(C) 2012 Malkie Spodek. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Descriptions and illustrations of the adult female and first-instar nymph of the kermesid Nidularia balachowskii Bodenheimer, based on the adult female lectotype and paralectotype (here designated), and new material collected from Israel are presented. A key for the identification of first-instar nymphs of Nidularia spp. is offered. Molecular identification of Nidularia balachowskii, using nucleotide sequences from the D2–D3 region of the 28S ribosomal gene, and the mitochondrial Cytochrome Oxidase I (COI) gene, is presented. Morphological and molecular analyses confirm that Nidularia balachowskii is closely related to other species within the Kermesidae. In Israel, this species develops only on Quercus ithaburensis and is univoltine. This is the first detailed report of Nidulariabalachowskii from Israel.
Scale insect, Quercus spp. , morphology, univoltine, monophagous, 28S, COI
Nidularia Targioni-Tozzetti, 1868 is one of ten genera of scale insects within the Kermesidae (Hemiptera: Coccoidea). Three species of Nidularia: Nidularia balachowskii Bodenheimer, 1941, Nidularia japonica Kuwana, 1918 and Nidularia pulvinata (Planchon, 1864), have been recorded so far only from the Palaearctic region (
The three species of Nidularia have been recorded as follows: Nidularia pulvinata from Algeria (
The family placement of Nidularia has changed over the years.
Most species of the Kermesidae appear to be univoltine (
The body of post-reproducing females may remain on the host tree for a year or more after the emergence of the first-instar nymphs (
The main synapomorphic characters of kermesid adult females have been summarized by several authors including:
Species of Nidularia share morphological and biological characteristics with other species of the Kermesidae. Like Kermes spp., Nidularia spp. are monophagous and develop on oak trees. They are known as ‘gall-like insects’ due to the size and body shape of the convex and sclerotized post-reproductive adult female (
Seven species of Kermesidae belonging to two genera, Kermes Boitard and Nidularia Targioni-Tozzetti, are recorded from Israel (
Between 2010 and 2012, we surveyed the scale insect fauna of various species of oak trees in Israel. Nidularia balachowskii was found on branches of Quercus ithaburensis over a wide range of oak forests in northern Israel. Although
Bodenheimer’s original description of Nidularia balachowskii contains a description and some illustrations of major characters in the adult female (
This redescription of Nidularia balachowskii is based on type material (see Material examined below), plus specimens from Israel collected by Bodenheimer and fresh material collected in Israel by us. Populations of Nidularia balachowskii from Quercus ithaburensis trees were studied and specimens were collected between 2010 and 2012 from the following nature reserves in northern Israel: Yehudiya Nature Reserve, Golan Heights (32°56'19"N, 35°39'56"E); Horshat Tal Nature Reserve, Upper Galilee (33°13'13.74"N, 35°37'45.65"E); Alonei Abba Nature Reserve, Lower Galilee (32°43'46.2"N, 35°10'18.47"E). Trees at each reserve were surveyed at least once a month and 150–200 branches (20–25 cm in length) were removed at each visit. The branches were taken back to the laboratory in large plastic bags and examined individually under a stereomicroscope for scale insects. Relevant specimens were slide-mounted for microscope examination using the protocol in Ben-Dov,
Illustrations of the adult female and the first-instar nymph of Nidularia balachowskii are generalizations of several specimens, showing the dorsum on the left and the venter on the right, with enlargements of important structures arranged around the main drawing. The enlarged structures are not drawn to the same scale. Terms for morphological features follow chiefly those of
Abbreviations of specimen depositories are as follows: BMNH -The Natural History Museum, London, U.K.; ICVI - Coccoidea Collection, Department of Entomology, Agricultural Research Organization, Bet Dagan, Israel; MNHN - Museum National d’ Histoire Naturelle, Paris, France; and TAU - Tel Aviv University Insect Collection, Israel.
Turkey: Lectotype female (ICVI), here designated, and paralectotype female (MNHN), 21 km at road from Mardin to Diyarbakir, on branches and twigs of Quercus sp. (Fagaceae), 13.ii.1939, F.S. Bodenheimer.
Additional non-type material from Turkey as follows: Van-Koçet Road (alt.1625 m) on Quercus sp., 19.vii.2005, B. Kaydan (Yuzuncu Yil University, Turkey 2056); Hakkari -Üzümcü Road (alt. 956 m) on Quercus sp., 15.ix.2005, B. Kaydan (Yuzuncu Yil Universty, Turkey 2343); Van-Hakkari Road (alt. 1266 m) on Quercus sp., 16.ix.2005, B. Kaydan (Yuzuncu Yil Universty, Turkey 2370); Hakkari-Doğan (alt. 1032 m) on Quercus sp., 22.v.2005, B. Kaydan (Yuzuncu Yil Universty, Turkey 2688); Bitlis River (alt. 797 m) on Quercus sp., 23.vi.2006, B. Kaydan (Yuzuncu Yil Universty, Turkey 3036); Bitlis-Kavakbaşı (alt. 1365) on Quercus sp. 30.v.2007, B. Kaydan (Yuzuncu Yil Universty, Turkey 3419).
