0.66±0.023). Margins almost subparallel, slightly divergent in basal half, evenly rounded to apex in apical third, maximal width of elytra posterior to midpoint.
Legs. 1st male protarsomere not dilated, without adhesive vestiture (Fig. 23).
Male genitalia. Median lobe of aedeagus (Fig. 54), with very narrow, elongate apex. Dorsal plate 1 short, pointed basally, with apical attenuation of moderate length. Dorsal sclerite 2 in a form of a separate structure, crosses plate 1 at apical third. Ventral sclerites slightly sclerotized. Right paramere rather long and moderately wide (Fig. 56). Left paramere without apical constriction (Fig. 55). Ring sclerite with long handle-like extension, widely rounded apically (Fig. 66).
Female genitalia. Gonocoxite 2 comparatively short, with moderately curved blade and rounded apex (Fig. 70). Laterotergite with 5-6 setae. Spermatheca atypical for genus (Fig. 75).
The species is known only from the type locality in the Sierra Aloapaneca Range, a part of the Sierra Madre de Oaxaca (Figs 77 and 94, black circle).
All beetles were collected at an elevation of 2600 m.
Externally, adults of Zapotecanillus kavanaughi are similar to those of Zapotecanillus iviei, Zapotecanillus pecki and Zapotecanillus montanus, described below, but males and females differ from those of these species in features of the median lobe and shape of the spermatheca, respectively. See also Fig. 90 for cladistic affinities.
http://zoobank.org/805794A0-A363-4C93-9041-C40DDBB9675D
http://species-id.net/wiki/Zapotecanillus_montanus
Figs 29, 48–50, 65, 77, 90, 94HOLOTYPE, male, in CMNH, point-mounted, labeled: \ MEXICO, Oaxaca, 52miles N of Oaxaca, Ber.202, sink litter, 17 May 1971 S.B.Peck collector\ CMNH \. PARATYPES (14 ex., 3♂4♀ were dissected), 6 ex. labeled same as a holotype; one female labeled: \ MEX. Oax., 52mi. N Oaxaca, 9500’ 17.V.71 S.Peck Ber.202, leaf lit.\ Anillinus (handwriting)\; 7 ex. labeled: \MEXICO: Oaxaca, 52miles N Oaxaca, 9500 ft., 25 May 1971, ex. litter in sinkhole, S.Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\ (deposited in CAS, CMNH).
The specific epithet is a Latin adjective from mons (= mountain), in the masculine form, meaning mountain-dwelling, and refers to the altitudinal data of the species locality.
Mexico, Oaxaca, 52 miles N of Oaxaca.
Recognition. Males of this new species are distinguished from those of other species of the genus by the combination of elongate habitus and shape of the median lobe (Fig. 48).
Size. Medium-sized for genus (SBL range 1.29–1.40 mm, mean 1.35±0.037 mm, n=7).
Habitus. Body form (Fig. 29) slightly convex, moderately elongate (WE/SBL 0.40±0.10), head of normal proportions for genus (WH/WPm 0.75±0.041), pronotum narrow compared to elytra (WPm/WE 0.70±0.012).
Color. Body monocolorous, rufotestaceous, appendages testaceous.
Microsculpture. Partially effaced on disc of pronotum.
Prothorax. Pronotum relatively short (LP/LE 0.38±0.009) and moderately transverse (WPm/LP 1.26±0.028), with margins rectilinear and markedly constricted posteriorly (WPm/WPp 1.40±0.028). Basal margin oblique laterally. Contour of posterior angles obtuse (115–125°) with 1–2 small denticles in front of the angles.
Elytra. Convex, not depressed along suture, of moderate width (WE/LE 0.69±0.016). Margins subparallel at middle, slightly divergent in basal third, evenly rounded to apex in apical third, maximal width of elytra at midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally.
Male genitalia. Median lobe of aedeagus (Fig. 48) with slightly elongate apex, angulately rounded at tip. Dorsal plate 1 short, pointed apically and basally. Dorsal plate 2 joined to plate 1 at its middle ventrally, where it forms a distinct protuberance. Ventral sclerites with pronounced sclerotization ventrally. Right paramere short and moderately wide (Fig. 50). Left paramere without apical constriction (Fig. 49). Ring sclerite with handle conically rounded apically (Fig. 65).
Female genitalia. Spermatheca standard for genus.
The species is known only from the type locality in the Sierra Juárez Range, a part of the Sierra Madre de Oaxaca (Figs 77 and 94, white quadrangle).
Specimens of this species were collected at 2900–3000m, which is the highest locality known among the Zapotecanillus species. The collection site was located in a limestone area with sinkholes and karst topography, covered with a pine-oak forest. Soil temperature at the time of collection was 48°F (S.Peck, pers. comm.).
Externally, adults of Zapotecanillus montanus are similar to those of Zapotecanillus kavanaughi, Zapotecanillus iviei and Zapotecanillus pecki, but males of Zapotecanillus montanus may be distinguished from those of the other species by the shape of the median lobe (Fig. 48). See also Fig. 90 for cladistic affinities.
http://zoobank.org/AFF220F9-B257-44DF-8A08-F07F9E6988F3
http://species-id.net/wiki/Zapotecanillus_pecki
Figs 30, 35, 51–53, 64, 77, 90, 94HOLOTYPE, male, in CMNH, point-mounted, labeled: \ MEXICO, Oaxaca, 3.5miles S of Suchixtepec, \ Ber.208, leaf litter, 3 June 1971 S.B. Peck collector\ CMNH\. PARATYPES (16 ex., 4♂2♀ were dissected), 6 ex. labeled same as a holotype; 6 ex. labeled: \ MEXICO, Oaxaca, 13 mi. N of Suchixtepec, 9500ft., ex. leaf litter, 4 June 1971, S. Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\; 4 ex. labeled: MEXICO, Oaxaca, 13.5 mi. S of Suchixtepec, 8000ft., ex. leaf litter, 3 June 1971, S. Peck\ THOMAS C. BARR COLLECTION 2011 Acc. No. 38014\ (deposited in CAS, CMNH).
