Citation: Albertoni FF, Krell F-T, Steiner J, Zillikens A (2014) Life history and description of larva and pupa of Platyphileurus felscheanus Ohaus, 1910, a scarabaeid feeding on bromeliad tissues in Brazil, to be excluded from Phileurini (Coleoptera, Scarabaeidae, Dynastinae). ZooKeys 389: 49–76. doi: 10.3897/zookeys.389.6888
The third instar larvae and the pupae of Platyphileurus felscheanus Ohaus, 1910 (Phileurini), recently synonymized with Surutu jelineki Endrődi, 1975 (Cyclocephalini), are described and illustrated, and some life history information is given. The larvae were collected and reared in bromeliads in rain forests of Santa Catarina state in southern Brazil. The systematic position of this monotypic genus is reassessed at the tribe level by considering larval and adult morphological characters. Both character sets, being described and illustrated, suggest the placement of Platyphileurus in the tribe Oryctini.
Surutu jelineki, Cyclocephalini, Bromeliaceae, beetle, third instar, dynastine tribe, classification
The rhinoceros beetle Platyphileurus felscheanus Ohaus, 1910 (Scarabaeidae: Dynastinae) was described twice as a new species, first under this name in the tribe Phileurini and 65 years later as Surutu jelineki by
The monotypic genus Platyphileurus Ohaus, 1910 is known only from Brazil (
Platyphileurus felscheanus can be recognized by its flat body, especially anteriorly, by the elytra being laterally dilated posteriorly, by lacking horns or tubercles on the head and pronotum, and by lacking a longitudinal furrow on the pronotum (Figs 26–28). With the anterior half of the body flatter than the posterior half and the flattened pronotum lacking a longitudinal furrow, Platyphileurus has an unusual appearance for a phileurine species. Its systematic position needs to be re-examined.
Here we describe the larva and pupa of Platyphileurus felscheanus repeatedly collected in bromeliad rosettes and reared to imagines, present data on their life history, and give new records on the occurrence of this bromelicolous species. We also explore whether characters of the immatures provide indications of the tribal placement of the genus.
Among the numerous insects recorded in bromeliad phytotelmata, beetles are typically represented by taxa with aquatic and semiaquatic habits. Larvae of Scirtidae are commonly found in bromeliad tanks (
Some few bromeliad associated species are Scarabaeidae (
Bromeliads were collected from five rain forest areas in Santa Catarina state, southern Brazil, specifically on Santa Catarina Island (municipality of Florianópolis). Two sites are secondary forest areas: 1) Unidade de Conservação Ambiental Desterro (UCAD) (27°31'52"S, 48°30'45"W), a 491 ha forest reserve of the Universidade Federal de Santa Catarina (
To sample the associated animals we collected 412 bromeliads of six species from March 2002 to March 2006: Nidularium innocentii Lem. (n=99), Aechmea lindenii (E. Morren) Baker (n=141), Aechmea nudicaulis Griseb. (n=61), Canistrum lindenii (Regel) Mez. (n=60), Vriesea vagans (L. B. Sm.) L. B. Sm. (n=39), and Hohenbergia augusta Mez. (n=12). Whole plants were cut off at the base and examined leaf by leaf in the laboratory (
All large beetle larvae and pupae found in the bromeliads were identified as scarabaeids. We prepared small rosettes of Nidularium innocentii from the innermost part of the plant (about 8–10 young leaves), washed with tap water to remove spiders and other predatory arthropods, or arranged freshly cut, clean bromeliad leaves to an artificial rosette in a funnel. Larvae and pupae were placed in the middle of the rosette and covered with 1–2 table spoons of humic leaf litter. The arrangement was covered with gauze and kept moist in the laboratory. Larvae were inspected about every second week to check their vitality and to replace the eaten up rosettes or leaves with new ones. The identification of Platyphileurus felscheanus was based on imagines obtained from these rearings.
In order to collect more specimens of Platyphileurus felscheanus and to learn about its life history, five additional bromeliads of the genera Aechmea Ruiz & Pav., Nidularium Lem. and Vriesea Beer. were collected between 2008 and 2012 in Florianópolis. In the field, we searched bromeliads for scarabaeid larvae. When a larva was present (n=6), the bromeliad was taken to the laboratory where it was kept upright in a plastic bucket. During the first days or weeks after collection the larvae were left in the bromeliads to observe their behavior. Thereafter, some bromeliad leaves were tied together in small rosettes and each larva was placed in the middle of this artificial rosette which were maintained in plastic pots (n=5). One larva was maintained in the original bromeliad until pupation. They were checked one to three times per week.
A cladistics analysis at tribal or generic level is beyond the scope of this paper. In full consideration that the current tribal classification rests on entirely typological foundations, we apply consistently phylogenetic reasoning sensu
Platyphileurus felscheanus Ohaus, 1910
The larval description is based on four third instar larvae with the following data: BRAZIL, Santa Catarina: UCAD, Florianópolis city, in Canistrum lindenii, 18.ii.2002, J. Steiner leg. (DMNS ZE.15759); dto., in Hohenbergia augusta (plant no. 17), 15.iv.2002, A. Zillikens leg. (DMNS ZE.15761); dto., in Aechmea lindenii (plant no. 329), 27.iv.2004, A. Zillikens leg. (DMNS ZE.15760); Santo Antônio de Lisboa, Florianópolis, in Aechmea nudicaulis (plant no. 318), 24.iii.2004, A. Zillikens leg. (DMNS ZE.15758).
Further larval material from which additional measurements were taken: BRAZIL: São Paulo: 1 third instar (MZSP 010.247): Salesópolis, Estação Biológica de Boracéia, Atlantic rain forest, 23°32'S, 45°51'W, 4–12.ix.2008, S.A. Casari and M. Duarte (MZC-016-Entomologia de Campo) leg. [NEW STATE RECORD]. Santa Catarina: 1 larva fixed (MZSP 010.246): Florianópolis city, Santinho, restinga, in Vriesea cf. friburgensis, 27°28'42.4"S, 48°23'6.8"W, 2.iii.2008, A.G. Martins and F.F. Albertoni leg. (Fig. 1); 1 larva fixed (MZSP 010.245): Florianópolis city, Santo Antônio de Lisboa, in Aechmea lindenii (plant NA20), 4.ii.2004, A.F. Cordeiro and M. Manfredini leg.; 3 larvae (MZSP 010.248): same locality and plant species (plant NA19), 2.iii.2004, A.F. Cordeiro and M. Manfredini leg.; 1 larva (MZSP 010.249): same locality and plant species (plant NA44), 5.iv.2004, A.F. Cordeiro and M. Manfredini leg.; 1 larva (MZSP 010.250): same locality and plant species (plant no. 362), 15.ix.2004, A. Zillikens and J. Steiner leg.; 1 larva (MZSP 010.251): Florianópolis city, UCAD, Atlantic rain forest, in Canistrum lindenii (plant no. 74), 16.v.2003, A. Zillikens and J. Steiner leg.
The pupal description is based on two pupae with the following data: BRAZIL: Santa Catarina: 1 female pupa (reared from larva) (MZSP 010.252) Florianópolis city, Pantano do Sul, “restinga arbórea” in Vriesea friburgensis Mez., 06.ii.2008, A.G. Martins and F.F. Albertoni leg. (illustrated and photographed); 1 male pupa (reared from larva) (MZSP 010.253): Florianópolis, Santo Antônio de Lisboa, in Aechmea sp., 23.iii.2011, A.G. Martins and F.F. Albertoni leg. (photographed).