Israel: adult female, Daphne Oaks (= current name Horshat Tal Nature Reserve), on Quercus sp., 1.v.1939, F.S. Bodenheimer, (ICVI C:4805). This was the first record of this species from Israel. Additional females and first-instar nymphs, all collected off Quercus ithaburensis by M. Spodek: Yehudiya Nature Reserve, 10.x.2010, 7.xi.2010, 11.i.2011, 6.ii.2011, 16.x.2011, 6.xi.2011, 3.vi.2012, (ICVI C:4891, C:4912, C:4945, C:4970, MC:587, C:4970, MC:690); Horshat Tal Nature Reserve, 30.v.2010, 14.ii.2012, 27.ii.2011, 13.iii. 2012, (ICVI MC:228, MC:614, MC:430, C:5131); Alonei Abba Nature Reserve, 11.1.2011, (ICVI MC:385). First-instar nymphs; Yehudiya Nature Reserve, 6.iii.2010, 20.iii.2011, 24.iii.2012, (ICVI MC:140, MC:460, MC:637); Horshat Tal Nature Reserve, 13.iii.2012, 15.iii.2012, (ICVI MC:635, MC:636).
Comparative material examined
Nidularia pulvinata: France: adult female and first-instar nymphs, Serignan, Vauctuse, on Quercus ilex, 18.v.1978, I. Foldi (ICVI C:4946); first-instar nymphs, Caumont (Avignon), on Quercus ilex, 11.iv.1978, D. Matile, J.P. Fabre, (MNHN 7337-4); Italy: adult femalePisa, on Quercus ilex, 5.iv.1988, D. Matile-Ferrero (MNHN10959, ICVI 5060)
Kermes quercus: Sweden: adult female, Skan, Near Lund on Quercus robur, 10.vi.2010, C.A Gertsson (ICVI C:4806); England: adult female , Wytham Wood, Berkshire on Quercus robur, 10.v.1965, S.C. Varley (BMNH 81-539); Poland; first-instar nymph, Warsaw on Quercus robur, 25.viii.1994, E. Podsiadlo (ICVI C:4798)
Kermes roboris: Hungary: adult female, Budapest, Plant Protection Institute-adjacent track on Quercus sp., 8.vi.1989, C.P. Malumphy (BMNH); England: adult female, Kent: Herne Bay on Quercus spp., 00.viii.1899, C.D Waterhouse (ICIV C:5071).
Specimens of the following adult female kermesidspecies, identified by YBD, were used in the molecular part of this study: Kermes nahalali Bodenheimer, Kermes echinatus Balachowsky, Kermes greeni Bodenheimer, Kermes quercus (Linnaeus), Kermes spatulatus Balachowskyand Nidularia balachowskii. Three specimens of each species were used as replicates except for Nidularia balachowskii, where six were used. Adult females, preserved in 96% ethanol, were examined under the stereomicroscope for the presence of hymenopteran parasitoid wasps prior to DNA extraction. Voucher specimens were slide-mounted using the cuticle of the actual specimens from which DNA was extracted. Slide mounting followed the protocol outlined in Ben-Dov,
Collection information and GenBank accession numbers for insect samples used in this study.
| Species name | Family | Voucher code | Host tree | Location collected | Date collected | Collector | GenBank Accession No. 28S | GenBank Accession No. COI |
|---|---|---|---|---|---|---|---|---|
| Kermes nahalali Bodenheimer | Kermesidae | C-5111 | Quercus ithaburensis | ISRAEL: Alonei Abba Reserve | 27.ii.2011 | M. Spodek | JX436134 | JX436113 |
| Kermes nahalali Bodenheimer | Kermesidae | C-5112 | Quercus ithaburensis | ISRAEL: Alonei Abba Reserve | 27.ii.2011 | M. Spodek | JX436135 | JX436114 |
| Kermes nahalali Bodenheimer | Kermesidae | C-5113 | Quercus ithaburensis | ISRAEL: Alonei Abba Reserve | 27.ii.2011 | M. Spodek | JX436136 | JX436115 |
| Kermes echinatus Balachowsky | Kermesidae | C-5114 | Quercus calliprinos | ISRAEL: Alonei Abba Reserve | 19.vi.2011 | M. Spodek | JX436137 | JX436116 |
| Kermes echinatus Balachowsky | Kermesidae | C-5115 | Quercus calliprinos | ISRAEL: Alonei Abba Reserve | 19.vi.2011 | M. Spodek | JX436138 | JX436117 |
| Kermes echinatus Balachowsky | Kermesidae | C-5116 | Quercus calliprinos | ISRAEL: Alonei Abba Reserve | 19.vi.2011 | M. Spodek | JX436139 | JX436118 |
| Kermes greeni Bodenheimer | Kermesidae | C-5117 | Quercus calliprinos | ISRAEL: Hanita | 8.vi.2011 | M. Spodek | JX436140 | JX436119 |