The specific epithet is a Latinized eponym in the genitive case, and is based on the surname of Stewart B. Peck, Professor in the Biology Department of Carleton University, Ottawa, Canada, the collector of the type series of this species.
Mexico, Oaxaca, 3.5miles S of Suchixtepec.
Males of this new species are distinguished from those of other species of the genus by the shape of the median lobe (Fig. 51).
Size. Medium-sized for genus (SBL range 1.32-1.38 mm, mean 1.35±0.026 mm, n=5).
Habitus. Body form (Fig. 30) slightly convex, moderately elongate (WE/SBL 0.41±0.08), head of normal proportions for genus (WH/WPm 0.73±0.011), pronotum narrow compared to elytra (WPm/WE 0.72±0.009).
Color. Body monocolorous, rufotestaceous, appendages testaceous.
Microsculpture. Microlines partially effaced on disc of pronotum.
Prothorax. Pronotum (Fig. 35) relatively short (LP/LE 0.41±0.012) and slightly transverse (WPm/LP 1.24±0.030), with margins slightly sinuate and distinctly constricted posteriorly (WPm/WPp 1.36±0.043). Basal margin bisinuate near posterior angles. Contour of posterior angles slightly obtuse (108–118°) with 1-2 small denticles in front of the angles.
Elytra. Slightly convex, not depressed along suture, of moderate width (WE/LE 0.70±0.022). Margins subparallel at middle, slightly divergent in basal half, evenly rounded to apex in apical half, maximal width of elytra at midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally.
Male genitalia. Median lobe of aedeagus (Fig. 51), with elongate apex, rounded at tip. Dorsal plate 1 long, pointed apically and basally. Dorsal plate 2 joined to plate 1 at its apical third, where it forms a pronounced biapical protuberance. Ventral sclerite faintly sclerotized, barely visible. Right paramere rather long and moderately wide, with additional (3rd) seta dorsally (Fig. 53). Left paramere without apical constriction (Fig. 52). Ring sclerite with short handle, widely rounded apically (Fig. 64).
Female genitalia. Spermatheca standard for genus.
The species is known only from the type locality in the Sierra Madre del Sur, in the surroundings of Suchixtepec (Figs 77 and 94, black flower).
Members of this species live at elevations of 8000-9500’ (2440-2900 m). At 8000’ (= 2440 m), beetles were collected in mixed pine-oak forest with Alnus, Carpinus, etc, and soil temperature at the time of collection was 56°F (S. B. Peck, pers. comm.).
Males of this species are easily distinguished from those of other members of the genus by the structure of the median lobe (Fig. 51) and setation of the right paramere (Fig. 53); and the geographical distribution of this species sets it apart from all its congeners. See also Fig. 90 for cladistic affinities.
http://zoobank.org/778A7293-CE0E-4EBB-B2E2-AEE3CB9857B2
http://species-id.net/wiki/Zapotecanillus_longinoi
Figs 31, 57–59, 67, 71, 76, 77, 90, 94HOLOTYPE, male, in KUNHM, point-mounted, labeled: \ MEXICO: Chiapas: Sierra Morena 16.16001°N, 93.60519°W, 1360m, 12-V-2008, ex. sifted leaf litter, 2° mesophil forest LLAMA08 Wa-A-01-1-all \ SM0833744 KUNHM-ENT \. PARATYPES (16 ex., 2♂3♀ were dissected), 9 ex. labeled same as a holotype, except barcode SM... numbers; 1 ex. labeled: \ MEXICO: Chiapas: Sierra Morena 16.15950°N, 93.60530°W, 1360m, 12-V-2008 sifted leaf litter, 2° mesophil forest LLAMA08 Wm-A-01-1 \ SM0839196 KUNHM-ENT \; 4 ex. labeled: \ MEXICO: Chiapas: Sierra Morena 16.15342°N, 93.60078°W, 1330m, 12-V-2008 ex. sifted leaf litter, 2° mesophil forest LLAMA08 Wa-A-01-2-all \ SM... KUNHM-ENT \; 2 ex. labeled: \ MEXICO: Chiapas, Mpio: Villa Corso, Ejido Sierra Morena, R. Biosfera La Sepultura, 16°09'10.6N, 93°35'25.1W, 1550m, 17–18.VII.2003, R. Anderson, mixed oak/pine forest litter, MEX1A03 110 \ SM... KUNHM-ENT \ (deposited in CAS, KUNHM).
The specific epithet is a Latinized eponym in the genitive case, and is based on the surname of John T. (Jack) Longino, Professor of the Biology Department of the University of Utah, and one of Co-PI’s of the LLAMA project, which provided the material on which the description of this species is based.
Mexico, Chiapas, Sierra Morena, 16.16001°N, 93.60519°W.
Adults of this new species can be distinguished from those of other species of the genus by the combination of small size and rufotestaceous color; and males can be further distinguished by the shape of the median lobe (Fig. 57).
