(C) 2013 Peter Michalik. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Michalik P, Ramírez MJ (2013) First description of the male of Thaida chepu Platnick, 1987 (Araneae, Austrochilidae) with micro-computed tomography of the palpal organ. ZooKeys 352: 117–125. doi: 10.3897/zookeys.352.6021
The male of the austrochilid spider Thaida chepu Platnick, 1987 is described for the first time. We analyzed the internal anatomy of the palpal organ by using micro-computed tomography to investigate the spermophor as well as the muscles and tendons in the cymbium and tibia in detail. As shown by our data, muscles 29 and 30 originate in the tibia and continue with tendons to the base of the bulb, which resembles the ancestral organization for the male palp of spiders; this condition has not been described for Araneomorphae until now. The 3D reconstruction of the spermophor confirms recent interpretations of the male palp sclerites within Austrochilidae.
Taxonomy, micro-CT, spermophor, palp
The family Austrochilidae consists of three genera with a very peculiar distribution. Whereas the genera Austrochilus Gertsch & Zapfe, 1955 (6 species) and Thaida Karsch, 1880 (2 species) are endemic to the forests of Central and Southern Chile and adjacent Argentina, the monotypic genus Hickmania Gertsch, 1958 is endemic to Tasmania (
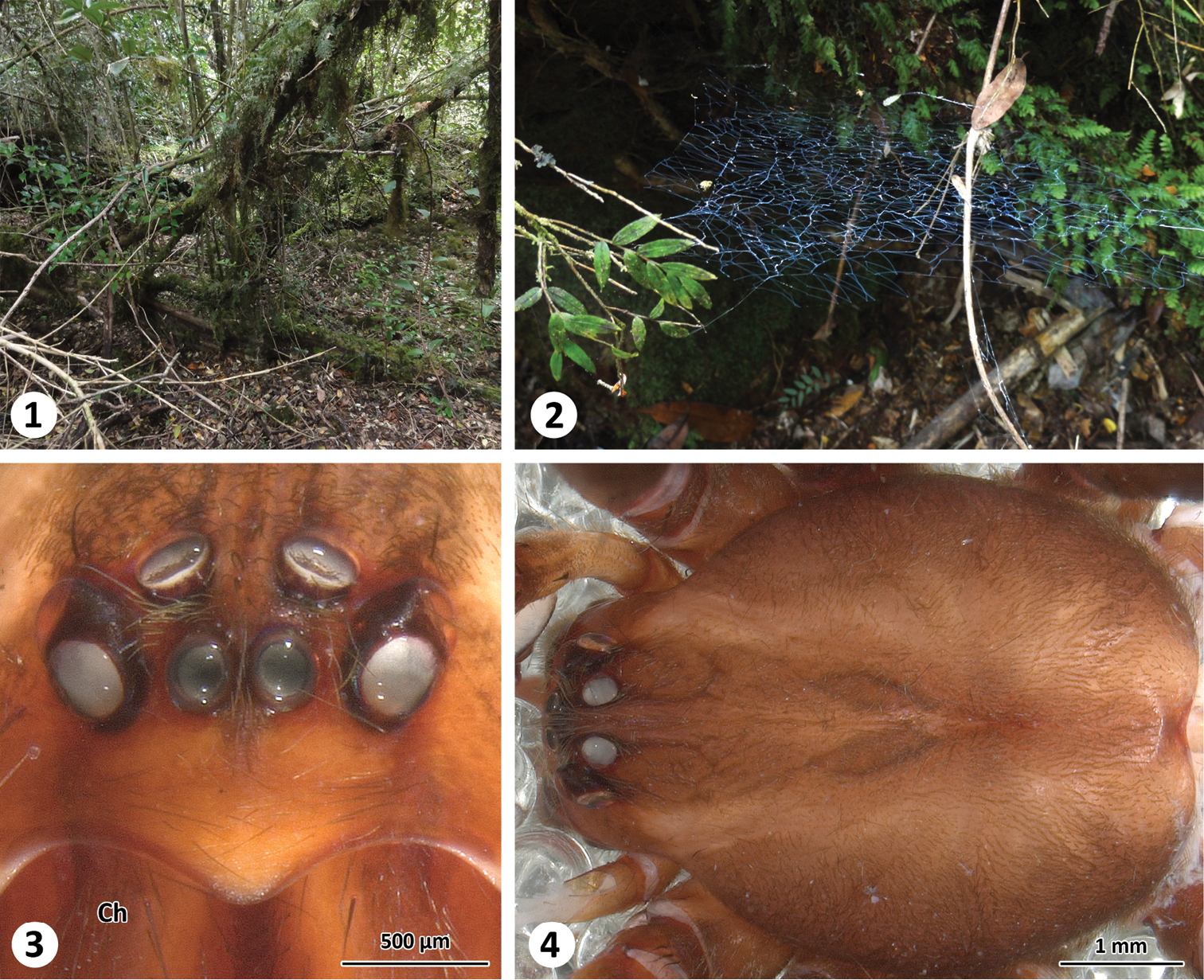
We collected a male of Thaida chepu close to the type locality in a wet lowland mixed forest at Lago Huillinco (Chiloé, Chile) (Fig. 1). The material was examined and documented (extended focal range images) in 80% ethanol using a Zeiss Discovery V20 stereo microscope with a Zeiss MCr camera. Editing of images to adjust brightness, contrast and color was performed using Adobe Photoshop CS4. Measurements (given in millimeters) were obtained from digital images using the IntMess module in the program Zeiss AxioVision 4.8 (Carl Zeiss MicroImaging GmbH, Göttingen, Germany). The style of the description is based on