| Kermes greeni Bodenheimer | Kermesidae | C-5118 | Quercus calliprinos | ISRAEL: Hanita | 8.vi.2011 | M. Spodek | JX436141 | JX436120 |
| Kermes greeni Bodenheimer | Kermesidae | C-5119 | Quercus calliprinos | ISRAEL: Hanita | 8.vi.2011 | M. Spodek | JX436142 | JX436121 |
| Kermes quercus (Linnaeus) | Kermesidae | C-5120 | Quercus robur | SWEDEN: Skan, near Lund | 10.vi.2010 | C. A. Gertsson | JX436143 | JX436122 |
| Kermes quercus(Linnaeus) | Kermesidae | C-5121 | Quercus robur | SWEDEN: Skan, near Lund | 10.vi.2010 | C. A. Gertsson | JX436144 | JX436123 |
| Kermes quercus (Linnaeus) | Kermesidae | C-5122 | Quercus robur | SWEDEN: Skan, near Lund | 10.vi.2010 | C. A. Gertsson | JX436145 | JX436124 |
| Kermes spatulatus Balachowsky | Kermesidae | C-5123 | Quercus ithaburensis | ISRAEL: Horshat Tal Reserve | 3.iv.2011 | M. Spodek | JX436146 | JX436125 |
| Kermes spatulatus Balachowsky | Kermesidae | C-5124 | Quercus ithaburensis | ISRAEL: Horshat Tal Reserve | 3.iv.2011 | M. Spodek | JX436147 | JX436126 |
| Kermes spatulatus Balachowsky | Kermesidae | C-5125 | Quercus ithaburensis | ISRAEL: Horshat Tal Reserve | 3.iv.2011 | M. Spodek | JX436148 | JX436127 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5126 | Quercusithaburensis | ISRAEL: Yehudiya Reserve | 11.i.2011 | M. Spodek | JX436149 | JX436128 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5127 | Quercus ithaburensis | ISRAEL: Yehudiya Reserve | 11.i.2011 | M. Spodek | JX436150 | JX436129 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5128 | Quercus ithaburensis | ISRAEL: Alonei Abba Reserve | 27.ii.2011 | M. Spodek | JX436151 | JX436130 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5129 | Quercus ithaburensis | ISRAEL: Alonei Abba Reserve | 27.ii.2011 | M. Spodek | JX436152 | JX436131 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5130 | Quercus ithaburensis | ISRAEL: Horshat Tal Reserve | 14.ii.2012 | M. Spodek | JX436153 | JX436132 |
| Nidularia balachowskii Bodenheimer | Kermesidae | C-5131 | Quercus ithaburensis | ISRAEL: Horshat Tal Reserve | 14.ii.2012 | M. Spodek | JX436154 | JX436133 |
| Kermes nakagawae Kuwana | Kermesidae | n/a | AB439525.1 | |||||
| Bambusaspis miliaris (Boisduval) | Asterolecaniidae | GU998966.1 | n/a | |||||
| Ceroplastes rubens Maskell | Coccidae | n/a | JQ795720.1 | |||||
| Paralecanium sp. | Coccidae | GU998968.1 | n/a | |||||
| Pelliculaspis celtis McDaniel | Diaspididae | GQ325525.1 | n/a | |||||
| Apiomorpha nookara Mills | Eriococcidae | n/a | JN863289.1 | |||||
| Eriococcus spurius (Modeer) | Eriococcidae | GU998969.1 | n/a | |||||
| Drosicha mangiferae (Green) | Monophlebidae | n/a | JF792882.1 | |||||
| Phenacoccus parvus Morrison | Pseudococcidae | n/a | JQ863289.1 | |||||
| Outgroup | ||||||||
| Acyrthosiphonpisum Harris | Aphididae | S50426.1 | EU701281.1 |
DNA was extracted from parasitoid-free adult females using the Cetyl trimethylammonium bromide (CTAB) method (
The PCR cycling conditions for 28S were 94°C for 4 min, followed by 35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1.5 min, with a final extension at 72°C for 4 min. The PCR cycling protocol for COI was 95°C for 7 min, followed by 40 cycles of 95°C for 1 min, 45°C for 1 min, and 72°C for 1.5 min, with a final extension at 72°C for 5 min. Each reaction was examined by electrophoresis and bands were visualized with UV light. PCR products were excised from the gel and purified using the Zymoclean Gel Extraction Kit (Zymo Research, Irvine, CA). Purified PCR products were sequenced in both the forward and reverse directions at Hy-Labs (Rehovot, Israel).
Sequence alignments for both 28S and COI gene sequences were performed with MUSCLE 3.7 (
http://species-id.net/wiki/Nidularia_balachowskii
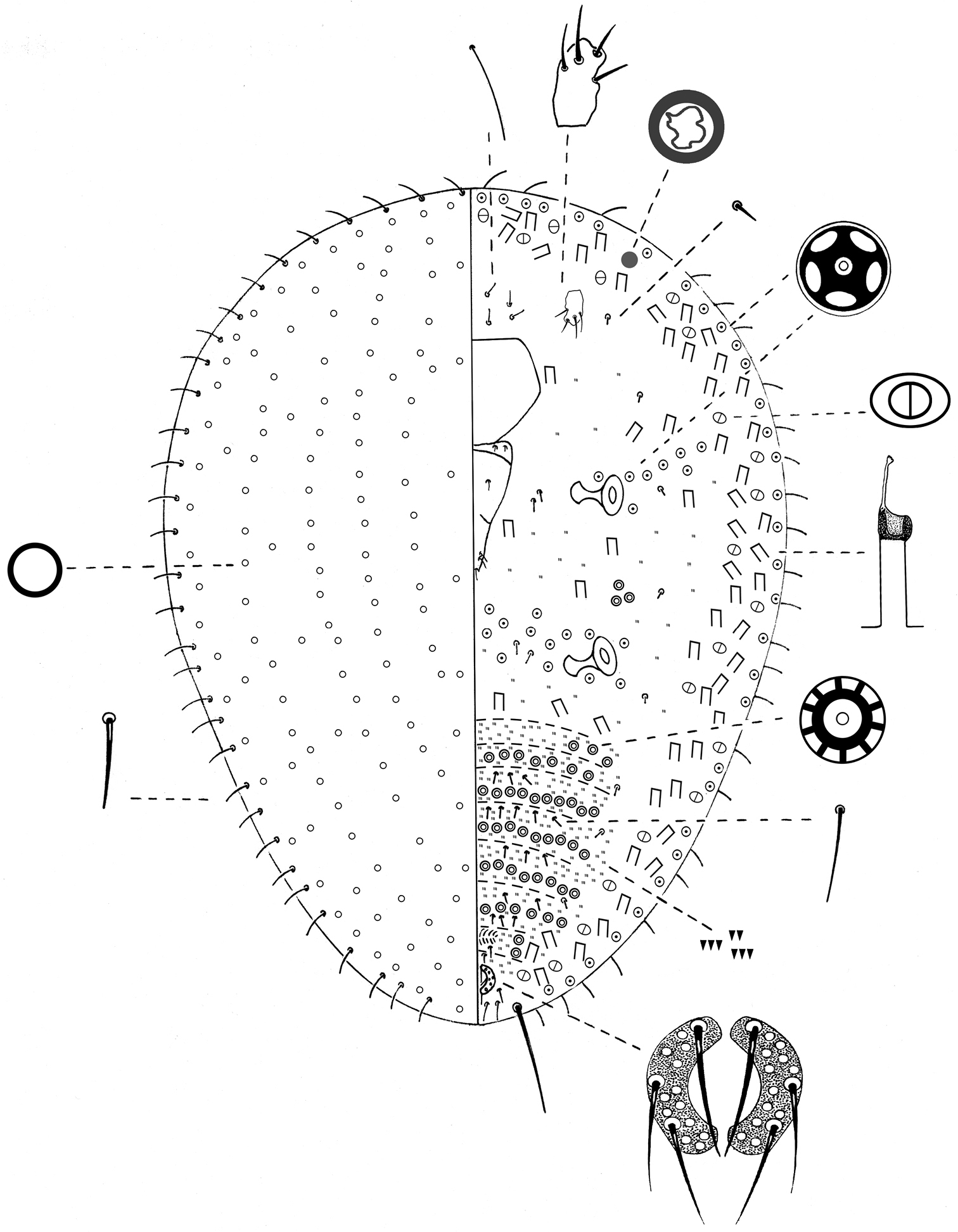