Size. Small sized for genus (SBL range 1.01-1.12 mm, mean 1.08±0.038 mm, n=12).
Habitus. Body form (Fig. 31) slightly convex, slightly elongate (WE/SBL 0.40±0.10), head of normal proportions for genus (WH/WPm 0.76±0.021), pronotum moderately wide compared to elytra (WPm/WE 0.76±0.014).
Color. Body monocolorous, rufotestaceous, appendages testaceous.
Microsculpture. Partially effaced on disc of pronotum.
Prothorax. Pronotum relatively short (LP/LE 0.41±0.010) and moderately transverse (WPm/LP 1.28±0.035), with margins rectilinear and moderately constricted posteriorly (WPm/WPp 1.35±0.037). Basal margin oblique laterally. Contour of posterior angles obtuse (114–125°) with 0-1 small denticles in front of the angles.
Elytra. Slightly convex, not depressed along suture, of moderate width (WE/LE 0.69±0.015). Margins nearly subparallel, slightly divergent in basal forth, evenly rounded to apex in apical third, maximal width of elytra near midpoint.
Legs. 1st male protarsomere markedly dilated apico-laterally.
Male genitalia. Median lobe of aedeagus (Fig. 57), with enlarged apex and neighboring part of ventral margin. Dorsal plate 1 small, pointed apically and narrowly rounded basally. Dorsal plate 2 located close to plate 1, in form of narrow stylet and shifted slightly apically. Ventral sclerites faintly sclerotized. Right paramere short and moderately wide (Fig. 59). Left paramere as in Fig. 58. Ring sclerite with short handle widely rounded apically (Fig. 67).
Female genitalia. Gonocoxite 2 comparatively short, with moderately curved blade and narrowly rounded apex (Fig. 71). Laterotergite with 7-8 setae. Spermatheca standard for genus (Fig. 76).
The species is known only from the type locality in the Sierra Madre de Chiapas (Figs 77 and 94, black triangles).
According to the label data, these beetles inhabit mesophyll and mixed oak/pine forests at low elevations.
In the structure of median lobe of males and geographical distribution, this species is only remotely related to its congeners. See also Fig. 90 for cladistic affinities.
Fig. 77
Material. MEXICO: Chiapas: 4km SE Custepec 15.71018°N, 92.92887°W, 2140m, 20-V-2008 ex. sifted leaf litter, cloud forest LLAMA08 Wa-A-03-1-all \ SM0832667 KUNHM-ENT \ (1♂); MEXICO: Chiapas: 4km SE Custepec 15.70673°N 92.93127°W, 2125m, 20-V-2008 ex: sifted leaf litter, cloud forest LLAMA08 Wa-A-03-2-all \ SM0821667 KUNHM-ENT \ (1♀), both in CAS.
Among the materials at hands, these two teneral specimens remain unidentified because of insufficient material for investigation. They were collected in the cloud forest near Custepec in the Sierra Madre de Chiapas (Fig. 77, white star). Both specimens resemble Zapotecanillus longinoi adults externally but are larger in size, and cannot be identified unambiguously. This locality represents the most southern point of the known range of Zapotecanillus.