Imagines of Platyphileurus felscheanus preserved: BRAZIL: Santa Catarina: 1 male and 1 female (reared from larvae) (LANUFSC): Campeche, Florianópolis, restinga, Aechmea nudicaulis (plant no. 235 and 236), 28.xi.2003, A.F. Cordeiro leg.; 1 female (reared from larva) (DMNS ZE.20187): UCAD, Florianópolis, in Aechmea lindenii on rock (plant no. 63), 14.x.2002 (emergence: 29.x.2002), A. Zillikens leg.; 1 male (reared from larva) (MZSP): Florianópolis, Santo Antônio de Lisboa, in Aechmea caudata Lindm., 26.iv.2008, A.G. Martins and F.F. Albertoni leg.; 1 female (reared from larva) (DMNS ZE.20188), same locality, in Aechmea lindenii, 5.vii.2004 (emergence: 8.x.2004), J. Steiner and A. Zillikens leg.; 1 female (reared from larva) (LANUFSC): same locality, in Aechmea sp., 23.iii.2011 (emerged: 09.xi.2011), A.G. Martins and F.F. Albertoni leg.; 1 male (LANUFSC): same locality, among the litter of Hohenbergia augusta, 27.xi.2012, F.F. Albertoni and J. Linemburg Jr. leg.
Additional material: Two last larval instar exuvia and one pupal exuvia (MZSP) of the reared Platyphileurus felscheanus larvae.
To assess the differences of apical setal patterns of pupae between different species of Dynastinae, pupae of the following species of Phileurini and Oryctini from the immature collection of MZSP were studied (for descriptions see
Homophileurus luederwaldti (Ohaus, 1910)
BRAZIL: São Paulo: One pupa reared from larva (genital ampulla damaged, sex not determined) (MZSP) Itanhaém, 12.i.1978, L.R. Fontes leg. in nest of Microcerotermes sp. (Isoptera).
Trioplus cylindricus (Mannerheim, 1829)
BRAZIL: São Paulo: One male pupa reared from larva (MZSP), São Paulo city, Cidade Universitária (USP), 09.i.1979, S.A. Vanin & C. Costa leg. in decaying tree trunk.
Strategus validus Fabricius, 1775
BRAZIL: São Paulo: One male pupa reared from larva (MZSP), Peruíbe city, 25–27.v.1982, exp. MZUSP leg.
Mystacella sp. (Diptera: Tachinidae: Exoristinae: Goniini).
BRAZIL, Santa Catarina: 2 imagines (reared from pupae), 2 puparia and 1 puparial exuvia of Mystacella sp. (MZSP) from 1 Platyphileurus felscheanus larva reared to pupa (MZSP): Florianópolis city, Pantano do Sul, “restinga arbórea”, in Vriesea friburgensis, 02.ii.2008, A.G. Martins and F.F. Albertoni leg.
Figs 1–13
Terminology after
Body length: 34–62 mm (x=46 mm; SD=12.5 mm; n=7 preserved specimens); dehydrated or otherwise contracted specimens measured as low as 26 mm.
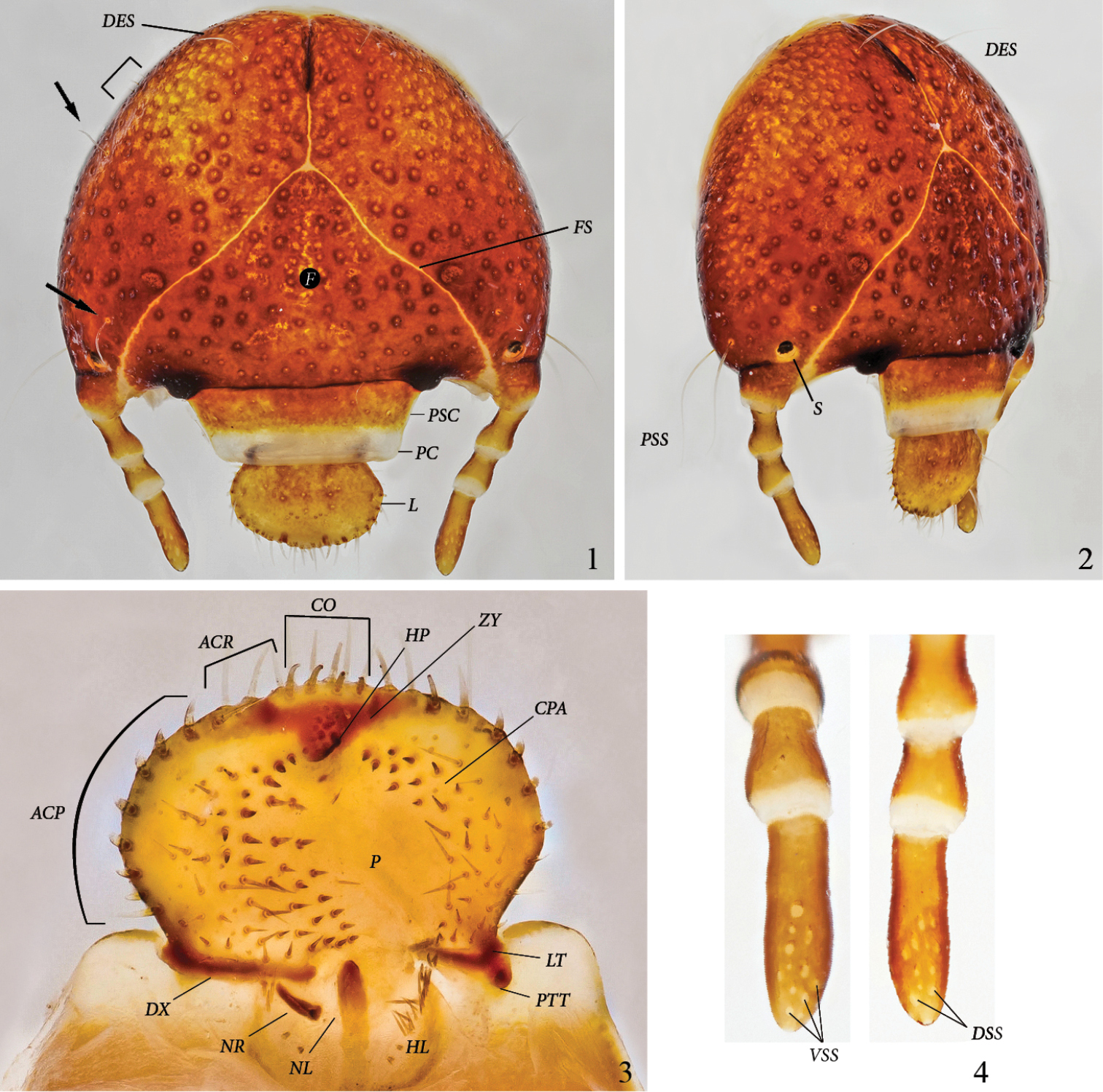
Cranium (Figs 1–2): Width of head capsule: 4.9–5.5 mm (x=5.2 mm; SD=0.2 mm; n=12). Reddish brown, strongly and moderately densely punctate. Light yellow sharp frontal suture (FS) reaches antennal base. Stemma (S) present at antennal base, close to frontal suture. Anterior angles of epicranium with 2 long, thin setae behind distal side of antennal base (PSS) (1 on dorsal, 1 on ventral side of epicranium). Up to 4 microscopic setae basoventrally of DES (often missing). A long, thin seta behind stemma close to frontal suture (arrow); 1 lateral, long, thin seta just behind middle of epicranium, and 1 lateral seta close to epicranial suture (arrow). Frons without setae.