General appearance. Young, pre-reproductive adult dorsum brownish and venter yellowish white; oval, soft and flat; 1.2–1.9 mm long and 0.6–0.9 mm wide. Dorsal surface covered with 5 longitudinal rows of rectangular wax plates, each plate about 0.25 mm long and 0.3 mm wide; median row with 11 plates, lateral row on each side of median row with 9–11 plates and marginal rows with 7–9 plates. The wax plates become gradually smaller in size towards anterior and posterior apices and lateral margin (Fig. 1). Post-reproductive female oval, moderately convex and sclerotized; 2.75–3.75 mm long, 2–3 mm wide and 0.8–1.8 mm high; 5 longitudinal rows of dark brown wax plates almost fused; with lighter brown wax in between rows of plates (Fig. 2).
Slide-mounted young adult female 0.8– 3 mm long, 0.5–2.5 mm wide (Fig. 3).
Margin. Marginal setae, pointed, 12–13 µm long; placed in a row of 30–36 setae. Stigmatic spines absent. Anal cleft absent.
Dorsum. Simple pores, circular, each with a sclerotized rim and 1 µm diameter; covering entire dorsum. Other pore types absent. Dorsal setae absent.
Venter. Eyes circular, 5–7 µm diameter, each placed anterolaterally to each antenna. Antennae each1-segmented, 15–25 µm long, 12–18 µm wide; eachbearing 2–6 fleshy setae. Legs absent. Clypeolabral shield 113–155 µm long, 113–125 µm wide. Labium 3-segmented, triangular, 100–125 µm long, 50–63 µm wide; labial setae as follows; basal segment with 2 pairs of hair-like setae, 9–20 µm long, median segment with 1 pair of hair-like setae, 11–13 µm long, apical segment with 6 setae; 2 apical setae, 9–12 µm long plus 4 hair-like; subapical setae, each 12–20 µm long. Spiracles subequal in size; each 42–60 µm long, 31–50 µm diameter of peritreme. Quinquelocular pores each 5 µm diameter; with 8–11 between mesothoracic spiracles and submarginal band of tubular ducts; 10–13 between the metathoracic spiracles; 2–4 laterad to each metathoracic spiracle; also in a single, complete submarginal band, 1 pore wide from head apex to anal ring; total number of pores per side about 50–71.Bilocular pores each about 3 µm wide, totaling 103–135 per side, dispersed within a submarginal tubular duct band. Multilocular pores each 7–8 µm in diameter with 9–10 loculi; in groups of 3 or 4 between each metathoracic and mesothoracic spiracle;in transverse bands across abdominal segments arranged as follows; segment III with 3–7, IV with 12–24, V with 14–28, VI with 15–35, VII with 18–25, VIII with 12–19, IX with 2–3 on each side of vulva. Tubular ducts dispersed in a complete submarginal band, 2–3 ducts wide, each duct with outer ductule 12–18 µm long and 5 µm wide, a sclerotized cup about 5 µm in diameter and inner ductule about 22–30 µm long; also scattered over thorax. Other ventral setae: with a group of 7 or 8 setae, each 7–13 µm long, anterior to clypeus; 1 pair, 7–8 µm long, posterior to each antenna; 8–12 setae, each 5–8 µm long, placed medially to each spiracle; 8 setae, 5–13 µm long, distributed in 1 longitudinal row placed medially to each marginal band of bilocular pores and tubular ducts; each abdominal segment with transverse rows of 4–10 setae, each 7–8 µm long, placed anterior to bands of multilocular pores. Microspines present on median and submedian areas of each abdominal segment, in 3–5 transverse rows; each spine about 1 µm long, also scattered on thorax. Anal ring located on venter, composed of 2semi-circles; diameter27–35 µm; each half circle bearing 3 pointed setae and 10–12 cells; anterior setae each 25–38 µm long, median and posterior setae each 15–25 µm long; 2 pairs of thin setae just anterior to anal ring, each 7–10 µm long, plus a pair of pointed setae postero-laterally to anal ring, separated by a space about double diameter of anal ring, each 10–13 µm long. Also1 pair of apical setae, each 65–68 µm long, and 4 setae, each 15–22 µm long, between apical setae, similar in structure to, but longer than, marginal setae.