Adults of the eight described species of this new genus are distinguished using the following key:
| 1 | Small (1.00–1.20 mm in length). Sierra Madre de Chiapas and Sierra Madre de Oaxaca within Sierra de Juárez | 2 |
| – | Large (greater than 1.25 mm in length). Sierra Madre de Oaxaca and Sierra Madre del Sur | 3 |
| 2 | Darker, brunneorufous (Fig. 25). Apex of median lobe unmodified (Fig. 39). Dorsal plate 1 larger (Fig. 39). Sierra Madre de Oaxaca | Zapotecanillus nanus |
| – | Lighter, rufotestaceous (Fig. 31). Apex of median lobe enlarged (Fig. 57). Dorsal plate 1 smaller (Fig. 57). Sierra Madre de Chiapas | Zapotecanillus longinoi |
| 3 | Less robust and more elongate, body monocolorous, either brunneorufous (Fig. 26) or rufotestaceous (Figs 27–30). Pronotum narrower (WPm/LP < 1.30). Pronotal basal margin oblique (Figs 33–34) or sinuous laterally (Fig. 35) | 4 |
| – | More convex and robust, body with brunneorufous pronotum and rufotestaceous elytra (Fig. 24). Pronotum wider, distinctly transverse (WPm/LP 1.33±0.022). Pronotal basal margin straight (Fig. 32). Sierra de Juárez | Zapotecanillus oaxacanus |
| 4 | Rufotestaceous beetles. Apex of median lobe elongate and narrow (Figs 45; 48, 51, 54). Dorsal plate 1 with long apical attenuation (Fig. 45), or pointed basally (Figs 48, 51, 54). Sierra Madre del Sur and Sierra Madre de Oaxaca within Sierra de Juárez and Sierra Aloapaneca | 5 |
| – | Brunneorufous beetles. Apex of median lobe short and broadly rounded (Fig. 42). Dorsal plate 1 long, rounded basally and with short apical attenuation (Fig. 42). Sierra de Juárez | Zapotecanillus ixtlanus |
| 5 | Pronotal basal margin bisinuate laterally (Fig. 35). Dorsal plate 2 enlarged basally in form of biapical protuberance (Fig. 51). Sierra Madre del Sur | Zapotecanillus pecki |
| – | Pronotal basal margin oblique laterally (Figs 33–34). Dorsal plate 2 of another shape. Sierra Madre de Oaxaca | 6 |
| 6 | Median lobe with elongate apex of normal size (Figs 45; 48). Spermatheca ball-shaped (Fig. 74). Sierra de Juárez | 7 |
| – | Median lobe with small and narrow apex (Fig. 54). Spermatheca fusiform with apical bulb enlargement (Fig. 75). Sierra Aloapaneca | Zapotecanillus kavanaughi |
| 7 | Pronotal baso-lateral margin with weak sinuation before posterior angles (Fig. 34). Dorsal plate 1 greater, rounded basally and with long apical attenuation (Fig. 45). Cerro Pelon, Sierra de Juárez | Zapotecanillus iviei |
| – | Pronotal baso-lateral margin rectilinear constricted towards posterior angles (as on Fig. 33). Dorsal plate 1 small, pointed basally and with rather short apical attenuation (Fig. 48). Sierra de Juárez to the South from Cerro Pelon | Zapotecanillus montanus |