Platyphileurus felscheanus Ohaus, 1910, third instar DMNS ZE.15758: 1 head capsule frontal, arrows indicate setae, half rectangle indicates three minute setae, DES – dorsal epicranial setae, F – frons, FS – frontal suture, L – labrum, PC – preclypeus, PSC – postclypeus 2 head capsule fronto-lateral view, DES – dorsal epicranial setae, PSS – post stemmatal setae, S – stemmata 3 epipharynx, ACP – acanthoparia, ACR – acroparia, CO – corypha, CPA – chaetoparia, DX - dexiotorma, HL – haptolochus, HP – haptomeral process, LT – laeotorma, NL – left nesium, NR – right nesium, P – pedium, PTT – pternotorma, ZY – zygum 4 antennae, ventral and dorsal view, respectively, VSS – ventral sensory spot, DSS – dorsal sensory spot. Photos: C Grinter.
Clypeus (Figs 1–2): Trapezoidal with straight sides. Postclypeus (PSC) orange brown, with punctures smaller and sparser than on cranium. One external seta on each side. Preclypeus(PC) white, without punctures.
Labrum (L) (Figs 1–2): Orange brown with lighter anterior margin. Broadly oval, with rounded, not angulate, lateral margins; with several discal points similar to those of postclypeus(PSC); without posterior labral setae, but with 1 or 2 lateral setae on each side, 1 in front of labral base and 1 close to anterior margin. Anterior margin slightly trilobate, with one seta each on shallow outer lobes and 2 setae on the stronger middle lobe.
Epipharynx (Fig. 3): Form transversely suboval, asymmetrical, with left lateral margin obtusely angulate in the middle. Right and left chaetoparia (CPA) with 53–60 and 33–48 setae, respectively; up to 10 sensilla among setae on each side. Acroparia (ACR) with 3 to 4 thick setae each. Left Acanthoparia (ACP) with 5 to 9 thick setae, anterior ones thicker and longer. Right acanthoparia with 6 to 9 thick setae, anterior ones thicker and longer. Pedium (P) extended to the left. Corypha (CO) with 10 thick setae. Zygum (ZY) brown, triangular, with ventral angle forming a blunt haptomeral process(HP). Laeotorma (LT) shorter than dexiotorma(DX). Pternotorma (PTT) blunt, rounded. Right nesium (NR) caudolaterally shifted and enlarged, forming a sharp, ventrally extending tooth. Left nesium (NL) caudally elongated with sense cone on anterior tip. Haptolachus (HL) without setae except left margin bearing about 20 thin, long setae. Crepis missing.
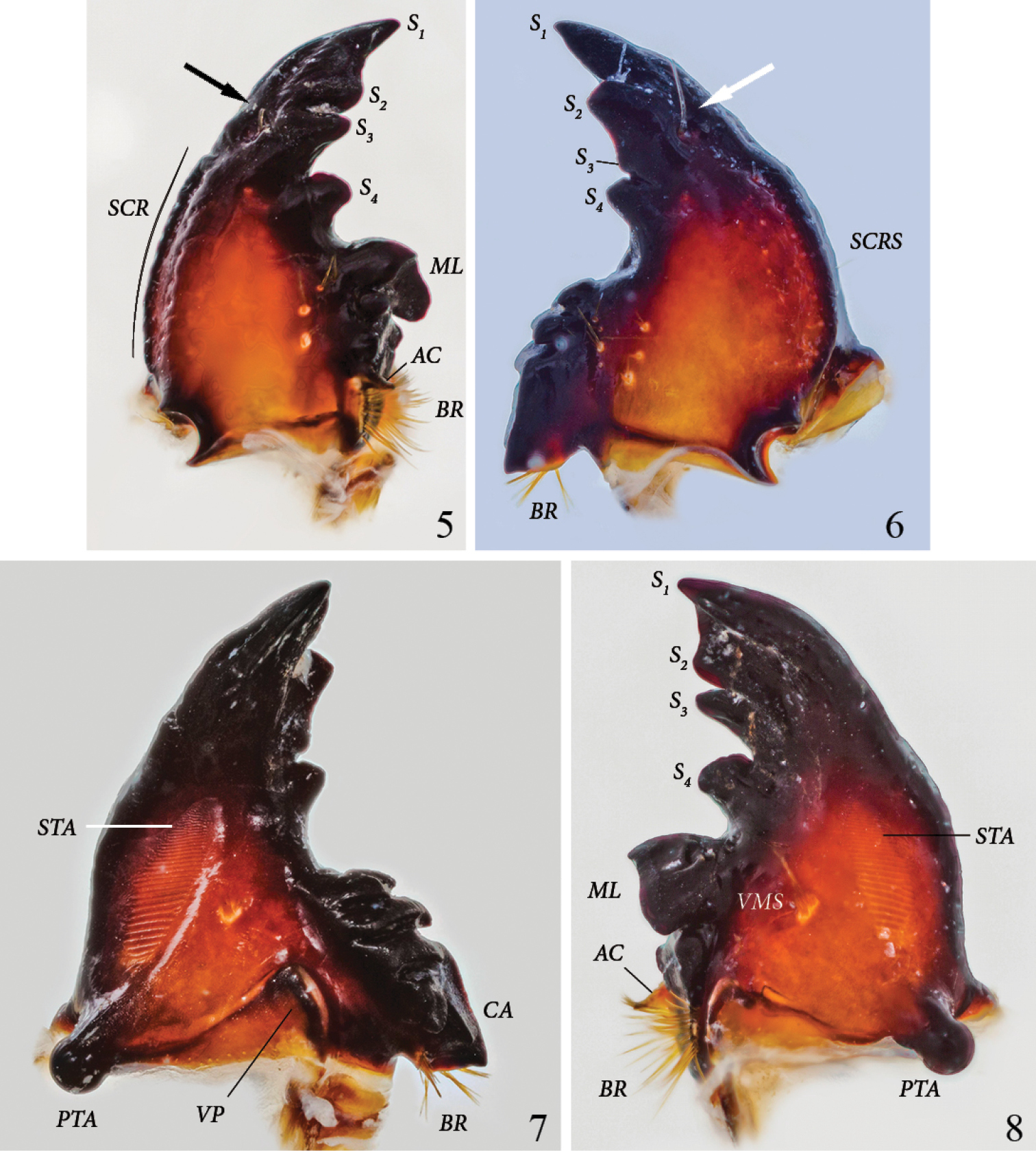
Left mandible (Figs 5, 8): Form falcate. Scissorial area with S1 and S2 distant but bridged by flat area forming broad apical blade, separated from S3 by acute scissorial notch. S4 of similar size as S3, blunt, of cylindrical appearance in ventral view, separated from S3 by acute and deep notch. Mandible dorsally with 1 long discal seta in front of labium at level of S3 (arrow). Outer margin convex. Scrobis (SCR) with 1 short, thin seta. Dorsal area adjacent to scrobis with 2 rows of 7 sensorial pits. Dorsal area adjacent to molar crown with 3 setae. Acia (AC) well developed, with brush of apical setae. Brustia (BR) with 12 long setae. Ventral surface with elongate-oval stridulatory area (STA) with about 30 narrowly separated, subparallel ridges. Molar area with a tuft of 8 ventral molar setae(VMS) (setae very close together and difficult to count). Molar lobe (ML) large, forming a dorsoventral ridge, not subdivided. Molar crown with 2 lobes. Postartis (PTA) large, spherical. Ventral process triangular with rounded tip.