Habitat (1), web (2) and somatic characters (frontal (3) and dorsal (4) view of prosoma) of Thaida chepu.
For the micro-CT analyses of the male palp, the sample was dehydrated in graded ethanol and stained with a 1% iodine solution for 12 hours. After washing in pure ethanol, the sample was scanned in ethanol with an Xradia MicroXCT-200 X-ray imaging system (Carl Zeiss X-ray Microscopy Inc., Pleasanton, USA) at 40 kV and 8 W using phase contrast (4.0 scintillator-objective lens unit, 15 s exposure time, 4.15 µm pixel size). The obtained data were processed using the 3D analysis software AMIRA v. 5.4.2 (Visage Imaging, Berlin, Germany). Virtual reconstruction of the spermophore was performed by delineation in each section (segmentation) and a smooth surface was computed using the surface editor. The image stack is stored in MorphDBase under creative commons attribution (CC-BY; ID: P_Michalik_20130729-M-4.1; https://www.morphdbase.de?P_Michalik_20130729-M-4.1).
ALE anterior lateral eye
AME anterior median eye
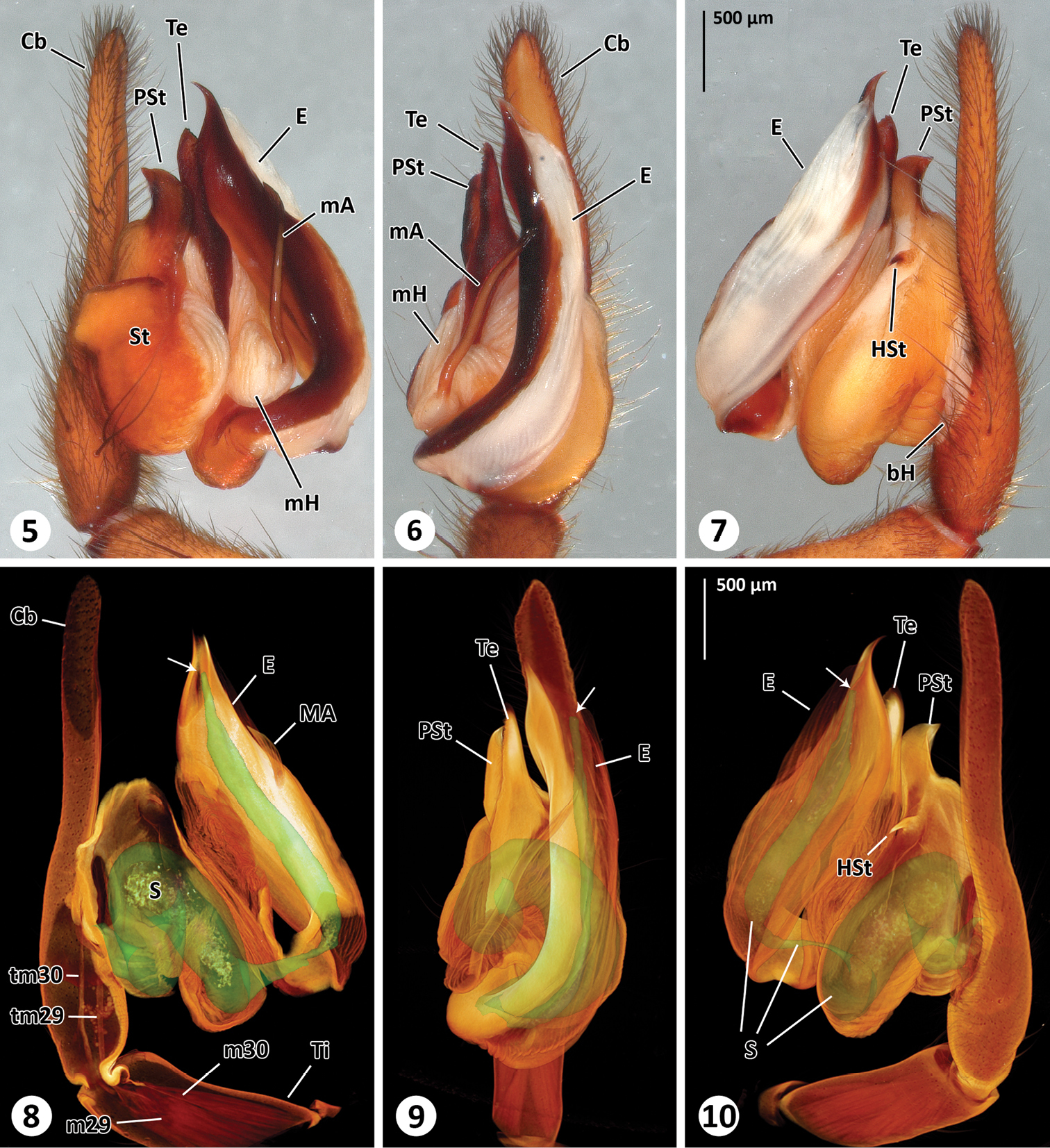
bH basal hematodocha
Cb cymbium
Ch chelicera
E embolus
HSt hook of subtegulum
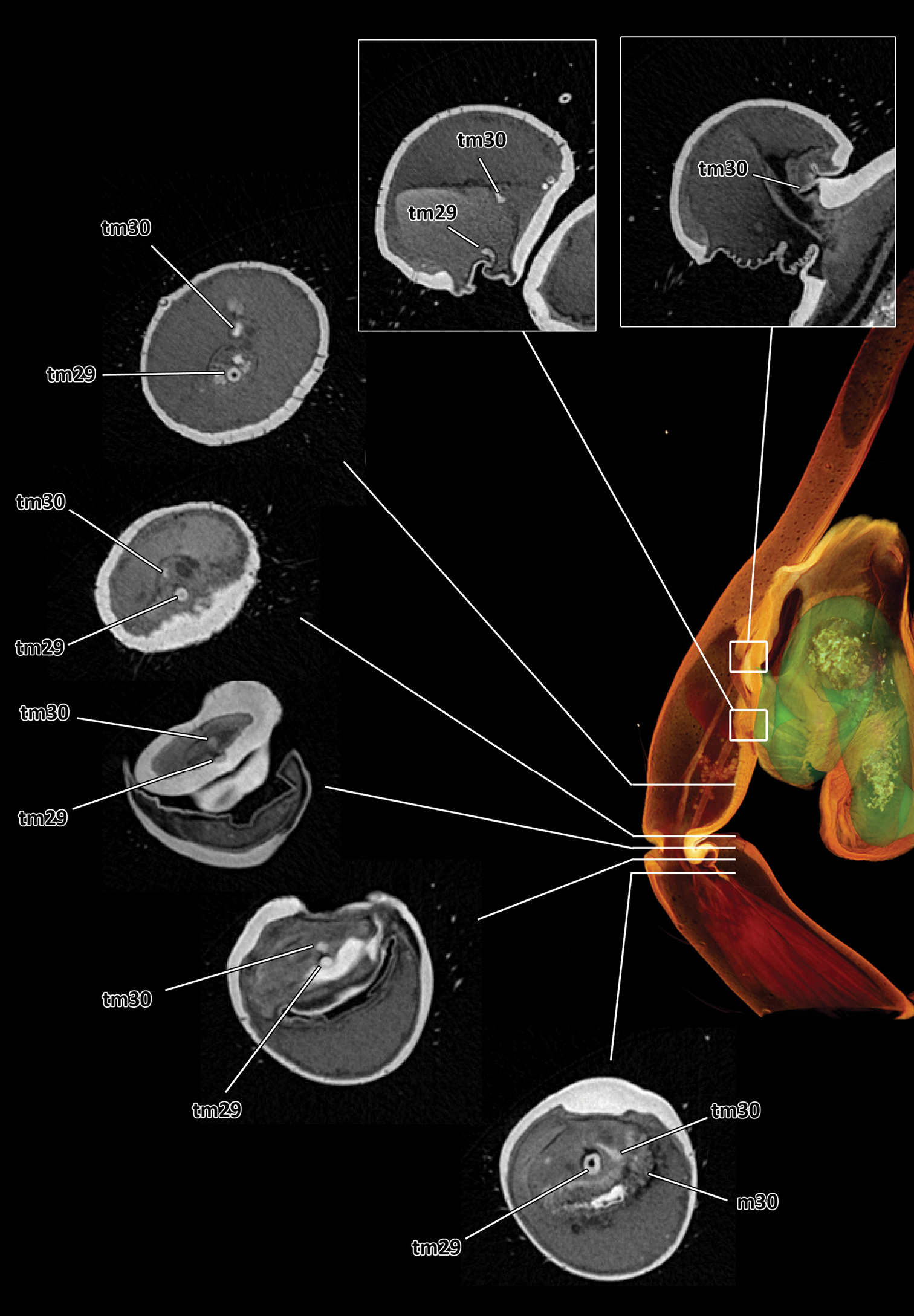
m29 muscle 29
m30 muscle 30
mA median apophysis
mH median hematodocha
MOQ median ocular quadrangle
PLE posterior lateral eye
PME posterior median eye
PSt process of subtegulum
S spermophor
St subtegulum
Te tegulum
tm29 tendon of muscle 29
tm30 tendon of muscle 30
ZIMG Zoologisches Institut und Museum Greifswald (Germany)
Subfamily Austrochilinae Zapfe 1955,
Genus Thaida Karsch 1880
CHILE: Region de Los Lagos (X), Chiloé province, Isla de Chiloé, Lago Huillinco, N margin, 4.6 km (air) ESE Cucao, 42.64117°S, 74.04763°W (GPS, ±100m), elev. 12 m (MJR-loc-86), 16 February 2012, 1 male, coll. K. Huckstorf, M. Izquierdo, P. Michalik, M. J. Ramirez, C. S. Wirkner (ZIMG II/28126).