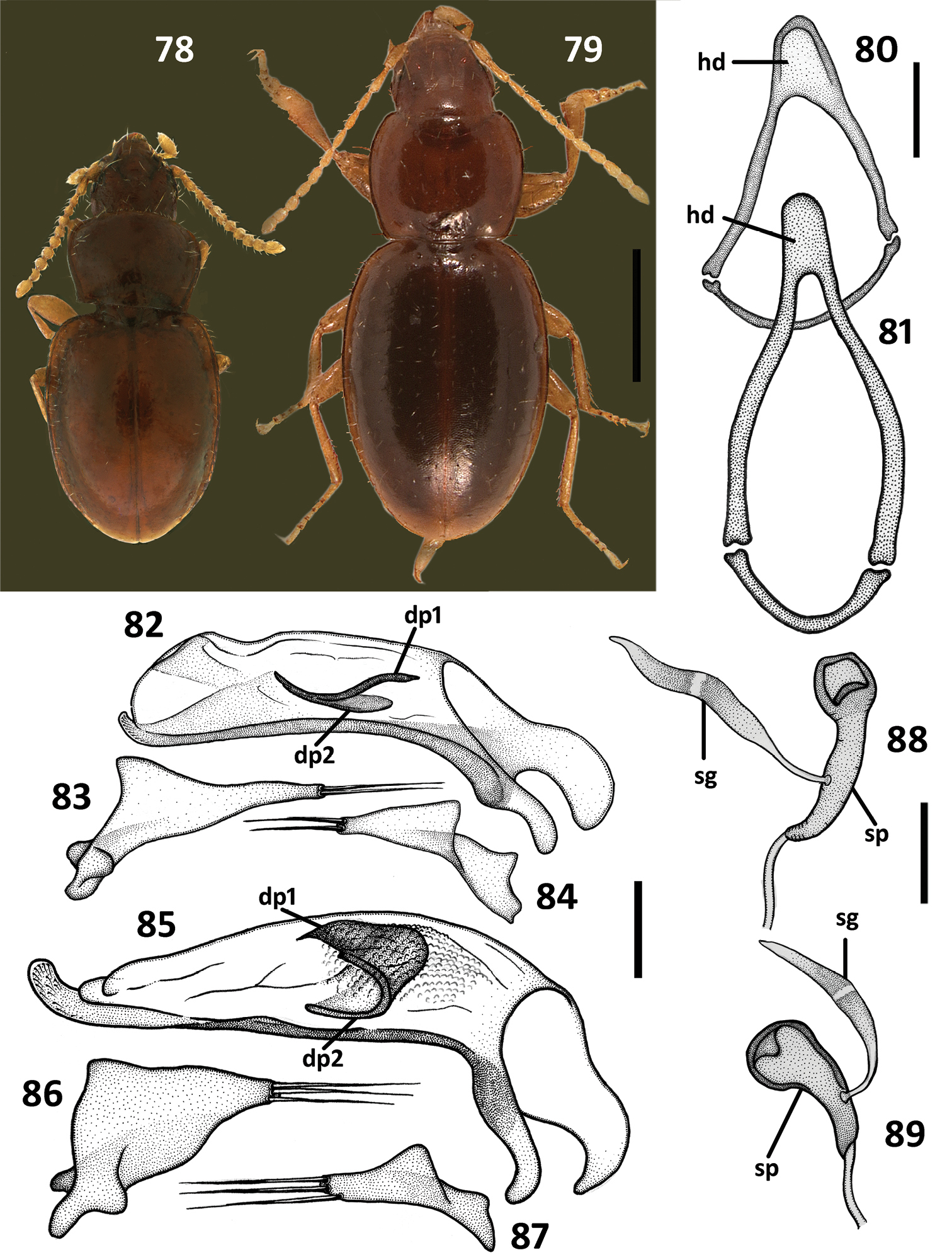
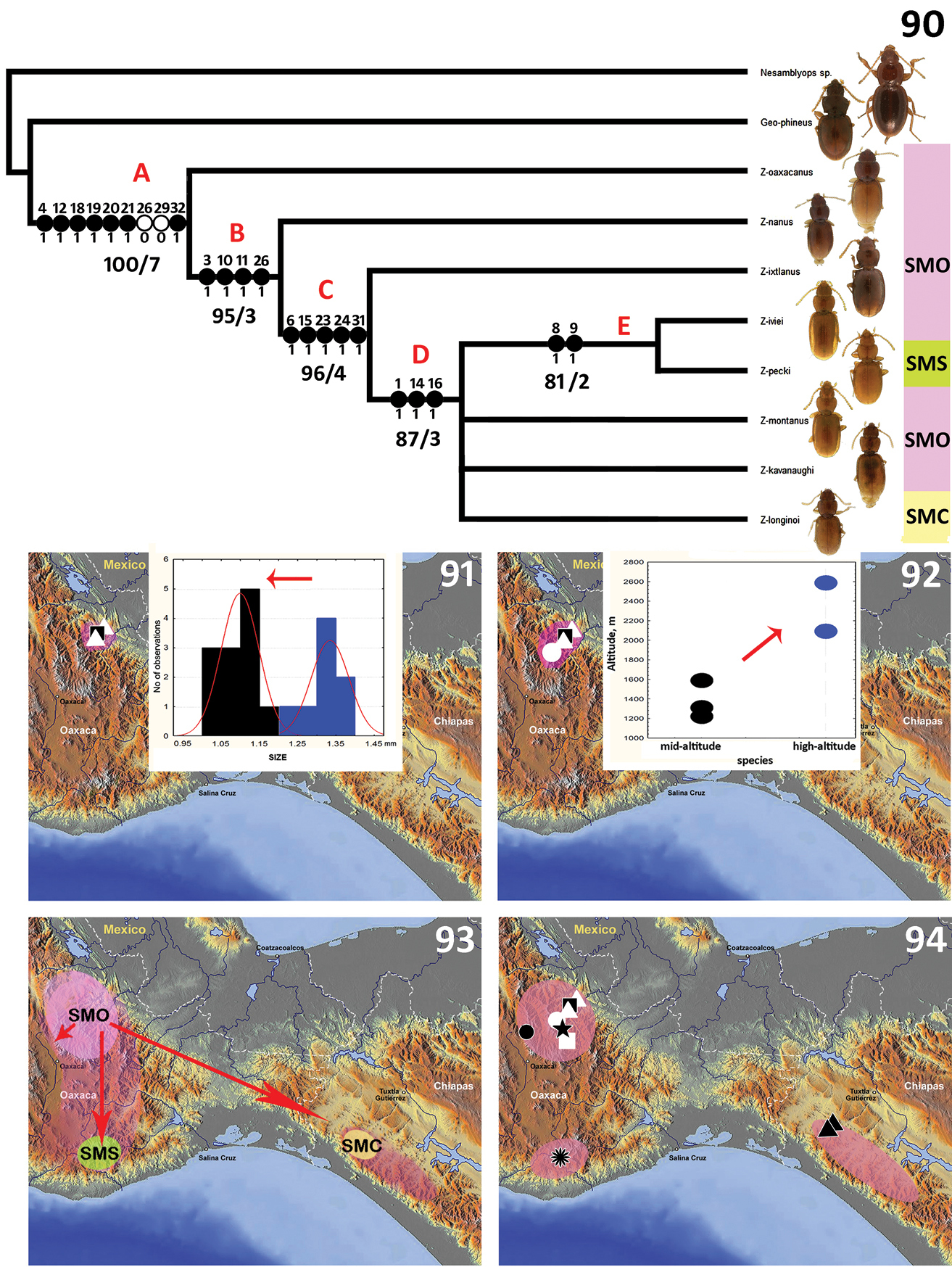
The parsimony analysis resulted in two most parsimonious trees (L=53; CI=0.74; RI=0.76); the 75% majority-rule consensus cladogram of these trees is presented in Fig. 90, with the characters and support values mapped on the corresponding internal branches. The main basal nodes of the cladogram are highly supported by Bootstrap and Bremer indices, whereas a part of the terminal nodes is inadequately supported, which results in collapsed branches. The Zapotecanillus species form a well-supported monophyletic group (clade A, bootstrap value=100). Their monophyly is supported by the derived states for characters 4 (labial mental suture), 12 (additional apicolateral pronotal setae), 18 (positions of 7, 8, and 9 pores of umbilicate series), 19 (elytral subapical sinuation), 20 (shape of the intercoxal process of the abdomen), 21 (shape of the metendoventrite) and 32 (shape of the spermatheca). Within the genus, a basal clade is presented by Zapotecanillus oaxacanus, which is morphologically the closest species to the outgroup taxa (the latter selected from the litter species of anilline genera Geocharidius and Nesamblyops, Figs 78 and 79, respectively). Clade B is characterized by few traits, highlighting changes in the species’ appearance – notably the proportional reduction in the size of pronotum (character 3) and the shifting of the pronotal hind angles in a forward direction (characters 10 and 11); also the apex of median lobe is getting smaller (character 26). Clade C includes species with derived shared characters, which intensify the habitual dissimilarity with outgroup taxa. The pronotum in members of these species is proportionally shorter (character 6) and the elytra are narrower (character 15); also, internal parts of the male genitalia, namely the ring sclerite (characters 23 and 24), and parameres (character 31), are reduced. Clade D includes the species that are most unlike the basal taxa externally. Members of species in this clade have slightly convex bodies (character 1) and are completely yellow in color (characters 14 and 16). Members of Clade E species share a narrow pronotum with a shallow sinuation of the lateral margins anterior to the hind angles (characters 8 and 9). Thus, the cladogram of species’ relationships primarily reflects the gradual changes in external characteristics from basal to terminal clades, incorporating some changes in genitalic structures. The trend in changes in external form on the cladogram (from ovoid and pigmented towards elongate and depigmented beetles) reflects evolutionary adaptations for a more endogean way of life.