Nidularia balachowskii Bodenheimer young adult female, general appearance.
Nidularia balachowskii Bodenheimer post-reproductive female, general appearance.
Nidularia balachowskii Bodenheimer adult female.
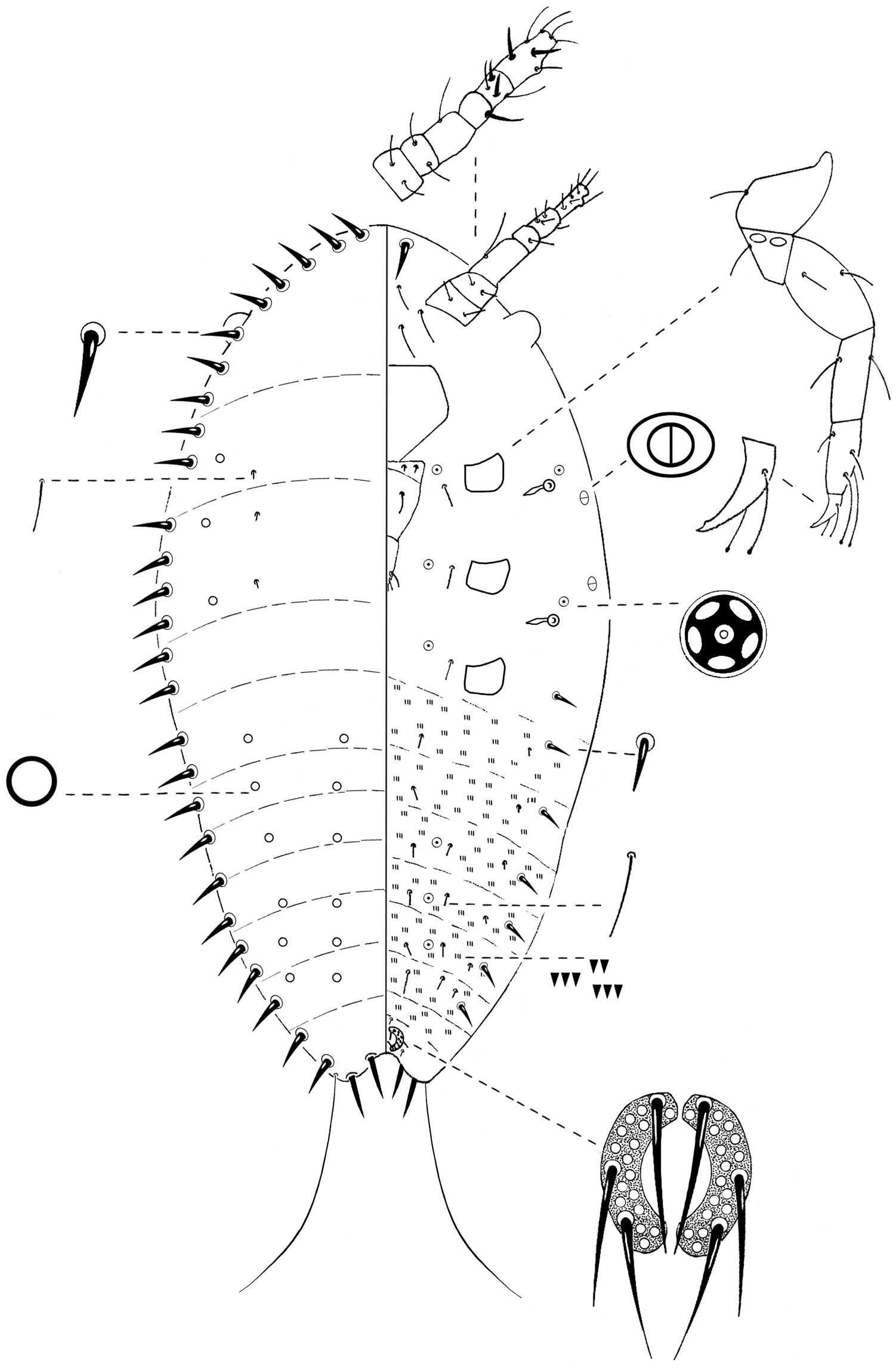
General appearance. Yellow-greyish, oval and tapering posteriorly 0.38–0.43 mm long and 0.2–0.3 mm wide.
Slide-mounted specimen. Oval, 0.42–0.53 mm long and 0.19–0.34 mm wide (Fig. 4).