Platyphileurus felscheanus Ohaus, 1910, third instar mouth parts DMNS ZE.15758: 5 left mandible dorsal view, arrow indicates setae, AC – acia, BR – brustia, ML – molar lobe, S1-4 – scissorial teeth, SCR – scrobis 6 right mandible dorsal view, arrow indicates setae, BR – brustia, S1-4 – scissorial teeth, SCRS – scrobis seta 7 right mandible ventral view, BR – brustia, CA – calx, PTA – postartis, STA – stridulatory area, VP – ventral process 8 left mandible ventral view, AC – acia, BR – brustia, ML – molar lobe, PTA – postartis, S1-4 – scissorial teeth, STA – stridulatory area, VMS – ventral molar setae. Photos: C Grinter.
Right mandible (Figs 6–7): Form falcate. Scissorial area with S2 separated from S1 by obtusely angled notch. S2 and S3 distant but bridged by flat area, S3 hardly developed as a denticle but with deep, acute notch separating it from S4. S4 triangular, as elevated as S2. Mandible with 1 long discal seta in front of labium at level of S3 (might be lacking or broken off) (Fig. 6, arrow). Outer margin convex. Scrobis with 1 short, thin seta(SCRS). Dorsal area of scrobis with an inner row of 5 and an outer row of 9 sensorial pits. Dorsal area adjacent to molar crown with a row of 4 white pits with 0, 4, 1, and 2 setae, respectively; discally with longitudinal row of 3 distinct, white pits with 3, 1, and 2 setae, respectively. Ventral surface with elongate oval, anteriorly tapering, stridulatory area (STA) with about 30, narrowly separated, subparallel ridges. Molar area with 5 ventral molar setae. Molar crown with 3 blunt ridges. Calx (CA) ventrally and dorsally ending in slightly blunt denticle. Brustia (BR) with about 15 setae. Postartis (PTA) large, spherical. Ventral process (VP) suboval, elongated laterally.
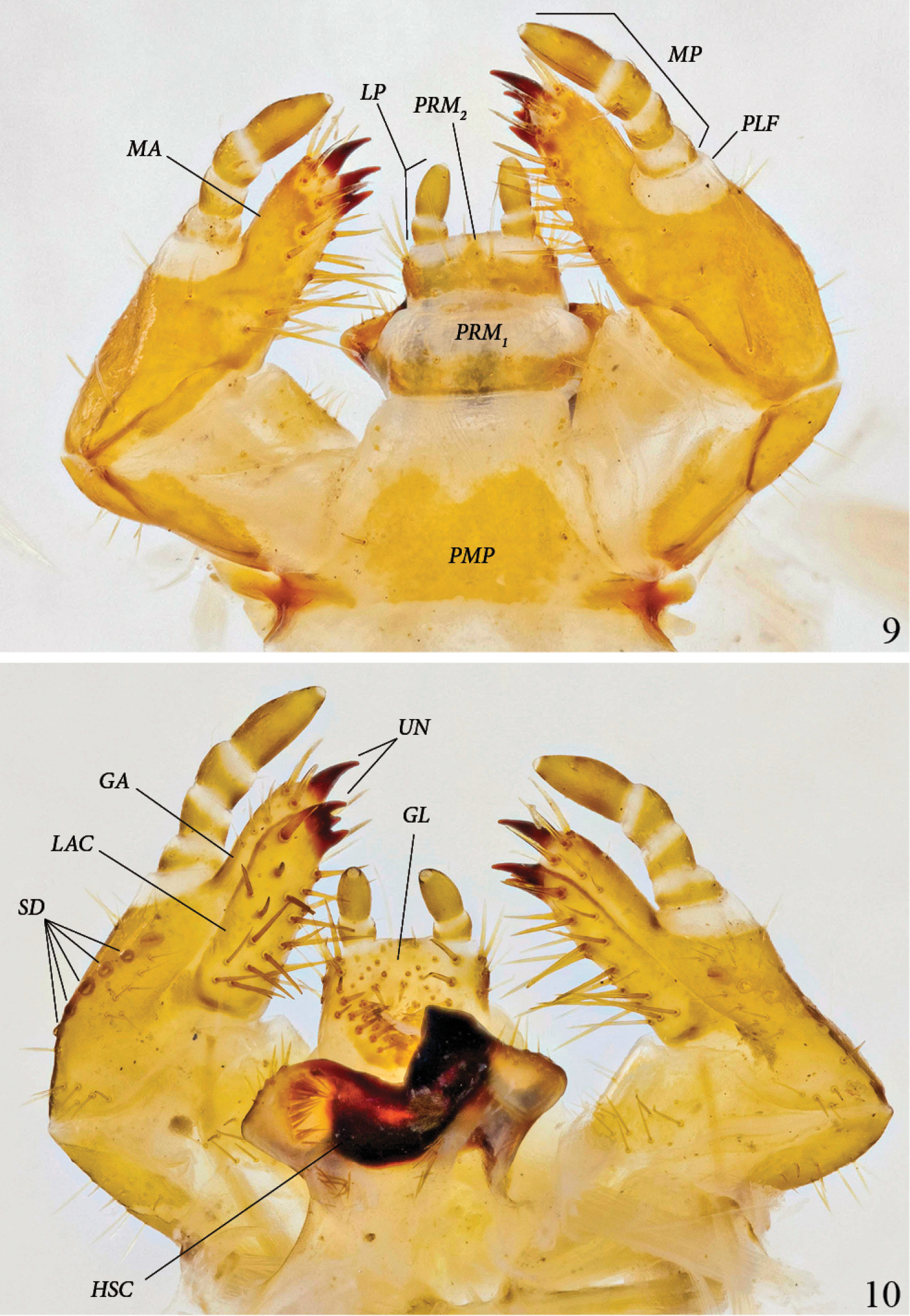
Maxilla and labium, ventral view (Fig. 9): Galea and lacinia fused, forming mala(MA). Ventral inner margin and apical area of mala with 7–8 strong, long setae, and another 5 or more on inner side of mala. Maxillary palpus (MP) 4-segmented; palpifer (PLF) white, membranous; spindle-shaped apical segment about twice as long as each preceding segment. Third segment with 2 strong, ventral setae. Mentum subdivided into 3 segments: yellow post-mentum (PMP) with one basolateral and one apicolateral seta on each side, white prementum 1 (PRM1) with orange base and 2 discal setae, orange prementum 2 (PRM2) with 2 setae on white base of each palpus. Labial palpus (LP) 2-segmented, spindle-shaped apical segment twice as long as basal segment.
Platyphileurus felscheanus Ohaus, 1910, third instar mouth parts DMNS ZE.15758: 9 maxilla and labium ventral view, LP – labial palpus, MA – mala, MP – maxillary palpus, PLF – palpifer, PMP – post-mentum, PRM1, 2 – Prementum 1 and 2 10 maxilla and labium dorsal view, GA – galea, GL – glossa, LAC – lacinia, HSC – hypopharyngeal sclerome, SD – stridulatory teeth, UN – uncus. Photos: C Grinter.
Maxilla and labium, dorsal view (Fig. 10): Galea and lacinia not fused. Galea (GA) with one apical uncus (UN); apicodorsal margin of galea with 3–4 setae. Lacinia (LAC) with 3 touching subterminal unci; dorsal surface of lacinia with 17–19 long, strong setae. Stridulatory area with 1 large, darker stridulatory tooth and a row of 4 to 6 smaller, rounded, sometimes more lightly colored teeth(SD), reaching longitudinally towards base of stipes. Glossa (GL) with 23–31 long and 12–13 very short setae. Hypopharyngeal sclerome (HSC) asymmetrical, with median rectangular incision, right side with one triangular, tooth-like process produced dorsally; left side neither elevated nor protruded. Left lateral lobe with 7 setae on margin, in middle with dense, longitudinal row of about 15 broad setae directed mesally and a few more basally; right lateral lobe with 8 setae on anterior margin and an oblique row of 6 setae basally at caudomedian border of hypopharyngeal sclerome.
