(C) 2012 Catherine A. Tauber. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Santocellus is a small Neotropical genus of leucochrysine lacewings that only recently was separated from Leucochrysa. Here, the features of the Leucochrysa risi Esben-Petersen holotype (a female) are described and shown to support the species’ transfer to Santocellus and the continued retention of the genus Santocellus as separate from Leucochrysa. The valid name for the species becomes Santocellus risi (Esben-Petersen, 1933), comb. n., and Santocellus bullata (Tauber, 2007) is recognized as a syn. n. of Santocellus risi. Currently, this species is reported only from Peru. An illustrated key is provided for distinguishing the known species in the genus Santocellus.
Santocellus, Leucochrysa, Neotropical, Leucochrysini, Peru
The Neotropical genus Santocellus (Neuroptera: Chrysopidae, Leucochrysini) was recently differentiatied from other leucochrysine genera on the basis of a subtle, but consistent, suite of adult and larval traits (
Herein, the features of the previously unknown female are described and illustrated; the results are used to examine the consistency of the female features among Santocellus species. In addition, a key with illustrations for identifying Santocellus species is provided. Methods for staining the abdomen and making measurements were those used previously (see
http://species-id.net/wiki/Santocellus_risi
Figs 1–3, 4C, 4D, 5E, 5FHead, thorax, wings (Figs 4C, 4D, 5E, 5F). Same as described for male (
Female abdomen (Figs 1C, 2, 3). Segments 1–7 long, slender; tergites shallow [ratio length : width = 8.6 (T5), 7.5 (T6), 4.0 (T7)], with slightly rounded margins, with brown circular spot mesally; T8 shorter, rounded, without brown spot. Sternites deep [ratio length : width = 1.5 (S5), 1.0 (S6), 1.4 (S7)], with dorsal margins slightly depressed (concave) mesally; tergites, sclerites with numerous, long, thin setae, dense microsetae, without microtholi. Pleural region with microsetae, P7 with long, thin setae; spiracles small, simple, with unenlarged atria.
Female genitalia. Callus cerci round to slightly oval, 0.10–0.15 mm in diameter, with 19–21 relatively thin trichobothria (longest ~0.13 mm long); cupuliform bases of variable diameter. Tergite 9 + ectoproct rounded, fused dorsally, blunt posteriorly, elongate, ventral section on each side enlarged into pair of bulbous lobes extending well below gonapophyses laterales; enlargement covered with dense, stout, upward-curving setae. Gonapophysis lateralis not large, occupying approximately one-half of posterior margin of abdomen; surface covered with robust, stout setae, especially on ventral half; interior membranous area not greatly expanded. Colleterial gland transparent, delicate, ovoid, small, mostly within gonapophyses laterales and T9+ect, not extending anteriorly much beyond bursa, but with numerous elongate accessory glands attached distally, with transparent, membranous tubule connecting to small reservoir; transverse sclerification short, narrow, receiving short duct from reservoir. Entire genital structure small, not much larger than subgenitale. Bursa copulatrix membranous, broad basally (near subgenitale), tapering and extending slightly into region above S7, folded dorsally, with slight longitudinal depression dorsally, connected ventrally to spermatheca via elongate dorsal slit on spermathecal velum and bursal duct at proximal tip of velum. Bursal duct very short, slender. Bursal glands not seen. Spermatheca doughnut shaped, tucked within distal end of bursa, with small, sail-like velum dorsally, small, V-shaped invagination ventrally. Spermathecal duct attached dorsally to distal end of spermatheca, short, sclerotized, extending into and out of subgenitale, with ~three curves, closely attached to membranes of bursa and subgenitale; terminus with long, dense setae. Subgenitale broad basally, rounded distally, nestled between ventral lobes of ectoproct, narrow in lateral view, with shallow ventral fold at attachment to S7, slightly deeper fold above, terminal process flat, long, extending almost full length of subgenitale, with pair of lobes at base, shallow crumena at rounded tip; membrane above subgenitale with crescent-shaped, lightly sclerotized lamellae.
Holotype (ZMCU) and a second female specimen (MCZ), with labels reading: [1] “El Campamiento Col. P?r?n? [“?” mine] PERU 1 July ’20”, [2] “Cornell Univ. Expedition Lot 569”, [3] “Leucochrysa (or Nodita) risi Esb-Petersen 1932 det. P.Adams 1974”. The locality data appear to refer to the Expedition’s Camp at Perené in the province of Chanchamayo, Junin, Peru, elevation 696 m (Cornell University Insect Collection Voucher Lot Series, Lot 569).