Margin. Marginal setae sharply spinose, pointed apically and slightly curved; each 9–15 µm long; in a distinct row of 26–33 setae on each margin.
Dorsum. Derm membranous; intersegmental lines observable. Simple pores, circular with a sclerotized rim, each 1 µm diameter; totaling about 30, scattered over entire dorsum in 4 longitudinal rows; 2 submarginal lines on thorax and abdomen and 2 medial lines on abdomen; with a single pair of submedial setae on each thoracic segment, each 5 µm long.
Venter. Eyes present as semi-circles near margin, diameter 12–15 µm. Antennae each 6-segmented, length 88–125 µm; segments III and VI longer than other segments; scape with 2 hair-like setae; pedicel with 2 hair-like setae; segment III with 1 long hair-like seta; IV with 1 fleshy seta; V with 1 fleshy seta, 2 hair-like setae and, 1 thick hair-like seta; apical segment with 2 fleshy setae and 5 hair-like setae. Legs well-developed; measurements of hind legs; (in µm): coxae 25–30, trochanter + femur 63–80, tibia 25–38, tarsus 25–60, claw 13–23; total leg length 158–213; trochanter with 2 oval, sensory pores on each side, each about 2–3 µm wide; setae present on each leg segment; tarsal digitules each 25–30 µm long, knobbed apically, extending beyond apex of claw; claw digitules knobbed apically, each 14–20 µm long, shorter than tarsal digitules; each claw with a single denticle near the tip. Clypeolabral shield well-developed; 75–90 µm long and 63–75 µm wide. Labium 3-segmented, triangular, 82–100 µm long and 35–38 µm wide; labial setae as follows; basal segment with 2 pairs of setae, each 10–15 µm long, median segment with 1 pair of hair-like setae on dorsal surface, each 10–20 µm long, apical segment with 3 pairs of hair-like setae, 12–17 µm long. Spiracles subequal in size; peritreme about 3–5 µm diameter; crescent shaped sclerosis 15–25 µm long. Bilocular pores, oval, each 2 µm long and 1 µm wide, with 1 present submarginally about level of each spiracle. Quinquelocular pores each 3 µm diameter, as follows; 1 just anterior to each spiracle; 1 medially to each coxa; 2 on each of abdominal segments IV, V, VI. Microspines present on median and submedian areas of each abdominal segment, in 2–4 transverse rows; each spine about 1 µm long. Setae 1 pair, similar in size and shape to marginal setae, between anterior apex of body and basal segments of antennae; 6 interantennal setae, each 20–30 µm long between basal segments of antennae and anterior apex of clypeus; 1 seta 8–15 µm long medially to each coxa; 2 longitudinal rows, each with 7–8 setae, similar in shape to marginal setae but shorter, each about 8 µm long, extending submarginally from laterad to metathoracic coxae to anterior margin of anal ring, no setae present on most posterior abdominal segment; 2 longitudinal rows of 6 setae, each about 5 µm long, located submarginally on abdomen; 2 longitudinal rows of 4 setae, each about 10 µm long, located submedially on abdomen; plus 2 longitudinal rows of 6 setae, about 17–18 µm long, located medially. Anal ring located ventrally; composed of 2 semi-circles; diameter 15–25 µm; each semi-circle with about 17 cells and 3 pointed setae, subequal in size, each 20–30 µm long. Also with a pair of setae, each 12–15 µm long, anterior to anal ring and 1 pair, each 15–25 µm long, postero-laterally to anal ring. Anal lobes well-developed; each lobe bearing 2 pointed setae, 12–15 µm long, distinctly thicker than marginal setae, plus 1 pair of long, apical setae 77–125 µm long.
Nidularia balachowskii Bodenheimer first-instar nymph.
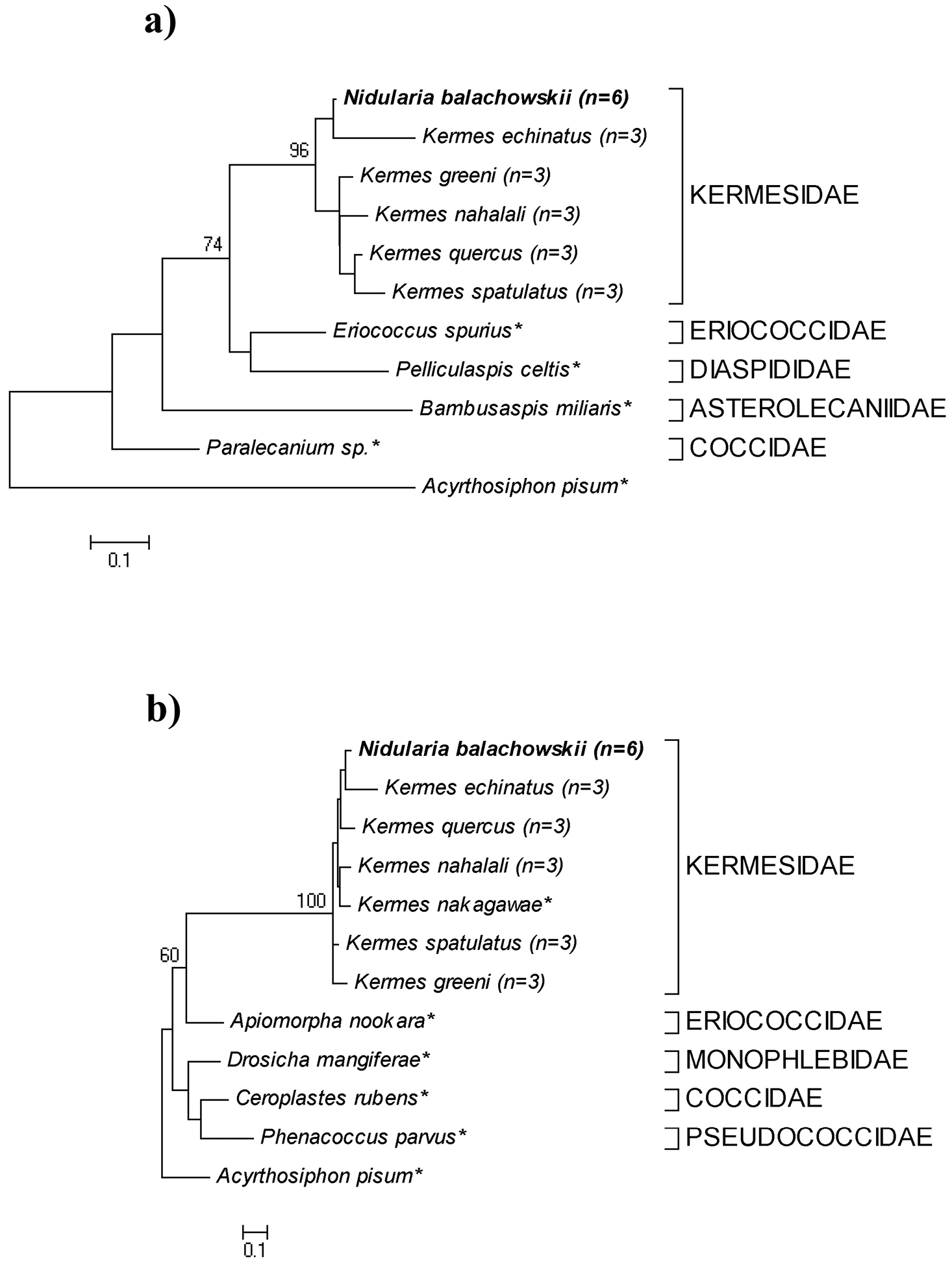