Similar to Thaida peculiaris by the clypeus about three times the diameter of the anterior median eyes (Fig. 3); males distinguished from Thaida peculiaris by the copulatory bulb, which has the median apophysis longer than the embolus (Figs 5–10; about half the length of the embolus in Thaida peculiaris,
Left male palp of Thaida chepu. 5–7 extended focal plane images of male palp in prolateral (5), ventral (6) and retrolateral (7) view 8–10 surface model of the spermophor superimposed on the volume rendering of the male palp to illustrate dimension and shape of the spermophor. The views correspond to Figs 5–7. The cymbium, subtegulum and tegulum are (partly) removed in Fig. 8 to show tendons and muscles. The arrows point to the opening of the embolus.
Male (ZIMG II/28126). Carapace 5.59 long and 4.38 wide; clypeal height 0.68 (in the middle about three times AME diameter in height; Fig. 3); coloration as depicted in Fig. 4. Eye sizes and interdistances: AME 0.24, ALE 0.31, PME 0.25, PLE 0.30; AME-AME 0.05, AME-ALE 0.15, PME-PME 0.26, PME-PLE 0.22, ALE-PLE 0.06; MOQ length 0.77, median ocular quadrangle width 0.78. Spination: femora: I d 1-0-1, p 2-3-3, r 2-3-3; II d 1-2-1, p 2-2-2, r 2-2-2; III d 1-2-1, p 1-2-2, r 2-2-2; IV d 2-2-1, p 0-1-2, r 0-1-3; tibiae: I p 2-3-2, v 3-3-4, r 2-2-2; II p 2-2-2, v 3-4-3, r 1-1-2; III d 1-0-1, p 0-2-2, v 1-1-4, r 0-2-1; IV missing; metatarsus: I p 3-1-2, v 1-1-1, r 2-2-2; II d 0-1-2, p 1-2-2, v 0-1-2, r 1-1-0; III p 1-1-2, v 2-3-2, r 2-2-1; IV missing. Palp (Figs 5-10): cymbium slender, median apophysis slender and spine-shaped with serrated tip, embolus broad with twisted, membranous flange and slit-like opening, membranous spermophor as depicted in Figs 8–10, m29 and m30 originating in tibia. The 3D reconstruction revealed that the spermophor fills most of the subtegulum and is flattened and thin within the embolus (Video 1). Abdomen missing.
Surface model of the spermophor superimposed on the volume rendering of the male palp. Video available for download in full resolution from https://www.pensoft.net/J_FILES/video/Michalik_Ramirez_Video_1.avi.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 8.82 | 7.67 | 5.95 | 7.3 | 2.99 |
| Patella | 2.02 | 1.83 | 1.54 | 1.69 | 0.92 |
| Tibia | 9.91 | 7.55 | 4.63 | missing | 1.43 |
| Metatarsus | 9.40 | 7.60 | 5.39 | missing | - |
| Tarsus | 3.68 | 3.04 | 2.17 | missing | 3.27 |
| Total | 33.84 | 27.71 | 19.69 | 8.62 |
The webs of Thaida chepu are very similar to those described for Thaida peculiaris Karsch 1880 and Austrochilusforsteri Grismado, Lopardo & Platnick, 2003 by
Based on the micro-CT data and manual segmentation of the spermophor we confirm the interpretation of the male palp sclerites especially with regard to the position of the embolus given by
Series of virtual cross sections through the left male palp of Thaida chepu showing the course of the two tendons.
As shown here micro-CT data can be used for precise and transparent descriptions (for details on the method and data handling see
We thank Christian Wirkner and Katharina Huckstorf (University Rostock, Germany) as well as Matias Izquierdo (MACN, Buenos Aires, Argentina) for sharing a fantastic time in the field. We thank Elisabeth Lipke (University Greifswald, Germany) for her help with the 3D data processing and Bernhard Huber (ZMFK Bonn, Germany) for helpful comments on the manuscript. This study was funded by the German Science Foundation (DFG Mi 1255/5-1), CONICET (PIP 112-200801-03209), ANPCyT (FONCyT, PICT-2007-01393) and the University of Greifswald (Germany). The material was collected under CONAF permit 027/2011.