Currently, this species is known only from three regions of Peru: Junin (~650 m) (new record), Pasco (~800 m) (
The genus Santocellus was described on the basis of a distinctive suite of larval and adult (male and female) character states. However, Santocellus risi (as bullata) was included in the genus only on the basis of its male characteristics (
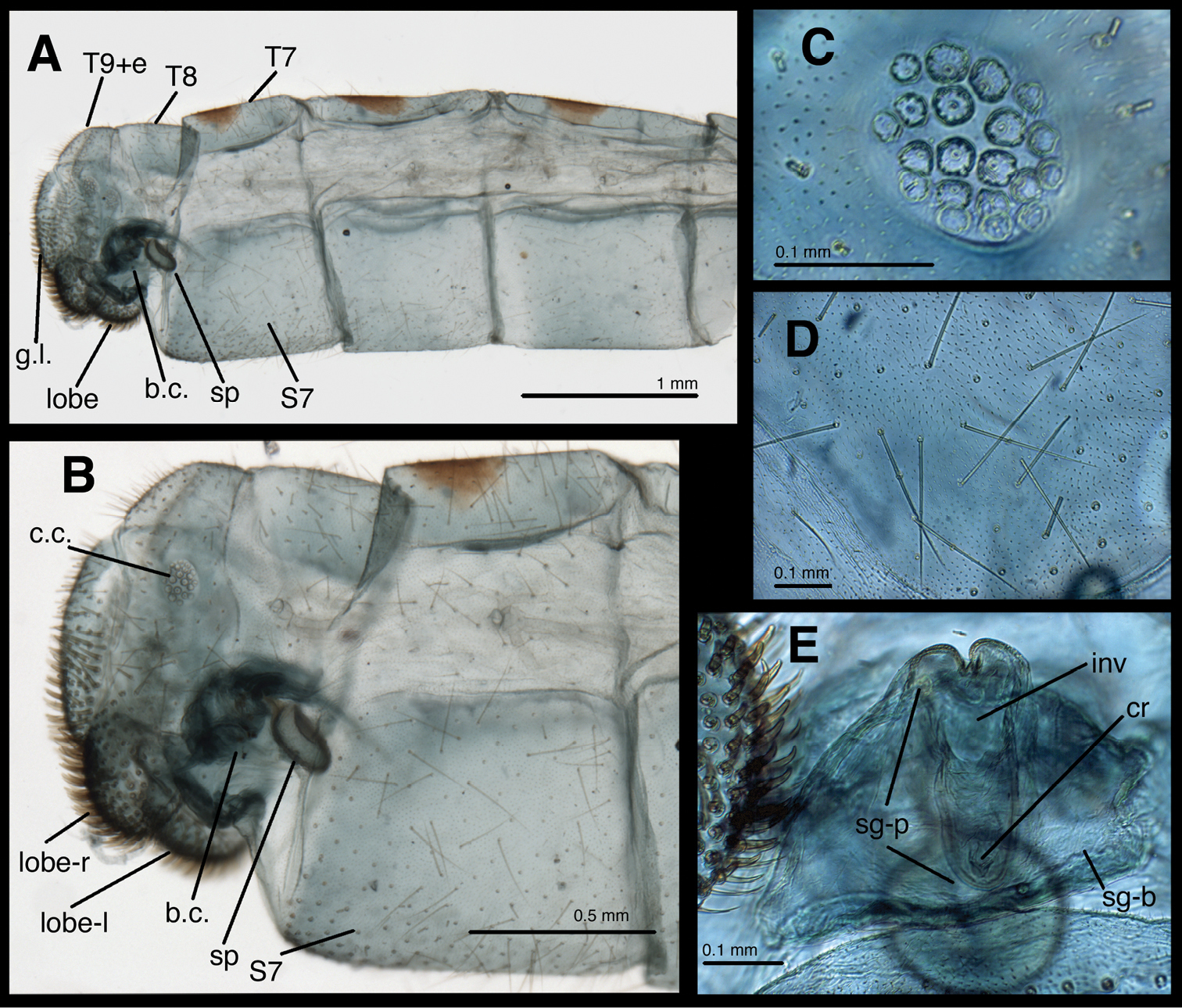
Leucochrysa risi Holotype [Female, Pasco, Peru; Zoological Museum of Copenhagen University; Photos by Niels P. Kristensen] A Head, thorax, dorsal B Head, frontal C Habitus, dorsal D Labels.