Structural features of the litter anilline species, chosen as an outgroup for the cladistic analysis of Zapotecanillus species. Geocharidius phineus Erwin 78 habitus 80 ring sclerite, dorsal aspect 82 median lobe, right lateral aspect 83 left paramere, left lateral aspect 84 right paramere, right lateral aspect 88 spermatheca. Nesamblyops sp. 79 habitus 81 ring sclerite, dorsal aspect 85 median lobe, right lateral aspect 86 left paramere, left lateral aspect 87 right paramere, right lateral aspect 89 spermatheca. dp1 – dorsal plate 1; dp2 – dorsal plate 2; hd – handle of round sclerite; sg – spermathecal gland; sp – spermatheca. Scale bars = 0.5mm (Figs 78–79), 0.1mm (Figs 80–81), 0.05mm (Figs 82–89).
Cladistic relationships and main events of the natural history of Zapotecanillus species, inferred from the cladogram. 90 75%-majority rule cladogram from the parsimony analysis: character states are shown only for nodes, forward changes as filled circles, and reversals as open circles; numbers under internal branches – indicate bootstrap/Bremer support indices; the letters B, C, and D above the nodes correspond to the maps 91, 92 and 93 below, illustrating evolutionary trends at the appropriate node; bar abbreviations, SMO – Sierra Madre de Oaxaca, SMS – Sierra Madre del Sur, SMC – Sierra Madre de Chiapas 91 syntopic miniaturization, blue bars – Zapotecanillus oaxacanus, black bars – Zapotecanillus nanus 92 altitudinal expansion, blue dots – Zapotecanillus ixtlanus, black dots – Zapotecanillus oaxacanus + Zapotecanillus nanus 93 regional dispersal, SMO – Sierra Madre de Oaxaca, SMS – Sierra Madre del Sur, SMC – Sierra Madre de Chiapas 94 modern distribution of species, Zapotecanillus oaxacanus – black quadrangle; Zapotecanillus nanus – white triangles; Zapotecanillus ixtlanus – white circle; Zapotecanillus iviei – black star; Zapotecanillus kavanaughi – black circle; Zapotecanillus montanus – white quadrangle; Zapotecanillus pecki – black flower; Zapotecanillus longinoi – black triangles.
New data enable us to discuss several taxonomical and evolutionary issues, despite the limited material available for Zapotecanillus.
Remarks on Geocharidius larva. Ten years ago, a description of the first-instar larva of Geocharidius was published (
Notes about Zapotecanillus. As previously mentioned, it is difficult to assess relationships of the new genus to the other North and Central American Anillina without a modern revision of the latter. Therefore, the conclusions drawn below should be treated as preliminary and speculative.
Externally, the absence of discal setae is a feature that members of this new genus share with those of the southern stock of genera, like Geocharidius, Mexanillus and Honduranillus Zaballos (
To confirm or reject the proposed phylogenetic relationship of Zapotecanillus within the North and Central American Anillina, the regional fauna requires further investigation, including analyses of additional morphological and, hopefully, molecular data.
Although cladistic analysis does not allow us to fully resolve phylogenetic relationships, some evolutional trends of Zapotecanillus, evident from the resulting cladogram, are worth examining.
Deviations from the form of the pronotum of litter species, such as the reduction in the overall size of the pronotum and forward shift of the hind angles (Fig. 90, clade B), reflect an increase in flexibility of the pronotal-elytral joint. A more flexible joint can potentially expand the number of accessible niches, enabling their bearers to live in a greater number of structurally different litter or soil interspace habitats. It seems that, among the litter-dwelling ancestors of Zapotecanillus, adaptations for living in a new environment were restricted by two major directions of species evolution.
The first direction, a syntopic habitat expansion, can be characterized as the intensification of local litter resource exploitation, presumably by means of niche differentiation. The structural complexity of the litter, undergoing decomposition, produces a graded series of overlapping planes interspersed with intertwined gaps, both of which tend to become smaller as one travels downwards toward the soil (
The second direction of evolution among Zapotecanillus species was connected with the altitudinal expansion of the genus and subsequent adaptations to the endogean way of life. In Clade C (Fig. 90), pronotal and elytral morphology have undergone additional modifications. Most of the species of this clade live at elevations above 2400m, as exemplified by Zapotecanillus ixtlanus (Fig. 92). The ability to live at high elevation implies adaptations to withstand daily and seasonal variations in temperature and humidity. One solution to this challenge is the acquisition of adaptations that facilitate vertical migrations, from the litter down into the soil and back to the litter again to track favorable and escape from unacceptable microclimatic shifts (e.g., regular frosts at high elevations of the Sierra de Juárez). Also, changes in forest communities along the elevation gradient can play an additional role in the evolution of species adaptations. In the Sierra Madre de Oaxaca, the humid montane cloud forests at elevations of 1200–1600m are characterized by the dominance of broadleaf tree species, such as Quercus, Liquidambar, Carpinus, and Fagus (
A review of the overall distribution of genus Zapotecanillus shows that species with endogean lifestyles are distributed across the whole range of the genus (Fig. 90, SMS, SMO, SMC), while litter-dwelling species are restricted to only the eastern slopes of the Sierra Madre de Oaxaca (Fig. 90, SMO). A priori, one might expect that litter-dwelling forms would be more broadly distributed than endogean forms, but within Zapotecanillus, this does not appear to be so. Perhaps additional litter-dwelling species of the genus remain undiscovered or have gone extinct in this region, but the extensive overall distribution of endogean species clearly suggests a role for them in the expansion and the shape of the modern geographical range of the genus. For instance, in the Sierra Madre de Chiapas, adults of Zapotecanillus longinoi and Zapotecanillus sp., both of the endogean morphological type, were collected at altitudes of 1330m to 2140m, which are approximately the same elevations at which low-altitude Zapotecanillus oaxacanus and Zapotecanillus nanus were collected in the Sierra de Juárez (Sierra Madre de Oaxaca). If a litter-dwelling ancestral Zapotecanillus species had dispersed from the Sierra Madre de Oaxaca to the Sierra Madre de Chiapas, one would expect members of the Chiapan descendant species to be similar in life-style and appearance to those of the Oaxacan ancestral form because low elevations are primarily occupied by litter species. However, adults of Zapotecanillus longinoi and Zapotecanillus sp., are flattened and depigmented, and quite different from the convex and pigmented litter-dwelling Zapotecanillus forms, as well as from litter-dwelling Geocharidius species. It is perhaps significant that Zapotecanillus longinoi and Zapotecanillus sp. members are syntopic with litter-dwelling species of Geocharidius in the Sierra Madre de Chiapas. It may be that the presence of two species of Zapotecanillus with endogean morphology at low elevations in the Sierra Madre de Chiapas represents a secondary occupation of the region by endogean forms, thus, supporting the idea that at least some endogean Zapotecanillus forms are capable of significant dispersal.