Antenna (Fig. 4): Four-segmented with fourth antennomere the longest, about 1.7 times as long as third, second antennomere slightly shorter than fourth, first antennomere slightly shorter than third but thicker than others. Terminal antennomere with 12–13 ventral sensory spots (VSS) and 13–15 dorsal sensory spots(DSS); apex with 1 sensory spot.
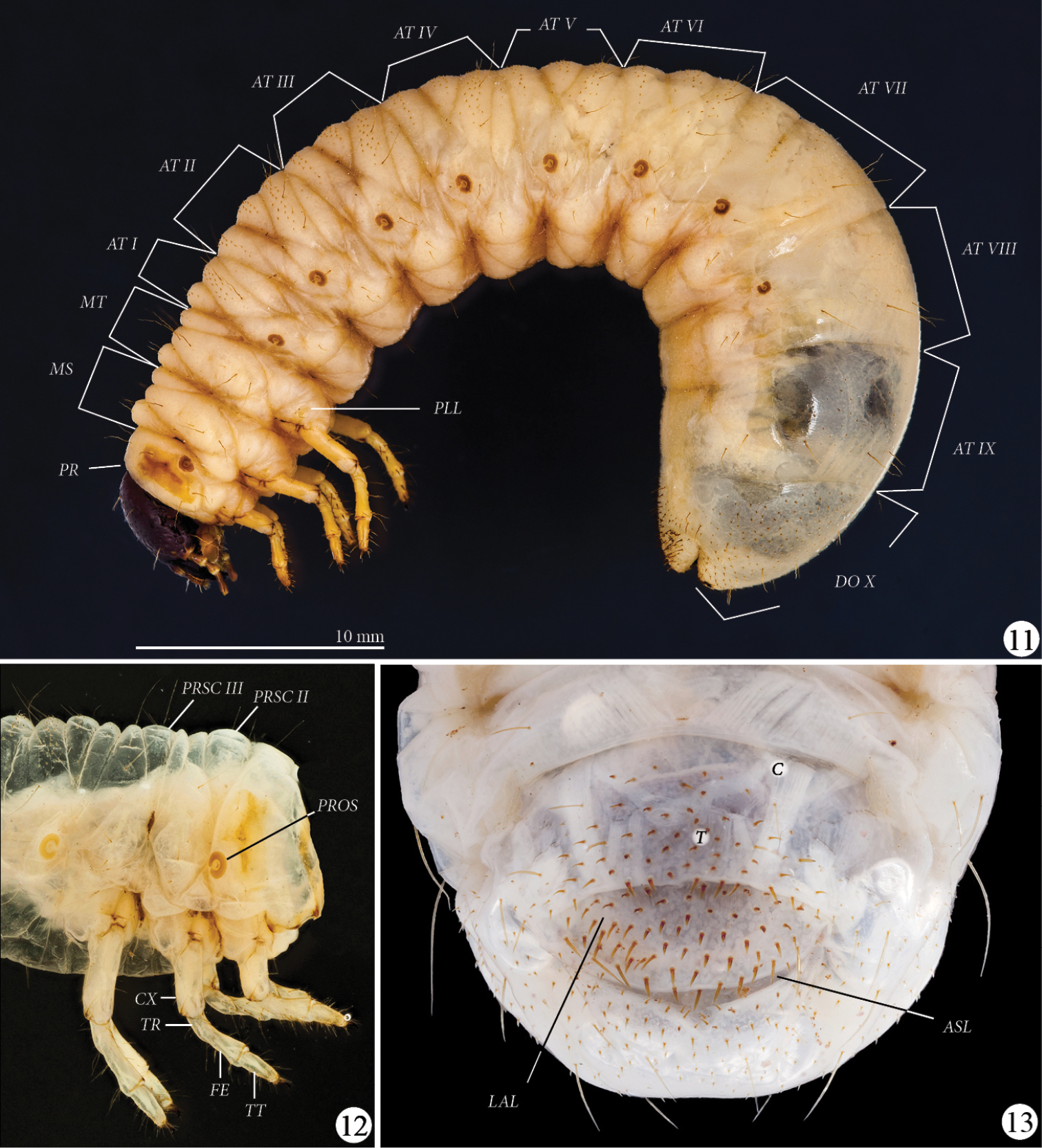
Thorax (Figs 11–12): Prothoracic spiracle (PROS) 0.48–0.54 mm wide, 0.76–0.80 mm long. Respiratory plate light brown, ovally C-shaped, with ends touching. Bulla barely prominent. Respiratory plate with about 35 holes across diameter at middle. Dorsum of pronotum (PR) and prescutum II (PRSC II) and III (PRSC III) each with 2 lateral, long, slender setae, otherwise glabrous.
Platyphileurus felscheanus Ohaus, 1910, third instar. 11 lateral view MZSP 010.245, AT I-IX – abdominal tergites I to IX, DO X – dorsum X, MS – mesothorax, MT – metathorax, PLL – pleural lobes, PR - prothorax 12 thorax and legs lateral view DMNS ZE.15758, CX – coxa, FE – femur, PROS – prothoracic spiracle, PRSC II – prescutum II, PRSC III – prescutum III, , TR – trochanter, TT – tibiotarsus 13 larval raster DMNS ZE.15760, ASL – anal slit, C – campus, LAL – lower anal lip, T – teges. Photos: FF Albertoni (11), C Grinter (12–13).
Legs (Figs 11–12): Tarsal claws falcate, all similarly curved and similar in size, with 1 basal seta and 1 seta in the middle of inner side. Tibiotarsus (TT) with 4 apical setae on outer side of the base of claw, and with 2 circular rows of 6 long setae. Femur (FE) with 2 circular rows of 4–5 long setae and accessory setae. Trochanter (TR) with 5–6 ventral setae. Coxae (CX) with 4 setae. Setae of legs light brown to transparent, thin.
Abdomen (Fig. 11): Spiracles of similar size as prothoracic spiracle; last one smaller; rounder than prothoracic spiracle. One long seta on stigma area behind each abdominal spiracle II to VII, no seta behind spiracles I and VIII. Pleural lobes (PLL) with 2 long setae. Pedal area with 1 central and 2 lateral long setae per segment. Abdominal tergites (AT) I–VII with many tiny, short, dark, spike-like setae, not arranged in rows. Abdominal tergite IX laterally with dark, sparse minute, spike-like setae. Dorsum X (DO X) completely covered with such setae.
Raster (Fig. 13): Surface without palidia. Campus (C) with 6 slender, moderately long setae. Teges (T) with about 110–115 shorter, thorn-like setae (some longer) anterior of transverse anal slit(ASL), slightly bent toward anal slit. Lower anal lip (LAL) with about 80–100 thinner, shorter, minute, thorn-like setae, some thin and long. Setae not arranged in any pattern.