Female abdomen of Leucochrysa risi [Female (mature), Pasco, Peru]. A Segments A5 to A9+ectoproct, lateral B Segments A7 to A9+ectoproct, lateral C Callus cerci (trichobothria missing) D Abdominal integument E Subgenitale, posterior. Abbreviations: b.c. bursa copulatrix; c.c. callus cerci; cr crumena of subgenitale; g.l. gonapophysis lateralis; inv, invagination below distal lobes of subgenitale; lobe setose lobe at ventral margin of ectoproct; lobe-l lobe on left side of body; lobe-r lobe on right side of body; sg-b base of subgenitale; sg-p elongate ventral process of subgenitale; sp spermatheca; S7 seventh sternite; T7, T8 seventh and eighth tergites; T9+e fused ninth tergite and ectoproct.
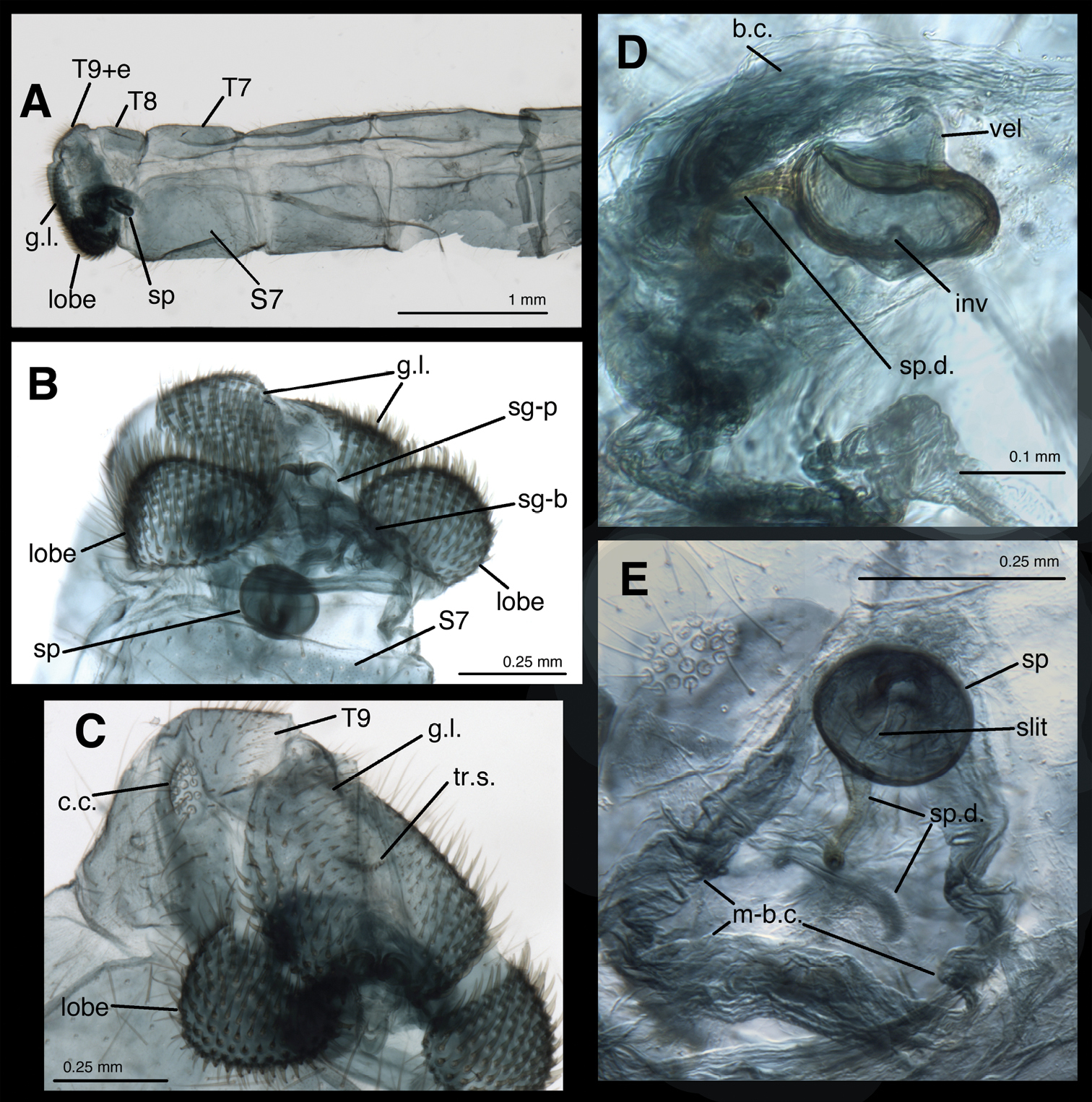
Female abdomen of Leucochrysa risi [Female (slightly teneral), Junin, Peru] A Segments A5 to A9+ectoproct, lateral B Abdominal terminus, posteroventral, showing enlarged, setose lobes on ventral margin of ectoproct. Note subgenitale nestled between left and right lobes, round spermatheca (with dorsal slit) above subgenitale C Abdominal terminus, ventrolateral, showing enlarged, setose lobes on ventral margin of ectoproct, setose surface of gonapophysis lateralis D Spermatheca below bursa copulatrix, lateral view E Spermatheca and spermathecal duct engulfed within membrane of bursa copulatrix, ventral view. Abbreviations: b.c. bursa copulatrix; c.c. callus cerci; g.l. gonapophysis lateralis; inv spermathecal invagination; lobe setose lobe on ventral margin of ectoproct; m-b.c. membrane of bursa