If we consider that the endogean way of life of the Zapotecanillus species was triggered by changes in microclimate parameters, then regional dispersal of the depigmented and only slightly convex Zapotecanillus species also could have been connected with certain climate changes. Such dispersal likely occurred during one or more of the Pleistocene glacial cycles, which enabled species with endogean life-styles to cross the Isthmus of Tehuantepec, and, perhaps, the Central Valleys of Oaxaca, and to establish populations in the Sierra Madre de Chiapas and the Sierra Madre del Sur, respectively (Fig. 93). Evidence that the Isthmus served as a corridor connecting Oaxaca and Chiapas Sierras during Pleistocene glaciations has been shown for some bird species (
The described sequence of events and exact mountain regions where the evolution and differentiation of Zapotecanillus took place are still debatable, given the paucity of data about the distribution and diversity of the genus and, thus, should be the subject of further investigations. For instance, the fauna of anillines is still unknown for such biogeographically important mountain ranges in Oaxaca as the Sierra de Zempoaltepec and the Sierra de Mijes. It is also still unknown how far to the north and west across the Oaxacan highlands Zapotecanillus species are distributed. These and many other questions await further investigation and discoveries.
I am greatly indebted to David H. Kavanaugh (Senior Curator of Entomology, California Academy of Sciences) for his efforts to accumulate all available material on Mesoamerican Anillina at the California Academy of Sciences and making it available for the treatment. Important loans were received from the University of Kansas Natural History Museum, Carnegie Museum of Natural History, Montana Entomology Collection and the U.S. National Museum of Natural History, through the help of Zachary H. Falin (Collections Manager), Robert L. Davidson (Collections Manager), Michael A. Ivie (Curator) and Terry L. Erwin (Curator) at those institutions, respectively. This paper is based in part on material obtained through sampling supported by the National Science Foundation through grant DEB-0640015 (J.T. Longino, R.S. Anderson, P.S. Ward, Principal Investigators). I thank the staff of Louisiana State Arthropod Museum, LSU, Baton Rouge, and the Director of that Museum, Christopher E. Carlton, personally for his diversified help, and especially the Louisiana State Arthropod Museum for permission to use the Museum’s equipment, without which most of the illustrations provided here could not have been made. I thank Jean DeMouthe (Geology Collections Manager, California Academy of Sciences, San Francisco, CA) for her assistance in creating many essential digital images for several species. I also appreciate help with SEM imaging provided by the staff of Microscopy Center at LSU School of Veterinary Medicine (Baton Rouge, LA) and by Scott Serrata from the SEM laboratory of the California Academy of Sciences (San Francisco, CA). I’m very thankful to Stewart B. Peck (Carleton University, Ottawa, Canada) for sharing with me the information about the collection data for Zapotecanillus montanus and Zapotecanillus pecki. I acknowledge Schlinger Postdoctoral Foundation for the fellowship at the California Academy of Sciences in 2012-2014. Finally, I am grateful to David H. Kavanaugh (California Academy of Sciences) for reviewing drafts of this manuscript and providing valuable suggestions for language improvement, and the anonymous reviewer for important editorial comments.