We obtained a total of forty-two nucleotide sequences from the 28S and COI genes from Nidularia balachowskii (six individuals for each gene) and from five adult female Palaearctic Kermesidae species (three individuals for each species for each gene). 28S gene sequences (~700 bp) and COI sequences (~400 bp) from all species were recovered and aligned with sequences of Coccoidea species representing different families (obtained from GenBank). All species for which multiple specimens were sampled showed no interspecies variation. The maximum likelihood analysis of both genes resulted in tree typologies that show that Nidularia balachowskii is a distinct species within the monophyletic Kermesidae. Nidularia balachowskii is grouped together with other kermesid species and not with the other Coccoidea (Figures 5a+b). The bootstrap value that represents the separation between species of Kermesidae and species from other Coccoidea families is higher in the 28S tree typology, 74, compared to 60 obtained from the COI sequences.
Sequence divergence based on Kimura 2-parameter pairwise distance, between Nidularia balachowskii and the other five Kermesidae species ranged from 0.16–0.19 in the 28S gene region. This range is compared to the 0.2–0.3 sequence divergence range between Nidularia balachowskii and species from the four other Coccoidea families. In the COI gene region, the sequence divergence between Nidularia balachowskii and the six other Kermesidae species ranged from 0.06–0.13 and between Nidularia balachowskii and the four species from other Coccoidea families had a sequence divergence range of 0.8–1. Both trees show a strong relationship between Nidularia balachowskii and Kermes echinatus, indicating that they are closer to each other than to the other Kermesidae species examined.
Maximum likelihood trees of 28S (a) and COI (b) nucleotide sequences of Nidularia balachowskii and other Coccoidea species. Acyrthosiphon pisum (Aphididae) sequences are used as outgroup species for both trees. Trees were constructed using K2P distance model and numerical values are bootstrap support, based on 1000 replicates (n= number of replicates, * = sequences derived from GenBank).
Observations about the life history of Nidularia balachowskii were made in three nature reserves in northern Israel: Alonei Abba Nature Reserve, Horshat Tal Nature Reserve and Yehudiya Nature Reserve. The predominant oak species growing in these reserves is Quercus ithaburensis. In Alonei Abba Reserve, Quercus calliprinos trees are also present but they are less common. In Israel, Nidularia balachowskii has only been found on the trunks and branches of Quercus ithaburensis, where Nidularia balachowskii is an oviparous and univoltine species.
Gravid females were observed on branches and trunks of trees throughout March, during which time they oviposited 200 to 250 (range from 10 specimens) whitish eggs. Each egg was about 0.4 mm long and 0.2 mm wide. Once all of the eggs have been laid and the brood chamber full of eggs, the female dies and the dorsum becomes sclerotized. The sclerotized, convex body of the dead, post-reproductive female may remain on the host tree for a year or more after first-instar emergence.
Eclosion of first-instar nymphs occurs inside the brood chamber and nymphs emerge from the cavity under the dead female body. This takes place from end of March and throughout April. Crawlers settle in bark crevices on branches and on the trunks of the trees. Young teneral females are found on the branches from June to February. The females continue feeding and increase in body size throughout this period. Feeding was confirmed by observations of honeydew elimination. By late February, the dorsum of the female begins to expand greatly, increasing in convexity and sclerotization. The ventral surface of the abdomen becomes concave, forming the brood chamber into which the eggs are deposited. The ovipositing female secretes a woolly, white wax that surrounds its body margin. No injury has been observed to the oak hosts by Nidularia balachowskii in Israel.