Figs 14–25, 34
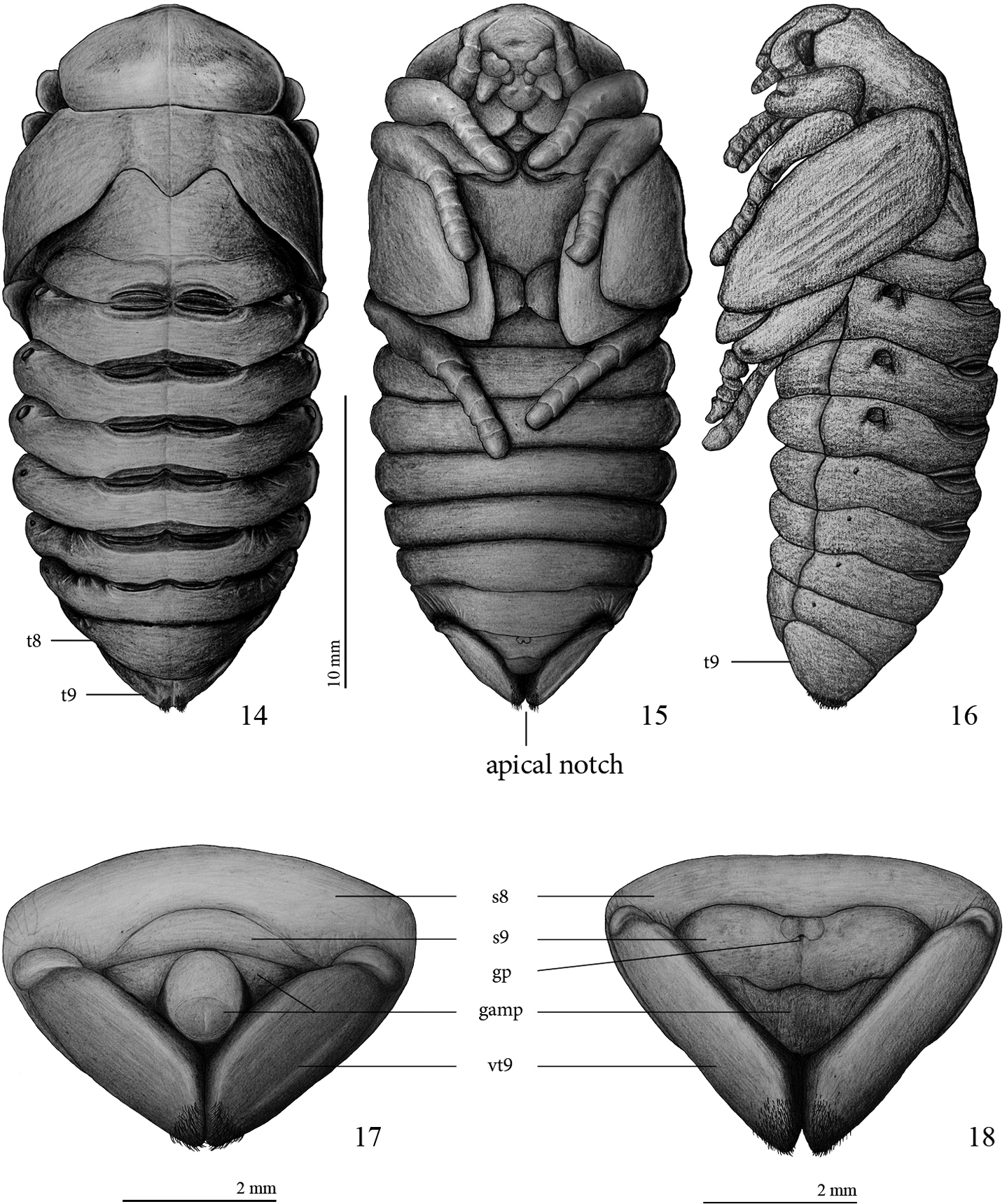
Female pupa (Figs 14–16, 18, 20, 22, 24–25, 34):
Length 23.9 mm; largest width 10.4 mm.
Adecticous; exarate; body oblong, smooth, apparently glabrous but with microsetae covering whole body (best seen at magnification > 50 ×), apex of tergite IX with dense tuft of setae seen in dorsal and ventral views; abdominal segments constitute almost two thirds of whole body; yellowish-brown before and after fixation, gin-traps and spiracular rings darker and more strongly sclerotized.
Head: epistomal suture incomplete at middle, clypeus with shallow depression, clypeolabral suture slightly marked.
Pronotum (Fig. 14): almost twice as wide as long; pentagonal, widest at middle, narrowed anteriorly, lateral margins rounded, medially with slightly transversal groove. Scutellum pentagonal, 1.2 times longer than wide.
Pterotheca (Figs 15–16): close to body, curved ventrally, extending posteriorly to second abdominal segment.
Platyphileurus felscheanus Ohaus, 1910, pupa: 14 female pupa dorsal view, t8, t9 – tergite 8 and 9, respectively 15 female pupa ventral view 16 female pupa lateral view 17 male pupa, ventral view of apex with genital ampulla 18 female pupa, ventral view of apex with genital ampulla. Legends: s8, s9 – sternite 8 and 9, respectively; gamp – genital ampulla; gp – genital pore, vt9 – ventralized tergite 9. Drawings: FF Albertoni.
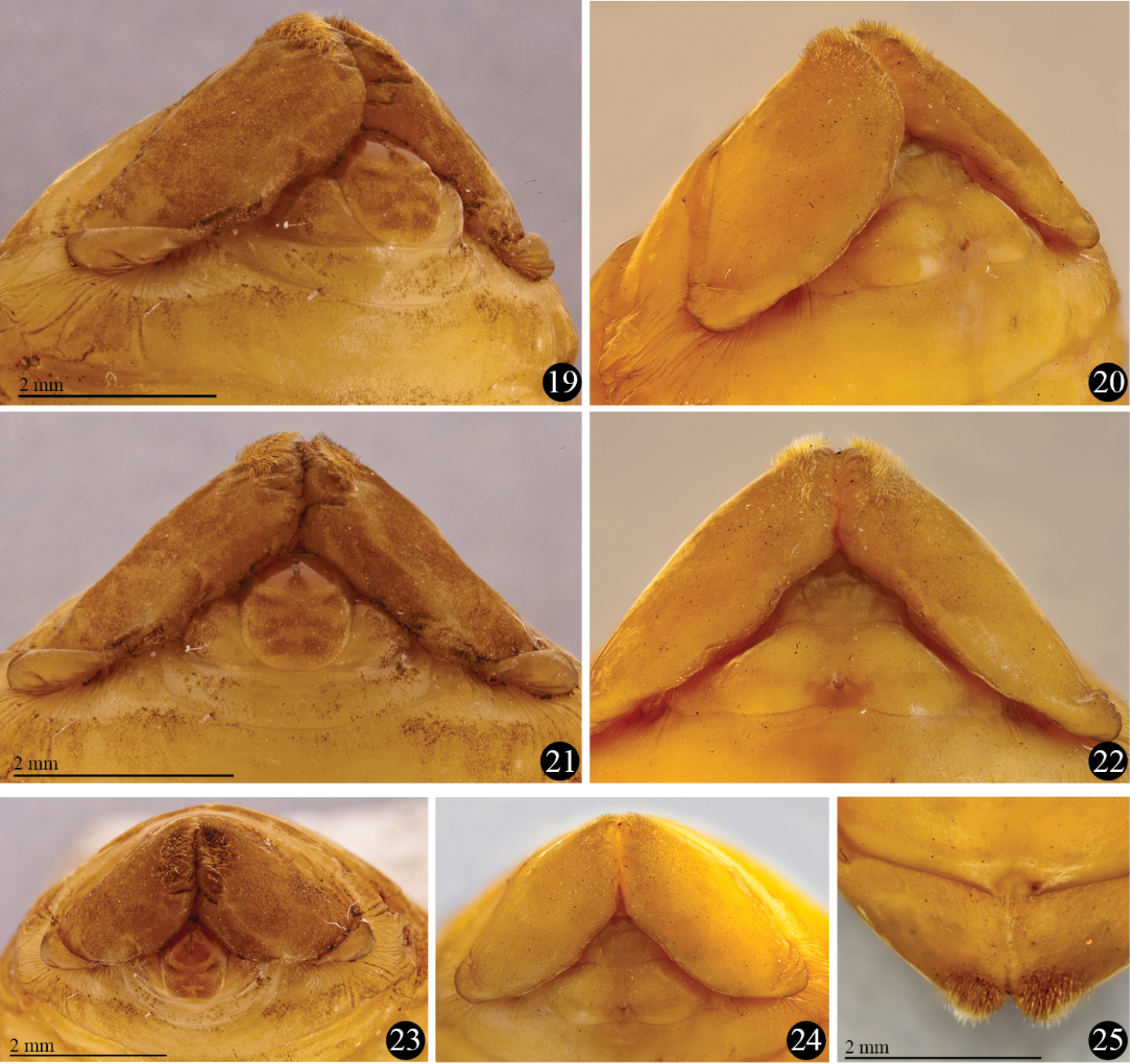
Abdomen: segments 1–7 widened transversally, about 6 times wider than long; segments 3, 4 and 5 widest; segment 8 longer on dorsal view than ventrally, about 4.5 times wider than long (Fig. 14); tergite 9 (t9) triangular, laterally extended to ventral side (see Figs 17–18) and with apical notch in ventral view; each side of apical notch covered with a tuft of yellowish, short, thin setae that do not extend ventrally, laterally, or dorsally (Figs 14–16, 18, 20, 22, 24–25); sternite 9 with genital pore; 4 pairs of functional spiracles, first pair almost completely concealed by pterothecae, other 3 pairs protruding about 0.2 mm (Figs 14, 16), 4 pairs of vestigial spiracles on laterotergites 5–8 (Fig. 16). Six pairs of gin-traps mid-dorsally in intersegmental region, the largest and most sclerotized between segments 1 and 2, those between segments 2 and 3, 3 and 4, 4 and 5, and 5 and 6 of same size, those between segments 6 and 7 smallest.