copulatrix; sg-b base of subgenitale; sg-p elongate ventral process of subgenitale; slit slit in dorsal surface of spermathecal velum, opening to bursal duct above (not visible); sp spermatheca; sp.d. spermathecal duct; S7 seventh sternite; T7, T8 seventh and eighth tergites; T9+e fused ninth tergite and ectoproct; tr.s. transverse sclerite; vel, spermathecal velum.
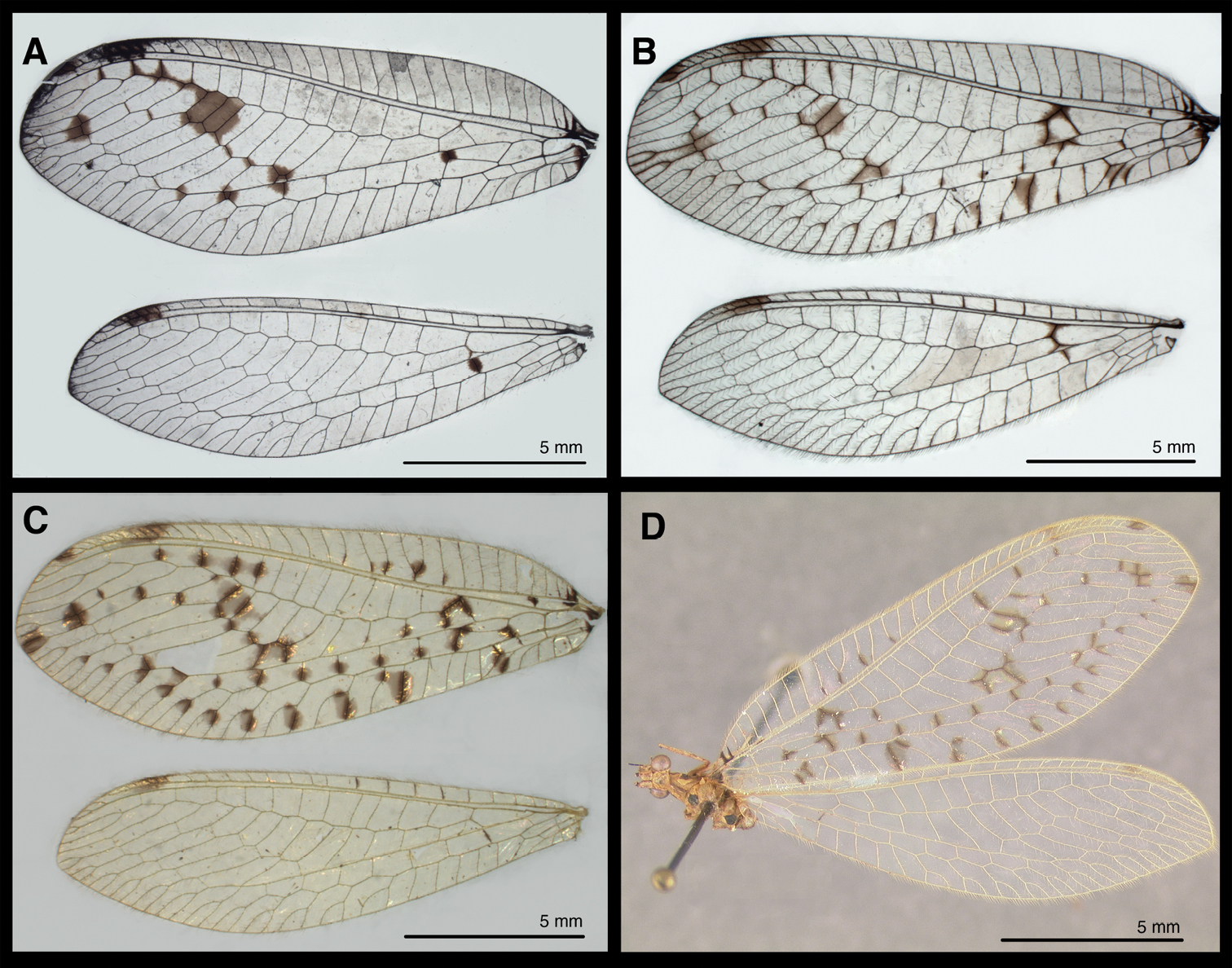
Wings of known Santocellus species. A Santocellus atlanticis [Female; Rio de Janeiro, Brazil] B Santocellus riodoce [Female; Espírito Santo, Brazil] C Santocellus risi [Male; Madre de Dios, Peru] D Santocellus risi [Female; Junin, Peru].
External features of known Santocellus species. Top row: Head and thorax, dorsal; Bottom row: Head, frontal A, B Santocellus atlanticis [Male; Rio Grande do Sul, Brazil] C, D Santocellus riodoce [Female; Espírito Santo, Brazil] E, F Santocellus risi [Male; Madre de Dios, Peru].
The key below is intended for identification without dissecting the specimens. For species-specific differences in male and female terminalia, see
| 1 | Membrane surrounding numerous crossveins of forewing with pustulate swellings (Figs 4C, 4D); meso- and metanotum each with large, dark brown to black, mesal spot (Fig. 5E) | Santocellus risi (Esben-Petersen) |
| – | Membrane of forewing smooth, without swellings (Figs 4A, 4B); mesonotum either largely or entirely dark brown to black, with or without light green areas (Figs 5A, 5C) | 2 |