Morphological characters and states used for cladistic analysis.
| External characters | |
| 1. | Body shape. 0, moderately convex (Figs 24–26, 78–79); 1, slightly convex (Figs 27–31). |
| 2. | Standardized body length. 0, larger, > 1.20mm; 1, smaller, <1.20mm. |
| 3. | Relative head width, ratio WH/WPm. 0, head narrower, with ratio <0.70; 1, head wider, with ratio > 0.71. |
| 4. | Labial mental suture. 0, present (Fig. 8); 1, absent (Fig. 7). |
| 5. | Paraglossae of ligula. 0, present (Fig. 7); 1, absent (Fig. 8). |
| 6. | Relative pronotal length, ratio LP/LE. 0, pronotum longer, ratio > 0.42; 1, pronotum shorter, ratio < 0.42. |
| 7. | Relative pronotal width, ratio WPm/WE. 0, pronotum wider, ratio > 0.80; 1, pronotum narrower, ratio < 0.78. |
| 8. | Pronotal width, ratio WPm/LP. 0, more transverse, ratio > 1.25; 1, less transverse, ratio < 1.25. |
| 9. | Pronotal basolateral sinuation. 0, absent (Figs 32–33); 1, shallow (Figs 34–35). |
| 10. | Pronotal basal margin. 0, straight (Fig. 32); 1, laterally oblique (Figs 33–34); 2, laterally sinuous (Fig. 35). |
| 11. | Pronotal hind angles. 0, nearly rectangular, <110⁰ (Figs 32, 35); 1, slightly obtuse, >115⁰ (Figs 33–34). |
| 12. | Additional apicolateral pronotal setae. 0, absent (Fig. 2); 1, present (Fig. 1). |
| 13. | Pronotal discal microsculpture. 0, with distinct mesh microsculpture; 1, with obsolete microsculpture, at most represented by fine parallel lines without mesh formation. |
| 14. | Pronotal coloration. 0, brown; 1, yellowish. |
| 15. | Elytral width, ratio WE/LE. 0, wider, ratio >0.71; 1, narrower, ratio <0.71. |
| 16. | Elytral coloration. 0, brown; 1, yellowish. |
| 17. | Discal pores on elytra. 0, present; 1, absent. |
| 18. | Position of 7, 8, and 9 pores of umbilicate series. 0, equidistant (Fig. 4); 1, 8 and 9 pores close to each other than to 7 (Fig. 3). |
| 19. | Elytral preapical sinuation. 0, elytra evenly tapered (Fig. 4); 1, elytra slightly sinuate subapically (Fig. 3). |
| 20. | Intercoxal process of 2nd ventrite. 0, triangular (Fig. 14); 1, apically truncated (Fig. 13). |
| 21. | Shape of metendosternite. 0, simple, without “arms” (Fig. 14); 1, cross-shaped, with long lateral “arms” (Fig. 13). |
| 22. | Male protarsomere. 0, noticeably expanded, with modified setae (Figs 20–21); 1, barely expanded, with modified setae (Fig. 22); 2, not expanded, without modified setae (Fig. 23). |
| Male genitalic characters | |
| 23. | Length of a handle of ring sclerite. 0, long (Figs 60–61, 66, 80–81), 1, short (Figs 62–65, 67). |
| 24. | Shape of a handle of ring sclerite. 0, subparallel (Figs 60–61, 66, 80–81); 1, conical (Figs 62–65, 67). |
| 25. | Median lobe shape. 0, bent dorsad from basal opening (Figs 36, 39, 42, 45, 48, 51, 85); 1, bent at basal opening (Figs 54, 57, 82). |
| 26. | Contour of the apex of median lobe, a lateral view. 0, straight, short, of moderate width (Figs 36, 42, 57); 1, straight, slightly elongated, of moderate width (Figs 39, 45, 48, 51); 2, straight, elongated and very narrow (Fig. 54); 3, upcurved, elongated and narrow (Figs 82, 85). |
| 27. | Modification of neighboring parts of apex of median lobe. 0, absent (Figs 36, 39, 42, 45, 48, 51, 54, 82, 85); 1, enlarged ventrally (Fig. 57). |
| 28. | State of dorsal plate 1, a lateral view. 0, long plate with rounded basal part (Figs 36, 39, 42, 45); 1, short plate with rounded basal part (Fig. 57); 2, long plate with pointed basal part (Fig. 51); 3, short plate with pointed basal part (Figs 48, 54); 4, long and curved stylet-shaped plate (Fig. 82); 5, triangular plate (Fig. 85). |
| 29. | State of dorsal plate 2, a lateral view. 0, adjoined to dorsal plate 1, like a small knob-like protuberance (Figs 36, 39, 42, 45, 48); 1, adjoined to dorsal plate 1, like a large biapical protuberance (Fig. 51); 2, like a separate structure either crossing or parallel to dorsal plate 1 (Figs 54, 57); 3, attached to dorsal sclerite 1 in form of a long appendix (Figs 82, 85). |
| 30. | Right paramere, seta number. 0, 3 setae (Figs 53, 87); 1, 2 setae (Figs 38, 41, 44, 47, 50, 56, 59, 84). |
| 31. | Left paramere, shape of apical third. 0, with attenuated apex (Figs 37, 40, 83, 86); 1, with evenly tapering sides to the tip (Figs 43, 46, 49, 52, 55, 58). |
| Female genitalic characters | |
| 32. | Spermatheca, shape. 0, elongated with apical bulb enlargement (Figs 75, 88–89); 1, spherical, ball-like (Figs 72–74, 76). |
Data matrix used for cladistic analysis.
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | |
| Nesamblyops sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 5 | 3 | 0 | 0 | 0 |
| Geocharidius phineus | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 0 | 4 | 3 | 1 | 0 | 0 |
| Zapotecanillus oaxacanus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Zapotecanillus nanus | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Zapotecanillus ixtlanus | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Zapotecanillus iviei | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Zapotecanillus montanus | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 1 | 1 | 1 |
| Zapotecanillus kavanaughi | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 2 | 0 | 3 | 2 | 1 | 1 | 0 |
| Zapotecanillus pecki | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 1 | 1 |
| Zapotecanillus longinoi | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 |