Platyphileurus felscheanus Ohaus, 1910, pupal apex showing genital ampulla, setose apex of ninth tergite and base of ventralized tergite 9: 19 male, ventro-lateral view 20 female, ventro-lateral view 21 male, ventral view 22 female, ventral view 23 male, frontal view 24 female, frontal view 25 female, dorsal view. Photos: FF Albertoni.
Female genital ampulla (gamp) (Figs 18, 20, 22, 24): at middle with 2 almost parallel lines slightly convergent anteriorly and 2 diagonal lines, 1 per side.
Male pupa (Figs 17, 19, 21, 23):
Length: 19.0 mm; largest width 10.1 mm.
Most characteristics as in female but basal part of ventral side of ninth tergite (vt9) with much stronger constrictions than in female (Figs 17–25). In males, they resemble 2 lateral lobes, whereas in females they are less folded (Figs 19–24). Sternite 9 (s9) anteriorly convex in males, in females with different form (see Figs 17–22).
Male genital ampulla (gamp) (Figs 17, 19, 21, 23) divided into 2 parts: a rounded structure and a narrow, trapezoidal band strongly emarginated at middle, surrounding partially the rounded structure; rounded structure with circular, glabrous area at apex, glabrous area with longitudinal, dark line impressed at apex.
In 14 ground-growing bromeliads, we collected 19 larvae of Platyphileurus felscheanus, 13 of them in Aechmea lindenii, three in Aechmea nudicaulis, one in Canistrum lindenii, one in Hohenbergia augusta, and one in Nidularium innocentii. The highest number of larvae in one bromeliad was four in a single Aechmea lindenii, but the majority of bromeliads had only one larva.
Second instars were found at the base of more external leaves of the rosette among leaf litter. No damage to bromeliad leaves near the larvae was observed. Third instars were always found in the center of the rosette with the surrounding leaves strongly damaged (Figs 32–33).
Coincidentally, when the bromeliad leaves started to dry and consequently to decompose, the larvae stopped feeding and started pupation. They built their pupal chamber in the center of the leaf rosettes or among leaves near the center, using leaf litter, twigs, and humus that had accumulated there. The larval exuvia was pushed to the rear of the pupal chamber (Fig. 34). Observations of the second phase are summarized in Table 1.
Results of second phase of study on Platyphileurus felscheanus life history.
| Pantano do Sul, restinga arborea |
Santinho/Mocambique, restinga arborea | Sto Antonio | ||||
|---|---|---|---|---|---|---|
| Bromeliad searched/ collected | 6 Vriesea friburgensis (Fig. 31) No damage to plant |
3 Aechmea spp. Plant senescent |
12 Aechmea caudata Plant apparently not injured. |
5 Aechmea sp. and 3 Nidularium sp. Plant apparently not injured. |
||
| Larvae found | 1 second instar larva (n° 1); 2.iv.2008. In outer leaf axils with much litter, but moved into the centre of rosette. Smaller than specimens n° 2 and 3. |
1 second instar larva (n° 2); 2.iv.2008. Outer leaf axils among leaf litter, but moved into the centre of rosette. Smaller than n° 3. |
1 second instar larva (n° 3); 2.iii.2008. Between dry external leaves, later moved slowly into the central rosette. Moulted on 7.iii.2008. |
1 third instar larva (n° 4) (Fig. 32) in Aechmea caudata 26.iv.2008. Down in center of rosette. |
1 third instar larva (n° 5) in Aechmea sp. 23.iii.2011. Down in center of rosette. |
1 third instar larva (n° 6) in Aechmea sp. Just next to the bromeliad of n° 5; Down in center of rosette. |
| Ate what? | Ate basal parts of leaves. | Ate white basal part of leaves. | Ate dry leaves; later basal part of green leaves and stalk. | Ate the basal parts of leaves. | Ate the basal (and green) parts of leaves. | Ate the basal (and green) parts of leaves. |
| Pupa | 6.ii.2009 starts pupal chamber, 10.ii pre-pupa, 15.ii. pupa, (fixed). | 25.xii.2008 pupa observed being parasitized by Tachinidae (Diptera) (Figs 36–37). (4 puparia of Mystacella sp.) (Fig. 35). | 22.ix.2008 larva fixed. | Before 15.ix.2008 | Before 04.x.2011. Fixed on 15.x.2011. |
On 08.x.2011. |
| Remarks | Female pupa. | 9.i.2009 emergence of 2 flies, fixed together with puparia. | Larva fixed with boiling water, preserved in ethanol 90%. | Male imago emerged 5.x.2008. Gnawed wood. Stayed most part of the time buried. Died 24.xii.2008. |
Male pupa. It seemed to pupate before final growing time and turns to smaller pupa than n° 1. |
pupa smaller than n°. 1 and 5 Female imago emerged on 10.xi.2011. Ate banana. Stayed most part of the time buried. Died on 9.xii.2011. |
Parasitism by Tachinidae flies was observed in one pupa (Fig. 35). As the bromeliad with the larva was kept open in the laboratory, we do not know whether parasitism occurred in the laboratory or in the field. The chamber of the affected pupa seemed crudely done, mainly built with plant fiber and missing a more consistent humus wall.