| 2 | Forewing with cells between Radial sector (Rs) and inner gradates 5-6 entirely filled with brown, Rs and all crossveins between Rs and Psm without dark clouding (Fig. 4A); mesoscutellum light green (Fig. 5A) | Santocellus atlanticis Tauber & Albuquerque |
| – | Forewing with cells between Radial sector (Rs) and inner gradates 5-6 only partially filled with brown, Rs and first two crossveins between Rs and Psm with dark clouding (Fig. 4B); mesoscutellum largely brown, posterior with small light green spot (Fig. 5C) | Santocellus riodoce Tauber |
It is a pleasure to thank N. P. Kristensen (Zoological Museum, University of Copenhagen), O. S. Flint, Jr. (U.S. National Museum of Natural History), and B. D. Farrell, P. D. Perkins and S. Cover (Museum of Comparative Zoology) for making specimens and/or images available for study; J. K. Liebherr (Cornell University) for information from the Cornell University Insect Collection Voucher Lot Series; J. D. Oswald (Texas A & M University) for access to the “Lacewing Digital Library” website and information from his database; the Mann Library at Cornell University and the Shields Library at the University of California, Davis, for cooperation and help; G. S. Albuquerque (GSA) for collaboration during our study at the MCZ; and M. J. Tauber (MJT) for his help, encouragement, and careful reading of the manuscript.
This work received support from the National Science Foundation (Grants INT-9817231, DEB-0542373, MJT, CAT), the National Geographic Society (MJT, CAT, GSA), the USDA/NRI (Competitive Grant 9802447, MJT, CAT), Regional Project W-3185, and Cornell University.