We compared our observations of the host plant and development of Nidularia balachowskii in Israel to
We observed that Nidularia balachowskii is an oviparous, univoltine species in Israel, similar to
Some morphological characters of adult female Nidularia balachowskii are compared with those of Nidularia pulvinata, Kermes quercus and Kermes roboris (type species of Kermes) in Table 2, in order to evaluate the generic and family placement of the former species. All four species possess the following synapomorphic traits: three-segmented labium, bilocular pores on the venter, simple pores on the dorsum, quinquelocular pores surrounding the spiracles, and tubular ducts on the venter.These characters are some of the synapomorphic characters of kermesid adult females that have been described by
Within the genus Nidularia, adult female Nidularia balachowskii share with Nidularia pulvinata the following characters: (i) one-segmented antennae; (ii) absence of legs; (iii) absence of setae-pore clusters on venter; (iv) ventral position of anal ring, and (v) an anal ring with setae and cells, whereas the two Kermes species have: four, five or six-segmented antennae, and possess legs, setae-pore clusters on venter, anal ring placed on dorsum, and an anal ring without setae and cells. Comparing Nidularia balachowskii and Nidularia pulvinata, the most obvious distinguishing feature is the presence of quinquelocular pores on the spiracle peritreme of Nidularia pulvinata.
Morphological characters of adult females of Nidularia balachowskii, Nidularia pulvinata, Kermes quercus and Kermes roboris.
| Character | Nidularia balachowskii | Nidularia pulvinata | Kermes quercus | Kermes roboris |
|---|---|---|---|---|
| antennal segments | 1 | 1 | 4 | 5–6 |
| labium segments | 3 | 3 | 3 | 3 |
| locular pores on spiracle peritreme | absent | present | absent | absent |
| locular pores surrounding spiracles | present | present | present | present |
| legs | absent | absent | present | present |
| bilocular pores on venter | present | present | present | present |
| simple pores on dorsum | present | present | present | present |
| setae -pore clusters on venter | absent | absent | present | present |
| tubular ducts on venter | 1 type | 1 type | 2 types | 2 types |
| anal ring location | ventral | ventral | dorsal | dorsal |
| anal ring shape | 2 semi- circles | 2 semi-circles | circular, complete | 2 semi-circles |
| anal ring cells | present | present | absent | absent |
| anal ring setae | 3 pairs setae | 3 pairs setae | setae absent, rare with 2 setae | setae absent, rare with 2 setae |
The morphological characters of first-instar nymphs of Nearctic kermesids were summarized by
The first-instar nymphs of Nidularia balachowskii share the following characters with other Kermesidae species: (i) six-segmented antennae; (ii) three-segmented labium; (iii) simple pores forming longitudinal lines on the dorsum (iv) dorsal setae; (v) anal ring with cells and setae; (vi) microspines in rows on abdominal segments, and (vii) bilocular pores on venter. This last character is sometimes overlooked.
Nidularia balachowskii can be distinguished from other Kermesidae species by the form of its marginal setae. Nidularia balachowskii has sharply spinose, apically pointed and slightly curved setae, each 9–15 µm long. This differs from Nidularia pulvinata which has hair-like setae (
| 1 | Quinquelocular pores absent on venter of abdomen | Nidularia pulvinata (Planchon) |
| – | Quinquelocular pores present on venter of abdomen | 2 |
| 2 | Marginal setae sharply spinose, apically pointed and slightly curved; quinquelocular pores, six, on venter of thorax only | Nidularia balachowskii Bodenheimer |
| – | Marginal setae setose and somewhat conical at base; quinquelocular pores, eight, on venter of head and thorax | Nidularia japonica Kuwana |
| * Characters of Nidularia japonica based on illustrations and descriptions by | ||
The DNA-sequence data for Nidularia balachowskii, six other species of Palaearctic Kermesidae, and species representing six other Coccoidea families showed that gene fragments of both COI and 28S separated Nidularia balachowskii from other Coccoidea species, and clearly placed Nidularia balachowskii in the Kermesidae. This study confirms that Nidularia balachowskii is a distinct species, clearly distinguishable from other closely-related kermesid species.
This work was funded by Keren Kayemeth LeIsrael (Project # 131-1621-11) and the Israel Taxonomy Initiative. This manuscript is part of the PhD thesis for the senior author. We thank those who helped us obtain specimens and slide material for this study: Carl-Axel Gertsson (Murarevägen 13, SE-227 30 Lund, Sweden), Bora Kaydan (Yuzuncu Yil Universty, Agriculture Faculty, Plant Protection Dept. Kampus, Van, Turkey); Jon Martin (BMNH); Imre Foldi and Daniele Matile-Ferrero (MNHN); Elzbieta Podsiadlo (Agricultural University of Warsaw, Department of Zoology, Ul. Ciszewskiego 8, 02-766 Warszawa, Poland). We also thank Adi Faigenboim, Ionit Iberkleid and Adi Kliot for phylogenetic analysis consultation and Alex Protasov for his photographic skills and technical support (Volcani Center, P.O. Box 6, Bet Dagan 50250, Israel). Collection permits for nature reserves were kindly provided by the Israel Nature and Parks Authority.