Generally, pupae of Scarabaeidae are of uniform appearance, mainly differing by adult characters such as horns. Usually they do not have pupa-specific ornamentations or setae for chaetotaxy analysis unlike pupae of other beetle families which might be the reason for the poor attention that scarabaeoid pupae have gained in the past (see
Several descriptions of pupae from Dynastinae tribes, such as Cyclocephalini, Phileurini, Oryctini point out the thin golden setae present at the apex of 9th abdominal segment but rarely mention the shape of the 9th tergite and the genital ampulla (Phileurini:
In a pupa of Homophileurus luederwaldti examined (length: 25.3 mm, largest width: 13.0 mm; Phileurini) the setation on the apex differs from that of Platyphileurus felscheanus by covering a much wider area, until the middle of the ventral part of t9, and the setae also extend slightly towards the dorsal side. The apical notch is more open than it is in Platyphileurus felscheanus. The genital ampulla region was damaged, and could not be analysed. In a male pupa of Trioplus cylindricus (length: 22.0 mm, largest width: 9.3 mm; Phileurini), the setae on the apical tergite are similar to the pupa of Homophileurus luederwaldti. They cover a larger area, spreading laterally towards the dorsal region, but almost do not change in density and sizes as in Homophileurus luederwaldti. The rounded structure of the genital ampulla is wider posteriorly and narrowed from middle to base, thus not rounded or elliptical. There are two longitudinal marks, one smaller anteriorly and the longer posteriorly. In a male pupa of Strategus validus (length: 60 mm, largest width: 27 mm; Oryctini), the rounded structure of the genital ampulla is elliptical with one longitudinal mark at the apex and thus, similar to that of Platyphileurus felscheanus. Tergite 9 has a transversal depression laterally (not present in Platyphileurus felscheanus); pubescence of uniform, small setae covering a wide area throughout the segment, ventrally covering almost the whole area extending laterally unto the edge of the lateral depression; and an apical notch connected mesally by V-shaped fold (absent in Platyphileurus felscheanus).
Furthermore, in Hemiphileurus elbitae
Even though the knowledge of pupae is much more limited than that of larvae, pupae do have some exclusive characters such as setae, spurs, tubercles and modified spiracles (
Dynastinae larvae frequently feed on decaying plant matter, especially wood (
Considering the place where we found the younger larvae, females most likely lay eggs into the external leaf axils that are rich in decaying plant matter and humus, and usually dryer than the water-filled central rosette. Our observations suggest that the larvae initially feed on dead decaying vegetal matter and then migrate into the center of the rosette to feed on the white basal part of living leaves. It is during this second phase that the larvae gain most weight.
The construction of pupal chambers in Dynastinae species is poorly documented. Larvae of Phileurus hospes Burmeister, 1847 built their pupal chamber only with humus from the decaying tree trunk in which the larvae were found (F. F. Albertoni pers. obs.). The termitophilous species Homophileurus luederwaldti, Actinobolus tribolus Luederwaldt, 1910 and Actinobolus radians Westwood, 1841 used their own faeces and soil from the termite nest to build their pupal chambers (
According to
Among the South American Coleoptera that are hosts of Tachinidae, Chrysomelidae and Scarabaeidae are the families with the highest numbers of parasitized species (
Since the species studied here was not only described as two species, Platyphileurus felscheanus and Surutu jelineki, but also as belonging to different tribes, namely Phileurini and Cyclocephalini, it is appropriate to explore whether larval characters can contribute to resolving its tribal classification.
Antennal sensory spots: Larvae of Cyclocephalini are characterized by 2, 3, or 4 sensory spots on the last antennomere plus an apical one (
Likewise, the species would have the highest number of antennal sensilla ever recorded in Phileurini larvae. So far, Homophileurus integer (Burmeister, 1847) had the highest number with 5 dorsal and 8 ventral sensory spots (
Larval mandibles: The shape of the larval left mandible of Platyphileurus resembles most the larval mandibles of some Oryctini species. Particularly the large, blunt scissorial denticle S4 is most commonly found in Oryctini, namely in Strategus splendens (Palisot de Beauvois, 1809) (
Other larval characters: According to
Adult characters: To exclude the possibility of Platyphileurus belonging to Oryctini, the adult mouthparts were examined. The mentum (labium) of Platyphileurus is triangular, basally broad, tightly tapered to a blunt, rounded, thin and slightly protruding tip (Fig. 29). The basis of the labial palps is almost visible, being slightly covered by the margin of the mentum only. This is different from the form of the mentum diagnostic for Phileurini, being broad and covering the basis of the labial palps completely. In fact, the mentum of Platyphileurus resembles broad menta of Oryctini or Pentodontini.
Imago of Platyphileurus felscheanus Ohaus, 1910. 26, 27 and 28 male habitus, dorsal, ventral and lateral, respectively 29 mentum DMNS ZE.20187 30 hind leg ventral and dorsal view respectively DMNS ZE.20188. Photos: FF Albertoni (26–28), C Grinter (29–30).
Platyphileurus felscheanus Ohaus, 1910, natural habitat: 31 bromeliad Vriesea friburgensis with leaf litter in open area of Atlantic Forest, Santa Catarina, Florianópolis 32 Aechmea sp., cylinder of the central leaves eaten by Platyphileurus felscheanus larva 33 larva on bromeliad leaf from the very center rosette 34 pupa in its pupal chamber built with pieces of leaves, twigs and humus 35 puparia of Mystacella sp. (Diptera: Tachinidae) and pupal exuvia of Platyphileurus felscheanus (arrow). Photo: A Zillikens (31), FF Albertoni (32–35).
Imago of Mystacella sp., parasite of Platyphileurus felscheanus. 36 dorsal view 37 lateral view. Photos: FF Albertoni.
Problems with the current tribal classification: In the current, typological classification, Oryctini are separated from Pentodontini by one variable adult character, the apex of the hind tibia, of which
In a caryological study
Tribal placement ofPlatyphileurus: The apex of the hind tibia in Platyphileurus (Fig. 30), being (weakly) dentate and not truncate, would place this genus in Oryctini or Dynastini, not in Pentodontini in the current sense. This is supported by the number of sensilla on the larval antenna and the shape of the left larval mandible. The slightly broader first tarsomere of the hind legs, together with the small body size being unusual for Dynastini, indicates that it rather belongs to Oryctini than to Dynastini in the current sense. The shape of the adult mentum, the large number of sensilla on the larval antenna, and the strong S4 of the left larval mandible are not found in Phileurini. We accordingly propose the transfer of Platyphileurus from Phileurini to Oryctini. We consider the flat body of Platyphileurus a convergence with the body shape of Phileurini, likely related to the unique habitat among the tight bromeliad leaves, where the larva pupates and that enable adults hiding between their leaves.
We thank Maike Hering de Queiroz (in memoriam), Diomário de Queiroz, and Aldaleia Sprada Tavares (UFSC) for granting access to the study sites; Fernando Vaz-de-Mello (Universidade Federal do Mato Grosso, Cuiabá, MT), Sergio Ide (Instituto Biológico, São Paulo, SP), and Brett Ratcliffe (University of Nebraska, Lincoln) for comments about the species synonym before it had been published and for further advice and discussions; André Ganzarolli Martins (UFSC) for the significant help in the field, Sônia A. Casari (MZSP) for general advice on the manuscript; Juares Fuhrmann (MZSP) for advice on the discussion of the genital ampulla, and two anonymous reviewers for important suggestions. Christopher Grinter (DMNS) took the exceptional photographs of the larva and the adult mentum and legs. This study is part of the project “Internal dynamics of rain forests: specificity of animal-plant interactions” and “Importância das bromélias para a manutenção da diversidade da fauna associada na Mata Atlântica”, a Brazilian-German scientific cooperation within the program “Science and technology for the Atlantic Rainforest”, funded by BMBF (01 LB 0205 A1); and CNPq (690143/01-1; 590040/2006-5). Research was authorized by IBAMA, permits 090/2005 and 260/2006, and by SISBIO, permits 12486-1, 12826-1, and 12826-2.